Key Points
Question
What is the potential upper bound of off-label use of erdafitinib in cancers with fibroblast growth factor receptor (FGFR) alterations?
Findings
In this cross-sectional study, an estimated 76.1% of patients with advanced cancer expressing FGFR2 or FGFR3 alterations could be eligible for off-label treatment with erdafitinib. A total of 29 completed studies evaluating FGFR-targeting drugs in 11 cancer types and 10 ongoing studies on erdafitinib for different oncological indications were identified.
Meaning
This study indicates that the potential for off-label use of FGFR inhibitors, such as erdafitinib, spans a number of cancer types and a large patient population.
This cross-sectional study estimates the potential upper bound of off-label use of erdafitinib to treat advanced cancer with fibroblast growth factor receptor (FGFR) gene alterations, compares it to the upper bound of on-label use in urothelial cancer, and reviews studies that may support off-label use.
Abstract
Importance
When a novel drug is granted accelerated approval, both its on-label and off-label uses must be taken into account.
Objectives
To estimate the potential upper bound of off-label use of erdafitinib to treat advanced cancer with fibroblast growth factor receptor gene (FGFR) alterations, compare it to the upper bound of on-label use in urothelial cancer, and to review studies that may support off-label use.
Design, Setting, and Participants
This cross-sectional study used frequency data on FGFR alterations by cancer type and the estimated number of deaths from all cancers for 2019 in the United States. Mortality statistics were used as surrogates for patients with advanced cancer. Analysis was conducted in May 2019.
Exposure
Percentage of patients with an FGFR2 or FGFR3 alteration.
Main Outcomes and Measures
Estimated number of patients with advanced cancer expressing an FGFR2 or FGFR3 alteration eligible for off-label use of erdafitinib by cancer type; number of studies investigating FGFR-targeting drugs for patients with cancer; and number of ongoing clinical trials on erdafitinib by cancer type.
Results
A total of 15 cancer types had reported FGFR alterations. Of 455 440 estimated patients who died of cancer in 2019, 17 019 (3.7%) were estimated to have FGFR2 or FGFR3 alterations. Of these patients, 12 955 (76.1%) could be eligible for off-label treatment with erdafitinib. A total of 29 completed studies evaluated FGFR-targeting drugs in 11 cancer types, and 10 ongoing studies are studying erdafitinib for different oncological indications.
Conclusions and Relevance
This study indicates that the potential for off-label use of FGFR inhibitors such as erdafitinib spans a number of cancer types and a large patient population. Systematic trials exploring off-label uses may be desirable for drugs that target clear, identifiable molecular alterations because this may be more efficient than off-label use in identifying clinical scenarios where the agent has activity.
Introduction
Erdafitinib was recently granted accelerated approval by the US Food and Drug Administration (FDA) for the treatment of patients with locally advanced or metastatic urothelial cancer with fibroblast growth factor receptor 2 (FGFR2) or FGFR3 gene mutations or fusions.1 Erdafitinib targets FGFR2 and FGFR3, receptors commonly expressed in metastatic urothelial cancer, and belongs to the more general class of tyrosine kinase inhibitors.2 It was the first FGFR-targeting drug approved by the FDA. The approval of erdafitinib was based on overall response rate (ORR) in 87 patients with FGFR2 and FGFR3 alterations from a single-group, phase 2, multicenter study.2,3 Among responders, median (interquartile range) duration of response was found to be 5.4 (4.2-6.9) months. The response rate varied considerably by alteration, with an ORR of 40.6% (26 of 64) for FGFR3 point mutations, 11.1% (2 of 18) for FGFR3 fusions, and 0% (0 of 6) for FGFR2 fusions.3
Urothelial cancer is not the only cancer type that harbors FGFR alterations, which may be found in breast cancer, non–small cell lung cancer, colorectal cancer, and endometrial cancer, among others.4 The availability of a drug targeting FGFR2 and FGFR3 alterations for 1 tumor type (ie, urothelial cancer) may encourage the off-label use in other types of cancers with these alterations. Patients with tumor types other than urothelial cancer already have access to erdafitinib through the expanded access program,5 and enthusiasm for precision therapies is high. Other studies have reported broad-based sequencing and off-label use of tyrosine kinase inhibitor paid for by insurers.6 Finally, empirical analyses show that molecularly targeted drugs are often recommended by expert panels for tumor types different from those that received approval.7
This study aimed to estimate the potential upper bound of off-label use of erdafitinib to treat other types of advanced cancer with FGFR alterations, determine an estimated ratio of off-label use to on-label use, and review studies that may support the benefit of off-label use.
Methods
Overview
In this cross-sectional study, we sought to estimate what percentage of FGFR2 and FGFR3 mutations and fusions were in approved vs unapproved tumor types for the drug erdafitinib. We also sought to document available, corroborative, or circumstantial evidence supporting the benefit of using erdafitinib to treat off-label tumor types.
Per Oregon Health and Science University human research protection program policy,8 this study did not require institutional review board approval as it did not involve personally identifiable data and all data are publicly available. This report followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Estimates
We extracted cancer-specific FGFR aberration frequency data by histology from Helsten et al.4 We obtained the estimated number of deaths from all cancers from the American Cancer Society: Cancer Facts and Figures 2019.9 Cancer types were included in the analysis if found in data sets from Helsten et al4 and Cancer Facts and Figures 2019.9 We used mortality statistics as a surrogate for incident presentation of advanced or metastatic cancer. To determine the upper-bound number of patients eligible for off-label use of erdafitinib by cancer type, the number of cancer deaths was multiplied by the percentage of patients who had an FGFR2 or FGFR3 mutation or fusion for each cancer type. This process was replicated for patients with any FGFR alteration. By determining the number of cancer patients in each cancer type with any FGFR alteration, we sought to offer a second, broader estimation of potential eligibility for off-label treatment with erdafitinib.
Off-label use was defined as any use of erdafitinib for cancer types other than urothelial cancer. We determined off-label eligibility specifically for FGFR2 and FGFR3 alterations because erdafitinib was approved for these alterations in urothelial cancer. Our methods were similar to prior analyses of the estimated, upper-bound effect of genome-guided therapies10 and immunotherapy checkpoint inhibitors11 in cancer medicine.
Studies Targeting FGFR Alterations in Other Cancer Types
To review studies that may be used to support off-label use of erdafitinib, we searched PubMed for studies investigating therapies targeting FGFR alterations in cancer types other than urothelial cancer. To search PubMed, we used the article type filters of case reports, clinical study, and clinical trial and searched the phrase FGFR with 1 of the following cancer types: carcinoma of unknown primary site, non–small cell lung cancer, pancreatic exocrine cancer, breast cancer, endometrial cancer, colorectal cancer, glioma, head and neck squamous cell cancer, gastric or gastroesophageal junction cancer, ovarian cancer, renal cell cancer, cholangiocarcinoma, all sarcomas, and melanoma. All studies investigating the use of an FGFR-targeting drug to treat cancer patients were included. Articles investigating mouse models or in vivo studies were excluded as well as articles assessing FGFR alterations in patients without investigating a FGFR-targeting treatment. Data collected included the title of study, study type (case report, case series, or phase 1, 2, or 3 trial), randomization, primary outcome, and number of participants.
For ongoing trials of erdafitinib, a search was made on ClinicalTrials.gov using the term erdafitinib. Results were filtered by excluding trials that were suspended, terminated, completed, or withdrawn. We also excluded studies in healthy patients and registered studies that did not have an intervention (eg, estimating eligibility). Searches of PubMed and ClinicalTrials.gov were made on June 6, 2019.
Statistical Analysis
We generated an estimate of the percentage of patients in the United States with cancer and FGFR alterations eligible for on-label and off-label treatment with erdafitinib by cancer type. All statistical analyses were conducted in Excel 2016 (Microsoft). The reviews of studies on targeting FGFR alterations and ongoing studies of erdafitinib were purely descriptive. Because all statistics are descriptive, no prespecified level of statistical significance was set. The study was conducted in May 2019.
Results
Estimation of Patients Eligible for Off-label Treatment With Erdafitinib, Based on FGFR2 and FGFR3 Alterations
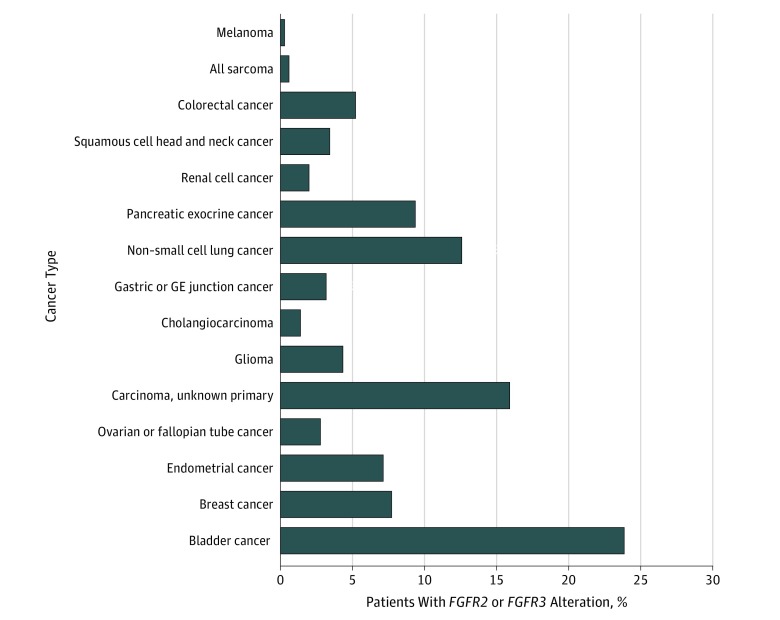
An estimated 455 440 individuals with cancer died in 2019. Of those, 17 019 (3.7%) were estimated to have either an FGFR2 or an FGFR3 alteration. A total of 15 cancer types, including urothelial cancer, had reported FGFR alterations (Table 1). Among the cancer types, urothelial cancer, carcinoma of unknown primary, non–small cell lung cancer, pancreatic exocrine cancer, and breast cancer had the highest number of patients with FGFR2 or FGFR3 alterations (urothelial cancer, 4064 of 17 670 patients [23.0%]; carcinoma of unknown primary sites, 2708 of 45 140 [6.0%]; non–small cell lung cancer 2140 of 142 670 [1.9%]; pancreatic exocrine cancer, 1601 of 45 750 [3.5%]; breast cancer, 1310 of 42 260 [3.1%]). We estimated that, of 17 019 patients with advanced cancer expressing FGFR2 or FGFR3 alterations, 12 955 (76.1%) could be eligible for off-label treatment with erdafitinib (Figure).
Table 1. Frequencies of FGFR Mutations and Fusions in the United States.
| Cancer Type | Estimated No. | ||
|---|---|---|---|
| Cancer Deaths, 2019 (N = 455 440) | Patients With FGFR Alteration (n = 35 536)a | Patients With FGFR2 or FGFR3 Alterations (n = 17 019)a | |
| Urothelial cancer | 17 670 | 5601 | 4064 |
| Carcinoma of unknown primary site | 45 140 | 3701 | 2708 |
| Non–small cell lung cancer | 142 670 | 7418 | 2140 |
| Pancreatic exocrine cancer | 45 750 | 2150 | 1601 |
| Breast cancer | 42 260 | 7353 | 1310 |
| Endometrial cancer | 12 160 | 1374 | 1216 |
| Colorectal cancer | 52 300 | 2301 | 889 |
| Glioma | 17 760 | 1350 | 746 |
| Head and neck squamous cell cancer | 21 720 | 999 | 586 |
| Gastric or GE junction cancer | 11 140 | 746 | 546 |
| Ovarian cancer | 13 980 | 1202 | 476 |
| Renal cell cancer | 14 770 | 679 | 340 |
| Cholangiocarcinoma | 3960 | 277 | 242 |
| Sarcoma, all | 6930 | 277 | 104 |
| Melanoma | 7230 | 108 | 51 |
Abbreviations: FGFR, fibroblast growth factor receptor gene; GE, gastroesophageal.
Based on percentages of FGFR alterations reported in Helsten et al.4
Figure. Estimated Number of Individuals With Fibroblast Growth Factor Receptor 2 (FGFR2) and FGFR3 Alterations Who Could Be Eligible for Off-label Use of Erdafitinib.
GE indicates gastroesophageal.
Estimation of Patients Eligible for Off-label Treatment With Erdafitinib Based on Any FGFR Alteration
We also estimated the percentage of patients eligible for treatment with erdafitinib among a pool of patients with any FGFR alteration (ie, not necessarily FGFR2 or FGFR3) (eFigure in the Supplement). Among 455 440 estimated patients, 35 536 (7.8%) had any FGFR alteration, and of these patients, 5601 (15.8%) had urothelial cancer. Therefore, if treatment were targeted at any FGFR alteration, an estimated 84.2% of the potential use of FGFR inhibitors, such as erdafitinib, would be off-label.
Studies Evaluating Erdafitinib and Other FGFR-Targeting Drugs in Various Cancer Types
We found 29 completed studies evaluating FGFR-targeting drugs in 11 of 14 cancer types analyzed in the off-label estimations (Table 2).12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38 There were 8 (27.6%) case reports, 1 (3.4%) case series, 9 (31.0%) phase 1 studies, 2 (6.9%) phase 1/2 studies, and 9 (31.0%) phase 2 studies. While 2 phase 2 trials (22.2%) were randomized, the rest were noncomparator trials. All phase 1 studies had primary outcomes of either safety or dosage. Phase 2 trials evaluated ORR (5 studies [55.5%]), progression-free survival (4 studies [44.4%]), or event-free survival (1 study [11.1%]) as their primary outcome.
Table 2. Studies Evaluating FGFR-Targeting Drugs in Other Cancer Types.
| Cancer Type | Study Title | Study Type | Patients, No. | Primary Outcome |
|---|---|---|---|---|
| Breast cancer | Pazopanib Sensitivity in a Patient With Breast Cancer and FGFR1 Amplification12 | Case report | 1 | NA |
| Breast cancer | Phase II, Randomized, Placebo-Controlled Study of Dovitinib in Combination With Fulvestrant in Postmenopausal Patients With HR+, HER2- Breast Cancer That Had Progressed During or After Prior Endocrine Therapy13 | Phase 2, randomized | 97 | PFS |
| Breast cancer | Phase I/IIa Study Evaluating the Safety, Efficacy, Pharmacokinetics, and Pharmacodynamics of Lucitanib in Advanced Solid Tumors14 | Phase 1/2, nonrandomized | 76 | Dosage |
| Breast cancer | Targeting FGFR With Dovitinib (TKI258): Preclinical and Clinical Data in Breast Cancer15 | Phase 2 nonrandomized | 81 | ORR |
| Non–small cell lung cancer | A Phase 1 Study of LY2874455, an Oral Selective Pan-FGFR Inhibitor, in Patients With Advanced Cancer16 | Phase 1, nonrandomized | 36 | Dosage |
| Non–small cell lung cancer | Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Kinase Inhibitor, in Patients With Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I, Dose-Escalation and Dose-Expansion Study17 | Phase 1, nonrandomized | 132 | Dosage |
| Non–small cell lung cancer | Efficacy and Safety of Dovitinib in Pretreated Patients With Advanced Squamous Non–Small Cell Lung Cancer With FGFR1 Amplification: A Single-Arm, Phase 2 Study.18 | Phase 2, nonrandomized | 26 | ORR |
| Endometrial cancer | Comprehensive Genomic Profiling Aids in Treatment of a Metastatic Endometrial Cancer19 | Case report | 1 | NA |
| Endometrial cancer | Phase I Dose-Escalation Study of JNJ-42756493, an Oral Pan–Fibroblast Growth Factor Receptor Inhibitor, in Patients With Advanced Solid Tumors20 | Phase 1, nonrandomized | 65 | Safety, dosage |
| Endometrial cancer | A Phase II Evaluation of Nintedanib (BIBF-1120) in the Treatment of Recurrent or Persistent Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study21 | Phase 2, nonrandomized | 32 | EFS |
| Endometrial cancer | A Phase II Trial of Brivanib in Recurrent or Persistent Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study.22 | Phase 2, nonrandomized | 43 | PFS, ORR |
| Colorectal cancer | A Phase I Dose–Escalation Study of Regorafenib (BAY 73–4506), an Inhibitor of Oncogenic, Angiogenic, and Stromal Kinases, in Patients With Advanced Solid Tumors23 | Phase 1, nonrandomized | 16 | Dosage |
| Glioma | Phase I Trial of Dovitinib (TKI258) in Recurrent Glioblastoma24 | Phase 1, nonrandomized | 12 | Safety |
| Glioma | Detection, Characterization, and Inhibition of FGFR–TACC Fusions in IDH Wild-Type Glioma25 | Case report | 2 | NA |
| Glioma | Phase II Trial of Triple Tyrosine Kinase Receptor Inhibitor Nintedanib in Recurrent High-Grade Gliomas26 | Phase 2, nonrandomized | 22 | PFS |
| Glioma | Phase II Open-Label Study of Nintedanib in Patients With Recurrent Glioblastoma Multiforme27 | Phase 2, nonrandomized | 25 | ORR |
| Gastric or GE junction cancer | Gastric Perforation Related to Concurrent Use of Nintedanib and Ramucirumab28 | Case report | 1 | NA |
| Gastric or GE junction cancer | A Randomized, Open-Label Study of the Efficacy and Safety of AZD4547 Monotherapy vs Paclitaxel for the Treatment of Advanced Gastric Adenocarcinoma With FGFR2 Polysomy or Gene Amplification29 | Phase 2, randomized | 71 | PFS |
| Gastric or GE junction cancer | A Phase 1 Study of LY2874455, an Oral Selective Pan-FGFR Inhibitor, in Patients With Advanced Cancer16 | Phase 1, nonrandomized | 36 | Dosage |
| Gastric or GE junction cancer | Acquired Resistance to LY2874455 in FGFR2-Amplified Gastric Cancer Through an Emergence of Novel FGFR2-ACSL5 Fusion30 | Case report | 1 | NA |
| Ovarian cancer | Tumors With AKT1E17K Mutations Are Rational Targets for Single Agent or Combination Therapy With AKT Inhibitors31 | Phase 1, nonrandomized | 41 | Safety |
| Renal cell cancer | Phase II Results of Dovitinib (TKI258) in Patients With Metastatic Renal Cell Cancer32 | Phase 2, nonrandomized | 67 | ORR |
| Renal cell cancer | Phase I Study of Dovitinib (TKI258), an Oral FGFR, VEGFR, and PDGFR Inhibitor, in Advanced or Metastatic Renal Cell Carcinoma33 | Phase 1, nonrandomized | 20 | Dosage |
| Renal cell cancer | A Phase I Dose-Escalation Study of Regorafenib (BAY 73-4506), an Inhibitor of Oncogenic, Angiogenic, and Stromal Kinases, in Patients With Advanced Solid Tumors23 | Phase 1, nonrandomized | 53 | Dosage |
| Renal cell cancer | FGFR Inhibitor Induced Peripheral Neuropathy in Patients With Advanced RCC34 | Case report | 1 | NA |
| Cholangiocarcinoma | Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients With FGFR2 Fusion-Positive Cholangiocarcinoma35 | Case series | 3 | NA |
| Sarcoma, all | Clinical Benefit of Pazopanib in a Patient With Metastatic Chondrosarcoma: A Case Report and Review of the Literature36 | Case report | 1 | NA |
| Melanoma | Calcinosis Cutis Dermatologic Toxicity Associated With Fibroblast Growth Factor Receptor Inhibitor for the Treatment of Wilms Tumor37 | Case report | 1 | NA |
| Melanoma | Phase I/II and Pharmacodynamic Study of Dovitinib (TKI258), an Inhibitor of Fibroblast Growth Factor Receptors and VEGF Receptors, in Patients With Advanced Melanoma38 | Phase 1/2, nonrandomized | 47 | Safety, ORR |
Abbreviations: AKT, protein kinase B; HER, human epidermal growth factor; HR, hormone receptor; IDH, isocitrate dehydrogenase; EFS, event-free survival; FGFR, fibroblast growth factor receptor; GE, gastroesophageal; NA, not applicable; ORR, overall response rate; PDGFR, platelet-derived growth factor receptor; PFS, progression-free survival; RCC, renal cell cancer; TACC, transforming acidic coiled-coil; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
There were 10 ongoing studies of erdafitinib being used for oncological indications registered on ClinicalTrials.gov, 2 (20.0%) of which included patients with urothelial cancer (eTable in the Supplement). There were 2 (20.0%) phase 1 studies, 2 (20.0%) phase 1/2 studies, 5 (50.0%) phase 2 studies, and 1 (10.0%) phase 3 study. One study (10.0%) was evaluating overall survival as its primary outcome; the rest were evaluating ORR (7 studies [70.0%]) or safety (3 studies [30.0%]). Two of the 10 trials (20.0%) were randomized.
Discussion
From our estimates, the number of patients with tumors exhibiting FGFR2 or FGFR3 alterations who may be potentially eligible for off-label treatment with an FGFR inhibitor is 3 times that of those eligible for on-label treatment. If interpretation of off-label use is understood more broadly to cover patients with any FGFR alteration, potential eligibility for off-label use would grow to be 5 times greater than on-label use. Alterations in FGFR are being studied in a number of cancers where these alterations are prevalent, and ORRs similar to that of erdafitinib in urothelial cancer may further encourage off-label treatments in other cancer types. Off-label use of targeted therapies is widely practiced in oncology.7,39 Erdafitinib was provisionally approved based on a surrogate outcome in a nonrandomized trial, and proof of clinical benefit will only be known after a confirmatory randomized trial investigating overall survival is conducted. The trial which led to the approval of erdafitinib found that adverse reactions of grade 3 or higher were reported by 67% of participants, and 46% were considered by investigators to be related to treatment.2 The FDA determined that the interim surrogate outcomes and toxic effects of erdafitinib were appropriate for patients with advanced urothelial cancer who had an FGFR2 or FGFR3 mutation or fusion. However, by approving this drug for this indication, the FDA has inevitably permitted off-label use of erdafitinib in many cancer types and among a considerably larger patient population.
Strengths and Limitations
There are strengths and limitations in our analysis. One strength is that we sought to capture the potential on-label and off-label use of a novel genome-targeted drug entering the US marketplace, and we estimated the ratio of potential on-label to off-label use. To our knowledge, this is the first article that seeks to do this at the time of product launch. Moreover, we compiled data that clinicians could use to justify off-label use (Table 2). We note that these studies are largely exploratory in nature and reliant on small uncontrolled studies, which are generally considered to be at the bottom of the hierarchy of medical evidence.40
There are also limitations. We determined cancer-specific FGFR aberration frequencies through an analysis that looked at next-generation sequencing from 1 company,4 which may not be wholly representative of population-level frequencies. We used cancer deaths to estimate cases of metastatic disease in each cancer type, which is an imperfect surrogate. Cancer deaths are not all because of metastatic disease, and as patients with metastatic disease live longer, the incidence of metastatic cancer cases outpaces the incidence of cancer deaths. Unfortunately, to our knowledge, there are currently no data on metastatic disease in the United States, as staging data are only recorded at diagnosis. Nevertheless, we found that cancer death data were the best available option to explore our study objective. A study using the Cancer Registry of Norway41 found that the majority of cancer deaths were because of metastatic disease, and the recorded numbers are likely underestimated because of underreporting of metachronous metastases. If data on metastatic status are collected in the US population in the future, we encourage researchers exploring this topic to use incidence of metastatic cancer instead.
We have almost certainly overestimated the number of cases of metastatic disease and, within that number, the number of patients who would be treated with an off-label drug. This assessment was not conducted to accurately approximate the number of patients who will be treated with erdafitinib but instead to determine the proportion of patients eligible for off-label use and to highlight the fact that the consequences of this accelerated approval reach far beyond the patient population it was intended to treat. Off-label drug use is practiced across almost all cancer types, most likely prescribed to metastatic cancer patients in a later line because of unapproved indication for specific tumors.42 Completed and ongoing trials on erdafitinib and other FGFR-targeting drugs in various cancers exemplify the interest around this type of targeted therapy, and the fact that erdafitinib is the first FGFR-targeting drug to be approved by the FDA43 makes it susceptible to off-label use for patients with FGFR alterations. Enthusiasm for precision oncology may fuel this extrapolation.44,45,46,47
Conclusions
This study found that the potential for off-label use of FGFR inhibitors such as erdafitinib spans a number of cancer types and a large patient population. Off-label use may be 3-fold higher than on-label use, based on population cancer statistics and the frequency of molecular alterations. Clinicians interested in off-label use may easily find case reports or series that may provide exploratory or circumstantial support for those choices. Because it may be tempting and plausible to use FGFR inhibitors for nonapproved uses, our analysis suggests that the size of this market share may be several times larger than the approved indication. Systematic trials to explore off-label uses may be desirable for drugs that target clear, identifiable molecular alterations because this may be more efficient than off-label use in identifying clinical scenarios where this agent has activity.
eFigure. Estimated Number of Individuals With Any FGFR Alteration and Percentage of Eligibility That Is Attributed to Approved or Off-label Use of FGFR Inhibitors
eTable. Ongoing Studies of Erdafitinib Registered Through ClinicalTrials.gov
References
- 1.US Food and Drug Administration FDA grants accelerated approval to erdafitinib for metastatic urothelial carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-erdafitinib-metastatic-urothelial-carcinoma. Accessed October 16, 2019.
- 2.Loriot Y, Necchi A, Park SH, et al. ; BLC2001 Study Group . Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):-. doi: 10.1056/NEJMoa1817323 [DOI] [PubMed] [Google Scholar]
- 3.Janssen Pharmaceutical Companies Balversa (erdafitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212018s000lbl.pdf. Accessed June 4, 2019.
- 4.Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016;22(1):259-267. doi: 10.1158/1078-0432.CCR-14-3212 [DOI] [PubMed] [Google Scholar]
- 5.Janssen Scientific Affairs. Expanded access program (EAP) for participants with advanced cancers and fibroblast growth factor receptor (FGFR) genetic alterations who have exhausted all treatment options. https://ClinicalTrials.gov/show/NCT03825484. Accessed October 16, 2019.
- 6.Sicklick JK, Kato S, Okamura R, et al. . Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med. 2019;25(5):744-750. doi: 10.1038/s41591-019-0407-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner J, Marquart J, Ruby J, et al. . Frequency and level of evidence used in recommendations by the National Comprehensive Cancer Network guidelines beyond approvals of the US Food and Drug Administration: retrospective observational study. BMJ. 2018;360:k668. doi: 10.1136/bmj.k668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oregon Health and Science University Human Research Protection Program Investigator Manual. https://ohsu.ellucid.com/documents/view/15225/?security=184610e97bae246351430a86e3f87992ad088dfc. Accessed October 31, 2019. [Google Scholar]
- 9.American Cancer Society Cancer Facts and Figures 2019. Atlanta, GA: American Cancer Society; 2019. [Google Scholar]
- 10.Marquart J, Chen EY, Prasad V. Estimation of the percentage of US patients with cancer who benefit from genome-driven oncology. JAMA Oncol. 2018;4(8):1093-1098. doi: 10.1001/jamaoncol.2018.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535-e192535. doi: 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng FT-F, Ou-Yang F, Lapke N, et al. . Pazopanib sensitivity in a patient with breast cancer and FGFR1 amplification. J Natl Compr Canc Netw. 2017;15(12):1456-1459. doi: 10.6004/jnccn.2017.7030 [DOI] [PubMed] [Google Scholar]
- 13.Musolino A, Campone M, Neven P, et al. . Phase II, randomized, placebo-controlled study of dovitinib in combination with fulvestrant in postmenopausal patients with HR+, HER2- breast cancer that had progressed during or after prior endocrine therapy. Breast Cancer Res. 2017;19(1):18-18. doi: 10.1186/s13058-017-0807-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soria J-C, DeBraud F, Bahleda R, et al. . Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol. 2014;25(11):2244-2251. doi: 10.1093/annonc/mdu390 [DOI] [PubMed] [Google Scholar]
- 15.André F, Bachelot T, Campone M, et al. . Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res. 2013;19(13):3693-3702. doi: 10.1158/1078-0432.CCR-13-0190 [DOI] [PubMed] [Google Scholar]
- 16.Michael M, Bang Y-J, Park YS, et al. . A phase 1 study of LY2874455, an oral selective pan-FGFR inhibitor, in patients with advanced cancer. Target Oncol. 2017;12(4):463-474. doi: 10.1007/s11523-017-0502-9 [DOI] [PubMed] [Google Scholar]
- 17.Nogova L, Sequist LV, Perez Garcia JM, et al. . Evaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol. 2017;35(2):157-165. doi: 10.1200/JCO.2016.67.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim SH, Sun J-M, Choi Y-L, et al. . Efficacy and safety of dovitinib in pretreated patients with advanced squamous non-small cell lung cancer with FGFR1 amplification: a single-arm, phase 2 study. Cancer. 2016;122(19):3024-3031. doi: 10.1002/cncr.30135 [DOI] [PubMed] [Google Scholar]
- 19.Dhami J, Hirshfield KM, Ganesan S, et al. . Comprehensive genomic profiling aids in treatment of a metastatic endometrial cancer. Cold Spring Harb Mol Case Stud. 2018;4(2):a002089. doi: 10.1101/mcs.a002089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabernero J, Bahleda R, Dienstmann R, et al. . Phase I dose-escalation study of JNJ-42756493, an oral pan–fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2015;33(30):3401-3408. doi: 10.1200/JCO.2014.60.7341 [DOI] [PubMed] [Google Scholar]
- 21.Dizon DS, Sill MW, Schilder JM, et al. . A phase II evaluation of nintedanib (BIBF-1120) in the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014;135(3):441-445. doi: 10.1016/j.ygyno.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell MA, Sill MW, Goodfellow PJ, et al. . A phase II trial of brivanib in recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014;135(1):38-43. doi: 10.1016/j.ygyno.2014.07.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mross K, Frost A, Steinbild S, et al. . A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18(9):2658-2667. doi: 10.1158/1078-0432.CCR-11-1900 [DOI] [PubMed] [Google Scholar]
- 24.Schäfer N, Gielen GH, Kebir S, et al. . Phase I trial of dovitinib (TKI258) in recurrent glioblastoma. J Cancer Res Clin Oncol. 2016;142(7):1581-1589. doi: 10.1007/s00432-016-2161-0 [DOI] [PubMed] [Google Scholar]
- 25.Di Stefano AL, Fucci A, Frattini V, et al. . Detection, characterization, and inhibition of FGFR–TACC fusions in IDH wild-type glioma. Clin Cancer Res. 2015;21(14):3307-3317. doi: 10.1158/1078-0432.CCR-14-2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norden AD, Schiff D, Ahluwalia MS, et al. . Phase II trial of triple tyrosine kinase receptor inhibitor nintedanib in recurrent high-grade gliomas. J Neurooncol. 2015;121(2):297-302. doi: 10.1007/s11060-014-1631-y [DOI] [PubMed] [Google Scholar]
- 27.Muhic A, Poulsen HS, Sorensen M, Grunnet K, Lassen U. Phase II open-label study of nintedanib in patients with recurrent glioblastoma multiforme. J Neurooncol. 2013;111(2):205-212. doi: 10.1007/s11060-012-1009-y [DOI] [PubMed] [Google Scholar]
- 28.Takahashi S, Murata S, Yoshino Y, Kobayashi Y, Nakamura M. Gastric perforation related to concurrent use of nintedanib and ramucirumab. Respirol Case Rep. 2018;7(1):e00383-e00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Cutsem E, Bang Y-J, Mansoor W, et al. . A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann Oncol. 2017;28(6):1316-1324. doi: 10.1093/annonc/mdx107 [DOI] [PubMed] [Google Scholar]
- 30.Kim SY, Ahn T, Bang H, et al. . Acquired resistance to LY2874455 in FGFR2-amplified gastric cancer through an emergence of novel FGFR2-ACSL5 fusion. Oncotarget. 2017;8(9):15014-15022. doi: 10.18632/oncotarget.14788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies BR, Guan N, Logie A, et al. . Tumors with AKT1E17K mutations are rational targets for single agent or combination therapy with AKT inhibitors. Mol Cancer Ther. 2015;14(11):2441-2451. doi: 10.1158/1535-7163.MCT-15-0230 [DOI] [PubMed] [Google Scholar]
- 32.Escudier B, Grünwald V, Ravaud A, et al. . Phase II results of dovitinib (TKI258) in patients with metastatic renal cell cancer. Clin Cancer Res. 2014;20(11):3012-3022. doi: 10.1158/1078-0432.CCR-13-3006 [DOI] [PubMed] [Google Scholar]
- 33.Angevin E, Lopez-Martin JA, Lin C-C, et al. . Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin Cancer Res. 2013;19(5):1257-1268. doi: 10.1158/1078-0432.CCR-12-2885 [DOI] [PubMed] [Google Scholar]
- 34.Loriot Y, Massard C, Angevin E, Lambotte O, Escudier B, Soria J-C. FGFR inhibitor induced peripheral neuropathy in patients with advanced RCC. Ann Oncol. 2010;21(7):1559-1560. doi: 10.1093/annonc/mdq237 [DOI] [PubMed] [Google Scholar]
- 35.Goyal L, Saha SK, Liu LY, et al. . Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017;7(3):252-263. doi: 10.1158/2159-8290.CD-16-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsavaris O, Economopoulou P, Kotsantis I, et al. . Clinical benefit of pazopanib in a patient with metastatic chondrosarcoma: a case report and review of the literature. Front Oncol. 2018;8:45-45. doi: 10.3389/fonc.2018.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arudra K, Patel R, Tetzlaff MT, et al. . Calcinosis cutis dermatologic toxicity associated with fibroblast growth factor receptor inhibitor for the treatment of Wilms tumor. J Cutan Pathol. 2018;45(10):786-790. doi: 10.1111/cup.13319 [DOI] [PubMed] [Google Scholar]
- 38.Kim KB, Chesney J, Robinson D, Gardner H, Shi MM, Kirkwood JM. Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma. Clin Cancer Res. 2011;17(23):7451-7461. doi: 10.1158/1078-0432.CCR-11-1747 [DOI] [PubMed] [Google Scholar]
- 39.Levêque D. Off-label use of targeted therapies in oncology. World J Clin Oncol. 2016;7(2):253-257. doi: 10.5306/wjco.v7.i2.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95(2)(suppl):2S-4S. doi: 10.1378/chest.95.2_Supplement.2S [DOI] [PubMed] [Google Scholar]
- 41.Dillekås H, Rogers MS, Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019;8(12):5574-5576. doi: 10.1002/cam4.2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saiyed MM, Ong PS, Chew L. Off-label drug use in oncology: a systematic review of literature. J Clin Pharm Ther. 2017;42(3):251-258. doi: 10.1111/jcpt.12507 [DOI] [PubMed] [Google Scholar]
- 43.Han DH. First-in-class, targeted therapy approved for metastatic bladder cancer. https://www.cancertherapyadvisor.com/home/cancer-topics/bladder-cancer/balversa-fda-approval-bladder-cancer-treamtment-risk/. Accessed October 16, 2019.
- 44.Prasad V. Perspective: the precision-oncology illusion. Nature. 2016;537(7619):S63. doi: 10.1038/537S63a [DOI] [PubMed] [Google Scholar]
- 45.Tannock IF, Hickman JA. Limits to personalized cancer medicine. N Engl J Med. 2016;375(13):1289-1294. doi: 10.1056/NEJMsb1607705 [DOI] [PubMed] [Google Scholar]
- 46.Fojo T. Precision oncology: a strategy we were not ready to deploy. Semin Oncol. 2016;43(1):9-12. doi: 10.1053/j.seminoncol.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 47.Prasad V, Fojo T, Brada M. Precision oncology: origins, optimism, and potential. Lancet Oncol. 2016;17(2):e81-e86. doi: 10.1016/S1470-2045(15)00620-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Estimated Number of Individuals With Any FGFR Alteration and Percentage of Eligibility That Is Attributed to Approved or Off-label Use of FGFR Inhibitors
eTable. Ongoing Studies of Erdafitinib Registered Through ClinicalTrials.gov