Abstract
Background
Previous studies indicated that long noncoding RNAs (lncRNAs) played vital roles in the development and progression of hepatocellular carcinoma (HCC). Recently, downregulation of lncRNA RP5‑833A20.1 has been observed in HCC tissues. However, the underlying mechanism by which RP5‑833A20.1 regulates the proliferation and apoptosis in HCC has not been investigated. Thus, this study aimed to investigate the role of RP5‑833A20.1 in the progression of HCC.
Methods
The levels of RP5‑833A20.1 in 30 pairs of HCC tissues and adjacent normal tissues were detected by RT-qPCR. In addition, the effects of RP5‑833A20.1 on cell proliferation, apoptosis and invasion were evaluated by CCK-8, flow cytometric, transwell assays, respectively. Meanwhile, the dual-luciferase reporter system assay was used to explore the interaction of RP5‑833A20.1 and miR-18a-5p in HCC.
Results
The level of RP5‑833A20.1 was significantly downregulated in HCC tissues and HCC cell lines. Downregulation of RP5‑833A20.1 markedly promoted the proliferation and invasion of Bel-7402 cells. In addition, overexpression of RP5‑833A20.1 notably inhibited the proliferation and invasion of Huh7 cells. Moreover, overexpression of RP5‑833A20.1 obviously induced the apoptosis of Huh7 cells via increasing the levels of Bax and active caspase 3, and decreasing the levels of Bcl-2, p-Akt and p-ERK. Meanwhile, in vivo experiments performed also indicated that overexpression of RP5-833A20.1 could inhibit the tumorigenesis of subcutaneous Huh7 xenograft in nude mice. Furthermore, bioinformatics and luciferase reporter assay identified that RP5-833A20.1 functioned as a competing endogenous RNA (ceRNA) for miR-18a-5p in HCC.
Conclusion
In this study, we found that RP5‑833A20.1 was downregulated in HCC tissues. In addition, RP5-833A20.1 could suppress the tumorigenesis in HCC through inhibiting Akt/ERK pathway by acting as a ceRNA for miR-18a-5p. Therefore, RP5-833A20.1 might be a valuable and potential biomarker and therapeutic target for the treatment of HCC.
Keywords: hepatocellular carcinoma, long noncoding RNA RP5‑833A20.1, microRNA-18a-5p, proliferation
Introduction
Hepatocellular carcinoma (HCC) is a malignant tumor, and one of the most common causes of cancer-related death worldwide.1,2 HCC is characterized by a high degree of aggressiveness and rapid growth.3 HBV or HCV infection, alcoholic or non-alcoholic steatohepatitis are the leading causes of HCC.4 At present, surgical resection and liver transplantation are the main radical therapies for HCC. However, most of the patients with HCC have had cirrhosis and distant metastasis when they were diagnosed.5 In addition, the five-year recurrence is up to 70% after surgical resection.6 Although several novel therapies were used to treat HCC in recent years, the prognosis of HCC has obviously not improved.7,8 Therefore, it is urgently to search novel therapeutic target for HCC.
Long noncoding RNAs (lncRNAs) are acknowledged as non-protein coding RNAs with more than 200 nucleotides in length.9 It has been shown that LncRNAs exert gene transcription regulatory function by epigenetic regulatory mechanism.10 In addition, lncRNAs play an important role in regulating proliferation, invasion and metastasis, angiogenesis, and viability in different tumors.11,12 Moreover, evidence showed that lncRNAs expression profiling could facilitate the development and progression of cancers, such as colorectal cancer, hepatocellular carcinoma, bladder cancer, suggesting that lncRNAs may act as effective therapeutic targets for the treatment of human cancers.13–15
Nuclear factor IA (NFIA) is a member of the NFI family, which is essential in astrocyte differentiation and gliogenesis in the central nervous system.16,17 LncRNA RP5‑833A20.1 is located in intron 2 of the NFIA gene.17 Previous studies indicated that RP5‑833A20.1 participated in the regulation of glioma and atherosclerosis.17,18 However, the role of lncRNA RP5‑833A20.1 in HCC remains unclear. The present study aimed to examine the role of RP5‑833A20.1 in the progression of HCC.
Materials And Methods
Patients
A total of 30 pairs of HCC tissues and corresponding adjacent non-cancerous tissues were obtained from patients with HCC. None of the patients received radiotherapy, biotherapy or systemic chemotherapy. The primary clinical and pathological features (including age, gender, tumor volume, distant metastasis, TNM stage) were obtained from the medical records. Written informed consent was obtained from all the patients. The study was approved by the Ethical Research Committee of Affiliated Hospital of Guizhou Medical University.
Cell Culture
The human immortalized liver cell line MIHA, and human liver tumor cell lines (SMMC-7221, Hun7 and Bel-7402) were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in Dulbecco modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 2 mM L-glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin at 37°C in an incubator with 5% CO2.
Quantitative Real‐time PCR (qRT-PCR)
Total RNA was extracted from the HCC tissues and HCC cell lines by TRIzol reagent (Takara Bio, Otsu, Japan) following the manufacturer’s instructions. First-strand complementary DNA (cDNA) was synthesized using 1 μg of total RNA and the PrimeScript RT reagent Kit (Promega, Madison, WI, USA). qRT-PCR was applied to measure the relative RNA level using the TaqMan Power SYBR Green PCR Mix kit (Thermo Fisher Scientific, Waltham, MA, USA). qPCR was performed using Applied Biosystems 7500 Real Time PCR System (Applied Biosystems, Foster City, CA, USA), under the following conditions: 10 min 95°C, followed by 40 cycles of 10 s 95°C, 30 s 60°C, and 30 72°C. The level of RP5‑833A20.1 was normalized to the level of h-actin using the 2−ΔΔCT method. H-actin, forward, 5ʹ-GTCCACCGCAAATGCTTCTA-3ʹ; reverse, 5ʹ-TGCTGTCACCTTCACCGTTC-3ʹ. RP5‑833A20.1, forward, 5ʹ- TCTCCAACAGGGTGAGGAAAC-3ʹ; reverse, 5ʹ- TCTGAGATGCTGGGTGCAAG-3ʹ.
Lentiviral Construction And Cell Infection
The RP5-833A20.1-shRNA1 and RP5-833A20.1-shRNA2 plasmids were obtained from Thermo Fisher Scientific. The sequences of targeting shRNA: RP5-833A20.1-shRNA1, 5ʹ-GCTCCACAGCATCTTTCTTAT-3ʹ; RP5-833A20.1-shRNA1, 5ʹ-GCACTGGGTTAGTTTCATAGA-3ʹ. The RP5-833A20.1 sequence was synthesized by GenePharma (Shanghai, China), and then sub-cloned into the pWPXL (lentiviral expression vector). The RP5-833A20.1-shRNA1, RP5-833A20.1-shRNA2, and pWPXL-RP5-833A20.1 plasmid were infected into 293T cells, respectively. After infection for 72hrs, the supernatant was collected, which was containing the retroviral particles.
Huh7 cells (4 × 105 cells/well) were seeded into cell plates (60 mm) overnight at 37°C. Cells were then infected with lenti-vector (NC), or lenti-RP5-833A20.1 (RP5-833A20.1-OE) supernatant, respectively, for 24hrs. Later on, virus-containing medium was replaced with fresh medium. Cell was then treated with puromycin (2.5 μg/mL, Thermo Fisher Scientific) to select stable Huh7 cells for another 48hrs.
Bel-7402 cells (4 × 105 cells/well) were seeded into cell plates (60 mm) overnight at 37°C. Cells were then infected with NC, RP5-833A20.1-shRNA1 or RP5-833A20.1-shRNA2 supernatant, respectively, for 24hrs. Later on, virus-containing medium was replaced with fresh medium. Cells were then treated with puromycin (2.5 μg/mL, Thermo Fisher Scientific) to select stable Bel-7402 cells for another 48hrs. QRT-PCR was used to determine the level of RP5-833A20.1 in HCC cells.
CCK-8 Assay
Cell viability was measured using cell counting kit-8 (CCK-8; KeyGen BioTECH) in accordance with the manufacturer’s instruction. Huh7 cells or Bel-7402 cells (5 × 103 cells per well) were seeded onto 96-well plates, respectively, and incubated at 37°C overnight. Then, cells were infected with RP5-833A20.1-OE or RP5-833A20.1-siRNA2 for 24, 48 and 72hrs, respectively. After that, 10 μL CCK-8 reagent was added into each well and incubated for another 2hrs. The optical density (OD) of each well was detected at 450 nm by using a microplate reader (Bio-Tek Instruments Inc., Winooski, VT, USA).
Immunofluorescence Staining Assay
Huh7 cells or Bel-7402 cells (5 × 103 cells per well) were plated onto 96-well plates at 37°C overnight. Then, cells were infected with RP5-833A20.1-OE or RP5-833A20.1-siRNA2 for 48hrs, respectively. After that, the cells were fixed with 4% paraformaldehyde for 20 mins. After that, the cells were incubated with primary antibodies anti-Ki67 (1:100, Abcam) at 4°C overnight, followed by incubation with secondary antibodies (1:1000, Abcam) at 37°C for 1hr. Nuclei were counterstained with DAPI for 30 mins. Finally, the cells were observed using a laser confocal microscope (Olympus CX23 Tokyo, Japan).
Cell Apoptosis Assay
The apoptosis of HCC cells was assessed using an Annexin V-FITC/PI Apoptosis kit (BioVision, Inc. Milpitas, CA, USA) according to the protocol. Briefly, HCC cells were collected, washed twice with PBS, and then stained with 5 μL Annexin V/propidium iodide (PI) solution for 30 mins in the dark at room temperature. Then, the number of apoptotic cells was analyzed by flow cytometer (BD Biosciences, Mountain View, CA, USA).
Cell Invasion Assay
The invasion of HCC cells was detected using 24-well transwell chambers (Corning New York, NY, USA) coated with Matrigel (BD Biosciences, NJ, USA). Huh7 cells (3×104 cells) were re-suspended in DMEM medium (200 μL) without 10% FBS and added into the upper compartment of the chamber. DMEM medium containing 10% FBS was applied to the lower compartment of the chamber to induce cell invasion. Twenty-four hours later, cells attached to the lower surface of the chamber were stained with 0.2% crystal violet. Then, cell images were photographed using a laser confocal microscope (Olympus). Five randomly selected fields of each membrane were counted.
Western Blot
BCA Protein Assay kit (GeneRay, Shanghai, China) was applied to determine the total protein concentration. The proteins in equal concentration were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto polyvinylidene difluoride (PVDF) membranes. After that, PVDF membranes were blocked with 5% nonfat milk for 1hr and then incubated with the primary antibodies overnight at 4°C. Anti-Bax (1:1000), anti-active caspase 3 (1:1000), anti-p-Akt (1:1000), anti-p-ERK (1:1000), anti-Bcl-2 (1:1000), anti-β-actin (1:1000). Antibodies were purchased from Abcam Cambridge (MA, USA). Later on, the membranes were washed three times with TBST and incubated with horseradish peroxidase-conjugated secondary antibody (1:5000, Abcam) at room temperature for 1hr. Finally, the membranes were detected using an Enhanced Chemiluminescence Detection System (Thermo Scientific, Carlsbad, CA, USA). β-Actin was used as an internal control.
Luciferase Reporter Assay
The putative miR-18a-5p target binding sequence in RP5-833A20.1 (GCACCUU) and its mutant of the binding sites (AGUUAGG) were separately synthesized and sub-cloned into the luciferase gene to establish two reporter plasmids: the wild-type (WT) and mutated-type (MT) reporter plasmids, respectively. WT-RP5-833A20.1 or MT-RP5-833A20.1 plasmid was co-infected with miR-18a-5p mimics and control, respectively, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Forty-eight hours later, the luciferase activities were detected using a Dual-Luciferase Reporter Assay System (Promega Madison, WI) according to the manufacturer’s instructions. The relative luciferase activity was normalized to renilla luciferase activity.
Animal Study
Male BALB/c nude mice (n = 9, 6–8 weeks old) were purchased from the Shanghai SLAC Animal Center (Shanghai, China). All animal experiments were approved by the Institutional Animal Care and Use Committee at Affiliated Hospital of Guizhou Medical University. Animals were randomized into three groups: blank, NC and RP5-833A20.1 group. RP5-833A20.1-OE Huh7 cells (5 × 106 cell per animal) were resuspended in 50 μL PBS and injected into the left flank of nude mice. Tumor volume was obtained by calipers every week and calculated by the following formula V = (length × width2)/2. After 5 weeks, 9 mice were sacrificed under anesthesia by following the recommended procedures of National Institutes of Health guide for the care and use of laboratory animals. Specimens of tumor tissues were fixed in 10% formaldehyde, and then subjected to TUNEL assay by using an apoptosis detection kit (Thermo Fisher Scientific, Waltham, MA, USA).
Immunohistochemistry Assay
Specimens of tumor tissues were incubated with Ki67 antibody (1:100, Abcam) overnight at 4°C. After that, the specimens were washed by PBS and incubated with secondary antibody HRP-conjugated goat anti-Rabbit IgG (Abcam) at room temperature for 30 mins. Then, diaminobenzidine (DAB, Sigma Aldrich (St. Louis, MO, USA) solution was used to re-dye according to the manufacturer’s specifications. Finally, Ki67 staining was observed using a laser confocal microscope (Olympus).
Statistical Analysis
All data were presented as mean ± standard deviation (SD) and analyzed with GraphPad Prism 7 (GraphPad Software, CA, USA). Comparisons were performed with one-way ANOVA followed by tukey’ test. Statistical significance was defined as P < 0.05.
Results
LncRNA RP5‑833A20.1 Was Downregulated In HCC Tissues
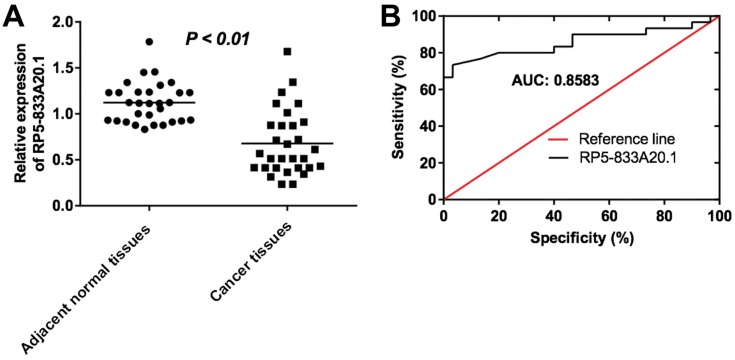
To explore the role of RP5-833A20.1 in HCC tumorigenesis, the level of RP5-833A20.1 in 30 samples of HCC tissues and adjacent tissues was assessed using qRT-PCR. As indicated in Figure 1A, low expression of RP5-833A20.1 was observed in HCC tissues compared to the adjacent tissues. In addition, to investigate the diagnostic performance of RP5-833A20.1 in HCC, ROC curves were used. The results showed that the AUC value was 0.8583, indicating that RP5-833A20.1 could discriminate patients with HCC and patients without HCC (Figure 1B). Moreover, RP5-833A20.1 expression correlated with several clinicopathological parameters, including tumor volume and lymph node metastasis (Table 1). These results suggested that RP5-833A20.1 was downregulated in HCC tissues, which was associated with poor prognosis of patient with HCC.
Figure 1.
LncRNA RP5‑833A20.1 was downregulated in HCC tissues. (A) The levels of RP5‑833A20.1 in HCC tissues from 30 pairs of patients of HCC were measured using qRT-PCR. (B) ROC curve analysis of RP5‑833A20.1.
Table 1.
LncRNA RP5-833A20.1 And Clinic-Pathological Parameters Of Patients With HCC
| Parameters | Number | RP5-833A20.1 | p value |
|---|---|---|---|
| Age | 0.360 | ||
| ≤ 50 | 12 | 0.753 ± 0.239 | |
| > 50 | 18 | 0.629 ± 0.398 | |
| Gender | |||
| Male | 18 | 0.654 ± 0.255 | 0.676 |
| Female | 12 | 0.710 ± 0.455 | |
| Tumor volume | |||
| ≤ 5 cm | 17 | 0.760 ± 0.362 | 0.021* |
| > 5 cm | 13 | 0.541 ± 0.136 | |
| Lymph node metastasis | 0.001** | ||
| N0 | 9 | 1.001 ± 0.411 | |
| N1-N3 | 21 | 0.540 ± 0.211 | |
| Distant metastasis | 0.191 | ||
| M0 | 11 | 0.945 ± 0.322 | |
| M1 | 19 | 0.587 ± 0.215 | |
| TNM stage | 0.066 | ||
| I–II | 8 | 0.876 ± 0.508 | |
| III–IV | 22 | 0.606 ± 0.249 |
Notes: Student’s t test, *P<0.05, **P<0.01.
Abbreviation: TNM, tumor-node-metastasis.
Overexpression Of RP5-833A20.1 Inhibited Proliferation And Invasion Of HCC Cells
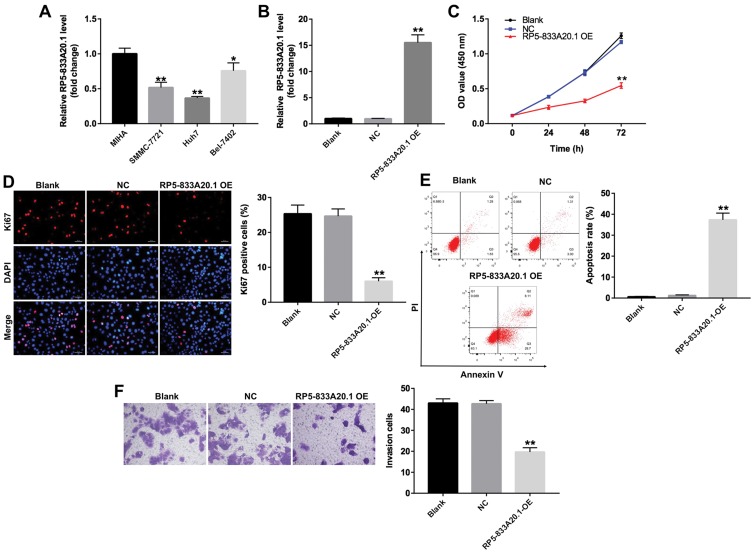
Next, qRT-PCR was used to detect the expression of RP5-833A20.1 in three human liver tumor cell lines, SMMC-7221, Hun7 and Bel-7402, and in human immortalized liver cell line MIHA, used as non-tumorigenic control. As indicated in Figure 2A, the level of RP5-833A20.1 was decreased the most in Huh7 cells, compared with that in MIHA cells. In addition, the expression of RP5-833A20.1 was slightly decreased in Bel-7402 cells, compared with that in MIHA cells (Figure 2A). To determine the function of RP5-833A20.1 in HCC, RP5-833A20.1 was overexpressed in Huh7 cells. As indicated in Figure 2B, the level of RP5-833A20.1 was significantly upregulated in Huh7 cells following infection with lenti-RP5-833A20.1. In addition, the results of CCK-8 assay and immunofluorescence assay showed that overexpression of RP5-833A20.1 markedly inhibited the proliferation of Huh7 cells (Figure 2C and D). Moreover, the apoptosis rates were obviously upregulated in Huh7 cells following infection with lenti-RP5-833A20.1, compared with the NC group (Figure 2E). Meanwhile, overexpression of RP5-833A20.1 significantly suppressed the invasion ability of Huh7 cells (Figure 2F). These results indicated that overexpression of RP5-833A20.1 could inhibit proliferation and invasion in Huh7 cells.
Figure 2.
Overexpression of RP5-833A20.1 inhibited proliferation and invasion of HCC cells. (A) Relative expressions of RP5‑833A20.1 in 4 cell lines including MIHA, SMMC-7721, Huh7 and Bel-7402 cells were detected by qRT-PCR. (B) Huh7 cells were infected with NC or lenti-RP5‑833A20.1 for 48hrs. The level of RP5‑833A20.1 in Huh7 cells was detected using qRT-PCR. (C) Huh7 cells were infected with NC or lenti-RP5‑833A20.1 for 0, 24, 48 and 72hrs. CCK-8 assay was used to detect cell viability. (D) Huh7 cells were infected with NC or lenti-RP5‑833A20.1 for 72hrs. Relative fluorescence expression levels were quantified by Ki67 and DAPI staining. (E) Apoptotic cells were detected with Annexin V and PI double staining. (F) Huh7 cells were infected with NC or lenti-RP5‑833A20.1 for 24hrs. Transwell invasion assay was used to detect cell invasion ability. *P<0.05, **P<0.01 vs NC group.
Downregulation Of RP5-833A20.1 Promoted Proliferation And Invasion Of HCC Cells
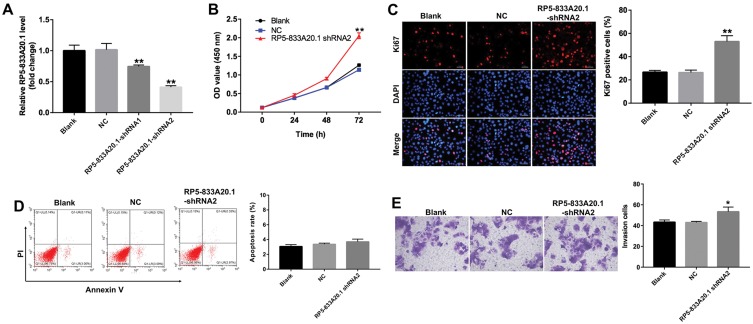
To further detect the function of RP5-833A20.1 in HCC, we used two different shRNAs (RP5-833A20.1-shRNA1 and RP5-833A20.1-shRNA2) to knock down its level in Bel-7402 cells. As shown in Figure 3A, the level of RP5-833A20.1 was significantly downregulated after infection with RP5-833A20.1-shRNA2, compared with NC group. In addition, downregulation of RP5-833A20.1 markedly promoted the proliferation of Bel-7402 cells (Figure 3B). Meanwhile, immunofluorescence assay showed that a significant increase of Ki67-positive cells was observed in RP5-833A20.1-shRNA2 group, compared with NC group (Figure 3C).Moreover, RP5-833A20.1-shRNA2 had very limited effect on the apoptosis of Bel-7402 cells, compared with the NC group (Figure 3D). Meanwhile, downregulation of RP5-833A20.1 significantly increased the invasion ability of Bel-7402 cells (Figure 3E). These data suggested that downregulation of RP5-833A20.1 could promote proliferation and invasion of Bel-7402 cells.
Figure 3.
Downregulation of RP5-833A20.1 promoted proliferation of HCC cells. (A) Bel-7402 cells were infected with NC, RP5‑833A20.1-shRNA1 or RP5‑833A20.1-shRNA2 for 48hrs. The level of RP5‑833A20.1 in Bel-7402 cells was detected using qRT-PCR. (B) Bel-7402 cells were infected with NC or RP5‑833A20.1-shRNA2 for 0, 24, 48 and 72hrs. CCK-8 assay was used to detect cell viability. (C) Bel-7402 cells were infected with NC or RP5‑833A20.1-shRNA2 for 72hrs. Relative fluorescence expression levels were quantified by Ki67 and DAPI staining. (D) Apoptotic cells were detected with Annexin V and PI double staining. (E) Bel-7402 cells were infected with NC or RP5‑833A20.1-shRNA2 for 24hrs. Transwell invasion assay was used to detect cell invasion ability. *P<0.05, **P<0.01 vs NC group.
MiR-18a-5p Was A Direct Binding Target Of RP5-833A20.1
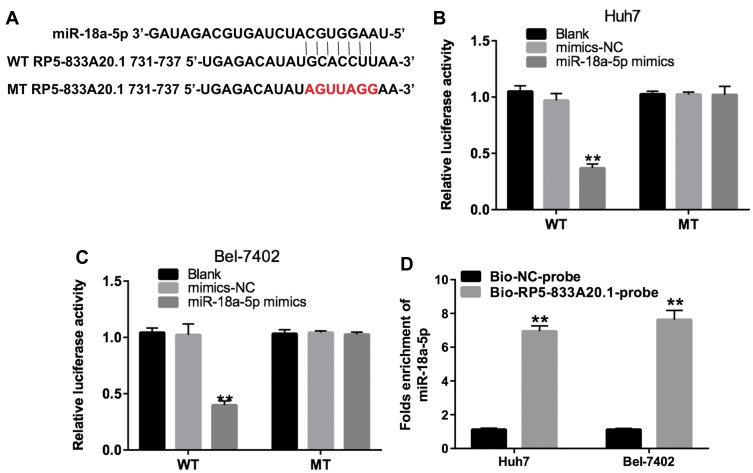
Previous study illustrated that lncRNAs function as competing endogenous RNAs by binding to miRNAs, which could liberate the target mRNA transcripts.19 Online bioinformatics tool miRDB was used to predict the potential miRNA binding regulated by RP5-833A20.1. The data indicated that miR-18a-5p might be a potential miRNA target of RP5-833A20.1 (Figure 4A). In addition, we conducted dual-luciferase reporter assay to identify whether RP5-833A20.1 was a functional target for miR-18a-5p. As illustrated in Figure 4B and C, miR-18a-5p inhibited the luciferase activity of RP5-833A20.1-WT, but it has not affected the luciferase activity of RP5-833A20.1-MT in Huh7 and Bel-7402 cells. Meanwhile, the results of pull-down assay indicated that RP5-833A20.1 was pulled down by miR-18a-5p significantly (Figure 4D). These data implied that miR-18a-5p could directly bind to the 3ʹUTR of RP5-833A20.1.
Figure 4.
MiR-18a-5p was a direct binding target of RP5-833A20.1. (A) Gene structure of RP5‑833A20.1 at the position of 731–737 indicates the predicted target site of miR-18a-5p in its 3ʹUTR. (B) The luciferase activity was detected following co-transfecting with WT/MT RP5‑833A20.1 plasmid and miR-18a-5p in Huh-7 cells using the dual-luciferase reporter assay. (C) The luciferase activity was detected following co-transfecting with WT/MT RP5‑833A20.1 plasmid and miR-18a-5p in Bel-7402 cells using the dual-luciferase reporter assay. (D) RP5‑833A20.1 pulled down by biotinylated miR-18a-5p in HCC cells was detected by qRT-PCR. **P<0.01 vs mimics NC group.
Overexpression Of RP5-833A20.1 Induced Apoptosis Of HCC Cells Via Inhibiting Akt/ERK Pathway
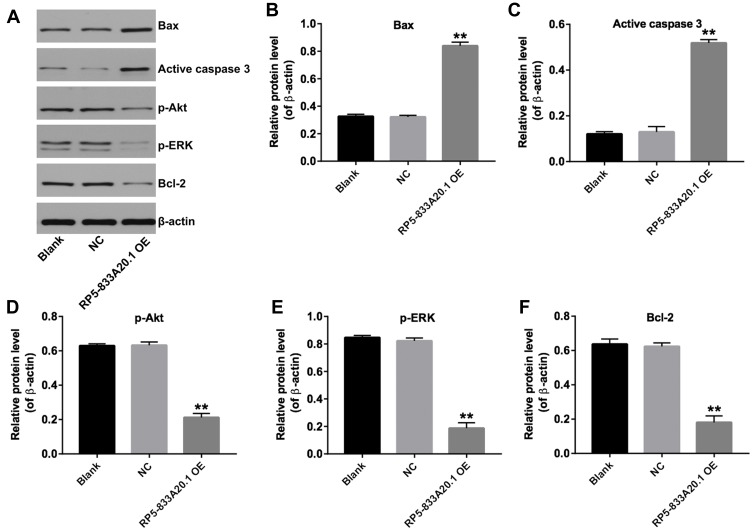
Next, we investigated the mechanisms underlying the role of RP5-833A20.1 in the progression of HCC. Akt and ERK signaling pathways have been known as the regulators of cell growth in HCC.20 As indicated in Figure 5A–F, overexpression of RP5-833A20.1 significantly upregulated the expressions of Bax, active caspase 3, and markedly downregulated the expressions of Bcl-2, p-Akt and p-ERK in Huh-7 cells. These data illustrated that overexpression of RP5-833A20.1 could induce the apoptosis of HCC cells via inhibiting Akt/ERK pathway.
Figure 5.
Overexpression of RP5-833A20.1 induced apoptosis of HCC cells via inhibiting Akt/ERK pathway. (A) Huh7 cells were infected with NC or lenti-RP5‑833A20.1 for 72hrs. Expression levels of Bax, active caspase 3, p-Akt, p-ERK and Bcl-2 in Huh-7 cells were detected with Western blotting. β-actin was used as an internal control. (B–F) The relative expressions of Bax, active caspase 3, p-Akt, p-ERK and Bcl-2 in Huh-7 cells were quantified via normalization to β-actin. **P<0.01 vs NC group.
Overexpression Of RP5-833A20.1 Inhibited The Tumorigenesis Of Huh7 Subcutaneous Xenograft In Vivo
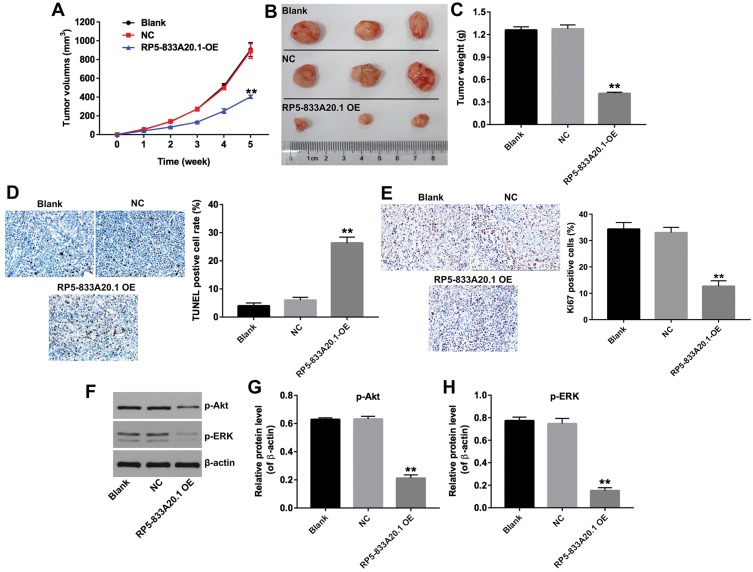
To investigate the role of RP5-833A20.1 on HCC tumorigenesis in vivo, Huh7 cells infected with lenti-RP5-833A20.1 were subcutaneously implanted into nude mice. As shown in Figure 6A and B, upregulation of RP5-833A20.1 markedly inhibited the tumor growth in xenografts. In addition, the tumor weight in RP5-833A20.1-OE group was lower compared to the NC group (Figure 6C). Moreover, TUNEL assay results indicated that upregulation of RP5-833A20.1 obviously induced apoptosis in RP5-833A20.1-OE group, compared with the NC group (Figure 6D). Furthermore, Ki67 Immunohistochemistry assay indicated that overexpression of RP5-833A20.1 markedly inhibited the cell proliferation in tumor tissues, compared with the NC group (Figure 6E). Meanwhile, overexpression of RP5-833A20.1 markedly downregulated the expressions of p-Akt and p-ERK in tumor tissues (Figure 6F–H). These results suggested that overexpression of RP5-833A20.1 could inhibit the tumorigenesis of Huh7 xenograft in vivo by inhibiting Akt/ERK pathway.
Figure 6.
Overexpression of RP5-833A20.1 inhibited the tumorigenesis of Huh7 subcutaneous xenograft in vivo. The nude mice were subcutaneously implanted with lenti-RP5‑833A20.1 infected Huh7 cells to establish tumor xenograft model. (A) Tumor volumes of xenograft were measured weekly. (B) HCC xenograft tumors were excised from xenografts and pictured after 5 weeks. (C) Weights of the tumors in each group were calculated. (D) TUNEL staining was used to detect the apoptosis of tumor tissues in each group. (E) Ki67 immunohistochemistry was used to detect the proliferation of tumor tissues in each group. (F) Expression levels of p-Akt and p-ERK in tumor tissues were detected with Western blotting. β-actin was used as an internal control. (G, H) The relative expressions of p-Akt and p-ERK were quantified via normalization to β-actin. **P<0.01 vs. NC group.
Discussion
In recent years, LncRNAs could be used as prognostic and individual diagnostic biomarkers in HCC.21 In the present study, for the first time, we found that RP5-833A20.1 was markedly downregulated in HCC tissues and HCC cell lines. In addition, overexpression of RP5-833A20.1 could induce apoptosis of HCC cells in vitro and in vivo. These findings indicated that RP5-833A20.1 was a tumor suppressor gene in HCC.
First, we found that overexpression of RP5-833A20.1 inhibited the proliferation and invasion in HCC cells through inducing apoptosis. These findings are in line with previous study, suggesting that the expression of RP5‑833A20.1 was downregulated in glioma tissues, and upregulation of RP5-833A20.1 could inhibit proliferation and metastasis in glioma U251 cells.17 On the contrary, the expression of RP5-833A20.1 was markedly increased in human acute monocytic leukemia macrophage-derived foam cells.18 It is possible that the difference might exist in the area of RP5-833A20.1 expressing profiles in different diseases. In addition, overexpression of RP5-833A20.1 could induce apoptosis of HCC cells in vitro and in vivo via inhibiting the Akt/ERK pathway. Wang et al found that LINC00707 promoted HCC progression via upregulation of the ERK/Akt pathway, which was consistent with our results.22 Taking the above data together, RP5-833A20.1 inhibited proliferation and induced apoptosis in HCC cells via downregulation of the Akt/ERK pathway.
Emerging evidence has indicated that lncRNAs function as competing endogenous RNAs for miRNAs to regulate the progression of tumor.19 In this study, the data stored in miRDB indicated that miR-18a-5p might be a potential miRNA binding target of RP5-833A20.1. On the other hand, luciferase reporter assay defined that miR-18a-5p was a binding target of RP5-833A20.1 in HCC cells. Previous studies found that miR-18a-5p was upregulated in osteosarcoma tissues, and potential acting as an oncogene in several human cancers.23,24 Fei et al found that lncRNA FER1L4 suppressed tumorigenesis in osteosarcoma by targeting miR-18a-5p.25 These results suggested that RP5-833A20.1 and miR-18-5p might be potential predictors for the treatment of patients with HCC. However, in the future, the mechanism of RP5‑833A20.1 and miR-18a-5p interaction in HCC needs to be further explored. Meanwhile, the mechanism by which RP5‑833A20.1 downregulates the Akt/ERK signaling pathway in HCC remains largely unknown, and the understanding of RP5‑833A20.1 remains to be extended in the future.
Conclusion
This study revealed that RP5-833A20.1 was downregulated in HCC tissues and cell lines. In addition, RP5-833A20.1 suppressed proliferation and invasion of HCC cells via sponging miR-18a-5p. Therefore, RP5-833A20.1 might be a valuable and potential biomarker and therapeutic target for the treatment of HCC.
Funding Statement
The study was funded by the Cooperation Project of the Science and Technology of Guizhou Province (2017, No. 7186) and the Cooperation Project of the Science and Technology of Guizhou Province (2018, No. 5779-31).
Disclosure
Zili Chen and Yifei Ma are co-first authors. The authors declare no competing financial interests.
References
- 1.Wang Y, Li W, Chen X, et al. MIR210HG predicts poor prognosis and functions as an oncogenic lncRNA in hepatocellular carcinoma. Biomed Pharmacother. 2019;111:1297–1301. doi: 10.1016/j.biopha.2018.12.134 [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Li Y, Jiang W, Li Q, Lan Y. The clinical significance of microRNA-122 in predicting the prognosis of patients with hepatocellular carcinoma: a meta-analysis validated by the cancer genome atlas dataset. Medicine (Baltimore). 2019;98(13):e14810. doi: 10.1097/MD.0000000000014810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin M, Wang J, Ji X, et al. MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):136. doi: 10.1186/s13046-019-1135-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Hassan W, Jabeen Q, Khan GJ, Iqbal F. Interdependent and independent multidimensional role of tumor microenvironment on hepatocellular carcinoma. Cytokine. 2018;103:150–159. doi: 10.1016/j.cyto.2017.09.026 [DOI] [PubMed] [Google Scholar]
- 5.Jin Q, Chen X, Zheng S. The security rating on local ablation and interventional therapy for Hepatocellular Carcinoma (HCC) and the comparison among multiple anesthesia methods. Anal Cell Pathol (Amst). 2019;2019:2965173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. [DOI] [PubMed] [Google Scholar]
- 7.Dutta R, Mahato RI. Recent advances in hepatocellular carcinoma therapy. Pharmacol Ther. 2017;173:106–117. doi: 10.1016/j.pharmthera.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo M. Systemic therapy for hepatocellular carcinoma:recent advances and future perspective. Nihon Shokakibyo Gakkai Zasshi. 2019;116(1):8–17. doi: 10.11405/nisshoshi.116.8 [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Li Y, Xu X. The long noncoding RNA cardiac hypertrophy-related factor plays oncogenic roles in hepatocellular carcinoma by downregulating microRNA-211. J Cell Biochem. 2019. [DOI] [PubMed] [Google Scholar]
- 10.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi: 10.1016/j.cell.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 11.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi: 10.1016/j.ccell.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arun G, Diermeier S, Akerman M, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30(1):34–51. doi: 10.1101/gad.270959.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai L, Li J, Dong Z, et al. Temporal expression and functional analysis of long non-coding RNAs in colorectal cancer initiation. J Cell Mol Med. 2019. doi: 10.1111/jcmm.2019.23.issue-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, Qin C, Yuan Z, Liu S. LncRNA PAPAS promotes hepatocellular carcinoma by interacting with miR-188-5p. J Cell Biochem. 2019. [DOI] [PubMed] [Google Scholar]
- 15.Luo H, Xu C, Le W, Ge B, Wang T. lncRNA CASC11 promotes cancer cell proliferation in bladder cancer through miRNA-150. J Cell Biochem. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CC, Chen PH, Ho KH, et al. The microRNA-302b-inhibited insulin-like growth factor-binding protein 2 signaling pathway induces glioma cell apoptosis by targeting nuclear factor IA. PLoS One. 2017;12(3):e0173890. doi: 10.1371/journal.pone.0173890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang CM, Hu YW, Nie Y, et al. Long non-coding RNA RP5-833A20.1 inhibits proliferation, metastasis and cell cycle progression by suppressing the expression of NFIA in U251 cells. Mol Med Rep. 2016;14(6):5288–5296. doi: 10.3892/mmr.2016.5854 [DOI] [PubMed] [Google Scholar]
- 18.Hu YW, Zhao JY, Li SF, et al. RP5-833A20.1/miR-382-5p/NFIA-dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler Thromb Vasc Biol. 2015;35(1):87–101. doi: 10.1161/ATVBAHA.114.304296 [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Wang Y, Wang L, et al. Long non-coding RNA AGAP2-AS1, functioning as a competitive endogenous RNA, upregulates ANXA11 expression by sponging miR-16-5p and promotes proliferation and metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):194. doi: 10.1186/s13046-019-1188-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Chen Z, Yu W, Zhou Q, et al. A novel lncRNA IHS promotes tumor proliferation and metastasis in HCC by regulating the ERK- and AKT/GSK-3beta-signaling pathways. Mol Ther Nucleic Acids. 2019;16:707–720. doi: 10.1016/j.omtn.2019.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He H, Chen D, Cui S, et al. Identification of a long noncoding RNAmediated competitive endogenous RNA network in hepatocellular carcinoma. Oncol Rep. 2019. doi: 10.3892/or.2019.7181 [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Luo Z, Yao T, Li W, Pu J. LINC00707 promotes hepatocellular carcinoma progression through activating ERK/JNK/AKT pathway signaling pathway. J Cell Physiol. 2019;234(5):6908–6916. doi: 10.1002/jcp.27449 [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, Li Z, Pan X, et al. Identification of miR-18a-5p as an oncogene and prognostic biomarker in RCC. Am J Transl Res. 2018;10(6):1874–1886. [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C, Peng K, Guo H, et al. miR-18a-5p promotes cell invasion and migration of osteosarcoma by directly targeting IRF2. Oncol Lett. 2018;16(3):3150–3156. doi: 10.3892/ol.2018.9032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fei D, Zhang X, Liu J, et al. Long noncoding RNA FER1L4 suppresses tumorigenesis by regulating the expression of PTEN targeting miR-18a-5p in osteosarcoma. Cell Physiol Biochem. 2018;51(3):1364–1375. doi: 10.1159/000495554 [DOI] [PubMed] [Google Scholar]