Abstract
Recent advances in targeted therapy and immunotherapy have once again raised the hope that a cure might be within reach for many cancer types. Yet, the majority of cancers are either insensitive to the therapies to begin with or develop resistance later on. Therapy with live tumor-targeting bacteria provides a unique option to meet these challenges. Compared to most other therapeutics, the effectiveness of tumor-targeting bacteria is not directly affected by the genetic makeup of a tumor. Bacteria initiate their direct antitumor effects from deep within the tumor, followed by innate and adaptive antitumor immune responses. As microscopic “robotic factories”, bacterial vectors can be reprogrammed following simple genetic rules or sophisticated synthetic bioengineering principles to produce and deliver anticancer agents based on clinical needs. Therapeutic approaches using live tumor-targeting bacteria can either be applied as a monotherapy or complement other anticancer therapies to achieve better clinical outcomes. In this Review, we summarize the potential benefits and challenges of this approach. We discuss how live bacteria selectively induce tumor regression and provide examples to illustrate different ways to engineer bacteria for improved safety and efficacy. Finally, we share our experience and insights on oncology clinical trials with tumor-targeting bacteria, including a discussion on regulatory issues.
There are a variety of cytotoxic agents that can kill cancer cells effectively. However, the conventional cytotoxic therapies often eliminate cancer cells at the expense of damaging the normal tissues, resulting in unacceptable toxicities. Therefore, eradication of cancer cells without causing collateral damage is the ultimate goal to all oncologists and cancer researchers. The persistent pursuit of that goal has recently led to two promising clinical advances – molecularly targeted therapy and immunotherapy. Molecularly targeted therapy aims at genes with specific genetic or epigenetic alterations in cancer cells, thus potentially minimizing side effects seen in patients treated with traditional chemotherapeutic agents1-6. In spite of its increased targeting precision against tumor cells, targeted therapy is far from perfect7. First, targeted therapeutic agents have a spectrum of their own toxicities, some of which are related to the normal functions of the target proteins8. Second, the small molecule inhibitors may not be sufficiently specific9. Third, resistance or relapse is often observed in patients treated with targeted therapy, resulting from intrinsic resistant genetic changes or selection for a subset of cancer cells with those changes10. Fourth, the majority of tumors do not carry currently actionable genetic changes. Immunotherapy can be seen as another “targeted” therapy which involves T cells reactive to tumor-specific neoantigens or tumor-associated antigens (TAAs). Recent clinical trials with immune checkpoint blockade have shown remarkable results including durable therapeutic effects on advanced metastatic cancers11,12. It is generally believed that sensitivity to checkpoint blockade is dependent on the neoantigen burdens of the tumor cells and immune infiltrates in the tumor microenvironment13. Unfortunately, the majority of the common cancers do not show abundant mutations and infiltrating immune cells, and consequently are insensitive to checkpoint blockade. Major efforts are being made to develop approaches that can sensitize these tumors to immunotherapy. In addition to molecular targets such as oncoproteins and neoantigens, unique pathological alterations at the tissue level can be exploited for tumor targeting. Tumor vasculature is generally irregularly-developed and chaotic, leading to insufficient infusion of oxygen and nutrients in areas within a solid tumor14,15. Cancer cells in these areas are dormant but viable16. They can be responsible for clinical relapse after chemo or radiation therapy, because the hypoxic areas are poorly accessible to systemically delivered therapeutics and oxygen is needed for effective radiation therapy. In addition, low oxygen levels affect the function of immune cells, contributing to immune privilege of solid tumors. Nevertheless, the necrotic/hypoxic regions provide a critical niche for bacteria to colonize.
There is a long history of observations that suggest natural bacterial infections can result in antitumor effects against malignant tumors. In 1813, Vautier reported that cancer patients who developed gas gangrene had tumor regressions17. Other historical accounts include observations by Busch (1866) that led Fehleisen (1883) and subsequently William B. Coley to experiment with the live infectious agent of erysipelas (later termed Group A Streptococcus or S. pyogenes) as a means of treating cancers18-20. Further pursuit of using bacteria to treat cancers was curtailed later on because of the focus of attention on the then novel chemo and radiation therapies. The enthusiasm for using live bacteria for cancer treatment has revived since the mid-1990s when the scientific community had a better understanding about the tumor microenvironment and recombinant DNA technology allowed generation of more potent and less toxic bacterial strains 21. Many bacterial strains have since been tested in animal models and shown preferential targeting of solid tumors, several of which have advanced to clinical trials21-27. The clinical development of live bacteria as therapeutic agents faces substantial hurdles mainly because of potential infection-associated toxicities. One successful example is the use of Bacillus Calmette-Guérin (BCG) in the treatment of bladder cancer28. BCG is a live attenuated strain of Mycobacterium bovis originally generated as a vaccine for tuberculosis. BCG therapy by intravesical administration was first documented in the 1970s and has since become an important treatment option for transitional-cell carcinoma in situ of the bladder29-31. It is believed that BCG’s therapeutic effect is mainly due to its immunomodulatory activity32-34. In this Review, we discuss the unique aspects of live tumor-targeting bacteria as therapeutic agents, focusing on some of the most investigated strains of Salmonella, Clostridium, and Listeria as examples. As an increasing number of therapeutic bacterial strains have advanced to the clinical stage, we also highlight issues associated with their clinical translation.
Live tumor-targeting bacteria
Intrinsic tumor-targeting.
Live bacteria “target” solid tumors using unique mechanisms. When administered systemically, therapeutic bacteria disseminate to both tumor and healthy tissues. Even though Salmonella has been shown to preferentially home to or are retained in the tumor microenvironment enriched in certain metabolites35, the initial amount of bacteria delivered to the tumor is usually not greater than that to the normal tissues36-38. However, bacteria in the circulation and other normal tissues are cleared within hours and days, respectively, while those in the tumor continue to proliferate, often to numbers greatly exceeding the colony forming units initially administered36,38-46. This selective colonization is likely the result of an immunosuppressive and biochemically unique microenvironment caused by pathologic changes associated with solid tumors35,47-51. Importantly, anaerobic bacteria do not colonize hypoxic or inflammatory lesions unrelated to neoplasia, as shown in experiments with obligate and facultative anaerobes, respectively38,52,53. Tumor-targeting of Listeria involves a different mechanism. Listeria is known to infect not only professional antigen-presenting cells (APCs) such as monocytes/macrophages and dendritic cells, but also myeloid-derived suppressor cells (MDSCs) that can deliver the bacteria selectively to tumor44,45. Listeria inside the tumor-infiltrating immunosuppressive MDSC is protected from immune clearance, but is rapidly eliminated from normal tissues that lack immune suppression. Obligate anaerobic bacteria such as clostridia are unable to survive in the oxygen-rich environment, thus further reinforcing their tumor-targeting specificity. Interestingly, germinated clostridia have also been observed within micro invasive lesions where necrosis was not evident as well as in the vicinity of neoplastic vessels in glioma models52,54, raising the possibility that these neoplastic structures provide sufficiently hypoxic, biochemically unique, and immunoprivileged microenvironment for bacterial colonization. As discussed in more detail below, facultatively anaerobic bacteria can be engineered such that their ability to survive in the normal tissues will be further diminished.
Tumor destruction by live bacteria.
Localized bacterial infection causes tumor regression through various mechanisms (FIG. 1). Bacteria have intrinsic antitumor activities, but different strains of bacteria or bacteria in different microenvironments may deploy distinct mechanisms to destroy solid tumors. In addition to the intrinsic antitumor effects, bacterial infection induces innate as well as adaptive immune responses against both tumor-colonizing bacteria and the tumor cells46-48,52,55-59. The host immune responses are more critical for the antitumor effects of bacteria such as Salmonella that are not sufficiently cytotoxic to tumor cells55,60. Numerous studies have suggested that both bacterium-intrinsic and host immune mechanisms are involved in tumor destruction (FIG. 1). The dominant mechanism is likely to vary depending on the bacterial species used in the therapy, the types of tumor being treated, and even the phases of the bacteria-host interaction. Importantly, bacteria can be genetically engineered to further enhance their antitumor activities in a variety of different ways, making them a versatile platform to deliver therapeutic payloads based on clinical needs.
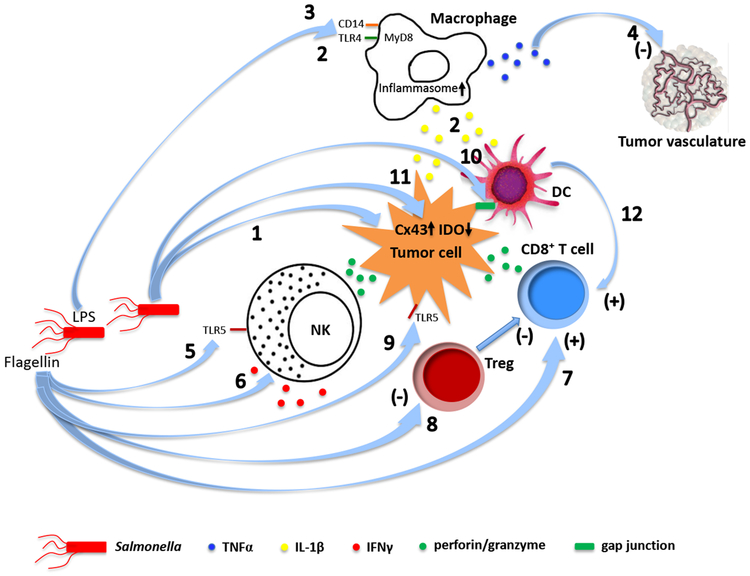
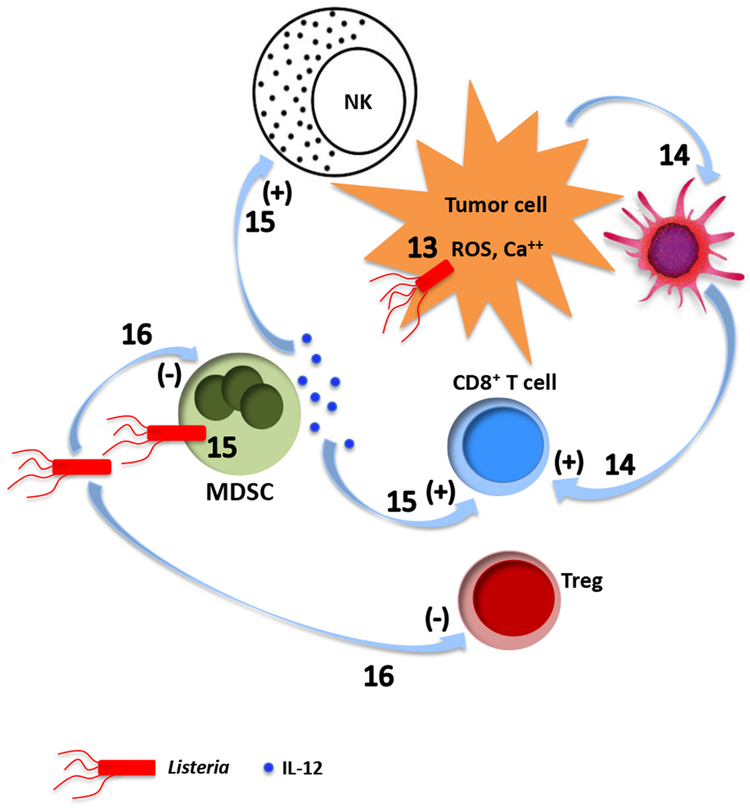
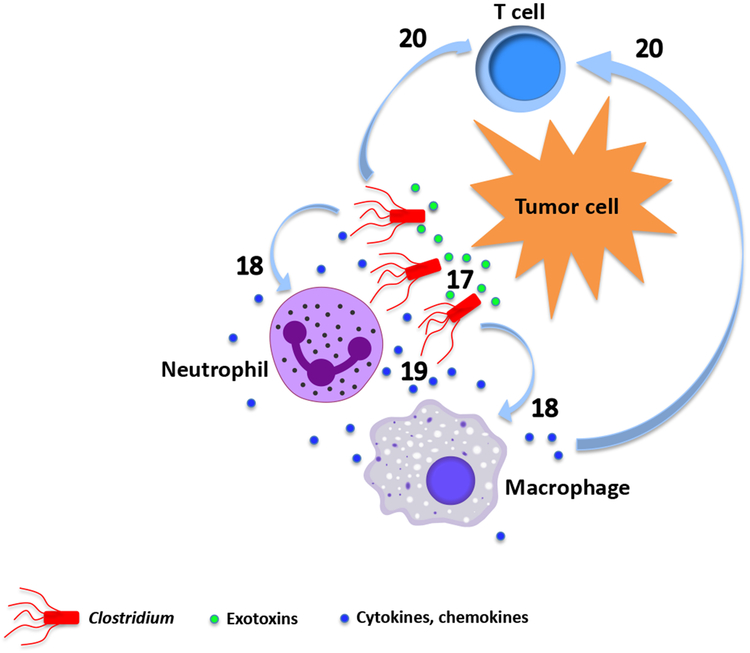
Fig. 1. Mechanisms of tumor destruction by live tumor-targeting bacteria.
Different bacterial species employ both shared and unique intrinsic mechanisms to destroy cancer. a. Salmonella. (1) Uncontrolled bacterial multiplication can lead to bursting of the invaded tumor cells184. Intracellular bacteria may also kill tumor cells by inducing apoptosis or autophagy184-188. (2) Macrophages and dendritic cells in Salmonella-colonized tumors secrete IL-1β responsible for the antitumor activity189. The elevated IL-1β secretion requires both LPS-induced TLR4 signaling and inflammasome activation in macrophage following phagocytosis of Salmonella-damaged tumor cells190. (3) LPS elicits TNFα expression through CD14, TLR4 and MyD88191,192, (4) leading to disruption of the tumor vasculature57. (5) Flagellin induces an NK cell-mediated antitumor response dependent on perforin193, as well as (6) release of IFNγ, a critical cytokine for both innate and adaptive immunity, from NK cells through a TLR-independent pathway involving IL-18 and Myd88194. (7) Flagellin also enhances a TLR5 and CD8+ T cell-dependent antitumor response in a peptide vaccine-based immunotherapeutic setting195, and (8) decreases frequency of CD4+CD25+ regulatory T cells (Treg)196. (9) In addition, flagellin can directly suppress tumor cell proliferation through TLR5 signaling197. (10) Salmonella induces upregulation of connexin 43 (Cx43)198,199,200 , leading to gap junction formation between tumor cells and dendritic cells (DC), which promotes transfer and cross-presentation of processed tumor antigenic peptides198. (11) Upregulation of Cx43 in tumor cells also reduces expression of the immunosuppressive indoleamine 2,3-dioxygenase (IDO)200. (12) Both tumor antigen cross-presentation by DC and decreased IDO further activate CD8+ T cells. b. Listeria. (13) Listeria can directly kill tumor cells through the nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase)-mediated production of reactive oxygen species (ROS) and intracellular calcium mobilization201. (14) The immunogenic tumor cell death caused by high levels of ROS activates CD8+ T cells responsible for eliminating both primary tumors and metastases201,202. (15) Listeria infects the immunosuppressive MDSC and alters a subpopulation of these cells into an immune-stimulating phenotype characterized by elevated production of IL-12, which is correlated with improved CD8+ T cell and NK cell responses45. (16) Listeria vaccine strains also inhibits MDSC and Treg124,203. c. Clostridia. (17) Direct tumor destruction is caused by a variety of exotoxins secreted by the colonizing clostridia, some of which (e.g. phospholipases, haemolysins, lipases) can damage membrane structures while others are internalized to interfere with critical cellular functions204-207. (18) Similar to infection by other bacterial species, the clostridial infection results in an initial accumulation of granulocytes and macrophages at the infection site 47,55. This first line of defense prevents the colonizing bacteria from invading into surrounding normal tissues as well as sufficiently perfused and oxygenized tumor regions48,52. (19) The cellular response results in elevated cytokines and chemokines that orchestrate a concerted immune response47,57. Clostridia can also trigger the release of TRAIL from neutrophils, killing cancer cells through activation of apoptosis208. (20) At later time points, adaptive immune cells including CD8+ T lymphocytes are recruited to fight cancer47.
Engineered bacteria
Bacteria can be attenuated for safety reasons or engineered to acquire enhanced antitumor activities. As discussed below, a large collection of engineered bacterial strains have been generated in laboratories around the world for a variety of purposes, all aimed at improving the therapeutic index when bacteria are used either alone or in combination with other cancer therapeutic approaches.
Improving safety.
The safety profile of a therapeutic bacterium can be improved by different approaches. For the known human pathogens, deletion of major virulence genes is often required to minimize their pathogenicity. An exceedingly toxic strain of Clostridium novyi (C. novyi) was converted to a considerably safer strain (C. novyi-NT) by deleting the gene for a lethal exotoxin61. Lipopolysaccharide (LPS) in the outer membrane of Gram-negative bacteria is one of the most potent TNFα stimulators and thus responsible for Gram-negative sepsis62. Deletion of the msbB gene from Salmonella resulted in loss of myristoylation of lipid A, a critical component of LPS, and minimized TNFα expression51. This modification reduced the toxicity of Salmonella by 10,000-fold. An attenuated strain of Salmonella named VNP20009 carrying this deletion was isolated and shown to be safe in clinical trials63,64. It should be noted that some of the virulence factors may also be responsible for the intrinsic antitumor activity of live bacteria. Whenever possible, attenuation should be achieved without substantially compromising the antitumor activity, unless the bacterial strain is used for the purpose of vaccination only. In this regard, the msbB-deficient Salmonella strain retained both tumor-targeting specificity and antitumor activity in the mouse B16F10 melanoma model51. Salmonella was also made defective in the synthesis of ppGpp (thus named ΔppGpp), a signaling molecule required for the induced expression of a number of virulence genes65. The ΔppGpp strain has a drastically improved safety profile. Interestingly, this strain is also defective in its ability to enter and replicate in the host cells, effectively turning it into an extracellular bacterium66.
Another way to improve safety is to generate auxotrophic mutants that cannot replicate efficiently in an environment where a particular nutrient required by the mutant strain is scarce. Salmonella A1-R represents such a strain, which is auxotrophic for leucine and arginine likely enriched in the tumor but not in normal tissues67. This strain, without further engineering, has shown selective tumor colonization as well as potent antitumor activity in a variety of mouse tumor models27,68. Listeria can be made safer by deleting prfA, the master virulence regulator gene69. However, prfA-deficient Listeria cannot escape into the cytosol of the infected cells, which would prevent the tumor antigens expressed by the vaccine strains from accessing the cytosol for processing and cell surface presentation. To maintain a sufficiently attenuated state while allowing cytosolic delivery of the tumor antigens, the prfA-deficient strains were engineered to express low levels of prfA and truncated Listeriolysin O (LLO) that can be fused with the antigens of choice for enhanced immunogenicity70,71. These strains are referred to as Lm-LLO, which have been used not only as vaccine strains, but also for tumor-targeted delivery of non-vaccine therapeutic payloads44,46,72. Attenuation of Listeria can also be achieved by deleting the virulence genes actA and inlB responsible for bacterial dissemination, creating strains known as LADD for Live Attenuated Double-Deleted73,74. Another method to generate attenuated Listeria strains involved insertional inactivation of the dal and dat genes required for the synthesis of bacterial cell wall75. The attenuated strains with these modifications are incapable of replication or spreading in vivo. Therefore, they are desired vaccine vectors, but not optimal for tumor-targeted delivery of non-vaccine antitumor payloads.
Increasing tumor targeting.
Obligate anaerobes have relatively high tumor specificity, thus resulting in minimal direct cytotoxicity to normal tissues38,39,76,77. In contrast, facultative anaerobes such as Salmonella and Listeria can survive and even proliferate in an oxygenated environment, causing direct damage to the normal tissues. For facultative anaerobes, improved tumor targeting could reduce their toxicity or enhance their efficacy without increasing toxicity. The αvβ3 integrin is overexpressed on activated endothelial cells and some cancer cells. A Salmonella strain displaying an integrin-binding RGD peptide on its outer membrane protein A (OmpA) showed a >1000-fold enrichment in the αvβ3 integrin-expressing U87MG and M21 xenografts compared to the control strain and an impressively enhanced antitumor activity in the MDA-MB-231 and MDA-MB-435 xenograft tumor models78. Bacteria have been engineered to target TAAs as well. Surface display of antibody fragments against the colorectal cancer-associated carcinoembryonic antigen (CEA) or lymphoma-associated antigen CD20 made the engineered Salmonella strains more effective in suppressing experimental tumors expressing these antigens79,80. Importantly, the anti-CD20 strain showed substantially reduced intracellular accumulation in the liver and spleen of the treated mice, while maintaining tumor accumulation80. Bacteria can also serve as a platform to display modular synthetic adhesins, where different adhesins can be chosen for targeting tumors expressing their specific ligands81.
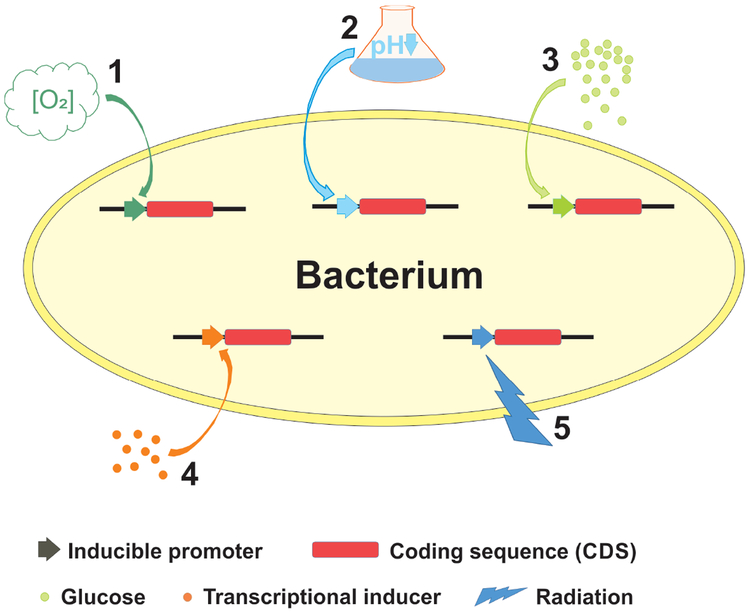
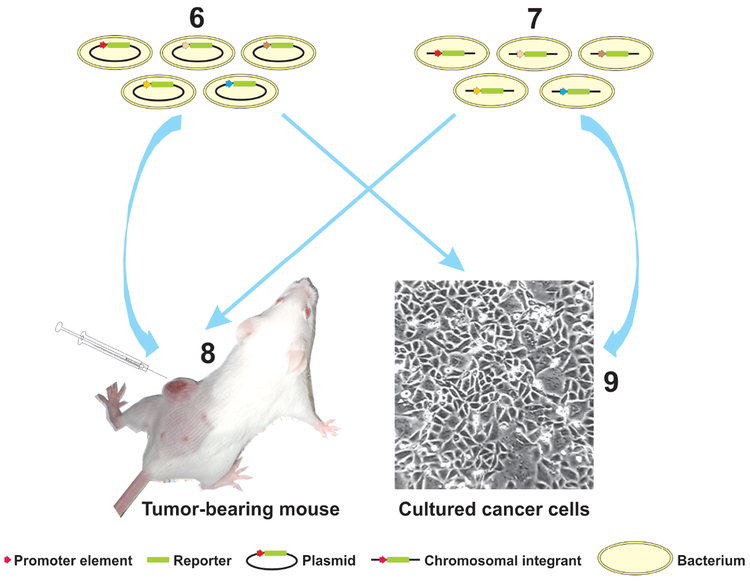
Gene promoters responsive to tumor-associated signals such as hypoxia have also been exploited for both targeted colonization and payload expression (FIG. 2a). In addition to promoters known to be induced by the tumor-associated factors, novel promoter elements activated in tumor microenvironment can be identified using unbiased large-scale screening methods such as those employing “promoter traps” (FIG. 2b). Promoters tightly regulated by exogenously applied chemical transcriptional triggers or by ionic radiation represent another means to control the expression of effector genes (FIG. 2a). While systemic administration of chemical triggers allows a temporal control, focused radiation can provide both temporal and spatial controls. It should also be noted that high-level constitutive expression of heterologous proteins can be a metabolic burden to the bacterial vector, resulting in decreased fitness and inefficient colonization82. Temporally controlled payload expression after a robust colonization has been established may be a good approach to address this problem.
Fig. 2. Inducible promoters used for targeted colonization and payload expression.
a. Various inducible promoters can be used for either tumor-selective expression or temporally or spatially controlled expression. (1) A Salmonella strain was engineered such that an essential gene was placed under the control of a hypoxia-inducible promoter, while expression of an inhibitory antisense RNA for this gene was activated by an oxygen-inducible promoter to minimize basal level expression in oxygenated normal tissues209. This strain showed a robust tumor colonization and greatly enhanced clearance from normal tissues, thus resulting in a substantially improved safety profile compared to the parental strain. Hypoxia-inducible promoters have also been used to direct the expression of effector genes such as those encoding cytotoxic proteins, which requires tighter control for safety reasons96. (2) Promoter elements responsive to low pH were among the ones identified to be active in tumor microenvironment in studies using “promoter traps” (see below)210. (3) A genetic circuit that can be triggered by glucose gradients often present in solid tumors has also been used to engineer bacteria211, enabling them to express antitumor proteins in metabolically more active tumor regions. (4) Exogenously applied transcriptional inducers such as L-arabinose, acetyl salicylic acid and doxycycline can tightly regulate the relevant inducible promoters introduced into bacteria97,99,100,212-215, providing a means to control the expression of effector genes in a temporal fashion. (5) Ionic radiation at as low as 2 Gy has also been shown to activate the recA promoter on a plasmid transfected into Clostridium216-218, raising the possibility to regulate effector gene expression with focused radiation treatment at clinically relevant doses (2 Gy is similar to a typical fractionated dose used in radiation therapy in an adjuvant setting for solid tumors). b. “Promoter traps” have been employed to identify promoter elements active in the tumor microenvironment210,219,220. “Promoter trap” libraries can be constructed by transforming bacteria with either (6) plasmids containing random genomic DNA fragments cloned upstream of a promoterless reporter gene, or (7) transposons containing a promoterless reporter gene which integrate randomly into the bacterial genome. These “promoter trap” libraries can be either (8) injected into experimental tumors or (9) co-cultured with tumor cells. Bacteria are then recovered and analyzed for reporter activities. Clones with high reporter activities are likely to contain promoter elements active in the tumor microenvironment.
Effector systems.
Attenuated bacteria alone often cannot eradicate solid tumors. Delivery of therapeutic payloads by tumor-targeted bacteria to augment their efficacy was first described in the mid-1990s83-87. Various effector systems have since been explored (TABLE 1). Here we briefly describe different strategies for payload delivery and effector systems categorized based on their antitumor mechanisms.
Table 1.
Effector systems
| Effector classes | Effectors or targets | Exemplary studies |
|---|---|---|
| Cytotoxic | Bacterial toxins, immunotoxins (e.g. Cytolysin A, S. aureus α-hemolysin, Pseudomonas exotoxin A, TGFα-PE38) | 96-102,221 |
| Apoptosis-inducing ligands (e.g. TNF-α, FasL, TRAIL, Azurin, Cp53, apoptin, Noxa BTD) | 89,106-110,214,222-224 | |
| Agents loaded into/onto bacteria (e.g. 188Re, 32P, doxorubicin, C3N4) | 44,46,111,112 | |
| Prodrug-converting enzymes | Thymidine kinase (TK), Cytosine deaminase (CD), Nitroreductase (NTR), Purine nucleoside Phosphorylase (PNP), Carboxypeptidase G2, YieF | 36,114-116,150,225-229 |
| Immunomodulators | Tumor antigens | 71,95,119,163,230-232 |
| Cytokines and chemokines (e.g. IL-2, IL-4, IL-12, IL-18, IFNγ, GM-CSF, Flt3L, LIGHT, CCL21) | 86,120,121,233-240 | |
| Others (e.g. heterologous flagellin, α-galactosylceramide, Immunodominant recall antigens) | 72,122 | |
| Targeting tumor stroma | Legumain, VEGFR2 (FLK-1), Endoglin (CD105), Thrombospondin-1, TEM8, PDGFRβ | 92,132,134,136-139,241-243 |
| Gene silencing | Silenced targets: IDO, STAT3, Bcl-2, MDM2, Survivin, MDR | 244-252 |
| Synthetic gene circuit | Quorum-sensing gene circuit for controlled payload production | 90,253 |
Different strategies for payload delivery.
The therapeutic payload can be delivered in the form of DNA, RNA or protein depending on their intended use and the type of delivery bacteria. In the majority of cases, bacteria are transformed with plasmids carrying gene expression cassettes that direct the expression of therapeutic proteins in the bacteria. The proteins need to be secreted from the bacteria to achieve their biological effects88. Alternatively, the vector strains can be engineered such that autolysis is induced for the release of therapeutic payload once a robust tumor colonization has been established89,90.
In addition to therapeutic proteins, DNA and RNA molecules can also be delivered to targeted cells. Intracellular bacteria can be engineered with DNA cassettes expressing therapeutic proteins under the control of mammalian promoters91-93. Biological activities of mammalian proteins often depend on correct folding and posttranslational modifications that may be absent in proteins produced in bacteria. Thus, one advantage of delivering DNA is to produce optimally active proteins by host cells. It should also be noted that proteins produced by intracellular bacteria and those produced by host cells may be targeted to different cellular compartments. In a study using Salmonella as a delivery vehicle, β-galactosidase expressed from a eukaryotic cassette induced substantially stronger immune responses than that expressed from a prokaryotic cassette94. A special category of therapeutic bacteria are DNA vaccine strains designed to deliver DNA to APCs. Vaccine strains delivering either DNA or protein are discussed in detail elsewhere95. Small hairpin RNA (shRNA) and small interfering RNA (siRNA) are popular forms of RNA used for gene silencing and their delivery by intracellular bacteria has been explored in multiple studies (TABLE 1).
Cytotoxic agents.
The most straightforward approach to enhance the antitumor activity would be to engineer bacterial vectors expressing cytotoxic agents. This approach requires the bacterial vectors to target tumors with sufficient specificity or the use of inducible promoters for a better control of gene expression to avoid toxicity to normal tissues. Several bacterial strains have been engineered to express the potent pore-forming bacterial toxin cytolysin A or S. aureus α-hemolysin under the control of promoters activated by hypoxia96, L-arabinose97-99, or doxycycline100, to ensure safety. An alternative method to increase safety involved expressing a chimeric protein with tumor growth factor alpha (TGFα), an epidermal growth factor receptor (EGFR) ligand, that targeted the Pseudomonas exotoxin A (ToxA, also referred to as PE) to EGFR overexpressed in many cancer types101,102. The chimeric protein was shown to selectively kill EGFR-positive cancer cells and retarded tumor growth in multiple mouse tumor models expressing EGFR.
Induction of tumor cell apoptosis is an attractive therapeutic approach, but systemic administration of apoptosis-inducing ligands such as TNF-α, Fas ligand (FasL), and TNF-related apoptosis-inducing ligand (TRAIL) is not feasible because of their toxicity or short circulating half-life103-105. To achieve sustained high levels of these proteins in the tumor microenvironment while avoiding systemic toxicity, several groups have engineered bacterial strains for their tumor-targeted delivery106-110. An attenuated Salmonella strain expressing FasL showed significant antitumor activities against both primary and metastatic mouse tumors in a Fas-dependent fashion108. In another elegant example, two separate inducible systems were used to drive the expression of the cytotoxic Cp53 peptide derived from the p53 protein and autolysis of the bacteria to release Cp53 for maximal killing89.
In addition to genetic engineering for expressing cytotoxic proteins, tumor-targeting bacteria have been used to deliver cytotoxic agents that can exert greater bystander effect on the surrounding uninfected tumor cells44,46,111,112. In one study, the high-energy beta emitter 188-Rhenium (188Re) was conjugated to a polyclonal antibody against Listeria followed by incubation of the radiolabeled antibody with an attenuated Listeria strain44. The resulting radioactive Listeria were accumulated in metastases after systemic administration and reduced the number of metastases by 90% in the Panc-02 metastatic mouse tumor model. Another innovative approach capitalized on the ability of some bacteria to generate cytotoxic NO from NO3−112. Upon photo-irradiation, photoelectrons were excited from the carbon-dot doped carbon nitride (C3N4) loaded onto the surface of Escherichia coli (E. coli) and transferred to E. coli NO-generating enzymes, resulting in substantially enhanced production of NO and tumor suppression. The focused photo-irradiation enabled targeted generation of NO.
Prodrug-converting enzymes.
Prodrug-converting enzymes were among the first effector systems engineered into tumor-targeting bacteria. Once expressed by the tumor-localized bacteria, these enzymes can metabolize their systemically administered innocuous substrates (prodrugs) and convert them into cytotoxic products. The major advantage for using prodrug-converting enzymes is that the cytotoxic products are small molecules able to diffuse farther inside the solid tumor and across the cell membrane, thus generating potent bystander effect. Tumor-targeting bacteria have been engineered to express several prodrug-converting enzymes (TABLE 1). Cytosine deaminase (CD) converts the non-toxic 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU), a first-line chemotherapeutic agent for metastatic colorectal cancer113. The VNP20009 Salmonella strain engineered to express E. coli CD showed clearly enhanced antitumor activity when combined with 5-FC in both mouse syngeneic and human xenograft colorectal tumor models114. Similarly, a C. sporogenes strain expressing H. influenza nitroreductase (NTR) had promising antitumor effect as well115. Bacterial NTR catalyzes conversion of the weak monofunctional DNA-alkylating agent CB1954 into a bifunctional DNA-alkylating derivative that can induce DNA crosslinks and apoptosis. Repeated administration of the NTR-expressing strain along with CB1954 achieved sustained tumor control in a mouse xenograft tumor model115. The efficacy of this effector system depends on robust and sustained tumor colonization by the delivering bacterial vector, which ensures continued high-level expression of the prodrug-converting enzyme115,116. It is worth noting that bacteria also carry endogenous enzymes capable of metabolically activating multiple prodrugs117,118.
Immunomodulators.
To further stimulate antitumor immunity, tumor-targeting bacteria have been engineered to express either tumor antigens or immunoregulatory factors. Live bacteria as vectors for tumor vaccination have been reviewed elsewhere71,95,119. In addition to vaccination with bacteria expressing tumor antigens, another approach to augment tumor immunogenicity could involve presenting the immunodominant T cell antigens from tetanus toxoid, poliovirus, or measles virus on the surface of tumor cells infected by intracellular tumor-targeting bacteria carrying expression cassettes for these antigens. The immune system in most individuals has seen these antigens earlier during childhood vaccinations, and thus has generated memory T cells. These T cells can be reactivated when seeing these antigens again, resulting in destruction of the infected tumor cells. Antigen spreading from the destructed tumor cells may also take place to induce an immune response against the uninfected tumor cells.
Engineered tumor-targeting bacteria have the ability to bring immunomodulatory proteins to the tumor microenvironment. A number of bacterial strains have been engineered to express immunoregulatory factors in the tumor microenvironment to boost antitumor immunity (TABLE 1). For example, a Salmonella strain expressing biologically active IL-2 was generated more than 20 years ago and showed enhanced antitumor activities dependent on natural killer (NK) cells and CD8+ T cells in the mouse MCA-38 hepatic metastasis model86,120. The IL-2-expressing Salmonella has also been tested in both canine and human clinical trials (discussed below under Clinical translation). Different bacterial species expressing other cytokines have been generated as well (TABLE 1). In addition to the classic cytokines and chemokines, other proteins with immunomodulatory activities have also been documented to have promising therapeutic effects when delivered by tumor-targeting bacteria. For instance, an attenuated Salmonella strain engineered to express LIGHT, a member of the TNF superfamily, showed considerable antitumor activities in subcutaneous as well as metastatic mouse tumor models121. These antitumor activities required both CD4+ and CD8+ T cells. Mobilization of natural killer T (NKT) cells could also enhance bacterial antitumor activity. In an interesting study with the 4T1 syngeneic mouse tumor model, α-galactosylceramide, a glycolipid that can activate NKT cells, was incorporated metabolically into Listeria and shown to help eliminate metastases and improve survival72. A recent study employed heterologous flagellin as a potent immunoregulator122. In this study, the Salmonella ΔppGpp strain engineered to secrete Vibrio vulnificus flagellin B displayed markedly improved ability for tumor control compared to the parental strain. Mechanistic studies showed that infection with the Gram-negative Salmonella activated the TLR4/MyD88 pathway, presumably by LPS present in the outer membrane of Gram-negative bacteria, resulting in a massive tumor infiltration of macrophages and neutrophils. Secreted heterologous flagellin triggered the TLR5 pathway and further shifted the tumor-infiltrating macrophages toward an M1 phenotype, which was associated with increased levels of tumoricidal mediators including interleukin 1 beta (IL-1β), TNF-α, and nitric oxide (NO).
Recent clinical success with immune checkpoint blockade has prompt a wave of preclinical and clinical studies combining checkpoint inhibitory antibodies with therapeutic bacteria or viruses123-125. These studies tested the hypothesis that intratumoral infection by the infectious agents could establish a more immunogenic microenvironment, thus sensitizing the tumors to checkpoint blockade. A more straightforward approach would be to generate bacterial strains secreting checkpoint inhibitors such as an anti-PD-1 antibody or a soluble PD-1 extracellular domain to bind and neutralize the T cell-inhibitory PD-L1 expressed by tumor cells. This approach is technically possible as functional single-chain antibodies have been produced from tumor-targeting bacterial strains126. These inhibitors can also be expressed by infected tumor cells when intracellular bacteria carrying expression cassettes with mammalian gene promoters and secretory signals are used. As the expression of the checkpoint inhibitors are targeted to tumors, this approach will not activate T cells in normal tissues, thus potentially minimizing toxicity associated with systemic checkpoint blockade127.
Targeting tumor stroma.
Tumor cells can evade the immune system by downregulating the expression of tumor antigens as well as proteins involved in antigen processing and cell surface presentation128. Targeting tumor vasculature required for tumor growth circumvents this problem and may be particularly beneficial for bacterial therapy. As discussed earlier, bacteria preferentially colonize necrotic/hypoxic tumor areas. Disruption of tumor vasculature with microtubule-destabilizing agents leads to destruction of the well-perfused tumor regions and expands bacterial colonization129-131. Bacteria themselves can be engineered to induce destruction of tumor vasculature. Several vaccine strains against critical components of the angiogenic tumor vessels have been constructed and tested in both prophylactic and therapeutic settings(TABLE 1)132-139. For example, a Salmonella DNA vaccine strain targeting vascular-endothelial growth factor receptor 2 (VEGFR2, also known as FLK-1) was able to break peripheral immune tolerance and elicit cytotoxic T cell (CTL)-mediated immunity against this self antigen expressed on proliferating endothelial cells, leading to effective protection against tumor challenges132. Another study with a Listeria vaccine strain further suggested that the antitumor activity induced by VEGFR2 vaccines is dependent on epitope spreading to a tumor antigen139. Other stromal components may be targeted as well.
Synthetic gene networks.
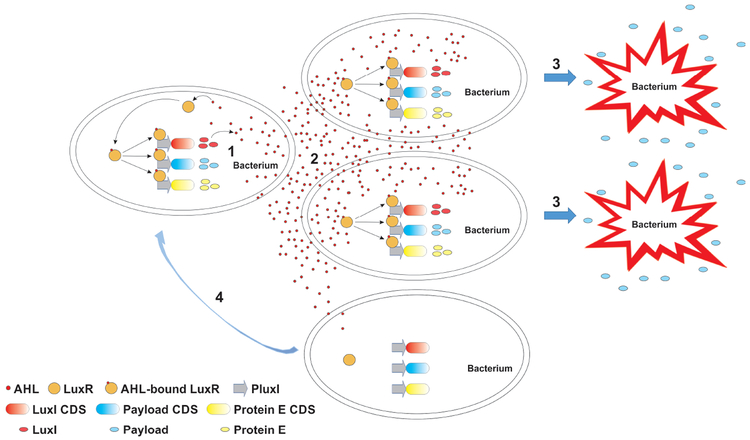
Both viruses and bacteria can be reprogrammed by genetic engineering, but bacteria can host heterologous DNA of considerably large sizes140, allowing for more sophisticated reprograming. The powerful recombinant DNA and synthetic biology technologies have even enabled recreation of viable bacterial cells by transplanting entire chemically synthesized genomes into recipient cells141,142. Therefore, bacteria have been dubbed “programmable robotic factories” at the microscopic scale21. Applying engineering (electrical engineering in particular) concepts, investigators have assembled biomolecular modules in bacteria to build genetic networks that can execute logical operations. Typical cis (e.g. promoters, enhancers) and trans (e.g. transcription factors, repressors) gene regulatory elements are employed and arranged in unique ways to form feedback and feedforward loops, called network motifs, with which the biological equivalents of electronic devices such as toggle switches, oscillators, and other sophisticated devices can be fabricated143-145. An elegant design using the quorum-sensing elements from Vibrio fischeri and Bacillus Thurigensis arranged to form negative feedback motifs enabled synchronized oscillations of gene expression in a population of bacterial cells146. In a subsequent study, this quorum-sensing gene circuit was modified to generate synchronized cyclical population self-control and anticancer drug delivery as the output90(FIG. 3). Once inside the tumor, the tumor-targeting Salmonella with this gene circuit underwent repeated cycles of population expansion and regression by autolysis in response to the density of bacterial cells. The lysis of the cells directly released the anticancer drug made by the bacteria. Thus, this gene circuit provided maximal release of the therapeutic payload through synchronized cell lysis and increased safety by maintaining the intratumoral bacterial population at a defined size, consequently minimizing the risk of a devastating systemic inflammatory response. This example illustrates the potential of gene networks to coordinate the behavior of bacteria at the population level in response to a particular environmental cue for an increased therapeutic index.
Fig. 3. Gene circuit for a transcriptional program regulating bacterial activities at the population level.
Illustrated is an example of a sophisticated gene circuit for a transcriptional program enabling synchronized population control and therapeutic payload release in repeated cycles. (1) The AHL-bound transcription factor LuxR interacts with and activates promoter PluxI that drives the expression of (from top to bottom) the AHL synthase LuxI to establish a positive feedback loop, the therapeutic payload, and the bacteriophage φX174 protein E to lyse the bacteria. CDS, coding sequence. (2) The AHL signaling molecules diffuse freely across the cell membranes, enabling synchronization of neighboring bacterial cells in the population for a concerted action. At low densities of the bacterial population, AHL molecules diffuse predominately out of bacterial cells, leaving the gene circuit inactive. Increased population density allows AHL molecules inside the majority of the bacterial cells to accumulate and reach a threshold concentration required to activate the gene circuit. (3) Synchronized activation of the transcriptional program leads to simultaneous lysis of bacterial cells in the population by protein E as well as a burst of therapeutic payload release. (4) The small number of bacteria surviving the lysis repopulate and kick off another cycle of lysis and payload release.
Experimental tumor models.
Preclinical animal study is a critical step toward clinical development of tumor-targeting bacteria. Colonization of tumor-targeting bacteria and subsequent antitumor activity can vary substantially among different preclinical models, because of the unique tumor microenvironment associated with particular tumor models. In addition to tumor histology, the method used to establish a tumor model can make a significant difference147. For instance, Listeria strain colonized the subcutaneously transplanted Panc02 pancreatic tumors and tumors spontaneously occurring in the genetically engineered KPC mice with comparable efficiencies46. However, the 4T1 mammary tumors transplanted subcutaneously to BALB/c mice were shown to support the colonization of two different attenuated Salmonella strains 10,000-fold more efficiently than the size-matched autochthonous mammary tumors spontaneously developed in transgenic BALB-neuT mice131. Interestingly, pretreatment with a vasculature-disrupting agent, shown to induce tumor necrosis, drastically improved tumor colonization in the autochthonous model. This example underscores the importance of identifying and employing the right tumor models for the assessment of both efficacy and toxicity that are truly relevant to human cancer patients. Perhaps a rational and hierarchical approach involving a variety of tumor models will help maximize the chance for the successful clinical development of a tumor-targeting bacterium-based therapeutic product 147.
Clinical translation
The number of published studies on bacterial cancer therapy have increased exponentially in recent years, many of which have shown promising results in experimental models21. Nevertheless, very few tumor-targeting bacteria have advanced to clinical stages. Model organisms share many genetic elements and biological pathways with humans, and yet fundamental differences exist. In addition, disease models lack the heterogeneity always seen in the patient population. Consequently, all experimental therapeutic approaches must pass the test in a patient population to show their clinical safety and utility. Translation of any novel therapeutic agent from the laboratory bench to the bedside would require enormous efforts, but is particularly challenging for live bacteria. Use of replication-competent bacteria in cancer therapy poses major challenges to both investigators and the regulatory authorities. Regulatory issues are among the most important issues that need to be addressed before a replication-competent bacterium can be applied to humans (Box 1).
Box 1. Regulatory considerations for clinical investigations of live tumor-targeting bacteria.
Distinct from conventional cancer treatment such as chemotherapies, targeted therapies or monoclonal antibody therapy, live tumor-targeting bacteria have its unique regulatory challenges. Detailed description of regulatory consideration and requirement is beyond the scope of this section. Listed below are some points for the sponsors to consider in initiation of clinical investigations using live bacteria for cancer treatment. The government regulatory agencies generally encourage the sponsors to consult the published guidance documents and engage with the regulatory agencies early in the development of live bacterium-based products.
- Preclinical study considerations.
- Preclinical proof-of-concept and safety studies are critically important for several reasons. They support the scientific rationale for proposed clinical studies, guide the selection of the initial clinical dose level, dose-escalation scheme, dosing schedule, and provide adequate safety information for the regulatory authorities to determine whether it is reasonably safe to conduct the proposed clinical trial.
- If previous human safety and activity data are available for a microbial vector used for gene therapy (MVGT) product including the live bacterium, additional extensive preclinical studies may not be necessary. However, to assess the relevance of the available data to specific product(s) previously administered to humans, adequate information regarding the manufacturing and characterization of the product(s) is required. In addition, sponsors should provide comprehensive activity and safety data from the previous human experience to support the safety of the proposed dosing of the MVGT product.
- Sponsors are encouraged to actively engage with the regulatory authorities early in product development to discuss above issues.
- Chemistry Manufacturing and Controls (CMC) considerations. The process of manufacturing live therapeutic bacteria is vastly more complex than that of the small molecules. Sponsors need to consider the following.
- The most optimal bacterial seed stock, banking system and the reagents used.
- The procedures in producing, purifying and harvesting live bacteria.
- The type of formulation of the final product.
- The tests for identity, purity and potency which face unique challenges for live bacteria as final products (also discussed in main text).
Pharmacokinetics and dose-response considerations. Live bacterial products do not follow typical patterns of pharmacokinetics and the dose-responses of conventional small-molecule drugs and proteins, thus posing challenges in determining the optimal starting dose and schedule for administration (also discussed in main text).
- Safety concerns. Safety is the major concern due to the infectious nature of the products, along with the concomitant medications and procedures for administering these products.
- Live bacterial products carry the risk of clinically significant infection/sepsis, especially in immunocompromised host. Administration of antibiotics post treatment, and in some cases, prolonged antibiotic administration may be needed to decrease this risk.
- For some products, it may be necessary that certain procedures are followed to administer these products. There are risks associated with these procedures. Thus, early clinical trials design would need to consider appropriate plans to mitigate these concerns.
Study population and study design. Discussed in main text.
- Relevant U.S. FDA guidance documents.
- “Recommendations for Microbial Vectors used for Gene Therapy” (September, 2016)182. This guidance focuses on the chemistry, manufacturing, and control (CMC) information that investigational new drug application (IND) sponsors should submit in an IND for MVGTs and provides an overview of preclinical and clinical considerations for these products. Many principles described in this guidance apply to microbial-based cancer therapies that are not genetically modified as well.
- “Preclinical Assessment of Investigational Cellular and Gene Therapy Products” (November, 2013)183. This guidance provides comprehensive recommendations regarding the selection of appropriate animal species and animal models of disease, as well as the overall design of preclinical proof-of-concept and toxicology studies for investigational products, including live bacterial products.
Challenges.
Given the unique nature of live engineered bacteria as therapeutic agents, a number of challenges should be considered. First, live genetically modified bacteria that carry antibiotic resistance genes or mobile genetic elements that can mediate horizontal gene transfer are generally not appropriate for clinical studies148. Chromosomal integration of the expression cassette without antibiotic selection markers provides a safer and more stable way for engineering149,150. Second, unlike small molecules or other non-viable clinical agents, live bacteria or bacterial spores cannot be sterilized either by heating or by filtering, which presents a major challenge for manufacturing Good Manufacturing Practices (GMP)-grade test articles. In addition, the conventional regulatory standard for sterility testing would not be feasible. Thus, production and purification in dedicated clean rooms following strict aseptic protocols with frequent in-process monitoring is the most practical way to ensure “sterility” (meaning no contamination from other live microorganisms). Although the final products cannot be demonstrated to be sterile, they should be assayed to be free from causative agents of other diseases or conditions, such as invasive bacterial pathogens listed by the Centers for Disease Control and Prevention (CDC)151 and specific pathogens described in the United States Pharmacopeia, Chapter 62 152, as appropriate. Third, live bacteria are proliferative in the target tissue and therefore, the effective (whether therapeutic or toxic) dose is not necessarily correlated with the administered dose. The effective dose depends more on the “quality” of the target tissue, which is defined by the accessibility, the extent of tumor necrosis/hypoxia, and the abundance of preexisting tumor-infiltrating inflammatory cells. These factors determine how easily the systemically administered bacteria can enter their target tissue and whether the target tissue can support a robust bacterial proliferation and spreading of the infection. The development of companion diagnostic approaches such as those based on angiography and hypoxia/necrosis imaging may help define the patient population that would benefit the most from bacterial therapy153-155. Additionally, germination and spreading of bacteria may be monitored directly by imaging the replicating bacteria156-158. It should also be noted that when low doses of bacteria are given, especially when administered systemically, the successful establishment of an infection in the target tissue is less predictable and may take much longer time to occur. This could pose a greater risk to the patients, because they are more likely to become less vigilant over time. Fourth, oncolytic bacterial therapy is a deliberate attempt to convert a tumor into a localized tumor-destructing infection, which may have serious consequences if not managed properly. The severity of the infection-associated toxicity generally correlates positively with the tumor size and the extent of necrosis/hypoxia inside the tumor that are important determinants for the robustness of the infection. As both therapeutic and toxic effects result from a robust infection, a carefully calculated balance is critical. Practically, this is difficult to achieve, because an antibiotic intervention too early would effectively eliminate the infection before an antitumor effect has been achieved, whereas a late intervention bears the risk of an unpredictable systemic inflammatory response. Effective management of the therapeutic infection requires experts across disciplines including oncologists, infectious disease specialists, and interventional radiologists or surgeons for managing abscess or non-abscess-forming infections that need invasive management. Therefore, when and how to intervene after an intratumoral infection has been established should be a team decision. Fifth, when a live biological agent is used in a clinical setting, its potential impact on public health and environment is always a concern and should be properly addressed.
Study population.
In general, for first-in-human (FIH) trials, risk and potential benefit need to be considered in the selection of the study subjects. Usually, subjects whose diseases are unresponsive or refractory to standard therapies are enrolled to the trials. For live bacterial products, additional considerations include intrinsic properties of the product and the concomitant therapies.
The underlying condition of the cancer patients might make them immunocompromised. They also may need to receive concomitant therapies to control their disease and some of these therapies (e.g., chemotherapy) can be immunosuppressive. To these patients, administering a live bacterial product may pose a significant risk of infection. Thus, in designing a FIH trial, the immune status of the patients and their prior / concomitant therapies need to be considered. Patients who are immunocompromised or receive concomitant immunotherapeutics may be excluded.
Certain patient conditions may particularly predispose patients to developing infections associated with administered live bacterial products because of their intrinsic properties. The following are examples to consider. (a) Bacteria in general, and anaerobic bacteria in particular, preferentially proliferate in necrotic tissues. Conditions such as brain abscess, diverticulitis or recent radiation treatment might promote the unintentional growth of these bacteria in non-target lesions, even though preclinical studies have shown that certain bacterial strains may not be able to gain access to the non-malignant lesions38,52,53. (b) Some live bacterial products have the potential to colonize foreign bodies such as artificial heart valves, joint replacement or implanted medical devices that may serve as reservoirs for these live products. Excluding patients with these conditions reduces the risk with these products.
Clinical Experience.
Several historical clinical observations with live antitumor bacteria have been documented as mentioned earlier. In recent years, carefully designed clinical trials for tumor-targeting bacteria have been conducted in both human patients and companion dogs with spontaneous tumors.
Canine studies.
Tumors developed spontaneously in companion dogs serve as an attractive model for human cancers159,160. These tumors resemble their human counterparts – originating from cells harboring naturally occurring mutations in hosts with heterogeneous genetic backgrounds. A few canine studies for tumor-targeting bacteria have been reported (TABLE 2). In one study, the Salmonella strain VNP20009 was given by intravenous (IV) infusion to 41 client-owned dogs with spontaneous tumors161. Complete and partial tumor responses were observed in 15% of the treated animals. Positive bacterial culture was obtained from tumor tissue in 42% of the cases; however this was not correlated with the administered doses. In another study, intratumoral injection of C. novyi-NT spores resulted in objective responses of target lesions in ~38% of 16 evaluable companion dogs54. Intriguingly, the objective response rate among the dogs with peripheral nerve sheath tumors was higher at ~57%. The numbers of dogs used in the trial were likely too small to achieve statistical significance, but should prompt further investigations to identify cancer types particularly sensitive to bacterial therapy. Tumor-targeting bacteria delivering therapeutic payloads have also been tested in canine patients. The Salmonella strain engineered to express IL-2 given at a neoadjuvant/adjuvant setting was combined with amputation and adjuvant doxorubicin to treat canine appendicular osteosarcoma86,162. The dogs in this study showed a significantly longer disease-free interval (DFI) when compared to historical controls treated with amputation and adjuvant doxorubicin, but not to those treated with amputation plus carboplatin and doxorubicin.
Table 2.
Clinical trials with engineered tumor-targeting bacteria
| Trial | Bacterial strain | No. patients treated/Cancer type | Treatment/Outcome | Reference/Recruitment Status |
|---|---|---|---|---|
| Canine trials with tumor-targeting strains | VNP20009 | 41 patients STS (AUS, FBS, RMS, HPC, MXS), melanoma, carcinomas, OSA, HSA, lymphoma, MCT |
IV infusion, 1.5×105 - 1×108 CFU/kg (dose escalation), 1 - 19 doses (mean=3) MTD 3×107 CFU/kg; tumor colonization observed in 42% cases; 4 CR, 2 PR |
Published161 |
| C. novyi-NT | 6 patients HSA, lingual SCC, OSA, nasal ACA, FBS |
IV infusion, 3×108 spores/kg, 3×107 spores/kg, 1 dose DLT (abscess formation) observed at 3×107 spores/kg; tumor abscess observed in 3 patients; 4 SD |
Published254 | |
| C. novyi-NT | 16 patients STS (PNST, RMS, FBS, MXS), OSAc, MCT, melanoma, SCS |
IT injection, 1×108 spores/dose, 1 - 4 doses Tumor abscess observed in 7 patients; 3 CR, 3 PR, 5 SD |
Published54 | |
| SalpIL2 (Salmonella χ4550 expressing IL-2) |
19 patients Appendicular OSA |
Neoadjuvant/adjuvant SalpIL2: PO, 1×105 - 1×109 CFU/patient (dose escalation), 6 (n=13), 4 (n=3), 3 (n=2), 1 (n=1) doses Amputation Adjuvant doxorubicin: IV, 30 mg/m2, 5 doses (n=13) No DLT observed; tumor colonization not evident in tumors from 5 patients assayed; DFI of patients treated with SalpIL2/doxorubicin significantly longer than comparison group treated with doxorubicin |
Published162 | |
| Human trials with tumor-targeting strains | VNP20009 | Phase 1 25 patients Melanoma, RCC |
30 min IV infusion, 1×106 - 1×109 CFU/m2 (dose escalation), 1 dose MTD 3×108 CFU/m2; tumor colonization observed in 3 patients in 2 highest dose cohorts; elevated circulating proinflammatory cytokines detected; objective tumor regression not observed |
Published63 |
| VNP20009 | 4 patients Melanoma |
4 hr IV infusion, 3×108 CFU/m2, 1 dose Treatment well tolerated; tumor colonization not evident; objective tumor response not observed |
Published64 | |
| TAPET-CD (VNP20009 expressing CD) |
3 patients Head & neck SCC, esophageal ACA |
TAPET-CD: IT injection, 3×106 CFU/m2, 1×107 CFU/m2, 3×107 CFU/m2 (dose escalation), multiple doses/cycles 5-FC: PO, 100 mg/kg/day divided thrice daily, multiple doses/cycles Tumor colonization evident in 2 patients; generation and accumulation of 5-FU observed in the 2 patients with TAPET-CD tumor colonization |
Published167 | |
| VNP20009 | Phase 1 Refractory, superficial solid tumors |
IT injection, dose escalation planned | Unpublished168, completed | |
| C. novyi-NT | Phase 1 2 patients Colorectal cancer |
IV infusion, 1×106 spores/kg, 1 dose | Unpublished170, terminated | |
| SalpIL2 (Salmonella χ4550 expressing IL-2) |
Phase 1 22 patients Liver metastases of solid tumors |
PO, 1×105 - 1×1010 CFU/dose (dose escalation planned) | Unpublished172, completed | |
| C. novyi-NT | Phase 1 5 patients Solid tumor malignancies |
IV infusion, 1×105 - 1×107 spores/kg (dose escalation planned), 1 dose | Unpublished173, terminated | |
| APS001F (B. longum expressing CD) |
Phase 1/2 75 patients (estimated) Advanced and/or Metastatic Solid Tumors |
APS001F ± maltose IV infusion/5-FC PO | Unpublished174, recruiting | |
| C. novyi-NT | Phase 1 24 patients Solid tumor malignancies |
IT injection, 1×104 - 3×106 spores/dose (dose escalation), 1 dose | Unpublished175, completed |
STS, soft tissue sarcoma; AUS, anaplastic/undifferentiated sarcoma; FBS, fibrosarcoma; RMS, rhabdomyosarcoma; HPC, hemangiopericytoma; MXS, myxosarcoma; OSA, osteosarcoma; HSA, hemangiosarcoma; MCT, mast cell tumor; SCC, squamous cell carcinoma; ACA, adenocarcinoma; PNST, peripheral nerve sheath tumor; OSAc, chondroblastic osteosarcoma; SCS, synovial cell sarcoma; RCC, renal cell carcinoma; PO, oral administration; CFU, colony-forming unit; MTD, maximum tolerated dose; DLT, dose-limiting toxicity; SAE, serious adverse event; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DFI, disease-free interval; OS, overall survival
Human studies.
A number of Listeria vaccine strains have been tested in clinical trials and some showed very encouraging results (TABLE 3)71,163,164. In comparison, human trials with tumor-targeting strains have been scarce. In addition to the historical human studies with live oncolytic bacteria18-20,165,166, a handful of human clinical trials have been reported and a few more registered with the federal regulatory authorities in more recent years (TABLE 2)54,63,64,167-175 (a search at EU Clinical Trials Register and UK Clinical Trials Gateway websites using relevant keywords did not return any result on trials with Clostridium, Salmonella, Listeria, Bidobacterium, Lactobacilli, or Escherichia).
Table 3.
Clinical trials with live attenuated Listeria monocytogenes (Lm)-based vaccines
| Vaccine name (attenuated strain) Combination agent |
Peptide antigen expressed | Trial type/No. patients treated/Cancer type | Treatment/Outcome | Reference/Recruitment Status |
|---|---|---|---|---|
| Lm-LLO-E7 (Lm-LLO) | HPV-16 E7 fused to LLO | Phase 1 15 patients (13 evaluable) Cervical cancer |
IV infusion, 1×109 CFU, 3.3×109 CFU or 1×1010 CFU/dose (dose escalation) ×2 All experienced flu-like syndrome, grade 3 AEs reported in 6 patients (40%), DLT (grade 2 diastolic hypotension) reported in 3 patients in the 1 × 1010 CFU group; Lm-LLO-E7 rapidly cleared from blood; Positive T cell response (IFNγ-ELISpot) specific to HPV-16 E7 peptides observed after the second dose on day 26 in one patient; 7 (53.8%) SD, 5 (38.5%) PD; 1 (7.7%) PR |
Published255 |
| ADXS11-001 (Lm-LLO) Cisplatin |
HPV-16 E7 fused to LLO | Phase 2 109 patients (69 evaluable) Cervical cancer |
ADXS11-001 only group (n=55): IV infusion of ADXS11-001 (1×109 CFU) on days 1, 29, 57 ADXS11-001 + cisplatin group (n=54): IV infusion of ADXS11-001 (1×109 CFU) on days 1, 85, 113, 141 and IV infusion of cisplatin (40 mg/m2) on days 29, 36, 43, 50, 57 Median OS comprable between the two groups, OS (two groups combined) 34.9% at 12 months and 24.8% at 18 months; most AEs mild to moderate, SAEs reported in two patients (CRS with one and bacterial peritonitis with septicemia in another) treated with ADXS11-001 monotherapy. |
Published256 |
| ANZ-100/CRS-100 (LADD) | None | Phase 1 9 patients Pancreatic cancer, colorectoal cancer, and melanoma all with liver metastases |
A single-dose IV infusion, 1 × 106, 3 × 107, 3 × 108 CFU/dose (dose escalation) Most frequent AEs: transient lymphopenia, hyperglycemia, hypophosphatemia, fever; MTD not reached; CD38 on NK cells increased after treatment; serum MCP-1 and MIP-1β elevated in patients after treatment at highest dose level, elevated IFN-γ and IL-12p70 also observed; ANZ-100 not detected in blood, stool, urine, or sputum at any time point |
Published257,258 |
| CRS-207 (LADD) | Mesothelin | Phase 1 17 patients Pancreatic cancer, mesothelioma, ovarian cancer, NSCLC |
IV infusion, 1 × 108, 3 × 108, 1 × 109, and 1 × 1010 CFU/dose x1-4 every 3 weeks Most frequent AEs: transient lymphopenia, hypophosphatemia, transaminitis, fever, chills/rigors, nausea, fatigue, hypotension; grade 2 CRS reported in 1 patient dosed at 1×1010 CFU; MTD 1×109; elevated MCP-1, MIP-1β and IP-10 for all dose levels observed; LLO-specific and mesothelin-specific T cell responses (IFNγ-ELISpot) detected; 37% of patients survived for 15 months; CRS-207 detected in blood cultures of 1 patient receiving 1×109 CFU at day 4 after 2nd IV infusion, CRS-207 not detected in all remaining blood, stool, or urine samples at any time point |
Published257,259 |
| CRS-207 (LADD) GVAX pancreas vaccine Cy |
Mesothelin | Phase 2 90 patients Pancreatic cancer |
Arm A (n=61): IV infusion of Cy (200 mg/m2) on day 1 of and ID injections of GVAX (5×108 cells) on day 2 of weeks 1 and 4; IV infusion of CRS-207 (109 CFU) on day 1 of weeks 7, 10, 13 and 16 Arm B (n=29): IV infusion of Cy (200 mg/m2) on day 1 of and ID injections of GVAX (5×108 cells) on day 2 of weeks 1, 4, 7, 10, 13 and 16 OS 6.1 months in arm A vs 3.9 months in arm B (hazard ratio 0.59; P=0.02); stabilized or reduced CA19-9 levels observed in 27% of patients in arm A and 9% of patients in arm B (two-sided P=0.08); An increase (P=0.042) in mesothelin-specific CD8+ T cells over baseline documented at week 20 in arm A only; Most frequent AEs in arm A: injection site reactions, nausea, vomiting, chills, fever, fatigue; grade 3 to 4 lymphopenia and transaminitis observed after CRS-207 infusion |
Published163,260 |
| ADXS11-001(Lm-LLO) | HPV-16 E7 fused to LLO | Phase 2 81 patients Cervical Intraepithelial Neoplasia |
IV infusion, 5×107, 3.3×108, or 1×109 CFU/dose x3 at 28 day intervals; placebo control arm: normal saline x3 at 28 day intervals | Unpublished261; terminated |
| ADXS11-001(Lm-LLO) | HPV-16 E7 fused to LLO | Phase 2 67 patients (estimated) Cervical cancer |
IV infusion on day 1, repeated every 28 days in the absence of disease progression or unacceptable toxicity. | Unpublished262; active, not recruiting |
| ADXS11-001(Lm-LLO) | HPV-16 E7 fused to LLO | Phase 1 2 patients HPV-16+, p16+OPSCC |
IV infusion, 3.3×108, 1×109, or 3.3×109 CFU/dose (dose escalation) x3 at 28 day intervals | Unpublished263,264; terminated |
| ADXS11-001(Lm-LLO) Ablative transoral robotic surgery |
HPV-16 E7 fused to LLO | Phase 2 30 patients (estimated) HPV+OPSCC |
IV infusion, 1×109 CFU/dose x2, administered ~33 and ~14 days before ablative transoral robotic surgery; control arm: surgery only | Unpublished265; recruiting |
| ADXS11-001(Lm-LLO) Chemoradiation (mitomycin, 5-FU and radiation) |
HPV-16 E7 fused to LLO | Phase 1/2 11 patients Anal cancer |
IV infusion, 1×109 CFU/dose x4, 1st dose administered 10-14 day before initiation of chemoradiation, 2nd dose administered at least 10 days after completion of chemoradiation; 2nd, 3rd and 4th doses administered at 28 day intervals | Unpublished266; terminated |
| ADXS11-001(Lm-LLO) | HPV-16 E7 fused to LLO | Phase 1/2 25 patients (estimated) HPV+ Cervical cancer |
1×1010 CFU/dose x3 at 28 day intervals | Unpublished267; active, not recruiting |
| ADXS11-001(Lm-LLO) MEDI4736 (durvalumab) |
HPV-16 E7 fused to LLO | Phase 1/2 66 patients (estimated) HPV+ Head & neck SCC or cervical cancer |
IV Infusion, ADXS11-001 plus MEDI4736 or MEDI4736 alone | Unpublished268; suspended |
| ADXS11-001(Lm-LLO) | HPV-16 E7 fused to LLO | Phase 2 55 patients (estimated) Anal or rectal cancer |
No information provided | Unpublished269; active, not recruiting |
| ADXS11-001(Lm-LLO) | HPV-16 E7 fused to LLO | Phase 3 450 patients (estimated) Cervical cancer |
Double-blind, placebo-controlled randomized study of ADXS11-001 administered in adjuvant setting after completion of cisplatin-based concurrent chemoradiotherapy; IV infusion administered every 3 weeks for 3 doses for the first 3 months, followed by treatment every 8 weeks for a total of 5 doses or until disease recurrence | Unpublished270; recruiting |
| ADXS11-001(Lm-LLO) Pemetrexed |
HPV-16 E7 fused to LLO | Phase 2 124 patients (estimated) HPV+ NSCLC |
Treatment arm: pemetrexed plus ADXS11-001 immunotherapy Control arm: pemetrexed only | Unpublished271; not yet recruiting |
| ADXS31-164 (Lm-LLO) | HER2/neu fused to LLO | Phase 1/2 12 patients HER2/neu+solid tumors |
Dose escalation | Unpublished272; active, not recruiting |
| ADXS-NEO (Lm-LLO) | Personalized neoepitopes fused to LLO | Phase 1 48 patients (estimated) Colorecal cancer, head & neck SCC, or NSCLC |
Dose escalation/de-escalation study with a standard 3 + 3 design; 3 dose levels planned: 1×109, 2×109, 4×109 CFU | Unpublished273; recruiting |
| CRS-207 (LADD) Pemetrexed and cisplatin |
Mesothelin | Phase 1b 60 patients Mesothelioma |
IV infusion of CRS-207 (1×109 CFU) with or without Cy (200 mg/m2) at weeks 1, 3, 23 and 26; maintenance CRS-207 vaccinations every 8 weeks starting at week 34 until disease progression; pemetrexed (500 mg/m2) and cisplatin (75 mg/m2) at weeks 5, 8, 11, 14, 17 and 20 | Unpublished274; active, not recruiting |
| CRS-207 (LADD) GVAX pancreas vaccine Cy |
Mesothelin | Phase 2b 303 patients Pancreatic cancer |
Cy/GVAX + CRS-207 arm: IV infusion of Cy (200 mg/m2) on day 1 of and ID injection of GVAX (5×108 cells) on day 2 of weeks 1 and 4; IV infusion of CRS-207 (1×109 CFU) on day 1 of weeks 7, 10, 13 and 16 CRS-207 arm: IV infusion of CRS-207 (1×109 CFU) on day 1 of weeks 1, 4, 7, 10, 13 and 16 |
Unpublished275; completed |
| CRS-207 (LADD) Epacadostat and pembrolizumab |
Mesothelin | Phase 1/2 126 patients (estimated) Ovarian, fallopian or peritoneal cancer |
Phase 1/arm 1: IV infusion of CRS-207 (1×109 CFU), oral administration of epacadostat (100 or 300 mg, twice a day) Phase 1/arm 2: IV infusion of CRS-207 (1×109 CFU) only Phase 2/arm 1: IV infusion of pembrolizumab (200 mg), IV infusion of CRS-207 (1×109 CFU) Phase 2/arm 2: IV infusion of pembrolizumab (200 mg), IV infusion of CRS-207 (1×109 CFU), oral administration of epacadostat (300 mg, twice a day) |
Unpublished276; active, not recruiting |
| CRS-207 (LADD) Pembrolizumab |
Mesothelin | Phase 2 35 patients (estimated) Mesothelioma |
IV infusion of pembrolizumab (200 mg) and CRS-207 (1×109 CFU) for 4 × 3-week cycles, followed by pembrolizumab treatment at 3 week intervals and CRS-207 treatment at 6 week intervals up to 24 months | Unpublished277; active, not recruiting |
| CRS-207 (LADD) Pembrolizumab |
Mesothelin | Phase 2 79 patients (estimated) Gastric, gastroesophageal junction, or esophageal cancer |
IV infusion of pembrolizumab (200 mg) and CRS-207 (1×109 CFU) for 4 × 3-week cycles, followed by pembrolizumab treatment at 3 week intervals and CRS-207 treatment at 6 week intervals up to 24 months | Unpublished278; active, not recruiting |
| ADU-623 (LADD) | EGFRvIII and NY-ESO-1 | Phase 1 11 patients Astrocytomas |
IV infusion, 1×108 or 1×109 CFU/dose (dose escalation) x4 at 21 day intervals | Unpublished279; active, not recuiting |
| ADU-214/JNJ-64041757 (LADD) | EGFRvIII and Mesothelin | Phase 1 42 patients (estimated) NSCLC |
IV infusion, 1×108 or 1×109 CFU/dose (dose escalation) at 21 day intervals | Unpublished280; recuiting |
| ADU-741/JNJ-64041809 (LADD) | Prostate-specific antigens | Phase 1 26 patients Prostate cancer |
IV infusion, 1×108 or 1×109 CFU/dose (dose escalation) at 21 day intervals | Unpublished281; active, not recuiting |
| pLADD (LADD) | Personalized neoepitopes | Phase 1 15 patients (estimated) Colorectal cancer |
IV infusion, 1×108 or 1×109 CFU/dose (dose escalation) at 21 day intervals | Unpublished282; recruiting |
AEs, adverse events; CRS, cytokine release syndrom; GVAX pancreas, granulocyte-macrophage colony-stimulating factor (GM-CSF)–secreting allogeneic pancreatic tumor cells; Cy, cyclophosphamide; ID, intradermal; HPV, human papillomavirus; OPSCC, oropharyngeal squamous cell carcinoma; NSCLC, non-small-cell lung carcinoma; 5-FU, fluorouracil
Historical studies with an oncolytic Clostridium strain have documented robust tumor colonization and tumor lysis in different cancer types165,166,176. Similarly, clinical signs of colonization have been observed in a large fraction of patients treated with either IV or intratumoral administration of C. novyi-NT spores in more recent Phase I trials170,173,175. Objective evidence of tumor response has also been shown in these trials. For example, extensive tumor destruction along with gas pockets, a signature sign for infection of the gas-forming clostridia, was observed by CT scan in a patient who received direct injection of C. novyi-NT spores into a metastatic shoulder lesion54,175. Biopsies of the lesion revealed extensive tumor necrosis and absence of viable tumor cells. Anaerobic culture of the biopsied material was positive for C. novyi-NT, suggesting its involvement in tumor destruction. However, these treatments with oncolytic bacteria alone failed to eradicate all cancer cells, which inevitably led to progression or relapse.
Attenuated Salmonella strains and their derivatives engineered to express therapeutic payloads have also been tested in early clinical trials63,64,167-169,171,172. Similar to the oncolytic Clostridium strains, Salmonella strains are reasonably tolerated in cancer patients. Unexpectedly, the Salmonella strains tested so far have yet to show the robust colonization and therapeutic benefit repeatedly observed in preclinical studies. The reason for this discrepancy is unclear, but over-attenuation has been proposed as a reason. It is worth noting that intratumoral-injected Salmonella expressing E. coli cytosine deaminase was able to colonize the target tumors and convert 5-FC to 5-FU inside the colonized lesions, resulting in a 3:1 tumor-to-plasma ratio of 5-FU167. This study demonstrated that bacteria colonizing human tumors can express significant amounts of functional enzymes.
Although small in number, these early human trials have already taught us a few important lessons. (1) The attenuated tumor-targeting bacteria are reasonably tolerated in human patients and toxicities observed are very similar to those seen in experimental animals. (2) Robust colonization is a prerequisite for significant clinical benefit. Future clinical studies may employ companion diagnostic approaches based on angiography and hypoxia/necrosis imaging to define a patient population potentially more sensitive to intratumoral bacterial colonization153-155. Alternatively, engineering bacteria to express proteins targeting tumor vasculature or combining bacteria with microtubule-destabilizing agents may help expand colonization in an otherwise less hypoxic tumor129,130,132-139.
Conclusions and future perspectives
Tumor-targeting bacteria are ideal vehicles to deliver therapeutic payloads because of their tumor selectivity and vast gene packaging capacity. This essentially unlimited gene packaging capacity would allow not only expression of large and multiple therapeutic proteins, but also engineering of bacteria with gene networks, enabling them to perform more sophisticated tasks in the fight against cancer. Despite the great therapeutic potential of engineered tumor-targeting bacteria, a successful cancer therapy is still likely to require combination approaches in the near future, because cancer heterogeneity, at both molecular and histologic levels, makes it very difficult to achieve cure with single anticancer agents. Bacteria thrive in necrotic and hypoxic tumor regions, but not in the highly perfused areas. The contrary is true for cytotoxic therapies, such as chemotherapeutic agents or radiation, which are often more effective against tumor cells in well perfused tumor areas177. Thus, bacteria and the cytotoxic therapies should synergize with each other for antitumor activities61,178-180. Tumor-targeting bacteria have further been shown to drive the G0/G1 to S/G2/M cell cycle transition of tumor cells, making them more susceptible to chemotherapy180,181. Conversely, therapies with small molecules targeting tumor vasculature can enlarge the hypoxic niche inside the solid tumor, consequently increasing bacterial colonization129-131, which is particularly important for tumors without extensive hypoxia. In addition, intratumoral bacterial infection can modulate antitumor immune response both systemically and in the tumor microenvironment (FIG. 1), making it attractive to combine live bacteria with other systemic immunotherapeutic approaches such as immune checkpoint blockade. With more rationally designed tumor-targeting bacteria entering clinical studies, therapy with these bacteria will hopefully become another powerful weapon in the arsenal for our fight against cancer in the near future.
Acknowledgments
This work was supported by The Virginia and D.K. Ludwig Fund for Cancer Research (SZ), BioMed Valley Discoveries, Inc. (SZ), Pancreatic Cancer Action Network grant PCAN-422247 (CG), and NIH grants CA062924 (SZ), CA199010 (CG), and GM098207 (DB).
Glossary list
- Germinated Clostridia
actively growing Clostridia germinated from clostridial spores
- Exotoxin
bacterial toxin secreted into the surroundings
- Gram-negative bacteria
bacteria including Salmonella unable to retain the crystal violet stain used in the Gram-staining method for bacterial differentiation
- Sepsis
a life-threatening complication associated with an infection triggering systemic inflammatory responses that can lead to tissue damage and organ failure
- Auxotrophic mutant
a mutant bacterial strain that has an additional nutritional requirement for growth compared to its parental strain
- Promoter traps
experimental approaches to identify particular promoters in a genome by using a promoterless reporter gene
- Autolysis
destruction of a cell through a mechanism present within the cell
- Bystander effect
referring in this article specifically to therapeutic effect on cells that are not infected by the bacteria
- Antigen spreading (or “epitope spreading”)
the expansion of an immune response to antigens that are not the original antigen targeted in the therapy
- Natural killer T (NKT) cells
a heterogeneous population of T cells that express an invariant αβ T-cell receptor and a number of cell surface molecules typically associated with natural killer cells
- Quorum-sensing
a bacterial cell-cell communication process that regulates gene expression in response to fluctuations in cell-population density
- Autochthonous tumors
tumors developed spontaneously, including those developed in genetically modified models or induced by chemical, viral, or physical carcinogens, as opposed to transplanted tumors
Footnotes
Competing interests: Under a licensing agreement between BioMed Valley Discoveries, Inc. and the Johns Hopkins University, SZ is entitled to a share of royalties received by the University on sales of products described in this article. SZ is also a Founding Scientific Advisor of Personal Genome Diagnostics, Inc. and a Founder of PapGene, Inc., companies focused on developing genetics-based cancer diagnostics. The terms of these arrangements are under ongoing management by the Johns Hopkins University in accordance with its conflict of interest policies. DB has financial interest in AviexTechnologies and Magna Therapeutics, and receives royalties from Yale University for technologies based on those described in this article.
References
- 1.Piccart-Gebhart MJ et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353, 1659–1672, doi: 10.1056/NEJMoa052306 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Romond EH et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353, 1673–1684, doi:353/16/1673 [pii] 10.1056/NEJMoa052122 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N Engl J Med (2004). [DOI] [PubMed] [Google Scholar]
- 4.Messersmith WA & Ahnen DJ Targeting EGFR in colorectal cancer. N Engl J Med 359, 1834–1836, doi: 10.1056/NEJMe0806778 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Bollag G et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467, 596–599, doi: 10.1038/nature09454 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaherty KT et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367, 1694–1703, doi: 10.1056/NEJMoa1210093 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widakowich C, de Castro G Jr., de Azambuja E, Dinh P & Awada A Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist 12, 1443–1455, doi: 10.1634/theoncologist.12-12-1443 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Lynch TJ Jr. et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist 12, 610–621, doi: 10.1634/theoncologist.12-5-610 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Imai K & Takaoka A Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 6, 714–727, doi: 10.1038/nrc1913 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Rotow J & Bivona TG Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 17, 637–658, doi: 10.1038/nrc.2017.84 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Topalian SL, Drake CG & Pardoll DM Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461, doi: 10.1016/j.ccell.2015.03.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postow MA, Callahan MK & Wolchok JD Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 33, 1974–1982, doi: 10.1200/JCO.2014.59.4358 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le DT et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science, doi: 10.1126/science.aan6733 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang J, Sawa T & Maeda H Factors and mechanism of “EPR” effect and the enhanced antitumor effects of macromolecular drugs including SMANCS. Adv Exp Med Biol 519, 29–49 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Jain RK Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol 31, 2205–2218, doi: 10.1200/JCO.2012.46.3653 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fluegen G et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat Cell Biol 19, 120–132, doi: 10.1038/ncb3465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mowday AM et al. Advancing Clostridia to Clinical Trial: Past Lessons and Recent Progress. Cancers (Basel) 8, doi: 10.3390/cancers8070063 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coley WB II. Contribution to the Knowledge of Sarcoma. Ann Surg 14, 199–220 (1891). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coley WB The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res, 3–11 (1991). [PubMed] [Google Scholar]
- 20.Nauts HC, Swift WE & Coley BL The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, M.D., reviewed in the light of modern research. Cancer Res 6, 205–216 (1946). [PubMed] [Google Scholar]
- 21.Forbes NS Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer 10, 785–794, doi: 10.1038/nrc2934 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bermudes D, Zheng LM & King IC Live bacteria as anticancer agents and tumor-selective protein delivery vectors. Current opinion in drug discovery & development 5, 194–199 (2002). [PubMed] [Google Scholar]
- 23.Theys J et al. Tumor-specific gene delivery using genetically engineered bacteria. Curr Gene Ther 3, 207–221 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarty AM Microorganisms and cancer: quest for a therapy. J Bacteriol 185, 2683–2686 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minton NP Clostridia in cancer therapy. Nat Rev Microbiol 1, 237–242 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Van Mellaert L, Barbe S & Anne J Clostridium spores as anti-tumour agents. Trends in microbiology 14, 190–196 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Hoffman RM Tumor-seeking Salmonella amino acid auxotrophs. Current opinion in biotechnology 22, 917–923, doi: 10.1016/j.copbio.2011.03.009 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Lamm DL BCG immunotherapy for transitional-cell carcinoma in situ of the bladder. Oncology (Williston Park) 9, 947–952, 955, discussion 955-965 (1995). [PubMed] [Google Scholar]
- 29.Morales A, Eidinger D & Bruce AW Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. The Journal of urology 116, 180–183 (1976). [DOI] [PubMed] [Google Scholar]
- 30.De Jager R et al. Long-term complete remission in bladder carcinoma in situ with intravesical TICE bacillus Calmette Guerin. Overview analysis of six phase II clinical trials. Urology 38, 507–513 (1991). [DOI] [PubMed] [Google Scholar]
- 31.Herr HW et al. Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol 13, 1404–1408 (1995). [DOI] [PubMed] [Google Scholar]
- 32.Bohle A, Gerdes J, Ulmer AJ, Hofstetter AG & Flad HD Effects of local bacillus Calmette-Guerin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. The Journal of urology 144, 53–58 (1990). [DOI] [PubMed] [Google Scholar]
- 33.Thalmann GN et al. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. The Journal of urology 164, 2129–2133 (2000). [PubMed] [Google Scholar]
- 34.Sharma P, Old LJ & Allison JP Immunotherapeutic strategies for high-risk bladder cancer. Semin Oncol 34, 165–172 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasinskas RW & Forbes NS Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res 67, 3201–3209, doi: 10.1158/0008-5472.CAN-06-2618 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Pawelek JM, Low KB & Bermudes D Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res 57, 4537–4544 (1997). [PubMed] [Google Scholar]
- 37.Forbes NS, Munn LL, Fukumura D & Jain RK Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Res 63, 5188–5193 (2003). [PubMed] [Google Scholar]
- 38.Diaz LA Jr. et al. Pharmacologic and toxicologic evaluation of C. novyi-NT spores. Toxicol Sci 88, 562–575 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Lambin P et al. Colonisation of Clostridium in the body is restricted to hypoxic and necrotic areas of tumours. Anaerobe 4, 183–188, doi: 10.1006/anae.1998.0161 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Clairmont C et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis 181, 1996–2002, doi:JID991140 [pii] 10.1086/315497 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Yu YA et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol 22, 313–320 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Min JJ et al. Noninvasive real-time imaging of tumors and metastases using tumor-targeting light-emitting Escherichia coli. Mol Imaging Biol 10, 54–61, doi: 10.1007/s11307-007-0120-5 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Weibel S, Stritzker J, Eck M, Goebel W & Szalay AA Colonization of experimental murine breast tumours by Escherichia coli K-12 significantly alters the tumour microenvironment. Cell Microbiol 10, 1235–1248, doi: 10.1111/j.1462-5822.2008.01122.x (2008). [DOI] [PubMed] [Google Scholar]
- 44.Quispe-Tintaya W et al. Nontoxic radioactive Listeria(at) is a highly effective therapy against metastatic pancreatic cancer. Proc Natl Acad Sci U S A 110, 8668–8673, doi: 10.1073/pnas.1211287110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH & Gravekamp C Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br J Cancer 108, 2281–2290, doi: 10.1038/bjc.2013.206 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandra D et al. 32-Phosphorus selectively delivered by listeria to pancreatic cancer demonstrates a strong therapeutic effect. Oncotarget 8, 20729–20740, doi: 10.18632/oncotarget.15117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agrawal N et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci U S A 101, 15172–15177 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westphal K, Leschner S, Jablonska J, Loessner H & Weiss S Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res 68, 2952–2960, doi: 10.1158/0008-5472.CAN-07-2984 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Lardner A The effects of extracellular pH on immune function. Journal of leukocyte biology 69, 522–530 (2001). [PubMed] [Google Scholar]
- 50.Joyce JA & Fearon DT T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80, doi: 10.1126/science.aaa6204 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Low KB et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol 17, 37–41. (1999). [DOI] [PubMed] [Google Scholar]
- 52.Staedtke V et al. Clostridium novyi-NT can cause regression of orthotopically implanted glioblastomas in rats. Oncotarget 6, 5536–5546, doi: 10.18632/oncotarget.3627 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu YA, Zhang Q & Szalay AA Establishment and characterization of conditions required for tumor colonization by intravenously delivered bacteria. Biotechnol Bioeng 100, 567–578, doi: 10.1002/bit.21785 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Roberts NJ et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med 6, 249ra111, doi: 10.1126/scitranslmed.3008982 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avogadri F et al. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res 65, 3920–3927, doi: 10.1158/0008-5472.CAN-04-3002 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Lee CH, Wu CL & Shiau AL Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin Cancer Res 14, 1905–1912, doi: 10.1158/1078-0432.CCR-07-2050 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Leschner S et al. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha. PLoS One 4, e6692, doi: 10.1371/journal.pone.0006692 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee CH, Hsieh JL, Wu CL, Hsu PY & Shiau AL T cell augments the antitumor activity of tumor-targeting Salmonella. Appl Microbiol Biotechnol 90, 1381–1388, doi: 10.1007/s00253-011-3180-z (2011). [DOI] [PubMed] [Google Scholar]
- 59.Kaimala S et al. Salmonella-mediated tumor regression involves targeting of tumor myeloid suppressor cells causing a shift to M1-like phenotype and reduction in suppressive capacity. Cancer Immunol Immunother 63, 587–599, doi: 10.1007/s00262-014-1543-x (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X et al. The genes slyA, STM3120 and htrA are required for the anticancer ability of VNP20009. Oncotarget 7, 81187–81196, doi: 10.18632/oncotarget.13217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dang LH, Bettegowda C, Huso DL, Kinzler KW & Vogelstein B Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A 27, 27 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beutler B & Rietschel ET Innate immune sensing and its roots: the story of endotoxin. Nature reviews. Immunology 3, 169–176, doi: 10.1038/nri1004 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Toso JF et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 20, 142–152 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heimann DM & Rosenberg SA Continuous intravenous administration of live genetically modified salmonella typhimurium in patients with metastatic melanoma. J Immunother 26, 179–180 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Na HS et al. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine 24, 2027–2034, doi: 10.1016/j.vaccine.2005.11.031 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Jeong JH et al. Salmonella enterica serovar gallinarum requires ppGpp for internalization and survival in animal cells. J Bacteriol 190, 6340–6350, doi: 10.1128/JB.00385-08 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao M et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A 102, 755–760, doi: 10.1073/pnas.0408422102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao M et al. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci U S A 104, 10170–10174, doi: 10.1073/pnas.0703867104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freitag NE, Rong L & Portnoy DA Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect Immun 61, 2537–2544 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gunn GR et al. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol 167, 6471–6479 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Wood LM & Paterson Y Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol 4, 51, doi: 10.3389/fcimb.2014.00051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh M et al. Direct incorporation of the NKT-cell activator alpha-galactosylceramide into a recombinant Listeria monocytogenes improves breast cancer vaccine efficacy. Br J Cancer 111, 1945–1954, doi: 10.1038/bjc.2014.486 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brockstedt DG et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A 101, 13832–13837, doi: 10.1073/pnas.0406035101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agarwal N, Sonpavde G & Sternberg CN Novel Molecular Targets for the Therapy of Castration-Resistant Prostate Cancer. Eur Urol, doi:S0302-2838(11)01412-6 [pii] 10.1016/j.eururo.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 75.Thompson RJ, Bouwer HG, Portnoy DA & Frankel FR Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires D-alanine for growth. Infect Immun 66, 3552–3561 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malmgren RA & Flanigan CC Localization of the Vegetative Form of Clostridium tetani in Mouse Tumors Following Intravenous Spore Administration. Cancer Res. 15, 473–478 (1955). [PubMed] [Google Scholar]
- 77.Moese JR & Moese G Oncolysis by Clostridia. I. Activity of Clostridium Butyricum (M-55) and Other Nonpathogenic Clostridia against the Ehrlich Carcinoma. Cancer Res 24, 212–216 (1964). [PubMed] [Google Scholar]
- 78.Park SH et al. RGD Peptide Cell-Surface Display Enhances the Targeting and Therapeutic Efficacy of Attenuated Salmonella-mediated Cancer Therapy. Theranostics 6, 1672–1682, doi: 10.7150/thno.16135 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bereta M et al. Improving tumor targeting and therapeutic potential of Salmonella VNP20009 by displaying cell surface CEA-specific antibodies. Vaccine 25, 4183–4192, doi: 10.1016/j.vaccine.2007.03.008 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Massa PE, Paniccia A, Monegal A, de Marco A & Rescigno M Salmonella engineered to express CD20-targeting antibodies and a drug-converting enzyme can eradicate human lymphomas. Blood 122, 705–714, doi: 10.1182/blood-2012-12-474098 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Pinero-Lambea C et al. Programming controlled adhesion of E. coli to target surfaces, cells, and tumors with synthetic adhesins. ACS synthetic biology 4, 463–473, doi: 10.1021/sb500252a (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galen JE & Levine MM Can a ‘flawless’ live vector vaccine strain be engineered? Trends in microbiology 9, 372–376 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Minton NP et al. Chemotherapeutic tumour targeting using clostridial spores. FEMS Microbiol Rev 17, 357–364 (1995). [DOI] [PubMed] [Google Scholar]
- 84.Sizemore DR, Branstrom AA & Sadoff JC Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science 270, 299–302 (1995). [DOI] [PubMed] [Google Scholar]
- 85.Fox ME et al. Anaerobic bacteria as a delivery system for cancer gene therapy: in vitro activation of 5-fluorocytosine by genetically engineered clostridia. Gene Ther 3, 173–178 (1996). [PubMed] [Google Scholar]
- 86.Saltzman DA et al. Attenuated Salmonella typhimurium containing interleukin-2 decreases MC-38 hepatic metastases: a novel anti-tumor agent. Cancer Biother Radiopharm 11, 145–153, doi: 10.1089/cbr.1996.11.145 (1996). [DOI] [PubMed] [Google Scholar]
- 87.Darji A et al. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell 91, 765–775 (1997). [DOI] [PubMed] [Google Scholar]
- 88.Russmann H et al. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science 281, 565–568 (1998). [DOI] [PubMed] [Google Scholar]
- 89.Camacho EM, Mesa-Pereira B, Medina C, Flores A & Santero E Engineering Salmonella as intracellular factory for effective killing of tumour cells. Sci Rep 6, 30591, doi: 10.1038/srep30591 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Din MO et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 536, 81–85, doi: 10.1038/nature18930 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hense M et al. Eukaryotic expression plasmid transfer from the intracellular bacterium Listeria monocytogenes to host cells. Cell Microbiol 3, 599–609 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Lee CH, Wu CL & Shiau AL Systemic administration of attenuated Salmonella choleraesuis carrying thrombospondin-1 gene leads to tumor-specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gene Ther 12, 175–184, doi: 10.1038/sj.cgt.7700777 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Fu W et al. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2’-deoxyriboside via Salmonella against murine tumors. Cancer Gene Ther 15, 474–484, doi: 10.1038/cgt.2008.19 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Darji A, zur Lage S, Garbe AI, Chakraborty T & Weiss S Oral delivery of DNA vaccines using attenuated Salmonella typhimurium as carrier. FEMS immunology and medical microbiology 27, 341–349 (2000). [DOI] [PubMed] [Google Scholar]
- 95.Toussaint B, Chauchet X, Wang Y, Polack B & Le Gouellec A Live-attenuated bacteria as a cancer vaccine vector. Expert Rev Vaccines 12, 1139–1154, doi: 10.1586/14760584.2013.836914 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Ryan RM et al. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther 16, 329–339, doi: 10.1038/gt.2008.188 (2009). [DOI] [PubMed] [Google Scholar]
- 97.Nguyen VH et al. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res 70, 18–23, doi: 10.1158/0008-5472.CAN-09-3453 (2010). [DOI] [PubMed] [Google Scholar]
- 98.St Jean AT, Swofford CA, Panteli JT, Brentzel ZJ & Forbes NS Bacterial delivery of Staphylococcus aureus alpha-hemolysin causes regression and necrosis in murine tumors. Molecular therapy : the journal of the American Society of Gene Therapy 22, 1266–1274, doi: 10.1038/mt.2014.36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hong H et al. Targeted deletion of the ara operon of Salmonella typhimurium enhances L-arabinose accumulation and drives PBAD-promoted expression of anti-cancer toxins and imaging agents. Cell Cycle 13, 3112–3120, doi: 10.4161/15384101.2014.949527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang SN et al. Engineering of bacteria for the visualization of targeted delivery of a cytolytic anticancer agent. Molecular therapy : the journal of the American Society of Gene Therapy 21, 1985–1995, doi: 10.1038/mt.2013.183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quintero D, Carrafa J, Vincent L & Bermudes D EGFR-targeted Chimeras of Pseudomonas ToxA released into the extracellular milieu by attenuated Salmonella selectively kill tumor cells. Biotechnol Bioeng 113, 2698–2711, doi: 10.1002/bit.26026 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lim D et al. Anti-tumor activity of an immunotoxin (TGFalpha-PE38) delivered by attenuated Salmonella typhimurium. Oncotarget 8, 37550–37560, doi: 10.18632/oncotarget.17197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hersh EM et al. Phase II studies of recombinant human tumor necrosis factor alpha in patients with malignant disease: a summary of the Southwest Oncology Group experience. J Immunother (1991) 10, 426–431 (1991). [DOI] [PubMed] [Google Scholar]
- 104.Rensing-Ehl A et al. Local Fas/APO-1 (CD95) ligand-mediated tumor cell killing in vivo. Eur J Immunol 25, 2253–2258, doi: 10.1002/eji.1830250821 (1995). [DOI] [PubMed] [Google Scholar]
- 105.Kelley SK et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther 299, 31–38 (2001). [PubMed] [Google Scholar]
- 106.Theys J et al. Stable Escherichia coli-Clostridium acetobutylicum shuttle vector for secretion of murine tumor necrosis factor alpha. Applied and environmental microbiology 65, 4295–4300 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoon WS, Chae YS, Hong J & Park YK Antitumor therapeutic effects of a genetically engineered Salmonella typhimurium harboring TNF-alpha in mice. Appl Microbiol Biotechnol 89, 1807–1819, doi: 10.1007/s00253-010-3006-4 (2011). [DOI] [PubMed] [Google Scholar]
- 108.Loeffler M, Le’Negrate G, Krajewska M & Reed JC Inhibition of tumor growth using salmonella expressing Fas ligand. J Natl Cancer Inst 100, 1113–1116, doi: 10.1093/jnci/djn205 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ganai S, Arenas RB & Forbes NS Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br J Cancer 101, 1683–1691, doi: 10.1038/sj.bjc.6605403 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen J et al. Salmonella-mediated tumor-targeting TRAIL gene therapy significantly suppresses melanoma growth in mouse model. Cancer Sci 103, 325–333, doi: 10.1111/j.1349-7006.2011.02147.x (2012). [DOI] [PubMed] [Google Scholar]
- 111.Zoaby N et al. Autonomous bacterial nanoswimmers target cancer. J Control Release 257, 68–75, doi: 10.1016/j.jconrel.2016.10.006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zheng DW et al. Optically-controlled bacterial metabolite for cancer therapy. Nat Commun 9, 1680, doi: 10.1038/s41467-018-03233-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Austin EA & Huber BE A first step in the development of gene therapy for colorectal carcinoma: cloning, sequencing, and expression of Escherichia coli cytosine deaminase. Mol Pharmacol 43, 380–387 (1993). [PubMed] [Google Scholar]
- 114.King I et al. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum Gene Ther 13, 1225–1233 (2002). [DOI] [PubMed] [Google Scholar]
- 115.Theys J et al. Repeated cycles of Clostridium-directed enzyme prodrug therapy result in sustained antitumour effects in vivo. Br J Cancer 95, 1212–1219 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu SC, Minton NP, Giaccia AJ & Brown JM Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther 9, 291–296 (2002). [DOI] [PubMed] [Google Scholar]
- 117.Lehouritis P, Stanton M, McCarthy FO, Jeavons M & Tangney M Activation of multiple chemotherapeutic prodrugs by the natural enzymolome of tumour-localised probiotic bacteria. J Control Release 222, 9–17, doi: 10.1016/j.jconrel.2015.11.030 (2016). [DOI] [PubMed] [Google Scholar]
- 118.Chen G et al. Tumor-targeting Salmonella typhimurium, a natural tool for activation of prodrug 6MePdR and their combination therapy in murine melanoma model. Appl Microbiol Biotechnol 97, 4393–4401, doi: 10.1007/s00253-012-4321-8 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Paterson Y, Guirnalda PD & Wood LM Listeria and Salmonella bacterial vectors of tumor-associated antigens for cancer immunotherapy. Semin Immunol 22, 183–189, doi: 10.1016/j.smim.2010.02.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saltzman DA et al. Antitumor mechanisms of attenuated Salmonella typhimurium containing the gene for human interleukin-2: a novel antitumor agent? J Pediatr Surg 32, 301–306 (1997). [DOI] [PubMed] [Google Scholar]
- 121.Loeffler M, Le’Negrate G, Krajewska M & Reed JC Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci U S A 104, 12879–12883, doi: 10.1073/pnas.0701959104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zheng JH et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med 9, doi: 10.1126/scitranslmed.aak9537 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Binder DC et al. Antigen-specific bacterial vaccine combined with anti-PD-L1 rescues dysfunctional endogenous T cells to reject long-established cancer. Cancer Immunol Res 1, 123–133, doi: 10.1158/2326-6066.CIR-13-0058 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mkrtichyan M et al. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer 1, 15, doi: 10.1186/2051-1426-1-15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ribas A et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 170, 1109–1119 e1110, doi: 10.1016/j.cell.2017.08.027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Groot AJ et al. Functional antibodies produced by oncolytic clostridia. Biochem Biophys Res Commun 364, 985–989, doi: 10.1016/j.bbrc.2007.10.126 (2007). [DOI] [PubMed] [Google Scholar]
- 127.Naidoo J et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26, 2375–2391, doi: 10.1093/annonc/mdv383 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Drake CG, Jaffee E & Pardoll DM Mechanisms of immune evasion by tumors. Advances in immunology 90, 51–81, doi: 10.1016/S0065-2776(06)90002-9 (2006). [DOI] [PubMed] [Google Scholar]
- 129.Theys J et al. Improvement of Clostridium tumour targeting vectors evaluated in rat rhabdomyosarcomas. FEMS immunology and medical microbiology 30, 37–41. (2001). [DOI] [PubMed] [Google Scholar]
- 130.Dang LH et al. Targeting Vascular and Avascular Compartments of Tumors with C. novyi-NT and Anti-Microtubule Agents. Cancer Biol Ther 3, 326–337 (2004). [DOI] [PubMed] [Google Scholar]
- 131.Drees JJ, Mertensotto MJ, Augustin LB, Schottel JL & Saltzman DA Vasculature Disruption Enhances Bacterial Targeting of Autochthonous Tumors. J Cancer 6, 843–848, doi: 10.7150/jca.12491 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Niethammer AG et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med 8, 1369–1375, doi: 10.1038/nm794 (2002). [DOI] [PubMed] [Google Scholar]
- 133.Luo Y, Markowitz D, Xiang R, Zhou H & Reisfeld RA FLK-1-based minigene vaccines induce T cell-mediated suppression of angiogenesis and tumor protective immunity in syngeneic BALB/c mice. Vaccine 25, 1409–1415, doi: 10.1016/j.vaccine.2006.10.043 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee SH et al. Endoglin (CD105) is a target for an oral DNA vaccine against breast cancer. Cancer Immunol Immunother 55, 1565–1574, doi: 10.1007/s00262-006-0155-5 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jarosz M et al. Therapeutic antitumor potential of endoglin-based DNA vaccine combined with immunomodulatory agents. Gene Ther 20, 262–273, doi: 10.1038/gt.2012.28 (2013). [DOI] [PubMed] [Google Scholar]
- 136.Wood LM et al. Targeting tumor vasculature with novel Listeria-based vaccines directed against CD105. Cancer Immunol Immunother 60, 931–942, doi: 10.1007/s00262-011-1002-x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruan Z et al. DNA vaccine against tumor endothelial marker 8 inhibits tumor angiogenesis and growth. J Immunother 32, 486–491, doi: 10.1097/CJI.0b013e3181a1d134 (2009). [DOI] [PubMed] [Google Scholar]
- 138.Kaplan CD et al. A novel DNA vaccine encoding PDGFRbeta suppresses growth and dissemination of murine colon, lung and breast carcinoma. Vaccine 24, 6994–7002, doi: 10.1016/j.vaccine.2006.04.071 (2006). [DOI] [PubMed] [Google Scholar]
- 139.Seavey MM, Maciag PC, Al-Rawi N, Sewell D & Paterson Y An anti-vascular endothelial growth factor receptor 2/fetal liver kinase-1 Listeria monocytogenes anti-angiogenesis cancer vaccine for the treatment of primary and metastatic Her-2/neu+ breast tumors in a mouse model. J Immunol 182, 5537–5546, doi: 10.4049/jimmunol.0803742 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shizuya H et al. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc Natl Acad Sci U S A 89, 8794–8797 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gibson DG et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 52–56, doi: 10.1126/science.1190719 (2010). [DOI] [PubMed] [Google Scholar]
- 142.Hutchison CA 3rd et al. Design and synthesis of a minimal bacterial genome. Science 351, aad6253, doi: 10.1126/science.aad6253 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Gardner TS, Cantor CR & Collins JJ Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342, doi: 10.1038/35002131 (2000). [DOI] [PubMed] [Google Scholar]
- 144.Elowitz MB & Leibler S A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338, doi: 10.1038/35002125 (2000). [DOI] [PubMed] [Google Scholar]
- 145.Khalil AS & Collins JJ Synthetic biology: applications come of age. Nat Rev Genet 11, 367–379, doi: 10.1038/nrg2775 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Danino T, Mondragon-Palomino O, Tsimring L & Hasty J A synchronized quorum of genetic clocks. Nature 463, 326–330, doi: 10.1038/nature08753 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Talmadge JE, Singh RK, Fidler IJ & Raz A Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol 170, 793–804, doi: 10.2353/ajpath.2007.060929 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mignon C, Sodoyer R & Werle B Antibiotic-free selection in biotherapeutics: now and forever. Pathogens 4, 157–181, doi: 10.3390/pathogens4020157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Martinez-Morales F, Borges AC, Martinez A, Shanmugam KT & Ingram LO Chromosomal integration of heterologous DNA in Escherichia coli with precise removal of markers and replicons used during construction. J Bacteriol 181, 7143–7148 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heap JT et al. Spores of Clostridium engineered for clinical efficacy and safety cause regression and cure of tumors in vivo. Oncotarget 5, 1761–1769, doi: 10.18632/oncotarget.1761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.CDC Active Bacterial Core surveillance (ABCs), https://www.cdc.gov/abcs/overview/background.html.
- 152.Microbiological examination of nonsterile products, https://hmc.usp.org/sites/default/files/documents/HMC/GCs-Pdfs/c62.pdf.
- 153.Kashiwagi N et al. Vascular supply with angio-CT for superselective intra-arterial chemotherapy in advanced maxillary sinus cancer. The British journal of radiology 83, 171–178, doi: 10.1259/bjr/16954991 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fleming IN et al. Imaging tumour hypoxia with positron emission tomography. Br J Cancer 112, 238–250, doi: 10.1038/bjc.2014.610 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Egeland TA, Gaustad JV, Galappathi K & Rofstad EK Magnetic resonance imaging of tumor necrosis. Acta Oncol 50, 427–434, doi: 10.3109/0284186X.2010.526633 (2011). [DOI] [PubMed] [Google Scholar]
- 156.Bettegowda C et al. Imaging bacterial infections with radiolabeled 1-(2’-deoxy-2’-fluoro-beta-D-arabinofuranosyl)-5-iodouracil. Proc Natl Acad Sci U S A 102, 1145–1150 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Diaz LA Jr. et al. Imaging of musculoskeletal bacterial infections by [124I]FIAU-PET/CT. PLoS One 2, e1007, doi: 10.1371/journal.pone.0001007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu G et al. Noninvasive imaging of infection after treatment with tumor-homing bacteria using Chemical Exchange Saturation Transfer (CEST) MRI. Magnetic resonance in medicine 70, 1690–1698, doi: 10.1002/mrm.24955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vail DM & MacEwen EG Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest 18, 781–792 (2000). [DOI] [PubMed] [Google Scholar]
- 160.Paoloni M & Khanna C Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer 8, 147–156, doi: 10.1038/nrc2273 (2008). [DOI] [PubMed] [Google Scholar]
- 161.Thamm DH et al. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin Cancer Res 11, 4827–4834, doi: 10.1158/1078-0432.CCR-04-2510 (2005). [DOI] [PubMed] [Google Scholar]
- 162.Fritz SE et al. A phase I clinical study to evaluate safety of orally administered, genetically engineered Salmonella enterica serovar Typhimurium for canine osteosarcoma. Vet Med Sci 2, 179–190, doi: 10.1002/vms3.32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Le DT et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 33, 1325–1333, doi: 10.1200/JCO.2014.57.4244 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Miles B, Safran HP & Monk BJ Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract 4, 10, doi: 10.1186/s40661-017-0047-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Heppner F & Möse JR The liquefaction (oncolysis) of malignant gliomas by a non pathogenic Clostridium. Acta Neurochir (Wien) 42, 123–125 (1978). [DOI] [PubMed] [Google Scholar]
- 166.Carey RW, Holland JF, Whang HY, Neter E & Bryant B Clostridial oncolysis in man. Eur. J. Cancer 3, 37–46 (1967). [Google Scholar]
- 167.Nemunaitis J et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther 10, 737–744, doi: 10.1038/sj.cgt.7700634 (2003). [DOI] [PubMed] [Google Scholar]
- 168.NCT00004216. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT00004216.
- 169.NCT00006254. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT00006254.
- 170.NCT00358397. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT00358397.
- 171.NCT00004988. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT00004988.
- 172.NCT01099631. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT01099631.
- 173.NCT01118819. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT01118819
- 174.NCT01562626. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT01562626.
- 175.NCT01924689. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT01924689.
- 176.Schmidt W, Fabricius EM & Schneeweiss U The tumour-Clostridium phenomenon: 50 years of developmental research (Review). Int J Oncol 29, 1479–1492 (2006). [PubMed] [Google Scholar]
- 177.Brown JM Tumor hypoxia in cancer therapy. Methods Enzymol 435, 297–321, doi:S0076-6879(07)35015-5 [pii] 10.1016/S0076-6879(07)35015-5 (2007). [DOI] [PubMed] [Google Scholar]
- 178.Bettegowda C et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proc Natl Acad Sci U S A 100, 15083–15088 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jiang SN et al. Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Molecular therapy : the journal of the American Society of Gene Therapy 18, 635–642, doi: 10.1038/mt.2009.295 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yano S et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle 13, 3958–3963, doi: 10.4161/15384101.2014.964115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Igarashi K et al. Tumor-targeting Salmonella typhimurium A1-R combined with recombinant methioninase and cisplatinum eradicates an osteosarcoma cisplatinum-resistant lung metastasis in a patient-derived orthotopic xenograft (PDOX) mouse model: decoy, trap and kill chemotherapy moves toward the clinic. Cell Cycle 17, 801–809, doi: 10.1080/15384101.2018.1431596 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Recommendations for Microbial Vectors used for Gene Therapy (September, 2016), https://www.fda.gov/downloads/Guidances/UCM466625.pdf.
- 183.Preclinical Assessment of Investigational Cellular and Gene Therapy Products (November, 2013), https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM376521.pdf.
- 184.Uchugonova A et al. Imaging the Different Mechanisms of Prostate Cancer Cell-killing by Tumor-targeting Salmonella typhimurium A1-R. Anticancer Res 35, 5225–5229 (2015). [PubMed] [Google Scholar]
- 185.Jia LJ et al. Oral delivery of tumor-targeting Salmonella for cancer therapy in murine tumor models. Cancer Sci 98, 1107–1112, doi: 10.1111/j.1349-7006.2007.00503.x (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ganai S, Arenas RB, Sauer JP, Bentley B & Forbes NS In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Ther 18, 457–466, doi: 10.1038/cgt.2011.10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee CH et al. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther 21, 309–316, doi: 10.1038/gt.2013.86 (2014). [DOI] [PubMed] [Google Scholar]
- 188.Zhou S et al. Suppression of pancreatic ductal adenocarcinoma growth by intratumoral delivery of attenuated Salmonella typhimurium using a dual fluorescent live tracking system. Cancer Biol Ther 17, 732–740, doi: 10.1080/15384047.2016.1177683 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kim JE et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1beta. Theranostics 5, 1328–1342, doi: 10.7150/thno.11432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Phan TX et al. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol Immunol 59, 664–675, doi: 10.1111/1348-0421.12333 (2015). [DOI] [PubMed] [Google Scholar]
- 191.Beutler B & Cerami A The biology of cachectin/TNF--a primary mediator of the host response. Annual review of immunology 7, 625–655, doi: 10.1146/annurev.iy.07.040189.003205 (1989). [DOI] [PubMed] [Google Scholar]
- 192.Dobrovolskaia MA & Vogel SN Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect 4, 903–914 (2002). [DOI] [PubMed] [Google Scholar]
- 193.Leigh ND et al. A flagellin-derived toll-like receptor 5 agonist stimulates cytotoxic lymphocyte-mediated tumor immunity. PLoS One 9, e85587, doi: 10.1371/journal.pone.0085587 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kupz A, Curtiss R 3rd, Bedoui S & Strugnell RA In vivo IFN-gamma secretion by NK cells in response to Salmonella typhimurium requires NLRC4 inflammasomes. PLoS One 9, e97418, doi: 10.1371/journal.pone.0097418 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nguyen CT et al. Flagellin enhances tumor-specific CD8(+) T cell immune responses through TLR5 stimulation in a therapeutic cancer vaccine model. Vaccine 31, 3879–3887, doi: 10.1016/j.vaccine.2013.06.054 (2013). [DOI] [PubMed] [Google Scholar]
- 196.Sfondrini L et al. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J Immunol 176, 6624–6630 (2006). [DOI] [PubMed] [Google Scholar]
- 197.Cai Z et al. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res 71, 2466–2475, doi: 10.1158/0008-5472.CAN-10-1993 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Saccheri F et al. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci Transl Med 2, 44ra57, doi: 10.1126/scitranslmed.3000739 (2010). [DOI] [PubMed] [Google Scholar]
- 199.Chang WW et al. Salmonella enhance chemosensitivity in tumor through connexin 43 upregulation. Int J Cancer 133, 1926–1935, doi: 10.1002/ijc.28155 (2013). [DOI] [PubMed] [Google Scholar]
- 200.Lin HC et al. The inhibition of indoleamine 2, 3-dioxygenase 1 by connexin 43. Int J Med Sci 14, 1181–1188, doi: 10.7150/ijms.20661 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kim SH, Castro F, Paterson Y & Gravekamp C High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res 69, 5860–5866, doi: 10.1158/0008-5472.CAN-08-4855 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jahangir A et al. Immunotherapy with Listeria reduces metastatic breast cancer in young and old mice through different mechanisms. Oncoimmunology 6, e1342025, doi: 10.1080/2162402X.2017.1342025 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wallecha A, Singh R & Malinina I Listeria monocytogenes (Lm)-LLO immunotherapies reduce the immunosuppressive activity of myeloid-derived suppressor cells and regulatory T cells in the tumor microenvironment. J Immunother 36, 468–476, doi: 10.1097/CJI.0000000000000000 (2013). [DOI] [PubMed] [Google Scholar]
- 204.Chagnon A, Hudon C, McSween G, Vinet G & Fredette V Cytotoxicity and reduction of animal cell growth by Clostridium M-55 spores and their extracts. Cancer 29, 431–434 (1972). [DOI] [PubMed] [Google Scholar]
- 205.Middlebrook JL & Dorland RB Bacterial toxins: cellular mechanisms of action. Microbiol Rev 48, 199–221 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bettegowda C et al. The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat Biotechnol 24, 1573–1580 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cheong I et al. A bacterial protein enhances the release and efficacy of liposomal cancer drugs. Science 314, 1308–1311 (2006). [DOI] [PubMed] [Google Scholar]
- 208.Shinnoh M et al. Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int J Oncol 42, 903–911, doi: 10.3892/ijo.2013.1790 (2013). [DOI] [PubMed] [Google Scholar]
- 209.Yu B et al. Explicit hypoxia targeting with tumor suppression by creating an “obligate” anaerobic Salmonella Typhimurium strain. Sci Rep 2, 436, doi: 10.1038/srep00436 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Flentie K et al. A bioluminescent transposon reporter-trap identifies tumor-specific microenvironment-induced promoters in Salmonella for conditional bacterial-based tumor therapy. Cancer Discov 2, 624–637, doi: 10.1158/21598290.CD-11-0201 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Panteli JT & Forbes NS Engineered bacteria detect spatial profiles in glucose concentration within solid tumor cell masses. Biotechnol Bioeng 113, 2474–2484, doi: 10.1002/bit.26006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Loessner H et al. Remote control of tumour-targeted Salmonella enterica serovar Typhimurium by the use of L-arabinose as inducer of bacterial gene expression in vivo. Cell Microbiol 9, 1529–1537, doi: 10.1111/j.1462-5822.2007.00890.x (2007). [DOI] [PubMed] [Google Scholar]
- 213.Stritzker J et al. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol 297, 151–162, doi: 10.1016/j.ijmm.2007.01.008 (2007). [DOI] [PubMed] [Google Scholar]
- 214.Jeong JH et al. Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium. PLoS One 9, e80050, doi: 10.1371/journal.pone.0080050 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Royo JL et al. In vivo gene regulation in Salmonella spp. by a salicylate-dependent control circuit. Nat Methods 4, 937–942, doi: 10.1038/nmeth1107 (2007). [DOI] [PubMed] [Google Scholar]
- 216.Nuyts S et al. Increasing specificity of anti-tumor therapy: cytotoxic protein delivery by non-pathogenic clostridia under regulation of radio-induced promoters. Anticancer Res 21, 857–861 (2001). [PubMed] [Google Scholar]
- 217.Nuyts S et al. Radio-responsive recA promoter significantly increases TNFalpha production in recombinant clostridia after 2 Gy irradiation. Gene Ther 8, 1197–1201, doi: 10.1038/sj.gt.3301499 (2001). [DOI] [PubMed] [Google Scholar]
- 218.Nuyts S et al. The use of radiation-induced bacterial promoters in anaerobic conditions: a means to control gene expression in clostridium-mediated therapy for cancer. Radiat Res 155, 716–723 (2001). [DOI] [PubMed] [Google Scholar]
- 219.Arrach N, Zhao M, Porwollik S, Hoffman RM & McClelland M Salmonella promoters preferentially activated inside tumors. Cancer Res 68, 4827–4832, doi: 10.1158/0008-5472.CAN-08-0552 (2008). [DOI] [PubMed] [Google Scholar]
- 220.Leschner S et al. Identification of tumor-specific Salmonella Typhimurium promoters and their regulatory logic. Nucleic Acids Res 40, 2984–2994, doi: 10.1093/nar/gkr1041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Swofford CA, St Jean AT, Panteli JT, Brentzel ZJ & Forbes NS Identification of Staphylococcus aureus alpha-hemolysin as a protein drug that is secreted by anticancer bacteria and rapidly kills cancer cells. Biotechnol Bioeng 111, 1233–1245, doi: 10.1002/bit.25184 (2014). [DOI] [PubMed] [Google Scholar]
- 222.Zhang HY et al. Tumor-targeted delivery of biologically active TRAIL protein. Cancer Gene Ther 17, 334–343, doi: 10.1038/cgt.2009.76 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang Y et al. Escherichia coli Nissle 1917 targets and restrains mouse B16 melanoma and 4T1 breast tumors through expression of azurin protein. Applied and environmental microbiology 78, 7603–7610, doi: 10.1128/AEM.01390-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Guan GF et al. Salmonella typhimurium mediated delivery of Apoptin in human laryngeal cancer. Int J Med Sci 10, 1639–1648, doi: 10.7150/ijms.6960 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yi C, Huang Y, Guo ZY & Wang SR Antitumor effect of cytosine deaminase/5-fluorocytosine suicide gene therapy system mediated by Bifidobacterium infantis on melanoma. Acta Pharmacol Sin 26, 629–634, doi: 10.1111/j.1745-7254.2005.00094.x (2005). [DOI] [PubMed] [Google Scholar]
- 226.Fu W, Lan H, Liang S, Gao T & Ren D Suicide gene/prodrug therapy using salmonella-mediated delivery of Escherichia coli purine nucleoside phosphorylase gene and 6-methoxypurine 2’-deoxyriboside in murine mammary carcinoma 4T1 model. Cancer Sci 99, 1172–1179, doi: 10.1111/j.1349-7006.2008.00808.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Stritzker J, Pilgrim S, Szalay AA & Goebel W Prodrug converting enzyme gene delivery by L. monocytogenes. BMC cancer 8, 94, doi: 10.1186/1471-2407-8-94 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Friedlos F et al. Attenuated Salmonella targets prodrug activating enzyme carboxypeptidase G2 to mouse melanoma and human breast and colon carcinomas for effective suicide gene therapy. Clin Cancer Res 14, 4259–4266, doi: 10.1158/1078-0432.CCR-07-4800 (2008). [DOI] [PubMed] [Google Scholar]
- 229.Barak Y et al. New enzyme for reductive cancer chemotherapy, YieF, and its improvement by directed evolution. Mol Cancer Ther 5, 97–103, doi: 10.1158/1535-7163.MCT-05-0365 (2006). [DOI] [PubMed] [Google Scholar]
- 230.Xiang R et al. An autologous oral DNA vaccine protects against murine melanoma. Proc Natl Acad Sci U S A 97, 5492–5497, doi: 10.1073/pnas.090097697 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Keenan BP et al. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology 146, 1784–1794 e1786, doi: 10.1053/j.gastro.2014.02.055 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chou CK, Hung JY, Liu JC, Chen CT & Hung MC An attenuated Salmonella oral DNA vaccine prevents the growth of hepatocellular carcinoma and colon cancer that express alpha-fetoprotein. Cancer Gene Ther 13, 746–752, doi: 10.1038/sj.cgt.7700927 (2006). [DOI] [PubMed] [Google Scholar]
- 233.Barbe S et al. Secretory production of biologically active rat interleukin-2 by Clostridium acetobutylicum DSM792 as a tool for anti-tumor treatment. FEMS Microbiol Lett 246, 67–73, doi: 10.1016/j.femsle.2005.03.037 (2005). [DOI] [PubMed] [Google Scholar]
- 234.Agorio C et al. Live attenuated Salmonella as a vector for oral cytokine gene therapy in melanoma. J Gene Med 9, 416–423, doi: 10.1002/jgm.1023 (2007). [DOI] [PubMed] [Google Scholar]
- 235.Yuhua L et al. Oral cytokine gene therapy against murine tumor using attenuated Salmonella typhimurium. Int J Cancer 94, 438–443 (2001). [DOI] [PubMed] [Google Scholar]
- 236.Zhang YL et al. Clostridium sporogenes delivers interleukin-12 to hypoxic tumours, producing antitumour activity without significant toxicity. Lett Appl Microbiol 59, 580–586, doi: 10.1111/lam.12322 (2014). [DOI] [PubMed] [Google Scholar]
- 237.Loeffler M, Le’Negrate G, Krajewska M & Reed JC IL-18-producing Salmonella inhibit tumor growth. Cancer Gene Ther 15, 787–794, doi: 10.1038/cgt.2008.48 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yoon W et al. Application of genetically engineered Salmonella typhimurium for interferon-gamma-induced therapy against melanoma. Eur J Cancer 70, 48–61, doi: 10.1016/j.ejca.2016.10.010 (2017). [DOI] [PubMed] [Google Scholar]
- 239.Yoon WS, Choi WC, Sin JI & Park YK Antitumor therapeutic effects of Salmonella typhimurium containing Flt3 Ligand expression plasmids in melanoma-bearing mouse. Biotechnol Lett 29, 511–516, doi: 10.1007/s10529-006-9270-9 (2007). [DOI] [PubMed] [Google Scholar]
- 240.Loeffler M, Le’Negrate G, Krajewska M & Reed JC Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol Immunother 58, 769–775, doi: 10.1007/s00262-008-0555-9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xiang R, Luo Y, Niethammer AG & Reisfeld RA Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol Rev 222, 117–128, doi: 10.1111/j.1600-065X.2008.00613.x (2008). [DOI] [PubMed] [Google Scholar]
- 242.Luo Y et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest 116, 2132–2141, doi: 10.1172/JCI27648 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Schmitz-Winnenthal FH et al. A phase 1 trial extension to assess immunologic efficacy and safety of prime-boost vaccination with VXM01, an oral T cell vaccine against VEGFR2, in patients with advanced pancreatic cancer. Oncoimmunology 7, e1303584, doi: 10.1080/2162402X.2017.1303584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Blache CA et al. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res 72, 6447–6456, doi: 10.1158/0008-5472.CAN-12-0193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Manuel ER et al. Salmonella-Based Therapy Targeting Indoleamine 2,3-Dioxygenase Coupled with Enzymatic Depletion of Tumor Hyaluronan Induces Complete Regression of Aggressive Pancreatic Tumors. Cancer Immunol Res 3, 1096–1107, doi: 10.1158/2326-6066.CIR-14-0214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang L et al. Intratumoral delivery and suppression of prostate tumor growth by attenuated Salmonella enterica serovar typhimurium carrying plasmid-based small interfering RNAs. Cancer Res 67, 5859–5864, doi: 10.1158/0008-5472.CAN-07-0098 (2007). [DOI] [PubMed] [Google Scholar]
- 247.Manuel ER et al. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer Res 71, 4183–4191, doi: 10.1158/0008-5472.CAN-10-4676 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yang N, Zhu X, Chen L, Li S & Ren D Oral administration of attenuated S. typhimurium carrying shRNA-expressing vectors as a cancer therapeutic. Cancer Biol Ther 7, 145–151 (2008). [DOI] [PubMed] [Google Scholar]
- 249.Jiang T et al. Enhanced therapeutic effect of cisplatin on the prostate cancer in tumor-bearing mice by transfecting the attenuated Salmonella carrying a plasmid co-expressing p53 gene and mdm2 siRNA. Cancer Lett 337, 133–142, doi: 10.1016/j.canlet.2013.05.028 (2013). [DOI] [PubMed] [Google Scholar]
- 250.Liu YB et al. Plasmid-based Survivin shRNA and GRIM-19 carried by attenuated Salmonella suppresses tumor cell growth. Asian J Androl 14, 536–545, doi: 10.1038/aja.2011.179 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li Z et al. Recombinant attenuated Salmonella typhimurium carrying a plasmid co-expressing ENDO-VEGI151 and survivin siRNA inhibits the growth of breast cancer in vivo. Mol Med Rep 7, 1215–1222, doi: 10.3892/mmr.2013.1308 (2013). [DOI] [PubMed] [Google Scholar]
- 252.Jiang Z et al. Using attenuated Salmonella typhi as tumor targeting vector for MDR1 siRNA delivery. Cancer Biol Ther 6, 555–560 (2007). [DOI] [PubMed] [Google Scholar]
- 253.Swofford CA, Van Dessel N & Forbes NS Quorum-sensing Salmonella selectively trigger protein expression within tumors. Proc Natl Acad Sci U S A 112, 3457–3462, doi: 10.1073/pnas.1414558112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Krick EL et al. Evaluation of Clostridium novyi-NT spores in dogs with naturally occurring tumors. Am J Vet Res 73, 112–118, doi: 10.2460/ajvr.73.1.112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Maciag PC, Radulovic S & Rothman J The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 27, 3975–3983, doi: 10.1016/j.vaccine.2009.04.041 (2009). [DOI] [PubMed] [Google Scholar]
- 256.Basu P et al. A Randomized Phase 2 Study of ADXS11-001 Listeria monocytogenes-Listeriolysin O Immunotherapy With or Without Cisplatin in Treatment of Advanced Cervical Cancer. Int J Gynecol Cancer 28, 764–772, doi: 10.1097/IGC.0000000000001235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Le DT et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res 18, 858–868, doi: 10.1158/1078-0432.CCR-11-2121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.NCT00327652. https://clinicaltrials.gov/ct2/show/NCT00327652.
- 259.NCT00585845. https://clinicaltrials.gov/ct2/show/NCT00585845.
- 260.NCT01417000. https://clinicaltrials.gov/ct2/show/NCT01417000.
- 261.NCT01116245. https://clinicaltrials.gov/ct2/show/NCT01116245.
- 262.NCT01266460. https://clinicaltrials.gov/ct2/show/NCT01266460.
- 263.NCT01598792. https://clinicaltrials.gov/ct2/show/NCT01598792.
- 264.ISRCTN47069182. http://www.isrctn.com/ISRCTN47069182.
- 265.NCT02002182. https://clinicaltrials.gov/ct2/show/NCT02002182.
- 266.NCT01671488. https://clinicaltrials.gov/ct2/show/NCT01671488.
- 267.NCT02164461. https://clinicaltrials.gov/ct2/show/NCT02164461.
- 268.NCT02291055. https://clinicaltrials.gov/ct2/show/NCT02291055.
- 269.NCT02399813. https://clinicaltrials.gov/ct2/show/NCT02399813.
- 270.NCT02853604. https://clinicaltrials.gov/ct2/show/NCT02853604.
- 271.NCT02531854. https://clinicaltrials.gov/ct2/show/NCT02531854.
- 272.NCT02386501. https://clinicaltrials.gov/ct2/show/NCT02386501.
- 273.NCT03265080. https://clinicaltrials.gov/ct2/show/NCT03265080.
- 274.NCT01675765. https://clinicaltrials.gov/ct2/show/NCT01675765.
- 275.NCT02004262. https://clinicaltrials.gov/ct2/show/NCT02004262.
- 276.NCT02575807. https://clinicaltrials.gov/ct2/show/NCT02575807.
- 277.NCT03175172. https://clinicaltrials.gov/ct2/show/NCT03175172.
- 278.NCT03122548. https://clinicaltrials.gov/ct2/show/NCT03122548.
- 279.NCT01967758. https://clinicaltrials.gov/ct2/show/NCT01967758.
- 280.NCT02592967. https://clinicaltrials.gov/ct2/show/NCT02592967.
- 281.NCT02625857. https://clinicaltrials.gov/ct2/show/NCT02625857.
- 282.NCT03189030. https://clinicaltrials.gov/ct2/show/NCT03189030.