Abstract
Prostate cancer incidence increases with age; along with many other cancers, it could be considered a disease of aging. Prostate cancer screening has led to a significant proportion of men diagnosed with low-grade, low-stage prostate cancer who are now more likely to choose an active surveillance strategy rather than definitive treatments. Definitive treatment, such as surgery and radiation therapy, is useful for high-grade disease; however, because of the low long-term risk of progression of a low-grade disease and side effects of surgery and radiation, these treatments are less commonly used for low-grade disease. While five alpha reductase inhibitors have been shown to reduce the risk of cancer detection on subsequent biopsies for men on active surveillance, no medications have been proven to prevent progression to high-grade disease. mTOR pathways have long been known to influence prostate cancer and are targets in various prostate cancer patient populations. Low-dose mTOR inhibition with rapamycin has shown promise in pre-clinical models of prostate cancer and appear to affect cellular senescence and immunomodulation in the aging population. We hypothesize that low-dose mTOR inhibition could reduce progression of low-grade prostate cancer patients, allowing them to remain on active surveillance.
Introduction
According to the United Nations, the number of people 60 years or older in 2012 was 809,743,000 (one out of nine), and that number is predicted to expand to 2,031,337,000 (one in five) by the year 2050.(1) This dramatic demographic shift will have a significant impact on healthcare for the elderly and will place a substantial financial burden on societies worldwide. Adult cancer can be considered a disease of aging as people over age 65 have an age-adjusted cancer mortality rate 15 times greater than young people.(2) In the case of prostate cancer, about 93% of prostate cancer deaths occur in men older than 65 years of age.(3) Development of malignant lesions involves activation of oncogenes and/or inactivation of tumor suppressor genes.(4) Like many other common cancers, prostate cancer has an addiction to a dysregulated (or up-regulated) mTOR (mechanistic or mammalian target of rapamycin)-mediated pro-cell growth state.(5)
Hypothesis
Low-dose rapamycin can slow the progression of low-grade prostate cancer by cellular senescence and immune function attenuation.
Implication
The confluence of current events provides a compelling rationale for a randomized clinical trial of low-dose rapamycin in patients with low-grade, low-stage prostate cancer who are managed with active surveillance with the objective to prevent disease progression and thus avoid the vast array of side effects of radiation or surgery.
Evaluation of Hypothesis
Normal mTOR Biology:
In healthy cells, mTOR coordinates energy-dependent anabolic activities with energy-producing catabolic processes for regulated cell growth (in mass). For cell growth, mTOR stimulates the production of proteins through downstream effectors S6K and ribosomal protein S6 (protein synthesis including ribosome biogenesis) and 4E-BPs (eIF4E-dependent mRNA translation).(6–9) For cell membranes during growth, mTOR signaling (under appropriate nutrient conditions) up-regulates lipogenesis and adipogenesis and inhibits lipolysis and β-oxidation.(10) For nucleic acids, growth-promoting signaling through mTOR (including proper nutrient levels) stimulates the synthesis of pyrimidines and purines.(11–13) mTOR also activates the pentose phosphate pathway to generate ribose and NADPH necessary for cell growth.(14) MTOR also regulates mitochondrial biogenesis and oxidative metabolismmTOR.(15) For the Warburg effect in cancer cells, mTOR stimulates glycolytic enzyme gene expression via HIF-1α.(14) Thus, the metabolic changes in cancer cells are a consequence of an addiction to an up-regulated mTOR system.(14–16)
mTOR functions within two complexes: mTORC1, which is sensitive to rapamycin, and mTORC2, which is insensitive to rapamycin in the short term but not in long-term treatments in vitro or in vivo.(17, 18) A recent discovery of the third complex in astrocytes indicates that more cell-type-specific complexes may lead to more discoveries.(19) As currently understood, mTORC1 controls most of the growth-promoting activities noted above. mTORC2, although less well characterized, has essential functions through interactions with ribosomes at the mitochondria-associated endoplasmic reticulum membrane to include activation of AGC kinase family members including Akt, SGK1 and PKC.(20) Association specifically with Raptor and mLST8 forms the core mTORC1 complex, while the association with Rictor and mLST8 form the core mTORC2. Other subunits (e.g., Deptor) contribute to the function of the complexes. Availability of mice carrying floxed alleles of both Raptor and Rictor genes provides opportunities to understand better the role of each complex in the development and progression of prostate cancer in mouse models that respond to rapamycin.
mTOR Inhibitors:
Rapamycin (assigned the generic name sirolimus) is marketed as Rapamune by Pfizer as an immunosuppressant for allograft rejection. Pharmaceutical companies developed derivative compounds (rapalogs) whose indications include allograft rejection, anti-cancer, and anti-restenosis. Since immunosuppression is a significant concern during long-term treatment, it is critical to understand rapamycin’s effect on the immune system. mTOR mediates profound effects on the immune system including differentiation. The primary cell populations affected include CD8+ T cells[22], CD4+ regulatory and non-regulatory T cells, and myeloid and B cells.(21, 22) Despite rapamycin perceived as an immunosuppressant, recent evidence indicates it can boost response to pathogens and antitumor immunity.(23, 24) Additionally, it was not adversely immunosuppressive in a mouse model of organ transplant.(25) Further, Mannick et al. reported that everolimus (a rapalog) improved influenza vaccine response in elderly humans.(26) An exhaustive study in mice by Hurez et al. concluded that chronic rapamycin is an immune modulator rather than a suppressant.(27)
A significant reason for rapamycin’s critical effects on the immune system (and the cells comprising all body organs) results from its influence on metabolism. As noted above concerning cell growth, mTOR effects on metabolism are especially crucial in metabolic organs such as the liver, muscle, and adipose tissue which are particularly sensitive to nutrients, energy and insulin/IGF-1, all three of which are the most critical inputs that control mTOR. In the liver, for example, the mTOR pathway plays a significant role in glucose homeostasis and hepatic lipogenesis.(28) In adipose tissue, mTORC1 plays many roles in metabolism including lipid synthesis, oxidation, transport, storage, and lipolysis, plus adipocyte differentiation and their function.(29) In addition to controlling metabolism, mTOR pathway may regulate mitochondrial oxidative capacity in muscle. Results from the knockout of tuberous sclerosis complex (TSC, leading to up-regulated mTORC1) in muscle suggest that alterations in mTORC1 signaling can affect whole-body metabolism.(30) In line with this observation, Kennedy and Lamming[33] have referred to mTOR as the “Grand conductor of metabolism and aging.” An example of how mTOR acts as a grand conductor is in maintaining energy balance. Translation is one of the most energy-consuming processes in cells (4 ATP equivalents per amino acid, or 20–30% of ATP) depending on the cell type, not counting rRNA transcription and AT needed for degradation of unused ribosome subunits.(31–34) To maintain energy balance, Morita et al. proposed a mTORC1-mediated feedback loop to mitochondria (activity and biogenesis) to boost ATP production needed for translation.(35) Finally, prostate epithelial cells undergo metabolic changes that likely contribute to transformation and progression. These changes were reviewed by Zadra et al., with notable alterations being increased aerobic glycolysis (in advanced disease), including increased de novo fatty acid, sterol biosynthesis and protein synthesis due to up-regulation of mTOR occurring in primary and advanced diseases.(36) mTORC1—and mTORC2—likely play a vital role in these metabolic alterations in PCa, as illustrated by its regulation of AMD1 in polyamine production, a hallmark of growing, proliferating tumor cells.(37) Metabolic changes also could be driven by androgens through the PTEN/PI3K/Akt/mTOR pathway.(38)
mTOR inhibition as an approach to prevent prostate cancer progression:
Calorie (or diet, e.g., protein) restriction, which coincidentally inhibits the mTOR system, increases life and health span.(39, 40) A low-fat, high-fiber diet and daily exercise lower insulin and reduce the growth of prostate primary epithelial cells.(41) In a castrate-resistant LUCaP23.1 prostate cancer model, protein restriction resulted in a 70% inhibition of tumor growth and down-regulation of the mTORC1 activity.(42) In the HiMyc mouse model of prostate cancer, calorie restriction significantly inhibited progression of PIN lesions to in situ and locally invasive adenocarcinomas and reduced mTORC1 signaling activity.(43) As an alternate approach, metformin is a mTOR inhibitor currently being studied in prostate cancer. The MAST (metformin active surveillance trial) study is evaluating whether this drug can delay time to progression of low-risk, early-stage prostate cancer. Although a promising approach, the majority of studies in humans are retrospective and extrapolate from diabetic populations.(44) Resveratrol, which extends lifespan in mice on a high-fat diet (but not on a regular diet), inhibits mTORC1 and prevents the development of high-grade prostatic intraepithelial neoplastic lesions through the involvement of SIRT1.(45–48) Some studies have shown that rapamycin may be an improvement over resveratrol and statins.(47)
Novel Treatment for low-grade prostate cancer:
Patients with aggressive forms of localized prostate cancer (i.e., higher stage and grade tumors) undergo for more aggressive therapies, such as surgery or radiation; there is little controversy with this approach. Conversely, low-risk prostate cancer is a more nuanced disease in which more minimally invasive methods are becoming preferred. Active surveillance is an increasingly-selected approach in the United States for patients with low-grade prostate cancer.(49) Active surveillance–monitoring protocols vary among institutions and providers, but this approach is now an acceptable option by the National Comprehensive Cancer Network (NCCN).(50) NCCN guidelines recommend a PSA every six months and repeat prostate biopsy no earlier than every 12 months or when clinically indicated. Diet and exercise investigations have led to recognition as essential adjuncts to modulate risk of cancer progression. In the randomized REDEEM clinical trial, dutasteride was associated with an absolute 10% reduction (38% vs. 28%) in cancer progression but had a higher risk of sexual side effects.(51) Due to the low absolute reduction, side effects, as well as an increased number of high-grade tumors, the medication did not receive FDA approval for this indication and is seldom employed in low-grade prostate cancer. The MAST (Metformin Active Surveillance Trial) is currently ongoing at the University of Toronto; no results are yet available. Phase 2 trials are in process for proscaVax (OncoBioMune Pharmaceuticals Inc., Baton Rouge, LA, USA), a prostate cancer vaccine to reduce cancer progression.
Rapamycin is a currently available medication with an extensive safety profile that has been used clinically since FDA registration in 1999. A pharmacodynamics neoadjuvant trial (n=32) utilizing rapamycin before prostatectomy reported limited side effects at a dose of 3mg or below, which includes high intra-prostatic drug concentrations.(52) While the study did show reduced S6 phosphorylation at serine 240/244 by IHC as an indirect measure of S6 kinase activity, other markers such as Ki67, AKT staining, and apoptosis were not evident. Nevertheless, the length of the study was very short (14 days) and may not have been sufficient time to see the real effects of the drug.
A primary focus of pharmaceutical intervention for low-grade prostate cancer is the side-effect profile. Usually, men diagnosed with early-stage/low-grade prostate cancer have undergone PSA screening and are otherwise healthy. These men are typically sexually active and would like to avoid aggressive therapy that could affect sexual and urinary function. Rapamycin at low doses would not be expected to result in either of these side effects. The primary concern of the use of mTOR inhibitors in a prevention-of-progression setting (i.e., in otherwise healthy men) would be the possible increased risk of infections. Reassuringly, such an increase in infectious events was not observed in everolimus-treated elderly patients by Mannick et al.(26) An additional benefit of rapamycin in this generally-older population is the potential for prevention of other age-related diseases. Despite getting the diagnosis of prostate cancer, the majority of men do not change their lifestyle habits while on active surveillance.(53) Rapamycin could be a therapeutic option in addition to encouraging a healthy lifestyle.
Since rapamycin complexed with FKBP12 chronically inhibits mTORC1 in preclinical settings, delays cancer development and progression, extends lifespan and reduces the risk of age-associated morbidities, we among others have suggested its use for prevention of diseases of aging such as prostate cancer. (Figure).(47, 54–61) By co-targeting aging, rapamycin has the potential to not only delay prostate cancer development/progression but to also delay other age-associated illnesses, resulting in longer overall survival. We posit that rapamycin is the first drug to have this potential. Supporting this concept are multiple pre-clinical studies that demonstrated that chronic rapamycin treatment (using a novel formulation eRapa) extends survival of wild-type mice (both sexes).(47, 59, 62) It is important to note that this formulation extends lifespan, even when started late in life (60 as measured in human years). Additionally, eRapa impedes multiple comorbidities of aging in both sexes.(59, 61, 63) As prostate cancer is a disease of aging, and as prostate cancer progression may be mTOR-modulated, there is a significant rationale for its use in delaying progression of low-grade prostate cancer. In addition to our work in cancer models (Rb1+/− and p53), other investigators have explored the potential of eRapa in the prevention of age-related diseases.(57, 58, 64) One age-related disease – urinary symptoms related to prostate enlargement - is often a reason why men with low-grade cancer ultimately opt for treatment, despite the lack of cancer progression. Intriguingly, one preclinical study found that rapamycin can prevent the continued growth of the prostate and likely reduces inflammation, potentially reducing bothersome urinary tract symptoms.(65)
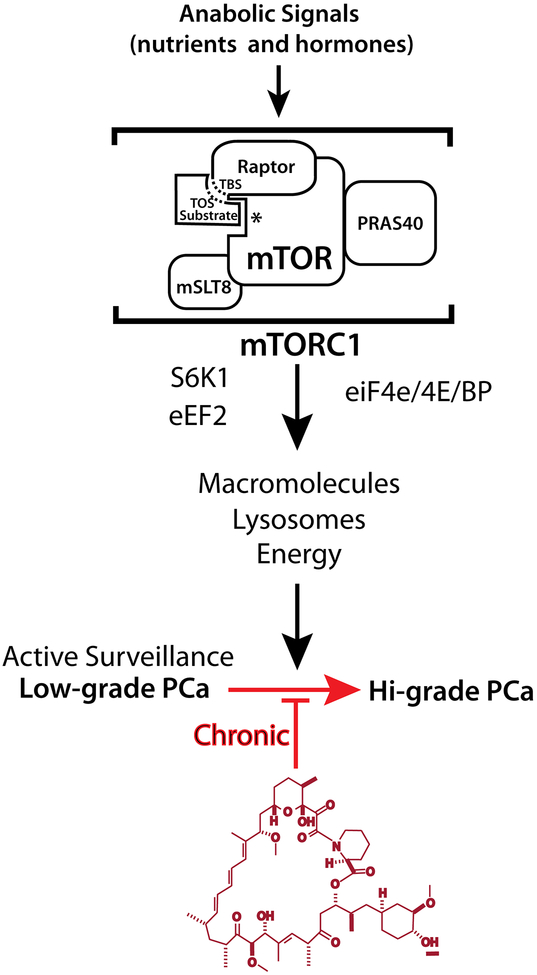
Figure.
Pathway to chronic rapamycin therapy in prostate cancer
Model: Chronic mTORC1 inhibition delays of PCa progression from active surveillance. mTORC1 and its downstream effectors promote tumor-cell growth (progression) in response to anabolic stimuli. Chronic inhibition of mTORC1 by drugs such as rapamycin would theoretically reduce the probability of conversion to Hi-grade PCa.
Relevant to this article, Saha et al., who examined the combined and separate effects of metformin and/or eRapa (14 ppm diet) in the same Hi-Myc mouse model discussed above, reported a reduction in mTORC1 activity consistent with an impressive reduction of prostate cancer progression.(66, 67) This effect of eRapa alone associates with both a reduction in mTORC1 signaling and a reduction of tissue inflammation. An important observation in this study was that eRapa “decreased prostate cancer progression without apparent toxicity.”(67) It is also important to note that the study by Saha et al. used what is now considered to be the minimal life-extending dose of eRapa (14 ppm diet).(68) We also observed a dose-response in the delay/prevention of polyposis in ApcMin/+ mice with the 14 ppm diets, extending survival almost to that of wild-type mice, while the 42 ppm diet resulted in a normal and—in some mice— a more significant than wild-type lifespan, which we housed in the same vivarium. These results merit further investigation to uncover the details of chronic eRapa effects on tumor and normal prostate.
Immune Response:
The MPAKT/Hi-Myc model also demonstrated an inflammatory response that could be modulated by rapamycin.(69, 70) Rapamycin regulates the inflammatory response and may be beneficial in men with low-grade/low-stage prostate cancer, depending on how rapamycin regulates the immune response. Rapamycin has been shown to repolarize macrophages to target cancer cells.(71) Rapamycin also suppresses a tumor TLR-4 based escape pathway in colon cancer cells.(72) Interestingly, the single Ig IL-1–related receptor (SIGIRR), which is a negative regulator of toll-like receptor 4 and IL-1–mediated activation of nuclear factor κ–light-chain enhancer of activated B cells, is specifically up-regulated in biochemically recurrent low-stage, low-grade prostate cancer undergoing prostatectomy.(73, 74) If rapamycin can suppress TLR-4 mediated pathways in inflammation, it may lead to prolonged time to progression in an active-surveillance population who also have low-grade/low-stage prostate cancer.
Potential Drawbacks
AKT Escape:
AKT pathway interacts with Myc pathway in prostate cancer. The MPAKT mouse model has shown to be extremely sensitive to rapamycin therapy. Several studies have used the Hi-Myc mouse model and showed a reduction of cancer progression.(67) However, Clegg et al. had shown experiments utilizing an MPAKT/Hi-Myc mouse model to demonstrate the MPAKT/Hi-Myc mice are resistant to rapamycin therapy.(69)
Androgen Receptor Up-regulation:
Wang et al. showed in 3 cell lines (LNCaP, C4–2, and LNAI) that androgen receptor had a two-fold increase in transcription activity, leading to a rise in hPSA luciferase in all cell lines treated with rapamycin.(75) Bicalutamide inhibited this effect, while a combination of bicalutamide and rapamycin resulted in apoptosis of prostate cancer cells. However, another study found that the mTOR pathway might be altered depending on the testosterone levels available to the cells. In a typical testosterone environment, mTOR activity may be reasonably robust, and when androgen therapy ensues, mTOR is weak but provides a survival mechanism to the prostate cancer cell.(76) Interestingly, rapamycin increased AR expression only in lower testosterone conditions. All testosterone concentrations induced PSA and KLK2 expression in LNCaP cells. The clinical implication of these data is that, while there is a possibility that PSA could increase, in normal testosterone environments, it did not correlate with an increase in AR expression. DHT did not affect the mTOR-AR signaling, so in the prevention or treatment of low-grade prostate cancer, based on these data, the combination of 5 alpha-reductase inhibitors to reduce DHT in the prostate would be unlikely to provide additional benefit.
Glucose Intolerance:
A significant concern with chronic rapamycin therapy is related to glucose metabolism, including hyperglycemia, glucose intolerance, and insulin resistance.(77) Indeed, rapamycin (Sirolimus) is already used as an immunosuppressant in human transplant recipients to prevent graft rejection with noted side effects of deranged lipid and glucose metabolism.(78) The main side effects noted in mice are severe glucose intolerance, hyperinsulinemia, and hyper-triglyceridemia thought to be due to increased hepatic glucose production, reduced skeletal muscle glucose uptake, adipose tissue PPAR activity, and reduced β-cell mass in the pancreas.(79, 80) Study subjects in a chronic rapamycin therapeutic trial would need careful monitoring for a diabetes-like syndrome. Some suggest that this is a natural side effect of calorie-restriction diets, causing historically referenced “starvation diabetes” characterized by glucose intolerance, decreased insulin, increased lipoproteins and ketones, gluconeogenesis and hepatic resistance to insulin, which is a reversible condition and not actual diabetes.(81) Glucose intolerance from calorie restriction does not typically lead to complications associated with diabetes, or such calorie restriction would not extend the healthy lifespan. As glucose intolerance is a reversible side effect and if identified, the medication may merely be stoppedidentified.(77, 82) Rather than eliminating rapamycin, some studies suggest that attenuation of these side effects by using a lower doserapamycin would be possible using intermittent therapy (weekly administration), using a rapalog (temsirolimus), or using Peroxisome-proliferator-activated receptor (PPARγ) inhibitor (rosiglitazone).(79, 83)
Immune suppression:
While at higher doses, rapamycin confers immunosuppression to prevent transplant graft rejection, lower doses do not exert this effect. Indeed, rapamycin has been used to enhance vaccine responses.(84–87) Mannick et al. suggest mTOR inhibition may be an immunostimulant at low doses in the elderly by regulating antigen-presenting cells and T-cell modulation.(26) Therefore, low-dose and/or intermittent rapamycin therapy may also increase immune function in the aging process, precisely at the point of prostate cancer’s highest prevalence. Rapamycin could also be used to augment new vaccines being produced such as Prostavac () or ProstAtak () for low-grade, low-stage prostate cancer patients electing an active surveillance strategy.
Conclusion
There are many potential benefits to low-dose rapamycin therapy. An early phase clinical trial should be conducted to begin the process of determining if rapamycin can delay progression of low-grade prostate cancer. The concern for increasing androgen receptor in cancer cell lines will require close monitoring with serial PSA and MRI imaging. However, low-grade prostate cancer would likely have the least risk of this concern, as compared to the risk in more aggressive forms of prostate cancer. Preclinical animal studies suggest that low-dose rapamycin may be useful as a single agent treatment to prevent prostate cancer progression and support the need for a human clinical trial.
Acknowledgments:
This material is based upon support in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Clinical Sciences Research. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Prostate Cancer Research Program under Award No. W81XWH-15-1-0441. ZDS laboratory supported by NCI CA193835. Opinions, interpretations, conclusions, and recommendations are those of the author are not necessarily endorsed by the Department of Defense. Additionally, we acknowledge the generous support of the Roger L. and Laura D. Zeller Charitable Foundation for their financial support of the corresponding author.
Footnotes
Conflicts of Interest: Under a licensing agreement between Rapamycin Holdings, Inc. and the University of Texas Health Science Center San Antonio, Z.D. Sharp and the university are entitled to milestone payments and royalty on sales of the rapamycin formulation (eRapa) cited in this paper. Ian M Thompson, Jr, MD serves as a consultant for Rapamycin Holdings, Inc.
References
- 1.Affairs UNDoEaS. Population Ageing and Development 2012 [Available from http://www.un.org/en/development/desa/population/publications/ageing/population-ageing-development-2012.shtml.]
- 2.Interdisciplinary Topics in Gerontology and Geriatrics. MExtermann M, editor: Karger; 2013. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 5.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23(6):744–55. [DOI] [PubMed] [Google Scholar]
- 6.Ekim B, Magnuson B, Acosta-Jaquez HA, Keller JA, Feener EP, Fingar DC. mTOR kinase domain phosphorylation promotes mTORC1 signaling, cell growth, and cell cycle progression. Mol Cell Biol. 2011;31(14):2787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauvin C, Koka V, Nouschi A, Mieulet V, Hoareau-Aveilla C, Dreazen A, et al. Ribosomal protein S6 kinase activity controls the ribosome biogenesis transcriptional program. Oncogene. 2014;33(4):474–83. [DOI] [PubMed] [Google Scholar]
- 8.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25(48):6416–22. [DOI] [PubMed] [Google Scholar]
- 9.Musa J, Orth MF, Dallmayer M, Baldauf M, Pardo C, Rotblat B, et al. Eukaryotic initiation factor 4E-binding protein 1 (4E-BP1): a master regulator of mRNA translation involved in tumorigenesis. Oncogene. 2016;35(36):4675–88. [DOI] [PubMed] [Google Scholar]
- 10.Caron A, Richard D, Laplante M. The Roles of mTOR Complexes in Lipid Metabolism. Annu Rev Nutr. 2015;35:321–48. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339(6125):1323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351(6274):728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French JB, Jones SA, Deng H, Pedley AM, Kim D, Chan CY, et al. Spatial colocalization and functional link of purinosomes with mitochondria. Science. 2016;351(6274):733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yecies JL, Manning BD. mTOR links oncogenic signaling to tumor cell metabolism. J Mol Med (Berl). 2011;89(3):221–8. [DOI] [PubMed] [Google Scholar]
- 16.Yecies JL, Manning BD. Transcriptional control of cellular metabolism by mTOR signaling. Cancer Res. 2011;71(8):2815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–68. [DOI] [PubMed] [Google Scholar]
- 19.Smithson LJ, Gutmann DH. Proteomic analysis reveals GIT1 as a novel mTOR complex component critical for mediating astrocyte survival. Genes Dev. 2016;30(12):1383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albert V, Hall MN. mTOR signaling in cellular and organismal energetics. Curr Opin Cell Biol. 2015;33:55–66. [DOI] [PubMed] [Google Scholar]
- 21.Chi H Regulation and function of mTOR signaling in T cell fate decisions. Nat Rev Immunol. 2012;12(5):325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30(5):218–26. [DOI] [PubMed] [Google Scholar]
- 23.Diken M, Kreiter S, Vascotto F, Selmi A, Attig S, Diekmann J, et al. mTOR inhibition improves antitumor effects of vaccination with antigen-encoding RNA. Cancer Immunol Res. 2013;1(6):386–92. [DOI] [PubMed] [Google Scholar]
- 24.Keating R, Hertz T, Wehenkel M, Harris TL, Edwards BA, McClaren JL, et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol. 2013;14(12):1266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, et al. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 2010;185(4):2004–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268):268ra179. [DOI] [PubMed] [Google Scholar]
- 27.Hurez V, Dao V, Liu A, Pandeswara S, Gelfond J, Sun L, et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell. 2015;14(6):945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013;23(1):53–62. [DOI] [PubMed] [Google Scholar]
- 29.Ricoult SJ, Manning BD. The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep. 2013;14(3):242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guridi M, Tintignac LA, Lin S, Kupr B, Castets P, Ruegg MA. Activation of mTORC1 in skeletal muscle regulates whole-body metabolism through FGF21. Sci Signal. 2015;8(402):ra113. [DOI] [PubMed] [Google Scholar]
- 31.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J. 1995;312 (Pt 1):163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granneman S, Tollervey D. Building ribosomes: even more expensive than expected? Curr Biol. 2007;17(11): R415–7. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy BK, Lamming DW. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016;23(6):990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77(3):731–58. [DOI] [PubMed] [Google Scholar]
- 35.Morita M, Gravel SP, Chenard V, Sikstrom K, Zheng L, Alain T, et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 2013;18(5):698–711. [DOI] [PubMed] [Google Scholar]
- 36.Zadra G, Priolo C, Patnaik A, Loda M. New strategies in prostate cancer: targeting lipogenic pathways and the energy sensor AMPK. Clin Cancer Res. 2010;16(13):3322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zabala-Letona A, Arruabarrena-Aristorena A, Martin-Martin N, Fernandez-Ruiz S, Sutherland JD, Clasquin M, et al. mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature. 2017;547(7661):109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66(15):7783–92. [DOI] [PubMed] [Google Scholar]
- 39.Lamming DW, Cummings NE, Rastelli AL, Gao F, Cava E, Bertozzi B, et al. Restriction of dietary protein decreases mTORC1 in tumors and somatic tissues of a tumor-bearing mouse xenograft model. Oncotarget. 2015;6(31):31233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126(9):913–22. [DOI] [PubMed] [Google Scholar]
- 41.Barnard RJ, Kobayashi N, Aronson WJ. Effect of diet and exercise intervention on the growth of prostate epithelial cells. Prostate Cancer Prostatic Dis. 2008;11(4):362–6. [DOI] [PubMed] [Google Scholar]
- 42.Fontana L, Adelaiye RM, Rastelli AL, Miles KM, Ciamporcero E, Longo VD, et al. Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget. 2013;4(12):2451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blando J, Moore T, Hursting S, Jiang G, Saha A, Beltran L, et al. Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. Cancer Prev Res (Phila). 2011;4(12):2002–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azvolinsky A Repurposing to fight cancer: the metformin-prostate cancer connection. J Natl Cancer Inst. 2014;106(2):dju030. [DOI] [PubMed] [Google Scholar]
- 45.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li G, Rivas P, Bedolla R, Thapa D, Reddick RL, Ghosh R, et al. Dietary resveratrol prevents development of high-grade prostatic intraepithelial neoplastic lesions: involvement of SIRT1/S6K axis. Cancer Prev Res (Phila). 2013;6(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68(1):6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314(1):80–2. [DOI] [PubMed] [Google Scholar]
- 50.Mohler JL, Armstrong AJ, Bahnson RR, D’Amico AV, Davis BJ, Eastham JA, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14(1):19–30. [DOI] [PubMed] [Google Scholar]
- 51.Fleshner NE, Lucia MS, Egerdie B, Aaron L, Eure G, Nandy I, et al. Dutasteride in localised prostate cancer management: the REDEEM randomised, double-blind, placebo-controlled trial. Lancet. 2012;379(9821):1103–11. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong AJ, Netto GJ, Rudek MA, Halabi S, Wood DP, Creel PA, et al. A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer. Clin Cancer Res. 2010;16(11):3057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liss MA, Schenk JM, Faino AV, Newcomb LF, Boyer H, Brooks JD, et al. A diagnosis of prostate cancer and pursuit of active surveillance is not followed by weight loss: potential for a teachable moment. Prostate Cancer Prostatic Dis. 2016;19(4):390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharp ZD, Curiel TJ, Livi CB. Chronic mechanistic target of rapamycin inhibition: preventing cancer to delay aging, or vice versa? Interdiscip Top Gerontol. 2013;38:1–16. [DOI] [PubMed] [Google Scholar]
- 55.Sharp ZD, Richardson A. Aging and cancer: can mTOR inhibitors kill two birds with one drug? Target Oncol. 2011;6(1):41–51. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy BK, Pennypacker JK. Drugs that modulate aging: the promising yet difficult path ahead. Transl Res. 2014;163(5):456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hasty P, Livi CB, Dodds SG, Jones D, Strong R, Javors M, et al. eRapa restores a normal life span in a FAP mouse model. Cancer Prev Res (Phila). 2014;7(1):169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Livi CB, Hardman RL, Christy BA, Dodds SG, Jones D, Williams C, et al. Rapamycin extends life span of Rb1+/− mice by inhibiting neuroendocrine tumors. Aging (Albany NY). 2013;5(2):100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69(2):119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nadon NL, Strong R, Miller RA, Nelson J, Javors M, Sharp ZD, et al. Design of aging intervention studies: the NIA interventions testing program. Age (Dordr). 2008;30(4):187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11(4):675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galvan V, Hart MJ. Vascular mTOR-dependent mechanisms linking the control of aging to Alzheimer’s disease. Biochim Biophys Acta. 2016;1862(5):992–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lesovaya EA, Kirsanov KI, Antoshina EE, Trukhanova LS, Gorkova TG, Shipaeva EV, et al. Rapatar, a nanoformulation of rapamycin, decreases chemically-induced benign prostate hyperplasia in rats. Oncotarget. 2015;6(12):9718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4(3):223–38. [DOI] [PubMed] [Google Scholar]
- 67.Saha A, Blando J, Tremmel L, DiGiovanni J. Effect of Metformin, Rapamycin, and Their Combination on Growth and Progression of Prostate Tumors in HiMyc Mice. Cancer Prev Res (Phila). 2015;8(7):597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaeberlein M Rapamycin and ageing: when, for how long, and how much? J Genet Genomics. 2014;41(9):459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clegg NJ, Couto SS, Wongvipat J, Hieronymus H, Carver BS, Taylor BS, et al. MYC cooperates with AKT in prostate tumorigenesis and alters sensitivity to mTOR inhibitors. PLoS One. 2011;6(3):e17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rebello RJ, Pearson RB, Hannan RD, Furic L. Therapeutic Approaches Targeting MYC-Driven Prostate Cancer. Genes (Basel). 2017;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weichhart T, Hengstschlager M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15(10):599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun Q, Liu Q, Zheng Y, Cao X. Rapamycin suppresses TLR4-triggered IL-6 and PGE(2) production of colon cancer cells by inhibiting TLR4 expression and NF-kappaB activation. Mol Immunol. 2008;45(10):2929–36. [DOI] [PubMed] [Google Scholar]
- 73.Bauman TM, Becka AJ, Sehgal PD, Huang W, Ricke WA. SIGIRR/TIR8, an important regulator of TLR4 and IL-1R-mediated NF-kappaB activation, predicts biochemical recurrence after prostatectomy in low-grade prostate carcinomas. Hum Pathol. 2015;46(11):1744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain S, Suklabaidya S, Das B, Raghav SK, Batra SK, Senapati S. TLR4 activation by lipopolysaccharide confers survival advantage to growth factor deprived prostate cancer cells. Prostate. 2015;75(10):1020–33. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Mikhailova M, Bose S, Pan CX, deVere White RW, Ghosh PM. Regulation of androgen receptor transcriptional activity by rapamycin in prostate cancer cell proliferation and survival. Oncogene. 2008;27(56):7106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y, Chhipa RR, Cheng J, Zhang H, Mohler JL, Ip C. Androgen receptor-mTOR crosstalk is regulated by testosterone availability: implication for prostate cancer cell survival. Anticancer Res. 2010;30(10):3895–901. [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y, Diaz V, Fernandez E, Strong R, Ye L, Baur JA, et al. Rapamycin-induced metabolic defects are reversible in both lean and obese mice. Aging (Albany NY). 2014;6(9):742–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stallone G, Infante B, Grandaliano G, Gesualdo L. Management of side effects of sirolimus therapy. Transplantation. 2009;87(8 Suppl):S23–6. [DOI] [PubMed] [Google Scholar]
- 79.Festuccia WT, Blanchard PG, Belchior T, Chimin P, Paschoal VA, Magdalon J, et al. PPARgamma activation attenuates glucose intolerance induced by mTOR inhibition with rapamycin in rats. Am J Physiol Endocrinol Metab. 2014;306(9):E1046–54. [DOI] [PubMed] [Google Scholar]
- 80.Houde VP, Brule S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59(6):1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blagosklonny MV. Rapamycin-induced glucose intolerance: hunger or starvation diabetes. Cell Cycle. 2011;10(24):4217–24. [DOI] [PubMed] [Google Scholar]
- 82.Schindler CE, Partap U, Patchen BK, Swoap SJ. Chronic rapamycin treatment causes diabetes in male mice. Am J Physiol Regul Integr Comp Physiol. 2014;307(4):R434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arriola Apelo SI, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, et al. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell. 2016;15(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jagannath C, Bakhru P. Rapamycin-induced enhancement of vaccine efficacy in mice. Methods Mol Biol. 2012;821:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turner AP, Shaffer VO, Araki K, Martens C, Turner PL, Gangappa S, et al. Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. Am J Transplant. 2011;11(3):613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson SC, Kaeberlein M. Rapamycin in aging and disease: maximizing efficacy while minimizing side effects. Oncotarget. 2016;7(29):44876–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McMichael AJ, Haynes BF. Influenza vaccines: mTOR inhibition surprisingly leads to protection. Nat Immunol. 2013;14(12):1205–7. [DOI] [PubMed] [Google Scholar]