Abstract
Diabetic retinopathy (DR), a major cause of blindness in working-age adults, is emerging as a major public health issue worldwide, in particular in low- and middle-income countries (LMIC). Traditionally, the management of DR has been on tertiary-level treatment (eg, laser, anti-VEGF injections and surgery) in specialized settings by highly trained ophthalmologists on individual patients. To win the war on DR, a paradigm shift in strategic focus and resources must be made from such tertiary treatment toward primary and secondary prevention, which are broader, more impactful, and cost-effective for the larger population. These include improving education and awareness of risk of DR among people initially diagnosed with diabetes, promoting behavioral modifications such as physical activity and medication adherence for improving glycemic and blood pressure control, setting up systematic screening programs for DR to detect the onset or progression of the disease, and implementing cost-effective, evidence-based policies and guidelines for managing DR. Additionally, there is a need to leverage on novel technology including the application of digital big data to predict people at risk of diabetes and DR, the use of wearable devices and smart phone apps, behavioral techniques including social media for self-management of diabetes, and telemedicine-based DR screening incorporating artificial intelligence (AI) to broaden access to screening in all settings. To turn the tide on the war on DR, we must reframe DR not only as a specific condition that can be managed by ophthalmologists, but fundamentally, as a preventable condition by shifting the weight of strategies from tertiary to secondary and primary battlegrounds.
Keywords: diabetic retinopathy, DR screening, guidelines, primary prevention, secondary prevention
Diabetes is increasing in an epidemic proportion worldwide affecting an estimated 425 million adults worldwide in 2017 and the number is expected to rise to 629 million by 2045.1 More than 80% of those with diabetes live in LMIC.
Of all the diabetes complications, DR exerts a significant public health and clinical impact for a number of reasons. One third of those with diabetes have DR, with 1 in 10 having vision-threatening levels of DR.2 Despite progress in understanding the disease and availability of new treatments, DR remains the most common cause of blindness in the economically active, working-age population, exerting a disproportionate impact on society. In 2010, DR ranked the fifth most common cause of blindness and visual impairment accounting for up to 4.5 million people to become blind or visually impaired.3 In parallel with the increase in diabetes prevalence and the aging of populations globally, the absolute burden of DR is substantial.
HOW DO WE TACKLE THIS CLEAR AND PRESENT DANGER?
In 1971, Richard Nixon, the President of the United States declared a “War on Cancer” to galvanize the medical and scientific community with an aim to develop new cancer therapies. The War on Cancer was partially successful. This strategic program provided increased governmental funding for cancer treatment research that led to improved understanding of the pathogenesis and novel treatments of some cancers.4 However, there was limited focus and attention to cancer prevention, and mortality owing to cancer was reduced by only 10% between 1985 and 2000, as opposed to the 50% goal set by the program. The oncology community subsequently realized that it may be unrealistic to simply find a magic bullet cure for cancer without an effort that is broader in community settings, and recommended a shift in focus from tertiary treatment to primary prevention.
WHAT WOULD A “WAR ON DR” LOOK LIKE AND CAN WE DRAW SOME USEFUL LESSONS FROM THE WAR ON CANCER?
Evidence suggests that the natural course of DR can favorably be altered by risk factor control, early detection and the appropriate cost-effective treatment. In the current arena, the ophthalmic community mostly focused on downstream tertiary level approaches in specialized settings focusing on treating individual patients. From the public health prevention perspective, the impact of downstream tertiary approaches is variable and much more expensive. Taking lessons from the War on Cancer, a shift in focus on upstream primary approaches targeted at the population and community, by promoting healthier life style and self-management of diabetes, and providing uniform, wider access to DR screening may be more cost-effective. The use of novel technology is also important. These include the use and application of digital big data to predict people at risk of diabetes, DR and vision loss, the use of self-monitoring wearable devices and smart phone, the application of modern psycho-behavioral techniques including nudges and social media influence for self-management of diabetes, and digital cloud-based telemedicine-based DR screening incorporating artificial intelligence (AI). In the current review, we review the burden of DR, prevention strategies, barriers and gaps, and outline strategies to overcome challenges to win the War on DR.
DIABETES IS A MAJOR HEALTH CRISIS GLOBALLY
Diabetes is one of the largest global health emergencies of the 21st century affecting 425 million adults aged 20 to 79 years worldwide in 2017 and is expected to increase to 629 million by 2045.1 If we take population aging into account and extend the age to 18 to 99 years, 451 million are affected currently and the number will increase to 693 million by 2045. Globally USD 727 billion is spent annually on diabetes-related health care. The worrying trend is half of the people with diabetes are undiagnosed. Even in high-income countries, more than one third are undiagnosed. Apart from diabetes, another 352 million adults worldwide are estimated to have prediabetes which could lead to diabetes in 5 years if they are undetected and intervened.
Nearly 80% of those with diabetes live in LMIC of which China and India share the major proportion. In 2017, the global prevalence of diabetes in adults aged 20 to 79 years was estimated to be 8.8%. Prevalence in China and India were estimated to be higher than the national average with 9.7% and 10.4%. Of the 429 million adults with diabetes, 114 million live in China and 73 million in India. The growing prevalence of diabetes in Asia is contributed by industrialization, urbanization, and migration associated with lifestyle changes leading to risk factors such as sedentary lifestyle and obesity. Owing to the rapid economic transition and acculturation, the Asian phenotype of diabetes has been shown to be different that Asians tend to develop diabetes at younger age (40–59 years vs >60 in Europeans), at lower levels of body mass index and at higher rates for the same amount of weight gain than whites.5 In addition, Asians also have high rate of clustering of cardiovascular risk factors (metabolic syndrome) and these characteristics increase the lifetime risk of diabetic complications in Asians.6
In smaller developed cities such as Singapore, the prevalence of diabetes follows the same trend as China and India with national average of 11% in 2017. By 2020, Singapore is estimated to have half a million people with diabetes which is expected to increase to 1 million by 2050. Unfortunately, Singapore ranked first in the incidence of type 2 diabetes-related end-stage renal disease and amputations worldwide. Consequent to the alarming rate of type 2 diabetes and its complications in Singapore, the Minister of Health declared a “War against type 2 diabetes” in 2016.
EPIDEMIOLOGY AND BURDEN OF DR
DR is the leading cause of preventable vision loss in working-age adults. Vision loss from DR could be because of proliferative DR (PDR) or diabetic macular edema (DME). A pooled meta-analysis involving 35 studies conducted in 2010 estimated global prevalence of any DR and PDR among patients with diabetes to be 35.4% and 7.5%, respectively. Individual studies involving type 2 diabetes showed prevalence of DR to be higher in the Western populations compared with Asians, for example, prevalence of DR was estimated to range from 28% to 40% in the United States compared with 16% to 23% in Asians. Corresponding estimates for vision-threatening DR (VTDR, including severe nonproliferative DR, PDR, and DME) were 4% to 8% in the United States and 4% to 5% in Asians. However, a systematic review of 72 studies concluded that DR prevalence may actually be higher in Asian countries and previously reported lower rates could be because of lower rate of detection of DR.7 In Singapore, prevalence of DR and VTDR was higher (33.9%, 8.9%) comparable to that of Western populations.
DME IS THE MOST FREQUENT CAUSE OF DR-RELATED BLINDNESS IN DEVELOPED COUNTRIES
Although prevalence of DR is increasing worldwide, epidemiological evidence from developed countries suggested a declining trend in the prevalence of blindness because of PDR in those with diabetes owing to improvement in management of diabetes, early detection of onset and progression of DR through regular screening for DR and timely treatment with laser photocoagulation. In 2009, a meta-analysis including 27,120 participants with diabetes reported the pooled incidence of PDR to have declined from 19.5% in 1975 to 1985 to 2.6% in 1986 to 2008 (2.6%).8 However, the decrease in prevalence of blindness owing to PDR in developed countries is offset by the increase in prevalence of blindness owing to DME. DR-related blindness accounted for 2.6% of all blindness in 2010 compared with 2.1% in 1990.3 In a recent study in the United States, Zhang et al9 showed that prevalence of clinically significant macular edema was twice as common as PDR (2.7% vs 1.5% in those with diabetes), suggesting DME to be a more common cause of visual loss in people with type 2 diabetes. Global prevalence of DME was estimated to be 6.8% in adults with diabetes aged 20 to 79 years2 with no significant difference between East and West.10 Prevalence estimates ranged from 2% in Canada to 7.6% in Australia in the West and from 1.4% in India to 7.6% in Singapore.11 It should be noted that most of the studies that reported DME prevalence used stereoscopic fundus photographs to assess the presence of DME which may have underestimated the cases of DME when compared with optical coherence tomography (OCT) which is more accurate for detecting the onset and progression of DME.
THERE REMAINS A LACK OF ROBUST, RELIABLE, AND ACCURATE ESTIMATES OF DR IN MANY COUNTRIES
Although many studies had reported on the prevalence of DR, there is still a lack of prevalence estimates in certain developing regions such as Eastern Europe, Africa, and Asia. Within the Asia-Pacific itself, estimates are not available from central Asia (eg, Armenia, Kazakhstan, and Mongolia), South East Asia (eg, the Philippines, Cambodia, Myanmar, and Vietnam) and Oceania (eg, Micronesia, Papua New Guinea, and Samoa) where prevalence of diabetes is high.12 Understanding the prevalence of DR in these regions is important for relevant authorities to estimate the needs of medical facilities and to plan resource allocation for managing the increasing number of patients with diabetes. Furthermore, studies assessing the prevalence of DME using fundus photographs are sparse. More studies assessing the presence of DME using OCT may provide accurate prevalence estimates and response to treatment.
RISK FACTORS OF DR ARE CONSISTENT ACROSS POPULATIONS
Despite the difference in prevalence of DR across populations, risk factors of DR are similar across populations. Duration of diabetes, hyperglycemia, and hypertension are the key risk factors consistently associated with DR in several epidemiological studies. The lifetime risk of developing DR is estimated to be 50% to 60% in those with type 2 and up to 90% in those with type 1 diabetes.13 DME shares similar risk factors with DR, but some differences have been reported. Although DR is more common in type 1 than in type 2 diabetes and prevalence of DR increases with increasing duration, DME is more common in type 2 diabetes and duration of diabetes is not linearly related to DME prevalence.14 Hyperglycemia is a well-known risk factor of both DR and DME in type 1 and type 2 diabetes.15–17 In the Singapore Indian Eye Study (SINDI), participants with diabetes and poor glycemic control (HbA1c ≥8%), had 4-fold increase risk of incident DR and progression of DR, compared with those with HbA1c <7%.16 In addition, mild and moderate levels of DR progressed despite reduction in mean HbA1c levels between baseline and follow-up which could be explained by the “legacy” effect or a “metabolic memory” phenomenon, because of epigenomic alterations resulting in sustained vascular dysfunction despite attainment of glycemic control.18 Similar findings were observed in studies conducted in Hong Kong17 and China, and together they confirm the “metabolic memory” phenomenon proposed first in the United Kingdom Prospective Diabetes Study (UKPDS) trial. These studies provide compelling evidence to support the need for early and sustained glycemic control for long-term reduction of complications including DR. Intensive glycemic control aiming to achieve near normal glycemia has been shown to prevent or delay the onset and progression of DR in several landmark clinical trials such as the Diabetes Control and Complications Trial (DCCT) in type 1 diabetes and the UKPDS in type 2 diabetes. However, as intensive glycemic control has been shown to increase the risk of hypoglycemia, rapid reductions in HbA1c should be avoided, in particular in elderly participants who are more prone to develop hypoglycemia. Similar to glycemic control, higher blood pressure (BP) has been shown to be associated with DR risk in several studies19–21 and tight control has been shown to be beneficial in reducing the risk of DR in the UKPDS trial and several antihypertensive medication trials.22 The consistency of association between HbA1c, BP, and DR in epidemiological studies and clinical trials across populations, suggest that a uniform strategy to manage glycemic and BP control is possible for all patients with diabetes irrespective of complications and population differences. Association between lipid control and DR risk is not clear. However, as both diabetes as well as DR increase the risk of cardiovascular disease, treatment of dyslipidemia is recommended in those with DR also to improve lipid control. As such, many guidelines, including the American Diabetes Association,23 have recommended HbA1c levels <7%, BP levels <130/80 mm Hg, and treatment of dyslipidemia as reasonable goals for all patients with diabetes to reduce the risk of both micro and macrovascular complications.
IMPLICATIONS OF THE INCREASING DR BURDEN TO PATIENTS AND HEALTH CARE SYSTEMS
With the rising prevalence of DR, blindness and vision impairment (VI) owing to DR will increase substantially in LMIC, increasing the need for eye care. Furthermore, with the aging population, it will become even more challenging to treat, which will put undue stress on the already strained health care system. Prevention therefore becomes crucial to tackle the increasing burden as evidence from high-income countries clearly shows blindness due to DR is preventable.
STRATEGIES TO WIN THE WAR ON DR
There are 3 major approaches to DR prevention (Fig. 1):
FIGURE 1.
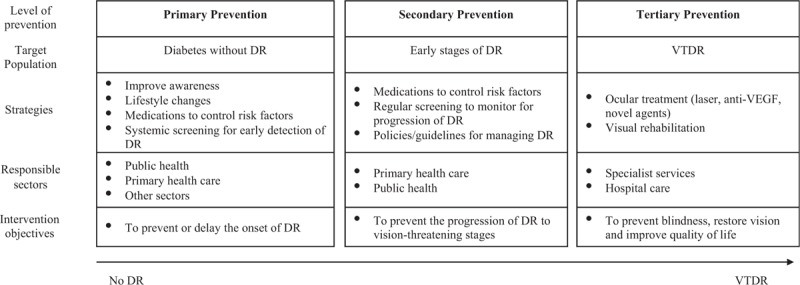
Prevention strategies for DR. Anti-VEGF indicates anti-vascular endothelial growth factor; DR, diabetic retinopathy; VTDR, vision-threatening DR.
Primary prevention aims to reduce the onset of DR in those with diabetes. Strategies include improving awareness of DR, risk factor reduction through lifestyle modifications, pharmacological interventions, and screening to detect onset of DR.
Secondary prevention aims to reduce the progression of DR in those with existing DR and focuses on preventing the progression of DR in patients with DR by continuing systemic risk factor control, regular screening to monitor progression of mild DR to vision-threatening stages, and development and implementation of evidence-based guidelines for managing DR.
Tertiary prevention focuses on treating blindness due to DR classically based on laser photocoagulation treatment and ocular surgery, but increasingly on the use of anti-vascular endothelial growth factor (anti-VEGF) for VTDR.
CURRENT DIABETES MANAGEMENT
Effective diabetic management is the hallmark of primary and secondary prevention of or delaying the onset of vascular complications including DR. Improvement in diabetes outcomes depends on a combination of factors at the patient level (eg, self-care, adherence to medication), provider level (eg, treatment modification), and the system level (eg, provision of screening services). In patients with diabetes, systemic risk factor control is the main goal of prevention. Some of the process measures to achieve this goal from the patient perspective include adherence to diabetic diet, adequate exercise, cessation of smoking, adherence to antidiabetic and antihypertensive medication therapy, regular self-monitoring of blood glucose, and regular monitoring of HbA1c, among others. From the provider perspective, prioritizing timely modification of therapy for those who did not achieve optimal glycemic goal, improving communication among primary care physician, endocrinologists, and specialists (eg, ophthalmologists), and care coordination and care supervision by a health care provider, for example, nurses. Effective diabetes management has been shown to reduce complications by 53% to 63%.24,25 Up to 40% of those with diabetes have been shown to be nonadherent to prescribed medications. Despite evidence showing the benefits of glycemic and BP control, achieving the recommended glycemic and BP goals were suboptimal in both LMIC and high-income countries.26–28 In the United States, nearly 50% of those with diabetes did not achieve glycemic goal of HbA1c <7% and BP target of <130/80 mm Hg.26 In Singapore, only 29% of Malay adults with diabetes achieved glycemic goal and 13% achieved recommended BP goal.28 Studies have also shown that patients younger than 65 years with diabetes are much less likely to achieve recommended targets of diabetes compared with those above 65 years.29
Several studies have identified barriers to and facilitators for diabetic management. A systematic review identified financial constraints faced by the patients and lack of access to health services and medications due to 2 important health system barriers to achieving good diabetes outcomes.30 Some of the identified barriers adhered to behavioral medications in Asians were poor health literacy, preference to traditional or alternative therapy, concerns about long-term safety of antidiabetic medications, and health beliefs such as viewing disease as a result of fate and the avoidance of visiting clinics.31 Although diabetes management in western countries highly relies on patient centeredness and self-empowerment, Asians tend to rely on authority.32
One of the main reasons of suboptimal glycemic control in patients with diabetes by treating physicians is “clinical inertia,” which prolongs suboptimal glycemic state in patients who are exposed to higher risk of vascular complications. It has been shown that up to 30% to 50% of patients experienced years of suboptimal glycemic control before treatment is accelerated.33 A combination of patient, physician, and system factors contribute to clinical inertia, for example, patients’ lack of understanding of the disease, fear of hypoglycemia due to adverse effect of drugs, lack of physician time and education, lack of individualized management guidelines and decision support aids, and poor communication between physician and staff.34
EDUCATION AND AWARENESS OF DR
Awareness of DR in those with diabetes and even with DR is suboptimal in both high-income countries and LMIC. Among those with diabetes and DR, 73% in the United States,35 and 83% in Singapore36 were unaware of their condition. 1 in 4 with VTDR was unaware of their DR status in Singapore. In India, it has been shown that nearly half of the people with diabetes visit ophthalmologists only after their vision is lost.37 In China, 67% of patients with diabetes who attended a specialist eye clinic for the first time were found to have VTDR.38 Awareness of DR among primary care physicians was also found to be suboptimal in some LMIC. The DR Barometer Study that assessed the level of awareness, and provision of screening and treatment for DR and DME in 2329 health care professionals involved in the management of adults with diabetes/DR/DME, including ophthalmologists from 41 countries, found that more than one third of diabetes specialists surveyed reported that they did not discuss eye care with their patients with diabetes and only over one third of ophthalmologists reported that they had sufficient information on diabetic eye complications.39
THE CENTRAL ROLE OF DR SCREENING
Routine DR screening of patients with diabetes for is an important strategy to identify patients at risk of developing sight-threatening DR and intervene promptly. Screening compliance has been shown to result in better visual outcomes.40 Thus, low screening uptake translates into lost opportunities in preventing visual loss because of DR. Adherence to screening recommendations is high in the West as systematic screening programmes are available at low or no cost. In the UK, from 2004 to 2005, 84% of those with known diabetes who were offered screening, had their eyes screened for DR.29 In Australia, 78% of nonindigenous Australians compared with 53% of indigenous Australians with diabetes had their eyes screened for DR.41 Contrary to the West, in LMIC where DR screening is offered as an opportunistic intervention, adherence to screening is suboptimal. In China, less than one third of patients with diabetes underwent screening for DR in the previous year.42
In high-income settings, barriers to DR screening identified were mostly related to process measures such as lack of coordination between physician and screening staff or lack of training, whereas in LMIC barriers were related to system measures such as lack of infrastructure, skilled manpower, and accessible eye centers, and poor transportation services. Lack of knowledge and awareness of DR was identified as the main barrier to DR screening uptake in all income settings.43 Younger individuals (aged 18–34 years) were least likely to attend screening with a higher risk of referable DR being found at first screen.44 Screening rate has been shown to improve after physician recommendation of the need for regular eye examination in patients with diabetes.45
THE IMPORTANCE OF COST-EFFECTIVE EVIDENCE-BASED DR GUIDELINES
It has been shown that much of the visual loss can be prevented by an adherence to care patterns recommended by guidelines. Data from serial National Health and Nutrition Examination survey (NHANES) surveys in the United States showed improvement in glycemic control after improved diabetes management and care.46 Evidence from trials focusing on 1 or 2 elements of recommended care showed the benefits of adherence to care recommendations. For example, in the Early Treatment Diabetic Retinopathy Study (ETDRS), those who undergo early photocoagulation for DR had lower rates of visual loss because of DR. Recently, a longitudinal study assessed adherence to recommended care for screening and secondary prevention including prescription drug use for glycemic, blood pressure, and lipid control, screening visits, ophthalmology visits, and so on using retrospective Medicare claims data in adults with diagnosed diabetes and with at least basic insurance coverage. The study showed that those who received guidelines-recommended levels of care had significantly lower rates of PDR, low vision, and blindness compared with those who did not receive recommended care despite being diagnosed with having diabetes.47 However, despite guidelines and having insurance coverage, uptake of recommended care remained low with only 15% of Medicare beneficiaries received recommended care.
Although implementing broad-based public health programs for diabetes care is feasible in high-income countries, it remains a serious challenge in LMIC, which are constrained by lack of trained eye care professionals, equipment, drugs, infrastructure, and so on. In many countries, the standard care pathway of DR is not clear.48 Although diabetes management guidelines are available in several countries, guidelines on DR management are limited. In a recent survey of 50 Asian countries, only 11 had some form of guidelines of which only 2 were specific to DR and the rest were for general diabetes care.49 Vision loss from DR can be prevented using broad-level public health strategies, but the strategies should take into account the resource-setting at country and population levels. With this goal in mind, the International Council of Ophthalmology (ICO) recently updated its guidelines on DR management with a specific focus on screening, referral, and follow-up schedules and timely treatment for DR tailored to suit different resource settings.48 ICO recommends the minimum screening examination which includes a screening vision examination and a retinal examination adequate for classifying DR status that can vary depending upon the resource setting. In high-income settings, a recommended screening method is visual acuity test with refraction and retinal photography, whereas in low- and intermediate-resource setting, screening should include visual acuity test using pin-hole and clinical retinal examination with pupil dilation. The ICO recommends uniform referral time for VTDR and DME: <1month for PDR and center-involving DME, and <3 months for severe nonproliferative DR and noncenter involving DME. However, for mild and moderate stages of DR, referral timings between resource settings can be different, 6 to 12 months and 3 to 6 months in high-income settings versus 1 to 2 years and 6 to 12 months in low- and intermediate-resource settings. Treatment-wise pan-retinal photocoagulation is recommended for PDR, and considering focal laser photocoagulation or anti-VEGF therapy for center-involving DME in low- and intermediate-resource settings. Whereas in high-income settings, the above strategies are also applied to treating severe nonproliferative DR and noncenter involving DME.
IS OUR CURRENT FOCUS AND STRATEGY ADEQUATE TO WIN THE WAR ON DR?
Currently, ophthalmologists and health care systems at all settings are focusing much of their efforts and investment on tertiary prevention as traditionally, the management of DR has been on tertiary-level treatment (eg, laser, anti-VEGF injections, and surgery) in specialized settings by highly trained ophthalmologists on individual patients. Although blindness owing to DR has declined in the past century because of early detection and treatment, incidence of DR is increasing in high-income countries. In LMIC, lack of resources and systems limits improving care for diabetes. These findings suggest that more efforts focusing on primary and secondary prevention are needed to reduce the onset and progression of DR.
Several important gaps remain in primary and secondary prevention strategies. Awareness of DR is suboptimal, and achieving optimal goals for glycemic and BP control is inadequate in high-income countries as well and also LMIC. Access to screening services is limited in many LMIC, whereas sustaining current screening programmes is questionable in high-income countries. Adherence to DR guidelines is suboptimal in high-income countries whereas implementation of DR guidelines is patchy in LMIC.
HOW CAN WE SHIFT THE FOCUS TO PRIMARY AND SECONDARY PREVENTION STRATEGIES?
Improving Self-Care Behavior
Improving self-care behaviors including adherence to recommended diet, adequate exercise, antidiabetic and antihypertensive medication, and self-monitoring of blood glucose are essential for improving diabetes management. Behavioral modifications need knowledge about the seriousness of the condition and motivation to make changes, which requires education and support. Patient empowerment is an important component and individual targets to discuss with the patient and to be agreed by the patient and the attending physician. Each health system needs to identify barriers from patient-, provider-, and system-perspectives, and address them to improve diabetes outcomes. Health education messages and behavioral modifications should be tailored to suit cultural beliefs and misconceptions.
Interventions based on behavioral economics using “nudges” or communication strategies that influence patients’ choices in a favorable way may help patients make complex decisions.50 Nudges may improve patients’ appropriateness of risk perception and attitude toward making lifestyle changes resulting in improved self-management of diabetes. Interventions such as providing personalized risk communication method using innovative metrics in an easily understandable way during routine primary care consultation have shown promising results in improving behavioral outcomes. For example, expressing the 10-year cardiovascular risk as an effective heart age may weaken diabetic patients’ optimistic bias of underestimating their risk of complications; and the impact of diabetes being poorly controlled on life expectancy expressed as, “hours of lifetime lost per day" calculated in comparison to a well-controlled individual have improved intentions to make lifestyle change in trials.51
More Effective Diabetes Management
Overcoming clinical inertia is a fundamental step in improving long-term care for individuals with type 2 diabetes. Consistent follow-up with patients, improved access to resources with targeted education, decision support aids, and patient feedbacks have been suggested to overcome clinical inertia. Diabetes management programmes should include process and outcome measures such as adherence to HbA1c testing, screening frequencies, treatment modifications, and intermediate outcomes like percentage of people who achieved target HbA1c, BP, weight loss, and so on to evaluate the success of the programme.
With the advancement in digital technology, internet, smart phones, and wearable devices greatly help facilitate diabetes management. Web-based health education materials like “Diabetes education online” “Diabetes Australia” provide patients with comprehensive information on understanding diabetes and self-management. Smartphone apps and wearable devices improve self-care by allowing people to track their health behaviors including diet, exercise, weight loss, sleep pattern, and health indicators such as blood sugar and blood pressure in real-time; and to improve medication adherence with features including automated schedules, alerts, and reminders.52 Virtual coaching platforms provide patients with personalized behavior modifications and help health professions make care decisions.
Improving Awareness of DR
Focused educational materials highlighting the importance of glycemic control in preventing eye complications and screening recommendations should be made available at primary and secondary care centers. As physician recommendation has been shown to improve screening rate, health care professionals’ knowledge about DR screening, pupil dilation, and referral schedules need to be strengthened using structured education programme. Good communication and care coordination between health care professionals and patients is essential to actively engage patients in DR prevention, recommending eye examination and positive reinforcement with negative screening results.
Increasing Availability and Effectiveness of DR Screening With Telemedicine and AI
Access to screening service and cost of screening are major barriers in many LMIC, as there are no systematic screening programmes in these countries. Telemedicine has revolutionized DR care in all settings. Manual grading is resource-intensive requiring trained and qualified graders. Telemedicine-based DR screening is well-established in the UK and is being deployed in several countries including China and India. In Singapore, telemedicine-based DR screening has now been successfully integrated into the National Diabetes Program. The Singapore Integrated DR Program (SiDRP) covers up to 200,000 people with diabetes in 18 primary care facilities in Singapore. Retinal photos taken at point-of-care are transmitted using teleophthalmology platform to a centralized reading center where a trained grader reads them remotely, and reports are sent back to primary care clinics on the same day mostly within an hour, which are then referred to ophthalmologists for referral if required. Telemedicine screening resulted in cost savings of SGD144/person in terms of direct cost only compared with the existing physician assessed model. Extrapolating this to the SiDRP population, this translated to lifetime savings of ∼ SGD 29 million.53 Telescreening using trained graders instead of ophthalmologists to read retinal images offers efficient and cost-effective screening solution to DR screening in all resource settings with improved coverage. Recent advances in telecommunications including ‘cloud’ storage, handheld fundus cameras, and automated DR screening systems offering excellent opportunities for expanding DR screening services to remote areas.
Current DR screening programmes requiring manual grading of DR are resource-intensive and are not sustainable in the long-run even in high-resource settings. Therefore, scaling and expanding it up to meet the growing diabetes epidemic is challenging. AI-based technology including deep learning systems (DLS) is gaining popularity in ophthalmology, in particular, in DR screening and automated segmentation of OCT images for diagnosis of DR and DME.54 Google health in the United States first showed the potential of an automated retinal image based DLS with accuracy ≥99% for detecting referable DR.55 In Singapore, a DLS called “SELENA” was able to identify other sight-threatening eye diseases such as age-related macular degeneration and glaucoma besides referable DR with accuracy of 94% in the development cohort and 90% to 98% accuracy in the external validation cohorts.56 Another DLS “IDX-DR” developed by Abramoff et al has been approved by the US Food and Drug Administration for detecting DR levels greater than mild in adults with diabetes without the need for interpretation by a clinician.57 A recent study conducted in Zambia which evaluated the performance of an AI-based DLS had accuracy >97% for detecting referable DR and DME58 rendering support for AI-based screening model to be applied to DR screening in resource-constrained settings. However, AI-application within low-resource settings might need more careful and comprehensive design and planning considering the availability of specialists, cost-effectiveness of the programme, and long-term patient outcomes. The performance of AI in DR screening is highly promising; however, several challenges still remain that limit its use in clinical medicine from image quality, identifying image features that are critical to image classification to large-scale implementation and medico legal implications.59
Innovative Approaches to Integrating Eye Health and Diabetes Management
To combat the increasing burden of DR and reverse the trend of blindness from DR, a collective approach integrating diabetes and eye care has been developed by the partners of the Global Diabetic Retinopathy Advocacy Initiative undertaken by leading nongovernment agencies in diabetes and eye care.60 Drawing on evidence from programs with promising results from 147 countries, the “Integrated care for diabetes and eye health: a global compendium of good practice” report provides guidance to policy makers, medical organizations, service providers, and social partners to address the challenges of blindness and vision loss from diabetes by ensuring services; support and information are available and accessible to all patients with diabetes. The guiding principles of the initiative are based on people-centered, equitable, evidence-based, and quality care achieved in a collaborative and cost-efficient manner through adequately trained, supported, and deployed health workforce within local regulatory frameworks.
Integrated care can involve a range of approaches to effectively bringing together and aligning management of diabetes with eye health care, but 4 broad categories of integrated care are identified by the group: integration of eye care into routine diabetes care/primary care including training primary care physicians and diabetes care professionals to raise the awareness of DR among patients, provision of eye heath examinations or screening services as part of routine diabetes care, identification of DR, advising patients on appropriate management strategies and making appropriate referral to secondary and tertiary care services; integration of diabetes into comprehensive/primary eye care including enhancing skills of eye health professionals such as ophthalmologists and optometrists to understand the consequences of DR, identification and management of DR, diabetes and its complications; horizontal and vertical integration of services aimed to improve referral or recall pathways within and across all levels of the health care system and establishment of innovative tactics to enhance the DR continuum of care; integration of DR policies, guidelines, and training into all relevant national health policies, such as inclusion of DR-specific policies in National Diabetes Plans, creation of National Action Plans for DR, standardization of care by implementing and ensuring adherence to a set of protocols by all secondary and tertiary points of care addressing DR. The compendium presented 17 international case studies that successfully adopted these models to address the challenges of DR covering both high- and low-middle-income countries from all regions like the United States, Australia, China, India, Mexico, Singapore, Iran, Peru, Zambia, and so on.
BIG DATA ANALYTICS IMPROVES PREDICTION OF DIABETES AND DR
Big data analytics combining data from several health providers enables clinicians to depict a new view of patient care processes by integrating different sources of information such as electronic medical records (EMR), claim data and pharmacy records among others, provide real-time data gathering greater accuracy and facilitating the building of decision support systems, predict future probabilities, prognosis, and population health management to improve patient care.61 Predictive models based on large amount of clinic data have been developed for use in management of diabetes and DR. A retrospective study using large number of EMR data in the United States found that EMR-derived phenotypes markedly improved the detection of diabetes even when records are incomplete, and are not recorded systematically across patients and practice locations.62 Applying the EMR-derived predictive algorithm to the current number of undiagnosed individuals in the United States, authors predict that an additional 400,000 patients with active, untreated diabetes would be identified compared with the conventional prescreening models. With regards to DR, several studies have published predictive models which focused on personalizing screening intervals such as extending or adjusting the screening interval in low-risk patients. Models identifying patients with diabetes at high risk of developing DR have the potential to be used in remote areas and in low-resource settings.63
Policy-Level Changes for DR Prevention
Increasing funding for primary and secondary prevention, creating an enabling environment to facilitate the adoption of healthy lifestyle, implementing diabetes and DR management guidelines that suit the system, providing infrastructure and skilled manpower for screening, improving access to screening, and integrating DR screening with existing disease management programmes, are the key changes required at policy level.
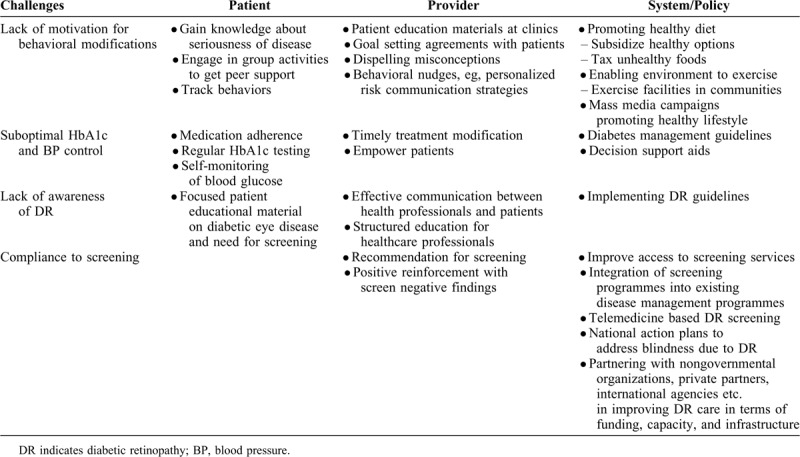
Table 1 summarizes the challenges associated with primary and secondary prevention of DR and proposed solutions to overcome the challenges.
Table 1.
Challenges and Solutions to Improve Primary and Secondary Prevention Strategies for DR
CONCLUSIONS
Our review suggests that several gaps remain in prevention strategies. Awareness of DR is low and risk factor control remains suboptimal across all settings. Early detection of DR by screening is variable across settings and is not even available in some low-resource settings. Adherence to or uptake of DR screening is inadequate in high-income countries. Implementation of DR guidelines and integration of DR in existing public health programmes are variable in LMIC. Although blindness owing to proliferative DR is declining in high-income countries owing to early detection and treatment of DR, incidence of DR is still on the rise in these countries, and blindness owing to DME is on the rise in high-income countries. Taking lessons from the failure of “War on Cancer” and the success of infectious disease control in the last century using a public health approach, to win the war on DR, rather than focusing more on expensive tertiary treatments which benefit a small proportion of patients, changing our focus to primary and secondary prevention, which benefits a larger proportion of the population is important. Interventions to improve self-care behavior in managing diabetes, awareness of DR among patients and providers, and provision of DR screening services should receive as much as or even more focus than tertiary prevention. However, adding primary and secondary prevention to the paradigm of DR prevention requires a change in the mindset of not only medical community and patients but a strong public health system and effective public health policies.
Footnotes
Conflicts of Interest Disclosures: W.T.Y. is a consultant & advisory board for Allergan, Bayer, Boehringer-Ingelheim, Genentech, Merck, Novartis, Oxurion (formerly ThromboGenics), Roche and co-founder of plano and EyRiS.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1. IDF Diabetes Atlas 8th Edition. 2017; https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html. [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leasher JL, Bourne RR, Flaxman SR, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care 2016; 39:1643–1649. [DOI] [PubMed] [Google Scholar]
- 4.Frieden TR, Myers JE, Krauskopf MS, Farley TA. A public health approach to winning the war against cancer. Oncologist 2008; 13:1306–1313. [DOI] [PubMed] [Google Scholar]
- 5.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301:2129–2140. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet 2010; 375:408–418. [DOI] [PubMed] [Google Scholar]
- 7.Ruta LM, Magliano DJ, Lemesurier R, et al. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet Med 2013; 30:387–398. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Mwamburi M, Klein R, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care 2009; 32:2307–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010; 304:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015; 2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan GS, Gan A, Sabanayagam C, et al. Ethnic differences in the prevalence and risk factors of diabetic retinopathy: The Singapore Epidemiology of Eye Diseases Study. Ophthalmology 2018; 125:529–536. [DOI] [PubMed] [Google Scholar]
- 12.Chua J, Lim CXY, Wong TY, Sabanayagam C. Diabetic retinopathy in the Asia-Pacific. Asia Pac J Ophthalmol (Phila) 2018; 7:3–16. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Moss SE. The Wisconsin epidemiological study of diabetic retinopathy: a review. Diabetes Metab Rev 1989; 5:559–570. [DOI] [PubMed] [Google Scholar]
- 14.Tan GS, Cheung N, Simo R, Cheung GC, Wong TY. Diabetic macular oedema. Lancet Diabetes Endocrinol 2017; 5:143–155. [DOI] [PubMed] [Google Scholar]
- 15.Chen MS, Kao CS, Fu CC, Chen CJ, Tai TY. Incidence and progression of diabetic retinopathy among non-insulin-dependent diabetic subjects: a 4-year follow-up. Int J Epidemiol 1995; 24:787–795. [DOI] [PubMed] [Google Scholar]
- 16.Kumari N, Bhargava M, Nguyen DQ, et al. Six-year incidence and progression of diabetic retinopathy in Indian adults: the Singapore Indian Eye study. Br J Ophthalmol 2019. [DOI] [PubMed] [Google Scholar]
- 17.Tam VH, Lam EP, Chu BC, Tse KK, Fung LM. Incidence and progression of diabetic retinopathy in Hong Kong Chinese with type 2 diabetes mellitus. J Diabetes Complications 2009; 23:185–193. [DOI] [PubMed] [Google Scholar]
- 18.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia 2015; 58:443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raman R, Ganesan S, Pal SS, Gella L, Kulothungan V, Sharma T. Incidence and progression of diabetic retinopathy in Urban India: Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS II), Report 1. Ophthalmic Epidemiol 2017; 24:294–302. [DOI] [PubMed] [Google Scholar]
- 20.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001; 44:156–163. [DOI] [PubMed] [Google Scholar]
- 21. Tudor SM, Hamman RF, Baron A, Johnson DW, Shetterly SM. Incidence and progression of diabetic retinopathy in Hispanics and non-Hispanic whites with type 2 diabetes. San Luis Valley Diabetes Study, Colorado. Diabetes Care. 1998;21:53–61. [DOI] [PubMed] [Google Scholar]
- 22.Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY. Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol 2016; 23:209–222. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes A. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37: suppl 1: S14–S80. [DOI] [PubMed] [Google Scholar]
- 24.Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 1999; 353:617–622. [DOI] [PubMed] [Google Scholar]
- 25.Macisaac RJ, Jerums G. Intensive glucose control and cardiovascular outcomes in type 2 diabetes. Heart Lung Circ 2011; 20:647–654. [DOI] [PubMed] [Google Scholar]
- 26.Ali MK, Bullard KM, Gregg EW. Achievement of goals in U.S. Diabetes Care, 1999–2010. N Engl J Med 2013; 369:287–288. [DOI] [PubMed] [Google Scholar]
- 27.Lian J, Liang Y. Diabetes management in the real world and the impact of adherence to guideline recommendations. Curr Med Res Opin 2014; 30:2233–2240. [DOI] [PubMed] [Google Scholar]
- 28.Sabanayagam C, Shankar A, Saw SM, et al. Prevalence of diabetes mellitus, glycemic control, and associated factors in a Malay population in Singapore. Asia Pac J Public Health 2009; 21:385–398. [DOI] [PubMed] [Google Scholar]
- 29.Gale R, Scanlon PH, Evans M, et al. Action on diabetic macular oedema: achieving optimal patient management in treating visual impairment due to diabetic eye disease. Eye (Lond) 2017; 31: suppl 1: S1–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bridging the Evidence Gap in Obesity Prevention: A Framework to Inform Decision Making. [press release]. 2010. [PubMed] [Google Scholar]
- 31.Sohal T, Sohal P, King-Shier KM, Khan NA. Barriers and facilitators for type-2 diabetes management in South Asians: a systematic review. PLoS One 2015; 10:e0136202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawton J, Ahmad N, Hanna L, Douglas M, Hallowell N. Diabetes service provision: a qualitative study of the experiences and views of Pakistani and Indian patients with type 2 diabetes. Diabet Med 2006; 23:1003–1007. [DOI] [PubMed] [Google Scholar]
- 33.Blonde L, Aschner P, Bailey C, et al. Gaps and barriers in the control of blood glucose in people with type 2 diabetes. Diab Vasc Dis Res 2017; 14:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okemah J, Peng J, Quinones M. Addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther 2018; 35:1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson DM. Diabetic retinopathy and age-related macular degeneration in the U.S. Am J Prev Med 2012; 43:48–54. [DOI] [PubMed] [Google Scholar]
- 36.Huang OS, Tay WT, Ong PG, et al. Prevalence and determinants of undiagnosed diabetic retinopathy and vision-threatening retinopathy in a multiethnic Asian cohort: the Singapore Epidemiology of Eye Diseases (SEED) study. Br J Ophthalmol 2015; 99:1614–1621. [DOI] [PubMed] [Google Scholar]
- 37.Lin S, Ramulu P, Lamoureux EL, Sabanayagam C. Addressing risk factors, screening, and preventative treatment for diabetic retinopathy in developing countries: a review. Clin Exp Ophthalmol 2016; 44:300–320. [DOI] [PubMed] [Google Scholar]
- 38.Sapkota R, Chen Z, Zheng D, Pardhan S. The profile of sight-threatening diabetic retinopathy in patients attending a specialist eye clinic in Hangzhou, China. BMJ Open Ophthalmol 2019; 4 (1):e000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavan D, Makaroff LE, da Rocha Fernandes J, et al. Global perspectives on the provision of diabetic retinopathy screening and treatment: survey of health care professionals in 41 countries. Diabetes Res Clin Pract 2018; 143:170–178. [DOI] [PubMed] [Google Scholar]
- 40.Zoega GM, Gunnarsdottir T, Bjornsdottir S, Hreietharsson AB, Viggosson G, Stefansson E. Screening compliance and visual outcome in diabetes. Acta Ophthalmol Scand 2005; 83:687–690. [DOI] [PubMed] [Google Scholar]
- 41.Foreman J, Keel S, Xie J, Van Wijngaarden P, Taylor HR, Dirani M. Adherence to diabetic eye examination guidelines in Australia: the National Eye Health Survey. Med J Aust 2017; 206:402–406. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Ding X, He M, et al. Use of eye care services among diabetic patients in urban and rural China. Ophthalmology 2010; 117:1755–1762. [DOI] [PubMed] [Google Scholar]
- 43.Piyasena M, Murthy GVS, Yip JLY, et al. Systematic review on barriers and enablers for access to diabetic retinopathy screening services in different income settings. PLoS One 2019; 14:e0198979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scanlon PH, Stratton IM, Leese GP, et al. Screening attendance, age group and diabetic retinopathy level at first screen. Diabet Med 2016; 33:904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dervan E, Lillis D, Flynn L, Staines A, O'Shea D. Factors that influence the patient uptake of diabetic retinopathy screening. Ir J Med Sci 2008; 177:303–308. [DOI] [PubMed] [Google Scholar]
- 46.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008; 31:81–86. [DOI] [PubMed] [Google Scholar]
- 47.Sloan FA, Grossman DS, Lee PP. Effects of receipt of guideline-recommended care on onset of diabetic retinopathy and its progression. Ophthalmology 2009; 116:1515–1521. 1521 e1511-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care: The International Council of Ophthalmology Recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 2018; 125:1608–1622. [DOI] [PubMed] [Google Scholar]
- 49.Wang LZ, Cheung CY, Tapp RJ, et al. Availability and variability in guidelines on diabetic retinopathy screening in Asian countries. Br J Ophthalmol 2017; 101:1352–1360. [DOI] [PubMed] [Google Scholar]
- 50.Fridman I, Hart JL, Yadav KN, Higgins ET. Perspectives on using decision-making nudges in physician-patient communications. PLoS One 2018; 13:e0202874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rouyard T, Leal J, Baskerville R, Velardo C, Salvi D, Gray A. Nudging people with type 2 diabetes towards better self-management through personalized risk communication: a pilot randomized controlled trial in primary care. Endocrinol Diabetes Metab 2018; 1:e00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graham GN, Ostrowski M, Sabina AB. Population health-based approaches to utilizing digital technology: a strategy for equity. J Public Health Policy 2016; 37: suppl 2: 154–166. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen HV, Tan GS, Tapp RJ, et al. Cost-effectiveness of a National Telemedicine Diabetic Retinopathy Screening Program in Singapore. Ophthalmology 2016; 123:2571–2580. [DOI] [PubMed] [Google Scholar]
- 54.Cheung CY, Tang F, Ting DSW, Tan GSW, Wong TY. Artificial Intelligence in Diabetic Eye Disease Screening. Asia Pac J Ophthalmol (Phila) 2019; 8:158–164. [DOI] [PubMed] [Google Scholar]
- 55.Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016; 316:2402–2410. [DOI] [PubMed] [Google Scholar]
- 56.Ting DSW, Cheung CY, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017; 318:2211–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abramoff MD, Lou Y, Erginay A, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci 2016; 57:5200–5206. [DOI] [PubMed] [Google Scholar]
- 58.Bellimo V, Lim Z, Lim G, et al. Artificial intelligence using deep learning to screen for referable and vision-threatening diabetic retinopathy in Africa: a clinical validation study. Lancet Digital Health 2019; 1:e35–e44. [DOI] [PubMed] [Google Scholar]
- 59.Ting DSW, Peng L, Varadarajan AV, et al. Deep learning in ophthalmology: the technical and clinical considerations. Prog Retin Eye Res 2019; 72:100759. [DOI] [PubMed] [Google Scholar]
- 60. Global Diabetic Retinopathy Advocacy Initiative. Integrated care for diabetes and eye health: A global compendium of good practice. Melbourne, Australia, 2018. [Google Scholar]
- 61.Talaei-Khoei A, Wilson JM. Identifying people at risk of developing type 2 diabetes: a comparison of predictive analytics techniques and predictor variables. Int J Med Inform 2018; 119:22–38. [DOI] [PubMed] [Google Scholar]
- 62.Anderson AE, Kerr WT, Thames A, Li T, Xiao J, Cohen MS. Electronic health record phenotyping improves detection and screening of type 2 diabetes in the general United States population: a cross-sectional, unselected, retrospective study. J Biomed Inform 2016; 60:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cichosz SL, Johansen MD, Hejlesen O. Toward big data analytics: review of predictive models in management of diabetes and its complications. J Diabetes Sci Technol 2015; 10:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


