Purpose:
The aim of this study is to evaluate whether trabeculectomy with antimetabolites or glaucoma drainage device (GDD) surgery is more likely to achieve an intraocular pressure (IOP) ≤10 mm Hg.
Design:
Retrospective, nonrandomized, cohort study of pseudophakic, primary glaucoma patients.
Methods:
53 pseudophakic patients underwent trabeculectomy and 65 received GDD at the University of Florida by one surgeon between 1993 and 2015. The main outcome measures were mean IOP and percentage of patients obtaining an IOP ≤10 mm Hg for up to 5 years postoperatively. A subgroup undergoing a first time glaucoma surgery was also analyzed because there were more redo glaucoma procedures in the GDD group.
Results:
Over 5 years, the mean annual IOP for the trabeculectomy eyes was between 6.9 and 7.8 mm Hg on an average of 0.2 medications, and that for GDD eyes was between 11.4 and 12.1 mm Hg on a mean of 1.6 to 1.9 medications (P < 0.002). A significantly higher percentage of trabeculectomy eyes than GDD eyes achieved a pressure of ≤10 mm Hg, for years 1 to 4 (P < 0.05). Visual acuity (VA) change was not statistically different between the groups, both for mean logMAR acuity and percentage of patients that lost ≥2 Snellen lines. Complication rates were similar between the groups. Postoperative VA change was similar for eyes achieving low IOP ≤5 mm Hg and those eyes with an IOP ≥10 mm Hg.
Conclusions:
Trabeculectomy provided significantly lower IOP for 5 years postoperatively in pseudophakic primary glaucoma patients, and was more likely to achieve an IOP ≤10 mm Hg.
Keywords: trabeculectomy, glaucoma drainage implant, intraocular pressure, pseudophakic primary glaucoma
It has been estimated that by the year 2020, there will be approximately 76 million people suffering from open angle glaucoma (OAG) or angle closure glaucoma.1 Being second only to cataract, glaucoma was the leading cause of blindness in the world in 2010.2 Trabeculectomy and GDD surgery remain the most popular glaucoma procedures in the United States despite the recent development of new microinvasive procedures.3 Medicare data show a downward trend in the number of trabeculectomies performed between 1994 and 2012 along with an increase in GDD procedures.3
A multicenter randomized clinical trial of patients conducted by the Tube Versus Trabeculectomy (TVT) Study group found that both procedures were associated with similar IOP reduction from baseline, and mean IOP at 5 years; the TVT reported an average IOP of 14.4 ± 6.9 mm Hg for tube surgery and 12.6 ± 5.9 mm Hg in the trabeculectomy group at 5 years (P = 0.12).4 A Kaplan-Meier analysis in the study showed a higher success rate for the GDD surgical group when an upper IOP limit of either 21 mm Hg, 17 mm Hg, or 14 mm Hg was selected (P = 0.002, 0.002, 0.017, respectively).
It is unclear, however, which procedure is more effective for patients who require very low IOPs. Compared with patients with early glaucoma, patients with advanced fixation-threatening glaucoma and those with normal tension glaucoma who are showing progression at low-normal IOPs are thought to require very low IOPs, even in the single digit range, to maintain their residual field of vision and prevent further progression of field loss.5–8
The patients recruited in the TVT study were either pseudophakic, had previous failed trabeculectomy, or both.9 Our study compares the efficacy of trabeculectomy with antimetabolite and GDD surgery in attaining pressures of ≤10 mm Hg in a similar diagnostic group of patients, all with primary glaucoma and pseudophakia, and with or without previous failed trabeculectomy.
In order to achieve similar diagnostic groups for the comparison in our retrospective study, we elected to focus specifically on pseudophakic, primary glaucoma eyes. This was done to avoid bias given the high likelihood of patients with secondary glaucomas, such as neovascular and uveitic glaucoma, being assigned to receive GDD surgery, and phakic patients with a primary glaucoma being assigned to receive a trabeculectomy. For a long time in clinical practice, as emphasized by the design and results of the TVT study 4,10,11, either surgical option was considered appropriate for pseudophakic, primary glaucoma eyes. Also, the largest group of patients in the TVT study were those with pseudophakic primary glaucoma, and the peer group designing the TVT study felt that it was reasonable to randomize these particular patients to either procedure.
METHODS
Ethical Statement
All research performed in this retrospective cohort study was in accordance with the University of Florida's Institutional Review Board (IRB) with protocol approval before initiation of the study. A waiver of informed consent was obtained from the IRB for the collection and use of these data. This study adhered to the tenets of the Declaration of Helsinki, and all federal and state laws.
Study Design and Patient Inclusion Process
This study is a retrospective chart review of glaucoma patients who underwent either trabeculectomy or GDD surgery with no other concurrent procedures by a single surgeon at a single site between September 1993 and June 2015. Initially, 744 patients were identified from hospital records using current procedural terminology (CPT) codes 66170 or 66172 for trabeculectomy and 66180 or 66185 for GDD implantation, respectively. We located 526 of these as physical records.
The total study population was then refined to only include patients who were pseudophakic and who had open angle primary glaucoma, including primary OAG, pseudoexfoliation glaucoma, pigmentary glaucoma, or low-tension glaucoma. In cases where patients had 2 eyes qualified for inclusion within the same surgical group, only the first eye that underwent surgery was included for analysis. 118 patients met these criteria, of whom 65 underwent GDD surgery and 53 underwent trabeculectomy (Tables 1 and 2).
TABLE 1.
Population Demographics
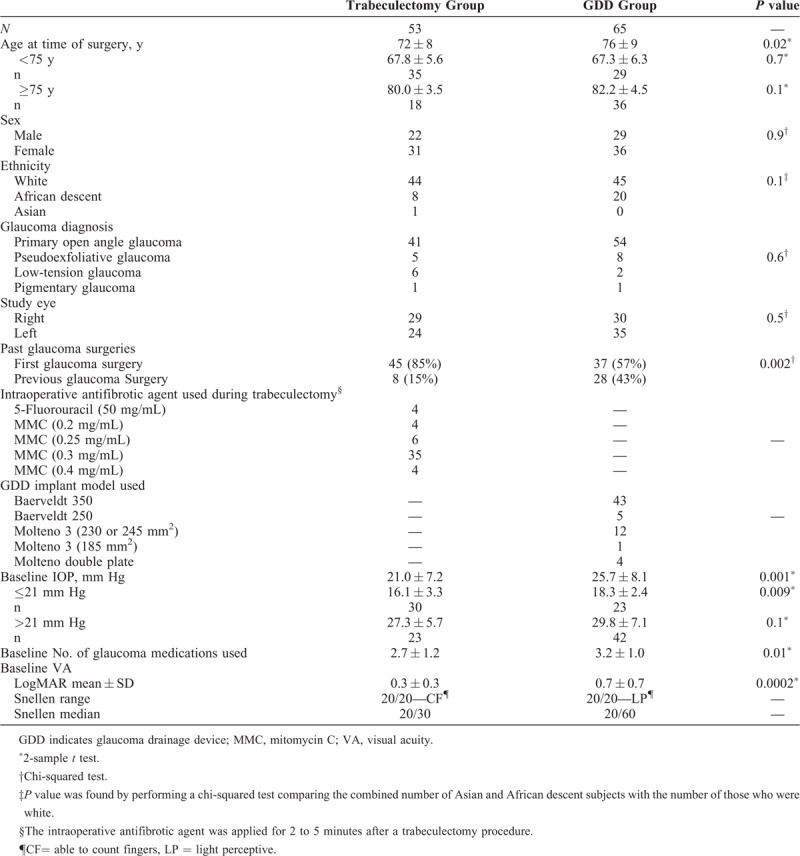
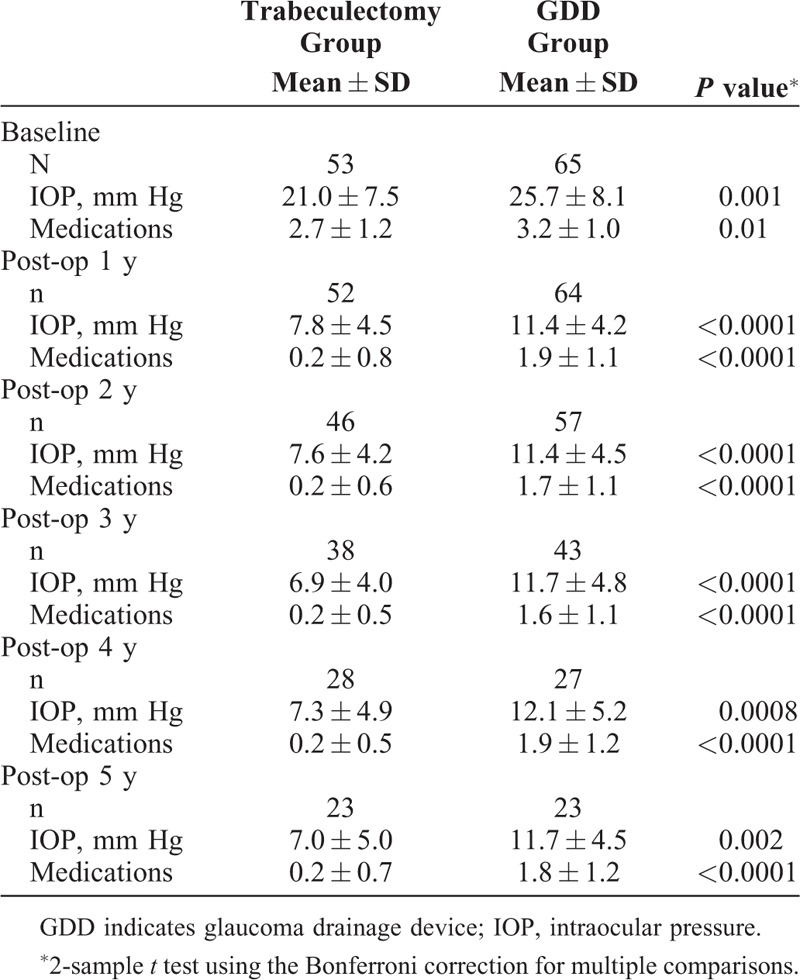
TABLE 2.
Average IOP and Medication Use in Trabeculectomy and GDD Patients
Of the 65 eyes that had GDD procedure, 48 eyes (74%) received Baerveldt implant (Abbott Laboratories, Inc. Abbott Park, IL), with the vast majority of these being Baerveldt 350 mm2 (43/48). The remaining 17 eyes (26%) had Molteno implant (Molteno Ophthalmic Ltd., Dunedin, NZ), with all but one of these having a surface area of 230 to 270 mm2 (Table 1). In the trabeculectomy group, 49 of 53 (92%) patients were treated intraoperatively with mitomycin-C (MMC) using concentrations between 0.2 and 0.4 mg/mL for 2 to 4 minutes (Table 1), with the remaining 4 receiving intraoperative 5-fluorouracil 50 mg/mL for 5 minutes instead. An ExPress Mini Glaucoma Shunt (Optonol Ltd., Neve Ilan, Israel) was used in 9 of the trabeculectomies.
Surgical Procedures
The GDD surgeries were mainly performed using the superotemporal quadrant and with a fornix based conjunctival flap. All were nonvalved implants and were temporarily occluded with either a 3–0 Supramid or a 4–0 Prolene internal stent plus a tightening 8–0 polygalactan suture (Vicryl) (Ethicon US, LLC, Bridgewater, NJ) or 9–0 Nylon ligature placed external to the tube. The flow along the tube was checked with balanced saline solution using a 30-gauge cannula to ensure complete blockage along the tube until the polygalactan suture dissolved at approximately 6 weeks postoperatively or the internal stent was pulled. The episcleral plate was sutured to the sclera behind the rectus muscle insertions using 9–0 nylon sutures, and the extrascleral portion of the tube was covered with a double layer pericardial patch in the earlier implant surgeries, and more recently with a hemicircular corneal patch graft. The tube was inserted into the anterior chamber using a 23-gauge needle tract, with the needle track starting 1 to 2 mm behind the limbus and orientated to try to keep the tube well back from the corneal endothelium and close to, or even touching, the iris. Venting slits were made in all tubes using a TG 140 – 8 needle attached to the 8–0 polygalactan suture, with the number adjusted according to aqueous flow. MMC was not used.
Trabeculectomy surgery utilized a limbus-based flap in >90% of cases with the incision line placed 11 to 13 mm superior to the limbus. 7 to 9 thinned Weck-cel pledgets (Beaver-Visitec International, Inc., Waltham, MA) saturated with MMC were placed in the subconjunvtival space, and a broad superior area of approximately 5 clock hours was treated from 9:30 am to 2:30 pm, with lifting of the conjunctival incision line away from the pledgets. The MMC was washed off copiously with about 50 mL of balanced saline solution, and use was made of this saline to hydrodissect inferiorly from the nasal and temporal quadrants to create a 360 degree bleb. A 2 to 3 mm rectangular, partial-thickness scleral flap was fashioned. The deep sclerectomy was created manually using a 75 blade and Vannas scissors rather than a scleral punch to have more control on the degree of overlap of the scleral flap over the sclerectomy. Releasable 10–0 nylon sutures were used in all cases to secure the flap and their tightness was adjusted to titrate aqueous flow before closure. In most cases, 2 releasable sutures were required but occasionally up to 4. The Tenon's capsule and conjunctiva were closed in 2 layers with continuous 8–0 polygalactan suture.
Data Collection and Main Outcome Measures
Baseline demographic information, including sex, race, age, study eye (right or left), glaucoma diagnosis, previous glaucoma surgery, the size and make of the implant used (for the GDD group), and the antifibrotic treatment applied to the trabeculectomy group were recorded. The main outcome measures of IOP, VA, and the number of glaucoma medications were obtained from visit notes from the presurgical and postsurgical periods. The data for baseline presurgical IOP and baseline medication use was determined from the average IOP and number of medications recorded at each glaucoma check-up visit between 42 days and 1 day before surgery, in accordance to the World Glaucoma Association (WGA) Guidelines.12 Baseline VA was determined by the best corrected VA measurement at the visit closest to the date of surgery. After these same guidelines, the data for each postoperative measurement was derived from the examination closest to the yearly time-point. To confirm the accuracy of the data collection, 40% of the patient records were reviewed for a second time by a separate investigator, and almost total concordance was noted.
Early and late postsurgical complications were recorded for each patient. Early complications were defined in accordance with the WGA Guidelines as up to 30 days after surgery and late as those occurring after this timepoint.12
For patients who suffered corneal edema requiring penetrating keratoplasty, or Descemet stripping endothelial keratoplasty, and for those who received corneal chelation procedure or yttrium-aluminum-garnet (YAG) capsulotomy, VA data were censored at the time of further surgery to avoid confounding the postoperative VA data. Patients who underwent further glaucoma surgery, such as diode cyclophotocoagulation (dCPC) procedure and additional GDD implant or trabeculectomy, were considered as failures, and their data were censored after that additional surgery (see Supplemental Digital Content 1, Appendix 1).
VA Analysis
VA is measured as the last complete line a patient could read on a Snellen chart, which ranges from 20/15 to 20/400 on the Snellen scale. If a patient has VA worse than 20/400, acuity is measured in ability to count fingers, detect hand motion, or perceive light.
For patients whose VA was measured as counting fingers, a Snellen value of 1/75 (20/1500) was used, as described by Schulze-Bonsel et al.13 In accordance with the WGA Guidelines, VA measured as hand motion or light perception was assigned Snellen values of 1/800 and 1/1600, respectively, then converted to LogMAR.12 After this, average values were calculated.12
Statistical Methods
The demographic characteristics of the sample patients were compared between the trabeculectomy group and the GDD group for any statistically significant differences using 2-sample t tests for continuous variables and chi-squared tests for independence for categorical variables. The mean IOP at each time point was analyzed between the trabeculectomy and GDD groups using 2-sample t-tests with the Bonferroni correction for multiple comparisons.
For the outcome of number of medications, a difference in means was assessed over time between the trabeculectomy and GDD groups using a 2-sample t -test. The change in mean VA from baseline between the GDD implant and the trabeculectomy was analyzed using a 2-sample t test. Statistical software used was the GraphPad statistical software package (GraphPad Software, Inc., La Jolla, CA) and base package in R Version 3.3.2 (R Core Team, 2016).14 For the sample size estimates, the software package G∗Power Version 3.1.9.2 (G∗Power, Universitat Dusseldorf, Germany) was used.15 The posthoc power analysis was conducted in G∗Power using the t test calculation for the difference in means between 2 independent groups (p. 49 in online user manual, accessed May 21, 2019). The general equation for the calculation of sample size is:
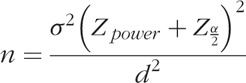 |
For this type of sample size calculation, the effect size was the difference in means between the 2 groups.
RESULTS
Demographics
Sex, ethnicity, glaucoma diagnosis, and study eye were not significantly different between the trabeculectomy and GDD groups (Table 1). However, the average age, number of eyes with previous trabeculectomy, baseline IOP, baseline mean number of medications, and baseline LogMAR VA were all significantly higher for the eyes in the GDD group than in the trabeculectomy group (Table 1). Previous literature suggests that the IOP outcome after glaucoma surgery may be influenced by previous glaucoma surgery, age, and preoperative IOP,16–19 which in our study were significantly different among treatment groups (Table 1). 28 (43%) of the 65 GDD surgeries had previous failed glaucoma surgery and 8 (15%) of the 53 trabeculectomy eyes (P = 0.002) (Table 1). A separate subanalysis was performed specifically for eyes that had no previous glaucoma surgery (Table 3 and Part B of Fig. 1) to allow for this disparity between groups, in case the GDD group may be biased toward more adverse results in eyes with previous surgical failure. Additionally, to more closely mirror the inclusion criteria of the TVT study, we specifically examined only those eyes that received a Baerveldt 350 mm2 drainage device, and only those trabeculectomies that received MMC as an antifibrotic agent and did not receive an ExPress shunt (Table 4).
TABLE 3.
Average IOP and Medication Use in Trabeculectomy and GDD Patients Who Had No Previous Glaucoma Surgery
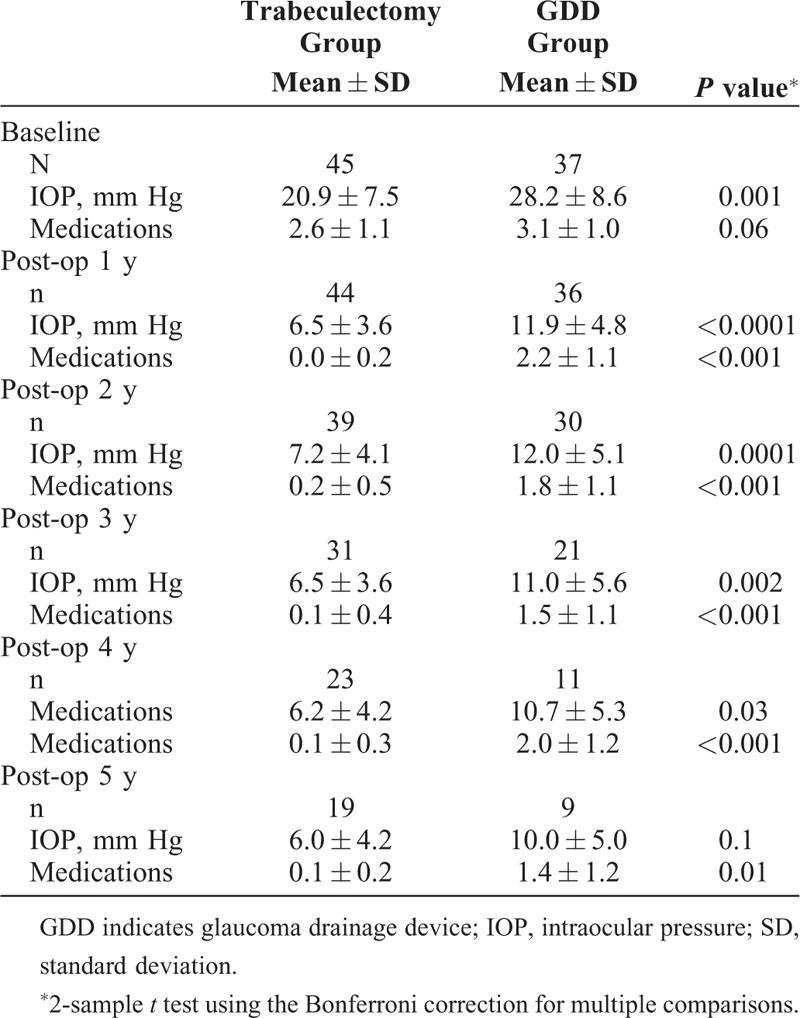
FIGURE 1.
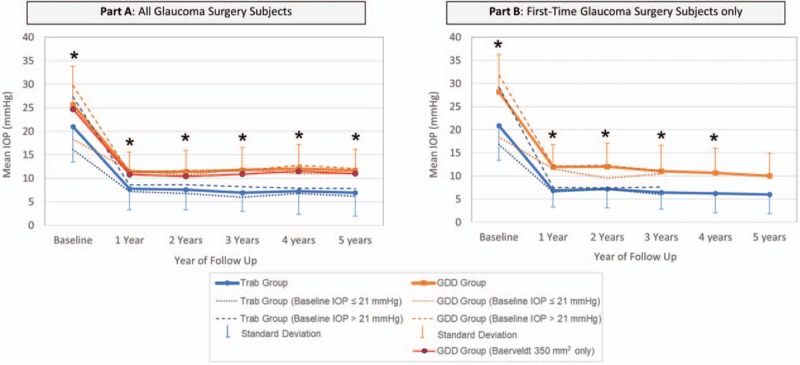
Mean IOP in trabeculectomy and GDD patients by year and by baseline IOP. A, (left): the difference between the mean IOP of the trabeculectomy and GDD group is statistically significantly different (∗) at baseline and years 1 through 5 of follow-up (P < 0.05 via 2-sample t test). The mean IOP of eyes receiving Baerveldt 350 mm2 implant is shown in red. B, (right): the difference between the mean IOP of the trabeculectomy and GDD group is statistically significantly different (∗) at baseline and years 1 through 4 of follow-up (P < 0.05 via 2-sample t test). GDD indicates glaucoma drainage device; IOP, intraocular pressure.
TABLE 4.
Average IOP and Medication Use in Trabeculectomy with MMC Eyes∗ and Baerveldt 350 mm2 Eyes
To create more comparable groups for age, we further divided our study population into subgroups of younger or older than 75 years, which were not statistically different in age (P = 0.7 and 0.1 by 2-sample t test, respectively) (Table 1 and Supplemental Digital Content 1, Appendix 2). Given the sample size for the patients in the 75 years or older group, there was 50% power to test for a statistically significant difference between mean ages for the GDD and trabeculectomy groups.
To help mitigate the difference in baseline IOPs between the trabeculectomy and GDD groups, the patients were subdivided into those with a baseline IOP ≤21 mm Hg (N = 30 and 23, respectively) and those with a baseline IOP >21 mm Hg (N = 23 and 42, respectively). In those eyes with baseline IOP >21 mm Hg, there was no statistically significant difference between trabeculectomy and GDD groups (P = 0.1). But for those eyes with a baseline IOP of ≤21 mm Hg, there remained a statistical difference (16.1 vs 18.3 mm Hg, respectively) (Table 1 and Part A of Fig. 1, and Supplemental Digital Content 1, Appendix 3).
IOP
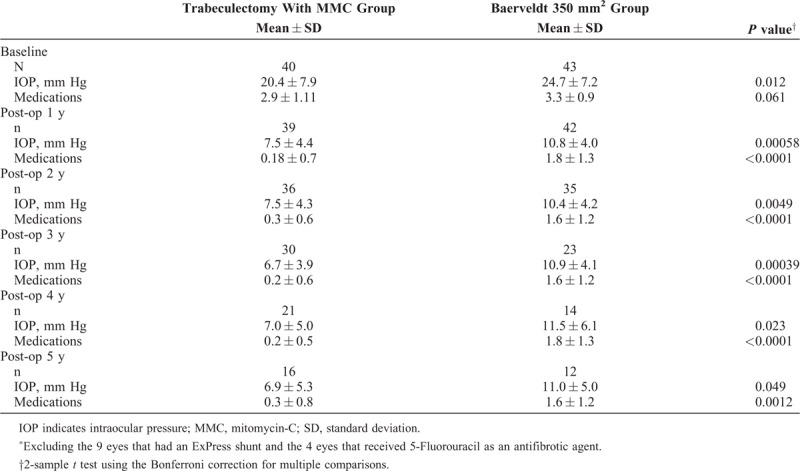
For the whole study population, mean IOP and medication use for all 5 years of follow-up were significantly lower in the trabeculectomy group than the GDD group (Table 2).
It is possible that the baseline IOP difference might influence postoperative results. As mentioned above, there was no significant baseline difference between the surgical groups in the IOP subgroup with a baseline IOP of >21 mm Hg (P = 0.1), but there was still a significant baseline difference between surgical groups in the IOP subgroup of baseline IOP ≤21 mm Hg (P = 0.009) (see Supplemental Digital Content 1, Appendix 3). When trabeculectomy eyes were compared with the GDD eyes in each baseline IOP subgroup (≤21 mm Hg and >21 mm Hg), the trabeculectomy pressures were still significantly lower than the GDD eyes at years 1 through 4 post-surgery for both subgroups, and at year 5 as well for the ≤21 mm Hg subgroup (see Supplemental Digital Content 1, Appendix 3, and Part A of Fig. 1).
There was a statistically significantly higher IOP at baseline in the GDD group for patients younger than 75 years, but there was no significant difference for those 75 years or older. The mean IOP was significantly lower for the trabeculectomy group in all 5 postoperative years for those patients 75 years and older, and for the first 3 years postoperatively in those younger than 75 years (see Supplemental Digital Content 1, Appendix 2).
A greater proportion of the trabeculectomy eyes achieved an IOP of ≤10 mm Hg GDD eyes, which was significant for the first 4 years after surgery (P < 0.05) (Fig. 2).
FIGURE 2.
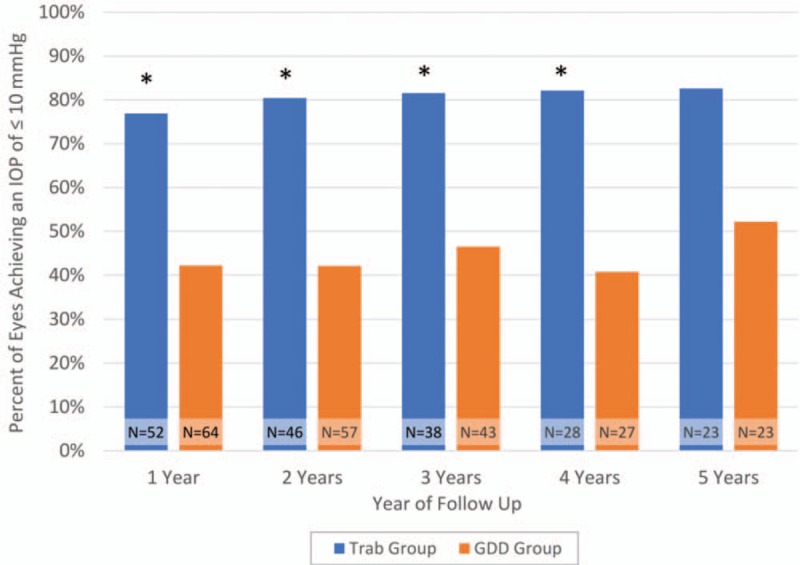
Percentage of IOP ≤ 10 mm Hg in trabeculectomy and GDD patients. There was a statistically significant difference (∗) in the number of eyes achieving IOP ≤10 mm Hg in the trabeculectomy and GDD groups at years 1 through 4 (P < 0.05 via chi-squared test). The difference was not significant at year 5 (P = 0.06 via chi-squared test). The total number of eyes in each surgical group at each year is displayed as “N = …”. GDD indicates glaucoma drainage device; IOP, intraocular pressure.
Glaucoma Medication Use
The mean number of medications used was 2.7 for the trabeculectomy group and 3.2 for the GDD group at baseline. Glaucoma medication use was significantly lower in the trabeculectomy group than the tube group at all 5 postoperative years, with the average number of medications being 0.2 at each year for the trabeculectomy group and ranging between 1.6 and 1.9 for the tube group over the 5 years (Table 2). The proportion of patients on no medications was significantly higher for all 5 years of follow-up for the trabeculectomy group than the tube group (Fig. 3). Figure 4 shows a comparison of the distribution of medication use at baseline and 5 years postoperatively in the trabeculectomy and GDD groups.
FIGURE 3.
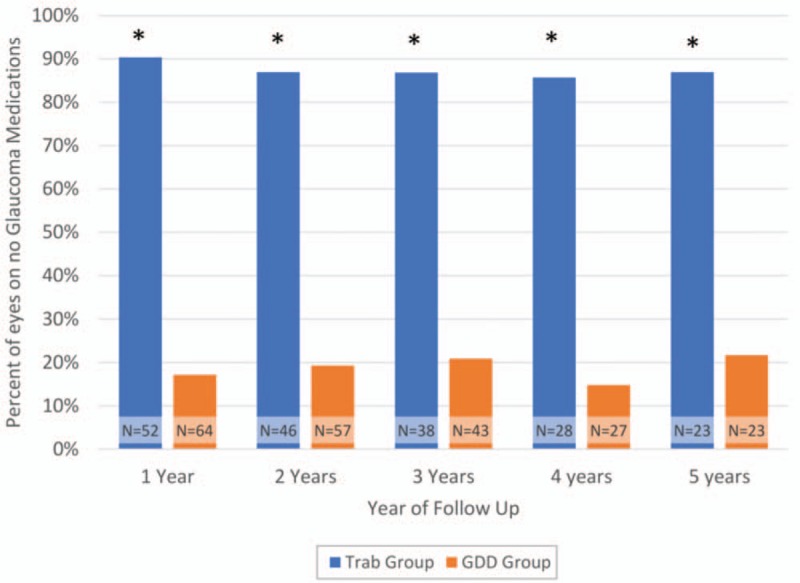
Percentage of patients on no glaucoma medications in trabeculectomy and GDD patients. There was a statistically significant difference (∗) in the number of eyes on no glaucoma medications in the trabeculectomy and GDD groups at postoperative years 1 through 5 (P < 0.05 via chi-squared test). The total number of eyes in each surgical group at each year is displayed as “N = …”. GDD indicates glaucoma drainage device.
FIGURE 4.
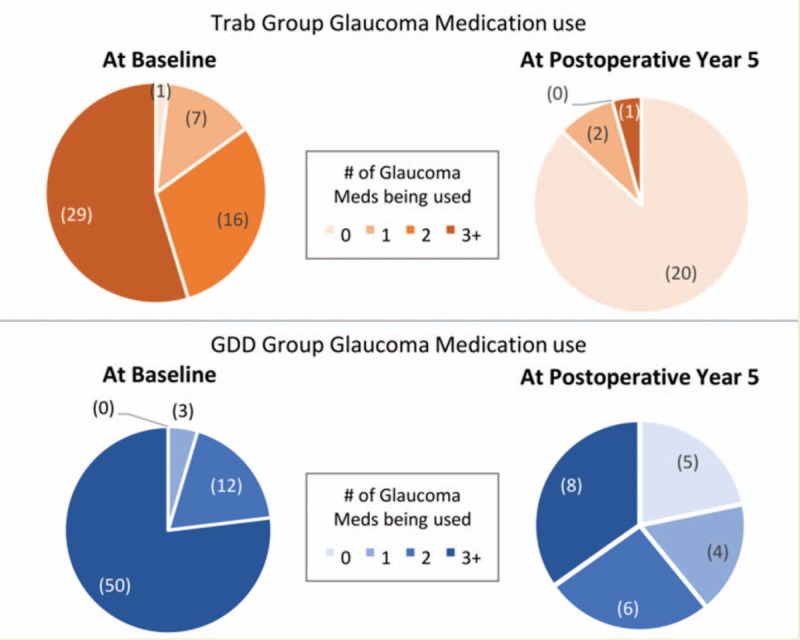
Medication use in trabeculectomy and GDD patients at baseline and at postoperative year 5. The level of medical therapy required for trabeculectomy and GDD group patients at baseline (upper and lower left respectively) and at postoperative year 5 (upper and lower right respectively) is displayed visually. The number of eyes on a certain number of medications is displayed in parentheses. GDD indicates glaucoma drainage device.
VA
At baseline, there was a significant difference in average logMAR VA between the trabeculectomy and GDD groups (P = 0.0002) as the trabeculectomy eyes had better initial vision (Table 1).
Our analysis specifically examined change from baseline in LogMAR VA, rather than absolute values at each postoperative timepoint. No statistically significant difference was found between surgical groups in change of mean VA from baseline at any timepoint during the 5 years of follow-up (Table 5).
TABLE 5.
Change From Baseline Visual Acuity in Trabeculectomy and GDD Patients
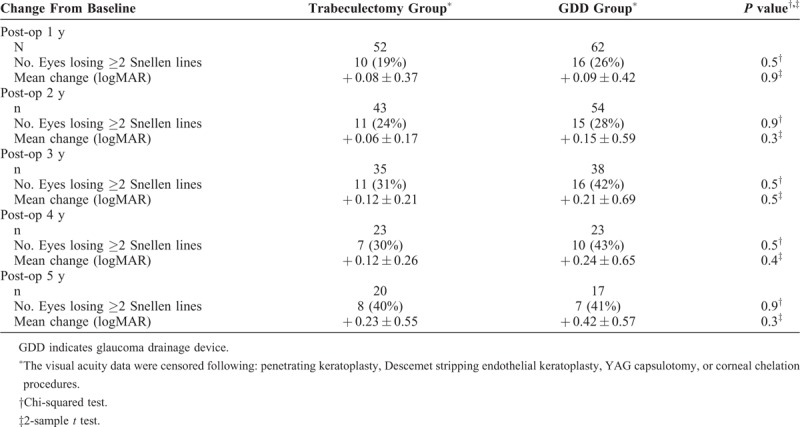
For the individual, a more clinically relevant decrease in VA was defined as a loss of 2 or more lines of Snellen vision from baseline. The incidence of this decrease of 2 or more Snellen lines was compared between groups, and no statistically significant difference was detected between the two surgeries (Table 5).
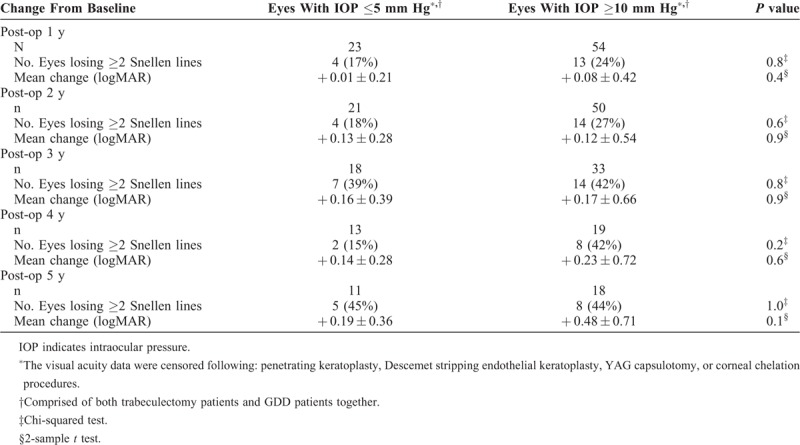
IOPs <6 mm Hg have been defined as failure by the World Glaucoma Congress12 and many studies9 because of the risk for macular hypotony and decreased VA. Change in LogMAR VA and loss of ≥2 Snellen lines of vision from baseline were analyzed for all eyes (combined trabeculectomy and GDD surgical groups) with an IOP of ≤5 mm Hg versus those with higher postoperative IOPs of ≥10 mm Hg. There was no statistically significant difference in either measurement of VA change between eyes with low single-digit IOP (IOP ≤5 mm Hg) and those whose postoperative pressures were within normal range (IOP ≥10 mm Hg) (Table 6).
TABLE 6.
Change From Baseline Visual Acuity in Eyes With An IOP of ≤5 mm Hg Versus an IOP of ≥10 mm Hg
Complications
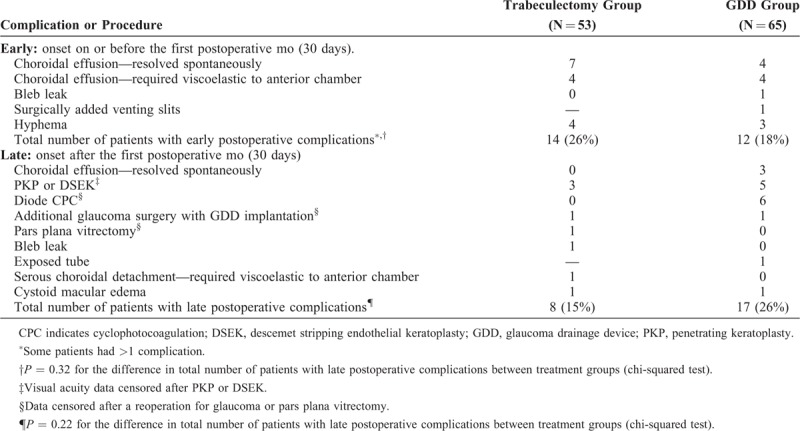
Early complications were similar between the trabeculectomy and GDD eyes. The commonest was choroidal effusion, many of which resolved spontaneously (Table 7). The overall frequency of late complications was also not statistically significant between groups, although more of the GDD eyes required an additional procedure for inadequate IOP control (Table 7). The incidence of corneal decompensation requiring a procedure was similar with 3 (6%) trabeculectomy eyes and 5 (8%) GDD eyes undergoing either penetrating keratoplasty or Descemet stripping endothelial keratoplasty (Table 7).
TABLE 7.
Postoperative Complications or Additional Procedures in the Trabeculectomy and GDD Groups
DISCUSSION
Our study is a nonrandomized, retrospective analysis of patients receiving glaucoma surgery between 1993 and 2015. The group has similarities to the types of eyes enrolled in the TVT study, except that all of the eyes in the present study were pseudophakic as opposed to 78% and 80% for the tube and trabeculectomy groups, respectively, in the TVT study.9 In the TVT study, a Baerveldt 350 mm2 GDD was used for all of the eyes randomized to receive a tube. The majority of eyes (43/65) in our GDD group were also Baerveldt 350 mm2 but the remainder had a mixture of smaller surface area Baerveldt 250 mm2 and Molteno (primarily 230–270 mm2) nonvalved implants (Table 1). Previous studies have indicated no difference in IOP control when comparing Baerveldt 350 mm2 with Baerveldt 250 mm2 implants20, and Meyer et al have shown that mid-sized implants including the Baerveldt 250 and Molteno 3 (230 and 245 mm2) gave similar results to the Baerveldt 350 mm2.21 We additionally performed a subanalysis (Table 4 and Part A of Fig. 1) which specifically examined only the Baerveldt 350 mm2 implants and the results were similar to the overall analysis using all implants, with the trabeculectomy eyes achieving lower IOPs at all timepoints throughout the 5 years of the study.
Baseline Characteristics
For our selected patients, the trabeculectomy and GDD groups were not statistically different in baseline characteristics, except for mean age (72 and 76 years, respectively), baseline mean IOP (21 and 26 mm Hg, respectively), baseline medication use (2.7 and 3.2 meds, respectively), and baseline mean LogMAR VA (0.3 and 0.7 LogMAR units, respectively). There were more eyes in the GDD group that had previous failed glaucoma filtration surgery, and therefore we also separately analyzed the subgroup of eyes that had had no previous glaucoma filtration surgery (Table 3 and Part B of Fig. 1). This group had no previous surgery and pseudophakia mirrored Stratum 1 in the TVT study, which was 44% of their enrolled eyes and their largest stratum.
IOP
Our results for the overall study cohort showed that the trabeculectomy group achieved lower mean IOP and had a significantly lower medication requirement than the tube group at all 5 postoperative years (Table 2). In contrast, the prospective TVT study did not find a significant difference in mean IOP between the groups at any of the 5 postoperative years, and only noted a significantly lower medication use in the trab group for the first 2 years.4 The Kaplan–Meier analysis of the TVT study, using a series of success definitions of either 6 to 21, 6 to 17, or 6 to 14 mm Hg, found a significantly higher success rate for the GDD group by all 3 definitions over 5 years.
The postoperative mean IOP achieved in our GDD group was similar to, and in fact slightly lower than, the GDD group in the TVT study4 with a range of 11.4 to 12.1 mm Hg over the 5 years when all the GDD models were considered (10.4 to 11.5 mm Hg if only the Baerveldt 350 mm2 eyes were analyzed) versus 12.5 to 14.4 mm Hg, respectively, over the 5 years of follow-up in the TVT GDD eyes. In the trabeculectomy group, however, our study showed much lower IOPs than the trabeculectomy eyes in the TVT study at all 5 postoperative years, with the range of mean IOPs from 6.9 to 7.8 mm Hg on a mean of 0.2 meds in our study versus 12.1 to 13.5 mm Hg on a mean of 0.5 to 1.2 meds in the TVT study. One difference between the TVT and our study is that the starting mean IOP was lower in our trabeculectomy cohort than that of the TVT study (21.0 vs 25.6 mm Hg). When we subselected those eyes with a starting IOP of ≥21 (with a preoperative mean IOP of 27.3 mm Hg), the mean IOP was 8.6 mm Hg at 1 year postoperative (N = 23) and 8.7 mm Hg at 2 years postoperative (N = 19) (see Supplemental Digital Content 1, Appendix 3). Also, when we compare the postoperative IOPs in the patients who were <21 mm Hg at baseline (mean IOP 16.1 mm Hg) with those ≥21 mm Hg at baseline (mean IOP 27.3 mm Hg), there was no significant difference in IOP at 1 through 5 years postoperative for both the GDD and trabeculectomy eyes (see Supplemental Digital Content 1, Appendix 4).
In contrast to the TVT study, a 5-year retrospective comparison study by Panarelli et al of patients at the University of Miami with primary glaucoma receiving either a primary Baerveldt GDD or trabeculectomy with MMC showed significantly lower mean IOP for the first year and medication use for the first 3 years in favor of the trabeculectomy eyes, but not thereafter.22 They found a significantly higher probability of complete success in the trabeculectomy group at 1, 2, and 3 years. The more recent prospective randomized primary tube versus trabeculectomy study, which examined phakic eyes with no previous ocular surgery, at 1-year follow-up agreed with our findings.23 Specifically, phakic eyes without previous glaucoma surgery had lower mean IOP, less medication use, and significantly higher success rates in the trabeculectomy group.23
Obtaining an IOP of ≤10 mm Hg
In our study, the trabeculectomy group was significantly more likely than the GDD group to obtain IOPs of ≤10 mm Hg and significantly fewer glaucoma medications. The importance of obtaining very low IOPs in progressive OAG is underscored in several studies. Oie et al6 followed 60 eyes of 60 patients with progressive normal tension glaucoma for a mean of 19.8 years with twice yearly Humphrey visual field tests. In the eyes that received trabeculectomy, they achieved a mean IOP of 9.1 mm Hg from a preintervention of 14.7 mm Hg, and the mean deviation (MD) slope decreased significantly from−0.86 to −0.19 dB/year. They examined 2 comparison groups treated with prostaglandins, one with an IOP reduction from 14.7 to 11.7 mm Hg, which demonstrated an MD slope reduction from −0.52 to −0.31 dB/yr and the other with only an 8% IOP reduction (14.4–13.2 mm Hg) in which there was no difference observed in the visual field MD slope.24 Naito et al examined 17 eyes with progressive normal tension glaucoma and found that 91.7% of eyes with single digit IOP post trabeculectomy with MMC showed improvement in MD slope, whereas only 20% of eyes with postoperative IOPs of ≥10 mm Hg showed this improvement.24 In another Japanese study of progressive normal tension glaucoma, the visual field MD change rate decreased to 0.25 dB/year from preoperative 0.70 dB/year when the IOP was reduced to 10.3 mm Hg from 15.7 mm HG.7 At the Bascom Palmer Institute in the United States, a similar finding of a reduction in mean MD slope to −0.25 dB/year from preoperative −1.05 dB/year after trabeculectomy surgery was noted in patients with progressive normal tension glaucoma when achieving a mean IOP reduction to 8.5 mm Hg from 13.1 mm Hg presurgery.8 Caprioli et al have shown that trabeculectomy can improve visual function25 and that eyes achieving the greatest reduction in IOP after surgery were more likely to show this improvement.
Of note, a number of patients, especially in our trabeculectomy group, achieved pressures of ≤5 mm Hg postoperatively. A failure criterion of IOP being too low was approved in a consensus meeting of the WGA in 2009 as part of a guideline on reporting glaucoma surgical results.12 An IOP of <6 mm Hg occurring on ≥2 consecutive occasions beyond 3 months postoperative was preselected to be a failure criterion in the TVT study.26 In contrast, Tseng et al in a retrospective, case-controlled study of primary trabeculectomies performed at a single center, showed that good vision may be maintained even in eyes with relative hypotony of ≤5 mm Hg. Using 3 separate failure criteria, the study noted no statistically significant differences in the rate of vision loss between hypotonous and nonhypotonous eyes.27 In our study, the VA of eyes in either the trabeculectomy or glaucoma tube group that achieved an IOP of ≤5 mm Hg was compared with those whose postoperative pressures were in the normal range of ≥10 mm Hg for each timepoint (Table 6). No statistically significant difference in either the change in mean LogMAR or loss of ≥2 lines of Snellen VA was found, again suggesting that low single-digit IOP may not be visually detrimental. Given the sample size, there is an 80% power to detect a potential 0.41 to 0.83 difference in logMAR between the group with an IOP of ≤5 mm Hg compared with eyes with an IOP of ≥10 mm Hg throughout all 5 years of follow-up.
Glaucoma Medication Use
In the TVT study, the number of medications for the trabeculectomy group was initially significantly lower than the tube group in years 1 and 2, but by years 3 through 5, there was no statistical difference between the groups. Our medication use in the trabeculectomy group was significantly lower than the tube group throughout all 5 years, and also lower than the tube group of the TVT study. The mean number of medications prescribed in our tube group was similar to or slightly higher than that described in the TVT study. It may be noted that in our study, there was a significantly higher baseline medication use in the tube group compared with our trabeculectomy group (3.2 vs 2.7 mean number of medications, P = 0.01) (Table 1), consisting of prostaglandins and the 3 categories of aqueous suppressants (beta blockers, alpha agonists, and carbonic anhydrase inhibitors). However, when the analysis is limited to those who have had no previous glaucoma surgery, the difference in mean medication use at baseline between the tube and trabeculectomy group patients becomes nonsignificant (P = 0.07) (Table 3). Postoperatively, glaucoma medications were not used commonly in the trabeculectomy eyes (0.2 types of medication on average), whereas, on average, nearly 2 classes of aqueous suppressants were still prescribed for most patients in the GDD group (Table 2).
If we specifically compare these pseudophakic patients who have had no previous glaucoma surgery with the 94 similar patients in Stratum 1 of the TVT study (who were also pseudophakic and had no prior surgery), it is notable that the GDD group had a similar complete success rate (defined as requiring no glaucoma medications) of 26% compared with 19% in our study. A large difference, however, is seen in the percentage of patients who were medication free for the trabeculectomy eyes, with the TVT 5-year complete success rate being 15%, as opposed to 85% in our study.
VA
Being a nonrandomized study, one of the weaknesses noted is that there was a baseline difference in VA between groups, with the GDD eyes having worse initial vision, despite all eyes being pseudophakic with primary glaucoma (Table 1). The primary VA outcome that was examined changed from baseline rather than absolute value of the postoperative mean LogMAR vision. Additionally, the incidence of significant visual decrease of ≥2 Snellen lines from baseline for an individual was analyzed. For both visual outcomes, there was no statistically significant difference between the trabeculectomy and drainage implant group (P > 0.05) (Table 5). Posthoc power analyses were conducted on the statistical tests investigating the change from baseline mean VA at each year of follow-up (Table 5). Given the sample size stated in Table 5, there is 80% power to detect a potential 0.35 to 0.70 difference in logMAR between the trabeculectomy and GDD groups throughout all 5 years of follow-up.
Complications
In the TVT study at 5 years of follow-up, a significantly larger number of trabeculectomy patients had early postoperative complications (P = 0.012), but the number of late complications was similar.28 In the trabeculectomy group of the TVT study, wound leak was the second most common early complication (11%), but there was none in our study. This difference might be related to a more stringent Seidel testing of the incision site in a prospective study, or to the fact that a limbal-based conjunctival incision was used in almost all the trabeculectomy surgeries in our study, whereas the majority of trabeculectomies in the TVT study employed a fornix-based approach. The TVT study noted corneal edema as the most common late complication for both groups. Late bleb leak was unique to the trabeculectomy group and occurred in 6 eyes in the TVT study, whereas tube erosion or obstruction was unique to the GDD group and occurred in 8 eyes. In the present study, the incidence of choroidal effusion was similar between the trabeculectomy group and the GDD group. Also, the incidence of postglaucoma surgery corneal grafts, although slightly higher in the tube group, was not statistically different (Table 7). Further procedures to control IOP were needed in 7 of the tube eyes but only 2 of the trabeculectomy eyes (Table 7).
LIMITATIONS
A limitation to consider in this study is that it is a retrospective, nonrandomized study, and that some differences exist in the demographics of 2 study groups. Subanalyses were performed to mitigate the effects of the most significant differences (ie, age, the occurrence of previous glaucoma surgery, and IOP at time of surgery). In trying to compare groups with as few confounding variables as possible, we limited our patient population to pseudophakic eyes with primary glaucoma diagnosis, although this may mean that the results are not applied to secondary glaucomas and phakic eyes. Another limitation of this study is that it reviews the records of 1 surgeon at 1 center, and therefore its results may not be generalizable to other surgeons and other patient populations. Lastly, a potential limitation of the study is that the trabeculectomies were performed with a limbus-based approach in nearly all cases. This is now a less common approach, but many studies worldwide have shown that long-term IOP and success rates are the same for either approach29–34.
CONCLUSIONS
In conclusion, if a low IOP of ≤10 mm Hg is required in a pseudophakic primary glaucoma eye, with or without previous glaucoma surgery, this study suggests that trabeculectomy with MMC is more likely to achieve this goal than a GDD surgery for up to 5 years of follow-up. Additionally, these eyes may require less adjunctive medication. The complication rates and VA loss postoperatively were similar between groups. Contrary to established guidelines, our study, similar to the one by Tseng et al,27 showed no evidence that patients who achieved a pressure of ≤5 mm Hg did any worse visually than those obtaining postoperative pressures in a higher range of ≥10 mm Hg. Previous studies have shown better visual field preservation in normal tension glaucoma patients with lower postoperative pressures. Therefore, it may be reasonable to set a more stringent IOP goal in patients with advanced glaucoma or progression at low-normal IOPs, given that there may not be a VA penalty.6
Supplementary Material
Footnotes
This study was supported in part by an unrestricted grant from Research to Prevent Blindness (New York, NY), to the Department of Ophthalmology, University of Florida.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014; 121:2081–2090. [DOI] [PubMed] [Google Scholar]
- 2.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012; 96:614–618. [DOI] [PubMed] [Google Scholar]
- 3.Arora KS, Robin AL, Corcoran KJ, et al. Use of Various Glaucoma Surgeries and Procedures in Medicare Beneficiaries from 1994 to 2012. Ophthalmology 2015; 122:1615–1624. [DOI] [PubMed] [Google Scholar]
- 4.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol 2012; 153:789–803e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Advanced Glaucoma Intervention Study (AGIS): 7 The advanced glaucoma intervention study (AGIS): 7. the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000; 130:429–440. [DOI] [PubMed] [Google Scholar]
- 6.Oie S, Ishida K, Yamamoto T. Impact of intraocular pressure reduction on visual field progression in normal-tension glaucoma followed up over 15 years. Jpn J Ophthalmol 2017; 61:314–323. [DOI] [PubMed] [Google Scholar]
- 7.Mataki N, Murata H, Sawada A, et al. Visual field progressive rate in normal tension glaucoma before and after trabeculectomy. Asia Pac J Ophthalmol (Phila) 2014; 3:263–266. [DOI] [PubMed] [Google Scholar]
- 8.Iverson SM, Schultz SK, Shi W, et al. Effectiveness of Single-digit iop targets on decreasing global and localized visual field progression after filtration surgery in eyes with progressive normal-tension glaucoma. J Glaucoma 2016; 25:408–414. [DOI] [PubMed] [Google Scholar]
- 9.Gedde SJ, Schiffman JC, Feuer WJ, et al. The tube versus trabeculectomy study: design and baseline characteristics of study patients. Am J Ophthalmol 2005; 140:275–287. [DOI] [PubMed] [Google Scholar]
- 10.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy study after one year of follow-up. Am J Ophthalmol 2007; 143:9–22. [DOI] [PubMed] [Google Scholar]
- 11.Gedde SJ, Schiffman JC, Feuer WJ, et al. Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol 2009; 148:670–684. [DOI] [PubMed] [Google Scholar]
- 12.Shaarawy TM, Sherwood MB, Grehn F. Kugler Publications, World Glaucoma Association Guidelines on Design and Reporting of Glaucoma Surgical Trials. Amsterdam: 2009. [Google Scholar]
- 13.Schulze-Bonsel K, Feltgen N, Burau H, et al. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Investig Ophthalmol Vis Sci 2006; 47:1236. [DOI] [PubMed] [Google Scholar]
- 14. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. Available at: http://www.r-project.org/. [Google Scholar]
- 15.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G∗Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009; 41:1149–1160. [DOI] [PubMed] [Google Scholar]
- 16.Law SK, Shih K, Tran DH, et al. Long-term outcomes of repeat vs initial trabeculectomy in open-angle glaucoma. Am J Ophthalmol 2009; 148:685–695. [DOI] [PubMed] [Google Scholar]
- 17.Chan MP, Grossi CM, Khawaja AP, et al. Associations with intraocular pressure in a large cohort: results from the UK biobank. Ophthalmology 2016; 123:771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazici A, Sen E, Ozdal P, et al. Factors affecting intraocular pressure measured by noncontact tonometer. Eur J Ophthalmol 2018; 19:61–65. [DOI] [PubMed] [Google Scholar]
- 19.Musch DC, Gillespie BW, Niziol LM, et al. Factors associated with intraocular pressure before and during 9 years of treatment in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology 2008; 115:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allan EJ, Khaimi MA, Jones JM, et al. Long-term efficacy of the baerveldt 250 mm2 compared with the baerveldt 350 mm2 implant. Ophthalmology 2015; 122:486–493. [DOI] [PubMed] [Google Scholar]
- 21.Meyer AM, Rodgers CD, Zou B, et al. Retrospective comparison of intermediate-term efficacy of 350 mm2 glaucoma drainage implants and medium-sized 230-250 mm2 implants. J Curr Glaucoma Pract 2017; 11:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panarelli JF, Banitt MR, Gedde SJ, et al. A Retrospective comparison of primary baerveldt implantation versus trabeculectomy with mitomycin c. Ophthalmology 2016; 123:789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gedde SJ, Feuer WJ, Shi W, et al. Treatment Outcomes In The Primary Tube Versus Trabeculectomy (PTVT) Study After One Year Of Follow-Up. In: American Glaucoma Society. Coronado, California. [Google Scholar]
- 24.Naito T, Fujiwara M, Miki T, et al. Effect of trabeculectomy on visual field progression in Japanese progressive normal-tension glaucoma with intraocular pressure <15 mmHg. PLoS One 2017; 12:e0184096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caprioli J, de Leon JM, Azarbod P, et al. Trabeculectomy can improve long-term visual function in glaucoma. Ophthalmology 2016; 123:117–128. [DOI] [PubMed] [Google Scholar]
- 26.Gedde SJ, Chen PP, Heuer DK, et al. The primary tube versus trabeculectomy study: methodology of a multicenter randomized clinical trial comparing tube shunt surgery and trabeculectomy with mitomycin c. Ophthalmology 2018; 125:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tseng VL, Kim CH, Romero PT, et al. Risk factors and long-term outcomes in patients with low intraocular pressure after trabeculectomy. Ophthalmology 2017; 124:1457–1465. [DOI] [PubMed] [Google Scholar]
- 28.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol 2012; 153:804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Haddad CE, Abdulaal M, Al-Moujahed A, et al. Fornix-based versus limbal-based conjunctival trabeculectomy flaps for glaucoma: findings from a cochrane systematic review. Am J Ophthalmol 2017; 174:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Haddad C, Abdulaal M, Al-Moujahed A, et al. Fornix-based versus limbal-based conjunctival trabeculectomy flaps for glaucoma. Cochrane Database Syst Rev 2015; CD009380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuroda U, Inoue T, Awai-Kasaoka N, et al. Fornix-based versus limbal-based conjunctival flaps in trabeculectomy with mitomycin C in high-risk patients. Clin Ophthalmol 2014; 8:949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalili MA, Diestelhorst M, Krieglstein GK. Langzeituntersuchung von 700 Trabekulektomien12. Klin Monbl Augenheilkd 2000; 217:1–8. [DOI] [PubMed] [Google Scholar]
- 33.el Sayyad F, el-Rashood A, Helal M, et al. Fornix-based versus limbal-based conjunctival flaps in initial trabeculectomy with postoperative 5-fluorouracil: four-year follow-up findings. J Glaucoma 1999; 8:124–128. [PubMed] [Google Scholar]
- 34.Robinson DI, Lertsumitkul S, Billson FA, et al. Long-term intraocular pressure control by trabeculectomy: a ten-year life table. Aust N Z J Ophthalmol 1993; 21:79–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


