Abstract
Goals:
We aimed to establish the epidemiological characteristics and documentation of diagnostic workup for gastroparesis (GP).
Background:
No study has used a national database to evaluate the prevalence, demographics, and associated comorbid conditions of GP, and document rates of proper diagnosis.
Materials and Methods:
This was a cross-sectional population-based study using the Explorys Platform to determine the prevalence of GP in a large and diverse population highly representative of the US population and to examine the diagnostic approach of GP. Data collected were individual characteristics from electronic medical records (EMRs) included age, ethnicity/race, sex, diagnostic report for esophagogastroduodenoscopy (EGD) and gastric emptying study (GES).
Results:
A total of 43,827,910 medical records were surveyed (1999 to 2014), of which 69,950 had a diagnosis of GP, yielding an overall prevalence of 0.16%. We identified 249,930 EMRs with type 1 diabetes mellitus (T1DM), and 2,940,280 EMR’s with type 2 diabetes mellitus (T2DM), of which 11,470 (4.59%) and 38,670 (1.31%) EMR’s had concurrent GP, respectively. The remainder 19,810 EMRs with a diagnosis of GP were classified as having idiopathic GP. In all three subgroups, women and Caucasians had the highest prevalence of GP. The diagnosis of GP was confirmed by both GES and EGD in 9,950 of patients (14.22%). For patients with T1DM, T2DM, or idiopathic GP, GP was confirmed by both diagnostic tests in 16.8%, 14.0%, and 13.2%, respectively.
Conclusions:
Our estimated rates of prevalence of GP in T1DM and T2DM indicate that GP is not a common clinical complication in these populations. Majority of EMRs that indicated a diagnosis of GP did not include any documentation of definitive diagnostic testing (EGD and/or GES).
Key Words: gastroparesis, diabetes mellitus, idiopathic, gastric emptying study
Gastroparesis (GP) is a syndrome characterized by delayed gastric emptying (GE) in the absence of mechanical gastric outlet obstruction.1 Although GP can be asymptomatic, it typically presents with postprandial fullness (early satiety), nausea, vomiting, and abdominal pain.2 To meet diagnostic criteria, symptoms must be present for >3 months, with symptom correlation, and documented delayed GE. Symptoms, especially abdominal pain, can be confused with many other gastrointestinal disorders, such as functional dyspepsia, in which a small minority of patients can have delayed GE, making diagnosis challenging.3 The reported prevalence of GP in the general population is quite variable, especially among presumed high-risk groups, including postoperative gastric surgery individuals and those with type 1 or type 2 diabetes mellitus (T1DM and T2DM). Published reports have estimated a prevalence of GP in the US population of 4%,4 30% in T2DM,5 and up to 58% in T1DM.6 This wide discrepancy may reflect differences in ascertainment when surveys are applied to the general population in contrast to those in tertiary referral centers.7–11 Large population-based studies are lacking, and the true prevalence of GP in the general population and in specific high-risk groups remains to be defined. Finally, the majority of patients with GP may not seek health care, contributing further to discrepancies in the prevalence of GP.7
The etiology of GP is diverse. The majority of patients diagnosed with GP are those with postoperative anatomic alterations, diabetes mellitus, and idiopathic disease.12 Presentation with nonspecific symptoms can often lead to an extensive medical evaluation, with inherent significant hospital costs, including hospital admission. A recent review of costs from a National Inpatient Sample database by Wadhwa et al13 described a significant increase in such costs from $13,350 per patient in 1997 to $34,585 per patient in 2013. Moreover, there has been a 138% increase in hospitalizations related to GP between 1995 and 2004.1 Beyond the medical costs, GP is a debilitating disease, associated with significant morbidity and mortality.14 GP imposes significant psychological distress and poor quality of life, with depression and/or anxiety in 24% of the GP population.15 For all of these reasons, it is important to conduct a cost-effective diagnostic approach to ensure proper management. A clinical presumptive diagnosis of GP requires verification by specific tests, including esophagogastroduodenoscopy (EGD) and a validated measure of GE [gastric emptying study (GES)]. As its initial description >40 years ago, scintigraphy assessment has been considered the standard modality used to diagnose GP.16–18
Current innovations in electronic medical records (EMRs) and search engines allow for the analysis of large samples to be surveyed using expanded databases. Explorys is a national database that links several hundreds of hospitals across the United States electronically, enabling the extraction of nonidentifiable data from medical records of millions of cases.
The aims of the study were to estimate the rate of prevalence of GP in patients with T1DM, T2DM, and in a diverse large population sample representative of the United States overall. In addition, we aim to evaluate and determine the current clinical tools used to diagnose GP, and if proper documentation is being reported by clinicians.
MATERIALS AND METHODS
A total of 43,827,910 medical records between 1999 to 2014 were surveyed using Explorys, a cloud-based platform, originally designed by the Cleveland Clinic Foundation (Cleveland, OH) in 1990, which was later acquired by IBM Watson (Armonk, NY). This platform is a large, HITECH and HIPAA-compliant, national database search engine with the capacity to survey EMRs. One major advantage of Explorys is its capacity to de-identify records to preserve anonymity. At the time of this study, Explorys had access to over 340 hospitals in the United States, comprising 22 different health care systems, from 1999 (introduction of Explorys) to 2014 (end date of the study). Explorys performs searches on a private cloud web-based system based on ICD codes, demographics, medications, and laboratory results, alone or in combination. It can also apply a temporal relationship to various diagnostic testing and diseases (ie, searching EGD or GES documentation before a diagnosis of GP).
We surveyed all patients with an active EMR in the given timeframe. Search terms for the survey included a diagnosis of GP using the ICD-9 library (536.3). We corroborated the concordance of diagnosis by reviewing the charts with this diagnosis from our own Hospital. Once identified, we obtained the following data from the records: age, sex, diabetes diagnosis (yes, no), type of diabetes (1, 2), ethnicity/race, EGD report (yes, no), and GES (yes, no). Rates of prevalence for GP were calculated for the population at large (numbers of all cases with GP as a percent of all medical records surveyed). We also calculated rates of prevalence segregating the population into specific subgroups: (1) patients with T1DM, (2) patients with T2DM and, (3) no history of diabetes, connective tissue disorders, neurological disorders or gastric surgery, including bariatric surgery (564.2), defined as idiopathic gastroparesis (IGP) in our study. Exclusion criteria for the cohort of T1DM, included T2DM and having any postgastric surgery status. Likewise, exclusion criteria for the subgroup of T2DM included those with T1DM and having any postgastric surgery status. Because the codiagnosis of diabetes represented an etiologic factor for GP, patients were required to have a diagnosis of diabetes (T1 or T2) before the diagnosis of GP.
Data Analysis
Our analysis was based on the presumption that the entire national database was at risk for GP. Rates of each subgroup of GP were calculated using a proportional analysis. Groups were compared using χ2 testing to estimate confidence intervals and P-values using statistical software (MedCalc).19 In addition, because Explorys rounds all numerical data to the nearest 10, statistical analysis could not be performed when a result was <10. A P-value <0.05 was considered to be statistically significant.
RESULTS
Of the 43,827,910 medical records surveyed 69,950 had a diagnosis of GP for a prevalence rate of 0.16%. From 249,930 EMRs that had a diagnosis of T1DM, 11,470 indicated a concurrent diagnosis of GP for a rate of prevalence within this population of 4.59%. Moreover, 2,940,280 EMRs were identified with the diagnosis of T2DM of which 38,670 also had a diagnosis of GP (1.31%). The remainder EMRs with a diagnosis of GP (19,810) had presumed IGP for a prevalence rate of 0.05%. Patients with T2DM comprised 55.3% of all patients with GP, those with T1DM 16.4%, and the rest were grouped as IGP (28.3%, Fig. 1).
FIGURE 1.
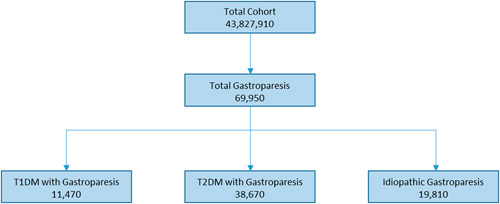
Flow diagram of the breakdown of patients with gastroparesis, represented by the total cohort. T1DM indicates type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
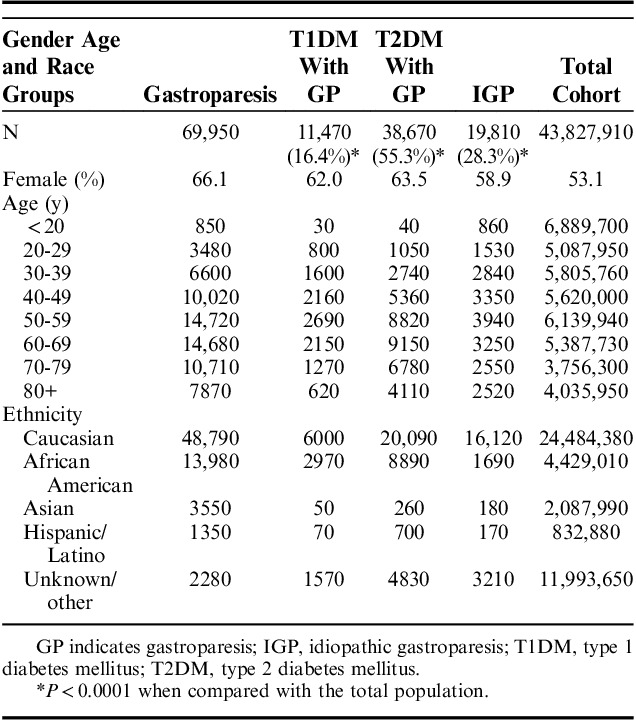
Caucasians had the highest rate of prevalence of GP in all cohorts, followed by African Americans (Table 1). Females had a higher prevalence of GP in all subgroups, accounting for a total of 66.1% of all GP patients identified. Within subgroups with a prior diagnosis of diabetes, there was also a predominance in females (62.0% in the T1DM population, 63.5% in T2DM) as well as in the idiopathic group (58.9%).
TABLE 1.
Demographics of Patients With GP as Compared With the Total Population
Although the absolute number of cases GP was greater in the T2DM group, this was largely due to the fact the prevalence of T2DM was much greater than the T1DM population or the IGP. However, when comparing the percentage of GP within each subgroup, we found a greater incidence of GP in the T1DM group (4.6%, P<0.0001). This was followed by the T2DM group, with 1.3% of patients with subsequent GP diagnosis (P<0.0001), as compared with the GP prevalence of 0.16% in the total population (Table 2). The prevalence rate of GP in T1DM was 3.5 times higher than GP prevalence in T2DM (95% confidence interval: 38.4%-39.4%, P<0.0001).
TABLE 2.
Clinical Characteristics of Patients With T1DM, T2DM, and Rate of Gastroparesis
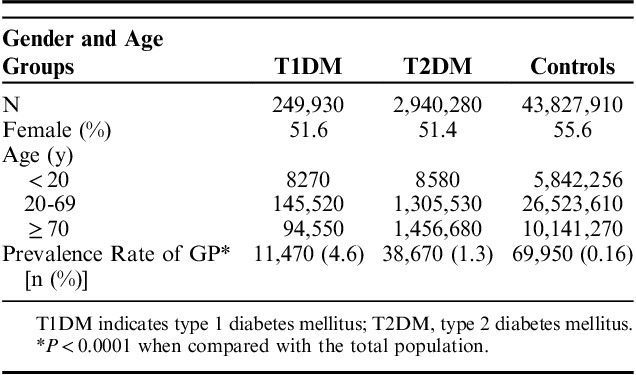
Figure 2 describes the age distribution of GP across all three subgroups. In patients younger than age 39 years, IGP incidence with the total GP group was highest. From ages 40 to 59, T1DM had the highest percentage of GP. T2DM was predominately associated with GP from age 60 to 80 years above which there was a higher percentage of patients with IGP.
FIGURE 2.
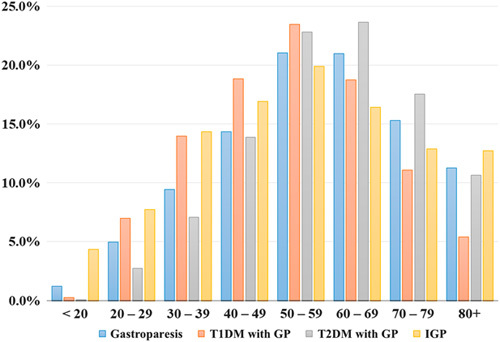
Incidence of GP with cohorts across all age groups. GP indicates gastroparesis; IGP, idiopathic gastroparesis; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
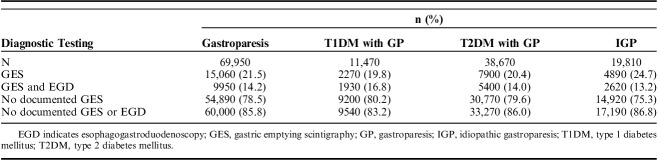
The second part of the study examined the accuracy of diagnosing GP, as reflected by the standard of care practice that requires the exclusion of gastric outlet obstruction using EGD, as well as the documentation of delayed GE using GES. We found that the vast majority of patients with diagnoses of GP in their EMR did not have adequate documentation of EGD or GES (85.8%). As shown in Table 3, of all the patients diagnosed with GP in all groups, only 21.5% had documentation of prior GES, and only 14.2% had both GES and EGD. In patients with T1DM, the rate of testing with EGD and GES was slightly higher than in the other subgroups (16.8%). The lowest rate of diagnostic testing was found in the IGP group, with only 13.2% of patients having undergone both GES and EGD.
TABLE 3.
Rates of Documented EGD and GES in Patients With GP
DISCUSSION
The true prevalence of GP is unknown. One community-based study in Olmsted County, MN described the incidence and prevalence of GP, as well as outcomes.7 This study used medical records in a single medical system to identify county residents with GP, further characterizing them into three distinct categories: (1) definite GP (delayed GE with scintigraphy+symptoms of >3 months’ duration); (2) probable GP (typical symptoms±food retention on EGD); and (3) possible GP (typical symptoms without scintigraphy or EGD OR asymptomatic patients with delayed GE). The authors identified 3604 GP cases, an incidence of 2.5 per 100,000 person-years for men and 9.8 per 100,000 person-years for women and concluded that GP is an uncommon disease.7 This study was confined to a single county in Minnesota and hence cannot be considered representative of the overall population in the Unites States.
Our epidemiological population-based study is the largest documented report of the prevalence of GP in current literature, not limited to one tertiary care center, nor a single community, but representative of the general population in the United States. Unlike most previous reports, our current study has utilized an extensive network of EMRs, and it supports the conclusion that the prevalence of GP is less common than previously reported.7 The selected search engine was designed to collect data without any identifier, including a hospital or clinic that holds the records. This allows for searches to be performed without requiring institutional review board approval but introduces the possibility of ascertainment bias by over or under-representation of academic/community/urban/rural hospitals. The likelihood that our data is biased based on the latter is unlikely due to the massive amount of hospitals and medical records researched.
Our study found a predominance of GP in the female population, 1.5 higher than in males, across all populations and ages. The demographics of our study allowed to contrast rates across the major ethnic groups in the United States. Caucasians have the highest prevalence of any type of GP and across all ages followed by African Americans. Within the groups with diabetes, the rate of prevalence was 4.6% in T1DM and 1.3% for T2DM, which contrasts to the prevalence of 0.16% in the total population. Our data is very consistent with the findings of Choung and colleagues that evaluated at the risk of GP in the diabetic population over a 10-year period. They reported an incidence of 5.2% in T1DM, 1.0% in T2DM, and 0.2% in individuals without diabetes.20 The higher prevalence found among the patients with diabetes may be due to a more intense diagnostic scrutiny in this population, in as much as GP is considered a chronic complication of diabetes.
Determination of the prevalence rate of any disease is contingent on precise identification of the disease using the appropriate diagnostic tools. In our study, we also gathered information on the clinical studies considered standard of care for diagnosis of GP, namely EGD to exclude gastric outlet obstruction, and GES to document delayed GE. We found remarkably low rates of diagnostic testing, especially in those patients considered to have IGP but also very low for patients with either T1DM or T2DM. This observation concurs with the study of Choung et al20 and indicates that most clinicians rely too much on symptoms alone for diagnosis of GP. These nonspecific symptoms (dyspepsia, abdominal pain, nausea, emesis) are also found in patients with other abnormalities, including cyclic vomiting syndrome, psychiatric disorders, somatoform disorders, peptic ulcer disease, and other gastrointestinal disorders.
The principal strength of the current study is the large sample size of over 43 million patients. The population-based study is representative of all patients with an EMR, and, owing to its size, is the largest documented cohort of GP in current literature. We found a very low rate of appropriate diagnostic testing. The latter may inflate the diagnosis of GP as the cardinal symptoms of GP (nausea, vomiting, and pain) are very common but nonspecific. Widespread use of medications such as opioids may also bias the diagnosis.21
Limitations include being a retrospective study. In addition, our results are dependent on accurate physician documentation into a patient’s EMR. However, it is important to note clinicians should document proper assessment and procedures into a patient’s EMR.
In summary, we found that GP is less common than reported previously in some studies,4–6 but is in agreement with other smaller community-based studies.1,7 As expected, higher prevalence rates were found in patients with diabetes, specifically T1DM. The rates of prevalence may be an overestimation as the diagnosis of GP is not been established with the recommended standard of care in many of the cases with this diagnosis. This may also be due to improper documentation in EMRs by clinicians and should encourage physicians to report and update a patient’s history properly. If indeed there is overdiagnosis of GP, the actual rate of prevalence of GP may even lower than our estimates, supporting the proposal to consider GP an orphan disease.
Footnotes
The authors declare that they have nothing to disclose.
REFERENCES
- 1.Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;1:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu N, Abell T. Gastroparesis updates on pathogenesis and management. Gut Liver. 2017;5:579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkman HP, Yates K, Hasler WL, et al. National Institute of Diabetes and Digestive and Kidney Diseases Gastroparesis Clinical Research Consortium. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying and gastroparesis severity. Gastroenterology. 2011;1:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–283. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz M, Harding PE, Maddox AF, et al. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151–159. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz M, Maddox AF, Wishart JM, et al. Relationships betweel oesophageal transit and solid and liquid gastric emptying in diabetes mellitus. Eur J Nucl Med. 1991;18:229–234. [DOI] [PubMed] [Google Scholar]
- 7.Jung H, Choung RS, Locke GR, et al. The incidence, prevalence and outcomes of patients with gastroparesis in Olmsted County, Minnesota from 1996–2006. Gastroenterology. 2009;4:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. [DOI] [PubMed] [Google Scholar]
- 9.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2007;103:313–322. [DOI] [PubMed] [Google Scholar]
- 10.Jones KL, Russo A, Stevens JE, et al. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–1269. [DOI] [PubMed] [Google Scholar]
- 11.Kong MF, Horowitz M, Jones KL, et al. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22:503–507. [DOI] [PubMed] [Google Scholar]
- 12.Parkman HP. Idiopathic Gastroparesis. Gastroenterol Clin North Am. 2015;1:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wadhwa V, Mehta D, Jobanputra Y, et al. Healthcare utilization and costs associated with gastroparesis. World J Gastroenterol. 2017;24:4428–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bielefeldt K. Gastroparesis: concepts, controversies, and challenges. Scientifica. 2012;2012:424802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodhouse S, Hebbard G, Knowles SR. Psychological controversies in gastroparesis: a systemic review. World J Gastroenterol. 2017;7:1298–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;3:753–776. [DOI] [PubMed] [Google Scholar]
- 17.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;6:1456–1462. [DOI] [PubMed] [Google Scholar]
- 18.Ajaj W, Goehde SC, Papanikolaou N, et al. Real time high resolution magnetic resonance imaging for the assessment of gastric motility disorders. Gut. 2004;9:1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman DG. Practical Statistics for Medical Research. London, UK: Chapman and Hall; 1991. [Google Scholar]
- 20.Choung RS, Locke GR, Schleck CD, et al. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;1:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;1:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]