Abstract
Tenosynovial giant cell tumour (TGCT) is a group of rare soft tissues neoplasia affecting synovial joints, bursae and tendon sheaths and is classified as localized type or diffuse type. The diffuse type (TGCT-D), also known as ‘pigmented villonodular (teno)synovitis’ is characterized by local aggressivity, with invasion and destruction of adjacent soft-tissue structures, and high local recurrence rate. Radical surgery remains the standard therapy while adjuvant radiotherapy may help to control local spread. Malignant TGCT is characterized by high rate of local recurrences and distant metastasis. Few cases of malignant TGCT and very few evidences on systemic therapies are described in the literature, so, to date, no systemic treatment is approved for this rare disease. We report the case of a malignant TGCT patient treated with many different systemic therapies, including chemotherapy and tyrosine-kinase inhibitors, and performed a review of the literature on the systemic treatment options of this rare tumour.
Keywords: chemotherapy, imatinib, malignant tenosynovial giant cell tumour, pazopanib, pigmented villonodular synovitis, tenosynovial giant cell tumour
Introduction
Tenosynovial giant cell tumour (TGCT) or giant cell tumour of the tendon sheath is a group of rare soft tissues neoplasia affecting synovial joints, bursae and tendon sheaths [1].
It is usually a benign tumour, predominant in young adults, especially females, with a median age of 40 years [2]. According to clinical presentation and biological behaviour, it is classified as localized type or diffuse type, the second one itself divided in a ‘benignant’ and ‘malignant’ form [3]. The localized form (TGCT-L) usually affects smallest joints, mainly fingers or wrists, and is a benign neoplasm, characterized by a low recurrence rate (10–20%) [4].
The diffuse type (TGCT-D), also known as ‘pigmented villonodular (teno)synovitis’, involves large joints (knee, hip, ankle, elbow) [5]. It is characterized by local aggressivity, with invasion and destruction of adjacent soft-tissue structures and high local recurrence rate (20–50%) [1]. The main treatment approach is radical surgery, while radiotherapy is used as adjuvant therapy, especially after partial resection, or as an alternative treatment when a complete resection cannot be performed [1,6].
Malignant TGCT can arise de novo as a primary malignancy or as malignant transformation of benign TGCT [7]. It is characterized by high rate of local recurrences and distant metastasis mainly in lungs and lymph nodes [8]. Radical surgical approach remains the best treatment of choice and adjuvant radiation therapy may help to control local spread.
Only a few cases of malignant TGCT and very few evidences on therapy options are described in the literature, so, to date, no systemic treatment is approved for this rare disease. In consideration of the limited data presented in the literature, we report the case of a malignant TGCT patient treated with many different systemic therapies and performed a review of the literature on the systemic treatment options of this rare tumour.
Case presentation
Our patient was a 41-year-old female Caucasian and her oncological history started with a self-finding of a progressive increasing mass in the upper left thigh from October 2016. In February 2017, she performed an MRI of the left thigh which confirmed the presence of a neoformation of 6 cm. The percutaneous biopsy performed in March 2017 lean to a diagnosis of ‘Giant cell tumour of soft tissues’. Histological examination revealed a mitotic index of 16/10, high power fields absence of necrosis, immunohistochemical positivity for CD68, p63, alpha-actin and desmin with negativity for CD45, mieloperossidasis, CD33, CD34, EMA, AE1/AE3 and SOX10. The total body computed tomography (TB CT) scan was negative for distant metastases.
In May 2017, a wide excision of the lesion of left thigh was performed and the histological examination reported the diagnosis of ‘Malignant Giant Cell Tumour of Tendon Sheaths’ of 7 cm diameter, with focal necrosis, a mitotic index of 26/10 HPF and focal vascular invasion.
In consideration of the diagnosis of soft tissue sarcoma and the stage of the disease, an adjuvant radiotherapy was planned for August 2017. However, in July 2017, the patient noticed a painful inguinal swelling, so a TB CT scan and an MRI of the left thigh were performed. Both imaging tests demonstrated a local recurrence in the field of the previous surgery [Diameter maximum (Dmax) 25 mm], the appearance of left obturatory and inguinal lymphadenopathies (Dmax 19 mm) and lung nodules (Dmax 8 mm). All the lesions were confirmed by a PET scan.
The adjuvant radiotherapy was omitted, and in September 2017, the patient underwent a first-line chemotherapy with Doxurubicin (75 mg/mq i.v. on day 1) and Olaratumab (15 mg/kg i.v. on day 1 and day 8) every three weeks, well tolerated. After two cycles of therapy, the TB CT scan and MRI of the left thigh revealed a numeric and dimensional increase of lungs nodules, of the lymphadenopathy (inguinal, external iliac and obturatory) (Fig. 1.1a–c) and of the soft tissue lesions of the left thigh (7 cm) (Fig. 2.1a–c). The patient also experienced a clinical progression with uncontrolled pain [Numerical Rating Scale (NRS) 6] at inguinal nodes.
Fig. 1.
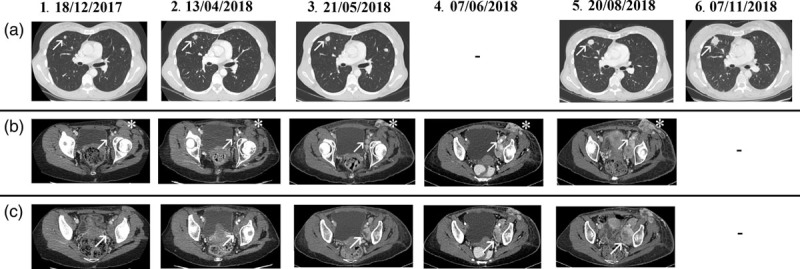
Major tumour lesions of the chest and lymphadenopathies. All CT scan are of the chest and the abdomen and the thighs, except for 07/06/2018 (CT scan of the abdomen and thighs) and 7 November 2018 (chest CT scan). (a) Chest. Multiple lung lesions, the major in the middle lobe of the right lung (arrow). (b) Left inguinal (asterisk) and external iliac lymphadenopathies (arrow). (c) Obturatory lymphadenopathies (arrow). ‘‘-’’ not performed. CT, computed tomography.
Fig. 2.
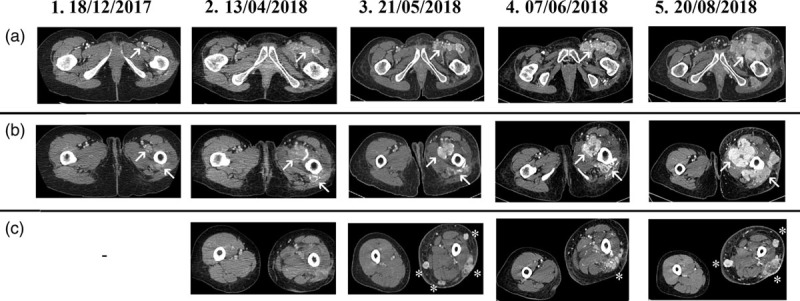
Major tumour lesions of the thighs. All CT scan are of the chest, the abdomen and the thighs, except for 7 June 2018 (CT scan of the abdomen and thighs). (a) Soft tissue lesions of the left groyne (arrow); (b) soft tissue lesions of the left thigh (arrows); (c) soft tissue lesions of the left upper leg (asterisks). CT, computed tomography.
In consideration of the low response to the chemotherapy, it was decided to propose an antiangiogenetic therapy, so in November 2017, the patient started a second-line therapy with Pazopanib 800 mg/mq and palliative radiotherapy (3DCRT, 30 Gy total in 10 fractions) on inguinal nodes with complete improvement of pain and no significant toxicity. After two months of Pazopanib, the TB CT scan and MRI of the left thigh showed a stable disease of the lymphadenopathies and the lung metastases, but a dimensional increased of the lesions of the thigh root and of the upper left leg with a worsening of the pain, so other 10 fractions of palliative radiotherapy (3DCRT, 30 Gy in 10 fractions/week) were performed concomitant to Pazopanib. Also this second RT cycle, associated to Pazopanib, was well tolerated with mild cutaneous toxicity (RTOG scale, G1).
After other three months of Pazopanib the TB CT scan and the MRI of the left thigh showed a mixed response with an increase in number of lung lesions (Dmax 18 mm), a minimal reduction of the left obturatory adenopathy with a more colliquate component (25 × 15 vs 30 × 18 mm2) and of the left inguinal lymphadenopathy (20 × 14 vs 26 × 15 mm2) (Fig. 1.2a–c). It showed also a dimensional increase in the lesions at the thigh root (30 vs 22 mm) and the numerical increase of the soft tissue nodulations of the upper left leg (Fig. 2.2a–c). Moreover, the patient experienced a worsening of the pain (NRS 7).
Therefore, we decided to offer a third-line therapy with Imatinib off-label despite the limited literature evidences. The therapy with Imatinib started in April 2018 and lasted only 24 days due to the severe worsening of the clinical status with an important left leg lymphoedema and a severe asthenia. A TB CT scan was therefore performed showing a dimensional and numeric increase in the multiple lung lesions (Dmax between 12 and 18 mm) and soft tissue nodulations of the left thigh (Dmax 49 × 37 and 61 × 23 mm2). Moreover, a dimensional increase in the left inguinal (22 × 39 mm2) and left iliac extern lymphadenopathy (12 × 22 mm2) (Figs. 1.3a–c and 2.3a–c).
In consideration of the rapid clinical and radiological progression of the disease and the disease entity, we opted for the reintroduction of the chemotherapy as any other sarcoma. In May 2018, the patient started a fourth-line of therapy with high-dose Ifosfamide (14 g/mq intravenous in continue infusion for 14 days every 28 days).
After two cycles of chemotherapy, the patient experienced severe asthenia, neutropenia, leucopoenia, anaemia (all toxicities of grade 3) and clinical progression of the lesions of the left groyne and thigh root, confirmed by a CT scan of the abdomen and thighs (Figs. 1.4b,c and 2.4a–c). The clinical progression and the drug intolerance led us to the decision of starting a fifth-line treatment with Trabectedine (1.5 mg/mq in 24 hours infusion) in June 2018.
Meanwhile, gene rearrangements (fusions) assessment of five genes and corresponding exon regions (ALK, ROS1, NTRK1, NTRK2, NTRK3) was performed with next generation sequencing test evaluating RNA extracted from FFPE tissue specimen. Sequencing target enrichment was accomplished, after reverse transcription, using an anchored multiplex PCR method [9]. No gene rearrangements were detected in region tested.
After three cycles of Trabectedine, which were well tolerated, the TB CT scan reported a substantial stable disease of the lung lesions (Fig. 1.5a), but a dimensional increase in the soft tissue lesions of the thigh root, partially confluents in a single mass (80 × 76 vs 49 × 42 mm2) (Fig. 2.5a–c). It showed also a dimensional increase in the left iliac lymphadenopathies (Dmax 60 × 37 vs 34 × 23 mm2), which were partially incorporated in the mass (Fig. 1.5b,c).
In September 2018, a sixth-line of treatment with Gemcitabine 1000 mg/mq (days 1–8 every 3 weeks) was started. After three cycles of chemotherapy, a clinical progression of the inguinal lymphadenopathies and thigh root lesions was observed. Only a chest CT scan was performed and showed a further increase of the lung lesions (Dmax 24 mm) (Fig. 1.6a).
The clinical status of the patient was progressively worsening with severe asthenia (grade 3), uncontrolled pain (NRS 10) and bleeding of the left groyne and thigh lesions and high fever. The patient died in October 2018, after 19 months from histological diagnosis and 16 months from the diagnosis of metastatic disease.
Discussion
Soft tissue sarcomas are a rare type of cancer and the malignant TGCT is an even more uncommon entity within this family. Differently from its benign variant, this disease is very aggressive with a high risk of local recurrence and distant metastases mainly to the lungs and, unlike many other sarcomas, to the lymph nodes [10]. As there is no standard treatment for such an aggressive and infrequent pathology, this case report has the purpose of illustrating the treatment options currently available.
The first and the most crucial treatment option is surgery as in localized malignant sarcomas a radical resection is proven to reduce mostly the local relapse but also, in a minimal percentage, the development of distant metastases [11]. A thoughtful balance between local control and function preservation in soft tissue sarcomas of the extremities is fundamental. However, we have to keep in mind that the systemic treatment options for the malignant TGCT are still a challenge.
Concerning the medical treatments, there are two big families to consider: chemotherapy and tyrosine-kinase inhibitors (TKIs) [12]. Doxorubicin is the mainstay of first-line chemotherapy in soft tissue sarcomas and can be associated with Ifosfamide when shrinkage is needed. In fact, this association demonstrated to improve response rate and progression-free survival, but no advantage was shown in overall survival (OS) [13,14]. At the time of our case, the association of Doxorubicin with Olaratumab was thought to improve OS based on a phase II study that led this drug to an early approval [15], so this combination chemotherapy was proposed to our patient. Unfortunately, at the half of January 2019, the preliminary results of the phase III study ANNOUNCE trial reported by a press release did not confirm the previous results and the drug approval was retired.
Beyond first-line therapy, there is no consensus and usually a histology-driven choice is made. Unfortunately, there is no proof of efficacy for any tailored chemotherapy for malignant TGCT but only active drugs in other sarcoma subtypes are available, as high-dose Ifosfamide, Trabectedine, Eribulin, Gemcitabine and Docetaxel as single agent or in combination.
In TGCT, there is also typically a reactive component with proliferation and recruitment of colony-stimulating factor 1 receptor (CSF1R)-expressing cells including macrophages, giant cells and osteoclasts [16–18]. In 30–60% of TGCT patients, CSF1 overexpression results from a t(1;2) translocation, which fuses the CSF1 gene to the collagen type VI a3 (COL6A3) promoter [19]. Systemic therapies targeting the CSF1/CSF1R signalling pathways have shown promising results as novel treatment [Brahmi]. Imatinib, a multy-TKI with an activity against CSF1R, was shown to be effective in several case reports [20,21] and in a retrospective series of patients with locally advanced or metastatic TGCT [19]. Based on the evidence of these studies, we requested Imatinib in off label but, unfortunately, our patient could not even complete one month of treatment as clinical progression occurred. Small molecule and mAb targeted therapies have been investigated as novel treatment options with promising results, particularly other multy-TKI with an activity against CSF1R, such as Nilotinib, Pazopanib, Pexidartinib, Sunitinib [2,16,22–27] and Emactuzumab [28]. Other molecular inhibitors are under investigation such as the mammalian target of rapamycin inhibitors and insulin growth factor receptor inhibitors [29].
The development of new CSF1R/CSF1 inhibitors or other molecular pathways-inhibitors represents a new avenue and the key treatment management of patients with TGCT. Meantime, in the clinical practice setting, the therapeutic management of malignant TGCT remains challenging.
Conclusion
New drugs targeting molecular pathway, especially CSF1/CSF1R axis, seem to have an activity on TGCT. Cytotoxic drugs currently remain the most effective available agents against this disease in clinical practice even if the response rate, duration of response and progression-free survival are still unsatisfactory. Malignant TGCT is, therefore, still an orphan disease with unmet medical needs.
Better understand of biological and molecular characteristics of this subtype of sarcoma and more clinical trials are needed in order to develop new therapies able to efficiently target specific pathways.
Acknowledgements
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This work was supported from Pharma Mar for the submission fees.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gouin F, Noailles T. Localized and diffuse forms of tenosynovial giant cell tumor (formerly giant cell tumor of the tendon sheath and pigmented villonodular synovitis) Orthop Traumatol Surg Res. 2017; 103:S91–S97 [DOI] [PubMed] [Google Scholar]
- 2.Brahmi M, Vinceneux A, Cassier PA. Current systemic treatment options for tenosynovial giant cell tumor/pigmented villonodular synovitis: targeting the CSF1/CSF1R axis Curr Treat Options Oncol. 2016; 17:10. [DOI] [PubMed] [Google Scholar]
- 3.Alexiev BA, Tumer Y, Yang GY. Malignant tenosynovial giant cell tumor with CDKN2A/B genomic alteration: a histological, immunohistochemical, and molecular study Hum Pathol. 2017; 63:144–148 [DOI] [PubMed] [Google Scholar]
- 4.Somerhausen NS, Fletcher CD. Diffuse-type giant cell tumor: clinicopathologic and immunohistochemical analysis of 50 cases with extraarticular disease Am J Surg Pathol. 2000; 24:479–492 [DOI] [PubMed] [Google Scholar]
- 5.Mastboom MJL, Verspoor FGM, Gelderblom H, van de Sande MAJ. Limb amputation after multiple treatments of tenosynovial giant cell tumour: series of 4 dutch cases Case Rep Orthop. 2017; 2017:7402570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temponi EF, Barros AAG, Paganini VO, Barbosa VAK, Badet R, Carvalho Júnior LH. Diffuse pigmented villonodular synovitis in knee joint: diagnosis and treatment Rev Bras Ortop. 2017; 52:450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Righi A, Gambarotti M, Sbaraglia M, Frisoni T, Donati D, Vanel D, Dei Tos AP. Metastasizing tenosynovial giant cell tumour, diffuse type/pigmented villonodular synovitis Clin Sarcoma Res. 2015; 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis) Am J Surg Pathol. 1997; 21:153–163 [DOI] [PubMed] [Google Scholar]
- 9.Zheng Z, Liebers M, Zhelyazkova B, Cao Y, Panditi D, Lynch KD, et al. Anchored multiplex PCR for targeted next-generation sequencing Nat Med. 2014; 20:1479–1484 [DOI] [PubMed] [Google Scholar]
- 10.Li CF, Wang JW, Huang WW, Hou CC, Chou SC, Eng HL, et al. Malignant diffuse-type tenosynovial giant cell tumors: a series of 7 cases comparing with 24 benign lesions with review of the literature Am J Surg Pathol. 2008; 32:587–599 [DOI] [PubMed] [Google Scholar]
- 11.Gronchi A, Lo Vullo S, Fiore M, Mussi C, Stacchiotti S, Collini P, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients J Clin Oncol. 2009; 27:24–30 [DOI] [PubMed] [Google Scholar]
- 12.In GK, Hu JS, Tseng WW. Treatment of advanced, metastatic soft tissue sarcoma: latest evidence and clinical considerations Ther Adv Med Oncol. 2017; 9:533–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borden EC, Amato DA, Rosenbaum C, Enterline HT, Shiraki MJ, Creech RH, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas J Clin Oncol. 1987; 5:840–850 [DOI] [PubMed] [Google Scholar]
- 14.Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, et al. ; European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial Lancet Oncol. 2014; 15:415–423 [DOI] [PubMed] [Google Scholar]
- 15.Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial Lancet. 2016; 388:488–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staals EL, Ferrari S, Donati DM, Palmerini E. Diffuse-type tenosynovial giant cell tumour: current treatment concepts and future perspectives Eur J Cancer. 2016; 63:34–40 [DOI] [PubMed] [Google Scholar]
- 17.Fiocco U, Sfriso P, Lunardi F, Pagnin E, Oliviero F, Scagliori E, et al. Molecular pathways involved in synovial cell inflammation and tumoral proliferation in diffuse pigmented villonodular synovitis Autoimmun Rev. 2010; 9:780–784 [DOI] [PubMed] [Google Scholar]
- 18.West RB, Rubin BP, Miller MA, Subramanian S, Kaygusuz G, Montgomery K, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells Proc Natl Acad Sci U S A. 2006; 103:690–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassier PA, Gelderblom H, Stacchiotti S, Thomas D, Maki RG, Kroep JR, et al. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis Cancer. 2012; 118:1649–1655 [DOI] [PubMed] [Google Scholar]
- 20.Blay JY, El Sayadi H, Thiesse P, Garret J, Ray-Coquard I. Complete response to imatinib in relapsing pigmented villonodular synovitis/tenosynovial giant cell tumor (PVNS/TGCT) Ann Oncol. 2008; 19:821–822 [DOI] [PubMed] [Google Scholar]
- 21.Stacchiotti S, Crippa F, Messina A, Pilotti S, Gronchi A, Blay JY, Casali PG. Response to imatinib in villonodular pigmented synovitis (PVNS) resistant to nilotinib Clin Sarcoma Res. 2013; 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. ; EORTC Soft Tissue and Bone Sarcoma Group; PALETTE study group. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial Lancet. 2012; 379:1879–1886 [DOI] [PubMed] [Google Scholar]
- 23.Nakayama R, Jagannathan JP, Ramaiya N, Ferrone ML, Raut CP, Ready JE, et al. Clinical characteristics and treatment outcomes in six cases of malignant tenosynovial giant cell tumor: initial experience of molecularly targeted therapy BMC Cancer. 2018; 18:1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor N Engl J Med. 2015; 373:428–437 [DOI] [PubMed] [Google Scholar]
- 25.Palmerini E, Staals EL, Maki RG, Pengo S, Cioffi A, Gambarotti M, et al. Tenosynovial giant cell tumour/pigmented villonodular synovitis: outcome of 294 patients before the era of kinase inhibitors Eur J Cancer. 2015; 51:210–217 [DOI] [PubMed] [Google Scholar]
- 26.Ravi V, Wang WL, Lewis VO. Treatment of tenosynovial giant cell tumor and pigmented villonodular synovitis Curr Opin Oncol. 2011; 23:361–366 [DOI] [PubMed] [Google Scholar]
- 27.Temple HT. Pigmented villonodular synovitis therapy with MSCF-1 inhibitors Curr Opin Oncol. 2012; 24:404–408 [DOI] [PubMed] [Google Scholar]
- 28.Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study Lancet Oncol. 2015; 16:949–956 [DOI] [PubMed] [Google Scholar]
- 29.Sikaria S, Heim-Hall J, Diaz EH, Williams RD, Sankhala KK, Laabs B, et al. Partial response of a rare malignant metastatic diffuse tenosynovial giant cell tumor with benign histologic features, treated with SCH 717 – 454, an insulin growth factor receptor inhibitor, in combination with everolimus, an MTOR inhibitor Targ Oncol. 2014; 9:73–79 [DOI] [PubMed] [Google Scholar]


