Abstract
Purpose of review
Asthma is a chronic inflammatory disease in which changes in macrophage polarization have been shown to contribute to the pathogenesis. The present review discusses the contribution of changes in macrophage function to asthma related to polarization changes and elaborates on possible therapeutic strategies targeting macrophage function and polarization.
Recent findings
Macrophage function alterations were shown to contribute to asthma pathology in several ways. One is by impaired phagocytosis and efferocytosis. Another is by changing inflammation, by altered (anti)inflammatory cytokine production and induction of the inflammasome. Finally, macrophages can contribute to remodeling in asthma, although little evidence is present in humans yet.
Novel therapeutic strategies targeting macrophages include dampening inflammation by changing polarization or by inhibiting the NLRP3 inflammasome, and by targeting efferocytosis. However, many of these studies were performed in animal models leaving their translation to the clinic for future research.
Summary
The present review emphasizes the contribution of altered macrophage function to asthma, gives insight in possible new therapeutic strategies targeting macrophages, and indicates which knowledge gaps remain open.
Keywords: efferocytosis, inflammation, innate immunity, phagocytosis
INTRODUCTION
Asthma is a heterogeneous chronic inflammatory disease of the lungs with many phenotypes that are all characterized by airway inflammation, reversible airflow obstruction, airway remodeling, and bronchial hyperresponsiveness. The precise characterization of these phenotypes is still evolving, but includes divisions based on the presence of allergy, increased numbers of eosinophils or neutrophils, time of disease onset, response to corticosteroids, and disease severity. In allergic asthma, allergens can trigger bronchoconstriction and this phenotype is associated with early-onset disease and increased eosinophil numbers in lung. Nonallergic asthma is associated with adult-onset disease and neutrophil infiltration. Patients with severe asthma can have a combination of characteristics and are usually corticosteroid resistant [1].
Although eosinophils and neutrophils are considered key determinants, the inflammatory profile in asthma is heterogeneous with various other cells involved. A role for macrophages in particular has long remained understudied, although it is now known that they can polarize into many different phenotypes and that these differentially polarized phenotypes are changed in asthma [2,3]. In the past, a bimodal subdivision of macrophage polarization was used, dividing macrophages in M1 and M2 macrophages in a similar fashion as used for T cells based on the expression of certain markers [4]. In this division M1 macrophages are associated with Th1 inflammation and considered pro-inflammatory and M2 macrophages are associated with Th2 inflammation and considered pro-repair and involved in wound healing. The bimodal subdivision has now been abandoned in favor of a model of a spectrum of polarization changes in macrophages that better illustrates the great variety in macrophage responses to stimuli [5]. A clear contribution of changes in macrophage polarization to asthma pathology has been shown by us and others [2,3,6,7▪,8,9]. Both in humans and mice with (experimental) asthma, more M1 and M2 macrophages (old nomenclature was then used) were found in lung tissue. However, polarization markers are indicative of certain functions and therefore in this review we will focus on the contribution of alterations in macrophage function to asthma. In addition, we will elaborate on novel experimental asthma therapies that target macrophage function.
Box 1.

no caption available
MACROPHAGES IN ASTHMA
Macrophages are abundantly present in lung tissue, but their contribution to asthma pathology seems to be through a change in function rather than number, as numbers of macrophages are unchanged in lung tissue of patients with asthma [2,9]. Similarly, no differences in macrophage percentages were found in bronchoalveolar lavage fluid of asthma and control subjects [8]. For sputum contradicting results have been found, with more [10], less [11], and similar [12] macrophage numbers in asthma versus controls. These differences may be because of asthma (pheno)type. In addition, location may explain why differences were found in sputum and not in lung tissue. Alveolar macrophages, resident on the alveolar epithelial surface, can be demonstrated in sputum, but interstitial macrophages, located within lung tissue, are not present in sputum and can only be detected in tissue sections/biopsies. Therefore, (certain types of) asthma may be accompanied by changes in numbers of alveolar macrophages and not interstitial macrophages.
CHANGES IN MACROPHAGE FUNCTION IN ASTHMA
Phagocytosis
As professional phagocytes, macrophages have the ability to recognize and eliminate pathogens and other foreign material, thereby playing an important role in innate immunity. In addition, several of the markers used to denote differentially polarized macrophages (e.g. CD64, CD163, CD206) are receptors important for phagocytosis. Therefore, changes in phagocytic behavior appear to be linked to asthma. Several studies have reported impaired phagocytosis of bacteria, yeasts, and particulate matter by airway macrophages and monocyte-derived macrophages from patients with asthma compared to controls (reviewed by Fricker et al.[13]). Recent findings also demonstrated less phagocytosis of Staphylococcus aureus by airway macrophages in children with neutrophil-predominant severe asthma as compared to severe asthma with few neutrophils [14]. Similarly, airway macrophages from patients with noneosinophilic asthma displayed lower phagocytic capacity of IgG-opsonized yeast than airway macrophages from patients with eosinophilic asthma [15].
However, studies appear to be inconsistent because sulfur colloid particle uptake by airway macrophages was higher in patients with mild asthma compared to healthy volunteers [16]. Furthermore, Kulkarni et al.[17▪] recently demonstrated that phagocytosis of S. aureus by alveolar macrophages from children with mild-to-moderate asthma was similar between patients with eosinophilic and noneosinophilic asthma and lower after allergen avoidance for 3 weeks.
These inconsistent findings for phagocytosis are also seen for CD163, a high-affinity scavenger receptor for hemoglobin that has been investigated in the context of asthma. More CD163+ macrophages were found in lungs of patients with asthma with fatal disease compared to control patients [18] and soluble CD163 in sputum was also higher in asthma compared to controls [19]. Moreover, in a mouse model of ovalbumin-induced experimental asthma, airway hyperresponsiveness and eosinophil counts were significantly lower in CD163-deficient mice compared to wild-type mice [18]. On the other hand, loss of CD163 expression in house dust mite-exposed mice was associated with enhanced eosinophilic inflammation, possibly through higher production of CCL24 by macrophages resulting in more recruitment of eosinophils [20]. The same investigators showed that CD163 can suppress CCL24 secretion by binding of the major house dust mite allergen, Derp1 [20].
Thus, although most studies suggest that asthma is associated with defective macrophage phagocytosis, contradicting results indicate that this may not be a general phenomenon of the disease. The phagocytic capacity may depend on the type of material that is being phagocytosed and dysfunctional phagocytosis is likely more pronounced during more severe forms of asthma, but exact mechanisms remain poorly understood. Further characterization of phagocytosis by macrophages and its effects on asthma symptoms is therefore warranted.
Efferocytosis
The phagocytic clearance of apoptotic cells is named efferocytosis. In contrast to phagocytosis, this process triggers anti-inflammatory behavior, mediated by receptors like Mer tyrosine kinase and executed by interleukin-10 (IL-10) production [21]. There are fewer IL-10+ macrophages in asthma, suggesting that efferocytosis may be impaired [2]. Indeed, Huynh et al.[22] described lower efferocytosis of apoptotic T cells by alveolar macrophages from patients with severe (but not mild-to-moderate) asthma compared to healthy volunteers. Findings from Simpson et al.[23] also showed that efferocytosis of apoptotic bronchial epithelial cells by sputum macrophages was impaired in patients with noneosinophilic asthma compared to eosinophilic asthma, suggesting this feature may be phenotype-specific. In contrast, recent work by Erriah et al.[24▪] demonstrated that efferocytosis of apoptotic granulocytes by monocyte-derived macrophages from patients with asthma was similar compared to healthy controls and also did not differ between noneosinophilic and eosinophilic asthma. This may be explained by the different origins of cells, as in contrast to monocyte-derived macrophages, lung macrophages are of fetal origin and more representative of niche effects [25–27].
Inflammation
Macrophages can contribute to asthmatic inflammation in several ways (Fig. 1). These include altered (anti)inflammatory cytokine/chemokine production, induction of the inflammasome, and altered cellular processes such as defective phagocytosis. More M1 and M2 macrophages were found in lung tissue of patients with asthma but whether having M1 or M2 macrophages is related to different phenotypes of asthma has not been investigated yet [2,10]. Interestingly, inhibiting either M1 or M2 polarization switched airway inflammation to more eosinophilic or neutrophilic inflammation respectively in mice [6,7▪,28], suggesting that M1-polarized macrophages lead to selective recruitment of neutrophils whereas M2-polarized macrophages recruit eosinophils. We are not aware of any studies investigating which macrophage-related chemokines may be responsible for this switch, but investigating this would be of major interest for understanding the development of different asthma phenotypes.
FIGURE 1.
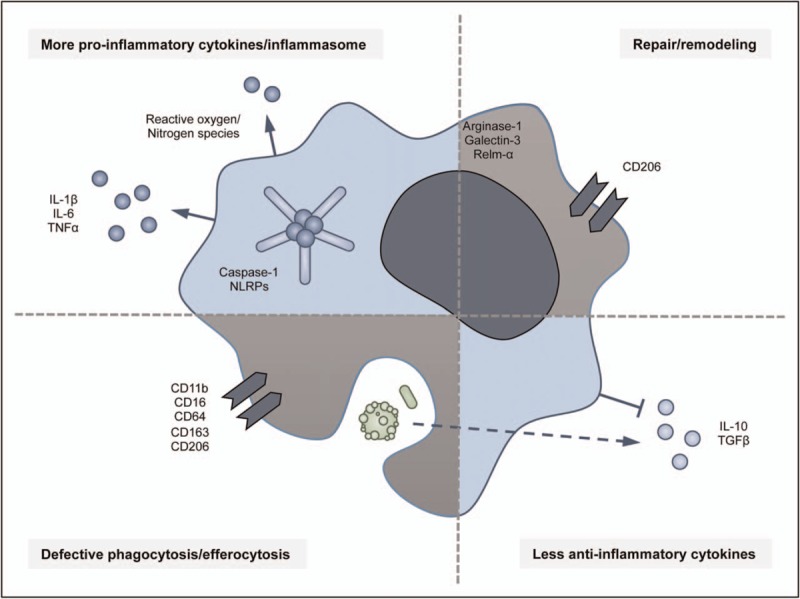
Summary of the contribution of macrophage functions to inflammation in asthma. Macrophages can contribute to asthma by inducing proinflammatory [29,30,31,34,57] and inhibiting anti-inflammatory cytokines [2,6,42], the inflammasome activation [32▪,33,34], changes in repair and remodeling [58–60], and defective phagocytosis and efferocytosis [14,15,16,22,23].
The changes in macrophage polarization found in asthma and its different phenotypes are indicative of changes in cytokine production by these cells. Macrophages contribute to chronic airway inflammation in asthma through production of proinflammatory cytokines such as TNFα and IL-1β. These cytokines are implicated in many types of asthma including neutrophilic, glucocorticoid-resistant [29,30], and allergen-induced atopic asthma [31].
Inflammasomes are a group of multiprotein intracellular complexes, which detect pathogenic microorganisms and sterile stressors and activate the production of pro-inflammatory cytokines like IL-1β [32▪,33]. Activation of inflammasomes in macrophages starts with endogenous or microbial stimuli that activate nucleotide-binding oligomerization domain-like receptors (NLRPs), of which many different types exist. These subsequently assemble, bind, and activate caspase-1 and active caspase-1 proteolytically cleaves pro-IL-1β to its active inflammatory form [34].
These inflammasomes and their product IL-1β are particularly interesting in the context of asthma. The NLRP3 inflammasome for instance has been associated with the development of asthma [33] and single-nucleotide polymorphisms in the NLRP1 gene were shown to activate the inflammasome in reaction to asthma-related stressors in a cohort of Brazilian children [32▪]. In addition, NLRPs that are mainly expressed in macrophages and neutrophils, are upregulated in neutrophilic asthma [32▪,34]. In fact, neutrophils were demonstrated to drive a proinflammatory phenotype in macrophages upon respiratory viral infection in mice by activating the NLRP3 inflammasome in alveolar macrophages [35]. Levels of NLRP3 have also been shown to correlate with asthma severity and steroid resistance [30,34,36▪▪].
Possible mechanisms for the higher levels of NLRP3 activation in asthma are among others apolipoprotein E, Charcot-Leyden crystals, and uric acid, all well known activators of the inflammasome [37,38]. Gordon et al.[39▪] showed that apolipoprotein E secretion by human macrophages from bronchoalveolar lavage fluid is higher after house dust mite exposure and may therefore be responsible for inflammasome activation. Charcot-Leyden crystals, that form spontaneously from galectin-10 proteins upon eosinophil lysis, were found to induce release of IL-1β in human monocyte-derived macrophages in an NLRP3-dependent manner and may therefore perpetuate and exacerbate eosinophilic airway inflammation [40▪]. Uric acid is abundantly produced by human airway epithelial cells when challenged with house dust mite [41] and can subsequently activate the inflammasome in macrophages. Taken together, a role for inflammasome activation in macrophages in asthmatic airway inflammation is indicated and may be an interesting target for therapy.
With respect to anti-inflammatory behavior, fewer macrophages expressing the anti-inflammatory cytokine IL-10 were found in lungs of patients with asthma compared to controls [2]. The loss of IL-10+ macrophages may contribute to the pathogenesis of asthma, as IL-10+ macrophages were shown to prevent neutrophilic asthma in mice [42]. Indeed, we recently showed that boosting the number of IL-10+ macrophages, by pretreating them with PGE2 before adoptive transfer, resulted in less airway inflammation in mice. In summary, stimulating IL-10 production or resolving behavior is currently unexplored avenue for novel asthma treatments [6].
Tissue remodeling
Airway remodeling is an important feature of asthma, characterized by structural airway abnormalities as a result of collagen deposition, elastic fiber remodeling, and goblet cell hyperplasia and contributing to mucus hypersecretion and airway hyperreactivity [43]. In mice, macrophages have been shown to play a role in repair and remodeling by detecting and eliminating damaged tissue and thereby inducing repair by regulating extracellular matrix production and by production of growth factors, matrix metalloproteinases, and their inhibitors.
However, there is little direct evidence of macrophages contributing to remodeling in patients with asthma. Several studies, including our own, investigated repair-associated macrophages (CD206+ macrophages) in airway wall biopsies or bronchoalveolar lavage fluid of asthma patients and controls [2,3,8,44]. We and others found that having more CD206+ macrophages was associated with more severe disease [2,3,44]. Although CD206+ macrophages are associated with remodeling, CD206 can affect collagen internalization and turnover in vitro[45,46], we did not find correlations between these macrophages and basement membrane thickness, goblet cell numbers, or collagen deposition in lungs of patients with asthma [2]. However, inhibition of M2 polarization in mice resulted in less collagen deposition around airways and more severe airway hyperresponsiveness, indicating collagen deposition may actually be a protective mechanism against airway hyperresponsiveness [7▪]. In conclusion, CD206+ M2 macrophages are associated with asthma but solid evidence of their exact role in remodeling in humans is lacking.
NEW THERAPEUTIC STRATEGIES TARGETING MACROPHAGES IN ASTHMA
Both novel and conventional asthma treatments affecting macrophage function have been extensively summarized by Fricker et al.[13] and include corticosteroids, macrolides, statins, phosphodiesterase inhibitors, leukotriene antagonists, and β-agonists. Here we have focused on the most recent findings in order to keep the reader up to date on the latest insights. However, most of the work has been done with in vitro or animal models, leaving their translation to the clinic for future research.
Targeting phagocytosis and efferocytosis
Phagocytosis and efferocytosis by alveolar macrophages is essential for lung homeostasis. Felton et al.[47▪▪] recently showed that alveolar macrophage efferocytosis contributed to the resolution of allergic airway inflammation in mice, thereby making apoptotic cell clearance a potential therapeutic strategy. Since this was found to be dependent on Mer tyrosine kinase, augmentation of Mer activity may provide a specific mechanism to target efferocytosis. Likewise, angiotensin-(1–7) was able to promote macrophage efferocytosis of apoptotic cells in an experimental mouse model of asthma [48]. This was associated with reduced eosinophil counts, an increase in apoptotic eosinophils, decreased nuclear factor-κB phosphorylation, and decreased airway remodeling and collagen deposition and therefore resulted in resolution of inflammation and restoration of homeostasis. Stimulation of efferocytosis could also be achieved by treatment with galectin-3 [21,24▪]. In humans, efferocytosis by monocyte-derived macrophages was found to be higher in patients with asthma upon treatment of the cells with galectin-3. Interestingly however, when stratifying for asthma phenotypes, this effect was only seen in noneosinophilic asthmatics and not in eosinophilic asthmatics. In addition, galectin-3 treatment did not result in increased efferocytosis in healthy controls.
Similarly, inhalation of hydrogen gas improved alveolar macrophage phagocytosis of Escherichia coli in ovalbumin-induced asthmatic mice and alleviated airway hyperresponsiveness and inflammation, possibly because of its antioxidant effects and via activation of nuclear factor erythroid 2-related factor two (Nrf2) [49].
Modulating inflammation
Reduction of inflammation is an important goal in the treatment of asthma. For example, corticosteroids have anti-inflammatory effects on macrophages by suppressing pro-inflammatory cytokines like IL-1β and enhancing IL-10 production [2,13]. Recently, a few more studies have described new therapeutic strategies to dampen inflammatory effects of macrophages in asthma. These include protostemonine and emodin, which were found to attenuate dust mite-induced, ragweed-induced, and aspergillus-induced asthmatic inflammation in a murine model and inhibit alternatively activated macrophage polarization [50,51]. Furthermore, the muscarinic M3 receptor antagonist tiotropium was shown to alleviate allergic airway inflammation through inhibition of resistin-like molecule-α and arginase-1 expressing (M2) macrophages in mice, further showing the importance of M2-polarized macrophages in asthma pathology [52]. Likewise, treatment of RAW 264.7 macrophages with the anti-inflammatory drug brevenal resulted in less expression of CD86 (M1-associated) and CD206 (M2-associated), suggesting a less active state, which resulted in less secretion of proinflammatory cytokines [53]. Other work in lung macrophages from mice indicated that pharmacological inhibition of sirtuin-2 not only resulted in less recruitment of inflammatory cells, but also led to less expression of chitinase-like protein three (YM1) and resistin-like molecule alpha, both markers associated with M2 polarization [54].
Another promising strategy for asthma treatment is targeting the NLRP3 inflammasome. Inhibition of the NLRP3 inflammasome by the cytokine release inhibitory drug CRID3 prevented airway hyperresponsiveness and inflammation in a mouse model of severe asthma, which was accompanied by lower expression of IL-1β, Th2 cytokines, and chemokines associated with eosinophils, neutrophils, and macrophages [36▪▪]. Other compounds found to inhibit NLRP3 include estrogen and some traditional herbs. Estrogen was found to suppress allergen-induced airway inflammation in mice by inhibiting NLRP3 transcription and activation [55] and traditional herbal recipes used for asthma treatment in Rwanda have recently been found to have inhibitory effects on the interactions between NLRP3 and caspase-1 [56].
CONCLUSION
This summary of recent literature shows that the altered function of macrophages found in asthma may contribute to disease pathology. Current therapies, primarily directed against inflammation, may also target macrophages and modulate macrophage function. New therapeutic strategies aiming at rescuing certain macrophage functions (e.g. efferocytosis) indeed coincide with reduced airway inflammation, although most of these studies still require translation to the clinic. Future studies are needed to pinpoint the actual consequences of macrophage (dys)function and macrophage-directed treatment in human asthma.
Acknowledgements
None.
Financial support and sponsorship
The authors gratefully acknowledge financial support by the Lung Foundation Netherlands through Consortium Grant 4.1.15.002.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
T. Anienke van der Veen and Linsey E.S. de Groot, contributed equally to this work.
REFERENCES
- 1.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012; 18:716–725. [DOI] [PubMed] [Google Scholar]
- 2.Draijer C, Boorsma CE, Robbe P, et al. Human asthma is characterized by more IRF5+ M1 and CD206+ M2 macrophages and less IL-10+ M2-like macrophages around airways compared with healthy airways. J Allergy Clin Immunol 2017; 140:280–283.e283. [DOI] [PubMed] [Google Scholar]
- 3.Melgert BN, ten Hacken NH, Rutgers B, et al. More alternative activation of macrophages in lungs of asthmatic patients. J Allergy Clin Immunol 2011; 127:831–833. [DOI] [PubMed] [Google Scholar]
- 4.Mills CD, Kincaid K, Alt JM, et al. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000; 164:6166–6173. [DOI] [PubMed] [Google Scholar]
- 5.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draijer C, Boorsma CE, Reker-Smit C, et al. PGE2-treated macrophages inhibit development of allergic lung inflammation in mice. J Leukoc Biol 2016; 100:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪.Draijer C, Robbe P, Boorsma CE, et al. Dual role of YM1+ M2 macrophages in allergic lung inflammation. Sci Rep 2018; 8:5105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that macrophage polarization determines asthma phenotype.
- 8.Girodet PO, Nguyen D, Mancini JD, et al. Alternative macrophage activation is increased in asthma. Am J Respir Cell Mol Biol 2016; 55:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melgert BN, Oriss TB, Qi Z, et al. Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol 2010; 42:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Chang Y, Bae B, et al. Innate immune crosstalk in asthmatic airways: innate lymphoid cells coordinate polarization of lung macrophages. J Allergy Clin Immunol 2019; 143:1769–1782.e1711. [DOI] [PubMed] [Google Scholar]
- 11.Demarche S, Schleich F, Henket M, et al. Detailed analysis of sputum and systemic inflammation in asthma phenotypes: are paucigranulocytic asthmatics really non-inflammatory? BMC Pulm Med 2016; 16:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busse PJ, Birmingham JM, Calatroni A, et al. Effect of aging on sputum inflammation and asthma control. J Allergy Clin Immunol 2017; 139:1808–1818.e1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fricker M, Gibson PG. Macrophage dysfunction in the pathogenesis and treatment of asthma. Eur Respir J 2017; 50: pii: 1700196. [DOI] [PubMed] [Google Scholar]
- 14.Grunwell JR, Stephenson ST, Tirouvanziam R, et al. Children with neutrophil-predominant severe asthma have proinflammatory neutrophils with enhanced survival and impaired clearance. J Allergy Clin Immunol Pract 2019; 7:516–525.e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexis NE, Soukup J, Nierkens S, et al. Association between airway hyperreactivity and bronchial macrophage dysfunction in individuals with mild asthma. Am J Physiol Lung Cell Mol Physiol 2001; 280:L369–375. [DOI] [PubMed] [Google Scholar]
- 16.Lay JC, Alexis NE, Zeman KL, et al. In vivo uptake of inhaled particles by airway phagocytes is enhanced in patients with mild asthma compared with normal volunteers. Thorax 2009; 64:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪.Kulkarni N, Kantar A, Costella S, et al. Macrophage phagocytosis and allergen avoidance in children with asthma. Front Pediatr 2018; 6:206. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that allergen avoidance results in reduced phagocytosis by airway macrophages from asthmatic children.
- 18.Tokunaga Y, Imaoka H, Kaku Y, et al. The significance of CD163-expressing macrophages in asthma. Ann Allergy Asthma Immunol 2019; 123:263–270. [DOI] [PubMed] [Google Scholar]
- 19.Zhi Y, Gao P, Li W, et al. Soluble CD163 levels and CD163+CD14+ monocyte/macrophage counts in patients with Asthma. Iran J Immunol 2018; 15:239–245. [DOI] [PubMed] [Google Scholar]
- 20.Dai C, Yao X, Gordon EM, et al. A CCL24-dependent pathway augments eosinophilic airway inflammation in house dust mite-challenged Cd163(-/-) mice. Mucosal Immunol 2016; 9:702–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zizzo G, Hilliard BA, Monestier M, et al. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol 2012; 189:3508–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huynh ML, Malcolm KC, Kotaru C, et al. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med 2005; 172:972–979. [DOI] [PubMed] [Google Scholar]
- 23.Simpson JL, Gibson PG, Yang IA, et al. Impaired macrophage phagocytosis in noneosinophilic asthma. Clin Exp Allergy 2013; 43:29–35. [DOI] [PubMed] [Google Scholar]
- 24▪.Erriah M, Pabreja K, Fricker M, et al. Galectin-3 enhances monocyte-derived macrophage efferocytosis of apoptotic granulocytes in asthma. Respir Res 2019; 20:1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that allergen avoidance results in reduced phagocytosis by airway macrophages from asthmatic children.
- 25.Guilliams M, De Kleer I, Henri S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 2013; 210:1977–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013; 38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013; 38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oriss TB, Raundhal M, Morse C, et al. IRF5 distinguishes severe asthma in humans and drives Th1 phenotype and airway hyperreactivity in mice. JCI Insight 2017; 2: pii: 91019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol 2008; 122:550–559.e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim RY, Pinkerton JW, Essilfie AT, et al. Role for NLRP3 inflammasome-mediated, IL-1beta-dependent responses in severe, steroid-resistant asthma. Am J Respir Crit Care Med 2017; 196:283–297. [DOI] [PubMed] [Google Scholar]
- 31.Gosset P, Tsicopoulos A, Wallaert B, et al. Increased secretion of tumor necrosis factor alpha and interleukin-6 by alveolar macrophages consecutive to the development of the late asthmatic reaction. J Allergy Clin Immunol 1991; 88:561–571. [DOI] [PubMed] [Google Scholar]
- 32▪.Leal VNC, Genov IR, Mallozi MC, et al. Polymorphisms in inflammasome genes and risk of asthma in Brazilian children. Mol Immunol 2018; 93:64–67. [DOI] [PubMed] [Google Scholar]; Shows that polymorphisms in inflammasome genes contribute to asthma development.
- 33.Liu Y, Gao X, Miao Y, et al. NLRP3 regulates macrophage M2 polarization through up-regulation of IL-4 in asthma. Biochem J 2018; 475:1995–2008. [DOI] [PubMed] [Google Scholar]
- 34.Simpson JL, Phipps S, Baines KJ, et al. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J 2014; 43:1067–1076. [DOI] [PubMed] [Google Scholar]
- 35.Peiro T, Patel DF, Akthar S, et al. Neutrophils drive alveolar macrophage IL-1beta release during respiratory viral infection. Thorax 2018; 73:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪▪.Rossios C, Pavlidis S, Hoda U, et al. Sputum transcriptomics reveal upregulation of IL-1 receptor family members in patients with severe asthma. J Allergy Clin Immunol 2018; 141:560–570. [DOI] [PubMed] [Google Scholar]; Shows that inflammasome activity is associated with severe asthma.
- 37.Braga TT, Forni MF, Correa-Costa M, et al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep 2017; 7:39884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kool M, Soullie T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med 2008; 205:869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪.Gordon EM, Yao X, Xu H, et al. Apolipoprotein E is a concentration-dependent pulmonary danger signal that activates the NLRP3 inflammasome and IL-1beta secretion by bronchoalveolar fluid macrophages from asthmatic subjects. J Allergy Clin Immunol 2019; 144:426–441.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that apolipoprotein E activates the inflammasome in alveolar macrophages from patients with asthma.
- 40▪.Rodriguez-Alcazar JF, Ataide MA, Engels G, et al. Charcot-Leyden crystals activate the NLRP3 inflammasome and cause IL-1beta inflammation in human macrophages. J Immunol 2019; 202:550–558. [DOI] [PubMed] [Google Scholar]; Shows that Charcot-Leyden crystals activate the inflammasome and thereby contribute to airway inflammation.
- 41.Huff RD, Hsu AC, Nichol KS, et al. Regulation of xanthine dehydrogensase gene expression and uric acid production in human airway epithelial cells. PLoS One 2017; 12:e0184260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawano H, Kayama H, Nakama T, et al. IL-10-producing lung interstitial macrophages prevent neutrophilic asthma. Int Immunol 2016; 28:489–501. [DOI] [PubMed] [Google Scholar]
- 43.Ito JT, Lourenco JD, Righetti RF, et al. Extracellular matrix component remodeling in respiratory diseases: what has been found in clinical and experimental studies? Cells 2019; 8:pii: E342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Zhu J, Zhang L, et al. Role of C/EBP homologous protein and endoplasmic reticulum stress in asthma exacerbation by regulating the IL-4/signal transducer and activator of transcription 6/transcription factor EC/IL-4 receptor alpha positive feedback loop in M2 macrophages. J Allergy Clin Immunol 2017; 140:1550–1561.e1558. [DOI] [PubMed] [Google Scholar]
- 45.Madsen DH, Leonard D, Masedunskas A, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol 2013; 202:951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Pomares L, Wienke D, Stillion R, et al. Carbohydrate-independent recognition of collagens by the macrophage mannose receptor. Eur J Immunol 2006; 36:1074–1082. [DOI] [PubMed] [Google Scholar]
- 47▪▪.Felton JM, Lucas CD, Dorward DA, et al. Mer-mediated eosinophil efferocytosis regulates resolution of allergic airway inflammation. J Allergy Clin Immunol 2018; 142:1884–1893.e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that Mer mediates efferocytosis by alveolar macrophages and contributes to the resolution of inflammation in mice.
- 48.Magalhaes GS, Barroso LC, Reis AC, et al. Angiotensin-(1-7) promotes resolution of eosinophilic inflammation in an experimental model of asthma. Front Immunol 2018; 9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang P, Wei S, Huang W, et al. Hydrogen gas inhalation enhances alveolar macrophage phagocytosis in an ovalbumin-induced asthma model. Int Immunopharmacol 2019; 74:105646. [DOI] [PubMed] [Google Scholar]
- 50.Song Y, Wu Y, Li X, et al. Protostemonine attenuates alternatively activated macrophage and DRA-induced asthmatic inflammation. Biochem Pharmacol 2018; 155:198–206. [DOI] [PubMed] [Google Scholar]
- 51.Song YD, Li XZ, Wu YX, et al. Emodin alleviates alternatively activated macrophage and asthmatic airway inflammation in a murine asthma model. Acta Pharmacol Sin 2018; 39:1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohta S, Jinno M, Tanaka A, et al. The effect of muscarinic M3 receptor blockage in development of M2 macrophages in allergic inflammation. J Allergy Clin Immunol 2019; 143:AB293. [DOI] [PubMed] [Google Scholar]
- 53.Keeler DM, Grandal MK, McCall JR. Brevenal, a marine natural product, is anti-inflammatory and an immunomodulator of macrophage and lung epithelial cells. Mar Drugs 2019; 17: pii: E184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee YG, Reader BF, Herman D, et al. Sirtuin 2 enhances allergic asthmatic inflammation. JCI Insight 2019; 4: pii: 124710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng C, Wu H, Wang M, et al. Estrogen ameliorates allergic airway inflammation by regulating activation of NLRP3 in mice. Biosci Rep 2019; 39: pii: BSR20181117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomani JCD, Gainkam LOT, Nshutiyayesu S, et al. An ethnobotanical survey and inhibitory effects on NLRP3 inflammasomes/Caspase-1 of herbal recipes’ extracts traditionally used in Rwanda for asthma treatment. J Ethnopharmacol 2018; 227:29–40. [DOI] [PubMed] [Google Scholar]
- 57.Baines KJ, Simpson JL, Wood LG, et al. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J Allergy Clin Immunol 2011; 127:153–160, 160.e151-159. [DOI] [PubMed] [Google Scholar]
- 58.Ge XN, Bahaie NS, Kang BN, et al. Allergen-induced airway remodeling is impaired in galectin-3-deficient mice. J Immunol 2010; 185:1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nair MG, Du Y, Perrigoue JG, et al. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med 2009; 206:937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puttur F, Gregory LG, Lloyd CM. Airway macrophages as the guardians of tissue repair in the lung. Immunol Cell Biol 2019; 97:246–257. [DOI] [PubMed] [Google Scholar]


