Objectives:
Despite the interest in scientific community, there is still poor evidence about pulsed radiofrequency (PRF) efficacy in the treatment of neuropathic pain. In order to determine whether high-voltage PRF and epidural adhesiolysis (PRF-EA) showed better results than epidural adhesiolysis alone (EA), a randomized, double-blind, comparative-effectiveness study was conducted in patients with chronic lumbosacral radiating pain and neuropathic features.
Materials and Methods:
A total of 41 patients were randomly allocated to 2 groups. Twenty-one patients were randomized to receive 2 cycles of 240 seconds high-voltage PRF followed by the injection of local anesthetics, hyaluronidase, and betamethasone, whereas 20 patients underwent sham stimulation followed by adhesiolysis. The treatment was delivered at the affected lumbosacral roots and patients, treating physicians and assessors were blinded to intervention.
Results:
A significant reduction of radiating pain was observed in mean Numeric Rating Scale score at follow-up. A change of −3.43 versus −1.75 (P=0.031) after 1 month and −3.34 versus −0.80 (P=0.005) after 6 months was reported in patients undergoing PRF-EA in comparison with EA, respectively. After 1 month, 57% of patients in the PRF-EA group experienced a pain reduction of ≥50% versus only 25% of patients allocated to EA (P=0.037). Improvement decreased to 48% in the PRF-EA group whereas only 10% of EA reported significant pain relief after 6 months (P=0.008).
Discussion:
High-voltage PRF of dorsal root ganglion delivered through multifunctional electrode provided significant pain relief and may be considered a valuable treatment in chronic lumbosacral radicular pain with neuropathic features.
Key Words: PRF, adhesiolysis, radiculopathy, neuropathic pain, NRS
Radiofrequencies are high-frequency electromagnetic waves that have been in use as a therapeutic tool since 1974.1 Pulsed radiofrequency (PRF) was developed as a non-neurodestructive or minimally neurodestructive technique alternative to continuous radiofrequency and used for the treatment of pain disorders.2 PRF currents may be delivered through a needle or a multifunctional electrocatheter with exposed tip whose temperature range is maintained between 40 and 42°C during the whole procedure, preventing massive cell destruction. The latter technique theoretically owns several advantages such as a closer stimulation of the dorsal root ganglion (DRG) and the chance to infuse medications into the epidural space.3–5 Although a vast amount of literature on needle-mediated PRF has been published, few randomized controlled studies with small populations and heterogenous painful conditions are available. Therefore, conclusions about PRF effectiveness are still controversial and guidelines about disorders that might benefit from the procedure are still lacking.
In the last 2 decades, the increased life expectancy has been related to higher incidence of aging-associated diseases such as low back pain (LBP) with or without radicular involvement. Individuals with low back and radiating leg pain may develop neuropathic symptoms with unsatisfactory response to medications and progression to chronic pain over the years.6 Neuropathic pain (NP) was defined by the International Association for the Study of Pain (IASP) in 1994 and revised in 2011, defined as a pain caused by a lesion or disease of the somatosensory nervous system. Recent studies reported the prevalence of probable NP, shifting from 6.5% to 11.8% in Europe and from 10% up to 15.7% in the United States.7 Among the available drugs for LBP, opioids prescription has significantly risen in the last years with several social and health concerns. The grow of opioid-related deaths has forced the scientific community to speculate about costs versus benefits and to acknowledge analgesic abuse as a major public health problem.8 Therefore, the discovery of effective and safe treatments may represent a crucial step forward either to improve quality of life and minimize risks in the management of patients with NP.
The aim of this study was to evaluate the effectiveness of electrocatheter-mediated high-voltage PRF in the treatment of lumbosacral radicular pain with neuropathic features in a double-blind, comparative effectiveness study.
MATERIALS AND METHODS
The protocol was approved by the Institutional Review Board from Santa Maria Maddalena Hospital and the local ethical committee. The procedure and its potential harms were fully explained to the patients and informed consent was obtained before enrollment. All the examinations and treatments have been performed in the Pain Medicine Unit of Santa Maria Maddalena Hospital, Occhiobello (RO), Italy.
Patients and Enrollment
A total of 183 patients were consecutively evaluated between September 2015 and November 2018. We recruited adults older than or equal to 18 years with the following features: (1) single leg-radiating pain (with or without LBP) confirmed by clinical examination and lasting for >6 month, (2) unresponsiveness to oral medications, physical therapy, or ultrasound-guided caudal or periradicular injections, (3) magnetic resonance imaging showing neural compression or spinal canal narrowing and/or electromyographic test suggestive of radiculopathy, and (4) the presence of definite or probable NP. In agreement with Treede and colleagues, neuropathic features were included if patient’s history was suggestive of a relevant lesion or disease affecting the somatosensory system and pain owned a distinct neuroanatomically plausible distribution confirmed by clinical examination and Douleur Neuropathique en 4 (DN4) questionnaire (ie, sensory negative or positive signs). Last, NP was confirmed as definite with a positive result from at least 1 diagnostic test such as MRI or electromyographic test.9–11
We adopted as exclusion criteria: (1) LBP more severe than radiating pain, (2) a possible or unlikely NP, (3) MRI not consistent with clinical symptoms, (4) pain improvement after previous treatments, (5) patients affected by central neurological impairment or peripheral distal neuropathies in the lower limbs, (6) certified psychiatric disorders, (7) radiculopathies with significant motor deficits requiring urgent surgery (eg, cauda equina syndrome), and (8) reported allergy to anesthetics.
The day of intervention, participants were randomized in a 1:1 ratio by computerized system and assigned to high-voltage PRF and epidural adhesiolysis (PRF-EA) or to epidural adhesiolysis alone (EA). Enrollment was performed by an investigator physician. Results of allocation were put in a sealed envelope and delivered to the only nurse with access to the PRF generator and aware of stimulation/sham progress. All the other health care professionals in the operating room were unaware of the treatment assignment. Likewise, clinical data were collected by an independent investigator blinded to randomization and treatment.
Treatments
Each patient received intravenous access and antibiotics administration before the procedure. The treatments were performed in a safe and quiet room with each patient lying on a fluoroscopy table in the prone position. PRF was delivered through a multifunctional Cosman catheter, an x-ray guidable and flexible, temperature-sensing electrode, and provided with an injection port. Its features allow the injection of drugs into epidural space, neural stimulation and safe temperature-controlled PRF treatment. Under local anesthesia, an introducer cannula was inserted through the sacral hiatus. The electrode was introduced through the needle, placed under fluoroscopic guidance into the lumbosacral epidural space and its active tip moved close to the dorsal root ganglion of interest. This area corresponded to the dorsal–cranial quadrant of the intervertebral foramen on lateral fluoroscopic image and midway into the pedicle column on anteroposterior view. The probe was connected to a generator (G4 System Cosman Medical Inc.) that delivered a 2-Hz motor stimulation with an output up to 2 V to rule out any motor nerve damage and a 50-Hz sensory stimulation with an output current <0.6 V. The latter caused a prolonged tingling sensation in the symptomatic areas to confirm the correct catheter position and serving as a sham stimulation in the EA group. If the pain affected more than a single nerve root the electrode was placed at a different segment and the procedure repeated with the same technique. The physician left the operating room before treatment and returned after PRF/sham was done to perform adhesiolysis.
PRF-EA Group
If impedance values were found to be coherent with the epidural space (ie, 200 to 400 Ω), PRF was started. Better results have been reported in previous studies if PRF was delivered by multiple cycles.12,13 Therefore, each patient in this group received PRF for 2 cycles of 240 seconds each at a frequency of 2-Hz (20 ms of current and 480 ms without stimulation resulting in 2 active phases/second), voltage between 65 and 80 V, and a tip temperature of 42°C. After 5 minutes, a 2 mL injection containing 0.25% of bupivacaine was delivered and, if no motor impairment occurred in the next 15 minutes, followed by the administration of 3 mL of the contrast medium iopamidol to observe the myelogram spreading to the nerve root of interest. The adhesiolysis was performed with the injection of hyaluronidase 900 units and 8 mg of betamethasone with a total volume of 5 mL (Fig. 1). If no major adverse event occurred the patient was sent to the recovery room.
FIGURE 1.
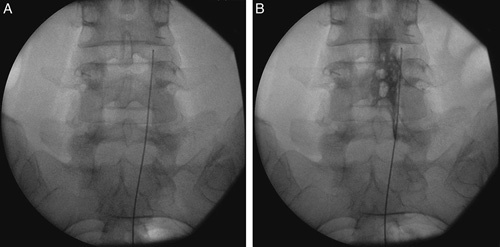
Imaging of multifunctional electrode positioning close to dorsal root ganglion in pulsed radiofrequency (A) and epidural adhesiolysis (B).
EA Group
The patient was told about PRF start and 2 cycles of sham sensory stimulation were done to give a tingling sensation radiating to the painful area. The catheter was placed using the same procedure as the PRF-EA group and sensory stimulation with no active treatment was delivered for 2 cycles of 240 seconds. Participant’s head cover with sterile prep and music in the operating room were used to facilitate blinding. Adhesiolysis was then performed with the same procedure described in the PRF-EA group.
Outcome Measures
Baseline features were recorded the day of the procedure whereas follow-ups were scheduled after 1 and 6 month. In patients with persistent pain, only dose adjustments of baseline medications were permitted after the procedures. Successful response to treatment was calculated at follow-up if pain reduction in Numeric Rating Scale (NRS) was ≥50% compared with baseline. An interview was done by phone after 3 months to confirm the persistence of pain improvement. If a negative outcome was reported, the patient exited the study and “last-observation-carried-forward” method was applied to calculate its subsequent data points.14
Primary outcomes were designated to be the pain intensity assessed with the 0 to 10 NRS and success rate at follow-up. As secondary outcomes, the impact of treatments on disability with the Oswestry Disability Index (ODI)15 and the burden on the quality of life with a reconstructed Italian version of the McGill Pain Questionnaire (QUID) consisting of 42 descriptors divided into 4 main pain rating index ranks (sensory, affective, evaluative, and mixed) whose sum gives the Total Pain Rating Index rank value (PRIr-T).16–18 By administering the DN4 questionnaire,11 it was assessed whether significant changes in neuropathic features occurred between the 2 groups after treatment.
Statistical Analysis
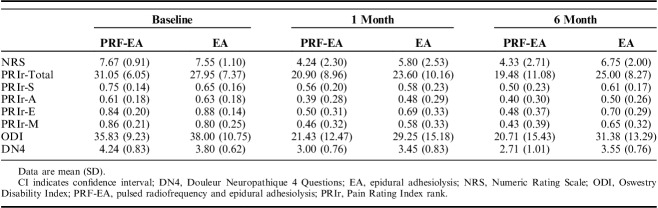
The data were analyzed by an “intention-to-treat” principle. A sample size of 18 patients in each group resulted appropriate to reach an 85% chance of detecting a difference of 2 points between treatments at follow-up. Mean baseline NRS pain score was 7.67 in PRF-EA and 7.55 in EA group with SD of 0.9 and 1.1. After treatment NRS was 4.24 and 4.33 in PRF-EA and 5.80 and 6.75 in EA at 1 and 6 months, respectively.
Descriptive statistics (mean±SD) were reported for NRS, QUID, ODI, and DN4 scores. t test and Mann-Whitney for continuous and χ2 and odds ratios (OR; 95% confidence interval [CI]) for dichotomous variables were used for comparisons between baseline and follow-ups. The Spearman rank correlation test was performed to quantify the association between changes in the pain scores. Simple and logistic regression were used to determine whether different variables might affect the outcome. P-values <0.05 were considered statistically significant.
RESULTS
After preliminary selection, 41 patients were randomized with allocation to PRF-EA or EA group. Baseline features and pain scores were similar except for sex differences in the 2 groups (P=0.043; Table 1). Positive symptoms (eg, paresthesia) in the affected limb were reported by 78% of the patients enrolled. Eight patients experienced temporary postoperative tingling sensation and mild numbness of the treated limb with no sequelae. Symptoms duration ranged between 30 minutes and 2 hours. Only 1 patient reported long-lasting symptoms with tingling of the treated limb for approximately 6 hours. Neither remarkable posttreatment adverse events or complications occurred. The effectiveness of blinding was investigated by asking to each participant to guess the allocation group and no significant difference was recorded between the 2 groups (P=0.647). Summary of patients progression through the study is displayed in Figure 2 and the effect on pain scores is summarized in Table 2.
TABLE 1.
Descriptive Statistics by Treatment Group
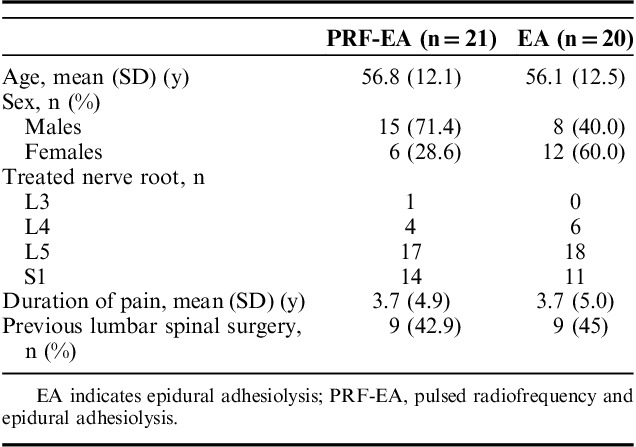
FIGURE 2.
Consort flow chart.
TABLE 2.
Difference in Pain Scores After Treatments
Primary Outcomes
At first follow-up PRF-EA showed better results if compared with EA group (with a change of −3.43 vs. −1.75; P=0.031) whereas benefits from adhesiolysis alone progressively decreased to nonsignificance after 6 months (−3.34 and −0.80; P=0.005; Fig. 3). After 1 month, 57% of the patients in the PRF-EA group experienced a pain reduction of ≥50% versus only 25% of the patients allocated to the EA group (OR, 4.00; 95% CI, 1.057-15.138; P=0.037). At 6 months, only 10% of those who underwent EA reported significant pain relief whereas the percentage was set at 48% in patients who received radiofrequency and adhesiolysis (OR, 8.12; 95% CI, 1.505-44.491; P=0.008).
FIGURE 3.
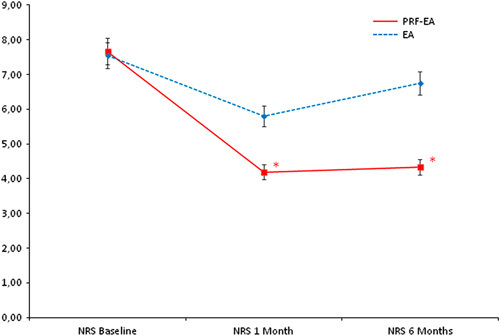
Comparison of mean NRS scores between the 2 groups at baseline, 1, and 6 months. *Statistical significance (P=0.031 and 0.005, respectively). EA indicates epidural adhesiolysis alone; NRS, Numeric Rating Scale; PRF-EA, pulsed radiofrequency and epidural adhesiolysis.
Secondary Outcomes
The Italian Pain Questionnaire showed significant pain improvement in the PRF-EA group at follow-up, but scores were not improved if compared to EA group (−10.15 vs. −4.35; P=0.396 at 1 month and −11.57 vs. −2.95; P=0.147 at 6 months; Fig. 4). ODI score detected higher improvement of disability due to PRF at 6-month follow-up only (−15.12 vs. −6.62; P=0.024), whereas after 1 month differences were not significant (−14.40 vs. −8.75; P=0.224; Fig. 5). If compared with adhesiolysis alone, the combined treatment was able to affect significantly DN4 score either at 1 (−1.24 vs. −0.35, P=0.030) or six months (−1.53 vs. −0.25; P=0.001; Fig. 6). In PRF-EA group a positive correlation was observed between NRS and DN4 scores at follow-ups (ρ=0.694, P=0.010 and ρ=0.686, P=0.010 at 1 and 6 months, respectively; Fig. 7). If factors associated with outcome were analyzed, the treatment success was not remarkably influenced by sex in the 2 groups (OR, 0.545; 95% CI, 0.152-1.955; P=0.352; Nagelkerke R2=0.029). Likewise, DN4 basal values were not correlated with outcome (OR, 1.332; 95% CI, 0.575-3.082; P=0.503; Nagelkerke R2=0.105).
FIGURE 4.
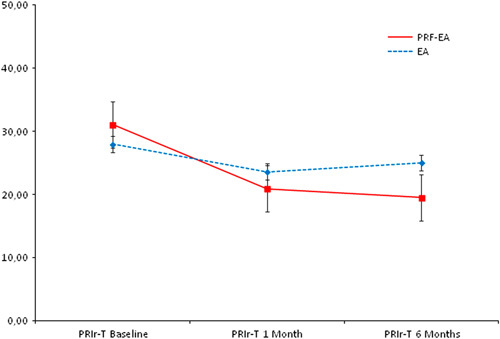
Comparison of mean Total Pain Rating Index rank (PRIr-T) scores between the 2 groups at baseline, 1, and 6 months. No statistical significance observed. EA indicates epidural adhesiolysis alone; PRF-EA, pulsed radiofrequency and epidural adhesiolysis; PRIr-T, Total Pain Rating Index rank.
FIGURE 5.
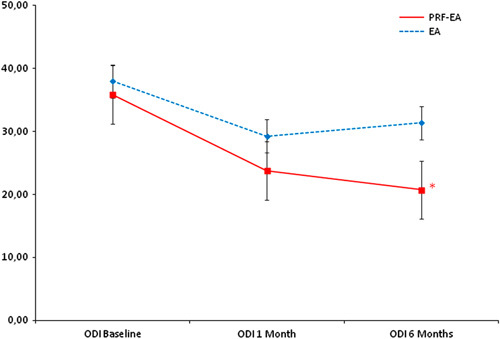
Comparison of mean Oswestry Disability Index (ODI) scores between the two groups at baseline, 1, and 6 months. *Statistical significance (reached at 6 months only, P=0.024). EA indicates epidural adhesiolysis alone; PRF-EA, pulsed radiofrequency and epidural adhesiolysis.
FIGURE 6.
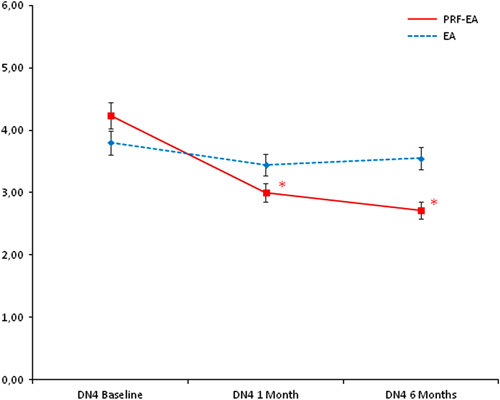
Comparison of mean Douleur Neuropathique en 4 Questions scores between the 2 groups at baseline, 1, and 6 months. *Statistical significance (P=0.030 and 0.001, respectively). DN4 indicates Douleur Neuropathique en 4; EA, epidural adhesiolysis alone; PRF-EA, pulsed radiofrequency and epidural adhesiolysis.
FIGURE 7.
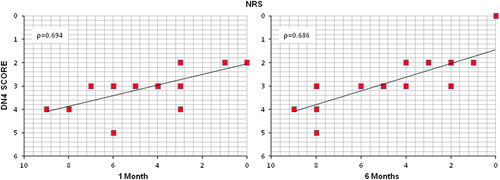
Correlation between Douleur Neuropathique en 4 Questions and Numeric Rating Scale scores in patients undergoing pulsed radiofrequency after 1 and 6 months. (P=0.010). DN4 indicates Douleur Neuropathique en 4.
DISCUSSION
The results of this study highlight the therapeutical impact of adding high-voltage PRF to EA and support its effectiveness in the treatment of lumbosacral NP. Pain relief in the PRF-EA group lasted longer if compared with patients undergoing adhesiolysis alone who experienced a drop off in benefits at 6 months. The pain improvement rates observed in this study were comparable with those reported in previous trials, although the range between studies may differ significantly.19,20
The McGill Pain Questionnaire failed to show a striking difference between PRF and adhesiolysis. Being PRIr-T the sum of indices that focus on affective-emotional and sensory pain perception, this result might be due to patients perception and global satisfaction rather than selective effectiveness on radicular pain.21 The positive impact of high-voltage PRF extended to ODI, but significance between groups was reached after 6 months only. Several randomized trials showed the effectiveness of percutaneous adhesiolysis in the treatment of chronic LBP and failed back surgery syndrome, with stronger evidence for short-term improvement.22,23 Therefore in our study, the lack of significant differences at first follow-up might be subsequent to the improvement of LBP, whereas after 6 month adhesiolysis benefits seem to fade away.16,24
Pain improvement may be either subsequent to PRF greater effectiveness in radiculopathy or to more sustained benefits in comparison with adhesiolysis. Nevertheless, significant differences were observed after 1 month already, pointing toward a more significant efficacy of PRF. The reason for the additive effect of PRF and adhesiolysis might lie in their different targets. Several studies described the role of the immune system in the development of NP, with a maladaptive mechanism inhibiting functional healing and promoting pathologic neuroinflammation and nerve damage. Prolonged nociceptive stimuli may trigger nerve sensitization and ectopic discharge which in turn contribute to long-lasting imbalance and support the development of neuropathic features such as hyperalgesia and allodynia.25–28 Because of their anti-inflammatory and immunosuppressant effects, steroids may antagonize the process leading to NP. Epidural steroids have moderate level of evidence for long-term pain relief in lumbar radiculopathies and in a recent meta-analysis the administration of perineural methylprednisolone or triamcinolone provided beneficial effects in chronic compression-related NP if compared to local anesthetics or conservative treatments. Despite small sample size and heterogenous disorders included in the studies, the reported success rates of 25% are meaningful considering that the patients evaluated did not respond to previous treatments.29,30
The available studies on PRF are mostly needle-mediated with only 1 prospective case series performed with a multifunctional electrode. Heterogenous enrollment criteria, population, devices, and stimulation protocols yielded to nonunivocal results.31–33 Few randomized controlled trials evaluated the effects of PRF on cervical or lumbar dorsal roots, comparing the treatment with transforaminal epidural steroid injection (TFESI) or with sham stimulation. In the latter case, PRF showed a better success rate in the treatment of cervicobrachial pain at 3 months follow-up only whereas small clinical improvements were reported in patients with chronic lumbar radicular pain.34,35 With respect to TFESI, no significant difference was observed between the 2 treatments although pain improved in both groups.36 In a similar way to the present study, TFESI was compared with TFESI + PRF in a different trial. Pain improvement was obtained with both treatments but significant NRS decrease with higher patient satisfaction was reported only in the PRF group after 2 and 3 months.37 The follow-up period never exceeded 6 months in all the trials and reports of PRF safety were satisfactory with mostly minor adverse effects, such as headache or postprocedure pain.
Some features of the present study need to be explained. First, we chose to treat patients with EA rather than TFESI, due to better results reported in people with chronic low back and radicular pain.38 Moreover in comparison with a needle-mediated approach, delivering PRF with a multifunctional flexible electrode allows a closer stimulation of the target and the chance to infuse medications into the epidural space. These features might increase neuromodulation of the dorsal root and improve the outcome without nerve injury, as postulated in previous studies.39 Pulsed radiofrequencies have shown positive effects against allodynia in animal models, therefore NP features might influence the response to treatment and need to be assessed before enrollment.40,41 If compared with conventional PRF, high-voltage stimulation showed better results in several NP models likely due to the generation of greater electric fields. In turn, this might be responsible for plastic changes in pain transmission pathways, accounting for slow onset and sustained effectiveness of high-voltage PRF.42–44
Current studies on lumbosacral pain often involve heterogenous study populations, due to not restrictive selection criteria. Nevertheless, this issue has been a matter of debate and has not yet yield to conclusive enrollment guidelines.45 More homogenous populations might improve diagnosis and therapeutical outcomes in future studies, improving our success rate in challenging disorders such as NP.46 Because PRF neuromodulation was reported to progressively fade away, effectiveness may vary significantly among studies with likely extended pain relief if treatments are repeated over time.47
Few issues and limitations of this study needs to be pointed out. In our opinion, the choice to compare PRF with a different treatment rather than placebo did not impair reliability of results. A small sample size might impair statistical power and lead to misinterpretation of results. Nevertheless, large population studies in analgesic trials have been proven to be challenging and hardly feasible. The sample size in our study provided us sufficiently high power to detect the treatment effects.48,49 The enrolled population featured patients with different pathologies, mostly with axial and radiating pain. If outcomes involved the evaluation of disability or subjective global satisfaction, the co-existence of LBP and radiculopathy might have affected our results, but excluding patients with LBP would extend duration of recruitment for years.50 Moreover, most of patients with chronic radiculopathies in the lower limbs reported axial pain at onset or in different stages of the disorder. Excluding patients with LBP might bias the results of the trial as the association of axial and radiating pain is the most common presentation in clinical practice. A female prevalence for chronic pain of neuropathic origin has been reported in epidemiological studies and can therefore explain sex difference in our study. Moreover, although being a potential risk factor in radicular pain, clinical improvement was not remarkably different between men and women at baseline and follow-up.6,51,52 Last, enrollment started almost 1 year before the revised NP clinical grading and therefore some of our patients were diagnosed with previous guidelines. However, we did not consider this to be a significant issue because differences between the 2 versions are mostly related to the “possible” NP allocation whereas patients enrolled in our study were in the “probable” or “definite” group.
CONCLUSIONS
This is the first randomized controlled trial showing the effectiveness of adding high-voltage PRF to EA in the treatment of NP due to chronic lumbosacral radiculopathy. If confirmed, these results might represent a significant step forward in the treatment of challenging NP syndromes, strengthening the importance of a broad consensus among pain physicians on the choice of devices and stimulation protocols. Therefore, the development of new randomized controlled trials testing long-term effects of PRF are encouraged to evaluate the contribution of the technique in pain management.
ACKNOWLEDGMENTS
The authors thankfully acknowledge Walter Rossi, Patrizia Silvegni, Sara Buzzoni, Mara Desiderati (RN, Santa Maria Maddalena Hospital, Pain Medicine Unit), and Camilla Olivieri (Advanced Algology Research) for their significant assistance in supporting this project.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Uematsu S, Udrarhelyi GB, Benson DW, et al. Percutaneous radiofrequency rhizotomy. Surg Neurol. 1974;2:319–325. [PubMed] [Google Scholar]
- 2.Byrd D, Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008;12:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigneri S, Sindaco G, Gallo G, et al. Effectiveness of pulsed radiofrequency with multifunctional epidural electrode in chronic lumbosacral radicular pain with neuropathic features. Pain Physician. 2014;17:477–486. [PubMed] [Google Scholar]
- 4.Martin DC, Willis ML, Mullinax LA, et al. Pulsed radiofrequency application in the treatment of chronic pain. Pain Pract. 2007;7:31–35. [DOI] [PubMed] [Google Scholar]
- 5.Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications—a review. Acta Neurochir (Wien). 2011;153:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torrance N, Smith BH, Bennett MI, et al. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281–289. [DOI] [PubMed] [Google Scholar]
- 7.DiBonaventura MD, Sadosky A, Concialdi K, et al. The prevalence of probable neuropathic pain in the US: results from a multimodal general-population health survey. J Pain Res. 2017;10:2525–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenblum A, Marsch LA, Joseph H, et al. Opioids and the treatment of chronic pain: controversies, current status and future directions. Exp Clin Psychopharmacol. 2008;16:405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. [DOI] [PubMed] [Google Scholar]
- 10.Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- 12.Huang JH, Galvagno S, Hameed H, et al. Occipital nerve pulsed radiofrequency treatment: a multi-center study evaluating predictors of outcome. Pain Med. 2012;13:489–497. [DOI] [PubMed] [Google Scholar]
- 13.Cohen SP, Peterlin BL, Fulton L, et al. Randomized, double-blind, comparative-effectiveness study comparing pulsed radiofrequency to steroid injections for occipital neuralgia or migraine with occipital nerve tenderness. Pain. 2015;156:2585–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghahreman A, Ferch R, Bogduk N. The efficacy of transforaminal injection of steroids for the treatment of lumbar radicular pain. Pain Med. 2010;11:1149–1168. [DOI] [PubMed] [Google Scholar]
- 15.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25:2940–2952; discussion 2952. [DOI] [PubMed] [Google Scholar]
- 16.De Benedittis G, Massei R, Nobili R, et al. The Italian Pain Questionnaire. Pain. 1988;33:53–62. [DOI] [PubMed] [Google Scholar]
- 17.Haas M, Nyiendo J. Diagnostic utility of the McGill Pain Questionnaire and the Oswestry Disability Questionnaire for classification of low back pain syndromes. J Manipulative Physiol Ther. 1992;15:90–98. [PubMed] [Google Scholar]
- 18.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. [DOI] [PubMed] [Google Scholar]
- 19.Abejón D, Garcia-del-Valle S, Fuentes ML, et al. Pulsed radiofrequency in lumbar radicular pain: clinical effects in various etiological groups. Pain Pract. 2007;7:21–26. [DOI] [PubMed] [Google Scholar]
- 20.Van Boxem K, van Bilsen J, de Meij N, et al. Pulsed radiofrequency treatment adjacent to the lumbar dorsal root ganglion for the management of lumbosacral radicular syndrome: a clinical audit. Pain Med. 2011;12:1322–1330. [DOI] [PubMed] [Google Scholar]
- 21.Wilkie DJ, Savedra MC, Holzemer WL, et al. Use of the McGill Pain Questionnaire to measure pain: a meta-analysis. Nurs Res. 1990;39:36–41. [PubMed] [Google Scholar]
- 22.Manchikanti L, Staats PS, Singh V, et al. Evidence-based practice guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2003;6:3–81. [PubMed] [Google Scholar]
- 23.Racz GB, Heavner JE, Trescot A. Percutaneous lysis of epidural adhesions evidence for safety and efficacy. Pain Pract. 2008;8:277–286. [DOI] [PubMed] [Google Scholar]
- 24.Epter RS, Helm S, II, Hayek SM, et al. Systematic review of percutaneous adhesiolysis and management of chronic low back pain in post lumbar surgery syndrome. Pain Physician. 2009;12:361–378. [PubMed] [Google Scholar]
- 25.Trescot AM, Chopra P, Abdi S, et al. Systematic review of effectiveness and complications of adhesiolysis in the management of chronic spinal pain: an update. Pain Physician. 2007;10:129–146. [PubMed] [Google Scholar]
- 26.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. [DOI] [PubMed] [Google Scholar]
- 27.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–264. [DOI] [PubMed] [Google Scholar]
- 28.Kopach O, Viatchenko-Karpinski V, Belan P, et al. Development of inflammation-induced hyperalgesia and allodynia is associated with the upregulation of extrasynaptic AMPA receptors in tonically firing lamina II dorsal horn neurons. Front Physiol. 2012;3:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sommer C, Leinders M, Üçeyler N. Inflammation in the pathophysiology of neuropathic pain. Pain. 2018;159:595–602. [DOI] [PubMed] [Google Scholar]
- 30.Mailis A, Taenzer P. Evidence-based guideline for neuropathic pain interventional treatments: spinal cord stimulation, intravenous infusions, epidural injections and nerve blocks. Pain Res Manag. 2012;17:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatia A, Flamer D, Shah PS. Perineural steroids for trauma and compression-related peripheral neuropathic pain: a systematic review and meta-analysis. Can J Anaesth. 2015;62:650–662. [DOI] [PubMed] [Google Scholar]
- 32.Teixeira A, Grandinson M, Sluijter ME. Pulsed radiofrequency for radicular pain due to a herniated intervertebral disc – an initial report. Pain Pract. 2005;5:111–115. [DOI] [PubMed] [Google Scholar]
- 33.Simopoulos TT, Kraemer J, Nagda JV, et al. Response to pulsed and continuous radiofrequency lesioning of the dorsal root ganglion and segmental nerves in patients with chronic lumbar radicular pain. Pain Physician. 2008;11:137–144. [PubMed] [Google Scholar]
- 34.Van Zundert J, Patijn J, Kessels A, et al. Pulsed radiofrequency adjacent to the cervical dorsal root ganglion in chronic cervical radicular pain: a double blind sham controlled randomized clinical trial. Pain. 2007;127:173–182. [DOI] [PubMed] [Google Scholar]
- 35.Shanthanna H, Chan P, McChesney J, et al. Pulsed radiofrequency treatment of the lumbar dorsal root ganglion in patients with chronic lumbar radicular pain: a randomized, placebo-controlled pilot study. J Pain Res. 2014;7:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DG, Ahn SH, Lee J. Comparative effectivenesses of pulsed radiofrequency and transforaminal steroid injection for radicular pain due to disc herniation: a prospective randomized trial. J Korean Med Sci. 2016;31:1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh W, Choi SS, Karm MH, et al. Treatment of chronic lumbosacral radicular pain using adjuvant pulsed radiofrequency: a randomized controlled study. Pain Med. 2015;16:432–441. [DOI] [PubMed] [Google Scholar]
- 38.Park Y, Lee WY, Ahn JK, et al. Percutaneous adhesiolysis versus transforaminal epidural steroid injection for the treatment of chronic radicular pain caused by lumbar foraminal spinal stenosis: a retrospective comparative study. Ann Rehabil Med. 2015;39:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Lee SH. Clinical effectiveness of percutaneous adhesiolysis versus transforaminal epidural steroid injection in patients with postlumbar surgery syndrome. Reg Anesth Pain Med. 2014;39:214–218. [DOI] [PubMed] [Google Scholar]
- 40.Pope JE, Deer TR, Kramer J. A systematic review: current and future directions of dorsal root ganglion therapeutics to treat chronic pain. Pain Med. 2013;14:1477–1496. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka N, Yamaga M, Tateyama S, et al. The effect of pulsed radiofrequency current on mechanical allodynia induced with resiniferatoxin in rats. Anesth Analg. 2010;111:784–790. [DOI] [PubMed] [Google Scholar]
- 42.Wan CF, Liu Y, Dong DS, et al. Bipolar high-voltage, long-duration pulsed radiofrequency improves pain relief in postherpetic neuralgia. Pain Physician. 2016;19:E721–E728. [PubMed] [Google Scholar]
- 43.Fang L, Tao W, Jingjing L, et al. Comparison of high-voltage- with standard-voltage pulsed radiofrequency of Gasserian Ganglion in the treatment of idiopathic trigeminal neuralgia. Pain Pract. 2015;15:595–603. [DOI] [PubMed] [Google Scholar]
- 44.Jia Y, Pan Y, Ren H, et al. Effectiveness and safety of high-voltage pulsed radiofrequency to treat patients with primary trigeminal neuralgia: a multicenter, randomized, double-blind, controlled study protocol. Pain Physician. 2018;21:469–481. [PubMed] [Google Scholar]
- 45.Perret DM, Kim DS, Li KW, et al. Application of pulsed radiofrequency currents to rat dorsal root ganglia modulates nerve injury-induced tactile allodynia. Anesth Analg. 2011;113:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shanthanna H. Investigating the effects of pulsed radiofrequency on dorsal root ganglion in chronic lumbar radicular pain patients: is it not important that we ask the right question, the right way, on an appropriate sample of patients? Pain Med. 2016;17:374–375. [DOI] [PubMed] [Google Scholar]
- 47.Jain KK. Current challenges and future prospects in management of neuropathic pain. Expert Rev Neurother. 2008;8:1743–1756. [DOI] [PubMed] [Google Scholar]
- 48.Shi Y, Wu W. Treatment of neuropathic pain using pulsed radiofrequency: a meta-analysis. Pain Physician. 2016;19:429–444. [PubMed] [Google Scholar]
- 49.Koh W, Shin J. Reply to: is it not important that we ask the right question, the right way, on an appropriate sample of patients? Pain Med. 2016;17:375–377. [DOI] [PubMed] [Google Scholar]
- 50.McKeown A, Gewandter JS, McDermott MP, et al. Reporting of sample size calculations in analgesic clinical trials: ACTTION systematic review. J Pain. 2015;16:199–206; e1–e7. [DOI] [PubMed] [Google Scholar]
- 51.Peul WC, Brand R, Thomeer RT, et al. Influence of gender and other prognostic factors on outcome of sciatica. Pain. 2008;138:180–191. [DOI] [PubMed] [Google Scholar]
- 52.Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an internet-based survey. J Pain. 2010;11:1230–1239. [DOI] [PubMed] [Google Scholar]