Abstract
This study investigated the clinical characteristics and somatosensory profiles of patients suffering from leprosy in Mumbai, India. A cross-sectional deep profiling study was conducted in 86 patients with leprosy (with and without pain) using an extensive battery of phenotyping measures including structured clinical examination, psychological state (General Health Questionnaire [GHQ-12]), and a quality-of-life condition-specific instrument (Brief Pain Inventory—short form). Quantitative sensory testing was performed according to the protocol of the German Research Network on Neuropathic Pain (DFNS) to assess the somatosensory profiles in the ulnar nerve innervation territory of all participants (dorsum of the hand). Reference data from 50 healthy Indian subjects were within the range of published DFNS values. Somatosensory profiles in leprosy patients with clinically or electroneurographically diagnosed neuropathy (with and without pain) revealed a profile of sensory loss to thermal and tactile stimuli combined with preservation of vibration and deep pressure detection. Sensory gain phenomena were not generally observed in patients with leprosy. In the group of subclinical neuropathy, a high degree of impaired thermal sensation was found, which could be clinically deployed to enhance identification of leprosy neuropathy at an early stage. Quantitative sensory testing can effectively document leprosy-associated neuropathy but does not distinguish between patients with or without pain. Patients with leprosy and neuropathic pain reported a poor quality of life and less psychological well-being compared with the pain-free patients with leprosy neuropathy.
Keywords: Leprosy, Mycobacterium leprae, Neuropathy, Pain, Neuropathic pain, Quantitative sensory testing, Sensory profile, Depression, Quality of life
1. Introduction
Leprosy is a chronic granulomatous infectious disease caused by Mycobacterium leprae, with more than 200,000 new leprosy cases detected annually, primarily in South Asia and Northern Brazil.75 The disease can induce neuropathy in peripheral nerve trunks, associated with sensory loss in skin lesions. The clinical presentation of leprosy is complex and determined by the host's immune response. Antimicrobial therapy can eliminate M. leprae, but immune-mediated reactions, including neuritis, can continue long after antimicrobial treatment has concluded, and neuropathic consequences might persist: While global access to cost-free mycobacterial-eliminating multidrug therapy has been a public health success, microbiologically cured patients continue suffering from long-term complications of permanent nerve damage including chronic neuropathic pain.23,31 Dealing with the disabling sequelae of leprosy imposes a significant problem, including mental health, and socioeconomic burden on developing countries and is often diagnostically unrecognised by health care practitioners in immigrants to developed countries.6
Leprosy neuropathy occurs before, during, or after multidrug therapy6,33 and affects around 65% of patients.59 Previous studies found 11% to 66%9,22–24,31,34,37,41,48,53 of these individuals to suffer from neuropathic pain,15,27 the wide range of its prevalence likely to be attributed to variations in study design. Neuropathic pain remains a treatment challenge with limited first-line treatment options which are of modest effectiveness and are of limited availability in low-resource settings.14,29
A comprehensive quantitative sensory testing (QST) according to the protocol of the German Research Network on Neuropathic Pain (DFNS) has been used in multiple studies to document sensory profiles in neuropathy associated with pain3,5,20,36,39,40,66 and predict sensory profile–associated treatment outcome,11,12 although it has not been hitherto applied in patients with leprosy. Moreover, a comprehensive QST approach has not been previously validated in any healthy Asian population, where normative values might differ from the existing data which were largely collated from studies conducted in Anglo-American populations in high-resource health care settings. Nevertheless, there are reports of selected sensory modalities being documented using a variety of methods in leprosy.13,34,60,64,65 A recent study, which measured pain symptoms in 169 Brazilian patients using the Neuropathic Pain Symptom Inventory questionnaire, demonstrated symptom heterogeneity of leprosy-associated neuropathic pain.42
In this study, we used the comprehensive and robustly validated QST battery according to the DFNS protocol46 to obtain normative data in a healthy Indian population. We then performed a deep profiling cross-sectional study on patients with leprosy, aiming to capture clinical features, pain, quality of life, and sensory profiles of patients with leprosy neuropathy.
2. Material and methods
The study protocol and recruitment procedures were approved by the following ethics committees in London and Mumbai: London School of Hygiene and Tropical Medicine Research Ethics Committee, ethical approval reference number 6181, 2013; Imperial College Research Ethics Committee (ICREC), ethical approval reference number ICREC_11_2_3, 2011; and Foundation for Medical Research Ethics Committee, IEC No _ FMR/IEC/LEP/04/2012. The study was conducted in adherence to the ethical guidelines of the most recent version of the Declaration of Helsinki.76 Participants gave informed consent.
2.1. Study design and participants
This cross-sectional study was conducted at The Bombay Leprosy Project (BLP) clinic and Foundation for Medical Research (FMR) both located in Mumbai, India. Blood sample analyses were performed by Metropolis Healthcare Ltd, Mumbai, India. There were 2 distinct substudies:
2.1.1. Collection of normative quantitative sensory testing data in local healthy volunteers
These data were to be compared with the DFNS normative values to evaluate whether the normative values for an Indian population were similar to that of the DFNS normative data assessed in a European population. Participants were invited from amongst the patients' relatives attending the leprosy clinic based at BLP and staff from the BLP and the FMR. Health status was established using a standardized questionnaire.19 All participants were asked to provide a urine sample for glucose testing and a blood sample for analysis of complete blood counts, blood glucose, thyroid function, vitamin B12 level, syphilis, and HIV serology, and, for women, a pregnancy test. Participants underwent a sensory assessment using the DFNS QST protocol.46,47 Participants were excluded if they had been diagnosed with, or suspected to potentially have, any neurological disease including different forms of peripheral neuropathy, cutaneous lesions in the tested site, systemic disease, chronic pain, or were taking medication during the time of study. Travel expenses were reimbursed for all volunteers, including those who did not meet the inclusion criteria.
2.1.2. Deep sensory profiling of patients with leprosy
Participants were adults (≥18 years of age) with confirmed leprosy irrespective of whether they were receiving, or had received, multidrug therapy or reported symptoms of a peripheral neuropathy in the ulnar nerve distribution. Exclusion criteria were as follows: a history of concomitant severe infection such as tuberculosis or any other serious underlying disease (cardiac, renal, or hepatic) that potentially might affect the study parameters; a history of other conditions associated with peripheral neuropathy such as previously known diabetes mellitus or nutritional deficiency (thiamine and vitamin B12 deficiency); other neurological or psychiatric disease; a history of regular, excessive intake of alcohol (for more than 10 years); pregnancy or lactation; history of thalidomide treatment; or insufficient level of communication (ie, lack of fluency in any of the 3 languages of the study: English, Hindi, or Marathi).
The study design consisted of a single clinical assessment appointment, where participants had detailed medical history taken by the investigator, recording the following: age, sex, time since leprosy symptoms first developed, the World Health Organization (WHO) and the Ridley–Jopling classification of their disease, history of multidrug therapy, history of previous leprosy-associated reactions, and family history of neuropathy. A detailed history of skin and nerve clinical symptoms and signs was recorded, including the number and morphology of skin lesions, the presence of peripheral oedema, and nerve tenderness. Participants were asked about presence and intensity of pain, pain characteristics, its duration, and pain treatment. All participants were interviewed, completed questionnaires, and underwent a structured neurological examination, followed by a detailed quantitative sensory testing using the DFNS QST protocol.
2.2. Case definitions
2.2.1. Leprosy
Patients had leprosy when they had one of the following signs: hypopigmented or reddish skin lesion with loss of sensation; thickened peripheral nerve to palpation, or presence of acid-fast bacilli in a slit-skin smear.6,73 This case definition includes patients who had yet to complete a full course of multidrug treatment, as well as patients who relapsed after completing a full course of treatment. It also includes mycobacteriologically cured subjects with late reactions.6,73
2.2.2. Subclinical neuropathy
Patients with leprosy as defined above and with no clinical evidence of mechanosensory and/or motor impairment in an area of the hand innervated by 1 or more nerve, but abnormal nerve conduction studies, were categorised as “subclinical neuropathy.” Electrophysiology data for such patients were usually available because they had previously participated in the “Treatment of Early Neuropathy in LEProsy” (TENLEP) study.71
2.2.3. Neuropathy
Patients with leprosy as defined above and clinical evidence of sensory or motor deficits were allocated to the “neuropathy” category: Mechanosensation was assessed using Semmes–Weinstein monofilaments (details below) and motor function using the modified Medical Research Council (MRC) scale.8 Sensory impairment was defined as not being able to perceive the 0.2-g monofilament at 2 points of 3 tested in each innervation territory of the hand. Motor impairment was defined by a decrease in voluntary muscle testing (VMT) score, by 1 point or more from the normal score of 5, using the modified MRC scale.
2.2.4. Painful neuropathy, pain distribution, and interference
Patients with leprosy and neuropathy as defined above and pain in the innervation territory of the neuropathic site were allocated to the “painful neuropathy” group. These patients were asked to mark the area of their pain on a body image template. The pain drawing of each patient was digitized and transformed into a colour-coded heat map of frequent pain areas, in which white areas mark body parts in which no patient felt spontaneous pain, while dark red areas indicate that most patients felt pain. The light-yellow areas represent body parts in which few patients experienced pain; orange and light-red areas represent a medium frequency of pain occurrence. Patients also filled in the short form of the Brief Pain Inventory (BPI), previously reported of in leprosy,23 consisting of 9 items measuring pain intensity and interference. Patients completed the Hindi/Marathi version of BPI questionnaire with the assistance of native language speaker in the presence of the investigator (O.M.O.H.).
2.3. Psychological and comorbidity measures
Psychological factors were assessed with the GHQ-12, which is an established instrument used to screen for the presence of mental distress.21 It has been validated in both in Indian and leprosy settings.4,16,28,49,52,63 Furthermore, the GHQ-12 has been demonstrated to be a valid screening in patients with leprosy neuropathic pain.23,31 The GHQ-12 version, which was validated in Hindi,16 was used with the kind permission of Professor Shiv Gautam, Gautam Institute of Behavioural Sciences and Alternative Medicine, India. It was scored using the Likert method, and the values of positive items were inverted.
2.4. Structured clinical examination
A structured clinical examination was conducted by a single medically qualified investigator (O.M.O.H.). The general physical examination, leprosy-specific parameters, and neurological assessment procedures are described in detail elsewhere.23,58,60 Mechanosensation was assessed using a standard set of coloured Semmes–Weinstein nylon monofilaments (nominal bending forces 0.05, 0.2, 2, 4, 10, and 300 g) (Estesiometro; SORRI-Bauru, Bauru, Brazil). Three sites per nerve in the upper limb were tested, and a threshold of 0.2 g was the normative reference point.59 Motor nerve function was assessed by VMT using the 0 to 5 modified (MRC) scale,8 which has been shown to be reliable in patients suspected of leprosy.45 An abnormal result was taken as a decrease in VMT score by 1 or more points from the normal score of 5 using the modified MRC scale.58,60 Leprosy-related disability was assessed using the WHO disability criteria, which defines grade 0 as no loss of sensation or visible deformity, grade 1 as loss of sensation without visible deformity, and grade 2 as presence of visible deformity.72
2.5. Quantitative sensory testing
The somatosensory profiles of healthy controls and patients with leprosy were measured using the DFNS QST protocol,46 which has been used to profile patients with a number of neuropathic pain conditions and fibromyalgia.3,5,20,25,36,40,43,54,57,66 In short, the protocol consists of 7 tests measuring 13 sensory parameters in the following standardised order: cold detection threshold (CDT), warm detection threshold (WDT), thermal sensory limen (TSL), the presence of paradoxical heat sensations, cold pain threshold, heat pain threshold, mechanical detection threshold (MDT), mechanical pain threshold, a stimulus–response function for mechanical pain sensitivity, dynamic mechanical allodynia, wind-up ratio, vibration detection threshold (VDT), and pressure pain threshold.
Standardized verbal instructions and questions of the DFNS QST protocol were translated into Hindi and Marathi using forward-translation and back-translation methods and WHO guidelines.74 All measures were performed on each subject by the same investigator (O.M.O.H.). Before commencing the study, the investigator (O.M.O.H.) successfully underwent the standard DFNS training and validation process.18 This includes a visit to a German training center and subsequent qualification by providing a data set from 18 healthy volunteers measured at Imperial College London which were compared with DFNS normative values.69
Quantitative sensory testing measurements were performed for all participants in the ulnar nerve innervated dorsum of the hand bilaterally because this has been reported as the most frequent site of leprosy-associated nerve damage.23 Reference data for these test areas are available35; all data were normalized to z-scores by subtracting the appropriate group mean of healthy subjects and dividing by their SD. Z-score sign was adjusted such that positive values indicate gain of function and negative values loss of function. The confidence interval of healthy control participants is represented by a z-score of 0 ± 1.96. In patients with bilateral pain, the most painful area was chosen as the test site. The pressure pain threshold for ulnar nerve innervation territories of the hand was assessed over the hypothenar eminence muscle. Vibration detection threshold was recorded over the bony prominence of the ulnar (styloid process).46,47
2.6. Statistical analysis
Statistical tests applied were paired and unpaired Student t tests; the Mann–Whitney U test; the Kruskal–Wallis test; and Pearson χ2 test. A P-value ≤0.05 was considered significant. Because there are no published reports of DFNS QST profiling in leprosy, the required sample size was calculated using MDT data reported in another case–control QST profiling study of another infection-related neuropathic pain condition, HIV-associated sensory neuropathy.40 This calculation revealed a minimum sample size of 15 was required per group for a power of 90%. In addition, we applied a recently suggested algorithm,5,68 which allocates patients into 1 of 3 sensory pathological phenotypes in addition to a “normal” profile. This method compares the full QST profile of each patient to 4 prototypic profiles that have been heuristically found in a large set of patients suffering from neuropathic pain and sorts each patient to the phenotype their QST profile is most similar to. These phenotypes are mainly characterized by (1) loss of thermal and mechanical detection and frequently paradoxical heat sensation (“sensory loss”), (2) intact sensory function, often combined with thermal hyperalgesia or allodynia (“thermal hyperalgesia”), (3) loss of thermal detection, but not mechanical detection, accompanied by mechanical hyperalgesia or allodynia (“mechanical hyperalgesia”), and (4) a mostly normal sensory profile resembling that of healthy subjects (“healthy sensory profile”).
3. Results
3.1. Healthy control participants
Between September 10, 2012, and April 30, 2013, a total of 56 local Indian volunteers were screened for the study of whom 6 subjects were excluded. Of these, 4 had a low blood vitamin B12 level, 1 was anaemic, and 1 was pregnant. Fifty healthy Indian participants, 28 women and 22 men, with mean age of 30 years (range 18–55 years), were recruited (Fig. 1). The normative data from the local population showed similar distribution with a mean z-score of 0.16 and a SD of 1.06 to those held in the DFNS database and no unexpected deviations in the distribution of abnormal values. We submitted the data to 2 published tests suggested by the DFNS to compare healthy participants with their normative data, showing no systematic deviation.35,69 As the DFNS normative data are more nuanced in being multicentric, age-, and sex-balanced, the DFNS normative data were used to calculate z-values, whereas the local group of healthy subjects were used for statistical comparisons with the leprosy patients (Table 1).
Figure 1.
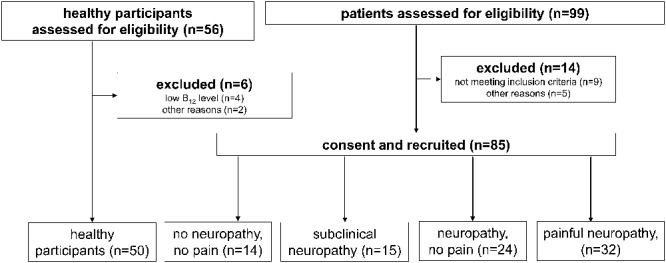
Flowchart of patient inclusion.
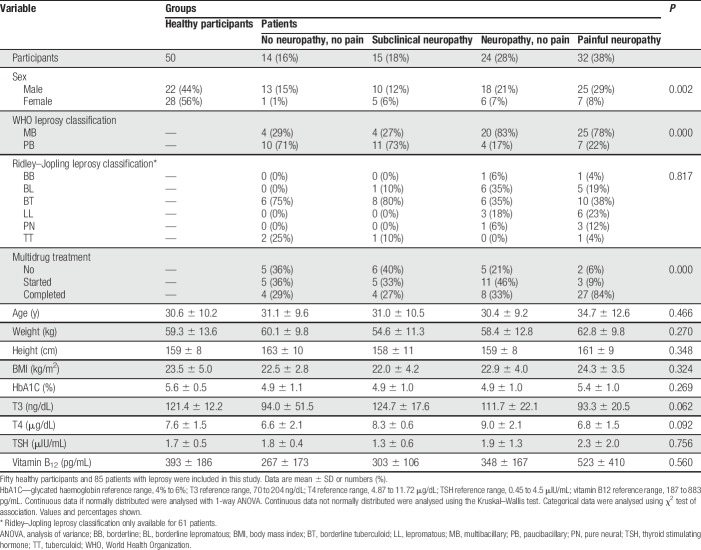
Table 1.
Demographic and clinical characteristics of participants by group.
3.2. Leprosy participants
Ninety-nine patients with leprosy were screened between October 12, 2012, and April 30, 2013, and 14 were either secondary excluded because it was uncovered that they did not fit the inclusion criteria on closer look (n = 9) or other reasons (n = 5); the remainder allocated into the 4 groups namely, no neuropathy, subclinical neuropathy, neuropathy, and painful neuropathy (Fig. 1). The demographic and clinical details of the study population including healthy volunteers are shown in Table 1. The groups were not significantly different with respect to age, weight, height, body mass index, or metabolic factors (thyroid hormone profiles, glucose, and vitamin B12 level). Interestingly, patients with neuropathy or painful neuropathy were significantly more frequently classified as multibacillary, whereas patients with no or subclinical neuropathy more often classified as paucibacillary. Patients with painful neuropathy had significantly more often completed multidrug treatment, indicating that pain is a long-term and late consequence of leprosy.
3.3. Pain and pain interference
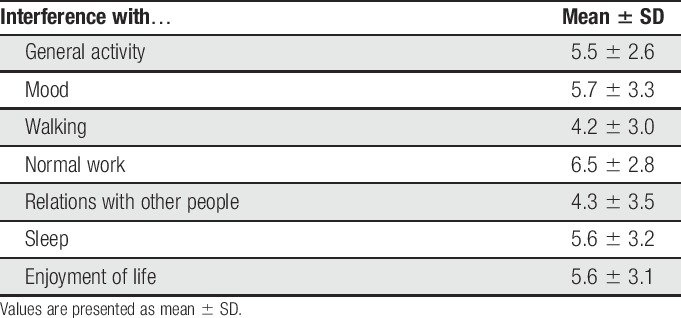
Patients with painful neuropathy (n = 32) mostly reported pain within the innervation territory of the ulnar nerve either bilaterally (n = 19, 59%) or unilaterally (n = 13, 41%). The most frequently reported sites of pain were the palmar aspect of the hand (n = 18, 56%) as the most frequent site of pain followed by the dorsal aspect of the hand (n = 14, 44%) (Fig. 2). The innervation territories of the ulnar nerve, the medial antebrachial cutaneous nerve, and the peroneal nerve were the most frequently associated with pain. Pain drawings in addition suggested painful distally symmetric neuropathy and knee pain. Of the 32 patients with leprosy and pain, 31 (97%) completed the Brief Pain Interference questionnaire. Pain interference levels were in the moderate-to-severe range (mean 4.2–6.5 across the selected daily life aspects). Pain was associated with substantial interference (≥4 on 0—10 scales) on average on all items (Table 2).
Figure 2.
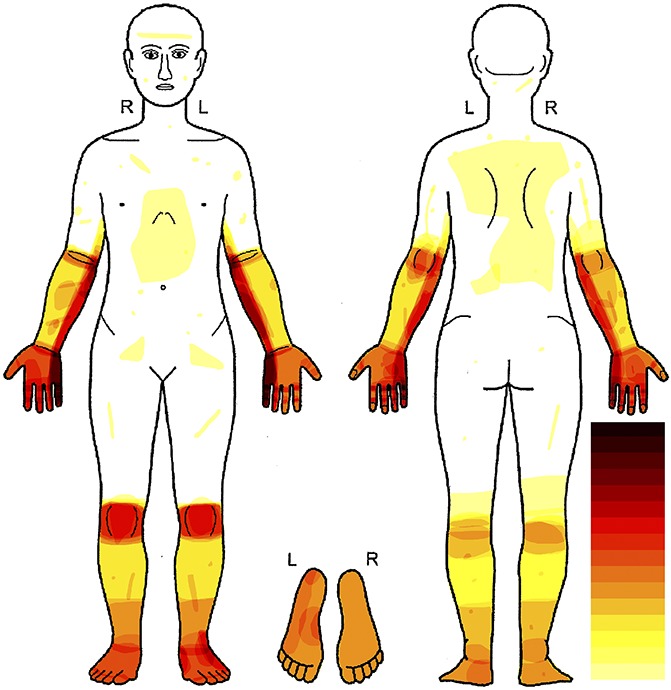
Distribution of pain in patients with leprosy. Body chart showing superimposed pain areas reported by all patients with pain (n = 32). The white areas mark body parts in which no patient felt spontaneous pain. In the dark red areas, most patients felt pain. The light-yellow areas represent body parts in which few patients experienced pain, and orange and light-red areas represent a medium frequency of pain occurrence. Most pain appeared in the ulnar territory, and a second hotspot was the knees of the patients.
Table 2.
BPI results for patients with painful neuropathy.
3.4. Psychological state (GHQ-12)
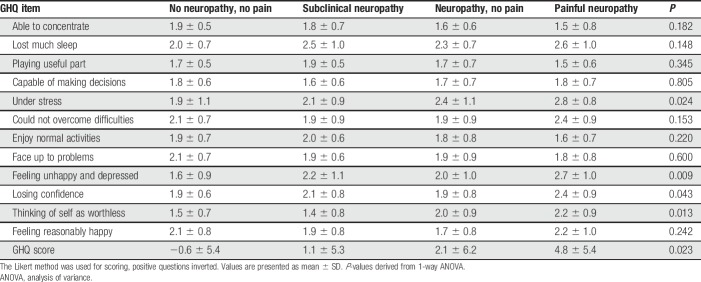
Approximately 85 (98.9%) patients completed the GHQ-12 questionnaires. Of these, 39 (46%) had a GHQ-12 score of 3 or more. The overall mean score for the GHQ-12 of the patients was 2.5 (SD 6.0). Participants with pain scored higher on the depression and anxiety parameters than those without pain. In patients with no or subclinical neuropathy, the proportion of patients scoring 3 or higher using the GHQ-12 was 8 (28%, mean 0.3, SD 4.1), compared with 11 (46%, mean 2.1, SD 6.2) in the painless neuropathy group and 20 (63%, mean 4.8, SD 5.4) in the painful neuropathy group (Table 3).
Table 3.
GHQ-12 results per group.
3.5. Quantitative sensory testing findings in patients with leprosy
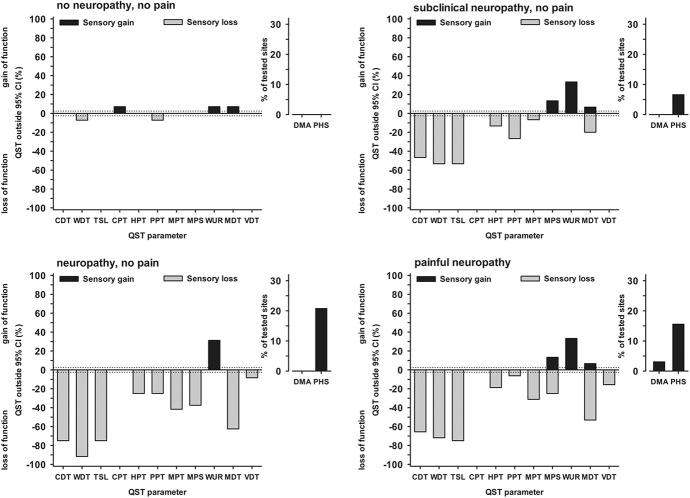
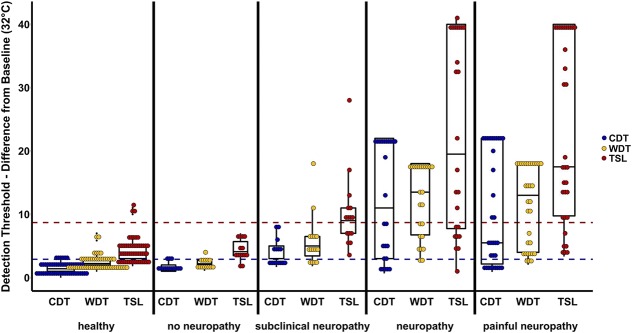
Patients who were categorized as having no neuropathy showed only mild sensory abnormalities, mostly within the normal range, regarding both the z-profile (Fig. 3) and number of abnormal values (Fig. 4). By contrast, within the patients with neuropathy, both subclinical and clinical as well as painless and painful, a striking loss of thermal detection function was seen in the z-profile (Fig. 3). The number of abnormally increased thermal detection thresholds amounted from more than 40% of patients (CDT in subclinical neuropathy) to more than 90% of patients (TSL in painless neuropathy, Fig. 4). Thermal detection thresholds (CDT, WDT, and TSL) for all participants are additionally plotted in detailed, individual data dot plots of raw temperature data (°C instead of z-values) in Figure 5.
Figure 3.
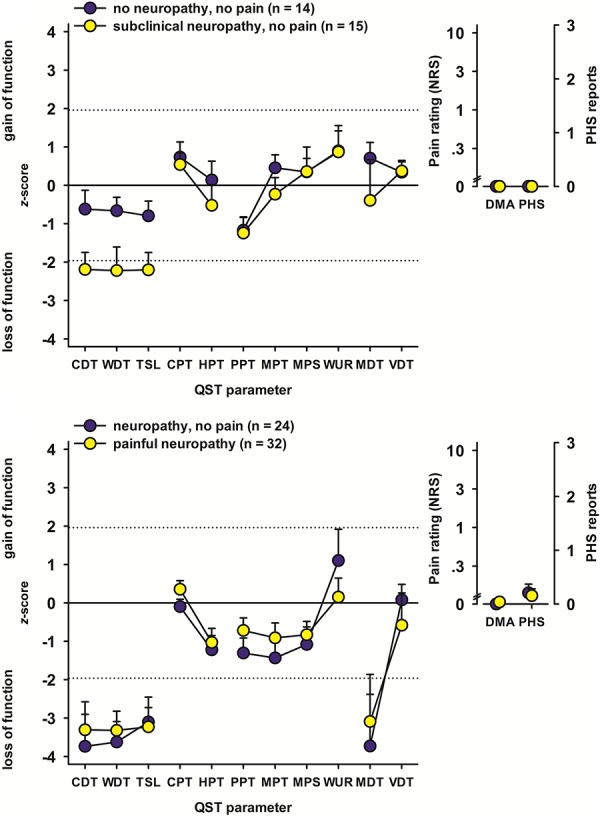
Sensory z-profiles. Sensory testing results were normalized to healthy subject reference data. Values below zero indicate loss of sensory function (ie, hypaesthesia); values above zero indicate gain of sensory function (ie, hyperalgesia). CDT, cold detection threshold; CPT, cold pain threshold; DMA, dynamical mechanical allodynia; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; NRS, numerical rating scale; PHS, paradoxical heat sensation; PPT, pressure pain threshold; QST, quantitative sensory testing; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio.
Figure 4.
Frequency of abnormal sensory findings per group. Patient data outside the 95% CI of healthy subjects were considered abnormal. Values below zero indicate significant loss of sensory function (ie, hypaesthesia); values above zero indicate significant gain of sensory function (ie, hyperalgesia). CDT, cold detection threshold; CI, confidence interval; CPT, cold pain threshold; DMA, dynamical mechanical allodynia; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PHS, paradoxical heat sensation; PPT, pressure pain threshold; QST, quantitative sensory testing; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio.
Figure 5.
Dot-box-plot of thermal detection thresholds per group. Difference from baseline (32°C) is presented. The dotted lines indicate the 95% quantile of the cold detection threshold (blue, overlying with the warm detection threshold 95% quantile) and the thermal sensory limen (red) of the healthy control group. Although the “no neuropathy” group is well within this quantile, more than half of the “subclinical neuropathy” group are beyond and could be identified by a simple thermal test. CDT, cold detection threshold; TSL, thermal sensory limen; WDT, warm detection threshold.
Although subclinical neuropathy differed from the other 2 neuropathy groups mainly by normal MDTs (which is unsurprising, as it is part of the definition of subclinical neuropathy), painless and painful neuropathy did not differ, neither in the z-profile (Fig. 4) nor in the number of abnormal values (Fig. 5). For both these groups, abnormal MDTs were frequently found (which is again part of the definition), although only in few cases, the VDT was elevated as well.
Patient groups differed in frequency of sensory phenotypes (Fig. 6). The “healthy” phenotype was frequently observed (29%) only in the group of patients with “no neuropathy” (Fig. 6). Two-thirds of the patients with subclinical neuropathy were classified into the “mechanical hyperalgesia” phenotype, which is characterized not only by mild mechanical hyperalgesia, but also by impaired thermal detection in combination with comparably intact mechanical detection.5 In the groups of patients with painless or painful neuropathy, the “sensory loss” phenotype was most frequent (75% and 59%, respectively, Fig. 6). Notably, leprosy patients with painful neuropathy had milder sensory deficits than those with painless neuropathy and more hyperalgesia, suggesting that a minimum number of surviving nociceptors may be required for pain and hyperalgesia to occur.
Figure 6.
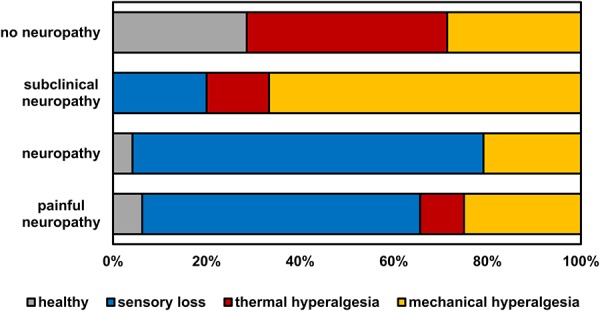
Frequency of sensory phenotypes per group. Although the sensory loss phenotype dominates in the 2 groups with clinical neuropathy, roughly two-thirds of the “subclinical neuropathy” group fall in the mechanical hyperalgesia phenotype, that is defined also by loss of small-fiber function.
4. Discussion
In this first report of detailed comprehensive quantitative sensory profiling in patients with leprosy, approximately 60% of patients with leprosy showed a loss of function of at least 1 sensory modality, a high number given the fact that sensory recovery is observed in both silent and overt leprosy neuropathy on early identification and intervention with steroids. Of particular interest is that vibration detection was preserved in almost all cases, while loss of tactile mechanosensation detection was prominent in patients with clinical neuropathy. Both assess large afferent fiber function,32 and it might be therefore expected that vibration results would parallel those for MDT. This has been reported, eg, in HIV, diabetic, and nonfreezing cold injury–induced neuropathy.40,43,54,57 This pattern of loss of CDT, WDT, and MDT with preservation of VDT has not been observed in the recently described 3 main sensory profiles of neuropathic pain.5 Therefore, its relevance could extend beyond the immediate realm of leprosy with regards to a novel sensory profile which could be exploited in testing the hypothesis that QST profiles may reflect underlying pain mechanism which can be exploited to predict individual responses to analgesic drugs.5–7,14,17,40,54,73 The increased wind-up ratio, which is rather uncommon, could fit into this concept as well. Still, the preserved vibration in this cohort could also be caused by the tuning fork being applied to bone, and there may be relative preservation of afferents innervating deep tissues such as bone rather than superficial cutaneous tissue. This could relate to the preference of M. leprae for cooler temperature and is also consistent with the relative preservation of deep tendon reflexes in leprosy and the normal deep pain thresholds in this neuropathy, which is different from other neuropathies. A third possible explanation would be a technical aspect because the vibration stimulus might be conducted through the bone to a nearby, unaffected area and detected there. A similar combination of loss of tactile detection and preserved vibration detection using the same QST protocol was shown in participants under A-fiber block, giving a similar explanation.67
Of potentially great clinical importance is the finding that leprosy nerve damage is associated with loss of function of small, thinly, or unmyelinated nerve fibers, which is at an early stage not detected by conventional field screening of mechanosensation with monofilaments.7,38,60 Disabling thermal sensory deficits is well known to experts in the field, but cold detection has been displaced by clinical electrophysiology in recent years, although these 2 tests assess different fiber populations. Our results confirm that the ability to detect thermal sensory deficit in low-resource settings with simple low cost instruments would be a major advance for early detection of leprosy-associated neuropathy, where electrophysiological or QST methods are not available.7,58,60 Figure 5 shows that no normalization of the data is necessary for this purpose, and a simple test such as acetone drop or spirit swabs for assessment of cool detection might add significant value. Acetone or alcohol drop cools the skin because of evaporation, regardless of climate, by roughly 2°; still, if it should be used for screening for signs of neuropathy, clinical sensitivity needs to be determined experimentally. Conversely, it may be possible to develop a cheap simple device that measures warm or cold detection thresholds, both methods might hold a potential for earlier diagnosis and thus treatment onset. For a resource-limited setting, heated or cooled test tubes or copper cylinders have been used previously.1,2 It seems to be certain in the light of these and previous findings1 that loss of thermal perception is an early sign of leprosy neuropathy, not detected by the use of the current mechanosensory-based screening methods.
Considering metabolic factors, in this cohort, the mean values of plasma concentration of vitamin B12 across the study groups were within the normal range, while 3 healthy volunteers had low levels of vitamin B12. The low vitamin B12 concentration observed in healthy participants might be attributed to Indian dietary habits because most volunteers followed a vegetarian diet, while patients to a large part were on vitamin supplementation, a reflection of routine practices at Indian clinical centers dealing with leprosy.
Participants with painful neuropathy reported interference from pain and reduced quality of life across most domains of the BPI and GHQ-12. These findings are comparable with the impact of neuropathic pain on quality of life in other infectious painful neuropathies,10 but pain in leprosy seems to be associated with greater disability and poor overall perception of general health.61 The pain distribution, with pain in the ulnar nerve innervation, but in addition to symmetric knee pain, might pose the question if nociceptive or nociplastic pain30 is present in addition to the localized, neuropathic pain as seen in patients with chemotherapy-induced and diabetic neuropathy.17,54 In addition, pain not related to the disease could be present and influencing this picture. Pain unrelated to neuropathy, outside the affected area, can still induce mechanical hyperalgesia in form of secondary hyperalgesia in a neuropathic region, and so, when used to grade neuropathic pain, it must be ascertained that mechanical hyperalgesia cannot be induced by pain unrelated to the neuropathy. Although we categorize our patients as “painful neuropathy,” we avoid the term neuropathic pain because, since this work was performed, a revised grading system of neuropathic pain has been published,15 which this work is probably consistent with but did not formally adhere to. A discussion of how the grading system could be operationalized in leprosy would be an important, but complicated issue outside of the remit of this study that should be addressed by a group of qualified experts on leprosy and neuropathic pain. People living with HIV who have a painful neuropathy had higher BPI interference scores compared to those with painless neuropathy.40 The here found differences between painful and painless neuropathy, especially for losing confidence or feeling depressed, fit well into the model of chronic pain in the new ICD-11 classification.56 These findings support the growing evidence that patients with neuropathic pain have an increased quality of life burden.26,44,50,51,55,62
This is the first report on the use of the DFNS QST battery in a resource-limited setting and the collection of normative data in a non-European setting and population, although there are data from a South American setting.70 The normative sensory parameters in the Indian population were compatible with those reported in European recruited subjects as well as the DFNS requirements of reference data for QST.35 However, as in HIV-associated neuropathy and diabetes,40,43,54 sensory profiling did not differ between patients with neuropathy who do and do not report pain.
To summarize, this study demonstrates that the somatosensory profile of patients with leprosy and leprosy-induced neuropathy is characterised by loss of function. Of interest is the loss of small-fiber function with preserved vibration detection as an early sign of neuropathy, before large-fiber impairment becomes clinically detectable. The involvement of small fibers in leprosy neuropathy has been shown before,34 demonstrating that this is likely not a random finding. Future work should refine the findings reported here to develop and validate a simple, low cost, and robust sensory diagnostic instrument for thermal sensory testing suitable for use in low-resource settings where leprosy is usually managed. This could include a simple clinical test for identification of early-stage small-fiber neuropathy such as cold or warm detection, which is not detected by the currently used routine sensory screening methods which rely on mechanosensory parameters.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgments
The authors thank the participants from the Bombay Leprosy Project and the healthy volunteers who had willingly shared their valuable time during the process of interviewing. The authors are grateful to the Foundation for Medical Research institute for providing laboratory facilities and equipment. The authors also thank Dr Peter Nicholls, Dr Steve Walker, Dr Tudor Phillips, Dr Juan Ramirez-Rozo, Dr S. Pandya, Ms. G Capadia, Dr Dhananjaya Saranath, and Dr S. Khadilkar who advised the authors during the preparation of the study protocol.
This work was funded by the Hospital and Homes of St. Giles and by BMBF (DFNS grants).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Antia NH, Mehta L, Shetty V, Irani PF. Clinical, electrophysiological, quantitative, histologic and ultrastructural studies of the index branch of the radial cutaneous nerve in leprosy. I. Preliminary report. Int J Lepr Other Mycobact Dis 1975;43:106–13. [PubMed] [Google Scholar]
- [2].Antia NH, Shetty VP. The peripheral nerve in leprosy and other neuropathies. New Delhi: Oxford University Press, 1997. [Google Scholar]
- [3].Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice ASC, Rolke R, Treede RD, Serra J, Toelle T, Tugnoli V, Walk D, Wallace MS, Ware M, Yarnitsky D, Ziegler D. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. PAIN 2013;154:1807–19. [DOI] [PubMed] [Google Scholar]
- [4].Bandyopadhyay G, Sinha S, Sen B, Sen G. Validity of General Health Questionnaire (GHQ-36/GHQ-12) in the psychiatric O.P.D. of a general hospital—a pilot study. Int J Soc Psychiatry 1988;34:130–4. [DOI] [PubMed] [Google Scholar]
- [5].Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice ASC, Segerdahl M, Serra J, Sindrup S, Sommer C, Tolle T, Vollert J, Treede RD. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. PAIN 2017;158:261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Britton WJ, Lockwood DN. Leprosy. Lancet 2004;363:1209–19. [DOI] [PubMed] [Google Scholar]
- [7].Cochrane RG, Davey TF. Leprosy in theory and practice. Bristol: John Wright & Sons Ltd., 1964. [Google Scholar]
- [8].Compston A. Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty's Stationery Office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O'Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain 2010;133:2838–44. [DOI] [PubMed] [Google Scholar]
- [9].Croft R. Neuropathic pain in leprosy. Int J Lepr Other Mycobact Dis 2004;72:171–2. [DOI] [PubMed] [Google Scholar]
- [10].Daniel HC, Narewska J, Serpell M, Hoggart B, Johnson R, Rice ASC. Comparison of psychological and physical function in neuropathic pain and nociceptive pain: implications for cognitive behavioral pain management programs. Eur J pain 2008;12:731–41. [DOI] [PubMed] [Google Scholar]
- [11].Demant DT, Lund K, Finnerup NB, Vollert J, Maier C, Segerdahl MS, Jensen TS, Sindrup SH. Pain relief with lidocaine 5% patch in localized peripheral neuropathic pain in relation to pain phenotype: a randomised, double-blind, and placebo-controlled, phenotype panel study. PAIN 2015;156:2234–44. [DOI] [PubMed] [Google Scholar]
- [12].Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. PAIN 2014;155:2263–73. [DOI] [PubMed] [Google Scholar]
- [13].Facer P, Mathur R, Pandya SS, Ladiwala U, Singhal BS, Anand P. Correlation of quantitative tests of nerve and target organ dysfunction with skin immunohistology in leprosy. Brain 1998;121:2239–47. [DOI] [PubMed] [Google Scholar]
- [14].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice ASC, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice ASC, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. PAIN 2016;157:1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gautam S, Nijhawan M, Kamal P. Standardisation of Hindi version of goldbergs general health questionnaire. Indian J Psychiatry 1987;29:63–6. [PMC free article] [PubMed] [Google Scholar]
- [17].Geber C, Breimhorst M, Burbach B, Egenolf C, Baier B, Fechir M, Koerber J, Treede RD, Vogt T, Birklein F. Pain in chemotherapy-induced neuropathy—more than neuropathic? PAIN 2013;154:2877–87. [DOI] [PubMed] [Google Scholar]
- [18].Geber C, Scherens A, Pfau D, Nestler N, Zenz M, Tolle T, Baron R, Treede RD, Maier C. Procedure for certification of QST laboratories. Schmerz 2009;23:65–9. [DOI] [PubMed] [Google Scholar]
- [19].Gierthmuhlen J, Enax-Krumova EK, Attal N, Bouhassira D, Cruccu G, Finnerup NB, Haanpaa M, Hansson P, Jensen TS, Freynhagen R, Kennedy JD, Mainka T, Rice ASC, Segerdahl M, Sindrup SH, Serra J, Tolle T, Treede RD, Baron R, Maier C. Who is healthy? Aspects to consider when including healthy volunteers in QST-based studies-a consensus statement by the EUROPAIN and NEUROPAIN consortia. PAIN 2015;156:2203–11. [DOI] [PubMed] [Google Scholar]
- [20].Gierthmuhlen J, Maier C, Baron R, Tolle T, Treede RD, Birbaumer N, Huge V, Koroschetz J, Krumova EK, Lauchart M, Maihofner C, Richter H, Westermann A. Sensory signs in complex regional pain syndrome and peripheral nerve injury. PAIN 2012;153:765–74. [DOI] [PubMed] [Google Scholar]
- [21].Goldberg DP. The detection of psychiatric illness by questionnaire. London: Oxford University Press, 1972. [Google Scholar]
- [22].Haanpaa M, Lockwood DN, Hietaharju A. Neuropathic pain in leprosy. Lepr Rev 2004;75:7–18. [PubMed] [Google Scholar]
- [23].Haroun OM, Hietaharju A, Bizuneh E, Tesfaye F, Brandsma JW, Haanpaa M, Rice ASC, Lockwood DN. Investigation of neuropathic pain in treated leprosy patients in Ethiopia: a cross-sectional study. PAIN 2012;153:1620–4. [DOI] [PubMed] [Google Scholar]
- [24].Hietaharju A, Croft R, Alam R, Birch P, Mong A, Haanpaa M. Chronic neuropathic pain in treated leprosy. Lancet 2000;356:1080–1. [DOI] [PubMed] [Google Scholar]
- [25].Hurtig IM, Raak RI, Kendall SA, Gerdle B, Wahren LK. Quantitative sensory testing in fibromyalgia patients and in healthy subjects: identification of subgroups. Clin J Pain 2001;17:316–22. [DOI] [PubMed] [Google Scholar]
- [26].Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology 2007;68:1178–82. [DOI] [PubMed] [Google Scholar]
- [27].Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice ASC, Treede RD. A new definition of neuropathic pain. PAIN 2011;152:2204–5. [DOI] [PubMed] [Google Scholar]
- [28].Jindal KC, Singh GP, Mohan V, Mahajan BB. Psychiatric morbidity among inmates of leprosy homes. Indian J Psychol Med 2013;35:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kamerman PR, Wadley AL, Davis KD, Hietaharju A, Jain P, Kopf A, Meyer AC, Raja SN, Rice ASC, Smith BH, Treede RD, Wiffen PJ. World Health Organization essential medicines lists: where are the drugs to treat neuropathic pain? PAIN 2015;156:793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice AS, Rief W, Sluka AK. Do we need a third mechanistic descriptor for chronic pain states? PAIN 2016;157:1382–6. [DOI] [PubMed] [Google Scholar]
- [31].Lasry-Levy E, Hietaharju A, Pai V, Ganapati R, Rice ASC, Haanpaa M, Lockwood DN. Neuropathic pain and psychological morbidity in patients with treated leprosy: a cross-sectional prevalence study in Mumbai. PLoS Negl Trop Dis 2011;5:e981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Light AR, Perl ER. Peripheral sensory systems. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF, editors. Peripheral neuropathy. Philadelphia: Saunders, 1993. p. 149–65. [Google Scholar]
- [33].Lockwood DN, Saunderson PR. Nerve damage in leprosy: a continuing challenge to scientists, clinicians and service providers. Int Health 2012;4:77–85. [DOI] [PubMed] [Google Scholar]
- [34].Lund C, Koskinen M, Suneetha S, Lockwood DN, Haanpaa M, Haapasalo H, Hietaharju A. Histopathological and clinical findings in leprosy patients with chronic neuropathic pain: a study from Hyderabad, India. Lepr Rev 2007;78:369–80. [PubMed] [Google Scholar]
- [35].Magerl W, Krumova EK, Baron R, Tolle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. PAIN 2010;151:598–605. [DOI] [PubMed] [Google Scholar]
- [36].Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. PAIN 2010;150:439–50. [DOI] [PubMed] [Google Scholar]
- [37].Malaviya GN. Neuropathic pain in leprosy patients. Int J Lepr Other Mycobact Dis 2005;73:34–5. [DOI] [PubMed] [Google Scholar]
- [38].Pandya SS, Bhatki WS. Severe pan-sensory neuropathy in leprosy. Int J Lepr Other Mycobact Dis 1994;62:24–31. [PubMed] [Google Scholar]
- [39].Pfau DB, Krumova EK, Treede RD, Baron R, Toelle T, Birklein F, Eich W, Geber C, Gerhardt A, Weiss T, Magerl W, Maier C. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): reference data for the trunk and application in patients with chronic postherpetic neuralgia. PAIN 2014;155:1002–15. [DOI] [PubMed] [Google Scholar]
- [40].Phillips TJ, Brown M, Ramirez JD, Perkins J, Woldeamanuel YW, Williams AC, Orengo C, Bennett DL, Bodi I, Cox S, Maier C, Krumova EK, Rice ASC. Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: a cross-sectional deep profiling study. PAIN 2014;155:1846–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Raicher I, Stump PR, Baccarelli R, Marciano LH, Ura S, Virmond MC, Teixeira MJ, Ciampi de Andrade D. Neuropathic pain in leprosy. Clin Dermatol 2016;34:59–65. [DOI] [PubMed] [Google Scholar]
- [42].Raicher I, Stump PR, Harnik SB, De Oliveira AB, Baccarelli R, Marciano LH, Ura S, Virmond Mda C, Teixeira AL, De Andrade DC. Neuropathic pain in leprosy: symptom profile characterization and comparison with neuropathic pain of other etiologies. Pain Rep 2018;3:e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Raputova J, Srotova I, Vlckova E, Sommer C, Uceyler N, Birklein F, Rittner HL, Rebhorn C, Adamova B, Kovalova I, Kralickova Nekvapilova E, Forer L, Belobradkova J, Olsovsky J, Weber P, Dusek L, Jarkovsky J, Bednarik J. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. PAIN 2017;158:2340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rice ASC, Smith BH, Blyth FM. Pain and the global burden of disease. PAIN 2016;157:791–6. [DOI] [PubMed] [Google Scholar]
- [45].Roberts AE, Nicholls PG, Maddali P, Van Brakel WH. Ensuring inter-tester reliability of voluntary muscle and monofilament sensory testing in the INFIR Cohort Study. Lepr Rev 2007;78:122–30. [PubMed] [Google Scholar]
- [46].Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [47].Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J pain 2006;10:77–88. [DOI] [PubMed] [Google Scholar]
- [48].Saunderson P, Bizuneh E, Leekassa R. Neuropathic pain in people treated for multibacillary leprosy more than ten years previously. Lepr Rev 2008;79:270–6. [PubMed] [Google Scholar]
- [49].Senturk V, Stewart R, Sagduyu A. Screening for mental disorders in leprosy patients: comparing the internal consistency and screening properties of HADS and GHQ-12. Lepr Rev 2007;78:231–42. [PubMed] [Google Scholar]
- [50].Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep 2012;16:191–8. [DOI] [PubMed] [Google Scholar]
- [51].Smith BH, Torrance N, Bennett MI, Lee AJ. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin J Pain 2007;23:143–9. [DOI] [PubMed] [Google Scholar]
- [52].Sriram TG, Chandrashekar CR, Isaac MK, Shanmugham V. The General Health Questionnaire (GHQ). Comparison of the English version and a translated Indian version. Soc Psychiatry Psychiatr Epidemiol 1989;24:317–20. [DOI] [PubMed] [Google Scholar]
- [53].Stump PR, Baccarelli R, Marciano LH, Lauris JR, Teixeira MJ, Ura S, Virmond MC. Neuropathic pain in leprosy patients. Int J Lepr Other Mycobact Dis 2004;72:134–8. [DOI] [PubMed] [Google Scholar]
- [54].Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice ASC, Bennett DL. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. PAIN 2016;157:1132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Torrance N, Lawson KD, Afolabi E, Bennett MI, Serpell MG, Dunn KM, Smith BH. Estimating the burden of disease in chronic pain with and without neuropathic characteristics: does the choice between the EQ-5D and SF-6D matter? PAIN 2014;155:1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasal S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyeny JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International classification of diseases (ICD-11). PAIN 2019;160:19–27. [DOI] [PubMed] [Google Scholar]
- [57].Vale TA, Symmonds M, Polydefkis M, Byrnes K, Rice ASC, Themistocleous AC, Bennett DLH. Chronic non-freezing cold injury results in neuropathic pain due to a sensory neuropathy. Brain 2017;140:2557–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].van Brakel WH, Nicholls PG, Das L, Barkataki P, Maddali P, Lockwood DN, Wilder-Smith E. The INFIR Cohort Study: assessment of sensory and motor neuropathy in leprosy at baseline. Lepr Rev 2005;76:277–95. [PubMed] [Google Scholar]
- [59].van Brakel WH, Nicholls PG, Das L, Barkataki P, Suneetha SK, Jadhav RS, Maddali P, Lockwood DN, Wilder-Smith E, Desikan KV. The INFIR Cohort Study: investigating prediction, detection and pathogenesis of neuropathy and reactions in leprosy. Methods and baseline results of a cohort of multibacillary leprosy patients in North India. Lepr Rev 2005;76:14–34. [PubMed] [Google Scholar]
- [60].van Brakel WH, Nicholls PG, Wilder-Smith EP, Das L, Barkataki P, Lockwood DN. Early diagnosis of neuropathy in leprosy-comparing diagnostic tests in a large prospective study (the INFIR cohort study). PLoS Negl Trop Dis 2008;2:e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].van Brakel WH, Sihombing B, Djarir H, Beise K, Kusumawardhani L, Yulihane R, Kurniasari I, Kasim M, Kesumaningsih KI, Wilder-Smith A. Disability in people affected by leprosy: the role of impairment, activity, social participation, stigma and discrimination. Glob Health Action 2012;5:18394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. PAIN 2014;155:654–62. [DOI] [PubMed] [Google Scholar]
- [63].Verma KK, Gautam S. Effect of rehabilitation on the prevalence of psychiatric morbidity among leprosy patients. Indian J Psychiatry 1994;36:183–6. [PMC free article] [PubMed] [Google Scholar]
- [64].Villarroel MF, Orsini MB, Grossi MA, Antunes CM. Impaired warm and cold perception thresholds in leprosy skin lesions. Lepr Rev 2007;78:110–21. [PubMed] [Google Scholar]
- [65].Villarroel MF, Orsini MB, Lima RC, Antunes CM. Comparative study of the cutaneous sensation of leprosy-suspected lesions using Semmes-Weinstein monofilaments and quantitative thermal testing. Lepr Rev 2007;78:102–9. [PubMed] [Google Scholar]
- [66].Vollert J, Attal N, Baron R, Freynhagen R, Haanpaa M, Hansson P, Jensen TS, Rice ASC, Segerdahl M, Serra J, Sindrup SH, Tolle TR, Treede RD, Maier C. Quantitative sensory testing using DFNS protocol in Europe: an evaluation of heterogeneity across multiple centers in patients with peripheral neuropathic pain and healthy subjects. PAIN 2016;157:750–8. [DOI] [PubMed] [Google Scholar]
- [67].Vollert J, Magerl W, Baron R, Binder A, Enax-Krumova EK, Geisslinger G, Gierthmuhlen J, Henrich F, Hullemann P, Klein T, Lotsch J, Maier C, Oertel B, Schuh-Hofer S, Tolle TR, Treede RD. Pathophysiological mechanisms of neuropathic pain: comparison of sensory phenotypes in patients and human surrogate pain models. PAIN 2018;159:1090–102. [DOI] [PubMed] [Google Scholar]
- [68].Vollert J, Maier C, Attal N, Bennett DLH, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmuhlen J, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Magerl W, Ramirez JD, Rice ASC, Schuh-Hofer S, Segerdahl M, Serra J, Shillo PR, Sindrup S, Tesfaye S, Themistocleous AC, Tolle TR, Treede RD, Baron R. Stratifying patients with peripheral neuropathic pain based on sensory profiles: algorithm and sample size recommendations. PAIN 2017;158:1446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Vollert J, Mainka T, Baron R, Enax-Krumova EK, Hullemann P, Maier C, Pfau DB, Tolle T, Treede RD. Quality assurance for Quantitative Sensory Testing laboratories: development and validation of an automated evaluation tool for the analysis of declared healthy samples. PAIN 2015;156:2423–30. [DOI] [PubMed] [Google Scholar]
- [70].von Bischhoffshausen S, Ivulic D, Alvarez P, Schuffeneger VC, Idiaquez J, Fuentes C, Morande P, Fuentes I, Palisson F, Bennett DLH, Calvo M. Recessive dystrophic epidermolysis bullosa results in painful small fibre neuropathy. Brain 2017;140:1238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wagenaar I, Brandsma W, Post E, van Brakel W, Lockwood D, Nicholls P, Saunderson P, Smith C, Wilder-Smith E, Richardus JH. Two randomized controlled clinical trials to study the effectiveness of prednisolone treatment in preventing and restoring clinical nerve function loss in leprosy: the TENLEP study protocols. BMC Neurol 2012;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].World Health Organization (WHO). WHO expert committee on leprosy. Sixth report. Geneva: World Health Organisation Technical Report Series, 1988;768. [PubMed] [Google Scholar]
- [73].World Health Organization (WHO). WHO expert committee on leprosy. Eighth report. Geneva: World Health Organization; 2012. Available at: http://apps.who.int/iris/bitstream/handle/10665/75151/WHO_TRS_968_eng.pdf?sequence=1&isAllowed=y. Accessed 8 November, 2018. [Google Scholar]
- [74].World Health Organization (WHO). Process of translation and adaptation of instruments. 2014. Available at: http://www.who.int/substance_abuse/research_tools/translation/en/. Accessed 8 November, 2018. [Google Scholar]
- [75].World Health Organization (WHO). Global leprosy situation, 2012. 2018. Reported Leprosy cases, as at 27 APRIL 2018, taken from Wkly Epidemiol Rec 17, 93, 221–228. Available at: http://www.who.int/wer/en/. Accessed 8 November, 2018.
- [76].World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]