Abstract
Summary:
Fracture-related infection (FRI) is a major complication in musculoskeletal trauma and one of the leading causes of morbidity. Standardization of general treatment strategies for FRI has been poor. One of the reasons is the heterogeneity in this patient population, including various anatomical locations, multiple fracture patterns, different degrees of soft-tissue injury, and different patient conditions. This variability makes treatment complex and hard to standardize. As these infections are biofilm-related, surgery remains the cornerstone of treatment, and this entails multiple key aspects (eg, fracture fixation, tissue sampling, debridement, and soft-tissue management). Another important aspect, which is sometimes less familiar to the orthopaedic trauma surgeon, is systemic antimicrobial therapy. The aim of this article is to summarize the available evidence and provide recommendations for systemic antimicrobial therapy with respect to FRI, based on the most recent literature combined with expert opinion.
Level of Evidence:
Therapeutic Level V. See Instructions for Authors for a complete description of levels of evidence.
Key Words: fracture-related infection, antibiotics, biofilm, suppressive therapy, antimicrobial therapy, consensus, fracture, infection
INTRODUCTION
The aim of this article is to summarize the available evidence and provide recommendations for systemic antimicrobial therapy with respect to fracture-related infection (FRI) patients, based on the most recent literature combined with expert opinion.1 For this purpose, organizations such as the AO Foundation, the European Bone and Joint Infection Society (EBJIS), the Orthopaedic Trauma Association (OTA), and the PRO-IMPLANT Foundation have collaborated in an FRI Consensus group. Many of the recommendations provided in this review are based on expert opinion because clinical data from controlled trials are not available, and the likelihood that studies for rationalizing the management of FRI will be conducted remains low.
Pathophysiology
Biofilm development on the surface of implants is the main hindrance of infection eradication. Bacteria growing in biofilms are proven to be up to 1000 times more resistant to antibiotics than in the planktonic state,2 and the antibiotic effect decreases even more with increasing age of biofilms.3 In periprosthetic joint infection (PJI), it was shown that failure rates of antibiotic therapy significantly increase with implant retention if symptoms have lasted for more than 4 weeks before treatment.4
In FRI, based on biofilm formation, different time-related classifications have been proposed (such as early, delayed, and late-onset infections vs. acute and chronic infections), but strong evidence to support one classification over another is lacking.3
Surgical Treatment Concepts
There are no randomized controlled trials on surgical treatment strategies and duration of systemic antimicrobial therapy in FRI. Prompt debridement should always be performed to reduce the bacterial load (bioburden) at the site of infection, thereby increasing the efficacy of antimicrobials and reducing the risk of developing antimicrobial resistance. Debridement will also reduce the local inflammatory response and risk of developing chronic infection/osteomyelitis. The application of local antimicrobials can be an important adjunct in the treatment of FRI and should be considered, especially in cases with a remaining bony defect or “dead space.”5 Systemic antimicrobial therapy in FRI is guided by 2 main surgical treatment concepts:
The first concept consists of Debridement, Antimicrobial therapy, and Implant Retention (DAIR).
The second consists of debridement, implant removal—in case the fracture has healed—or exchange (in 1 or multiple stages), combined with antimicrobial therapy.
Based on these concepts, treatment duration and mode of administration will be discussed below (Fig. 1).
FIGURE 1.
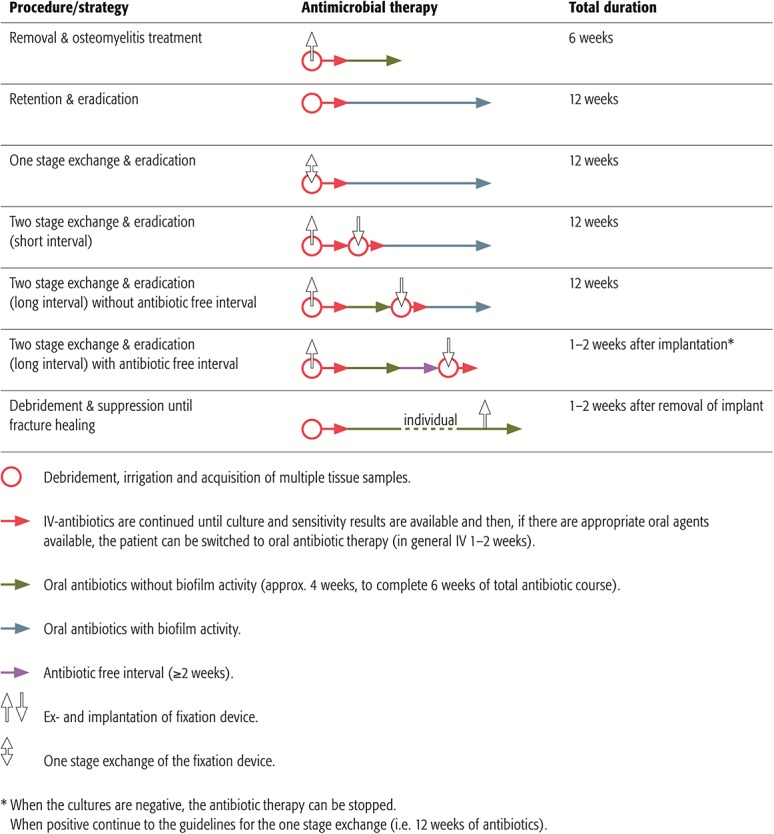
Duration of antimicrobial therapy according to treatment strategy.
Removal of the Implant in Case of Fracture Consolidation
When FRI occurs after the fracture healed and a debridement has been performed with complete removal of the implant, the treatment duration can be extrapolated from that for acute osteomyelitis. Four to 6 weeks of intravenous (IV) antibiotics has been standard practice for many years.6,7 The prolonged use of IV antibiotics was based on recommendations by Waldvogel et al8 in 1970, with beta-lactams being the main available class of antibiotics for IV and oral routes. The key factor however is to achieve high enough drug levels in blood and bone, rather than the route of administration. Newer oral agents with better bioavailability and acceptable bone penetration are now available. The OVIVA trial compared intravenous with oral antibiotics and demonstrated noninferiority of oral antibiotics.9,10 In this trial, patients in the oral arm received up to 1 week of IV antibiotics. It is therefore recommended that patients only receive empiric broad-spectrum IV antibiotics until culture and sensitivity results are available, and then, if there are appropriate bioavailable oral agents (Table 1), they can be switched to oral antibiotics to complete a period of 6 weeks from implant removal.7,9,10
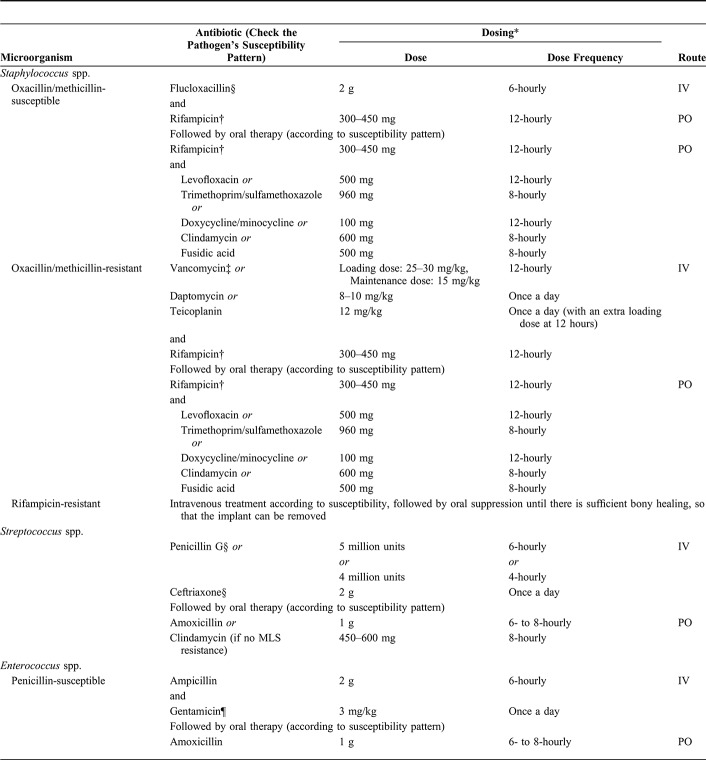
TABLE 1.
Antimicrobial Therapy for Fracture-Related Infection
Debridement, Antimicrobial therap, and Implant Retention
No published data are available regarding the duration of antimicrobial treatment in FRI with a retained implant. Treatment is likely to eradicate the infection only if a biofilm-active antibiotic agent is used. Biofilm activity has been clearly demonstrated only for rifampicin combinations against staphylococci and fluoroquinolones against Gram-negative bacteria.11–13 When biofilm-active therapy is possible, a total treatment duration of 12 weeks is recommended, with an initial IV treatment until the patient (eg, polytrauma or intensive care unit patient) and the soft tissue are settled and full antibiotic susceptibility results of the cultures are available.9 As previously mentioned, IV treatment can be as short as 1 week or even less. When no biofilm-active antibiotics can be given due to resistance, patient intolerance, or potential drug interactions, infections are classified as “difficult-to-treat” and may not be eradicated by antibiotics as long as the implants are retained. In this situation, FRI can likely be suppressed but not eradicated. Similarly, if the first debridement takes place more than 4 weeks after the start of symptoms and the implant cannot be removed/exchanged, antimicrobial therapy may not achieve eradication of the established older biofilms and again may be suppressive rather than eradicative. If a suppressive approach is chosen, antimicrobials should be continued until the fracture is consolidated to the extent that the implant can be removed without compromising stability.14 One to 2 weeks of continuation after removal is indicated. Importantly, an initially suppressive approach will not be successful if fracture healing is estimated to be very unlikely (eg, instability of the construct) or if no signs of fracture healing can be found over the course of suppressive treatment. There is a lack of published data comparing different suppressive regimens in FRI. As guidance, suppressive therapy needs to be tailored to the susceptibility of the responsible pathogen(s), should have low propensity to induce resistance, should be orally available, and the dosage and antibiotic class should be able to control the infection with minimal side effects and toxicity.
Debridement with One- or Two-Stage Exchange of the Implant
A 1- or 2-stage exchange is performed if patients without bone union do not qualify for eradicative or suppressive treatment with implant retention. A total duration of 12 weeks of biofilm-active therapy is recommended in a 1-stage exchange with intravenous treatment until full antibiotic susceptibility results of the cultures are available.
A 2-stage exchange with an implant-free interval at the site of infection can be required if no biofilm-active antimicrobial is available, surgical factors are unfavorable (eg, soft-tissue status), or suppressive therapy is not an adequate option. This requires temporary stabilization of the fracture, with the type of fixation being at the discretion of the treating surgeon. During the implant-free interval, 6 weeks of antibiotic treatment without biofilm activity is recommended, starting with intravenous treatment until full antibiotic susceptibility results of the cultures are available. After the first 6 weeks of treatment, 2 different approaches exist with no consensus and a lack of data on the optimal continuation therapy. One approach consists of an immediate reimplantation followed by further 6 weeks of biofilm-active treatment. The other allows a 2-week antibiotic-free interval before reimplantation to see whether clinical signs of infection reoccur and to enable regrowth of potential residual bacteria. If no clinical signs of infection occur and tissue cultures from reimplantation stay negative, the antibiotics that were restarted after implantation can be stopped (Fig. 1).
It should be acknowledged that in cases of FRI, there is often the presence of a fracture (ie, instability). A period of 6 weeks without fracture stability is not advisable. External fixation could be used, although chances are higher of developing an infection (ie, pin tracts) after a period of long-term external fixation followed by internal fixation. Therefore, in some cases, a two-stage exchange with a short interval can be considered. Here, a period of 1–2 weeks of temporary fracture fixation, again the type of fixation is at the discretion of the treating surgeon, is advisable until culture results are known and targeted systemic and local antibiotic therapy can be started. The total treatment duration remains 12 weeks.
Cases of segmental bone resection without internal fixation (eg, ring fixator) are an exception. Here, all dead and infected bone should be removed, and the multidisciplinary team needs to decide whether a long course of antibiotic treatment is necessary. It may be sufficient to give 2 weeks of antimicrobial therapy to eradicate the residual contamination in the soft tissue.
The experts acknowledge that there are multiple different treatment strategies available, with a lack of evidence supporting one over the other (Fig. 1). In addition, scientific data on duration of antimicrobial treatment in FRI remain scarce. They suggest that every patient receives an individualized treatment plan that is confirmed by the multidisciplinary team. Furthermore, future studies are warranted to give better insights into the optimal treatment strategy.
Empirical Antibiotic Therapy
In case of suspected FRI, antibiotics should not be started before the initial surgical debridement unless the patient is septic according to the 2016 international definition of sepsis.15 Multiple tissue samples for microbiology and histopathology should be taken intraoperatively, using separate sterile instruments for each sample at the start of the debridement.16 If there are confirmatory signs of FRI (eg, pus) or there is a high suspicion due to the presence of suggestive signs,16,17 empiric intravenous antimicrobial therapy should be started immediately after perioperative tissue sampling. Empiric antimicrobial treatment should be continued until the microbiology results are available and then reassessed.17 The choice of empiric therapy depends on the local epidemiology of antibiotic resistance rates, antibiotic formularies, and risk factors of each individual patient (ie, previous antibiotics, comorbidities, allergies, previous hospitalizations, previous debridements at the same site, and previously recovered pathogens). Initially, empiric therapy should be broad-spectrum, including a lipopeptide or a glycopeptide and an agent covering Gram-negative bacilli; thereafter, it should be adapted according to culture results as soon as possible.
All patients who are started on IV antibiotics should be tested for baseline inflammatory markers, full blood count, electrolytes, and liver and renal function tests and should be monitored at least once weekly (depending on host status) in the acute phase of their illness as common side effects of high-dose IV antibiotics include bone marrow suppression, hepatitis, and nephritis. The multidisciplinary team plays an important role in assessing these side effects.
Targeted Antimicrobial Therapy
Treatment of Staphylococcus Species
Staphylococcus aureus
In methicillin-susceptible Staphylococcus aureus (MSSA), IV flucloxacillin is the first choice initially. Garzoni et al studied the interactions of continuous flucloxacillin and oral rifampicin in 15 patients. The combination with rifampicin increased flucloxacillin levels (by 44.5%); however, in monotherapy, the plasma-free drug level of flucloxacillin also exceeded the minimal inhibitory concentration (MIC) for S. aureus by several fold.18 In other studies, a lowered serum level of flucloxacillin was observed.19 The expert group recommends a dose of 2-g flucloxacillin every 6 hours. When S. aureus is methicillin-resistant (MRSA), IV vancomycin is recommended initially. Careful monitoring for nephrotoxicity is essential, with therapeutic serum values between 15 and 20 mg/L. However, both glycopeptides (vancomycin and teicoplanin) and daptomycin demonstrate low cure rates when given as monotherapy in experimental implant-associated infection.20,21 In cases of vancomycin allergy, daptomycin can be considered.
Coagulase-Negative Staphylococci
Coagulase-negative staphylococci such as Staphylococcus epidermidis are treated like MRSA if they are methicillin-resistant (MRSE). Methicillin susceptibility testing may be difficult in some strains. Expression of resistance is affected by test conditions and resistance is often heterogeneous, with only a proportion of cells showing resistance. If mecA is absent, strains can be treated as for MSSA.
General Principles
For Staphylococcus species, the curative treatment of choice with implants in situ is a combination of rifampicin with a second active antibiotic after the initial IV period. Rifampicin is a bactericidal agent active against growing and nongrowing staphylococci in biofilms.22–25 However, monotherapy with rifampicin leads to rapid emergence of resistance.11 The risk of superinfection with (a) rifampicin-resistant strain(s) increases when the bacterial load is high and the wound is oozing or drains are present.26,27 The recommended dose of oral rifampicin is 300–450 mg twice or 600–900 mg once daily started after debridement and when the wound has sealed.27 The expert group recommends liver function tests at baseline and thereafter if there are clinical concerns.28
Once the wound is stable, drains are out, and microbiology results are available, it is recommended to switch to an oral antibiotic in combination with rifampicin. The first choice is a fluoroquinolone. Patients should be warned about adverse effects including Achilles tendon tendinitis and photosensitivity. Second-line antimicrobials combined with rifampicin include sulfamethoxazole/trimethoprim in high dose (960 mg, 8 hourly). Other second-line agents include doxycycline or minocycline and fusidic acid. In some cases, where there is resistance or intolerance to the other agents, oral pristinamycin can be used.29,30 The dosages for the aforementioned antimicrobials are summarized in Table 1.
Linezolid in monotherapy is an alternative agent. In animal models, the success rate of monotherapy appears inferior to a combination of rifampicin and linezolid. The combination therapy results in 75%–95% clearance of planktonic MRSA and a cure rate of 50%–60% with implants.31 Rifampicin however may reduce the levels of linezolid and may lead to subinhibitory concentrations.32 Further studies are needed regarding combination therapy with rifampicin and linezolid. If this regimen is used, it is important to check lactate and full blood counts weekly, as linezolid can cause severe reversible pancytopenia, lactate acidosis, and irreversible neurotoxicity.33 It also interacts adversely with many psychiatric drugs risking hypertensive crises and serotonin syndrome (serotonin uptake inhibitors).
Monotherapy with fluoroquinolones is not recommended for staphylococcal infections because of the rapid emergence of resistance and high treatment failure rate.34,35 Moxifloxacin has a lower MIC for staphylococci than levofloxacin and ciprofloxacin. In addition, it has the advantage that no dose adjustment is necessary in case of renal insufficiency.36 Studies have shown that moxifloxacin has a lower risk of emergence of antimicrobial resistance than other fluoroquinolones.35,37–39 In addition, studies on tuberculosis have shown a lowering of moxifloxacin serum levels by 30% when used in combination with rifampicin.40 Wouthuyzen-Bakker et al39 observed a success rate of 89% using a moxifloxacin/rifampicin combination in patients with early acute PJI caused by MSSA. More studies are necessary before recommending moxifloxacin/rifampicin as the preferred first-line per oral therapy.
The use of clindamycin in combination with rifampicin as an oral alternative is controversial. Bernard et al showed a dramatic reduction of clindamycin serum concentration when used in combination with rifampicin. Rifampicin is a potent cytochrome P450 inducer, while clindamycin is metabolized through CYP3A4, a member of this cytochrome P450 system and one of the main enzymes involved in the metabolism of drugs. This interaction enhances the elimination of clindamycin in combination with rifampicin.41 However, since clindamycin can be given at a high dose (600–900 mg every 6–8 hours), therapy with this antibiotic may be sufficient.42
Treatment of Streptococcus Species
The expert group recommendations for streptococcal infections are based on the IDSA PJI guidelines.34 Beta-lactam antibiotics are the agent of choice, although it is known that antibiotics that target the cell wall may be ineffective once the initial phase has passed, due to the slower growth rate of adherent bacteria, during which cell wall synthesis is reduced.43 However, poor efficacy of these beta-lactams has not been demonstrated in streptococcal implant-associated infections.13,44–46 Intravenous beta-lactam therapy should be given for up to a week followed by oral therapy with amoxicillin (Table 1).
After prompt debridement, it is recommended (if there is no history of penicillin allergy) to start with IV benzyl penicillin 4 million units (2.4 g) every 4 hours (24 MU/day) or 5 million units (3.0 g) every 6 hours (20 MU/day). When the MIC value is <0.1 mg/L, the dosage can be adjusted to 12–18 MU/day. If there is a history of nonimmediate, nonsevere penicillin allergy (eg, a delayed rash), an alternative is ceftriaxone or cefotaxime (Table 1). In case of penicillin allergy, trimethoprim/sulfamethoxazole, doxycycline, and clindamycin are alternatives, depending on susceptibility testing.
Despite good activity of rifampicin against planktonic streptococci, it has been observed in clinical studies that it has no activity on streptococcal biofilms.47,48 Therefore, despite some studies showing a beneficial effect of rifampicin, the expert group does currently not recommend rifampicin for streptococcal FRI infections.27,45
Treatment of Enterococcus Species
If enterococci (especially Enterococcus faecalis) are sensitive to ampicillin or amoxicillin, this should be the initial agent of choice, with up to a week intravenous therapy followed by oral amoxicillin.
For ampicillin-resistant enterococci, vancomycin and daptomycin are the first choices. Resistance to vancomycin is increasing (vancomycin-resistant enterococci). Apart from daptomycin or oral linezolid, often no other alternative options are available. Pristinamycin may be considered for treatment of E. faecalis, although evidence supporting this is limited for enterococcal implant-related infections.29,30,33,49
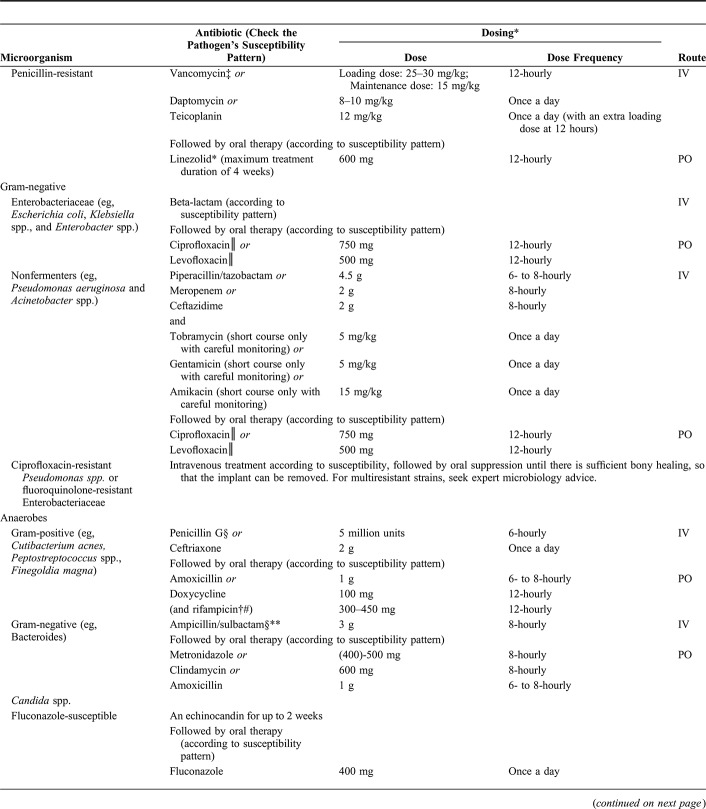
IV fosfomycin has extensive tissue penetration in inflamed tissue and bone. In an experimental model of foreign-body infection, the combination of fosfomycin and gentamicin showed good biofilm activity against E. faecalis.50 Further studies are needed before IV fosfomycin can be recommended as a standard therapy in enterococcal FRI, although it may be considered in selected cases.51 No safety data exist on long-term oral administration of fosfomycin. This route of administration is therefore not recommended for bone and joint infections.
Treatment of Enterobacteriaceae
If Enterobacteriaceae are cultured, an anti–Gram-negative agent should be administered (ideally a narrower beta-lactam agent if possible) in line with the antibiogram. Once susceptibilities are available and the wound is dry, an oral fluoroquinolone may be appropriate as monotherapy. Fluoroquinolones are able to eliminate Gram-negative rods in young biofilms and have excellent bioavailability. Unfortunately, selection of resistance to fluoroquinolones is common when there is a high bioburden, so they should only be started after surgical debridement.12,13 Furthermore, the risk of superinfection with selected fluoroquinolone-resistant strains from the skin microbiome is highest during the early postoperative period, when the patient has drains and an oozing wound.
When there is fluoroquinolone resistance, oral sulfamethoxazole/trimethoprim in high dose (800/160 mg, 8 hourly) or continuation of beta-lactam agents is recommended. Sulfamethoxazole/trimethoprim has poor biofilm activity by which it is regarded as a suppressive rather than curative therapy.52
IV fosfomycin showed biofilm activity against Escherichia coli in vitro and in an experimental model of foreign-body infection, and may be considered as an additional antibiotic against Gram-negative FRI in selected cases, caused by susceptible bacteria.53
For some carbapenemase-producing Enterobacteriaceae, other agents such as tigecycline, intravenous fosfomycin, or colistin, in combination treatment, may need to be considered. Expert microbiology advice should be sought for any of these infections.
Treatment of Nonfermenters
For nonfermenters such as Pseudomonas aeruginosa, fluoroquinolones are not recommended as initial treatment after debridement because of the high rate of resistance that can develop. Although the bactericidal action of beta-lactams appears inferior to fluoroquinolones,54 beta-lactam antibiotics such as piperacillin/tazobactam, cefepime, ceftazidime, or a carbapenem (except ertapenem) should be used as the initial therapy. There is some in vitro evidence of a decrease in the rate of bacterial killing with a high inoculum, and so, the addition of an aminoglycoside for 2–5 days can be considered.55
If P. aeruginosa is resistant to fluoroquinolones, there are no other suitable oral agents and beta-lactams need to be continued intravenously according to susceptibility testing.
Pseudomonas spp. and other nonfermenters such as Acinetobacter spp. may be multiresistant but sensitive to colistin. Colistin should be used in combination therapy with careful monitoring of renal function and trough serum dosages, as it is associated with a high risk of nephrotoxicity. Tigecycline may be an alternative for some organisms, but not for P. aeruginosa because of intrinsic resistance to the agent. Expert microbiology advice should be sought for any of these infections.
Treatment of Anaerobes
In the group of Gram-positive anaerobes, Cutibacterium (previously Propionibacterium) acnes is most frequently isolated).56 Cutibacterium acnes is highly susceptible to a wide range of antibiotics except metronidazole; however, resistance is emerging against macrolides, clindamycin, doxycycline, or minocycline and trimethoprim-sulfamethoxazole.57–60 In biofilm-related infections, vancomycin, levofloxacin, and clindamycin are less effective, while rifampicin, daptomycin, and ceftriaxone have demonstrated biofilm activity in animal models. In combination, rifampicin and levofloxacin show good efficacy.57,61 We recommend starting with benzylpenicillin or ceftriaxone, followed by oral treatment of rifampicin in combination with amoxicillin, doxycycline, or quinolones. For other Gram-positive anaerobes (eg, Finegoldia magna, Peptostreptococcus species, and Clostridium species), there are no studies to guide treatment. Penicillin (or ceftriaxone) would be a good initial therapy, and treatment should be guided by speciation, susceptibilities, and microbiological advice.
Gram-negative anaerobes should be treated with IV ampicillin/sulbactam or amoxicillin/clavulanic acid based on availability, followed by oral metronidazole.
Treatment of Candida Species
Little is published regarding the treatment of FRI caused by Candida or any other fungal species. There are no published guidelines even for PJI. Azzam et al62 suggested that debridement with implant retention is not sufficient to control the infection. Although a few studies have shown a successful outcome with debridement and antifungal therapy,63–65 the IDSA guidelines recommend removal of the implant in PJI. In cases of FRI, we also recommend removal of all foreign material. Various authors have studied the use of antifungals in Candida biofilms. Echinocandins have the ability to treat Candida biofilms and may have a better fungicidal and anti-biofilm action than azoles.66,67 Amphotericin B has as good activity, but is more toxic than echinocandins and can cause renal dysfunction.67–69 When the Candida spp. is sensitive to fluconazole, this is a useful oral follow-on treatment.62,67 It is recommended to continue treatment until bone consolidation is achieved and removal of the implant is possible without compromising stability.
Treatment of Polymicrobial Infections
A high percentage of patients with FRI are diagnosed with a polymicrobial infection. Studies indicate that this can be up to one-third of the FRI population.70,71 Furthermore, within the group of polymicrobial infections, there seems to be a high resistance rate of up to 30%. Therefore, broad-spectrum empiric therapy in FRI should be strongly considered.70 Treatment should be tailored to the individual patient and culture results, as described in the sections above. Expert microbiology advice should be sought for any of these infections.
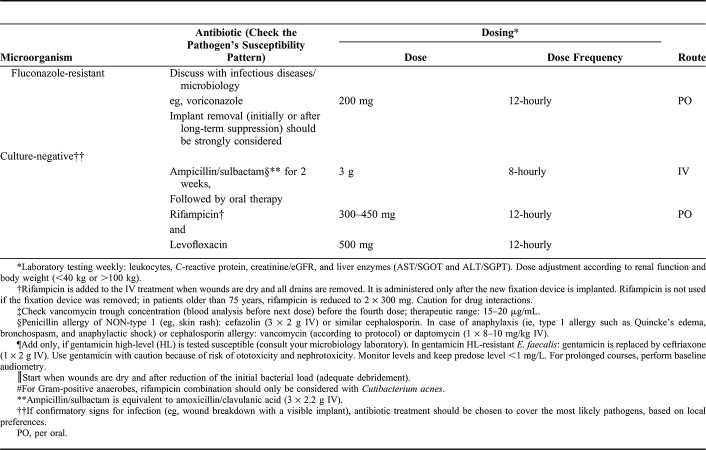
Treatment of Culture-Negative Infections
The exact rate of culture-negative infections in fracture care is unknown. Previous studies published rates that varied between 1% and 16%.72–74 However, the rate of undetected, culture-negative FRIs may be higher, especially in nonunions.75,76 Reasons that cultures remain negative are previous antibiotic treatment before tissue sampling, too few samples (ie, <3 tissue samples) taken during surgery, incorrect localization of sampling, or infections with difficult-to-grow/-to detect organisms. Adequate treatment of culture-negative FRI is severely hampered since antibiotics cannot be targeted to a specific pathogen. Therefore, additional methods to increase pathogen detection, such as implant sonication or molecular techniques (ie, polymerase chain reaction), should be considered in such cases.75,77
If a confirmed FRI stays culture-negative, antibiotic treatment should be chosen to cover the most likely pathogens (Table 1). If cultures are negative but the diagnosis of the FRI is not confirmed, the preferred option is to withhold empirical antibiotic treatment to observe the clinical evolution, and to eventually repeat tissue sampling if possible, this allows the avoidance of antibiotic toxicity and the risk of selecting antibiotic resistance.
Outpatient Parenteral Antibiotic Therapy
The use of outpatient parenteral antibiotic therapy (OPAT) has increased in recent years. It has several advantages, such as shortening the hospital stay, diminishing the risk of nosocomial infections, and lower costs.78 The most useful agents for OPAT are those that can be administered once daily. Intravenous agents that can be given once daily include ceftriaxone, teicoplanin, daptomycin, and ertapenem. Vancomycin can be used in OPAT, but should be administered twice daily with a lengthy infusion each time. Flucloxacillin infusions, using elastomeric pumps, have been used successfully in the community for MSSA infections. Elastomeric pumps can also be used for other antimicrobial agents such as, vancomycin, piperacillin/tazobactam, and cefepime.79
Not every hospital has the resources to organize OPAT therapy. Therefore, the expert group has no specific recommendations for its use in FRI. Where possible an early switch to highly bioavailable, well-tolerated oral antibiotics is the preferred option.
CONCLUSION
Research focusing on systemic antibiotic therapy in FRI is scarce, and attention primarily focuses on PJI. To improve the overall outcome of patients with FRI, there is an urgent need for standardized recommendations for antimicrobial therapy. This review focuses on delivering these recommendations (Table 2), in combination with optimal treatment pathways for FRI patients (Fig. 1) based on up-to-date scientific evidence and expert opinion.
TABLE 2.
Key Recommendations on Antimicrobial Therapy

ACKNOWLEDGMENTS
This article was developed by the FRI consensus group [supported by the AO Foundation, Orthopaedic Trauma Association (OTA), Pro-Implant Foundation, and the European Bone and Joint Infection Society (EBJIS)].
The authors specifically thank the Anti-Infection Task Force (AOTK System; Claas Albers) and the Clinical Priority Program Bone Infection (AOTrauma; Philipp Buescher) for their support of the consensus meetings that were convened in 2016 (Davos, Switzerland) and 2018 (Zürich, Switzerland). Furthermore, the authors thank Jolien Onsea (department of Trauma Surgery, University Hospitals Leuven) and Lois Wallach (AOTK System) for their assistance in preparing and proofreading this article.
Members of the FRI consensus group: Willem-Jan Metsemakers, MD, PhD (Chair); Department of Trauma Surgery, University Hospitals Leuven, Leuven, Belgium; William T. Obremskey, MD, MPH (Chair); Department of Orthopaedic Surgery and Rehabilitation, Vanderbilt University Medical Center, Nashville, TN; Martin A. McNally, MD, FRCS(Orth) (Chair); The Bone Infection Unit, Nuffield Orthopaedic Centre, Oxford University Hospitals, Oxford, United Kingdom; Nick Athanasou, MD, PhD, MRCP, FRCPath; The Bone Infection Unit, Nuffield Orthopaedic Centre, Oxford University Hospitals, Oxford, United Kingdom; Bridget L. Atkins, MD, MBBS, MSc, FRCP, FRCPath; The Bone Infection Unit, Nuffield Orthopaedic Centre, Oxford University Hospitals, Oxford, United Kingdom; Olivier Borens, MD, PhD; Orthopedic Department of Septic Surgery, Orthopaedic-Trauma Unit, Department for the Musculoskeletal System, CHUV, Lausanne, Switzerland; Melissa Depypere, MD; Department of Laboratory Medicine, University Hospitals Leuven, Belgium; Henrik Eckardt, MD; Department of Orthopaedic and Trauma Surgery, University Hospital Basel, Switzerland; Kenneth A. Egol, MD; Department of Orthopedic Surgery, NYU Langone Orthopedic Hospital, New York, NY; William Foster, MD; Department of Orthopaedic Surgery, Virginia Commonwealth University Richmond, VA; Austin T. Fragomen, MD; Hospital for Special Surgery, Limb Lengthening & Complex Reconstruction Service, New York, NY; Geertje A.M. Govaert, MD, PhD; Department of Trauma Surgery, University of Utrecht, University Medical Center Utrecht, Utrecht, the Netherlands; Sven Hungerer, MD; Department of Joint Surgery and Arthroplasty, Trauma Center Murnau, Murnau Germany and Paracelsus Medical University (PMU) Salzburg, Austria; Stephen L. Kates, MD; Department of Orthopaedic Surgery, Virginia Commonwealth University Richmond, VA; Richard Kuehl, MD; Department of Infectious Diseases and Hospital Epidemiology, University Hospital of Basel, Switzerland; Leonard Marais, MD, PhD; Department of Orthopaedics, School of Clinical Medicine, University of KwaZulu-Natal, Durban, South Africa; Ian Mcfadyen, MD; Department of Orthopaedic Surgery, University Hospitals of North Midlands, Stoke-on-Trent, United Kingdom; Mario Morgenstern, MD; Department of Orthopaedic and Trauma Surgery, University Hospital Basel, Switzerland; T. Fintan Moriarty, PhD; AO Research Institute Davos, Switzerland; Peter Ochsner, MD; Medical University Basel, Switzerland; Alex Ramsden, MD; The Bone Infection Unit, Nuffield Orthopaedic Centre, Oxford University Hospitals, Oxford, United Kingdom; Michael Raschke, MD, PhD; Department of Trauma Surgery, University Hospital of Münster, Germany; R. Geoff Richards, PhD; AO Research Institute Davos, Switzerland; Carlos Sancineto, MD; Department of Orthopaedics, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina; Charalampos Zalavras, MD, PhD; Department of Orthopaedic Surgery, Keck School of Medicine, University of Southern California, Los Angeles; Eric Senneville, MD, PhD; Department of Infectious Diseases, Gustave Dron Hospital, University of Lille, France; Andrej Trampuz, MD; Center for Musculoskeletal Surgery, Charité—Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany; Michael H.J. Verhofstad, MD, PhD; Department of Trauma Surgery, Erasmus University Medical Centre, Rotterdam, the Netherlands; and Werner Zimmerli, MD; Interdisciplinary Unit for Orthopedic Infections, Kantonsspital Baselland, Liestal, Switzerland.
Footnotes
The source of funding with respect to hosting the consensus meeting was the AO Foundation (AOTK system and AOTrauma). Open access publication was funded by the AO Foundation and the Orthopaedic Trauma Association (OTA).
The authors report no conflict of interest.
The FRI Consensus group writing committee members are included in the “Acknowledgments” section.
M. Depypere first authorship.
A. Trampuz last (senior) authorship.
Members of the FRI Consensus Group are listed in Acknowledgments section.
REFERENCES
- 1.Ivy MI, Thoendel MJ, Jeraldo PR, et al. Direct detection and identification of prosthetic joint infection pathogens in synovial fluid by metagenomic shotgun sequencing. J Clin Microbiol. 2018;56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jhajharia K, Parolia A, Shetty KV, et al. Biofilm in endodontics: a review. J Int Soc Prev Community Dent. 2015;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metsemakers WJ, Kuehl R, Moriarty TF, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury. 2018;49:511–522. [DOI] [PubMed] [Google Scholar]
- 4.Barberan J, Aguilar L, Carroquino G, et al. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am J Med. 2006;119:993.e997–910. [DOI] [PubMed] [Google Scholar]
- 5.Metsemakers WJ, Fragomen AT, Moriarty TF, et al. Evidence-based recommendations for local antimicrobial strategies and dead space management in Fracture-Related Infection. J Orthop Trauma. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. [DOI] [PubMed] [Google Scholar]
- 7.Kavanagh N, Ryan EJ, Widaa A, et al. Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin Microbiol Rev. 2018;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282:198–206. [DOI] [PubMed] [Google Scholar]
- 9.Li HK, Scarborough M, Zambellas R, et al. Oral versus intravenous antibiotic treatment for bone and joint infections (OVIVA): study protocol for a randomised controlled trial. Trials. 2015;16:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Widmer AF, Gaechter A, Ochsner PE, et al. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin Infect Dis. 1992;14:1251–1253. [DOI] [PubMed] [Google Scholar]
- 12.Aboltins CA, Dowsey MM, Buising KL, et al. Gram-negative prosthetic joint infection treated with debridement, prosthesis retention and antibiotic regimens including a fluoroquinolone. Clin Microbiol Infect. 2011;17:862–867. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Pastor JC, Munoz-Mahamud E, Vilchez F, et al. Outcome of acute prosthetic joint infections due to gram-negative bacilli treated with open debridement and retention of the prosthesis. Antimicrob Agents Chemother. 2009;53:4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS. 2017;125:353–364. [DOI] [PubMed] [Google Scholar]
- 15.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govaert GAM, Kuehl R, Atkins BL, et al. Diagnosing fracture-related infection: current concepts and recommendations. J Orthop Trauma. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metsemakers WJ, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury. 2018;49:505–510. [DOI] [PubMed] [Google Scholar]
- 18.Garzoni C, Uckay I, Belaieff W, et al. In vivo interactions of continuous flucloxacillin infusion and high-dose oral rifampicin in the serum of 15 patients with bone and soft tissue infections due to Staphylococcus aureus—a methodological and pilot study. Springerplus. 2014;3:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donlan RM. Role of biofilms in antimicrobial resistance. Asaio J. 2000;46:S47–S52. [DOI] [PubMed] [Google Scholar]
- 20.Eisenstein BI, Oleson FB, Jr, Baltz RH. Daptomycin: from the mountain to the clinic, with essential help from Francis Tally, MD. Clin Infect Dis. 2010;50(suppl 1):S10–S15. [DOI] [PubMed] [Google Scholar]
- 21.Saleh-Mghir A, Muller-Serieys C, Dinh A, et al. Adjunctive rifampin is crucial to optimizing daptomycin efficacy against rabbit prosthetic joint infection due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:4589–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Helou OC, Berbari EF, Lahr BD, et al. Efficacy and safety of rifampin containing regimen for staphylococcal prosthetic joint infections treated with debridement and retention. Eur J Clin Microbiol Infect Dis. 2010;29:961–967. [DOI] [PubMed] [Google Scholar]
- 23.Giulieri SG, Graber P, Ochsner PE, et al. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection. 2004;32:222–228. [DOI] [PubMed] [Google Scholar]
- 24.Widmer AF, Frei R, Rajacic Z, et al. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J Infect Dis. 1990;162:96–102. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerli W, Widmer AF, Blatter M, et al. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537–1541. [DOI] [PubMed] [Google Scholar]
- 26.Achermann Y, Eigenmann K, Ledergerber B, et al. Factors associated with rifampin resistance in staphylococcal periprosthetic joint infections (PJI): a matched case-control study. Infection. 2013;41:431–437. [DOI] [PubMed] [Google Scholar]
- 27.Sendi P, Zimmerli W. Antimicrobial treatment concepts for orthopaedic device-related infection. Clin Microbiol Infect. 2012;18:1176–1184. [DOI] [PubMed] [Google Scholar]
- 28.Aboltins CA, Page MA, Buising KL, et al. Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid. Clin Microbiol Infect. 2007;13:586–591. [DOI] [PubMed] [Google Scholar]
- 29.Cooper EC, Curtis N, Cranswick N, et al. Pristinamycin: old drug, new tricks? J Antimicrob Chemother. 2014;69:2319–2325. [DOI] [PubMed] [Google Scholar]
- 30.Valour F, Boibieux A, Karsenty J, et al. Pristinamycin in the treatment of MSSA bone and joint infection. J Antimicrob Chemother. 2016;71:1063–1070. [DOI] [PubMed] [Google Scholar]
- 31.Niska JA, Shahbazian JH, Ramos RI, et al. Vancomycin-rifampin combination therapy has enhanced efficacy against an experimental Staphylococcus aureus prosthetic joint infection. Antimicrob Agents Chemother. 2013;57:5080–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoyo I, Martinez-Pastor J, Garcia-Ramiro S, et al. Decreased serum linezolid concentrations in two patients receiving linezolid and rifampicin due to bone infections. Scand J Infect Dis. 2012;44:548–550. [DOI] [PubMed] [Google Scholar]
- 33.Rubinstein E, Isturiz R, Standiford HC, et al. Worldwide assessment of linezolid's clinical safety and tolerability: comparator-controlled phase III studies. Antimicrob Agents Chemother. 2003;47:1824–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 35.Metzler K, Hansen GM, Hedlin P, et al. Comparison of minimal inhibitory and mutant prevention drug concentrations of 4 fluoroquinolones against clinical isolates of methicillin-susceptible and -resistant Staphylococcus aureus. Int J Antimicrob Agents. 2004;24:161–167. [DOI] [PubMed] [Google Scholar]
- 36.Landersdorfer CB, Kinzig M, Hennig FF, et al. Penetration of moxifloxacin into bone evaluated by Monte Carlo simulation. Antimicrob Agents Chemother. 2009;53:2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bispo PJ, Alfonso EC, Flynn HW, et al. Emerging 8-methoxyfluoroquinolone resistance among methicillin-susceptible Staphylococcus epidermidis isolates recovered from patients with endophthalmitis. J Clin Microbiol. 2013;51:2959–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noguchi N, Okihara T, Namiki Y, et al. Susceptibility and resistance genes to fluoroquinolones in methicillin-resistant Staphylococcus aureus isolated in 2002. Int J Antimicrob Agents. 2005;25:374–379. [DOI] [PubMed] [Google Scholar]
- 39.Wouthuyzen-Bakker M, Tornero E, Morata L, et al. Moxifloxacin plus rifampin as an alternative for levofloxacin plus rifampin in the treatment of a prosthetic joint infection with Staphylococcus aureus. Int J Antimicrob Agents. 2018;51:38–42. [DOI] [PubMed] [Google Scholar]
- 40.Nijland HM, Ruslami R, Suroto AJ, et al. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis. 2007;45:1001–1007. [DOI] [PubMed] [Google Scholar]
- 41.Bouazza N, Pestre V, Jullien V, et al. Population pharmacokinetics of clindamycin orally and intravenously administered in patients with osteomyelitis. Br J Clin Pharmacol. 2012;74:971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernard A, Kermarrec G, Parize P, et al. Dramatic reduction of clindamycin serum concentration in staphylococcal osteoarticular infection patients treated with the oral clindamycin-rifampicin combination. J Infect. 2015;71:200–206. [DOI] [PubMed] [Google Scholar]
- 43.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. [DOI] [PubMed] [Google Scholar]
- 44.Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013;56:182–194. [DOI] [PubMed] [Google Scholar]
- 45.Lora-Tamayo J, Senneville E, Ribera A, et al. The not-so-good prognosis of streptococcal periprosthetic joint infection managed by implant retention: the results of a large multicenter study. Clin Infect Dis. 2017;64:1742–1752. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Pardo D, Pigrau C, Lora-Tamayo J, et al. Gram-negative prosthetic joint infection: outcome of a debridement, antibiotics and implant retention approach. A large multicentre study. Clin Microbiol Infect. 2014;20:O911–O919. [DOI] [PubMed] [Google Scholar]
- 47.Akgun D, Trampuz A, Perka C, et al. High failure rates in treatment of streptococcal periprosthetic joint infection: results from a seven-year retrospective cohort study. Bone Joint J. 2017;99-B:653–659. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez Moreno M, Trampuz A, Di Luca M. Synergistic antibiotic activity against planktonic and biofilm-embedded Streptococcus agalactiae, Streptococcus pyogenes and Streptococcus oralis. J Antimicrob Chemother. 2017;72:3085–3092. [DOI] [PubMed] [Google Scholar]
- 49.Ruparelia N, Atkins BL, Hemingway J, et al. Pristinamycin as adjunctive therapy in the management of Gram-positive multi-drug resistant organism (MDRO) osteoarticular infection. J Infect. 2008;57:191–197. [DOI] [PubMed] [Google Scholar]
- 50.Oliva A, Furustrand Tafin U, Maiolo EM, et al. Activities of fosfomycin and rifampin on planktonic and adherent Enterococcus faecalis strains in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2014;58:1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dijkmans AC, Zacarias NVO, Burggraaf J, et al. Fosfomycin: pharmacological, clinical and future perspectives. Antibiot (Basel). 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Widmer AF, Wiestner A, Frei R, et al. Killing of nongrowing and adherent Escherichia coli determines drug efficacy in device-related infections. Antimicrob Agents Chemother. 1991;35:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corvec S, Furustrand Tafin U, Betrisey B, et al. Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-beta-lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrob Agents Chemother. 2013;57:1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka G, Shigeta M, Komatsuzawa H, et al. Effect of the growth rate of Pseudomonas aeruginosa biofilms on the susceptibility to antimicrobial agents: beta-lactams and fluoroquinolones. Chemotherapy. 1999;45:28–36. [DOI] [PubMed] [Google Scholar]
- 55.Bulitta JB, Ly NS, Yang JC, et al. Development and qualification of a pharmacodynamic model for the pronounced inoculum effect of ceftazidime against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renz N, Mudrovcic S, Perka C, et al. Orthopedic implant-associated infections caused by Cutibacterium spp. - a remaining diagnostic challenge. PLoS One. 2018;13:e0202639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Achermann Y, Goldstein EJ, Coenye T, et al. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27:419–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crane JK, Hohman DW, Nodzo SR, et al. Antimicrobial susceptibility of Propionibacterium acnes isolates from shoulder surgery. Antimicrob Agents Chemother. 2013;57:3424–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moon SH, Roh HS, Kim YH, et al. Antibiotic resistance of microbial strains isolated from Korean acne patients. J Dermatol. 2012;39:833–837. [DOI] [PubMed] [Google Scholar]
- 60.Nakase K, Nakaminami H, Noguchi N, et al. First report of high levels of clindamycin-resistant Propionibacterium acnes carrying erm(X) in Japanese patients with acne vulgaris. J Dermatol. 2012;39:794–796. [DOI] [PubMed] [Google Scholar]
- 61.Furustrand Tafin U, Corvec S, Betrisey B, et al. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56:1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azzam K, Parvizi J, Jungkind D, et al. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am. 2009;91(suppl 6):142–149. [DOI] [PubMed] [Google Scholar]
- 63.Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13:707–712. [DOI] [PubMed] [Google Scholar]
- 64.Fukasawa N, Shirakura K. Candida arthritis after total knee arthroplasty—a case of successful treatment without prosthesis removal. Acta Orthop Scand. 1997;68:306–307. [DOI] [PubMed] [Google Scholar]
- 65.Simonian PT, Brause BD, Wickiewicz TL. Candida infection after total knee arthroplasty. Management without resection or amphotericin B. J Arthroplasty. 1997;12:825–829. [DOI] [PubMed] [Google Scholar]
- 66.Chang CC, Slavin MA, Chen SC. New developments and directions in the clinical application of the echinocandins. Arch Toxicol. 2017;91:1613–1621. [DOI] [PubMed] [Google Scholar]
- 67.Rex JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:662–678. [DOI] [PubMed] [Google Scholar]
- 68.Cobo F, Rodriguez-Granger J, Sampedro A, et al. Candida prosthetic joint infection. A review of treatment methods. J Bone Joint Infect. 2017;2:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koutserimpas C, Samonis G, Velivassakis E, et al. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61:266–269. [DOI] [PubMed] [Google Scholar]
- 70.Jorge LS, Fucuta PS, Oliveira MGL, et al. Outcomes and risk factors for polymicrobial posttraumatic osteomyelitis. J Bone Joint Infect. 2018;3:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trampuz A, Zimmerli W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury. 2006;37:59–66. [DOI] [PubMed] [Google Scholar]
- 72.Morgenstern M, Athanasou NA, Ferguson JY, et al. The value of quantitative histology in the diagnosis of fracture-related infection. Bone Joint J. 2018;100-B:966–972. [DOI] [PubMed] [Google Scholar]
- 73.Sheehy SH, Atkins BA, Bejon P, et al. The microbiology of chronic osteomyelitis: prevalence of resistance to common empirical anti-microbial regimens. J Infect. 2010;60:338–343. [DOI] [PubMed] [Google Scholar]
- 74.Kuehl R, Tschudin-Sutter S, Morgenstern M, et al. Time-dependent differences in management and microbiology of orthopaedic internal fixation-associated infections: an observational prospective study with 229 patients. Clin Microbiol Infect. 2019;25:76–81. [DOI] [PubMed] [Google Scholar]
- 75.Gille J, Wallstabe S, Schulz AP, et al. Is non-union of tibial shaft fractures due to nonculturable bacterial pathogens? A clinical investigation using PCR and culture techniques. J Orthop Surg Res. 2012;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palmer MP, Altman DT, Altman GT, et al. Can we trust intraoperative culture results in nonunions? J Orthop Trauma. 2014;28:384–390. [DOI] [PubMed] [Google Scholar]
- 77.Street TL, Sanderson ND, Atkins BL, et al. Molecular diagnosis of orthopedic-device-related infection directly from sonication fluid by metagenomic sequencing. J Clin Microbiol. 2017;55:2334–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mansour O, Heslin J, Townsend JL. Impact of the implementation of a nurse-managed outpatient parenteral antibiotic therapy (OPAT) system in Baltimore: a case study demonstrating cost savings and reduction in re-admission rates. J Antimicrob Chemother. 2018;73:3181–3188. [DOI] [PubMed] [Google Scholar]
- 79.Voumard R, Gardiol C, Andre P, et al. Efficacy and safety of continuous infusions with elastomeric pumps for outpatient parenteral antimicrobial therapy (OPAT): an observational study. J Antimicrob Chemother. 2018;73:2540–2545. [DOI] [PubMed] [Google Scholar]