OBJECTIVES:
The nonsystemic antibiotic rifaximin is indicated for irritable bowel syndrome with diarrhea (IBS-D) in adults; however, determinants of response remain unclear. The utility of lactulose breath testing (LBT) in predicting response to rifaximin was examined.
METHODS:
Adults with IBS-D received open-label rifaximin 550 mg 3 times daily for 2 weeks, followed by a 4-week posttreatment assessment period. Thirteen centers prospectively participated in this substudy. LBT was conducted before (day 1) and after (day 14) therapy (breath samples obtained every 15 minutes; up to 240 minutes). Patient response (decrease from baseline of ≥30% in abdominal pain and ≥50% decrease in frequency of mushy/watery stool), symptom improvement, and the relationship of clinical outcomes to LBT results were assessed.
RESULTS:
A total of 93 patients were included; 62 (66.7%) had positive baseline LBT results. Overall, 48.4% (45/93) of patients responded to rifaximin; of these, 59.7% (37/62) had a positive baseline LBT vs 25.8% (8/31) with a negative LBT (P = 0.002; odds ratio 4.3, 95% confidence interval, 1.5–12.7). Patients with a positive baseline LBT result experienced significantly greater improvement from baseline in 6 of 7 individual IBS symptoms. LBT results after rifaximin therapy did not correlate with clinical response in the 86 patients with evaluable breath tests (P = 0.21); however, patients whose LBT results normalized after rifaximin had the highest response rate of 76.5% (13/17).
DISCUSSION:
A positive baseline LBT result predicted a higher likelihood of response to rifaximin in IBS-D, suggesting a gut microbiome modulatory mechanism of action for rifaximin.
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal disorders and, based on Rome criteria, can be categorized by the predominant stool type: IBS with diarrhea (IBS-D), IBS mixed type, and IBS with constipation (1). There are a number of hypotheses for the etiology of IBS, and changes in the gut microbiome appear to play an important role (2,3). Rifaximin has been shown to improve abdominal pain, stool consistency, bloating, and bowel movement (BM) urgency (4–7) and is indicated in the United States for the treatment of IBS-D in adults (8).
Although rifaximin is classified as a nonsystemic antibiotic (8), the exact mechanism of action by which rifaximin exerts its beneficial effects is unclear. The debate over its mechanism of action is heightened by stool sequencing data of patients with IBS or inflammatory bowel disease, which suggest minimal changes in the stool microbiome with the administration of rifaximin (9–11). Such findings suggest that the mechanism of action of rifaximin may involve targeting selected bacterial strata and preventing mucosal inflammation that could lead to increased mucosal permeability, or through changes in the small intestinal microbiome, rather than the colonic microbiome (12–15).
Multiple studies on drugs that are effective in IBS have failed to identify a robust and clinically relevant determinant of response (16). One goal of therapy should be a tailored approach to the management of IBS. A goal of maximizing symptom improvement would be highly valued, as this can potentially decrease direct and indirect health care costs (16,17). Symptoms similar to those observed in IBS-D are frequently reported in patients with small intestinal bacterial overgrowth (SIBO), and a North American consensus statement has standardized the preparation, performance, and interpretation of lactulose breath testing (LBT) in the diagnosis of SIBO (18). In the current study, the utility of breath testing to predict the response to rifaximin in the treatment of IBS-D and the potential to add granularity around the mechanism of action of rifaximin in IBS were evaluated.
METHODS
Patients
A subgroup of 13 centers from the phase 3 TARGET 3 trial (targeted, nonsystemic antibiotic rifaximin gut-selective evaluation of treatment for IBS-D; ClinicalTrials.gov ID: NCT01543178) prospectively participated in the LBT substudy. Patients included in the substudy met all eligibility requirements for the TARGET 3 main study. Details on the study design and patient population for the TARGET 3 trial have been previously described (6). Briefly, patients aged 18 years or older with a diagnosis of IBS-D (Rome III criteria) were eligible if they had inadequate relief of global IBS symptoms and bloating during a 10-day placebo screening phase. Patients were required to have a minimum baseline severity of symptoms for study entry, including average abdominal pain ≥3 (scale 0–10: 0 = no pain; 10 = worst possible pain), bloating ≥3 (scale 0–6: 0 = not at all; 6 = a very great deal), and loose stools for ≥2 days a week (Bristol Stool Scale [BSS] type 6 or 7) (6). Exclusion criteria included use of antidiarrheals, antispasmodics, narcotics, prokinetic drugs, drugs indicated for IBS, or probiotics during the placebo-screening phase and any antibiotic within 14 days before providing informed consent. The substudy was approved by all institutional review boards and ethics committees at participating sites, and all patients provided written informed consent. Both investigators and patients were blinded to LBT results.
Study design and LBT
Eligible patients received open-label rifaximin 550 mg 3 times daily for 2 weeks followed by a 4-week posttreatment period to assess response (6). Response was defined as a composite endpoint of simultaneously achieving weekly response criteria for abdominal pain (≥30% decrease from baseline in mean weekly pain score) and stool consistency (≥50% decrease from baseline in number of days/week with BSS type 6 or 7 stool) during ≥2 of the first 4 weeks after treatment. Average daily symptom scores for abdominal pain, bloating, IBS symptoms, BSS score, stool frequency, days/week with loose/watery BMs, and days/week with BM urgency were determined during the first 4 weeks after treatment. During an additional 18 weeks, responders were followed up for recurrence (<30% decrease from baseline in mean weekly pain score or <50% decrease from baseline in number of days/week with BSS type 6 or 7 stool for ≥3 weeks of a consecutive, rolling 4-week period). Safety was evaluated as previously described (6). LBT was conducted at baseline (before [day 1]) and after the 2-week course of rifaximin (day 14; with +5-day flexibility to day 19).
Preparation for breath testing included avoidance of highly fermentable products or a heavy meal the night before testing (24-hours before test) and a 12-hour fasting period to minimize baseline residual fermentation products. Breath testing was conducted using a commercially available mail-in kit (Commonwealth Labs, Salem, MA). Patients provided end-alveolar air by exhaling continuously to end expiration into prelabeled containers. Sampling was performed at baseline and every 15 minutes for up to 240 minutes after the ingestion of 10-g lactulose powder dissolved in 4 ounces (∼120 mL) of water. Breath samples were then extracted and analyzed centrally for hydrogen (H2), methane (CH4), and carbon dioxide (CO2) levels using gas chromatography. To standardize for alveolar levels, H2 and CH4 levels were corrected to the expected alveolar CO2 concentration (18).
Gas measurements were available for all breath tests, and the results were classified into the following 6 categories: normal (no excessive CH4 or H2 concentration rise); H2-positive (H2 rise of ≥20 parts per million [ppm] within 90 minutes); CH4-positive (CH4 of ≥10 ppm at any timepoint); H2- and CH4-positive (meeting both H2 and CH4 criteria described above); flatline test (nonmethane fixed-hydrogen producers); and elevated baseline (H2 ≥ 20 ppm at baseline) (18). The breath test results were excluded from the analysis if they had ≥2 undetectable H2/CH4/CO2 gas measurements within the first 90 minutes after already having an H2 measurement of >10 ppm. Undetectable gas measurements implied poor sample collection and substantial room air contamination.
Data analysis
The Student t test was used to assess the effect of baseline demographics and clinical symptoms on response to open-label rifaximin. Logistic regression was used for assessment of confounders and modifying effects of age and sex. The Pearson χ2 test was used to compare categorical variables. When patients were categorized into 3 groups, between-group differences of continuous variables were assessed using analysis of variance. The Bonferroni method was used to adjust for potential type 1 errors. Area under the concentration-time curve (AUC) for H2 was calculated by adding the CO2-adjusted H2 levels within the first 90 minutes of the breath test. AUC levels with >2.5 interquartile below the first quartile or above the fourth quartile were considered outliers.
The significance level was set at 5%. Meaningful assessments of the breath test subtype effect, multivariate analysis, and postobservation phase data could not be performed because of the small number of patients in strata. All statistical analyses were performed using a standard software package (Stata V. 15.0; StataCorp LLC, College Station, TX). Because of the exploratory nature of the substudy, no sample size calculation was conducted.
RESULTS
Patient population
Of 98 patients with LBT data, 93 met the criteria for evaluable results and were analyzed (Table 1). The breath test was positive in 62 (66.7%) patients who were categorized as follows: H2-positive (n = 43), CH4-positive (n = 5), H2- and CH4-positive (n = 5), flatline (n = 3), or elevated H2 at baseline (n = 6). Demographics and baseline characteristics were similar between patients with positive vs negative baseline LBT results (Table 1).
Table 1.
Demographics and baseline characteristics
Response to rifaximin
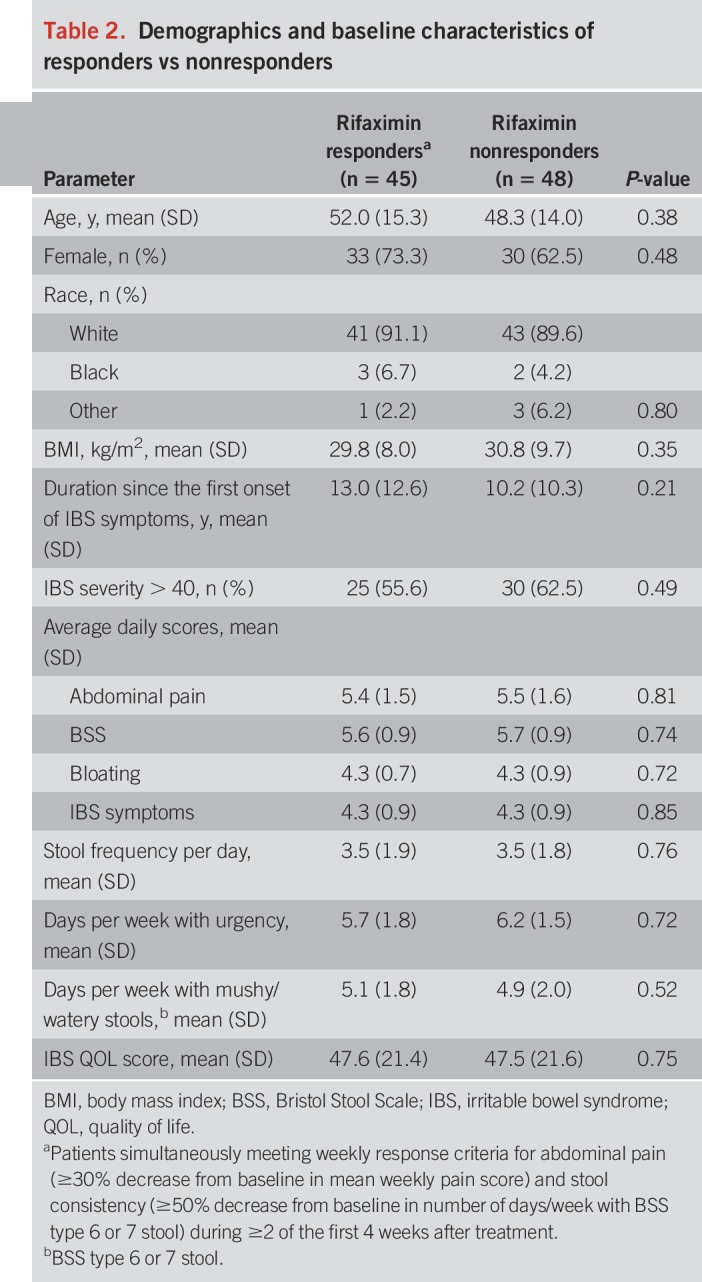
Of the 93 patients, 45 (48.4%) met the criteria for clinical response to the 2-week course of rifaximin, which was similar to the response rate of 44.1% (6) seen in the overall TARGET 3 efficacy-evaluable population (n = 2,438; P = 0.40). There were no significant differences in baseline demographics or IBS-D symptom profile between responders and nonresponders in the current study, and none of the baseline parameters predicted response to therapy (Table 2).
Table 2.
Demographics and baseline characteristics of responders vs nonresponders
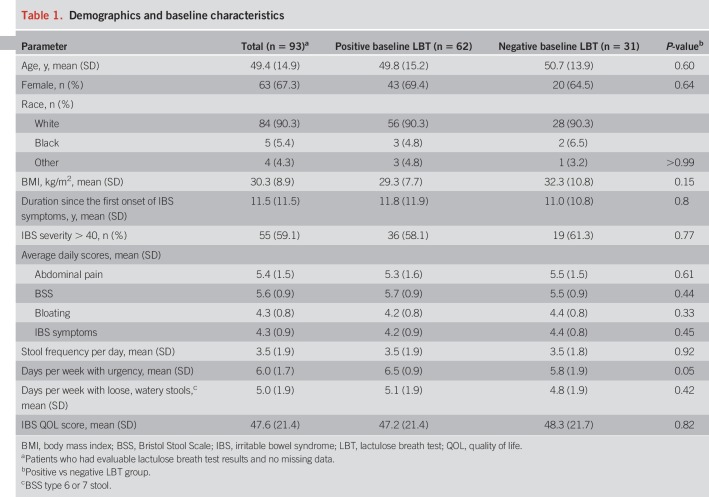
A significantly greater percentage of patients with IBS-D who had a positive baseline LBT responded to open-label rifaximin vs those with a negative baseline LBT (Figure 1). Overall, 48.4% (45/93) of patients responded to rifaximin (composite endpoint): 37 had a positive baseline LBT (37/62 [59.7%]), and 8 patients had a negative LBT (8/31 [25.8%]; Figure 1). The odds of patients with a positive baseline LBT responding to rifaximin (for composite endpoint) were 4.3 times higher (95% confidence interval, 1.5–12.7; P = 0.002) than those for patients with a negative baseline LBT. Age (P = 0.80) and sex (P = 0.61) did not have a confounding or modifying effect on predictability of response by breath testing. The AUC for H2 decreased significantly in rifaximin responders (−53.9 ± 107.4 ppm) compared with nonresponders (+2.3 ± 129.5 ppm; P = 0.04). An insufficient number of patients had excessive CH4 for a meaningful analysis of CH4 AUC changes.
Figure 1.
Responders* to treatment with rifaximin categorized by baseline LBT results. *Patients simultaneously meeting weekly response criteria for abdominal pain (≥30% decrease from baseline in mean weekly pain score) and stool consistency (≥50% decrease from baseline in number of days/week with BSS type 6 or 7 stool) during ≥2 of the first 4 weeks after treatment (6). BSS, Bristol Stool Scale; LBT, lactulose breath test.
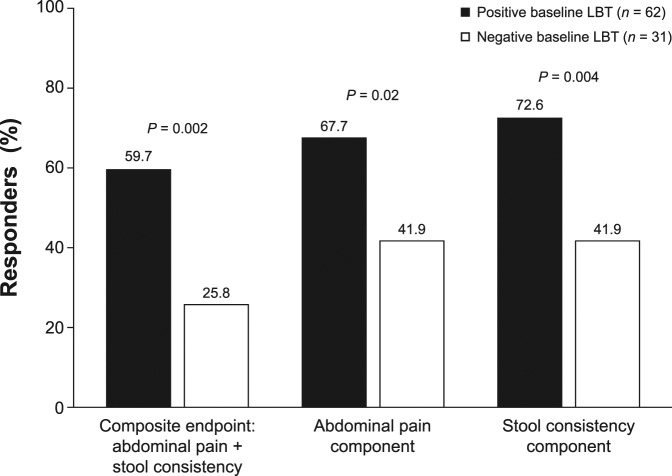
For the individual components of the composite endpoint, a significantly greater percentage of patients with a positive baseline LBT were responders for abdominal pain or stool consistency (Figure 1). Patients with a positive baseline LBT had significantly greater improvement from baseline in 6 of the 7 individual IBS-D symptoms assessed vs patients with a negative baseline LBT (Table 3).
Table 3.
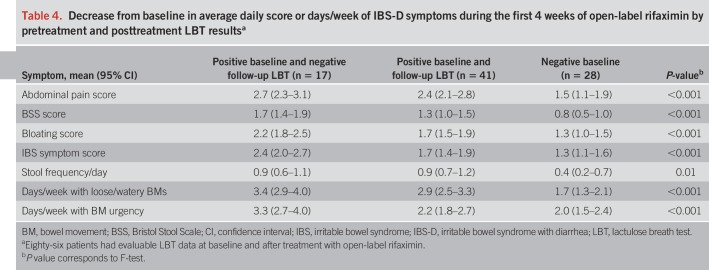
Decrease from baseline in average daily score or days/week of IBS-D symptoms during the first 4 weeks after open-label rifaximin therapy, by baseline LBT resulta
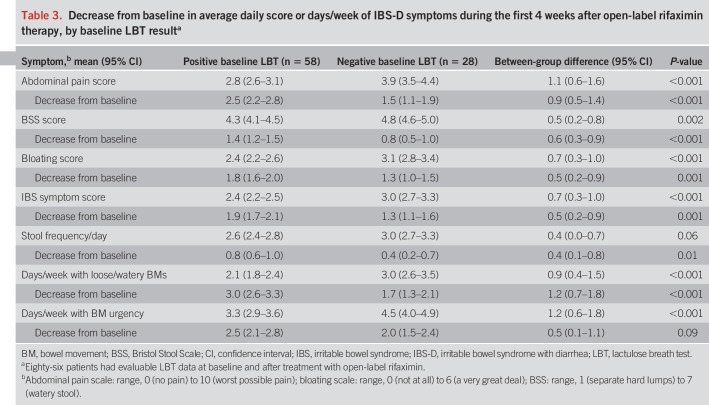
After rifaximin therapy, 86 patients had evaluable breath test results (n = 58 positive baseline LBT; n = 28 negative baseline LBT). Of the 58 patients with a positive baseline LBT, 17 (29.3%) had normalization of their LBT after treatment. Thirteen of the 17 patients (76.5%) with an abnormal baseline LBT that normalized after treatment responded (Figure 2). Furthermore, as compared with patients with a negative baseline LBT, patients who had a positive LBT at baseline that normalized after treatment also showed a greater improvement in all 7 individual IBS-D symptoms evaluated (Table 4). When response to rifaximin was assessed solely based on posttreatment LBT results, the presence or absence of a normal LBT did not correlate with the clinical response to rifaximin (P = 0.21).
Figure 2.
Responders* to rifaximin categorized by pretreatment and posttreatment LBT results. Fifty-eight patients with a positive baseline LBT had evaluable LBT data after treatment with open-label rifaximin; 31 patients had a negative baseline LBT. *Patients simultaneously meeting weekly response criteria for abdominal pain (≥30% decrease from baseline in mean weekly pain score) and stool consistency (≥50% decrease from baseline in number of days/week with BSS type 6 or 7 stool) during ≥2 of the first 4 weeks after treatment (6). BSS, Bristol Stool Scale; LBT, lactulose breath test.
Table 4.
Decrease from baseline in average daily score or days/week of IBS-D symptoms during the first 4 weeks of open-label rifaximin by pretreatment and posttreatment LBT resultsa
Symptom recurrence after response to therapy
Of the 93 patients, 45 responded to rifaximin and entered the 18-week observation phase for assessment of symptom recurrence. Average time to recurrence was 94.8 ± 38.6 days; 7 (15.6%) of the 45 patients did not experience symptom recurrence by the end of the 18-week observation phase. The difference in the rate of recurrence in patients with a positive baseline LBT vs those with a negative baseline LBT was not significant (64.8% vs 75.0%; P = 0.58). Posttreatment LBT (P = 0.11) or the combination of pre/post LBT results did not predict recurrence (P = 0.33).
Safety
Of the 93 patients, 40 patients reported ≥1 adverse event (AE) during the open-label phase (2 weeks of treatment, 4 weeks of evaluation, and up to 18 weeks of observation). The most common AEs were upper respiratory tract related (i.e., rhinitis, sinusitis, or upper respiratory tract infection) and occurred in 15 of the 40 patients; all instances were considered by the investigator to be unrelated to study drug. Among patients who reported ≥1 AE, there was no significant difference based on pretreatment LBT results (46.8% [29/62; positive LBT] vs 35.5% [11/31; negative LBT]; P = 0.30) or posttreatment LBT (34.0% [17/50; normalized LBT] vs 47.2% [17/36; positive LBT]; P = 0.63). Three patients (1 of 31 patients with a negative baseline LBT [3.2%] and 2 of 62 patients [3.2%] with a positive baseline LBT) experienced an AE of headache, which was considered a drug-related AE. One patient experienced an AE identified as right knee pain of severe intensity with swelling; it was considered by the investigator to be unrelated to study drug.
DISCUSSION
In this substudy of a large, multicenter trial of a 2-week course of rifaximin to treat IBS-D, breath testing appeared to be an important predictor of response to treatment using the US Food and Drug Administration (FDA) endpoints along with abdominal pain, bloating, BM frequency, BM urgency, and stool consistency. Response to rifaximin was seen in 25.8% of patients with a negative LBT, whereas the response rate increased to 59.7% with a positive baseline LBT (number needed to treat [NNT] of 2.9). Furthermore, patients with an abnormal baseline LBT that normalized after rifaximin treatment had a response rate of 76.5%.
During the past 2 decades, there has been growing interest in the role of the gut microbiome in human health and disease (19). The first drug to receive FDA approval as a gut microbiome–based therapy was rifaximin. Rifaximin has demonstrated superiority to placebo in improving abdominal pain, stool consistency, and bloating symptoms (4–6,20).
Despite rifaximin being classified as a nonsystemic antibiotic, there has been some debate on the exact mechanism of action of this agent. This is further confounded by stool microbiome data revealing a near lack of impact on the stool microbiome in IBS (9–11). Because of these findings, researchers have speculated that rifaximin may be acting more proximal to the colon (e.g., small intestine), or that it may have an effect independent of traditional antibiotic activity. What is clear from phase 3 data is that a 2-week course of rifaximin has the potential for long-term benefit (e.g., 35.6% of responders did not experience symptom recurrence during 22 weeks of follow-up in TARGET 3) (6). This supports the concept that rifaximin was treating the underlying IBS pathophysiology in a subgroup of patients and not just providing drug-induced symptom control.
Concurrently, data from published studies using breath testing, culture, and sequencing (21) have supported the possibility that at least some of the symptoms of IBS can be explained as part of a small bowel microbiome condition and may be part of the spectrum of SIBO (21). Nevertheless, there remains controversy about the role of breath testing in IBS and even whether breath testing represents SIBO (22). To address these issues, a North American Consensus group published its summation on breath testing in 2017 (18). Importantly, the group recognized the limitations of breath testing but provided statements to standardize breath test use and interpretation based on the expanding literature. Since 1999, there has been growing evidence to suggest that IBS is associated with the presence of SIBO (23,24). This is based primarily on breath testing data. A meta-analysis demonstrated that patients with IBS were more likely to have positive breath test results compared with healthy controls among age- and sex-matched studies, suggesting that some patients with IBS may have alterations in the gut microbiota relative to healthy individuals (25). Controversy continues despite small bowel culture data (26,27) and even deep sequencing results (28). Most of the controversy surrounds the use of breath testing, sampling error/contamination of small bowel aspirates, and lack of a true “gold standard” for diagnosis of SIBO. Nevertheless, the current study supports breath testing as a predictor of response in patients with IBS-D.
The current study is unique for a number of reasons. This is the first study examining breath testing in a multicenter-registered trial of IBS-D using the new FDA endpoint. Furthermore, breath sample analysis was centralized, and participants and investigators were blinded to the test results. In the current study, although baseline breath testing results predicted response to therapy, they did not predict time to recurrence of symptoms in patients who initially responded to therapy. This underlines the complexity of gut microbiome balance in patients with IBS-D and various factors that control it. Studies assessing the gut microbiome in regular intervals after response to therapy are needed to shed more light on the utility of breath testing in determination of clinical symptom recurrence. This effect was further demonstrated among patients with an initial abnormal breath test that was normalized by rifaximin. Patients with a positive LBT did not experience more AEs. Hence, breath testing can improve the NNT without any change in number needed to harm.
The study has a number of limitations. The breath test substudy, which included 93 patients, represented a subpopulation of the larger TARGET 3 trial (n = 2,438) (6). However, participants were selected from several study sites. Furthermore, as breath samples were analyzed centrally, patients and study personnel were blinded to the breath test results. The TARGET 3 trial was designed to assess the efficacy of repeat treatment with rifaximin, with patients who responded and then experienced recurrence enrolled in a randomized, double-blind, placebo-controlled phase (6). The LBT substudy sample size was too small and thus not powered for examination of the effect of breath testing through the double-blind portion of the trial. Breath testing was conducted using mail-in breath testing kits. Although this technique ensures concealment of the blinding procedure and allows for central processing of samples, it may be associated with poor sample collection. The current LBT analyses used strict inclusion criteria and excluded samples with poor collection technique. In addition, meaningful assessments of CH4 AUC and potential differences in rifaximin response between CH4 and H2 producers could not be performed because too few patients had positive CH4 levels. This is not surprising, given that excessive CH4 excretion is not a common finding in patients with IBS-D (29). Similarly, the number of patients with elevated baseline breath test results was too small to allow for a meaningful analysis of this subgroup of patients. Finally, dichotomizing test results with continuous measurements into normal and abnormal results decreases the accuracy of the tests, especially with values close to cutoff limits. Studies with a larger sample size would thus be needed to determine the values of specific rises or declines in gas measurements.
The findings of the current study have potential clinical implications. First, these data suggest that the mechanism of action of rifaximin may indeed be on the gut microbiome, based on changes in fermentation on breath testing. This also enhances the possibility of modifying the gut microbiome to treat IBS-D. Although not addressed in the current study, there is evidence that rifaximin has modest effects on the colonic microbiome, affecting microbial richness and diversity (10,11). Therefore, the findings of the current study open the possibility that the small bowel microbiome may have some importance in the pathophysiology of IBS-D (9–11). Although breath testing is the strongest known predictor of response to rifaximin in patients with IBS-D (NNT = 2.9), it did not predict response in all patients, suggesting that rifaximin may have another mechanism of action. This underscores the heterogeneity of IBS and the need to develop biomarkers that can help further stratify IBS subtypes based on the underlying pathophysiology.
In conclusion, this study demonstrates that breath testing may be an important predictor of response to rifaximin in the treatment of IBS-D. More specifically, the greatest likelihood of improvement was predicted by having a baseline “abnormal” LBT, which was normalized by rifaximin in patients with positive H2 breath test results. This is the first multicenter trial in IBS-D to demonstrate the utility of baseline breath testing as a predictor of response to rifaximin, and the data may be consistent with a gut microbiome modulatory mechanism of action for rifaximin.
CONFLICTS OF INTEREST
Guarantor of the article: Ali Rezaie, MD, FRCP(C), MEpi.
Specific author contributions: A.R. and M.P. conducted the analyses. A.R. wrote the first draft of the manuscript. All authors critically revised the manuscript and approved the final version of the article.
Financial support: This trial was supported by Salix Pharmaceuticals, Bridgewater, NJ, in collaboration with academic investigators and the US Food and Drug Administration. An editorial consultant was paid by Salix Pharmaceuticals to assist in revising and editing the manuscript. All authors had full access to the data, approved the final published manuscript, and affirmed the integrity of the data and analyses.
Potential competing interests: A.R. reports serving as a consultant for and receiving research grants from Salix Pharmaceuticals and serving as a consultant for GutHub. Z.H. is an employee of Salix Pharmaceuticals. R.M. reports being a speaker for Salix Pharmaceuticals. M.P. reports serving as a consultant for and receiving research grants from Salix Pharmaceuticals. In addition, Cedars-Sinai Medical Center has a licensing agreement with Salix Pharmaceuticals and Gemelli Biotech. A.R. and M.P. have equity in Gemelli Biotech.
Study Highlights.
WHAT IS KNOWN
✓ Rifaximin is efficacious and well-tolerated for irritable bowel syndrome with diarrhea (IBS-D) in adults.
✓ Predictors of response to rifaximin are lacking.
WHAT IS NEW HERE
✓ Positive baseline lactulose breath test results increase the likelihood of IBS-D symptom improvement in response to rifaximin.
✓ Baseline characteristics and symptom profiles of patients with IBS-D do not predict response to rifaximin.
✓ Rifaximin may have a positive modulatory effect on the small intestinal microbiome.
ACKNOWLEDGEMENTS
Technical editorial assistance was provided, under the direction of the authors, by Mary Beth Moncrief, PhD, and Sujata Swaminathan, PhD, Synchrony Medical Communications, LLC, West Chester, PA. Funding for this support was provided by Salix Pharmaceuticals, Bridgewater, NJ.
REFERENCES
- 1.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150:1393–407. [DOI] [PubMed] [Google Scholar]
- 2.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA 2015;313:949–58. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy PJ, Cryan JF, Dinan TG, et al. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J Gastroenterol 2014;20:14105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharara AI, Aoun E, Abdul-Baki H, et al. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol 2006;101:326–33. [DOI] [PubMed] [Google Scholar]
- 5.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011;364:22–32. [DOI] [PubMed] [Google Scholar]
- 6.Lembo A, Pimentel M, Rao SS, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 2016;151:1113–21. [DOI] [PubMed] [Google Scholar]
- 7.Menees SB, Maneerattannaporn M, Kim HM, et al. The efficacy and safety of rifaximin for the irritable bowel syndrome: A systematic review and meta-analysis. Am J Gastroenterol 2012;107:28–35. [DOI] [PubMed] [Google Scholar]
- 8.Xifaxan® (Rifaximin) Tablets, for Oral Use. Salix Pharmaceuticals: Bridgewater, NJ, 2018. [Google Scholar]
- 9.Maccaferri S, Vitali B, Klinder A, et al. Rifaximin modulates the colonic microbiota of patients with Crohn's disease: An in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother 2010;65:2556–65. [DOI] [PubMed] [Google Scholar]
- 10.Acosta A, Camilleri M, Shin A, et al. Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Clin Transl Gastroenterol 2016;7:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fodor AA, Pimentel M, Chey WD, et al. Rifaximin is associated with modest, transient decreases in multiple taxa in the gut microbiota of patients with diarrhoea-predominant irritable bowel syndrome. Gut Microbes 2019;10:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauritano EC, Gabrielli M, Lupascu A, et al. Rifaximin dose-finding study for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2005;22:31–5. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Lee HR, Low K, et al. Rifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBS. Dig Dis Sci 2008;53:169–74. [DOI] [PubMed] [Google Scholar]
- 14.Darkoh C, Lichtenberger LM, Ajami N, et al. Bile acids improve the antimicrobial effect of rifaximin. Antimicrob Agents Chemother 2010;54:3618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu D, Gao J, Gillilland M, III, et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014;146:484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol 2018;113:1–18. [DOI] [PubMed] [Google Scholar]
- 17.Schiller LR. Evaluation of chronic diarrhea and irritable bowel syndrome with diarrhea in adults in the era of precision medicine. Am J Gastroenterol 2018;113:660–9. [DOI] [PubMed] [Google Scholar]
- 18.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American consensus. Am J Gastroenterol 2017;112:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet 2017;18:690–9. [DOI] [PubMed] [Google Scholar]
- 20.Pimentel M, Park S, Mirocha J, et al. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: A randomized trial. Ann Intern Med 2006;145:557–63. [DOI] [PubMed] [Google Scholar]
- 21.Ghoshal UC, Shukla R, Ghoshal U. Small intestinal bacterial overgrowth and irritable bowel syndrome: A bridge between functional organic dichotomy. Gut Liver 2017;11:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut 2011;60:334–40. [DOI] [PubMed] [Google Scholar]
- 23.Rezaie A, Pimentel M, Rao SS. How to test and treat small intestinal bacterial overgrowth: An evidence-based approach. Curr Gastroenterol Rep 2016;18:8. [DOI] [PubMed] [Google Scholar]
- 24.Attar A, Flourié B, Rambaud JC, et al. Antibiotic efficacy in small intestinal bacterial overgrowth-related chronic diarrhea: A crossover, randomized trial. Gastroenterology 1999;117:794–7. [DOI] [PubMed] [Google Scholar]
- 25.Shah ED, Basseri RJ, Chong K, et al. Abnormal breath testing in IBS: A meta-analysis. Dig Dis Sci 2010;55:2441–9. [DOI] [PubMed] [Google Scholar]
- 26.Pyleris E, Giamarellos-Bourboulis EJ, Tzivras D, et al. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: Relationship with irritable bowel syndrome. Dig Dis Sci 2012;57:1321–9. [DOI] [PubMed] [Google Scholar]
- 27.Posserud I, Stotzer PO, Björnsson ES, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007;56:802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giamarellos-Bourboulis EJ, Tang J, Pyleris E, et al. Molecular assessment of differences in the duodenal microbiome in subjects with irritable bowel syndrome. Scand J Gastroenterol 2015;50:1076–87. [DOI] [PubMed] [Google Scholar]
- 29.Pimentel M, Mayer AG, Park S, et al. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci 2003;48:86–92. [DOI] [PubMed] [Google Scholar]