Purpose of review
Diabetic kidney disease is a growing problem leading to end-stage kidney disease but also atherosclerotic cardiovascular disease and heart failure. Aldosterone is a key risk factor promoting inflammation and fibrosis causing cardio-renal failure. Current options and challenges with mitigating the risk of aldosterone are reviewed.
Recent findings
More aggressive renin–angiotensin–aldosterone system (RAAS) blockade can be maintained in individuals with hyperkalemia if new potassium binders are added. Aldosterone synthase inhibitors may lower aldosterone without causing hyperkalemia. Novel nonsteroidal mineralocorticoid receptor antagonists (MRA) are able to lower proteinuria and markers of heart failure, with limited potassium problems and without renal impairment. Ongoing clinical trials are evaluating the safety and potential benefits of nonsteroidal MRAs on progression of renal disease and development of cardiovascular outcomes in type 2 diabetes and kidney disease.
Summary
Aldosterone is an important driver of inflammation and fibrosis leading to renal and cardiovascular complications. MRA lower albuminuria but data showing prevention of end-stage kidney disease are lacking. Side effects including hyperkalemia have previously prevented long-term studies in diabetic kidney disease but new treatment strategies with potassium binders, aldosterone synthase inhibitors and nonsteroidal MRA have been developed for clinical testing.
Keywords: aldosterone, diabetic kidney disease, mineralocorticoid receptor antagonist, novel therapy, renin–angiotensin–aldosterone system
INTRODUCTION
Globally one in 11 has diabetes, and numbers are increasing [1]. Controlling multiple risk factors improve outcome [2]. US population-based data demonstrated a 28% reduction in risk for end-stage kidney disease (ESKD) in diabetes from 1990 to 2010, but individuals referred for ESKD treatment increased from 20 000 to 50 000 during this period [3]. Increasing prevalence of diabetes, reduced mortality from cardiovascular disease (CVD) before ESKD, and increased eligibility for treatment of ESKD has contributed, but it also reflects a need for better prevention and treatment of diabetic kidney disease (DKD) [4▪], the leading cause of ESKD. In addition to the risk for ESKD, DKD is associated with a significant increased risk for CVD including atherosclerotic disease and heart failure [5]. At the age of 30, DKD shortens life span by 16 years [6].
This development has occurred during 30 years with increased focus on treatment of hypertension in DKD [7] and in particular implementation of reno-protective treatments with blockade of the renin–angiotensin system (RAS) with angiotensin converting enzyme inhibitors (ACEi) [8] or angiotensin II receptor blockers (ARB) [9,10].
The benefits of ACEi and ARBs in chronic kidney disease (CKD) has been ascribed to reduction in systemic and intraglomerular blood pressure (BP) and proteinuria, but there has been increasing focus on benefits of reduction in aldosterone due to the deleterious effect of overactivation of mineralocorticoid receptors by aldosterone in kidney and heart disease resulting in fibrosis and inflammation [11]. In addition to the effect on mineralocorticoid receptors in the classic location of the distal nephron, these effects are mediated through mineralocorticoid receptors on smooth muscle cells, endothelium, fibroblasts, podocytes and inflammatory cells [12▪▪]. Recently the cardiorenal syndrome was revisited suggesting factors like diabetes and hypertension activating fibrosis and inflammation as a common driver for cardiorenal damage [13▪]. Long-term treatment is often followed by aldosterone breakthrough or escape either because of insufficient blockade of RAS or concomitant increases in potassium [14].
In heart failure with reduced ejection fraction blocking aldosterone with mineralocorticoid receptor antagonists (MRAs) using spironolactone [15] and eplerenone [16] reduced mortality, and in resistant hypertension in diabetes effects on BP were documented [17]. In CKD, including DKD, short-term studies demonstrated a promising reduction in albuminuria when MRAs were add-on to ACEi or ARBs, but with a three-fold increased withdrawal because of hyperkalemia [18]. Hyperkalemia and concern of reduction in renal function has precluded long-term studies targeting aldosterone in CKD. In recent years, new opportunities for reducing the damaging effect of aldosterone have appeared, and this review will summarize opportunities and challenges for lowering aldosterone as seen in Table 1, either by mitigating the risk of hyperkalemia with potassium binders during treatment with RAS inhibitor and steroidal MRA treatment, or use of nonsteroidal MRAs, so far found to have less increase in potassium or inhibition of aldosterone synthase. All have been tested in clinical studies, and the first phase 3 studies are ongoing with the nonsteroidal MRA finerenone (Fig. 1).
Table 1.
Different strategies to reduce the damaging effects of aldosterone in diabetic kidney disease: options, problems and potential solutions
| Target | Intervention and effects | Results | Challenges | Potential solutions |
| Angiotensin-converting enzyme | ACE inhibition [8] ↓Angiotensin II and its effects | ↓BP and proteinuria Long-term benefit on outcomes in DKD demonstrated | Alternative Ang II formation Aldosterone breakthrough Hyperkalemia | Combine with Chymase inhibitor [24▪] Aldosterone-specific intervention (MRA/ASI) Diet, Loop diuretics New potassium binders [39,40] |
| Angiotensin II receptor 1 | ARB [9,10] Block effect mediated from AngIIR stimulation | ↓ BP and proteinuria Long-term benefit on outcomes in DKD demonstrated | Aldosterone breakthrough Hyperkalemia | Combine with Aldosterone-specific intervention (MRA/ASI) Diet, loop diuretics New potassium binders |
| Mineralocorticoid receptor | Mineralocorticoid receptor antagonist [14,15,16,17,18] Blocks MR-mediated effects of aldosterone | ↓proteinuria and BP/resistant hypertension Only surrogate renal outcome data in DKD ↓mortality in heart failure with systolic dysfunction | Spironolactone: hormonal side effects (gynecomastia) Hyperkalemia Increase in aldosterone may increase nongenomic aldosterone effects | Increase selectivity (eplerenone or nonsteroidal MRA) Diet, loop diuretics New potassium binders Nonsteroidal MRA Aldosterone Synthase Inhibition |
| Mineralocorticoid receptor | Nonsteroidal mineralocorticoid receptor antagonist [44,45] Blocks MR mediated effects of aldosterone | ↓ Proteinuria ↓NTproBNP in heart failure with systolic dysfunction and CKD Only surrogate renal outcome data in DKD | Hyperkalemia (less frequent than for steroidal MRA) ↑Aldosterone may increase nongenomic aldosterone effects Not clinically available | Diet, loop diuretics New potassium binders Aldosterone synthase inhibition Tested in ongoing phase III trials FIGARO and FIDELIO |
| Aldosterone synthase | Aldosterone synthase inhibition [48,49] ↓aldosterone (MR and non-MR mediated effects affected) | Lowers aldosterone and blood pressure Limited clinical data | Difficult to develop (selectivity, duration of action) Not clinically available | Needs further development (ongoing preclinical studies) |
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blockers; ASI, aldosterone synthase inhibition; BP, blood pressure; CKD, chronic kidney disease; DKD, diabetic kidney disease; MR, mineralocorticoid receptor; MRA, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal pro-brain natriuretic peptide.
FIGURE 1.
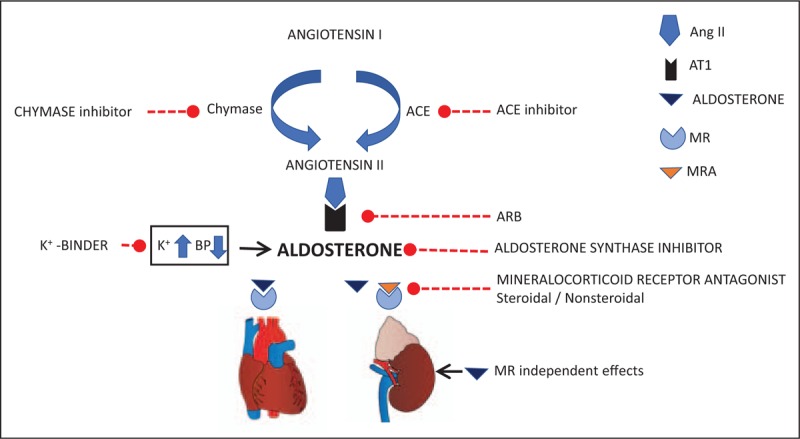
Opportunities to mitigate the effect of aldosterone. Aldosterone drives fibrosis and inflammation, and reducing angiotensin II is made from angiotensin I from angiotensin converting enzyme or chymase activity, and can be reduced with angiotensin converting enzyme inhibitors or chymase inhibitors. Angiotensin II and potassium stimulates production of aldosterone and angiotensin II receptor blockers blocks the angiotensin II receptor type 1. Aldosterone formation can also be mitigated by inhibition of aldosterone synthesis, and the mineralocorticoid receptor mediated effects blocked with steroidal or nonsteroidal mineralocorticoid receptor antagonists. Hyperkalemia is a limiting side effect with several options but may be treated potassium binders.
Box 1.
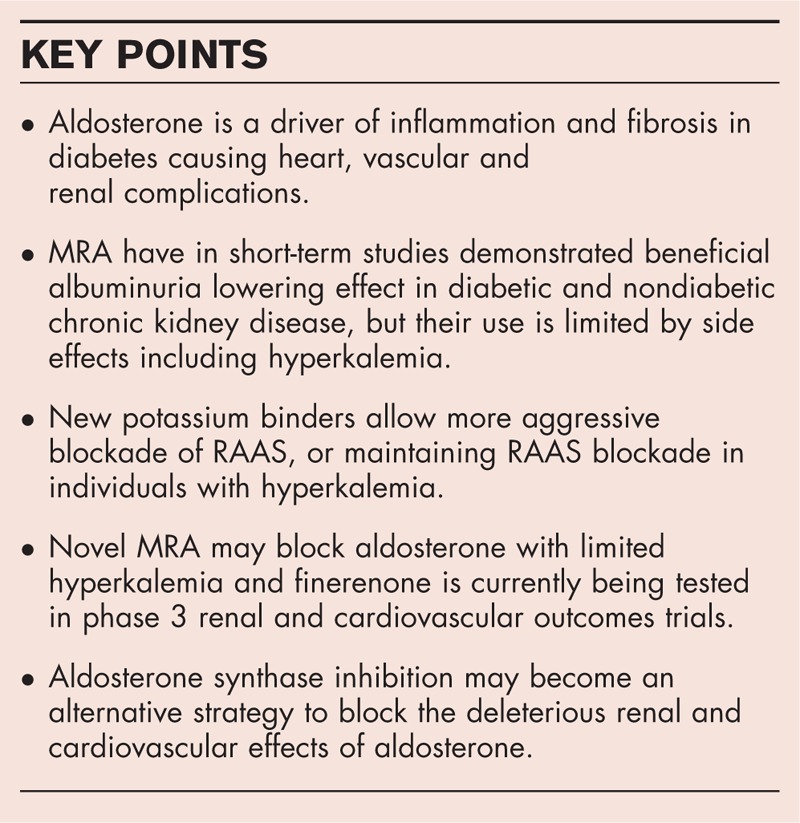
no caption available
STANDARD OF CARE INHIBITION OF THE RENIN–ANGIOTENSIN SYSTEM
Until recent data from studies of sodium glucose transporter 2 inhibitors or glucagon like peptide 1 receptor agonists were presented [19,20▪▪], the standard of care in DKD for almost 20 years have been blockade of RAS with ACEi or ARB on top of glucose management. Although this improves renal as well as cardiovascular outcomes (development of doubling of serum creatinine or ESKD, and hospitalization for heart failure) up to 50% of individuals have been reported to reach the primary endpoint after 4 years in the treated group, albeit these data come from a study completed almost 20 years ago [10].
Hyperkalemia was a problem in the RENAAL study with losartan as monotherapy. Indeed 38% of individuals had elevated potassium (>5.0 mmol/l) after 6 months compared with 23% in the placebo group, and elevated potassium was associated with an increased risk of renal outcomes [21].
The insufficient effect on renal and cardiovascular outcome is explained by RAS blockade being incomplete. ACEi can be bypassed by angiotensin II formation from chymases, and angiotensin II type 1 receptor blockade may be incomplete. This led to exploration of dual blockade with a combination of ACEi and ARBs. In DKD dual blockade was able to reduce proteinuria compared with single agent intervention but did not provide long-term renal benefit in the VA nephron D study, which was stopped for futility and side effects including hyperkalemia [22]. Addition of renin inhibition was also not efficient long-term as reported in the ALTITUDE study, and the proportion of patients with hyperkalemia (serum potassium level, ≥6 mmol/l) was significantly higher in the aliskiren group than in the placebo group (11.2 vs. 7.2%), (P < 0.001) [23].
Chymase inhibitors were developed to address the insufficient blockade of angiotensin II formation during ACEi. Fulacimstat is a first in class agent for clinical use tested in patients with left ventricular (LV) dysfunction [24▪]. This agent is now being evaluated for safety and its antiproteinuric effect in a 6 months study in individuals with type 2 diabetes (T2D) and a clinical diagnosis of DKD (NCT03412006).
During long-term blockade of RAS aldosterone escape or breakthrough is seen [14]. In a study of type 1 diabetes (T1D) individuals with DKD treated with losartan for 3 years the decline in GFR (Cr-EDTA plasma clearance) was 5.0 ml/min/1.73 m2/year in patients with aldosterone escape, compared with 2.4 in individuals without [25]. In contrast the AMADEO study [26], could not in a post hoc analysis find association between aldosterone breakthrough at 6 months and change in GFR between 6 and 12 months in a large cohort of type 2 diabetic individuals with DKD [27]. This could potentially be explained by difference in follow-up or lack of a common definition of breakthrough.
Hyperglycemia may via the formation of succinate binding to a renal succinate receptor activate renin [28] and recently an association between hyperglycemia, elevated renin and aldosterone was demonstrated in T2D [29].
STEROIDAL MINERALOCORTICOID RECEPTOR ANTAGONISTS
The correlation between aldosterone levels and breakthrough with decline in GFR supports aldosterone as a target for intervention in DKD in individuals on ACEi or ARB. The steroidal MRA spironolactone is potent but nonselective causing gynecomastia. Spironolactone was investigated in doses 25–50 mg/day in combination with ACEi or ARBs in individuals with T1D or T2D, microalbuminuria, macroalbuminuria or nephrotic range albuminuria in 16 studies lasting 2–18 months demonstrating a significant 39% reduction in albuminuria with the major concern being a more than six-fold increased risk for hyperkalemia particularly in individuals with impaired renal function [30]. Similar data were found in meta-analyses including diabetic and nondiabetic CKD [18]. A recent 72 weeks intervention study with four arms combining irbesartan 150 or 300 mg daily with spironolactone 20 mg or placebo confirmed long-term 30% antiproteinuric effect and safety in elderly with T2D and DKD [estimated glomerular filtration rate (eGFR) > 45 ml/min/1.73 m2] when spironolactone was add-on to irbesartan [31]. Prevention of DKD (onset of microalbuminuria and loss of eGFR) in T2D, with spironolactone is being tested in the 3-year PRIORITY study including normoalbuminuric patients with high risk for DKD determined from a urinary proteomics-based risk pattern for CKD (CKD273). The study includes only individuals with GFR more than 60 ml/min/1.73 m2, to reduce the risk of hyperkalemia [32].
Significantly in hemodialysis patients the composite cardiovascular outcome death from cardiocerebrovascular (CCV) events, aborted cardiac arrest and sudden cardiac death was reduced with long-term low-dose spironolactone with a hazard ratio of 0.42 (95% confidence interval 0.26–0.78). Death from CCV events occurred in 4.0% of patients in the spironolactone group and in 11.7% of patients in the control group [33].
Eplerenone is a second-generation, more selective but less potent steroidal MRA, with documented benefits in heart failure with reduced ejection function [16]. Studies with eplerenone as add-on to ACE inhibition in type 2 diabetic individuals with DKD demonstrated similar antiproteinuric effects, but potassium problems led to a recommendation against eplerenone in DKD [34,35]. Long-term studies aiming to prevent progression of established DKD have not been carried out and therefore it is not known if the beneficial effect on albuminuria translates into prevention of ESKD. The association between reduction in albuminuria after onset of antihypertensive therapy and long-term preservation of renal function was demonstrated many years ago [36] and was recently discussed at a National Kidney Foundation arranged conference on endpoints in renal trials in 2018 with participation of Food and Drug Adminitration (FDA) and European Medicines Agency (EMA), where comprehensive data from observational studies including 693 000 individuals and intervention studies with almost 30 000 participants were presented supporting this surrogate endpoint. It was demonstrated how a 30% decrease in albuminuria compared with placebo will provide an average hazard ratio for the hard clinical endpoint of 0.68 [37,38▪]. Still not all interventions lowering albuminuria in phase II trials have documented renoprotection in phase III trials [22,23].
CIRCUMVENTION OF HYPERKALEMIA COULD ALLOW THERAPY INTENSIFICATION
Successful management of hyperkalemia could facilitate treatment with ACE is or ARBs, as well as MRA add-on. To prevent hyperkalemia, it is important with dietary advice including sufficient fluid intake, cessation of potassium increasing comedication and potentially increasing dose of loop diuretics. Regular monitoring should follow potassium levels, and unfortunately the problem often remains. The potassium binder sodium polystyrene sulfonate has been used to treat acute hyperkalemia, whereas long-term treatment has been limited by side effects.
New potassium binders patiromer and sodium zirconium cyclosilicate (ZS-9) were approved by FDA and EMA as they are efficient in lowering elevated serum Potassium. Side effects are less with these new agents allowing long-term treatment. Patiromer permitted continued RAS blockade for 52 weeks in patients with DKD and hyperkalemia in the AMETHYST-DN study [39] even in the subgroup with heart failure [40].
Implementation has been limited by lack of outcome studies showing benefits on endpoints such as progression of kidney disease or heart failure hospitalization, as well as costs. It is important to consider the costs related to hyperkalemia which is associated with hospitalizations and poor outcome. A Danish population-based study of healthcare costs related to hyperkalemia included patients with a first incident of CKD (n = 78 372) during 2005–2011. Among all patients experiencing a first high-potassium event (serum potassium level >5.0 mmol/l), healthcare costs were compared during 6 months before and 6 months after the high-potassium event. Overall, 17 747 (23%) CKD patients with a first high-potassium event were identified. In CKD patients, overall mean costs were euro 5518 higher 6 months after vs. before first high potassium, whereas euro 441 higher in matched CKD patients without high potassium, yielding high-potassium-associated costs of euro 5077 [41]. Nevertheless, it remains to be demonstrated that treating hyperkalemia is cost-effective.
NOVEL NONSTEROIDAL MINERALOCORTICOID RECEPTOR ANTAGONISTS
Although spironolactone lowers BP in resistant hypertension [17] and in CKD [18], and the physiological role of aldosterone is linked to control of potassium and volume, studies in heart failure and CKD found benefits without lowering of BP. The beneficial impact probably relates more to the antifibrotic and anti-inflammatory of blocking aldosterone. It has been suggested that compounds with a profile including the potential beneficial effects on progression of kidney disease and heart diseases, but excluding the adverse effects of hyperkalemia could be developed. Novel nonsteroidal MRAs may have this profile [42,43]. Finerenone (BAY 94 8862) is the most advanced in its development. The difference in mode of action of finerenone compared with steroidal MRAs is explained by the different physiochemical properties affecting tissue distribution and cellular penetration, mode of binding to the mineralocorticoid receptor and blockade or recruitment of tissue-selective or ligand-specific cofactors leading to differential gene expression [43].
Finerenone is more selective and has higher affinity to the mineralocorticoid receptor than spironolactone and eplerenone [43] The MinerAlocorticoid Receptor Antagonist Tolerability Study (ARTS) study evaluated different doses of finerenone for 28 days in 393 patients with chronic heart failure with reduced LV ejection fraction and CKD stage 3. Compared to spironolactone there was a lesser increase in serum potassium, at a comparable reduction in N-terminal pro-brain natriuretic peptide and albuminuria, and a significantly lower rate of hyperkalemia (5.3 vs. 12.7%) and renal impairment (3.8 vs. 28.6%) [44]. The MinerAlocorticoid Receptor Antagonist Tolerability Study in Diabetic Nephropathy (ARTS-DN) study evaluated different doses of finerenone and placebo for 90 days in 823 patients with T2D and albuminuria (urinary albumin creatinine ratio (UACR) ≥ 30 mg/g) on RAS blockade. Finerenone led to a dose-dependent reduction in albuminuria. Adverse events were similar to placebo. Hyperkalemia and subsequent discontinuation of study drug occurred in 1.8% of patients receiving finerenone compared with no patients on placebo. No differences in the incidence of an eGFR decrease of at least 30% were seen between the groups. With finerenone, reductions in UACR (21–38%) were significantly greater than with placebo. However, it should be noted that there was no active comparator in the ARTS-DN study [45].
These data support the assumption that the antiproteinuric effect can be maintained with a limited effect on serum potassium. Whether this translates into prevention of cardiovascular and renal events is currently being tested in individuals with T2D and CKD in the FIGARO (NCT02545049) and FIDELIO (NCT02540993) studies. FIGARO is a randomized double-blind phase III study of cardiovascular morbidity and mortality in T2D with DKD and microalbuminuria, and the primary endpoint is time to first occurrence of cardiovascular death or nonfatal myocardial infarction (MI) or stroke or hospitalization for heart failure. Secondary endpoints include onset of kidney failure, a sustained decrease of eGFR at least 40% or renal death. The study has randomized 7437 individuals and is expected to end in July 2021. FIDELIO is a randomized double-blind phase III study of progression of kidney disease in 5734 individuals and the primary endpoint is time to first occurrence of onset of kidney failure, a sustained decrease of eGFR at least 40% or renal death. Secondary endpoints include cardiovascular death or nonfatal MI or stroke or hospitalization for heart failure. The study is expected to end in May 2020. These companion studies will be the first to test if the antiproteinuric effect of aldosterone blockade translates safely into prevention of progression of renal disease and prevention of cardiovascular events including heart failure. The similarities in intervention, design and endpoints will make it possible to analyze the combined cohort covering the range of DKD in type 2 diabetes from early to late stages.
ALDOSTERONE SYNTHASE INHIBITION
An alternative strategy for lowering the aldosterone-related risk for fibrotic heart and kidney disease is aldosterone synthase inhibitors [46]. This may work differently than classical mineralocorticoid receptor blockade and potentially better, as aldosterone not only confers mineralocorticoid receptor mediated classical ‘genomic’ effects with mineralocorticoid receptor but also ‘nongenomic’ and mineralocorticoid receptor independent effects leading to fibrosis [47]. These nongenomic effects may actually be enhanced during treatment with MRAs as they increase aldosterone levels, but their importance is debated [46].
The clinical development has been complicated by difficulties in obtaining sufficient selectivity blocking aldosterone synthase, but not cortisol synthase as seen with LC1699, the first aldosterone synthase inhibitor for clinical use [48].
Next-generation aldosterone synthase inhibitors were more selective for aldosterone synthase. In two phase 1 studies with a selective aldosterone synthase inhibitor LY3045697 from Eli Lilly the suppression of aldosterone was only temporary, potentially due to upregulation of aldosterone synthesis with ongoing therapy [49]. The most selective inhibitors in preclinical studies comes from Boehringer Ingelheim (BI 689648 and BI 689794) having more than 300-fold selectivity [50]. This treatment option remains to be developed for clinical studies in DKD.
CONCLUSION
For decades there has been interest in the potential cardio-renal protective effect of reducing aldosterone effects in DKD. This has been difficult because of side effects. Recent years have provided significant developments with new agents to treat hyperkalemia allowing more aggressive renin–angiotensin–aldosterone system blockade, and new ways to block the mineralocorticoid receptor with less side effect or inhibit aldosterone synthesis. The nonsteroidal MRA finerenone is being tested in two large phase III studies addressing if this is a safe and efficacious new treatment option in DKD.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
P.R. is on the Steering Committee of the following clinical trials: CADA-DIA (Bayer), Fidelio (Bayer) Figaro (Bayer), DAPA-CKD (Astra Zeneca), and FLOW (Novo-Nordisk); The Steno Diabetes Center Copenhagen has received fees for consultancy and/or speaking from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Eli Lilly, Mundi, Novo Nordisk and Sanofi Aventis. F.P. has served as a consultant, on advisory boards or as an educator for Astra Zeneca, Novo Nordisk, Sanofi, Mundipharma, MSD, Boehringer Ingelheim, Novartis, and Amgen, and has received research grants to the Steno Diabetes Center Copenhagen from Novo Nordisk, Amgen, and Astra Zeneca M.F.-M. declares no conflicts.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017; 128:40–50.. [DOI] [PubMed] [Google Scholar]
- 2.Andresdottir G, Jensen ML, Carstensen B, et al. Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes Care 2014; 37:1660–1667.. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med 2014; 370:1514–1523.. [DOI] [PubMed] [Google Scholar]
- 4▪.Koye DN, Magliano DJ, Reid CM, et al. Trends in incidence of ESKD in people with type 1 and type 2 diabetes in Australia, 2002–2013. Am J Kidney Dis 2019; 73:300–308.. [DOI] [PubMed] [Google Scholar]; This is an important study of trends in end-stage kidney disease in diabetes mellitus.
- 5.Gilbert RE, Connelly K, Kelly DJ, et al. Heart failure and nephropathy: catastrophic and interrelated complications of diabetes. Clin J Am Soc Nephrol 2006; 1:193–208.. [DOI] [PubMed] [Google Scholar]
- 6.Wen CP, Chang CH, Tsai MK, et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int 2017; 92:388–396.. [DOI] [PubMed] [Google Scholar]
- 7.Parving HH, Smidt UM, Hommel E, et al. Effective antihypertensive treatment postpones renal insufficiency in diabetic nephropathy. Am J Kidney Dis 1993; 22:188–195.. [DOI] [PubMed] [Google Scholar]
- 8.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.. [DOI] [PubMed] [Google Scholar]
- 9.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.. [DOI] [PubMed] [Google Scholar]
- 10.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.. [DOI] [PubMed] [Google Scholar]
- 11.Bauersachs J, Jaisser F, Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 2015; 65:257–263.. [DOI] [PubMed] [Google Scholar]
- 12▪▪.Barrera-Chimal J, Girerd S, Jaisser F. Mineralocorticoid receptor antagonists and kidney diseases: pathophysiological basis. Kidney Int 2019; 96:302–319.. [DOI] [PubMed] [Google Scholar]; This is an excellent review of the pathophysiological basis for the importance of aldosterone and its effects in kidney disease.
- 13▪.Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation 2018; 138:929–944.. [DOI] [PubMed] [Google Scholar]; Introduces a revised definition of cardiorenal syndrome that can support targeted intervention.
- 14.Sato A, Saruta T. Aldosterone breakthrough during angiotensin-converting enzyme inhibitor therapy. Am J Hypertens 2003; 16:781–788.. [DOI] [PubMed] [Google Scholar]
- 15.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.. [DOI] [PubMed] [Google Scholar]
- 16.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.. [DOI] [PubMed] [Google Scholar]
- 17.Oxlund CS, Henriksen JE, Tarnow L, et al. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus: a double blind randomized clinical trial. J Hypertens 2013; 31:2094–2102.. [DOI] [PubMed] [Google Scholar]
- 18.Currie G, Taylor AH, Fujita T, et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol 2016; 17:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41:2669–2701.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380:2295–2306.. [DOI] [PubMed] [Google Scholar]; This is a seminal article reporting the first dedicated renal outcome study with the SGLT2 inhibitor canagliflozin in type 2 diabetic individuals with proteinuria.
- 21.Miao Y, Dobre D, Heerspink HJ, et al. Increased serum potassium affects renal outcomes: a post hoc analysis of the reduction of endpoints in NIDDM with the angiotensin II antagonist losartan (RENAAL) trial. Diabetologia 2011; 54:44–50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.. [DOI] [PubMed] [Google Scholar]
- 23.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.. [DOI] [PubMed] [Google Scholar]
- 24▪.Dungen HD, Kober L, Nodari S, et al. Safety and tolerability of the chymase inhibitor fulacimstat in patients with left ventricular dysfunction after myocardial infarction-results of the CHIARA MIA 1 trial. Clin Pharmacol Drug Dev 2018; doi: 10.1002/cpdd.633. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; This is the first clinical study with a chymase inhibitor.
- 25.Schjoedt KJ, Andersen S, Rossing P, et al. Aldosterone escape during blockade of the renin–angiotensin–aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia 2004; 47:1936–1939.. [DOI] [PubMed] [Google Scholar]
- 26.Bakris G, Burgess E, Weir M, et al. Telmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathy. Kidney Int 2008; 74:364–369.. [DOI] [PubMed] [Google Scholar]
- 27.Moranne O, Bakris G, Fafin C, et al. Determinants and changes associated with aldosterone breakthrough after angiotensin II receptor blockade in patients with type 2 diabetes with overt nephropathy. Clin J Am Soc Nephrol 2013; 8:1694–1701.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peti-Peterdi J. High glucose and renin release: the role of succinate and GPR91. Kidney Int 2010; 78:1214–1217.. [DOI] [PubMed] [Google Scholar]
- 29.Griffin TP, Wall D, Browne GA, et al. Associations between glycaemic control and activation of the renin–angiotensin–aldosterone system in participants with type 2 diabetes mellitus and hypertension. Ann Clin Biochem 2018; 55:373–384.. [DOI] [PubMed] [Google Scholar]
- 30.Hou J, Xiong W, Cao L, et al. Spironolactone add-on for preventing or slowing the progression of diabetic nephropathy: a meta-analysis. Clin Ther 2015; 37:2086–2103.. e2010. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Liu P, Chen X, et al. Effects of different doses of irbesartan combined with spironolactone on urinary albumin excretion rate in elderly patients with early type 2 diabetic nephropathy. Am J Med Sci 2018; 355:418–424.. [DOI] [PubMed] [Google Scholar]
- 32.Tofte N, Lindhardt M, Adamova K, et al. Characteristics of high- and low-risk individuals in the PRIORITY study: urinary proteomics and mineralocorticoid receptor antagonism for prevention of diabetic nephropathy in Type 2 diabetes. Diabet Med 2018; 35:1375–1382.. [DOI] [PubMed] [Google Scholar]
- 33.Lin C, Zhang Q, Zhang H, Lin A. Long-term effects of low-dose spironolactone on chronic dialysis patients: a randomized placebo-controlled study. J Clin Hypertens (Greenwich) 2016; 18:121–128.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epstein M, Buckalew V, Altamirano J, et al. Eplerenone reduces proteinuria in type II diabetes mellitus: implications for aldosterone involvement in the pathogenesis of renal dysfunction. J Am Coll Cardiol 2002; 39:249–1249.. [Google Scholar]
- 35.Epstein M, Buckalew V, Martinez FA, et al. Antiproteinuric efficacy of eplerenone, enalapril, and eplerenone/enalapril combination therapy in diabetic hypertensives with microalbuminuria. Am J Hypertens 2002; 15:A24. [Google Scholar]
- 36.Rossing P, Hommel E, Smidt UM, Parving H-H. Reduction in albuminuria predicts a beneficial effect on diminishing the progression of human diabetic nephropathy during antihypertensive treatment. Diabetologia 1994; 37:511–516.. [DOI] [PubMed] [Google Scholar]
- 37.Coresh J, Heerspink HJL, Sang Y, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol 2019; 7:115–127.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪.Heerspink HJL, Greene T, Tighiouart H, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 2019; 7:128–139.. [DOI] [PubMed] [Google Scholar]; These important data report on the link between changes in albuminuria and long-term hard renal outcomes.
- 39.Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: The AMETHYST-DN randomized clinical trial. JAMA 2015; 314:151–161.. [DOI] [PubMed] [Google Scholar]
- 40.Pitt B, Bakris GL, Weir MR, et al. Long-term effects of patiromer for hyperkalaemia treatment in patients with mild heart failure and diabetic nephropathy on angiotensin-converting enzymes/angiotensin receptor blockers: results from AMETHYST-DN. ESC Heart Fail 2018; 5:592–602.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim K, Thomsen RW, Nicolaisen SK, et al. Healthcare resource utilisation and cost associated with elevated potassium levels: a Danish population-based cohort study. BMJ Open 2019; 9:e026465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dojki FK, Bakris G. Nonsteroidal mineralocorticoid antagonists in diabetic kidney disease. Curr Opin Nephrol Hypertens 2017; 26:368–374.. [DOI] [PubMed] [Google Scholar]
- 43.Kolkhof P, Nowack C, Eitner F. Nonsteroidal antagonists of the mineralocorticoid receptor. Curr Opin Nephrol Hypertens 2015; 24:417–424.. [DOI] [PubMed] [Google Scholar]
- 44.Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel nonsteroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. European Heart Journal 2013; 34:2453–2463.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 2015; 314:884–894.. [DOI] [PubMed] [Google Scholar]
- 46.Weldon SM, Brown NF. Inhibitors of aldosterone synthase. Vitam Horm 2019; 109:211–239.. [DOI] [PubMed] [Google Scholar]
- 47.Chen D, Chen Z, Park C, et al. Aldosterone stimulates fibronectin synthesis in renal fibroblasts through mineralocorticoid receptor-dependent and independent mechanisms. Gene 2013; 531:23–30.. [DOI] [PubMed] [Google Scholar]
- 48.Amar L, Azizi M, Menard J, et al. Aldosterone synthase inhibition with LCI699: a proof-of-concept study in patients with primary aldosteronism. Hypertension 2010; 56:831–838.. [DOI] [PubMed] [Google Scholar]
- 49.Sloan-Lancaster J, Raddad E, Flynt A, et al. LY3045697: results from two randomized clinical trials of a novel inhibitor of aldosterone synthase. J Renin Angiotensin Aldosterone Syst 2017; 18:1470320317717883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyers K, Cogan DA, Burke J, et al. Dihydrobenzisoxazole-4-one compounds are novel selective inhibitors of aldosterone synthase (CYP11B2) with in vivo activity. Bioorg Med Chem Lett 2018; 28:979–984.. [DOI] [PubMed] [Google Scholar]


