Abstract
Arsenic is a metalloid found in groundwater as a byproduct of soil/rock erosion and industrial and agricultural processes. This xenobiotic elicits its toxicity through different mechanisms, it has been identified as a toxicant that affects virtually every organ or tissue in the organism. In the central nervous system exposure to arsenic can induce cognitive dysfunction, it has been linked to several neurological disorders, including neurodevelopmental alterations, and is considered a risk factor for neurodegenerative disorders. However, the exact mechanisms involved are still unclear. In this review, we aim to appraise the neurotoxic effects of arsenic and the molecular mechanisms involved. First, we discuss the epidemiological studies reporting on the effects of arsenic in intellectual and cognitive function during development as well as studies showing the correlation between arsenic exposure and altered cognition and mental health in adults. The neurotoxic effects of arsenic and the potential mechanisms associated with neurodegeneration are also reviewed including data from experimental models supporting epidemiological evidence of arsenic as a neurotoxicant. Next, we focused on recent literature regarding arsenic metabolism and the molecular mechanisms that begin to explain how arsenic damages the central nervous system; including, oxidative stress, energy failure and mitochondrial dysfunction, epigenetics, alterations in neurotransmitter homeostasis and synaptic transmission, cell death pathways and inflammation. Outlining the specific mechanisms by which arsenic alters the cell function is key to understand the neurotoxic effects that convey cognitive dysfunction, neurodevelopmental alterations and neurodegenerative disorders.
Keywords: iAs, redox homeostasis, oxidative stress, metalloid, neurotoxicity
Introduction
Arsenic (As) is an element cataloged as a metalloid, because it has both metal and nonmetal properties. This metalloid is also classified as a xenobiotic because it does not have physiological functions but exerts toxic effects on humans. The principal route of exposure to this metalloid is through the food and contaminated water. In the World, there are a number of regions where the levesl of As in drinking-water exceeds the recommended exposure limits. At least 140 million people in 50 countries have been drinking water containing As at levels above 10 μg/L (world Health Organization [WHO] provisional guideline). As is naturally present at high levels in the groundwater of a number of countries, including Argentina, Bangladesh, Chile, China, India, Mexico, and the United States of America. As has been identified as a toxicant that affects virtually every organ or tissue in the organism, its deleterious effects include, skin lesions, different types of cancer, as well as cardiovascular, respiratory and gastrointestinal effects. In the nervous system this metalloid can induce peripheral neuropathies, encephalopathy, neurobehavioral alterations, and has also been associated to neurodegeneration. Although there are toxicological and epidemiological studies that show the neurotoxic effects of As, this topic is still evolving [1-3].
Environmental and anthropogenic sources of Arsenic
As is present in soil, air and water, it is widely distributed in the Earth’s crust being the 12th most abundant element in the human body. As compounds can be categorized as inorganic (iAs) and organic. iAs occurs in two oxidation states, arsenite +3 (iAsIII) and arsenate +5 (iAsV). Nowadays iAs is used as a wood preservative, in agricultural chemicals, as an alloying element, in the electronics industry, and it has been used to treat leukemia. At least 140 million people in 50 countries have been exposed to iAs at levels above the WHO recommended exposure limits (10 ppb). In contaminated areas iAs concentration can exceed 1,000 ppb in groundwater [4, 5].
Toxic effects of Arsenic in the Nervous System
Gestational and developmental effects
A clear understanding of the gestational and developmental neurotoxicity of iAs is still missing. However, toxicological and epidemiological reports have demonstrated that exposure to iAs during development affects intellectual and cognitive function (Table 1). Furthermore, a reduction in full-scale intelligence quotient (IQ) and memory have been linked to iAs exposures even below current safety recommendations [6, 7]. Children exposed to iAs concentrations ranging from 5 to 50 ppb in water, from Mexico [8, 9], the US [10] and Bangladesh [11] showed neurobehavioral alterations in verbal abilities, cognitive function, IQ, motor skills and long term memory. Gestational exposure to sodium arsenite (NaAsO2) increases its accumulation in the mice offspring’s brain and impairs learning and memory processes [12, 13]. Additionally, prenatal exposure of mice to NaAsO2 produces behavioral impairments as well as abnormal formation of the prelimbic cortex in the adult offspring [14].
Table 1.
Toxic effects of Arsenic in the Nervous System
| Human studies |
Exposure [iAs] |
Symptoms / Effects | Ref |
|---|---|---|---|
| Adults 37.6 y (Bangladesh) |
Chronic 14.1 ± 3.27 y < 129 μg/L, 130-264 μg/L and > 265 μg/L |
⇓ Activity of the plasma cholinesterase enzyme with increasing levels of iAs exposure. This enzyme is involved in both liver and brain function and neurotoxicity | [101] |
| Adults 40 y (India) |
Cross-sectional study (5 y) Decrease in the exposure from 190.1 to 37.94 μg/L |
⇑ Neuropathy, conjunctivitis and respiratory distress | [17] |
| Adults 44 years |
Single dose 0.25-20 mg/L |
Peripheral neuropathy ⇓ motor conduction velocity Marked abnormalities of sensory nerve action potentials | [20] |
| Adults 43 years (India) |
Chronic 128.95 ± 65.54 μg/L |
Peripheral neuropathy ⇑ Senescence associated miRNAs and PMP22, a specific target of miR-29a | [102] |
| Adults (Myanmar) |
Subchronic (7 m) ≥ 10 mg/L 10-50 mg/L < 50 mg/L |
Weakness and chronic numbness or pain Peripheral nerve disturbances | [18] |
| Adults 20 - 50 y |
Cross-sectional study (2 y) Decrease in the exposure from 115 to 0.025 μg/L |
⇑ Subclinical sensory neuropathy (elevated toe vibration threshold). | [19] |
| Children 8-11 y (Bangladesh) |
Chronic 10 μg/L |
⇓ Motor function (fine manual control and body coordination) | [11] |
| Children 7.61 years (Mexico) |
Chronic 62.9 ± 0.03 μg As / g creatinine |
⇓ Verbal IQ, verbal comprehension and long term memory | [8] |
| Children 7 years (Mexico) |
Chronic > 50 μg/L |
⇓ visual/spatial, verbal and motor abilities | [9] |
| Children 9.67 years (USA) |
Chronic (7.3 y) > 5 μg/L |
⇓ Full scale IQ, perceptual reasoning, working memory and verbal comprehension | [10] |
| Mice | Exposure [iAs] |
Symptoms / Effects | Ref |
| C3H | Gestational 85 mg/L |
60 weeks of age: Behavioral inflexibility, abnormal formation and disarrangement of the prelimbic cortex | [14] |
| CD-1 | Gestational 20 mg/L |
PND 1 (whole brain): ⇑ GSSG, xCT, EAAT3, LAT1 PND 15 (hippocampus): ⇑ GSH, xCT, EAAT3; ⇓ GLT1, NMDA (NR2A-B) / AMPA receptors PND 15 (cortex): ⇑ xCT, EAAT3; ⇓ NR2A PND 90 (hippocampus): ⇓ xCT, NR2B, GluA1, GluA2 PND 15, 90 : ⇓ spatial memory (LTP) |
[12, 13] |
| C57BL/6 | Gestational 100 mg/L |
PND 21 (striatum): ⇓ NMDA receptors, VGLUT2 and mGluR2 | [103] |
| Swiss Webster | Chronic (1 m) → geriatric age 0.10 mg/L |
Striatum and cortex: ⇑ protein ubiquitination, LC3 levels, tyrosine hydroxylase and synuclein accumulation | [31] |
| 3xTgAD model | Gestational → 6 m 3 mg/L |
Behavioral impairment Hippocampus: ⇓ ATP and complex I; ⇑ oxidant state Cortex: ⇑ antioxidant response Frontal cortex and hippocampus: ⇑ amyloid β and phosphorylated tau |
[104] |
| Rats | Exposure [iAs] |
Symptoms / Effects | Ref |
| Wistar | Gestational → 4 m 3 mg/L |
Behavioral deficits Whole brain : ⇑ AD biomarkers (RAGE, Aβ and BACE1 activity) |
[26] |
| Acute (9 h) 15 and 20 mg/kg |
Disappearance of neurofilament and fibroblast proteins Changes in cytoskeletal composition |
[21] | |
| Subchronic (30 d) 10 mg/kg |
Sural nerves of the right hind limb: ⇑ Lipid peroxidation, slower nerve conduction velocity; ⇓ conduction area, myelin thickness, area and perimeter of axons |
[23] | |
| Experimental Model |
Exposure [iAs] |
Symptoms / Effects | Ref |
|
Zebrafish (Danio rerio) |
Subchronic (96 h) 0.05, 5, 15 mg/L |
⇑ Locomotor activity and Anxiety Whole brain : ⇓ ATP, ADP and AMP hydrolysis (ectonucleotidases activities) |
[105] |
| Chronic (90 d) 50 μg/L |
Whole brain: ⇓ GSH; ⇑ antioxidant response (Nrf2, HO1, NQO1, Sod, Cox1, GPx1, Cat); ⇑ apoptosis | [106] | |
| Subchronic (96 h) 1, 10, 100 μg/L |
Impaired long term memory and learning ⇑ protein oxidation | [107] | |
| Larval, juvenile, and adult stage (4 h → 150 days postfertilization) 50, 500 μg/L |
Impaired motor function, associative learning and sensorimotor response. | [108] | |
|
Nematode (Caenorhabditis elegans) |
100 μM | Deficits in the structure of sensory neurons ⇓ Locomotor activity,⇑ ROS production |
[109] |
Abbreviations: AD, Alzheimer's disease; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; APP, amyloid precursor protein; BACE-1, β-secretase; Cat, catalase; COX, cytochrome c oxidase; d, days; γ-GCS, gamma-glutamylcysteine synthetase; GPx1, glutathione peroxidase 1; h, hours; HO1; heme oxygenase1, iAs: inorganic arsenic; IQ, intelligence quotient; ; IL, interleukin; m, months of age; NMDA, glutamate/N-methyl-D-aspartate; NQO1, NADPH dehydrogenase quinone 1; Nrf2, nuclear factor (erythroid-derived 2)-like 2; p62, autophagic receptor sequestosome-1, p62/SQSTM1; PMP22, peripheral myelin protein 22; PND, postnatal day; RAGE, advanced glycation end products; ROS, reactive oxygen species; SOD, superoxide dismutase; TNF-α, tumor necrosis factor alpha; xCT, cystine/glutamate exchanger system; y, years of age.
Adults
Epidemiological reports on the effect of iAs exposure on adults are limited (Table 1). A series of studies have shown a significant correlation between iAs exposure and altered adult cognition and mental health [15, 16]. High levels of iAs in drinking water and/or urine in adults have been associated with peripheral nerve disturbances, decreased peripheral nerve conduction velocity, peripheral neuropathy, and altered sensory function [2, 17-19]. After ingestion of a single dose of iAs, four patients developed a peripheral neuropathy showing reduction of motor conduction velocity and marked abnormalities of sensory nerve action potentials [20]. Disappearance of neurofilament and fibroblast proteins in the sciatic nerves occurred in rats exposed to iAs [21, 22]. Moreover, iAs exposure in rats increases oxidative damage, demyelination, and morphological alterations in axons of peripheral nerves, suggesting that these changes can lead to decreased transmission of information between peripheral sensory organs to the central nervous system (CNS) [23].
Neurodegeneration
Several association studies have failed to demonstrate that exposure to iAs alone causes neurodegeneration. However, the neurotoxic effects of iAs can correlate or synergize with the molecular mechanisms associated with neurodegeneration (oxidative stress, mitochondrial dysfunction and inflammation). In a case-control study, an increased risk to develop AD was associated with urinary levels of iAs (high), dimethylarsinic acid (DMAV, low) and selenium (Se, low) [24], iAs can induce vascular injury and dementia in vivo [25]. In rats, chronic iAs exposure induces behavioral deficits accompanied by higher levels of advanced glycation end products (RAGE), increased amyloid-β production and β-secretase (BACE-1) activity [26]. iAs exacerbates amyloid-β and phosphorylated Tau immunostaining in transgenic Alzheimer’s disease mouse models and this correlated with bioenergetic dysfunction and changes in redox metabolism [27]. The pro-amyloidogenic effects of iAs seem to be increased when combined with other heavy metals, and these effects seem to correlate with oxidative damage and neuroinflammation [28]. Accordingly, iAs increases the levels of pro-inflammatory cytokines in astrocytes that correlate with higher levels of amyloid precursor protein (APP) and BACE-1 [29], iAs can synergize with dopamine to trigger neurotoxicity [30]. iAs also induces the accumulation of α-synuclein (the pathological biomarker of Parkinson’s disease) accumulation and oligomerization with an overall increase in biomarkers markers of proteotoxic stress, but did not trigger neurodegeneration in vivo [31]. These findings suggest that iAs exposure may increase the susceptibility of developing neurodegeneration.
Arsenic metabolism
Transport
iAs can be transported across the blood brain barrier (BBB) and enter the brain. iAsIII uses aqua(glycerol)porins (AQP), organic anion transporters and glucose transporters (GLUT) to enter the cell. While iAsV is transported through phosphate transporters and then, inside the cell, is reduced to iAsIII (Figure 1a) [32-34]. Once inside the brain iAs is methylated and accumulated, with the highest accumulation in the pituitary gland [35]. iAs metabolites are exported through the multidrug resistance proteins (MRP1, MRP2 or MRP4) (Figure 1d) [36-38]. Our results (unpublished data) together with other studies have shown that astrocytes can be resistant to iAsIII through the MRP proteins [39]. Interestingly, knockout mouse for the P-glycoprotein showed a higher accumulation of iAs in the brain [40].
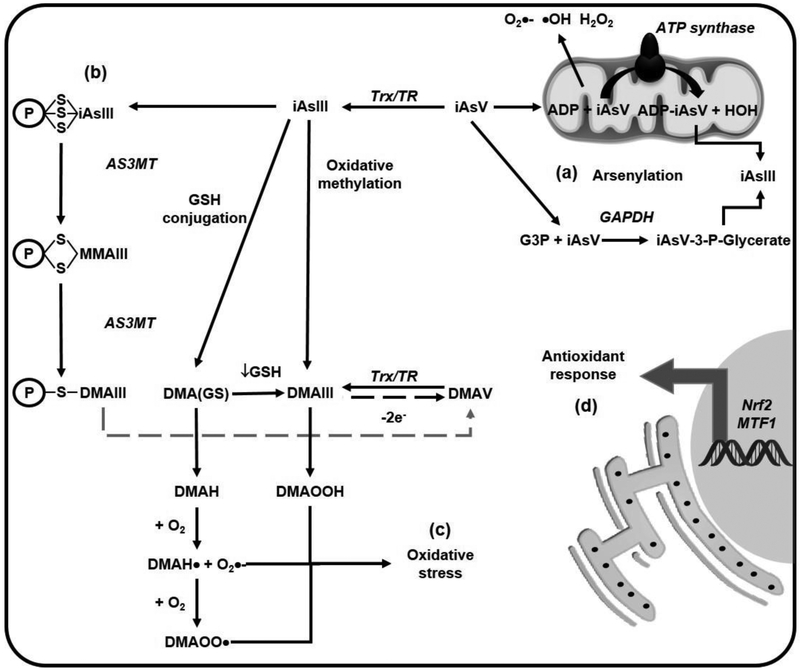
Figure 1. iAs metabolism.
(a) Arsenite (iAsIII) enters the cells through aqua(glycerol)porins (AQP) and the glucose transporters (GLUT), while arsenate (iAsV) does it through phosphate transporters. iAs is methylated through different mechanisms: (b) Oxidative methylation refers to the reduction of iAsV to iAsIII by the thioredoxin (Trx) / trx reductase (TR) system, and its subsequent methylation by the arsenite methyltransferase (AS3MT). Monomethyl arsonous acid (MMAIII), monomethylarsonic acid (MMAV); dimethylarsinous acid (DMAIII) and dimethylarsinic acid or cacodylic acid (DMAV) are the metabolites formed, (c) The glutathione (GSH) conjugation mechanism is based on the formation of GSH complexes with AsIII resulting in the formation of arsenic triglutathione [As(GS)3] by glutathione S-transferases (GSTs). The As(GS)3 is subsequently methylated by AS3MT to form monomethylarsinic GSH [MMA(GS)2] and dimethylarsinic GSH [DMA(GS)]. (d) iAs metabolites are exported through the multidrug resistance proteins (MRP1, MRP2 or MRP4)
Redox metabolism and methylation
Once inside the cell iAsIII can be methylated by different mechanisms. The first mechanism is the oxidative methylation that is mediated by arsenite methyltransferase (AS3MT) that uses S-adenosylmethionine (SAM) as a co-substrate. iAsIII is methylated to monomethylarsonic acid or arsonate (MMAV) that is reduced to monomethylarsonous acid (MMAIII) and then methylated again to dimethylarsinic acid (DMAV) [2, 41]. DMAV reduction generates dimethylarsinous acid (DMAIII) (Figure 1b). The pentavalent arsenicals (iAsV, MMAV and DMAV) can be reduced, through a process mediated by the thioredoxin/thioredoxin reductase (Trx/TR) system, although the antioxidant glutathione (GSH) seems to also increase their methylation by an unidentified mechanism [42].
Another mechanism for iAs methylation is via its conjugation with GSH where the GSH complexes generated are known as As triglutathione [As(GS)3]. This conjugation can occur non-enzymatically or enzymatically via the glutathione-S transferases GSTO1, GSTM1 or GSTP1 [36, 41]. The As(GS)3 complexes are methylated by the AS3MT to form monomethylarsinic diglutathione [MMA(GS)2] and then again to generate dimethylarsinic GSH [DMA(GS)]. When GSH levels are low, the conjugates can be hydrolyzed and then oxidized to generate MMAV and DMAV (Figure 1c) [41].
iAsV has been shown to replace phosphate in several metabolic pathways in a process called arsenylation where the end product is the reduction of iAsV to iAsIII as the arsenylated byproduct is more readily reduced than iAsV, increasing iAs toxicity. iAsV uncouples oxidative phosphorylation and ATP formation in the mitochondria by binding to ADP via the ATP-synthase. Likewise, arsenylation during glycolysis can impair carbon flux and ATP production. Furthermore, iAsV reacts with glucose generating glucose 6-arsenate an analog of glucose 6-phosphate that can act as an inhibitor of the hexokinase. iAsV is arsenylated by a number of different enzymes [43-45]; therefore, alterations in central carbon metabolism, energy failure and mitochondrial dysfunction can also be consequences of iAsV toxicity (Figure 2a).
Figure 2. Alterations in mitochondrial function and redox balance induced by iAs.
(a) Arsenate (AsV) can replace phosphate in several metabolic pathways (arsenylation) where the end product is the reduction of AsV to arsenite (AsIII). AsV uncouples oxidative phosphorylation and ATP formation in the mitochondria by binding to ADP via the ATP-synthase. AsV is also arsenylated by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) to generate 1-arsenato-3-phospho-D-glycerate (iAsV-3-P-glycerate) from glyceraldehyde 3-phosphate (G3P). (b) iAsIII binds to protein thiols (coenzyme A, lipoic acid and zinc finger domain containing proteins such as metallothionenin [MT]) and is methylated while still conjugated to these proteins, (c) Dimethylarsinic GSH [DMA(GS)] can form dimethylarsine (DMAH) and react with molecular oxygen (O2) to form DMAH radical (DMAH•) and the DMAH peroxyl radical (DMAOO•). Dimethylarsinous acid (DMAIII) reacts with O2 and forms dimethylated arsenic peroxide (DMAOOH). These peroxyl radicals lead to lipid peroxidation and protein carbonylation (oxidative stress), (d) AsIII induces an antioxidant response via nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and metal-responsive transcription factor-1 (MTF1).
It is important to mention that while the primary organ involved in iAs methylation is the liver, significant amounts of iAs and methylated As forms can be found in mice exposed to iAs. iAs crosses the blood-brain barrier (BBB) and is methylated in different brain regions that express AS3MT [35, 46]. Potentially, methylated iAs species in circulation can also cross the BBB but the mechanisms involved have not been studied. During development, iAsV or iAsIII might be accumulated in the brain more readily when the blood brain barrier is still not fully developed. In addition, iAs has been reported alter BBB gap junction formation as well [47, 48].
Thiol-binding properties
Recently, a third mechanism for iAsIII metabolism was proposed suggesting that first iAsIII binds to protein thiols and then it is methylated. This idea is supported by the findings that iAsIII has more affinity for protein thiols than for GSH [49]. iAsIII binds to thiol containing molecules and protein-cysteine thiols that can lead to enzyme inactivation. Moreover, the iAsIII-GSH complexes can bind to protein thiols, so the export of iAs- GSH adducts from the cell is an important process of detoxification [50]. Dithiol molecules together with proteins that have adjacent cysteines have been reported to bind iAsIII (Figure 2b) [51-55].
Molecular mechanisms of toxicity
Mitochondrial Dysfunction and Oxidative Stress
In the cell, a major source of reactive oxygen species (ROS) is the electron leak from the mitochondrial electron transport chain. Under physiological conditions, the steady-state levels of mitochondrial ROS are maintained by antioxidant systems, but in damaged or aged an increased ROS formation occurs [56-58]. Other sources of ROS / reactive nitrogen species (RNS) are the nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidases (NOX) and the NO synthases (NOS). [59, 60] and to a lesser extent, the activity of enzymes such as xanthine oxidases, cyclooxygenases, cytochrome p450 enzymes, lipoxygenases, myeloperoxidases and the protein folding machinery in the endoplasmic reticulum (ER) [56, 57, 61]. Physiological levels of these reactive species participate in signaling pathways, however, an imbalance between an increase in the levels of ROS/RNS and their metabolism or detoxification leads to a state known as oxidative stress. When oxidative stress is generated it can lead to oxidative modifications of biomolecules associated with the loss of function of proteins, damage to organelles and even cell death [57, 58, 61, 62]. To prevent the generation of oxidative stress, cells have antioxidant compounds that can be classified as enzymatic and non-enzymatic [56-58, 61].
Due to the high levels O2 consumption, the low content of antioxidants defenses, together with elevated levels of lipids and fatty acids, the brain is remarkably sensitive to oxidative damage. [63, 64]. In brain regions of animal models and in glial cell and neuron cultures exposed to iAs an increase in oxidative stress has been shown [65-69]. Mitochondria are a primary source of ROS formation induced by As [70, 71]. Accordingly, it has been shown that chronic iAs exposure increases mitochondrial oxidative stress in the rat brain by damaging the mitochondrial complexes I, II, and IV followed by increased ROS formation, protein carbonylation and lipid peroxidation [72]. Furthermore, the levels of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and the mitochondrial transcription factor A (TFAM) are reduced by iAs exposure leading to a decrease in mitochondrial biogenesis [73]. Dimethylarsine (DMAH) and peroxyl radicals can also be generated by iAs and in turn mediate lipid peroxidation and the accumulation of oxidized byproducts. Moreover, the metabolites MMAIII and DMAIII appear to be more potent toxicants because their ability to produce radicals (Figure 2c) [74].
iAs also activates the antioxidant transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2), through a non-canonical pathway where inhibition of autophagy leads to the accumulation of the ubiquitin-binding protein/adaptor autophagic receptor sequestosome-1, also known as p62 (p62/SQSTM1) that sequesters the Nrf2 suppressor kelch-like ECH-associated protein 1 (Keap1) (Figure 2d) [75], iAsIII toxicity can be counteracted by the transcriptional regulation of metallothionein I via the metal-activated transcription factor 1 (MTF1) (Figure 2d) [76].
Epigenetics
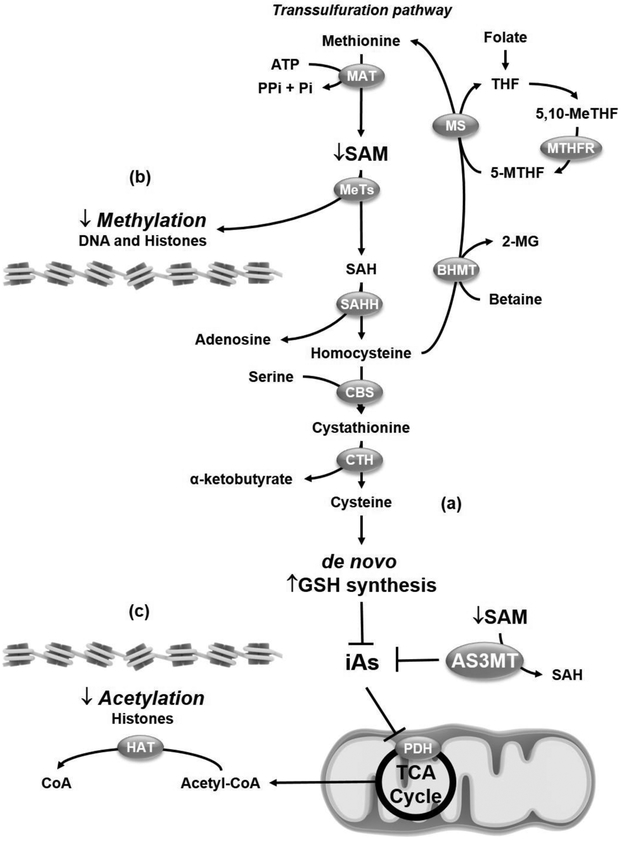
Both iAs biotransformation by AS3MT and the salvaging of sulfur to maintain adequate redox buffering capacity via the transsulfuration pathway consume SAM (Figure 3a). SAM is also required for methylation-dependent epigenetic changes such as DNA-methylation and histone modification (Figure 3b). Thus, iAs exposure is known to affect the epigenetic control of gene expression. Several studies have investigated the epigenetic effects or iAs exposure in the brain during development. DNA methylation typically acts to repress gene transcription. In an epidemiological study, >2000 genes were identified with iAs-associated differences in DNA methylation in newborn cord blood. These changes were located primarily within CpG islands positioned within the first exon (regions of DNA susceptible for cytosine methylation to 5-methylcytosine, where a cytosine is followed by a guanine nucleotide), the 5' untranslated region and 200 bp upstream of the transcription start site suggesting a significant association with changes in gene expression. iAs–induced changes in gene methylation were related to changes in gestational age and head circumference [77]. Genes involved in neuroplasticity have altered methylation patterns during developmental exposure of rats to iAs [78]. Interestingly, brain tissue from weaned rats treated with iAs showed suppressed levels of DNA methyltransferases (DNMTs) and ten-eleven translocations (TETs), enzymes involved in DNA methylation and demethylation processes, respectively. These changes correlated with impaired memory and learning abilities. Importantly, SAM levels where unchanged suggesting that oxidative stress was primarily involved in the perturbations to DNA methylation processes [79].
Figure 3. Epigenetic changes induced by iAs.
(a) iAs detoxification by arsenite methyltransferase (AS3MT) and glutathione (GSH) requires adenosylmethionine (SAM) consumption. In the transsulfuration pathway methionine is converted to SAM in an ATP-dependent reaction catalyzed by methionine adenosyltransferase (MAT). Subsequently, S-adenosylhomocysteine (SAH) is generated via the activity of a number of methyltransferases (MeTs). SAH is hydrolyzed by SAH hydrolase (SAHH) to adenosine and homocysteine. Homocysteine is conjugated with serine to provide cystathionine by the action of cystathionine beta synthase (CBS). Finally, cystathionine-γ-lyase (CTH, or γ-cystathionase) catalyzes the conversion of cystathionine to cysteine, which is used for de novo GSH synthesis, (b) If homocysteine is converted to cysteine and cannot be recycled re-methylated to methionine via the activities of betaine hydroxy methyltransferase (BHMT) or methionine synthase (MS) the SAM pool is depleted. SAM depletion will alter both DNA and histone methylation and the epigenetic signature in the brain that determines the expression of genes that might affect the neurodevelopmental processes. (c) Similarly, inhibition of pyruvate dehydrogenase (PDH) by iAs will impair Acetyl-CoA synthesis and as a consequence the acetylation of histones by histone acetyltransferases (HAT) and the epigenetic regulation of genes. CoA, coenzyme A; 5,10-MeTHF, 5,10-methylene tetrahydrofolate; 2-MG, 2-methyl glycine; 5-MTHF, 5-methyl tetrahydrofolate; MTHFR, methyl tetrahydrofolate reductase; Pi, phosphate; PPi, diphosphate; TCA, tricarboxylic acid.
Post-translational modification of histones modifies gene expression by altering chromatin structure and as a consequence DNA accessibility to the transcription machinery. Methylation and demethylation of histones turns genes "off" and "on,” respectively (Figure 3b). On the other hand, acetylation of histones is known to increase the expression of genes. iAs alters mitochondrial function and metabolism, more specifically, it impairs pyruvate dehydrogenase (PDH) activity [80, 81], which catalyzes the oxidation of pyruvate to acetyl-CoA (Figure 3c). Acetyl-CoA is a required substrate for histone acetylation and it is also expected that iAs exposure will affect histone acetylation. In mice prenatally exposed to iAs, hypo-acetylation at histone 3K9 (H3K9) which correlated with impaired spatial and episodic memory [82]. In contrast, other studies have reported a sex-specific regulation of H3K9 acetylation and methylation in response to developmental iAs exposure [83].
Alterations in neurotransmitter homeostasis and synaptic transmission
iAs neurotoxicity has also been linked to changes in neurotransmitter metabolism leading to changes in the synaptic transmission [12, 13]. Realgar, an As sulfide mineral, and source of highly toxic iAs, increases extracelluar glutamate levels and induces excitotoxicity and changes in GLT-1 and glutamate / N-methyl-D-aspartate (NMDA) receptor levels in rat hippocampus [84]. Subtoxic iAs exposure downregulates the GluA1 subunit of the glutamate / α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor [85]. Gestational exposure of mice to iAs induces alters synaptic plasticity (longterm potentiation [LTP]), learning and memory that were associated with an increase in extracellular glutamate levels and downregulation of both AMPA and NMDA receptor subunits [12, 13], iAs also decreases the expression levels of α7 nicotinic receptors in rats [86], and alters development of the cholinergic and dopaminergic systems [87].
Arsenic (In microglia, iAs activates the cystine/glutamate exchanger system (xCT) to increase extracellular glutamate levels [88], whereas in astrocytes iAs decreases the expression and activity levels of glutamine synthetase (GS) and glutamate transporters (GLAST/EAAT1 and GLT-1/EAAT2) [66, 89]. Notably, an increase in GSH levels and Nrf2 activity but no oxidative stress, was also shown [66].
Cell death pathways
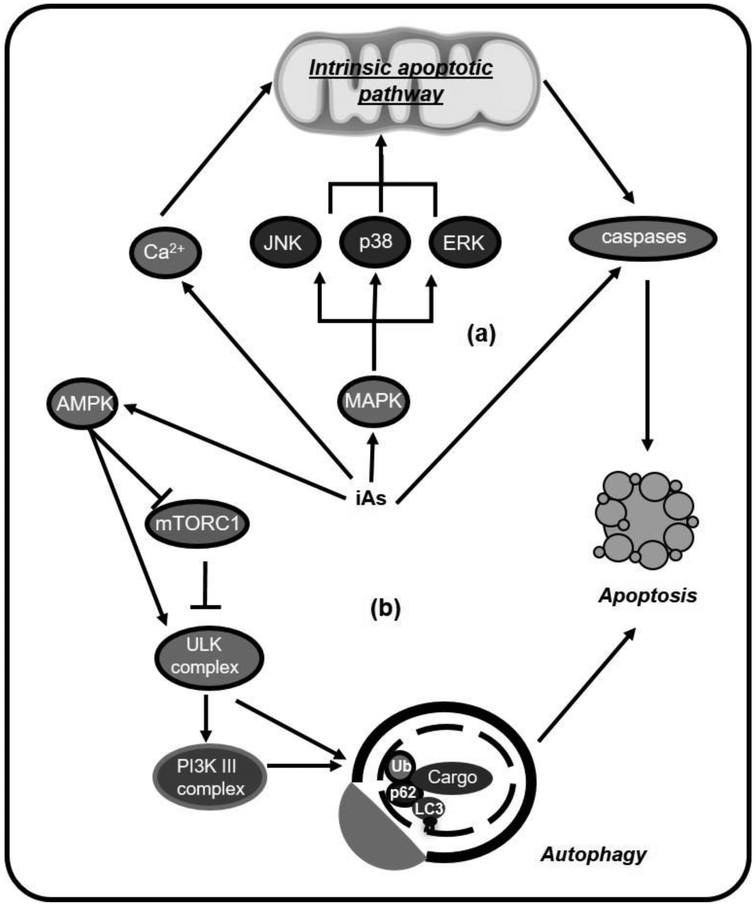
We will now discuss the activation of neural death pathways by iAs. iAs and its methylated metabolites induces caspase-dependent apoptosis in neurons and neuroblastoma cells through the mitogen-activated protein kinase (MAPK) signaling, that can involve the extracellular-signal-regulated kinase 2 (ERK2), p38 or c-Jun N-terminal kinases (JNK) [90, 91], Apoptosis induced by iAs can also be triggered by Ca2+ (Figure 4a) [92]. In vivo, iAs has been reported to trigger apoptosis in cerebral cortex [93]. Interestingly, iAs-induced apoptosis in hippocampal neurons seems to also me mediated by the antagonism of neurotrophic signaling [94].
Figure 4. Mechanisms of iAs-induced cell death in neuronal cells.
iAs neurotoxicity has been related to the activation of different cell death pathways. (a) Exposure to iAs and its methylated metabolites induces caspase-dependent apoptosis, involving the activation of the mitogen-activated protein kinase (MAPK) pathways, specifically c-Jun N-terminal kinase (JNK), p38 and extracellular-signal-regulated kinase (ERK) which are associated with the intrinsic mitochondrial apoptotic pathway. iAs also triggers intracellular calcium (Ca2+) increase that mediates apoptosis. (b) iAs-induced cell death is regulated by autophagy activation through the activation of the AMP-dependent protein kinase (AMPK) and inhibition of the mechanistic (or mammalian) target of rapamycin (mTOR).
Autophagy is a homeostatic process in which double–membraned autophagosomes engulf cellular components to be subsequently degraded upon fusion with lysosomes. Autophagosome cargo degradation preserves cellular homeostasis and viability via the turnover of damaged organelles and biomolecules whose prevalence or accumulation within cells can lead to deleterious effects. In most cases, induction of autophagy in response to stress acts as a pro-survival mechanism, but several examples have been reported where autophagy mediates cell death [95]. iAs treatment during development induces autophagy in the mouse brain via inhibition of phosphoinositide 3-kinase (PI3K) / Akt / mechanistic target of rapamycin (mTOR) signaling [48]. Inhibition of autophagy decreases the toxic effects of iAs in glioblastoma cell lines [96]. We have observed that iAs induced apoptosis in cortical astrocytes is regulated by the AMP-dependent protein kinase (AMPK) / mTOR signaling pathways and autophagy activation (unpublished data) (Figure 4b). In a recent study, intra-hippocampal injection of low concentrations of NaAsO2 (5 and 10 nM) enhanced autophagy and diminished apoptosis, while higher concentrations (100 nM) of NaAsO2 showed the opposite effect. At low concentration iAs facilitated the acquisition of spatial learning and the authors suggested that this phenomenon could be attributed to the induction of neuronal autophagy [97].
Inflammation
iAs induces strong inflammatory responses in the brain. In rat hippocampus, cultured microglia and astrocytes, iAs treatment increases the expression levels of the pro-inflammatory cytokines interleukin 1 beta (IL-1β), IL-6, interferon gamma (IFNγ) and TNFα [29, 98, 99]. Importantly, released cytokines can further mediate neuronal toxicity [99]. Chronic exposure with iAs also induces nitrosative stress by activating the inducible NOS (iNOS) in the brain. [100].
Conclusions and Perspectives
Understanding the neurotoxic effects of iAs is an important issue since iAs exposure is a public health concern around the World. The epidemiological reports undoubtedly show that iAs affects intellectual and cognitive function during development and in adults. Moreover, while iAs cannot be considered by itself a trigger of neurodegeneration there is evidence that suggests that it can increase the susceptibility to develop neurodegenerative disorders. While there are toxicological and epidemiological studies that show the neurotoxic effects of iAs the mechanisms involved are still unclear. In this review, we have summarized the current state of knowledge in regards to iAs metabolism and transport, and our current understanding of the molecular mechanisms involved in its neurotoxic effects. As summarized elsewhere [3], it is clear that more research is needed to clearly identify the mechanisms involved in the alterations in neurotransmission and cognitive/behavioral functions associated with developmental iAs exposure and the integrated role of neural cell populations (neurons and glia). In particular, a detailed exploration of the changes in epigenetic signatures and metabolism is still missing (bioenergetics, redox and neurotransmitter), which has the enormous potential to be used as biomarkers of disease mechanisms. Furthermore, the effect of aggregate exposures to the neurotoxicity of iAs has been poorly studied.
Acknowledgements
This work was supported by the National Institutes of Health Grant P20RR17675 Centers of Biomedical Research Excellence (COBRE), the Research Council and the Office of Research at the University of Nebraska-Lincoln.
List of abbreviations:
- Aβ
amyloid beta peptide
- AD
Alzheimer's disease
- Akt
Protein Kinase B
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- AMPK
AMP-dependent protein kinase
- APP
amyloid precursor protein
- AQP
aqua(glycerol)porins
- As
arsenic
- As(GS)3
arsenic triglutathione
- As2O3
arsenic trioxide
- AS3MT
arsenite methyltransferase
- AsIII
arsenite
- AsV
arsenate
- BACE-1
β-secretase
- BBB
blood brain barrier
- BHMT
betaine hydroxy methyltransferase
- Ca
calcium
- Cd
Cadmium
- CBS
cystathionine beta synthase
- CNS
Central Nervous System
- CoA
coenzyme A
- COX-2
cyclooxygenase-2
- CTH
cystathionine-γ-lyase or γ-cystathionase
- Cys
cysteine
- DMAIII(GS)
dimethylarsinic GSH
- DMAIII
dimethylarsinous acid
- DMAV
dimethylarsinic acid
- DMAH
dimethylarsine
- DMA•
dimethylarsine radical
- DMAOO•
dimethylarsine peroxyl radical
- DMAOOH
dimethylated arsenic peroxide
- EAAT
excitatory amino acid transporter
- ER
endoplasmic reticulum
- ERK
extracellular-signal-regulated kinase
- γ-GCL
gamma-glutamylcysteine ligase (or synthase; γ-GCS)
- G3P
glyceraldehyde 3-phosphate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GLUT
glucose transporters
- GS
glutamine synthetase
- GSH
glutathione
- GSTs
glutathione-S transferases
- HAT
histone acetyltransferases
- iAs
inorganic As
- iAsV-3-P-glycerate
1-arsenato-3-phospho-D-glycerate
- IFNγ
Interferon gamma
- IQ
intelligence quotient
- IL-1β
interleukin 1 beta
- iNOS
inducible NOS
- JNK
c-Jun N-terminal kinase
- Keap1
kelch-like ECH-associated protein 1
- Pb
Lead
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- MAT
methionine adenosyltransferase
- 5,10-MeTHF
5,10-methylene tetrahydrofolate
- MeTs
methyltransferases
- 2-MG
2-methyl glycine
- MMA(GS)2
monomethylarsinic diglutathione
- MMAIII
momomethylarsonous acid
- MMAV
monomethylarsonic acid
- MRPs
multidrug resistance proteins
- MS
methionine synthase
- MT
metallothionenin
- MTF1
metal-responsive transcription factor-1
- 5-MTHF
5-methyl tetrahydrofolate
- MTHFR
methyl tetrahydrofolate reductase
- mTOR
mechanistic (or mammalian) target of rapamycin
- NaAsO2
sodium arsenite
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDA
glutamate/N-methyl-D-aspartate
- NMDAR
glutamate/N-methyl-D-aspartate receptor
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOX
NADPH oxidases
- NQO1
NADPH dehydrogenase quinone 1
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- p62
autophagic receptor sequestosome-1, p62/SQSTM1
- PD
Parkinson's disease
- PDH
pyruvate dehydrogenase
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PI3K
class III phosphatidylinositol 3-kinase
- PNS
peripheral nervous system
- Pi
phosphate
- PPi
diphosphate
- PTGEs
prostaglandin E synthase
- RAGE
advanced glycation end products
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SAH
s-adenosylhomocysteine
- SAHH
SAH hydrolase
- SAM
S-adenosylmethionine
- SOD
superoxide dismutases
- TCA
tricarboxylic acid
- TFAM
mitochondrial transcription factor A
- TNF-α
tumor necrosis factor alpha
- TR
Trx reductase
- Trx
thioredoxin
- xCT
cystine/glutamate exchanger system
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest statement
The authors declare that they have no conflict of interest.
References
- 1.Garza-Lombo C, Posadas Y, Quintanar L, Gonsebatt ME and Franco R (2018) Antioxid Redox Signal 28:1669–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou S, Harper C, Ingerman L, Llados F, Colman J, Chappell L, Osier M, Odin M and Sage G (2007) Toxicological profile for arsenic. The Agency, Altanta, GA [Google Scholar]
- 3.Carlin DJ, Naujokas MF, Bradham KD, Cowden J, Heacock M, Henry HF, Lee JS, Thomas DJ, Thompson C, Tokar EJ, Waalkes MP, Birnbaum LS and Suk WA (2016) Environ Health Perspect 124:890–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandal BK and Suzuki KT (2002) Talanta 58:201–235 [PubMed] [Google Scholar]
- 5.Gundert-Remy U, Damm G, Foth H, Freyberger A, Gebel T, Golka K, Rohl C, Schupp T, Wollin KM and Hengstler JG (2015) Arch Toxicol 89:2219–2227 [DOI] [PubMed] [Google Scholar]
- 6.Tolins M, Ruchirawat M and Landrigan P (2014) Ann Glob Health 80:303–314 [DOI] [PubMed] [Google Scholar]
- 7.Farzan SF, Karagas MR and Chen Y (2013) Toxicol Appl Pharmacol 272:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderon J, Navarro ME, Jimenez-Capdeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I, Borja-Aburto V and Diaz-Barriga F (2001) Environ Res 85:69–76 [DOI] [PubMed] [Google Scholar]
- 9.Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, Garcia-Vargas G, Del Carmen Caamano M, Cebrian ME and Stoltzfus RJ (2007) Environ Health Perspect 115:1371–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wasserman GA, Liu X, Loiacono NJ, Kline J, Factor-Litvak P, van Geen A, Mey JL, Levy D, Abramson R, Schwartz A and Graziano JH (2014) Environ Health 13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, Sultana R, Sultana R, Islam T, Levy D, Mey JL, van Geen A, Khan K, Kline J, Ahsan H and Graziano JH (2011) Environ Health Perspect 119:1665–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-Chavez LA, Rendon-Lopez CR, Zepeda A, Silva-Adaya D, Del Razo LM and Gonsebatt ME (2015) Front Cell Neurosci 9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson-Mora J, Escobar ML, Rodriguez-Duran L, Massieu L, Montiel T, Rodriguez VM, Hernandez-Mercado K and Gonsebatt ME (2018) Arch Toxicol 92:1037–1048 [DOI] [PubMed] [Google Scholar]
- 14.Aung KH, Kyi-Tha-Thu C, Sano K, Nakamura K, Tanoue A, Nohara K, Kakeyama M, Tohyama C, Tsukahara S and Maekawa F (2016) Front Neurosci 10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong YS, Song KH and Chung JY (2014) J Prev Med Public Health 47:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyler CR and Allan AM (2014) Curr Environ Health Rep 1:132–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul S, Das N, Bhattacharjee P, Banerjee M, Das JK, Sarma N, Sarkar A, Bandyopadhyay AK, Sau TJ, Basu S, Banerjee S, Majumder P and Giri AK (2013) J Expo Sci Environ Epidemiol 23:156–162 [DOI] [PubMed] [Google Scholar]
- 18.Mochizuki H, Phyu KP, Aung MN, Zin PW, Yano Y, Myint MZ, Thit WM, Yamamoto Y, Hishikawa Y, Thant KZ, Maruyama M and Kuroda Y (2019) Environ Health Prev Med 24:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafeman DM, Ahsan H, Louis ED, Siddique AB, Slavkovich V, Cheng Z, van Geen A and Graziano JH (2005) J Occup Environ Med 47:778–784 [DOI] [PubMed] [Google Scholar]
- 20.Le Quesne PM and McLeod JG (1977) J Neurol Sci 32:437–451 [DOI] [PubMed] [Google Scholar]
- 21.Vahidnia A, Romijn F, Tiller M, van der Voet GB and de Wolff FA (2006) Hum Exp Toxicol 25:667–674 [DOI] [PubMed] [Google Scholar]
- 22.Vahidnia A, Romijn F, van der Voet GB and de Wolff FA (2008) Chem Biol Interact 176:188–195 [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Chavez E, Segura B, Merchant H, Jimenez I and Del Razo LM (2007) J Neurol Sci 258:104–110 [DOI] [PubMed] [Google Scholar]
- 24.Yang YW, Liou SH, Hsueh YM, Lyu WS, Liu CS, Liu HJ, Chung MC, Hung PH and Chung CJ (2018) Toxicol Appl Pharmacol 356:8–14 [DOI] [PubMed] [Google Scholar]
- 25.Sharma B and Sharma PM (2013) Toxicol Appl Pharmacol 273:180–188 [DOI] [PubMed] [Google Scholar]
- 26.Nino SA, Martel-Gallegos G, Castro-Zavala A, Ortega-Berlanga B, Delgado JM, Hernandez-Mendoza H, Romero-Guzman E, Rios-Lugo J, Rosales-Mendoza S, Jimenez-Capdeville ME and Zarazua S (2018) Chem Res Toxicol 31:13–21 [DOI] [PubMed] [Google Scholar]
- 27.Nino SA, Morales-Martinez A, Chi-Ahumada E, Carrizales L, Salgado-Delgado R, Perez-Severiano F, Diaz-Cintra S, Jimenez-Capdeville ME and Zarazua S (2019) ACS Chem Neurosci 10:323–336 [DOI] [PubMed] [Google Scholar]
- 28.Ashok A, Rai NK, Tripathi S and Bandyopadhyay S (2015) Toxicol Sci 143:64–80 [DOI] [PubMed] [Google Scholar]
- 29.Escudero-Lourdes C, Uresti-Rivera EE, Oliva-Gonzalez C, Torres-Ramos MA, Aguirre-Banuelos P and Gandolfi AJ (2016) Neurochem Res 41:2559–2572 [DOI] [PubMed] [Google Scholar]
- 30.Shavali S and Sens DA (2008) Toxicol Sci 102:254–261 [DOI] [PubMed] [Google Scholar]
- 31.Cholanians AB, Phan AV, Ditzel EJ, Camenisch TD, Lau SS and Monks TJ (2016) Toxicol Sci 153:271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres-Avila M, Leal-Galicia P, Sanchez-Pena LC, Del Razo LM and Gonsebatt ME (2010) Environ Res 110:443–447 [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P and Rosen BP (2002) Proc Natl Acad Sci U S A 99:6053–6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calatayud M, Barrios JA, Velez D and Devesa V (2012) Chem Res Toxicol 25:446–453 [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Pena LC, Petrosyan P, Morales M, Gonzalez NB, Gutierrez-Ospina G, Del Razo LM and Gonsebatt ME (2010) Environ Res 110:428–434 [DOI] [PubMed] [Google Scholar]
- 36.Leslie EM, Haimeur A and Waalkes MP (2004) J Biol Chem 279:32700–32708 [DOI] [PubMed] [Google Scholar]
- 37.Yoshino Y, Yuan B, Kaise T, Takeichi M, Tanaka S, Hirano T, Kroetz DL and Toyoda H (2011) Toxicol Appl Pharmacol 257:198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukalek CB, Swanlund DP, Rousseau RK, Weigl KE, Marensi V, Cole SP and Leslie EM (2016) Mol Pharmacol 90:127–139 [DOI] [PubMed] [Google Scholar]
- 39.Tadepalle N, Koehler Y, Brandmann M, Meyer N and Dringen R (2014) Neurochem Int 76:1–11 [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Liu Y, Powell DA, Waalkes MP and Klaassen CD (2002) Toxicology 170:55–62 [DOI] [PubMed] [Google Scholar]
- 41.Watanabe T and Hirano S (2013) Arch Toxicol 87:969–979 [DOI] [PubMed] [Google Scholar]
- 42.Ding L, Saunders RJ, Drobna Z, Walton FS, Xun P, Thomas DJ and Styblo M (2012) Toxicol Appl Pharmacol 264:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas DJ (2010) Toxicol Sci 117:249–252 [DOI] [PubMed] [Google Scholar]
- 44.Hughes MF (2002) Toxicol Lett 133:1–16 [DOI] [PubMed] [Google Scholar]
- 45.Nemeti B, Regonesi ME, Tortora P and Gregus Z (2010) Toxicol Sci 117:270–281 [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez VM, Del Razo LM, Limon-Pacheco JH, Giordano M, Sanchez-Pena LC, Uribe-Querol E, Gutierrez-Ospina G and Gonsebatt ME (2005) Toxicol Sci 84:157–166 [DOI] [PubMed] [Google Scholar]
- 47.Golmohammadi J, Jahanian-Najafabadi A and Aliomrani M (2019) Biol Trace Elem Res 189:172–179 [DOI] [PubMed] [Google Scholar]
- 48.Manthari RK, Tikka C, Ommati MM, Niu R, Sun Z, Wang J, Zhang J and Wang J (2018) Arch Toxicol 92:3255–3275 [DOI] [PubMed] [Google Scholar]
- 49.Rehman K and Naranmandura H (2012) Metallomics 4:881–892 [DOI] [PubMed] [Google Scholar]
- 50.Mizumura A, Watanabe T, Kobayashi Y and Hirano S (2010) Toxicol Appl Pharmacol 242:119–125 [DOI] [PubMed] [Google Scholar]
- 51.Zhang HN, Yang L, Ling JY, Czajkowsky DM, Wang JF, Zhang XW, Zhou YM, Ge F, Yang MK, Xiong Q, Guo SJ, Le HY, Wu SF, Yan W, Liu B, Zhu H, Chen Z and Tao SC (2015) Proc Natl Acad Sci U S A 112:15084–15089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen S, Li XF, Cullen WR, Weinfeld M and Le XC (2013) Chem Rev 113:7769–7792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, Sun X, Mobarak C, Gandolfi AJ, Burchiel SW, Hudson LG and Liu KJ (2014) Chem Res Toxicol 27:690–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qu W and Waalkes MP (2015) Toxicol Appl Pharmacol 282:267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang YY, Kuo TC, Hsu CH, Hou DR, Kao YH and Huang RN (2012) Arch Toxicol 86:911–922 [DOI] [PubMed] [Google Scholar]
- 56.Halliwell B and Cross CE (1994) Environ Health Perspect 102 Suppl 10:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olsen LF, Issinger OG and Guerra B (2013) Redox Rep 18:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forman HJ (2016) Free Radic Biol Med 97:398–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Culotta VC, Yang M and O'Halloran TV (2006) Biochim Biophys Acta 1763:747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schrader M and Fahimi HD (2006) Biochim Biophys Acta 1763:1755–1766 [DOI] [PubMed] [Google Scholar]
- 61.Finkel T (2011) J Cell Biol 194:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reczek CR and Chandel NS (2015) Curr Opin Cell Biol 33:8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel M (2016) Trends Pharmacol Sci 37:768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salim S (2017) J Pharmacol Exp Ther 360:201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia-Chavez E, Jimenez I, Segura B and Del Razo LM (2006) Neurotoxicology 27:1024–1031 [DOI] [PubMed] [Google Scholar]
- 66.Castro-Coronel Y, Del Razo LM, Huerta M, Hernandez-Lopez A, Ortega A and Lopez-Bayghen E (2011) Toxicol Sci 122:539–550 [DOI] [PubMed] [Google Scholar]
- 67.Negishi T, Takahashi M, Matsunaga Y, Hirano S and Tashiro T (2012) J Neuropathol Exp Neurol 71:468–479 [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Duan X, Li J, Zhao S, Li W, Zhao L, Li W, Nie H, Sun G and Li B (2016) Neurochem Res 41:2119–2128 [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Chavez E, Santamaria A, Diaz-Barriga F, Mandeville P, Juarez BI and Jimenez-Capdeville ME (2003) Brain Res 976:82–89 [DOI] [PubMed] [Google Scholar]
- 70.Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ and Valko M (2011) J Appl Toxicol 31:95–107 [DOI] [PubMed] [Google Scholar]
- 71.Flora SJ (2011) Free Radic Biol Med 51:257–281 [DOI] [PubMed] [Google Scholar]
- 72.Prakash C, Soni M and Kumar V (2015) Biol Trace Elem Res 167:121–129 [DOI] [PubMed] [Google Scholar]
- 73.Prakash C and Kumar V (2016) Chem Biol Interact 256:228–235 [DOI] [PubMed] [Google Scholar]
- 74.Zamora PL, Rockenbauer A and Villamena FA (2014) Chem Res Toxicol 27:765–774 [DOI] [PubMed] [Google Scholar]
- 75.Lau A, Zheng Y, Tao S, Wang H, Whitman SA, White E and Zhang DD (2013) Mol Cell Biol 33:2436–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He X and Ma Q (2009) J Biol Chem 284:12609–12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rojas D, Rager JE, Smeester L, Bailey KA, Drobna Z, Rubio-Andrade M, Styblo M, Garcia-Vargas G and Fry RC (2015) Toxicol Sci 143:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez L, Jimenez V, Garcia-Sepulveda C, Ceballos F, Delgado JM, Nino-Moreno P, Doniz L, Saavedra-Alanis V, Castillo CG, Santoyo ME, Gonzalez-Amaro R and Jimenez-Capdeville ME (2011) Neurochem Int 58:574–581 [DOI] [PubMed] [Google Scholar]
- 79.Du X, Tian M, Wang X, Zhang J, Huang Q, Liu L and Shen H (2018) Environ Pollut 234:590–600 [DOI] [PubMed] [Google Scholar]
- 80.Samikkannu T, Chen CH, Yih LH, Wang AS, Lin SY, Chen TC and Jan KY (2003) Chem Res Toxicol 16:409–414 [DOI] [PubMed] [Google Scholar]
- 81.Schiller CM, Fowler BA and Woods JS (1977) Environ Health Perspect 19:205–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cronican AA, Fitz NF, Carter A, Saleem M, Shiva S, Barchowsky A, Koldamova R, Schug J and Lefterov I (2013) PLoS One 8:e53478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tyler CR, Hafez AK, Solomon ER and Allan AM (2015) Toxicol Appl Pharmacol 288:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huo TG, Li WK, Zhang YH, Yuan J, Gao LY, Yuan Y, Yang HL, Jiang H and Sun GF (2015) Mol Neurobiol 51:980–994 [DOI] [PubMed] [Google Scholar]
- 85.Maekawa F, Tsuboi T, Oya M, Aung KH, Tsukahara S, Pellerin L and Nohara K (2013) Neurotoxicology 37:197–206 [DOI] [PubMed] [Google Scholar]
- 86.Monaco NM, Bartos M, Dominguez S, Gallegos C, Bras C, Esandi MDC, Bouzat C, Giannuzzi L, Minetti A and Gumilar F (2018) Neurotoxicology 67:37–45 [DOI] [PubMed] [Google Scholar]
- 87.Chandravanshi LP, Gupta R and Shukla RK (2019) Biol Trace Elem Res 189:118–133 [DOI] [PubMed] [Google Scholar]
- 88.Singh V, Gera R, Kushwaha R, Sharma AK, Patnaik S and Ghosh D (2016) Sci Rep 6:30601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao F, Liao Y, Jin Y, Li G, Lv X and Sun G (2012) Toxicol In Vitro 26:24–31 [DOI] [PubMed] [Google Scholar]
- 90.Namgung U and Xia Z (2001) Toxicol Appl Pharmacol 174:130–138 [DOI] [PubMed] [Google Scholar]
- 91.Lu TH, Tseng TJ, Su CC, Tang FC, Yen CC, Liu YY, Yang CY, Wu CC, Chen KL, Hung DZ and Chen YW (2014) Toxicol Lett 224:130–140 [DOI] [PubMed] [Google Scholar]
- 92.Florea AM, Splettstoesser F and Busselberg D (2007) Toxicol Appl Pharmacol 220:292–301 [DOI] [PubMed] [Google Scholar]
- 93.Yen CC, Ho TJ, Wu CC, Chang CF, Su CC, Chen YW, Jinn TR, Lu TH, Cheng PW, Su YC, Liu SH and Huang CF (2011) Arch Toxicol 85:565–575 [DOI] [PubMed] [Google Scholar]
- 94.Pandey R, Rai V, Mishra J, Mandrah K, Kumar Roy S and Bandyopadhyay S (2017) Toxicol Sci 159:137–158 [DOI] [PubMed] [Google Scholar]
- 95.Doherty J and Baehrecke EH (2018) Nat Cell Biol 20:1110–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li C, Liu Y, Liu H, Zhang W, Shen C, Cho K, Chen X, Peng F, Bi Y, Hou X, Yang Z, Zheng Z, Wang K, Wang X, Zhang J, Zhong C, Zou H, Zhang X and Zhao S (2015) Cell Physiol Biochem 35:1303–1316 [DOI] [PubMed] [Google Scholar]
- 97.Bonakdar Yazdi B, Khodagholi F, Shaerzadeh F, Sharifzadeh A, Ahmadi R, Sanati M, Mehdizadeh H, Payandehmehr B, Vali L, Jahromi MM, Taghizadeh G and Sharifzadeh M (2017) Eur J Pharmacol 796:54–61 [DOI] [PubMed] [Google Scholar]
- 98.Firdaus F, Zafeer MF, Waseem M, Ullah R, Ahmad M and Afzal M (2018) Biomed Pharmacother 102:1152–1160 [DOI] [PubMed] [Google Scholar]
- 99.Mao J, Yang J, Zhang Y, Li T, Wang C, Xu L, Hu Q, Wang X, Jiang S, Nie X and Chen G (2016) Toxicol Appl Pharmacol 303:79–89 [DOI] [PubMed] [Google Scholar]
- 100.Saha S, Sadhukhan P, Mahalanobish S, Dutta S and Sil PC (2018) J Nutr Biochem 55:26–40 [DOI] [PubMed] [Google Scholar]
- 101.Ali N, Hoque MA, Haque A, Salam KA, Karim MR, Rahman A, Islam K, Saud ZA, Khalek MA, Akhand AA, Hossain M, Mandal A, Karim MR, Miyataka H, Himeno S and Hossain K (2010) Environ Health 9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chatterjee D, Bandyopadhyay A, Sarma N, Basu S, Roychowdhury T, Roy SS and Giri AK (2018) Environ Pollut 233:596–603 [DOI] [PubMed] [Google Scholar]
- 103.Sung K, Kim M, Kim H, Hwang GW and Kim K (2019) Biol Trace Elem Res 187:224–229 [DOI] [PubMed] [Google Scholar]
- 104.Nino SA, Morales-Martinez A, Chi-Ahumada E, Carrizales L, Salgado-Delgado R, Perez-Severiano F, Diaz-Cintra S, Jimenez-Capdeville ME and Zarazua S (2018) ACS Chem Neurosci, doi 10.1021/acschemneuro.8b00278 [DOI] [PubMed] [Google Scholar]
- 105.Baldissarelli LA, Capiotti KM, Bogo MR, Ghisleni G and Bonan CD (2012) Comp Biochem Physiol C Toxicol Pharmacol 155:566–572 [DOI] [PubMed] [Google Scholar]
- 106.Sarkar S, Mukherjee S, Chattopadhyay A and Bhattacharya S (2014) Ecotoxicol Environ Saf 107:1–8 [DOI] [PubMed] [Google Scholar]
- 107.de Castro MR, Lima JV, de Freitas DP, Valente Rde S, Dummer NS, de Aguiar RB, dos Santos LC, Marins LF, Geracitano LA, Monserrat JM and Barros DM (2009) Comp Biochem Physiol C Toxicol Pharmacol 150:337–342 [DOI] [PubMed] [Google Scholar]
- 108.Dipp VR, Valles S, Ortiz-Kerbertt H, Suarez JV and Bardullas U (2018) Zebrafish 15:575–585 [DOI] [PubMed] [Google Scholar]
- 109.Yu CW and Liao VH (2014) Metallomics 6:1824–1831 [DOI] [PubMed] [Google Scholar]