Abstract
Van der Waals heterostructures (vdWHs) leverage the characteristics of two-dimensional (2D) material building blocks to create a myriad of structures with unique and desirable properties. Several commonly employed fabrication strategies rely on polymeric stamps to assemble layers of 2D materials into vertical stacks. However, the properties of such heterostructures frequently are degraded by contaminants, typically of unknown composition, trapped between the constituent layers. Such contaminants therefore impede studies of the intrinsic properties of heterostructures and hinder their application. Here, we use the photothermal induced resonance (PTIR) technique to obtain infrared spectra and maps of the contaminants down to a few attomoles and with nanoscale resolution. Heterostructures comprised of WSe2, WS2, and hBN layers were found to contain significant amounts of polydimethylsiloxane (PDMS) and polycarbonate, corresponding to the stamp materials used in their construction. Additionally, we verify that an atomic force microscope-based “nano-squeegee” technique is an effective method for locally removing contaminants by comparing spectra within as-fabricated and cleaned regions. Having identified the source of the contaminants, we demonstrate that cleaning PDMS stamps with isopropanol or toluene prior to vdWH fabrication reduces PDMS contamination within the structures. The general applicability of the PTIR technique for identifying the sources corrupting vdWHs provides valuable guidance for devising mitigation strategies (e.g., stamp cleaning or pre-/post-treatments) and enhances capabilities for producing materials with precisely engineered properties.
Keywords: van der Waals heterostructures, 2D materials, interlayer contaminants, nanoscale spectroscopy, photothermal induced resonance, IR spectroscopy
Graphical Abstract
INTRODUCTION
Two-dimensional (2D) materials are composed of layers that are weakly bound to each other via van der Waals forces. This characteristic enables facile mechanical isolation, pick up, and transfer of few or single layers from bulk crystals. The family of 2D materials includes metals such as graphene,1 semiconductors such as the transition metal dichalcogenides (TMDs),2 and insulators such as hexagonal boron nitride (hBN).3 Individually, single layers of these materials attract much attention due to their diverse and exceptional electronic, optical, and magnetic properties.4–6 Notably, the absence of out-of-plane covalent bonding enables restacking of multiple individual layers in any sequence without regard for lattice match, providing an unprecedented opportunity to create synthetic heterostructures with tailored properties.7–9 These stacks, referred to as van der Waals heterostructures (vdWHs), can provide novel functionalities and open new opportunities in optoelectronics,2 valleytronics,10 and catalysis,11 while revealing new physics.12
A common fabrication route for vdWHs involves the pick-up, transfer, and release of selected layers onto a target substrate. This process is already achievable with a variety of techniques: the poly-methyl methacrylate (PMMA) carrying layer,13 the wedge transfer,14 the polydimethylsiloxane (PDMS) deterministic all-dry transfer,15 and the van der Waals pick-up transfer16 methods. However, these stacking methods unintentionally trap material, typically of unknown composition, between the layers, even when carried out in nominally clean environments (e.g., glove boxes or at reduced pressures). These trapped contaminants obstruct intimate contact between the layers and often lead to the formation of numerous sub-surface aggregates, which appear as protrusions (“bubbles” or “blisters”) and other topographic irregularities in vdWHs.17 Previously, it was reported that the bubbles observed in vdWHs constructed using the PMMA carrying layer method contained amorphous hydrocarbons, as would be expected in the case of PMMA contamination.18 However, little is known about the contaminants trapped by other transfer methods. These contaminants preclude the formation of pristine interfaces, modulate material properties, hinder interlayer interactions (e.g., charge and energy transfer),19 and promote degradation of delicate 2D materials such as black phosphorus.20,21
Common strategies for limiting contamination and for promoting intimate layer contacts include control of the transfer and peeling speed or thermal annealing. Annealing promotes contaminant aggregation through Ostwald ripening of the bubbles and diffusion of contaminants out of the vdWHs through the edges,22 thereby leaving behind wider, possibly pristine regions, but leads to unpredictable contaminant distributions. Recently, controllable, bubble-free regions in vdWHs were prepared either by using the tip of an atomic force microscope (AFM) probe to sweep (“squeegee”) an area of interest23,24 or by stacking the 2D materials under vacuum.25 However, the fabrication of “clean” interfaces using typical transfer procedures remains a significant challenge that must be resolved to fully realize the potential of vdWHs in widespread applications. Consequently, novel analytical methods capable of both high chemical sensitivity and nanoscale resolution are necessary to identify the minute quantities of interlayer contaminants. Such knowledge will provide valuable guidance for devising mitigation strategies that improve the efficacies of vdWH fabrication and for producing precisely engineered nanostructures.
Here, we leverage photothermal induced resonance (PTIR),26,27 a chemically sensitive scanning probe technique to identify the chemical compositions of nanoscale contaminants trapped within vdWHs fabricated using polymeric stamps; i.e., the most common transfer techniques. The close correspondence between PTIR and well-known far-field infrared (IR) spectra,28–30 enables the identification of PDMS or polycarbonate (PC) contaminants, matching the stamp materials used in vdWH fabrication. As few as ≈ 10−18 mol of contaminants were detected in single nanobubbles. We verify that using an AFM-based nano-squeegee technique is a generally effective, albeit low-throughput, method to create contaminant-free areas in vdWHs without thermal annealing. Additionally, having identified the trapped impurities and their likely sources, we find that washing PDMS stamps with isopropanol or toluene prior to pick-up is effective at reducing the amount of polymeric residue transferred by the stamp. Based on the wide applicability of PTIR,26,27 we believe that our approach will be generally useful to engineer cleaner vdWHs and to characterize nanostructures with precisely controlled properties.
RESULTS AND DISCUSSION
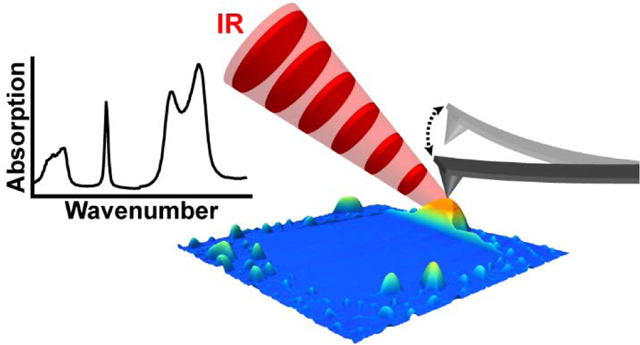
Infrared micro-spectroscopy is a diffraction-limited technique widely used to identify materials and chemical impurities but lacks the sensitivity and spatial resolution to characterize the nanoscale contaminants studied here. By contrast, PTIR,26,27 also known as AFM-IR, bypasses light diffraction limitations using a sharp AFM probe as a near-field mechanical transducer, thereby enabling IR characterization with nanoscale resolution and high sensitivity. In PTIR, a pulsed, wavelength-tunable, monochromatic laser (Figure 1b) illuminates a portion of the sample (≈ 50 μm diameter) centered around an AFM tip in contact with the sample. Within the illuminated area, the sample constituents that absorb the laser pulse undergo rapid thermal expansion and, among those areas, only the portion of the sample directly beneath the AFM probe contributes to the PTIR signal. The sample expansion dynamics can be captured directly using ultrasensitive, custom-made nanophotonic probes,31 but this expansion is too fast for conventional AFM cantilevers, which instead are kicked into oscillations akin to a struck tuning fork. Because the cantilever oscillation amplitude is proportional to the local absorption coefficient in the sample,28 in a first approximation, PTIR enables identification of materials27 and chemical groups32 by comparison with far-field IR spectral databases. Also, since the PTIR signal derives from the rapid sample expansion rather than the slower heat diffusion,33 spatial resolutions as high as ≈ 20 nm34,35 in contact mode or ≈ 10 nm in tapping-mode36 are possible. Other recent PTIR innovations include the extension to the visible spectral range35 and operation in a water environment.37,38 Due to these characteristics, PTIR impacts an ever-growing number of applications including, polymer science,39,40 photovoltaics,41,42 plasmonics,43,44 geology,45 biology,46 and medicine,47,38,36 as discussed in recent reviews.26,27 For 2D materials, PTIR has been applied to characterize the polariton distribution in hBN nanostructures48,49 and to characterize the functional groups in graphene oxide.50
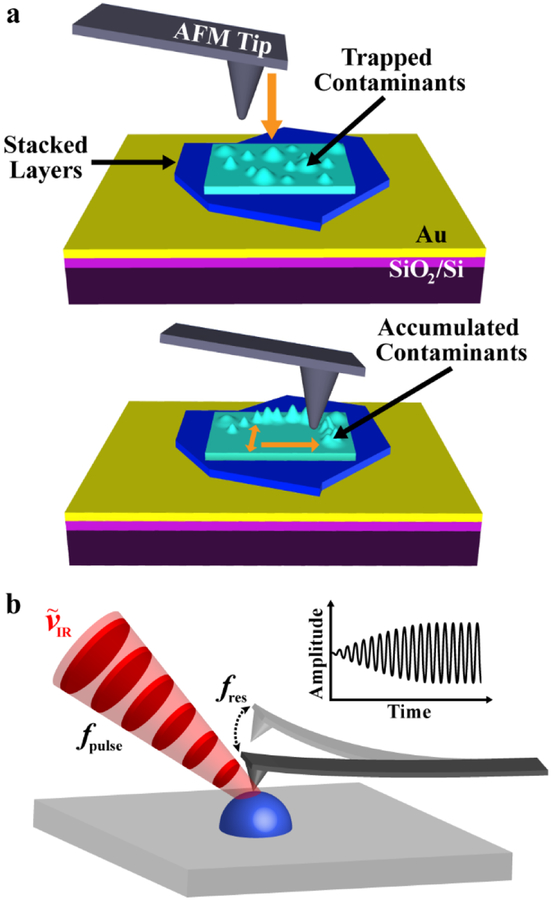
Figure 1.
Photothermal induced resonance (PTIR) and nano-squeegee techniques identify the chemical composition of nanoscale contaminants. (a) Schematic illustrating the nano-squeegee technique, which uses the tip of an atomic force microscope probe to sweep trapped contaminants to the periphery of the scanned region. (b) Schematic illustrating PTIR measurements. When infrared (IR) laser pulses are absorbed by the sample, the sample heats up and expands, thereby kicking the cantilever into oscillation. The laser pulse repetition rate (fpulse) is tuned to match one of the cantilever contact-resonant frequencies (fres), leading to enhancement of the oscillation amplitude. The wavenumber (ṽ) of the impinging IR light is varied to obtain sample absorption spectra.
In this work, we resonantly excite the AFM cantilever by tuning the laser pulse repetition rate to match one of the cantilever contact-resonance frequencies, thereby amplifying PTIR signal-to-noise ratio by the cantilever’s quality (Q) factor, as shown previously.34 To maintain the cantilever resonant excitation during mapping, a phase-locked loop is used to tie the laser repetition rate to the cantilever oscillation frequency.36 Using such an excitation scheme, the temperature rise is small (typically less than 1 K)34 and the thin (< 100 nm) samples analyzed here thermalize back to room temperature between pulses.31
Heterostructures were assembled from combinations of hBN, tungsten diselenide (WSe2) and tungsten disulfide (WS2). Assemblies were obtained using one of three common fabrication techniques that rely on polymeric stamps: (1) PDMS all-dry transfer,51 (2) water-assisted PDMS pick-up and transfer,51–54 and (3) polymer-assisted dry pick-up and transfer.55 While the first two methods employed bare PDMS stamps, the third method used PC-coated PDMS stamps to create 2D materials-based vdWHs. These techniques are described in detail in the Supporting Information (Figures S1–S3). In brief, polymeric stamps were used to pick up and then re-deposit sheets of hBN or TMDs onto complementary flakes through both wet and dry surface-contact processes. The resulting vdWHs exhibited numerous rounded topographic protrusions (e.g., see Figure 2), typically a tell-tale sign of contaminants trapped between the stacked layers.18,24 The contaminant bubble distribution is driven by competing forces: van der Waals interactions drawing adjacent layers together thereby squeezing contaminants into pockets, while the mechanical stress arising from deformations of the 2D layers favors unstrained layers with uniformly distributed contaminants.56
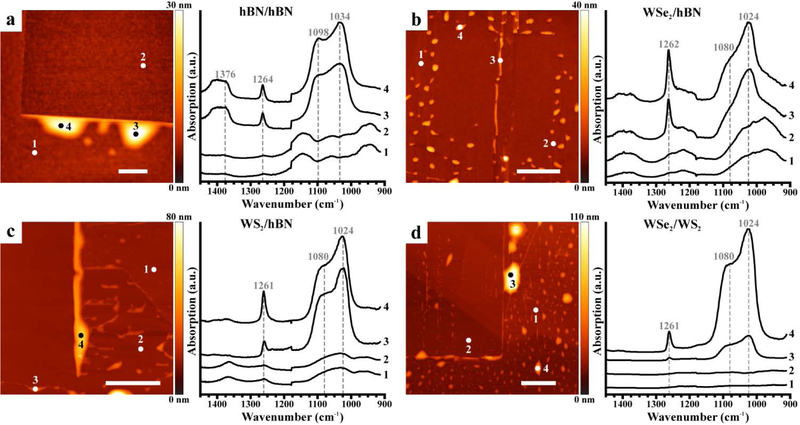
Figure 2.
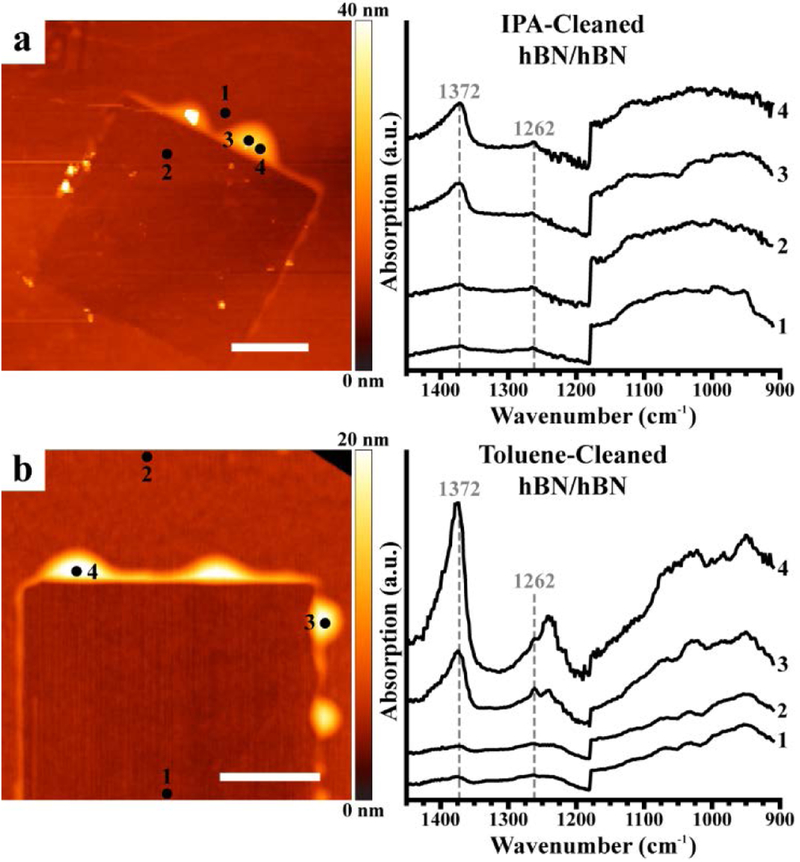
Photothermal induced resonance (PTIR) spectra identify contaminants in van der Waals heterostructures (vdWHs) prepared using the water-assisted PDMS pick-up and transfer method. Representative topographic images (left) and PTIR absorption spectra (right) obtained at the marked locations on vdWHs fabricated by overlaying flakes of (a) hBN/hBN, (b) WSe2/hBN, (c) WS2/hBN, and (d) WSe2/WS2. Scale bars represent 2 μm.
After assembly, the vdWHs were subjected to a nano-squeegee flattening process reported previously.24 This process, which moves contaminants towards the periphery of a scanning probe raster pattern creates both larger, contaminant-filled bubbles and contaminant-free regions (Figure 1a). For our purposes, both effects are advantageous as they provide areas with enriched contaminant concentrations (i.e., easier to detect), and control regions where little-to-no PTIR signal is expected. Additionally, locally modified regions of the sample exist side-by-side with as-prepared regions, enabling spectroscopic and topographic comparison.
First, we characterize vdWHs fabricated using the water-assisted PDMS pick-up and transfer method. Representative PTIR absorption spectra (Figure 2) were obtained within and around the nano-squeegeed region of the vdWHs by positioning the probe tip either atop or away from trapped contaminants (bubbles), identified by AFM topography maps. The spectra collected on the nanocontaminants within hBN/hBN vdWHs (Figure 2a) showed prominent absorption peaks at around 1034 cm−1, 1098 cm−1 (Si–O–Si stretches), and 1264 cm−1 (Si–CH3 asymmetric deformation), characteristic of PDMS.57,58 An additional broad peak at about 1376 cm−1 is related to hBN phonon-polaritons and possibly to the in-plane phonon mode of hBN.48,49,59 Spectra measured on the contaminants trapped within TMD-based vdWHs constructed with the same fabrication method (Figure 2b–d) showed similarly prominent peaks (1024 cm−1, 1080 cm−1, and about 1262 cm−1), which we assign to PDMS. Small sample-to-sample differences in the peak positions and band shapes are ascribed to differences in the local environments and, consequently, to different intermolecular interactions60 between the nanocontaminants and the 2D materials sandwiching them. The nanocontaminant PTIR absorption spectra closely resemble the spectra obtained on macroscopic slabs of PDMS measured via attenuated total reflectance Fourier transform IR (ATR-FTIR) spectroscopy (see Figure S5 in the Supporting Information). In contrast, spectra measured at locations without obvious contamination (i.e., away from bubbles or within the nano-squeegeed region) were notably weaker across their entire spectral range. These spectra, emphasized in Figure S8, however, show broad features that can be attributed for the most part to imperfect background compensation. We attribute the broad, weak features at about 1000 cm−1 to SiO2, which is present as a thin layer in the probe tip and the underlying substrate.
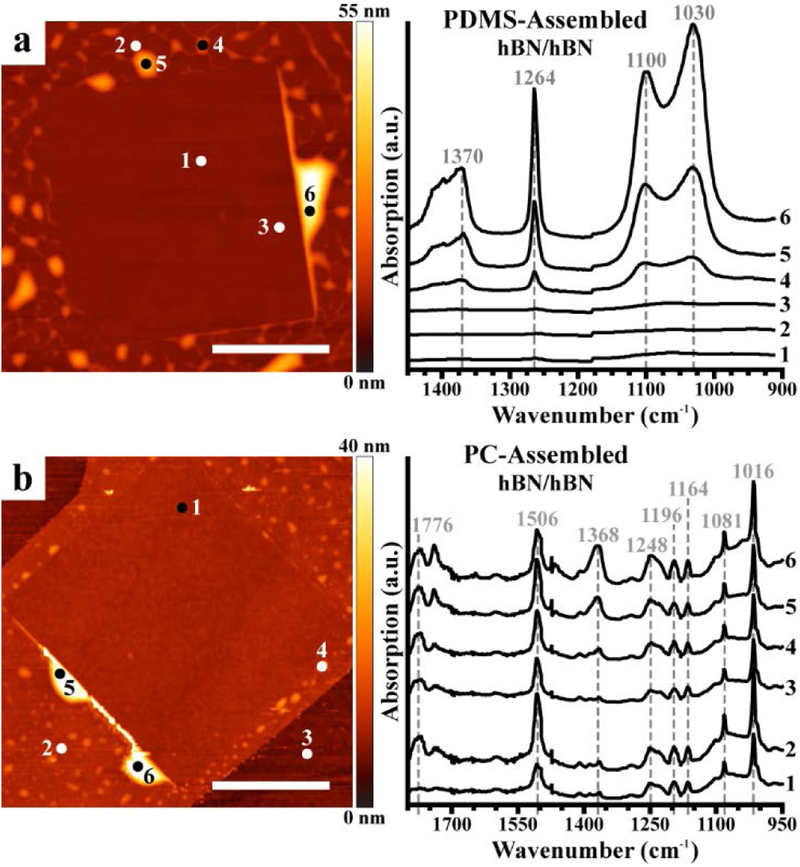
Next, we analyze representative PTIR absorption spectra obtained on vdWHs fabricated using dry, solvent-free transfer methods (Figure 3). Heterostructures assembled using these methods also exhibited notable topographic features suggestive of trapped contaminants within the stacks of 2D materials. This observation is surprising because the stamps do not touch the contaminated interface during fabrication and no solvent, which might facilitate transport of polymeric residue from the stamp, is used in the process. The spectra of the trapped contaminants within vdWHs assembled using the PDMS all-dry transfer method (Figure 3a) show the strong absorption peaks characteristic of PDMS. We note that the stamps directly contact only the outward facing surfaces of the 2D materials, suggesting the polymer contaminants are itinerant even when using solvent-free methods. In the case of the PDMS stamps, the contamination is attributed to low molecular weight volatile fractions released by the stamp, which adsorb on the exposed surface of the bottom 2D layer and become trapped when depositing the top layer.61,62 By contrast, spectra measured on vdWHs assembled using PC-coated stamps (Figure 3b) exhibited very different absorption bands. Among the many features evident in these spectra, the prominent peaks at about 1016 cm−1, 1368 cm−1, and 1506 cm−1, are characteristic of O–C–O symmetric deformation, CH3 symmetric deformation, and aromatic C=C stretch vibrations, respectively.63,64 These absorption spectra (Figure 3b) show remarkable similarities with ATR-FTIR (Figure S5) and PTIR (Figure S6) spectra measured on PC reference thin films and, notably, lack the characteristic PDMS absorption peaks observed in Figures 2 and 3a. These observations suggest that the contaminants derive only from the exposed stamp surface (PC in this case). Prominent PC absorption peaks were measured both on and away from contaminant bubbles, including inside the nano-squeegeed region and the exposed underlying hBN layer. However, these peaks were absent in spectra measured far (> 1 mm) from the stamp-contacted regions around the vdWHs. As such, for the PC-coated stamps, the contamination likely is promoted by the higher temperatures used in the fabrication process and incomplete removal of PC by solvents after stacking.65
Figure 3.
Photothermal induced resonance (PTIR) spectra identify contaminants in van der Waals heterostructures (vdWHs) prepared using ‘dry’ transfer methods. Topography (left) and PTIR spectra (right) obtained at the marked locations on vdWHs assembled by overlapping flakes of hexagonal boron nitride (hBN/hBN) using the (a) PDMS all-dry transfer method and (b) polymer-assisted dry pick-up and transfer method, which employs stamps coated in polycarbonate (PC). Scale bars represent 5 μm.
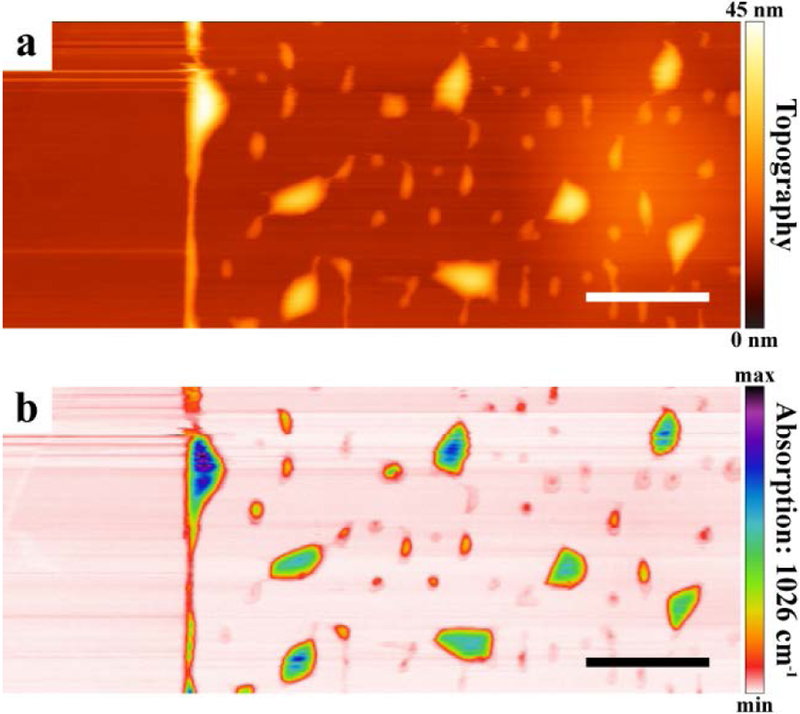
Absorption maps were obtained on vdWHs to assess the relative abundance of contaminants within the scanned region by monitoring absorption at a set wavelength. Figure 4 shows that a PTIR absorption map at 1026 cm−1 (Si–O–Si stretch of PDMS) corresponds well to the simultaneously measured topography of a WSe2/hBN vdWH, prepared using the water-assisted PDMS transfer method, indicating that most contaminant bubbles contain PDMS. These observations clearly suggest that the contaminants consist of PDMS derived from the stamps used in vdWH fabrication. We can also conclude that the regions surrounding the bubbles are largely pristine and free from contaminants. Determining the volumes of contaminant bubbles from the AFM topography maps (Figure S12), and assuming each is filled entirely with PDMS with bulk density of 13 mol∙L−1,66 we estimate that some of the smaller bubbles contain as few as ≈ 1.8 amol of PDMS (see Supporting Information for details). Due to the convolution of the AFM probe tip and contaminant bubble geometries, these measurements overestimate the true bubble sizes and, as such, the amount of material detected shall be considered an upper bound.
Figure 4.
Photothermal induced resonance (PTIR) absorption imaging reveals the nanocontaminant distribution within van der Waals heterostructures (vdWHs). Simultaneously measured (a) topography and (b) PTIR relative absorption maps (Si–O–Si stretch of PDMS, 1026 cm−1) obtained on vdWHs consisting of WSe2/hBN flakes assembled using the water-assisted PDMS pick-up and transfer method. Scale bars represent 1 μm.
Based on these findings, to reduce the stamp-derived contaminants, we devise a stamp cleaning procedure aimed at removing unpolymerized material or other soluble residue from PDMS stamps, prior to vdWH assembly. The PDMS stamps were cleaned with one of four solvents: isopropanol, acetone, toluene, and hexane, and then dried before use. As seen in Figures 5 and S7, vdWHs made using cleaned PDMS stamps (dry transfer) still possess topographic features characteristic of trapped contaminants, with the nano-squeegee process promoting closer contact between the hBN flakes while creating bubbles and a small step around the periphery of the scanned region, which sometimes appears topographically lower than the surrounding region. Without thermal annealing (not used in this work), the top-most 2D layer likely floats on a thin, uniform layer of material, usually < 2 nm thick, separating it from the underlying 2D layer as discussed previously.24 However, for the structures prepared using stamps cleaned with isopropanol (Figure 5a) and toluene (Figure 5b), the spectra obtained from these bubbles do not show any evidence of PDMS. Instead, broad absorption peaks around 1000 cm−1 and 1372 cm−1 show that the only detectable signals derive from the SiO2 and hBN layers. Since the bubble sizes are comparable to those observed in Figures 2–4, but no PDMS or other foreign material is detected, we conclude that either the bubbles are empty or are only partially filled with PDMS contamination in amounts below the detection limit. Another possibility is that the bubbles may be filled with contaminant material that do not possess absorbance features in the examined spectral range, as might be expected for inorganic materials. By contrast, the PTIR spectra of vdWHs prepared with stamps cleaned with acetone or hexane (Figure S7) showed the prominent characteristic PDMS absorption peaks, suggesting that those solvents were ineffective at reducing or preventing contamination. Additional details regarding stamp cleaning and the characterization of vdWHs assembled from stamps cleaned with other solvents are available in the Supporting Information. These experiments suggest that the abundance and identity of contaminants in vdWHs cannot be assessed based on AFM topography alone and that composition-sensitive methods capable of nanoscale resolution should be employed when possible.
Figure 5.
Cleaning polydimethylsiloxane (PDMS) stamps using isopropanol (IPA) and toluene reduces contaminants in van der Waals heterostructures (vdWHs). Topography (left) and photothermal induced resonance absorption spectra (right) obtained at the marked locations on vdWHs fabricated using the all-dry transfer method by overlaying two flakes of hexagonal boron nitride (hBN/hBN) using PDMS stamps pre-cleaned with (a) IPA and (b) toluene. Scale bars represent 2 μm.
CONCLUSIONS
In summary, PTIR absorption spectra and maps enable chemical identification and real space visualization of the nanoscale contaminants commonly found in vdWHs. Clear evidence on vdWHs assembled from 2D materials with different compositions shows that the contaminants consist of residue transferred from the surface of polymeric stamps (PDMS or PC in this work) used in the heterostructure fabrication. Notably, polymer contamination between layers occurs even when ‘dry’ fabrication methods are used. We found that cleaning PDMS stamps with isopropanol or toluene prior to use reduced the amount of PDMS contaminants trapped within vdWHs. The measurements presented here suggest that the assessment of contaminants in vdWHs should not be conducted based on AFM topographic images alone but, rather, aided by nanoscale composition-sensitive methods, such as PTIR. This analytical approach, coupled with the nano-squeegee procedure, is demonstrated here on vdWHs created with a variety of commonly used fabrication methods, stamps materials, and 2D materials pairs, highlighting its broad applicability to the widespread challenge posed by contaminants trapped between 2D materials, independent from their chemical and mechanical properties. We believe that knowledge of the contaminant composition obtained with the methods presented here will aid better understanding of vdWH properties, which are affected by these impurities, as well as to guide improvements to fabrication techniques to produce intrinsic, contaminant-free heterostructures with precisely tuned properties.
METHODS
PDMS Stamp Preparation.
PDMS stamps were prepared using commercially available silicone elastomer kits.67 The two components (elastomer and curing agent) were mixed in a 10:1 ratio by weight and then degassed under vacuum for 20 min. Next, the mixture was spun onto a clean, polished silicon wafer and then cured on a hot plate at 80 °C for 30 min. Cured PDMS films (≈ 250 μm thick) were cut into rectangular pieces of varying sizes before use.
PDMS Stamp Cleaning.
Only for specific experiments indicated in the manuscript, PDMS stamps were cleaned by immersion in a large excess (> 2000× their volume) of one of four solvents: isopropanol, acetone, toluene, and hexane. The stamps remained immersed for a period of one week while under continuous agitation. Afterward, the stamps were dried for > 24 h in air before use.
Polycarbonate-Coated PDMS Stamp Preparation.
A PC polymer solution (6 % mass fraction) was prepared by dissolving solid PC in chloroform. The solution was quickly spread over a SiO2/Si substrate and the solvent allowed to evaporate, thereby leaving a thin film of PC (≈ 200 nm) coating the surface. These PC/SiO2/Si substrates were then contacted by PDMS stamps, approximately 1 mm × 2 mm in size. A sharp blade was used to cut the PC films around the periphery of the PDMS stamps. Before removing the stamps, we carefully removed the surrounding PC films using tweezers and then applied drops of deionized water to the edges of the stamps. The water permeated under the PC films, enabling easy removal of PC-coated PDMS stamps. Finally, the PC/PDMS stamps were blown dry with nitrogen gas and placed on glass slides with their PC films exposed (PC/PDMS/glass).
2D Material Deposition.
Flakes of hBN were deposited on PDMS, Au-coated SiO2, and SiO2 surfaces by mechanical exfoliation from a bulk crystal.68 Monolayers (and sparingly few bi- and multilayers) of WSe2 and WS2 were deposited on SiO2/Si substrates via chemical vapor deposition.69 In one case (Figure 2d), an exfoliated flake of WS2, ≈ 20 nm, was used as the bottom-most layer in a vdWH.
Van der Waals Heterostructure Fabrication.
Flakes of various 2D materials (hBN and TMDs) were transferred from their original substrates and stacked to create vdWHs. Flakes were selected with the aid of an optical microscope and manipulated using a custom-built transfer stage. Three transfer processes were used in this study (PDMS All-Dry Transfer, Water-Assisted PDMS Pick-up and Transfer, Polymer-Assisted Dry Pick-up and Transfer), which are described in the Supporting Information.
Nano-squeegee.
An AFM-based nano-squeegee24 procedure was employed to prepare regions of vdWHs with clean, contaminant-free interfaces between stacked 2D materials (see Figure 1a). A relatively dull (or large-diameter) probe tip was used to groom the surface through repeated raster scans, thereby coercing the contaminant bubbles to move to the periphery of the scanned area. The minimum force applied by the probe to induce motion of the trapped contaminants depends on the stiffness (thickness and Young’s modulus) of the top, contacted 2D layer. Here, we used the probe in contact mode and first brought the tip to engage on the sample surface while continuously scanning a single line in the area of interest. By gradually increasing the force applied by the tip, the measured topography of the line scan would change; for instance, the apparent heights of the bubbles would decrease with greater tip-surface forces until the feature finally disappeared, indicating tip-induced migration of contaminants. After attaining the requisite force, scanning the area of interest resulted in clearing away the contaminant originally present.
Photothermal Induced Resonance.
Gold-coated cantilevers with a nominal spring constant between 0.07–0.4 N/m and nominal first resonance frequency in air of 13 ± 4 kHz were used in the PTIR experiments. The probes were operated in contact mode and the PTIR laser was set to match the third or fourth contact-resonant frequency of the cantilever (≈ 450 kHz or ≈ 700 kHz, respectively). Incident from a quantum cascade laser array with tunable repetition rate (1 kHz to 2000 kHz) and wavelength (910 cm−1 to 1905 cm−1) laser light was polarized parallel to the plane of incidence (p-polarized). Maps of PTIR absorption were obtained using a phase-locked loop with 50–100 kHz bandwidth (centered around the laser repetition rate) to maintain the resonance excitation of the cantilever throughout the image. Absorption spectra were obtained by sweeping the laser wavelength while monitoring the cantilever oscillation amplitude. Absorption maps were obtained by illuminating the sample with a constant wavelength while moving the probe across the sample surface.
Supplementary Material
ACKNOWLEDGMENTS
J.J.S. acknowledges support under the Corporative Research Agreement between the University of Maryland and the National Institute of Standards and Technology, Award 70NANB14H209, through the University of Maryland. H.-J.C. acknowledges support from an American Society for Engineering Education fellowship. M.R.R. and S.V.S. acknowledge support from National Research Council fellowships.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.9b06594.
Mass changes of cleaned PDMS stamps, van der Waals heterostructure fabrication methods, molecular structures, spectral and image processing procedures, attenuated total internal reflectance Fourier transform infrared spectra, additional photothermal induced resonance absorption maps, spectra, and topographs, nanocontaminant quantity estimates (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Novoselov KS; Jiang D; Schedin F; Booth TJ; Khotkevich VV; Morozov SV; Geim AK Two-Dimensional Atomic Crystals. Proc. Natl. Acad. Sci. U.S.A 2005, 102 (30), 10451–10453. 10.1073/pnas.0502848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Wang QH; Kalantar-Zadeh K; Kis A; Coleman JN; Strano MS Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol 2012, 7 (11), 699–712. 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- (3).Zhang K; Feng Y; Wang F; Yang Z; Wang J Two Dimensional Hexagonal Boron Nitride (2D-HBN): Synthesis, Properties and Applications. J. Mater. Chem. C 2017, 5 (46), 11992–12022. 10.1039/C7TC04300G. [DOI] [Google Scholar]
- (4).Mas-Ballesté R; Gómez-Navarro C; Gómez-Herrero J; Zamora F 2D Materials: To Graphene and Beyond. Nanoscale 2011, 3 (1), 20–30. 10.1039/C0NR00323A. [DOI] [PubMed] [Google Scholar]
- (5).Novoselov KS; Mishchenko A; Carvalho A; Neto AHC 2D Materials and van Der Waals Heterostructures. Science 2016, 353 (6298), aac9439 10.1126/science.aac9439. [DOI] [PubMed] [Google Scholar]
- (6).Zhang Y; Rubio A; Lay GL Emergent Elemental Two-Dimensional Materials beyond Graphene. J. Phys. D: Appl. Phys 2017, 50 (5), 053004 10.1088/1361-6463/aa4e8b. [DOI] [Google Scholar]
- (7).Novoselov KS; Neto AHC Two-Dimensional Crystals-Based Heterostructures: Materials with Tailored Properties. Phys. Scr 2012, T146, 014006 10.1088/0031-8949/2012/T146/014006. [DOI] [Google Scholar]
- (8).Liu Y; Weiss NO; Duan X; Cheng H-C; Huang Y; Duan X Van Der Waals Heterostructures and Devices. Nat. Rev. Mater 2016, 1, 16042 10.1038/natrevmats.2016.42. [DOI] [Google Scholar]
- (9).Hamann DM; Hadland EC; Johnson DC Heterostructures Containing Dichalcogenides-New Materials with Predictable Nanoarchitectures and Novel Emergent Properties. Semicond. Sci. Technol 2017, 32 (9), 093004 10.1088/1361-6641/aa7785. [DOI] [Google Scholar]
- (10).Lee G-H; Cui X; Kim YD; Arefe G; Zhang X; Lee C-H; Ye F; Watanabe K; Taniguchi T; Kim P; Hone J Highly Stable, Dual-Gated MoS2 Transistors Encapsulated by Hexagonal Boron Nitride with Gate-Controllable Contact, Resistance, and Threshold Voltage. ACS Nano 2015, 9 (7), 7019–7026. 10.1021/acsnano.5b01341. [DOI] [PubMed] [Google Scholar]
- (11).Deng D; Novoselov KS; Fu Q; Zheng N; Tian Z; Bao X Catalysis with Two-Dimensional Materials and Their Heterostructures. Nat. Nanotechnol 2016, 11 (3), 218–230. 10.1038/nnano.2015.340. [DOI] [PubMed] [Google Scholar]
- (12).Cao Y; Fatemi V; Fang S; Watanabe K; Taniguchi T; Kaxiras E; Jarillo-Herrero P Unconventional Superconductivity in Magic-Angle Graphene Superlattices. Nature 2018, 556 (7699), 43–50. 10.1038/nature26160. [DOI] [PubMed] [Google Scholar]
- (13).Dean CR; Young AF; Meric I; Lee C; Wang L; Sorgenfrei S; Watanabe K; Taniguchi T; Kim P; Shepard KL; Hone J Boron Nitride Substrates for High-Quality Graphene Electronics. Nat. Nanotechnol 2010, 5 (10), 722–726. 10.1038/nnano.2010.172. [DOI] [PubMed] [Google Scholar]
- (14).Schneider GF; Calado VE; Zandbergen H; Vandersypen LMK; Dekker C Wedging Transfer of Nanostructures. Nano Lett 2010, 10 (5), 1912–1916. 10.1021/nl1008037. [DOI] [PubMed] [Google Scholar]
- (15).Castellanos-Gomez A; Buscema M; Molenaar R; Singh V; Janssen L; Zant H. S. J. van der; Steele GA Deterministic Transfer of Two-Dimensional Materials by All-Dry Viscoelastic Stamping. 2D Mater 2014, 1 (1), 011002 10.1088/2053-1583/1/1/011002. [DOI] [Google Scholar]
- (16).Wang L; Meric I; Huang PY; Gao Q; Gao Y; Tran H; Taniguchi T; Watanabe K; Campos LM; Muller DA; Guo J; Kim P; Hone J; Shepard KL; Dean CR One-Dimensional Electrical Contact to a Two-Dimensional Material. Science 2013, 342 (6158), 614–617. 10.1126/science.1244358. [DOI] [PubMed] [Google Scholar]
- (17).Pizzocchero F; Gammelgaard L; Jessen BS; Caridad JM; Wang L; Hone J; Bøggild P; Booth TJ The Hot Pick-up Technique for Batch Assembly of van Der Waals Heterostructures. Nat. Commun 2016, 7, 11894 10.1038/ncomms11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Haigh SJ; Gholinia A; Jalil R; Romani S; Britnell L; Elias DC; Novoselov KS; Ponomarenko LA; Geim AK; Gorbachev R Cross-Sectional Imaging of Individual Layers and Buried Interfaces of Graphene-Based Heterostructures and Superlattices. Nat. Mater 2012, 11 (9), 764–767. 10.1038/nmat3386. [DOI] [PubMed] [Google Scholar]
- (19).Alexeev EM; Catanzaro A; Skrypka OV; Nayak PK; Ahn S; Pak S; Lee J; Sohn JI; Novoselov KS; Shin HS; Tartakovskii AI Imaging of Interlayer Coupling in van Der Waals Heterostructures Using a Bright-Field Optical Microscope. Nano Lett 2017, 17 (9), 5342–5349. 10.1021/acs.nanolett.7b01763. [DOI] [PubMed] [Google Scholar]
- (20).Kuriakose S; Ahmed T; Balendhran S; Bansal V; Sriram S; Bhaskaran M; Walia S Black Phosphorus: Ambient Degradation and Strategies for Protection. 2D Mater 2018, 5 (3), 032001 10.1088/2053-1583/aab810. [DOI] [Google Scholar]
- (21).Huang Y; Qiao J; He K; Bliznakov S; Sutter E; Chen X; Luo D; Meng F; Su D; Decker J; Ji W; Ruoff RS; Sutter P Interaction of Black Phosphorus with Oxygen and Water. Chem. Mater 2016, 28 (22), 8330–8339. 10.1021/acs.chemmater.6b03592. [DOI] [Google Scholar]
- (22).Purdie DG; Pugno NM; Taniguchi T; Watanabe K; Ferrari AC; Lombardo A Cleaning Interfaces in Layered Materials Heterostructures. Nat. Commun 2018, 9 (1), 5387 10.1038/s41467-018-07558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Lindvall N; Kalabukhov A; Yurgens A Cleaning Graphene Using Atomic Force Microscope. J. Appl. Phys 2012, 111 (6), 064904 10.1063/1.3695451. [DOI] [Google Scholar]
- (24).Rosenberger MR; Chuang H-J; McCreary KM; Hanbicki AT; Sivaram SV; Jonker BT Nano-“Squeegee” for the Creation of Clean 2D Material Interfaces. ACS Appl. Mater. Interfaces 2018, 10 (12), 10379–10387. 10.1021/acsami.8b01224. [DOI] [PubMed] [Google Scholar]
- (25).Kang K; Lee K-H; Han Y; Gao H; Xie S; Muller DA; Park J Layer-by-Layer Assembly of Two-Dimensional Materials into Wafer-Scale Heterostructures. Nature 2017, 550 (7675), 229–233. 10.1038/nature23905. [DOI] [PubMed] [Google Scholar]
- (26).Centrone A Infrared Imaging and Spectroscopy Beyond the Diffraction Limit. Annu. Rev. Anal. Chem 2015, 8 (1), 101–126. 10.1146/annurev-anchem-071114-040435. [DOI] [PubMed] [Google Scholar]
- (27).Dazzi A; Prater CB AFM-IR: Technology and Applications in Nanoscale Infrared Spectroscopy and Chemical Imaging. Chem. Rev 2017, 117 (7), 5146–5173. 10.1021/acs.chemrev.6b00448. [DOI] [PubMed] [Google Scholar]
- (28).Dazzi A; Glotin F; Carminati R Theory of Infrared Nanospectroscopy by Photothermal Induced Resonance. J. Appl. Phys 2010, 107 (12), 124519 10.1063/1.3429214. [DOI] [Google Scholar]
- (29).Lahiri B; Holland G; Centrone A Chemical Imaging Beyond the Diffraction Limit: Experimental Validation of the PTIR Technique. Small 2013, 9 (3), 439–445. 10.1002/smll.201200788. [DOI] [PubMed] [Google Scholar]
- (30).Ramer G; Aksyuk VA; Centrone A Quantitative Chemical Analysis at the Nanoscale Using the Photothermal Induced Resonance Technique. Anal. Chem 2017, 89 (24), 13524–13531. 10.1021/acs.analchem.7b03878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Chae J; An S; Ramer G; Stavila V; Holland G; Yoon Y; Talin AA; Allendorf M; Aksyuk VA; Centrone A Nanophotonic Atomic Force Microscope Transducers Enable Chemical Composition and Thermal Conductivity Measurements at the Nanoscale. Nano Lett 2017, 17 (9), 5587–5594. 10.1021/acs.nanolett.7b02404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Katzenmeyer AM; Canivet J; Holland G; Farrusseng D; Centrone A Assessing Chemical Heterogeneity at the Nanoscale in Mixed-Ligand Metal–Organic Frameworks with the PTIR Technique. Angew. Chem., Int. Ed. 2014, 53 (11), 2852–2856. 10.1002/anie.201309295. [DOI] [PubMed] [Google Scholar]
- (33).Katzenmeyer AM; Holland G; Chae J; Band A; Kjoller K; Centrone A Mid-Infrared Spectroscopy beyond the Diffraction Limit via Direct Measurement of the Photothermal Effect. Nanoscale 2015, 7 (42), 17637–17641. 10.1039/C5NR04854K. [DOI] [PubMed] [Google Scholar]
- (34).Lu F; Jin M; Belkin MA Tip-Enhanced Infrared Nanospectroscopy via Molecular Expansion Force Detection. Nat. Photonics 2014, 8 (4), 307–312. 10.1038/nphoton.2013.373. [DOI] [Google Scholar]
- (35).Katzenmeyer AM; Holland G; Kjoller K; Centrone A Absorption Spectroscopy and Imaging from the Visible through Mid-Infrared with 20 nm Resolution. Anal. Chem 2015, 87 (6), 3154–3159. 10.1021/ac504672t. [DOI] [PubMed] [Google Scholar]
- (36).Wieland K; Ramer G; Weiss VU; Allmaier G; Lendl B; Centrone A Nanoscale Chemical Imaging of Individual Chemotherapeutic Cytarabine-Loaded Liposomal Nanocarriers. Nano Res 2019, 12 (1), 197–203. 10.1007/s12274-018-2202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Jin M; Lu F; Belkin MA High-Sensitivity Infrared Vibrational Nanospectroscopy in Water. Light Sci. Appl 2017, 6 (7), e17096 10.1038/lsa.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Ramer G; Ruggeri FS; Levin A; Knowles TPJ; Centrone A Determination of Polypeptide Conformation with Nanoscale Resolution in Water. ACS Nano 2018, 12 (7), 6612–6619. 10.1021/acsnano.8b01425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Morsch S; Liu Y; Lyon SB; Gibbon SR Insights into Epoxy Network Nanostructural Heterogeneity Using AFM-IR. ACS Appl. Mater. Interfaces 2016, 8 (1), 959–966. 10.1021/acsami.5b10767. [DOI] [PubMed] [Google Scholar]
- (40).Tri PN; Prud’homme RE Nanoscale Lamellar Assembly and Segregation Mechanism of Poly(3-Hydroxybutyrate)/Poly(Ethylene Glycol) Blends. Macromolecules 2018, 51 (1), 181–188. 10.1021/acs.macromol.7b02019. [DOI] [Google Scholar]
- (41).Strelcov E; Dong Q; Li T; Chae J; Shao Y; Deng Y; Gruverman A; Huang J; Centrone A CH3NH3PbI3 Perovskites: Ferroelasticity Revealed. Sci. Adv 2017, 3 (4), e1602165 10.1126/sciadv.1602165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Chae J; Dong Q; Huang J; Centrone A Chloride Incorporation Process in CH3NH3PbI3–xClx Perovskites via Nanoscale Bandgap Maps. Nano Lett 2015, 15 (12), 8114–8121. 10.1021/acs.nanolett.5b03556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Katzenmeyer AM; Chae J; Kasica R; Holland G; Lahiri B; Centrone A Nanoscale Imaging and Spectroscopy of Plasmonic Modes with the PTIR Technique. Adv. Opt. Mater 2014, 2 (8), 718–722. 10.1002/adom.201400005. [DOI] [Google Scholar]
- (44).Mancini A; Giliberti V; Alabastri A; Calandrini E; De Angelis F; Garoli D; Ortolani M Thermoplasmonic Effect of Surface-Enhanced Infrared Absorption in Vertical Nanoantenna Arrays. J. Phys. Chem. C 2018, 122 (24), 13072–13081. 10.1021/acs.jpcc.8b03808. [DOI] [Google Scholar]
- (45).Kebukawa Y; Kobayashi H; Urayama N; Baden N; Kondo M; Zolensky ME; Kobayashi K Nanoscale Infrared Imaging Analysis of Carbonaceous Chondrites to Understand Organic-Mineral Interactions during Aqueous Alteration. Proc. Natl. Acad. Sci. U.S.A 2019, 116 (3), 753–758. 10.1073/pnas.1816265116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Dazzi A; Prater CB; Hu Q; Chase DB; Rabolt JF; Marcott C AFM–IR: Combining Atomic Force Microscopy and Infrared Spectroscopy for Nanoscale Chemical Characterization. Appl. Spectrosc 2012, 66 (12), 1365–1384. 10.1366/12-06804. [DOI] [PubMed] [Google Scholar]
- (47).Ruggeri FS; Longo G; Faggiano S; Lipiec E; Pastore A; Dietler G Infrared Nanospectroscopy Characterization of Oligomeric and Fibrillar Aggregates during Amyloid Formation. Nat. Commun 2015, 6, 7831 10.1038/ncomms8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Brown LV; Davanco M; Sun Z; Kretinin A; Chen Y; Matson JR; Vurgaftman I; Sharac N; Giles AJ; Fogler MM; Taniguchi T; Watanabe K; Novoselov KS; Maier SA; Centrone A; Caldwell JD Nanoscale Mapping and Spectroscopy of Nonradiative Hyperbolic Modes in Hexagonal Boron Nitride Nanostructures. Nano Lett 2018, 18 (3), 1628–1636. 10.1021/acs.nanolett.7b04476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ciano C; Giliberti V; Ortolani M; Baldassarre L Observation of Phonon-Polaritons in Thin Flakes of Hexagonal Boron Nitride on Gold. Appl. Phys. Lett 2018, 112 (15), 153101 10.1063/1.5024518. [DOI] [Google Scholar]
- (50).Liu Z; Nørgaard K; Overgaard MH; Ceccato M; Mackenzie DMA; Stenger N; Stipp SLS; Hassenkam T Direct Observation of Oxygen Configuration on Individual Graphene Oxide Sheets. Carbon 2018, 127, 141–148. 10.1016/j.carbon.2017.10.100. [DOI] [Google Scholar]
- (51).Chuang H-J; Chamlagain B; Koehler M; Perera MM; Yan J; Mandrus D; Tománek D; Zhou Z Low-Resistance 2D/2D Ohmic Contacts: A Universal Approach to High-Performance WSe2, MoS2, and MoSe2 Transistors. Nano Lett 2016, 16 (3), 1896–1902. 10.1021/acs.nanolett.5b05066. [DOI] [PubMed] [Google Scholar]
- (52).Xu M; Liang T; Shi M; Chen H Graphene-Like Two-Dimensional Materials. Chem. Rev 2013, 113 (5), 3766–3798. 10.1021/cr300263a. [DOI] [PubMed] [Google Scholar]
- (53).Hanbicki AT; Chuang H-J; Rosenberger MR; Hellberg CS; Sivaram SV; McCreary KM; Mazin II; Jonker BT Double Indirect Interlayer Exciton in a MoSe2/WSe2 van Der Waals Heterostructure. ACS Nano 2018, 12 (5), 4719–4726. 10.1021/acsnano.8b01369. [DOI] [PubMed] [Google Scholar]
- (54).Jia H; Yang R; Nguyen AE; Alvillar SN; Empante T; Bartels L; Feng PX-L Large-Scale Arrays of Single- and Few-Layer MoS2 Nanomechanical Resonators. Nanoscale 2016, 8 (20), 10677–10685. 10.1039/C6NR01118G. [DOI] [PubMed] [Google Scholar]
- (55).Zomer PJ; Guimarães MHD; Brant JC; Tombros N; van Wees BJ Fast Pick up Technique for High Quality Heterostructures of Bilayer Graphene and Hexagonal Boron Nitride. Appl. Phys. Lett 2014, 105 (1), 013101 10.1063/1.4886096. [DOI] [Google Scholar]
- (56).Khestanova E; Guinea F; Fumagalli L; Geim AK; Grigorieva IV Universal Shape and Pressure inside Bubbles Appearing in van Der Waals Heterostructures. Nat. Commun 2016, 7, 12587 10.1038/ncomms12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Groza A; Surmeian A Characterization of the Oxides Present in a Polydimethylsiloxane Layer Obtained by Polymerisation of Its Liquid Precursor in Corona Discharge. J. Nanomater 2015, 2015, 1–8. 10.1155/2015/204296. [DOI] [Google Scholar]
- (58).Lin-Vien D; Colthup NB; Fateley WG; Grasselli JG Organosilicon Compounds In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Lin-Vien D, Colthup NB, Fateley WG, Grasselli JG, Eds.; Academic Press: San Diego, 1991; pp 251–261. 10.1016/B978-0-08-057116-4.50021-3. [DOI] [Google Scholar]
- (59).Geick R; Perry CH; Rupprecht G Normal Modes in Hexagonal Boron Nitride. Phys. Rev 1966, 146 (2), 543–547. 10.1103/PhysRev.146.543. [DOI] [Google Scholar]
- (60).Centrone A; Brambilla L; Zerbi G Adsorption of H2 on Carbon-Based Materials: A Raman Spectroscopy Study. Phys. Rev. B 2005, 71 (24), 245406 10.1103/PhysRevB.71.245406. [DOI] [Google Scholar]
- (61).Andersson LHU; Hjertberg T Silicone Elastomers for Electronic Applications. I. Analyses of the Noncrosslinked Fractions. J. Appl. Polym. Sci 2003, 88 (8), 2073–2081. 10.1002/app.12027. [DOI] [Google Scholar]
- (62).Glasmästar K; Gold J; Andersson A-S; Sutherland DS; Kasemo B Silicone Transfer during Microcontact Printing. Langmuir 2003, 19 (13), 5475–5483. 10.1021/la026558x. [DOI] [Google Scholar]
- (63).Schnell H Chemistry and Physics of Polycarbonates; Interscience Publishers: New York, 1964; Vol. 9. [Google Scholar]
- (64).Silverstein RM; Webster FX; Kiemle D Spectrometric Identification of Organic Compounds, 7th Edition; Wiley, 2005. [Google Scholar]
- (65).Lin Y-C; Lu C-C; Yeh C-H; Jin C; Suenaga K; Chiu P-W Graphene Annealing: How Clean Can It Be? Nano Lett 2012, 12 (1), 414–419. 10.1021/nl203733r. [DOI] [PubMed] [Google Scholar]
- (66).Chemical Retrieval on the Web. Poly(dimethylsiloxane) https://polymerdatabase.com/polymers/Polydimethylsiloxane.html (accessed Mar 1, 2019).
- (67).Lee JN; Park C; Whitesides GM Solvent Compatibility of Poly(Dimethylsiloxane)-Based Microfluidic Devices. Anal. Chem 2003, 75 (23), 6544–6554. 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]
- (68).Wang Z; Tang Z; Xue Q; Huang Y; Huang Y; Zhu M; Pei Z; Li H; Jiang H; Fu C; Zhi C Fabrication of Boron Nitride Nanosheets by Exfoliation. Chem. Rec 2016, 16 (3), 1204–1215. 10.1002/tcr.201500302. [DOI] [PubMed] [Google Scholar]
- (69).McCreary KM; Hanbicki AT; Jernigan GG; Culbertson JC; Jonker BT Synthesis of Large-Area WS2 Monolayers with Exceptional Photoluminescence. Sci. Rep 2016, 6, 19159 10.1038/srep19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.