Abstract
Purpose:
Cisplatin, a commonly used chemotherapeutic, results in tinnitus, the phantom perception of sound. Our purpose was to identify the clinical and genetic determinants of tinnitus among testicular cancer survivors (TCS) following cisplatin-based chemotherapy.
Experimental Design:
TCS (n= 762) were dichotomized to cases (moderate/severe tinnitus; n=154) and controls (none; n=608). Logistic regression was used to evaluate associations with comorbidities and SNP dosages in GWAS following quality control and imputation (covariates: age, noise exposure, cisplatin dose, genetic principal components). Pathway over-representation tests and functional studies in mouse auditory cells were performed.
Results:
Cisplatin-induced tinnitus (CisIT) significantly associated with age at diagnosis (P=0.007) and cumulative cisplatin dose (P=0.007). CisIT prevalence was not significantly greater in 400 mg/m2-treated TCS compared to 300 (P=0.41), but doses >400 mg/m2 (median 580, range 402–828) increased risk by 2.61-fold (P<0.0001). CisIT cases had worse hearing at each frequency (0.25–12 kHz, P<0.0001), and reported more vertigo (OR=6.47; P<0.0001) and problems hearing in a crowd (OR=8.22; P<0.0001) than controls. Cases reported poorer health (P=0.0005) and greater psychotropic medication use (OR=2.4; P=0.003). GWAS suggested a variant near OTOS (rs7606353, P=2×10−6) and OTOS eQTLs were significantly enriched independently of that SNP (P=0.018). OTOS overexpression in HEI-OC1, a mouse auditory cell line, resulted in resistance to cisplatin-induced cytotoxicity. Pathway analysis implicated potassium ion transport (q=0.007).
Conclusions:
CisIT associated with several neuro-otological symptoms, increased use of psychotropic medication, and poorer health. OTOS, expressed in the cochlear lateral wall, was implicated as protective. Future studies should investigate otoprotective targets in supporting cochlear cells.
Terms: cisplatin, hearing loss, tinnitus, ototoxicity, GWAS, adverse drug events, genetic architecture
INTRODUCTION
Approximately 9.6% of the U.S. population experiences tinnitus, the phantom perception of sound characterized by persistent ringing, buzzing, beeping, or hissing in the ears (1). Several studies have linked tinnitus to increased anxiety, depression, insomnia, and reduced productivity and quality of life (QoL) (2, 3). Nevertheless, the pathogenic mechanisms of this disorder remain poorly understood (4), and no pharmacological agents are approved to treat tinnitus (5); treatment is typically limited to cognitive behavioral therapy and management of associated anxiety (5, 6). Despite its etiological ambiguity, several risk factors have been shown to contribute to the development of tinnitus, including age, hearing loss, and the administration of ototoxic drugs (7, 8).
Cisplatin is one of the most commonly used chemotherapeutics, and is known for its potent ototoxic effects. It can lead to irreversible bilateral hearing loss in up to 80% of patients, with many experiencing permanent tinnitus (9–11). Although few studies have rigorously assessed tinnitus following cisplatin-based chemotherapy, approximately 40% of patients appear to experience at least some degree of tinnitus [6]. This is substantially higher than the rate observed in the general U.S. population, and in testicular cancer patients who did not receive cisplatin-based chemotherapy (12%; [9]). Severe cases of tinnitus are also markedly increased in cisplatin-treated patients when compared to the general population (22% vs. 1–2%) (4) (9). One study of 1,409 testicular cancer survivors (TCS) noted that 19% of TCS treated with 100–400 mg/m2 cisplatin exhibited symptoms of moderate/severe tinnitus, with that prevalence reaching 42% in the dose-intensive cisplatin chemotherapy group [9], suggesting a dose-dependent effect. It has been previously demonstrated that cancer survivors with neuropathy, hearing loss, and tinnitus were more likely to report poorer quality of life (QoL) than those with neuropathy alone (12). Another investigation confirmed worse perceived stress among cancer survivors with tinnitus (13).
Despite this documented effect on QoL, little is known about the risk factors and mechanisms of cisplatin-induced tinnitus (CisIT). The subjective nature of the disorder and lack of model systems hamper the elucidation of its pathophysiology. While genotype-phenotype associations promise to identify underlying molecular alterations, to our knowledge, no genome-wide association study (GWAS) of CisIT has yet been conducted. We previously characterized ototoxicity in our North American cohort of TCS receiving homogeneous cisplatin-based treatment. Among 488 TCS, 40% experienced some degree of tinnitus and 15.8% reported moderate/severe tinnitus. Further, tinnitus was significantly correlated with reduced hearing at each frequency examined (P < 0.001) (9). In this study, we comprehensively evaluate the role of clinical characteristics and modifiable risk factors on the development of tinnitus after cisplatin-based chemotherapy in a cohort of 762 TCS, and identify genetic determinants of CisIT through GWAS. In addition, we conduct pathway analyses and in vitro experiments to validate and contextualize our results.
METHODS
Patients and Study Design
All patients were enrolled in the Platinum Study, which includes eight cancer centers in the United States and Canada. Eligibility criteria included: men diagnosed with histologically or serologically confirmed germ cell tumor (GCT), age <55 years at diagnosis and >18 years at enrollment, treatment with first-line cisplatin-based chemotherapy regimen for either initial GCT or recurrence after active surveillance, with no antecedent chemotherapy for another primary cancer, no salvage chemotherapy, and routine follow-up at the participating site. All patients were disease-free at the time of clinical evaluation and provided written consent for study participation, access to medical records, and genotyping. Study procedures were approved by the Human Subjects Review Board at each institution. The studies were conducted in accordance with the U.S. Common Rule.
Assessments
Patient data were either ascertained at clinical follow-up or abstracted from medical records according to a standardized protocol (Figure S1). Data abstracted from medical charts included age at GCT diagnosis, GCT tumor characteristics, treatment regimen with cumulative dose and number of cycles, and BMI at the initiation of treatment. Data collected at clinical evaluation included age and BMI, audiometric studies, self-reported questionnaires, and genotyping. Pure-tone air conduction thresholds were measured bilaterally on a subset of 602 survivors at the frequency range 0.25–12 kHz to quantify hearing. The geometric mean of hearing thresholds across cisplatin-affected frequencies (4–12 kHz) was used to quantify cisplatin-induced hearing loss as previously described (9). Self-reported ototoxicity and neurotoxicity were assessed with two validated questionnaires: the EORTC-Chemotherapy Induced Peripheral Neuropathy 20 item questionnaire (CIPN20) and the Scale for Chemotherapy-Induced Neurotoxicity (SCIN)(14).
Hypertension, hypercholesterolemia, use of psychotropic medication (antidepressants, anxiolytics, and antipsychotics), overall health, tobacco use, alcohol consumption, and noise exposure were also ascertained at follow-up as patient-reported outcomes. Hypertensive subjects indicated receiving a diagnosis of high blood pressure and taking prescription medication for it at evaluation. Hypercholesterolemia subjects answered “yes, current” to the question: “Have you ever taken prescription medication for high cholesterol?” The use of psychotropic medication was assessed as in Fung et al. (15), and was based on whether a patient had been taking psychotropic medication for at least one month at the time of clinical evaluation. Self-reported health was rated on a poor-excellent scale in response to the question “How would you rate your health?” Values were numerically converted for association testing (poor/fair = 1, good = 2, very good = 3, excellent = 4). Exposure to noise was assessed using two questions: “Have you ever had a job where you were exposed to loud noise for 5 or more hours a week? (Loud noise means noise so loud that you had to speak in a raised voice to be heard)” and “Outside of a job, have you ever been exposed to steady loud noise or music for 5 or more hours a week? (Examples are noise from power tools, lawn mowers, farm machinery, cars, trucks, motorcycles, or loud music)”. After numeric conversion (Yes = 1, No = 0), overall noise exposure was computed as the sum of noise exposure in and outside of work. Alcohol consumption was assessed as the self-reported response to the question “During the past year, how many drinks of alcoholic beverage have you consumed on average? (1 drink = 12 oz. beer [1 can or bottle], 4 oz. glass of wine, 1 mixed drink or shot of liquor)” with the following options: Rarely/never (0), 1–3/month (1), 1/week (2), 2–4/week (3), 5–6/week (4), 1/day (5), 2–3/day (6), 4–5/day (7), and 6+/day (8). Tobacco use was assessed as the response to the following two questions: “Have you EVER smoked cigarettes?” with “Yes” (1) and “No” (0) options and “Do you CURRENTLY smoke cigarettes?”
Genotyping
Genotyping was done as previously described (16, 17). DNA was extracted from peripheral blood mononuclear cells. SNPs were genotyped in two phases at the RIKEN Center for integrative Medical Science (Yokohama, Japan). Set 1 was genotyped on the HumanOmniExpressExome-8v1-2_A chip (Illumina, San Diego, CA) and set 2 was genotyped on the InfiniumOmniExpressExome-8v1-3_A chip (Illumina, San Diego, CA). Samples were plated with inter- and intra-plate duplicates at random. Standard quality control (QC) was implemented in PLINK, and is outlined in Figure S2. Sample-level QC criteria included: sample call rate > 0.99, pairwise identity by descent < 0.125, coefficient of inbreeding F < 6 standard deviations from the mean, and genetically European as determined by principal component analysis (performed using SMARTPCA). SNP-level QC included: call rate > 0.99, and Hardy-Weinberg equilibrium (Chi-squared P > 1 × 10−6). QC was first performed in each set independently and then the two sets were merged followed by additional QC. QC for batch effects was performed using a batch pseudo-GWAS where variants associated with batch pseudo-phenotype (10 genotypic PC-adjusted P<10−4) were excluded. SNPs and samples passing QC criteria comprised the input set for imputation with EAGLE phasing, performed on the Michigan Imputation Server using the Haplotype Reference Consortium (18–20). SNPs with imputation R2 < 0.8, MAF < 0.01, and INFO scores > 1.05 or < 0.3 were excluded. Only subjects who passed QC were included in the current study (Figure S2).
Case-Control Definition
Using SCIN (14), TCS were dichotomized to tinnitus cases/controls based on responses to the question: “Have you had in the last 4 weeks: Ringing or buzzing in the ears?” Cases responded “quite a bit/very much”, and controls responded “not at all”. Those answering “a little” were not included. We labeled “not at all”, “a little”, and “quite a bit/very much” as none, mild, and moderate/severe tinnitus. TCS were also asked: “Do you have: ringing and buzzing in the ears?” Tinnitus cases responding “no” to this question were excluded from analysis.
Genome-Wide Analyses
GWAS was done in PLINK v1.9 (21, 22) with logistic regression assuming additive effects, with cumulative cisplatin dose, age at diagnosis, self-reported noise exposure, and the first 5 genetic principal components (SMARTPCA (23)) as covariates. We performed imputation of missing covariates to avoid sample exclusion. Eight individuals with missing age at diagnosis were assigned an imputed age calculated as age at clinical follow-up minus median follow-up duration (5.2 years). Twelve individuals were missing values for cumulative cisplatin dose received, but each of these patients had information on the cycles of cisplatin received. Since one cycle of cisplatin is equivalent to 100 mg/m2, these patients were assigned an imputed cumulative dose by multiplying the number of cycles they received by 100 (i.e. 3 cycles × 100 = 300 mg/m2). No subjects were missing data on noise exposure. Genome-wide significance was set to P<5 × 10−8. A list of expression quantitative trait loci (eQTLs) was extracted from GTEx v7 using all 48 tissues with eQTL analysis (24). Conditional analysis was performed by adjusting for the lead single nucleotide polymorphism (SNP) as a covariate. SNP family enrichment was assessed using the empirical Brown’s combined p-value (25). Narrow-sense heritability was estimated with a genetic relationship matrix variance component model as implemented by GCTA with the same covariates as GWAS (26). Pathway over-representation analysis was performed using Gene Ontology (27, 28) pathways with the g:Profiler package(29) at a significance threshold of FDR q < 0.05 after attributing genes the p-value of the most significantly associated SNP in or within 25 kilobases of gene start/end sites (GENCODE GRCh37.p12).
Gene Expression and Toxicity in Cell Lines
OTOS expression data in different cancer cell lines was obtained from the Cancer Cell Line Encyclopedia (CCLE) (30). Drug sensitivity to cisplatin and other antineoplastic agents, measured as the area under the dose-response curve, was obtained from the Genomics of Drug Sensitivity in Cancer Project (31). Spearman correlation and linear regression was performed between expression and sensitivity of cancer cell lines with non-missing OTOS expression in R 3.3.2.
Statistical Analysis
Data are presented with medians (ranges). Age was modeled continuously and in quartiles (ordinal). Cisplatin dose was treated continuously and in dose categories: <300, 300, 400, and >400 mg/m2. Prevalence ratios (PR) were calculated to compare tinnitus prevalence by treatment or allele group, with normal approximation for 95% confidence intervals (CI) and χ2 p-values reported. Logistic models were constructed to assess associations (odds ratios [OR]) with tinnitus in univariable and multivariable contexts. All tests were two-sided at a significance threshold of P<0.05. Results are presented as OR/PR (95% CI). All statistical analyses were performed in R 3.3.2 and plotted with ggplot2 (32) unless otherwise specified.
Cell Culture
HEI-OC1, a mouse auditory cell line derived from the inner ear organ of Corti, was cultured in complete media of DMEM (high glucose, L-glutamine and pyruvate) with 10% FBS (Corning Inc.; Tewksbury, MA). L929, a mouse fibroblast cell line, was cultured in complete media of EMEM with 10% FBS and 1% penicillin/ streptomycin. Cells were passaged 3-times/week, plated at 1 × 106 cells/T25 culture flask, and grown in a humidified chamber. HEI-OC1 was grown at 33°C with 10% CO2 and L929 at 37°C with 5% CO2. The authenticity of the cell lines was confirmed by IDEXX BioResearch (Columbia, MO) by measurement for interspecies contamination or misidentification, Case # 26495–2018 (HEI-OC1) and #32923–2018 (L929). Cell lines were also assessed for mycoplasma contamination using a MycoAlert Mycoplasma Detection Kit (Lonza; Rockland, ME) at the time of acquisition and prior to performing in vitro experiments.
Plasmid Preparation
OTOS protein vector, pOTOS (pPM-N-D-C-His; cat# PV401573) and blank control (pControl; PV001) vector were purchased from Applied Biological Materials, Inc. (Richmond, BC), with the blank control vector sequence being verified by the supplier. The plasmids were amplified into competent DH5α bacteria (Life Technologies Corporation; Grand Island, NY), expanded using 50μg/ml kanamycin selection, and isolated using PureYield Plasmid Midiprep (Promega Corporation; Madison, WI). Confirmation of OTOS cDNA sequence was performed at the University of Chicago DNA Sequencing Core.
Cell Viability Assay
After 24 hours of plating each cell line at 31,250 cells/cm2 into 96-well plates, the cells were transfected in Opti-MEM (Life Technologies) with the addition of a complexed DNA mixture consisting of 1 part Lipofectamine 3000 (Fisher Scientific) with 3 μg pOTOS or pControl, as per manufactures’ instructions. Transfection continued for 24 hours. Cisplatin (Sigma-Aldrich) was dissolved in a darkened hood using 0.9% saline. The solution was vortexed for 1 minute, sonicated for 10 minutes with no heat, and then filtered through a 0.2 μm syringe filter to obtain a 10 mM stock solution. Transfection media was exchanged for 5, 7.5, 10, 25 or 50 μM cisplatin in complete media or vehicle control. At 48 or 72 hours post-drug treatment, 100 uL CellTiter-Glo (Promega Corporation) was added to each well, as per manufacturer’s instructions. 135 μL of each well was transferred to a white Costar assay plate (Fisher Scientific) and luminescence was measured on a Synergy-HT plate reader (BioTek Corporation; Broadview, IL). Four independent experiments were performed and within an experiment, each concentration was evaluated in triplicate. IC50 values were calculated through non-linear regression of the log cisplatin concentration vs. normalized response curve using Prism6 software.
Quantitative PCR.
Cells were plated at 31,250 cells/cm2 into 12-well plates for qPCR collections. After 24, 48, 72, and 96 hours of transfection (corresponding to 0, 24, 48, and 72 hours after cisplatin treatment), cells were pelleted following a brief trypsinization and stored at −80°C. RNA was isolated using the RNeasy Plus Kit (Qiagen; Germantown, MD) followed by reverse transcription of 200ng RNA into cDNA with the High Capacity cDNA reverse transcription kit (Fisher Scientific). Mouse OTOS (Mm01292235_g1) expression was tested in HEI-OC1 and L929 cells, and was found to be minimally expressed. Expression of human OTOS was determined with qPCR using Taqman primer OTOS (Hs00964785_m1) and compared to mouse Actβ (Mn00607939_s1), the endogenous control. Comparative ΔΔCT method was used to determine fold change of human OTOS at each timepoint. Four independent experiments were performed and each sample was run in triplicate on a Life Technology Viia7 PCR machine.
RESULTS
Cohort Characteristics
Of 1,029 TCS who passed GWAS QC criteria, 608 (59.1%), 265 (25.8%), and 154 (15.0%) reported none, mild, and moderate/severe tinnitus, respectively. Subjects were dichotomized to cases (moderate/severe) and controls (none). Two cases were excluded for inconsistent responses. Cohort characteristics are provided in Table 1. Median age at diagnosis and evaluation was 31 (15–54) years and 39 (18–75) years, respectively. Median follow-up time was 5.2 (1–37) years. 91.6% were treated with either BEP (n=444, 58.3%) or EP (n=254, 33.3%). The remaining 64 (8.4%) received vinblastine (n=6), carboplatin (n=5) or ifosfamide (n=45) in combination with cisplatin, or had missing cisplatin dose data (n=12).
Table 1.
Demographic Features, Clinical Characteristics, and Patient-Reported Outcomes of 762 Male Germ Tumor Survivors According to Tinnitus Status
| Characteristic | All Participants (n=762) | Tinnitus Controls (n=608) | Tinnitus Casesa (n=154) |
|---|---|---|---|
| Age at diagnosis (years)b | |||
| Median (range) | 31 (15–54) | 30 (15–54) | 35 (16–52) |
| <20 | 43 (5.7%) | 35 (5.8%) | 8 (5.2%) |
| 20–29 | 300 (39.8%) | 249 (41.5%) | 51 (33.1%) |
| 30–39 | 243 (32.2%) | 196 (32.7%) | 47 (30.5%) |
| 40–55 | 168 (22.2%) | 120 (20.0%) | 48 (31.2%) |
| Age at assessment (years) | |||
| Median (range) | 39 (18–75) | 38 (18–71) | 43 (20–75) |
| <20 | 6 (0.8%) | 5 (0.8%) | 1 (0.6%) |
| 20–29 | 134 (17.6%) | 115 (18.9%) | 19 (12.3%) |
| 30 to 49 | 274 (36.0%) | 230 (37.8%) | 44 (28.6%) |
| 40 to 49 | 202 (26.5%) | 150 (24.7%) | 52 (33.8%) |
| 50 to 59 | 123 (16.1%) | 88 (14.5%) | 35 (22.7%) |
| ≥60 | 23 (3.0%) | 20 (3.3%) | 3 (2.0%) |
| Time since therapy completion (years)c | |||
| Median (range) | 5.2 (1–37) | 5.1 (1–37) | 6.2 (1–35) |
| ≤1 | 3 (0.4%) | 1 (0.2%) | 2 (1.3%) |
| >1 and ≤5 | 362 (48.0%) | 293 (48.8%) | 69 (44.8%) |
| >5 and ≤10 | 180 (23.9%) | 145 (24.2%) | 35 (22.7%) |
| >10 and ≤20 | 159 (21.1%) | 127 (21.2%) | 32 (20.8%) |
| >20 | 50 (6.6%) | 34 (5.7%) | 16 (10.4%) |
| Educational level | |||
| Secondary school or less | 95 (12.6%) | 62 (10.3%) | 33 (21.9%) |
| Post-secondary school training | 166 (22.0%) | 129 (21.4%) | 37 (24.5%) |
| College graduate | 334 (44.2%) | 276 (45.7%) | 58 (38.4%) |
| Post graduate training/degree | 160 (21.2%) | 137 (22.7%) | 23 (15.2%) |
| Chemotherapy regimend | |||
| BEP | 444 (58.3%) | 355 (58.4%) | 89 (57.8%) |
| EP | 254 (33.3%) | 205 (33.7) | 49 (31.8%) |
| Other | 64 (8.4%) | 48 (7.9%) | 16 (10.4%) |
| Number of cycles, platinum-based chemotherapye | |||
| ≤2 | 15 (2.0%) | 13 (2.2%) | 2 (1.3%) |
| 3 | 333 (44.0%) | 272 (45.1%) | 61 (39.6%) |
| 4 | 389 (51.4%) | 307 (50.9%) | 82 (53.2%) |
| >4 | 20 (2.6%) | 11 (1.8%) | 9 (5.8%) |
| Cumulative cisplatin dose (mg/m2)f | |||
| Median | 400 (100–828) | 400 (200–800) | 400 (100–827.6) |
| < 300 | 41 (5.5%) | 32 (5.4%) | 9 (5.9%) |
| 300 | 300 (40.0%) | 248 (41.5%) | 52 (34.0%) |
| >300 and <400 | 21 (2.8%) | 15 (2.5%) | 6 (3.9%) |
| 400 | 358 (47.7%) | 287 (48.1%) | 71 (46.4%) |
| > 400 | 30 (4.0%) | 15 (2.5%) | 15 (9.8%) |
| Reduced hearing in past four weeksg | |||
| Not at all | 555 (73.1%) | 521 (85.7%) | 34 (22.5%) |
| A little | 127 (16.7%) | 71 (11.7%) | 56 (37.1%) |
| Quite a bit/very much | 77 (10.1%) | 16 (2.6%) | 61 (40.4%) |
| Problems hearing in crowdsh | |||
| Yes | 222 (30.6%) | 124 (21.2%) | 98 (69.0%) |
| No | 505 (69.4%) | 461 (78.8%) | 44 (31.0%) |
| Requires hearing aidi | |||
| Yes | 2 (0.3%) | 2 (0.3%) | 0 (0%) |
| No | 741 (99.7%) | 602 (99.7%) | 139 (100%) |
| Noise exposure | |||
| None | 430 (56.4%) | 365 (60.0%) | 65 (42.2%) |
| Work related OR other exposure | 213 (28.0%) | 163 (26.8%) | 50 (32.5%) |
| Work related AND other exposure | 119 (15.6%) | 80 (13.2%) | 39 (25.3%) |
| Audiometrically assessed hearing lossj,k | |||
| None/mild | 238 (39.4%) | 220 (45.4%) | 18 (15.0%) |
| Moderate | 93 (15.4%) | 75 (15.5%) | 18 (15.0%) |
| Moderately severe | 129 (21.4%) | 103 (21.3%) | 26 (21.7%) |
| Severe/profound | 144 (23.8%) | 86 (17.8%) | 58 (48.3%) |
| Peripheral neuropathyl | |||
| None | 373 (49.0%) | 329 (54.1%) | 44 (28.6%) |
| Mild | 281 (36.9%) | 215 (35.4%) | 66 (42.9%) |
| Severe | 108 (14.1%) | 64 (10.5%) | 34 (28.6%) |
| Persistent vertigo or dizzinessm | |||
| Yes | 36 (4.9%) | 15 (2.5%) | 21 (14.3%) |
| No | 702 (95.1%) | 576 (97.5%) | 126 (85.7%) |
| Self-reported healthn | |||
| Excellent | 134 (17.6%) | 121 (20.0%) | 13 (11.7%) |
| Very good | 317 (41.7%) | 264 (43.6%) | 53 (45.5%) |
| Good | 266 (35.0%) | 196 (32.3%) | 70 (34.4%) |
| Poor/fair | 43 (5.7%) | 25 (4.1%) | 18 (8.4%) |
| Medication for anxiety, psychosis, and/or depressiono | |||
| Yes | 57 (10.1%) | 38 (8.3%) | 19 (17.75%) |
| No | 510 (89.9%) | 422 (91.7%) | 88 (82.2%) |
| Hypertension and on medication | |||
| Yes | 106 (14.0%) | 69 (11.6%) | 37 (24.2%) |
| No | 651 (86.0%) | 535 (89.9%) | 116 (75.8%) |
| Smoking statusp | |||
| Ever smoker | 303 (39.9%) | 235 (38.8%) | 68 (44.2%) |
| Never smoker | 457 (60.1%) | 371 (61.2%) | 86 (55.8%) |
| Alcohol consumptionq | |||
| ≤4 drinks per week | 487 (64.2%) | 384 (63.4%) | 103 (66.9%) |
| 5–7 drinks per week | 172 (22.7%) | 140 (24.3%) | 32 (20.8%) |
| ≥2 drinks per day | 100 (13.2%) | 81 (12.3%) | 19 (12.3%) |
Abbreviations: BEP, bleomycin, etoposide, and cisplatin, EP, etoposide, and cisplatin, NA not applicable
NOTE: Data presented as number (%) unless otherwise noted
Restricted to patients who reported “quite a bit’ or ‘very much” tinnitus. Patients who reported “a little” tinnitus (n=265) are excluded from the table and all analyses.
Category excludes 8 participants for whom age at diagnosis was not recorded.
Category excludes 8 participants for whom this variable was not stated.
BEP category includes patients who received only bleomycin, etoposide, and cisplatin; EP includes patients who received only etoposide and cisplatin. The other category includes patients who received other antineoplastic agents, including ifosfamide (n=45), vinblastine (n=6), and carboplatin (n=5), or who were missing dose data (n=12).
Category excludes 5 participants for whom number of cycles was not stated.
Category excludes 12 participants for whom complete dose data were not available.
Category excludes 3 participants who did not answer this question.
Category excludes 35 participants who did not answer this question.
Category excludes 19 participants who did not answer this question.
Category excludes 158 participants who did not complete audiometric testing.
ASHA criteria defined hearing loss severity as the following: mild: 21 to 40 dB; moderate: 41 to 55 dB; moderately severe: 56 to 70 dB; severe: 71 to 90 dB; and profound: > 90 dB; for at least one tested frequency for either ear (https://www.asha.org/public/hearing/Degree-of-Hearing-Loss)
Following conversion of the Likert scale: “none, a little, quite a bit, very much” to a 0–3 numeric scale, each individual was assigned a summary statistic for the sensory subscale (Cronbach (α = 0.88) and the motor subscale (α = 0.78) by taking the mean of the response in the subscale: none (mean = 0), mild (0 < mean ≤ 1), severe (mean > 1) (16, 17).
Category excludes 24 participants who did not answer this question.
Category excludes 2 participants who did not answer this question.
Category excludes 195 who did not answer this question. Participants could report more than one psychotropic medication including anxiolytics, antipsychotics, and antidepressants. Psychotropic medications investigated include: alprazolam (n=8), aripiprazole (n=2), amphetamine + dextroamphetamine (n = 14), amantadine (n = 1), buproprion (n = 9), carbidopa/levodopa (n = 1), citalopram (n = 8), clonazepam (n = 13), desvenlafaxine (n = 1), diazepam (n = 1), duloxetine (n = 8), escitalopram (n = 19), fluoxetine (n = 5), fluvoxamine (n = 1), hydroxazine (n = 1), lisdexamfetamine (n = 4), lorazepam (n = 1), methylphenidate (n = 13), nortriptyline (n = 2), olanzapine (n = 2), paroxetine (n = 5), pramipexole (n = 1), sertraline (n = 7), trazodone (n = 8), valproate (n = 1), venlafaxine (n = 10).
Category excludes 2 participants for whom this outcome was not stated.
Category excludes 3 participants for whom this outcome was not stated.
Diagnosis and Treatment Risk Factors
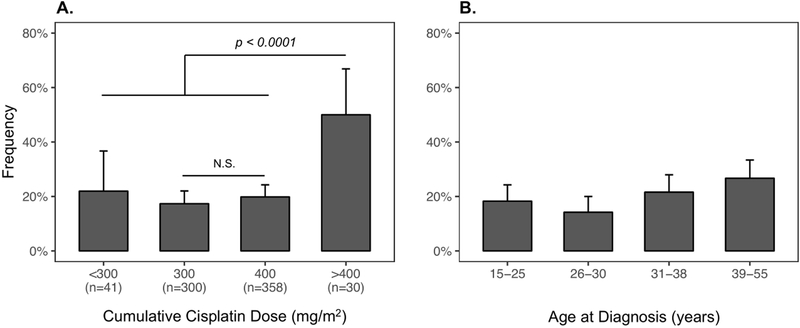
Cumulative cisplatin dose was significantly associated with tinnitus (OR per 100 mg/m2=1.38, 95% confidence interval (CI): 1.1–1.7, P=0.007). Tinnitus risk for 400 mg/m2-treated TCS (n=358) was not significantly greater than those receiving 300 mg/m2 (n=300) (OR=1.14, 95% CI: 0.8–1.6, P=0.41). However, survivors who received >400 mg/m2 (n=30, median dose=580 mg/m2, range=402–828 mg/m2) were at 2.61(95% CI: 1.8–3.9)-fold increased risk compared to those receiving ≤400 mg/m2 (P<0.0001, Figure 1A). Tinnitus was not associated with cumulative doses of bleomycin (OR=1.01, P=0.13), etoposide (OR=1.02, P=0.33), or ifosfamide (OR=1.06, P=0.87) when cumulative cisplatin dose was included as a covariate. Three of five TCS receiving both carboplatin (median dose=1800 mg/m2, range=5–3,646 mg/m2) and cisplatin (median dose=200 mg/m2, range=200–527 mg/m2) had tinnitus (OR=7.37, 95% CI: 1.2–58.5, P=0.034). Tinnitus was associated with increasing age at diagnosis as a continuous variable (OR by decade=1.31, 95% CI: 1.1–1.6, P=0.006) and by quartile (OR=1.22, P=0.013, Figure 1B). Age at evaluation was also associated with tinnitus (OR=1.30, 95% CI: 1.1–1.5, P=0.002). No association with follow-up duration was observed (age-adjusted OR=0.99, P=0.57).
Figure 1. Tinnitus Frequency by Cumulative Cisplatin Dose and Age at Testicular Cancer Diagnosis (quartiles).
A. Bar plot showing the frequency of tinnitus by cumulative cisplatin dose group (<300, 300, 400, and >400 mg/m2) illustrates that the significant association between dose and tinnitus (logistic regression OR = 1.38, 95% CI: 1.1–1.7, P=0.007) is due to the significantly increased risk of tinnitus in patients treated with doses > 400 mg/m2 (post-hoc Chi-squared P < 0.0001). The number of subjects per category is presented on the x-axis under the dose group label. B. Bar plot representing the frequency of tinnitus by age at diagnosis quartile shows positive association with cisplatin risk (OR by decade = 1.31, 95% CI: 1.1–1.6, P = 0.006). Error bars represent the binomial 95% CIs for each subgroup. N.S = not significant.
Associations with Self-Reported Health and Neuro-Ototoxic Symptoms
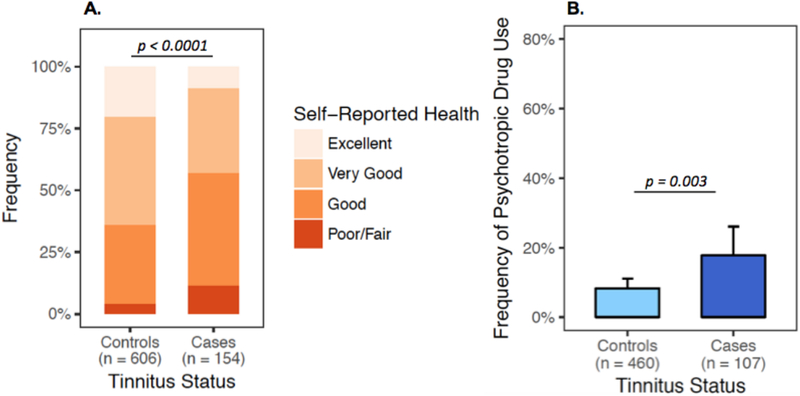
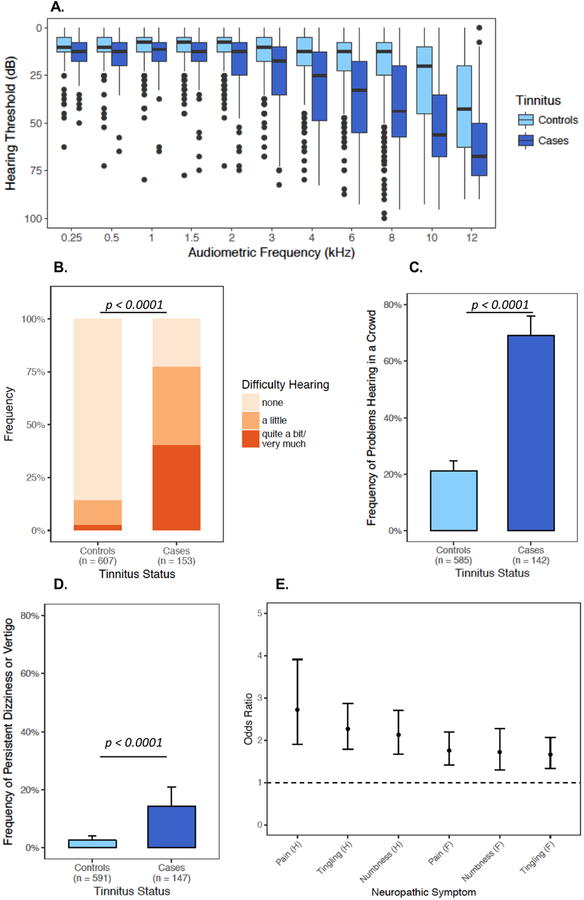
Self-reported overall health was significantly lower in cases than in controls (OR=0.54, 95% CI: 0.4–0.7, P<0.0001, Figure 2A). Tinnitus cases also reported using psychotropic medications more frequently than controls (OR=2.40, 95% CI: 1.3–4.4, P=0.003, Figure 2B). Audiometry was performed on 602 (78.4%) subjects. TCS with tinnitus had significantly worse hearing at every frequency examined (0.25 kHz–12 kHz, P<0.0001, Figure 3A). Tinnitus was also associated with self-reported hearing loss (OR=6.38, 95% CI: 4.9–8.5, P<0.0001, Figure 3B), problems hearing in a crowd (OR=8.28, 95% CI: 5.5–12.5, P<0.0001, Figure 3C), and persistent dizziness or vertigo (OR=6.40, 95% CI: 3.2–12.9, P<0.0001, Figure 3D), as well as symptoms of sensory neuropathy (OR range 1.66–2.72, P-values<0.0001, Figure 3E).
Figure 2. Self-reported Health and Use of Psychotropic Medications by Tinnitus Status.
A. Bar plot of the distribution of self-reported health according to tinnitus status. Patients were asked: “How would you rate your overall health?” and responded on a poor-excellent ordinal scale, which was converted to a numeric scale (0 = poor/fair, 1 = good, 2 = very good, 3 = excellent). Logistic regression revealed significant negative correlation between tinnitus status and self-reported health (OR=0.54, 95% CI: 0.4–0.7, P<0.0001). B. Bar plot of psychotropic medication use by tinnitus status shows significantly higher prevalence of psychotropic medication use in tinnitus cases (OR= 2.4, 95% CI: 1.3–4.4, P=0.003). Patients were dichotomized to “Yes” and “No” psychotropic medication use based on receiving medications from a list of frequently prescribed antidepressants, anxiolytics, and antipsychotics (Supplemental Methods, (15)). The number of subjects per category is presented on the x-axis. Error bars represent the binomial 95% CIs for each subgroup.
Figure 3. Associations of Tinnitus with Subjective and Objective Hearing Loss, Problems Hearing, Vertigo, and Neurological Symptoms.
A. Box plot of audiometric hearing thresholds (y-axis, dB) across all tested audiometric frequencies (x-axis, kHz) showing significantly worse hearing in tinnitus cases at each frequency (P < 0.0001 at each frequency). Box centers indicate medians, hinges indicate interquartile regions (IQRs), and whiskers indicate 1.5x IQRs. Points outside the range of 1.5x IQR are shown. Bar plots of self-reported B. difficulty hearing (OR = 6.36, 95% CI: 4.8–8.5, P < 0.0001), C. problems hearing in a crowd (OR = 8.2, 95% CI: 5.5–12.5, P<0.0001), and D. persistent dizziness or vertigo (OR = 6.40, 95% CI: 3.2–12.9, P<0.0001). Error bars represent the binomial 95% CIs for subgroup. E. Forest plot showing the odds ratio (OR, center points) and 95% confidence intervals (error bars) of the association between tinnitus and neurotoxic symptoms from the EORTC-CIPN20. “H” denotes hands/fingers. “F” denotes feet/toes. Responses to EORTC-CIPN20 items were converted from a none-very much Likert scale to a numerical ordinal scale (0–3). Associations in B-E were evaluated using logistic regression adjusted for age at diagnosis.
Associations with Modifiable Risk Factors
Tinnitus was significantly associated with hypertension (age-adjusted OR=2.07, 95% CI: 1.3–3.4, P=0.003), and hypercholesterolemia (age-adjusted OR=1.96, 95% CI: 1.2–3.2, P=0.009). In an age at clinical examination-adjusted model with both variables, tinnitus was significantly associated with hypertension (OR=1.77, 95% CI: 1.02–3.0, P=0.039), but not hypercholesterolemia (OR=1.6, 95% CI: 0.9–2.8, P=0.09). Tinnitus was associated with noise exposure at work (age-adjusted OR=1.93, 95% CI: 1.3–2.8, P=0.0005), and outside of work (age-adjusted OR=2.28, 95% CI: 1.6–3.3, P<0.0001). Risk was also associated with chronic (>15 years) smoking (age-adjusted OR=2.07, 95% CI: 1.2–3.8, P=0.005), but not increased among ever smokers (OR=1.26, 95% CI: 0.9–1.7, P=0.20) or with alcohol consumption (OR=0.92, 95% CI: 0.5–1.5, P=0.12). Association with chronic smoking remained significant after adjusting for age, hypertension, and noise exposure (OR = 1.79, 95% CI: 1.01–1.04, P=0.04).
Genome-Wide Association Study
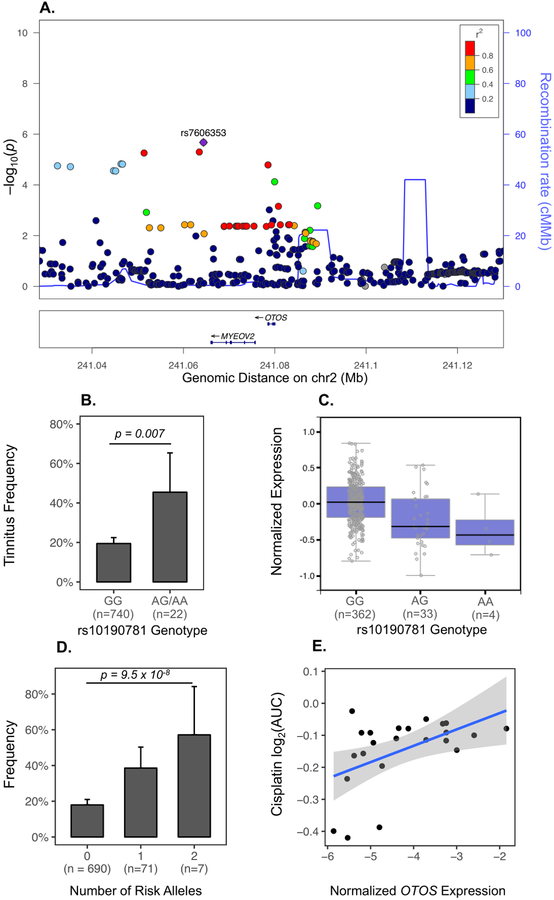
Using GCTA’s linear mixed model approach (26), we found that additive SNP effects explained a large fraction of CisIT variance (h2=0.81±0.42, P=0.006). In GWAS, no SNP met genome-wide significance (Table S1, Figure S3). The first independent signal identified (rs7532231, OR = 0.45, P = 1.1×10−6) is 116 kilobases upstream of the lncRNA RP11–364B6.1. The second (rs6671895, OR = 4.32, P = 1.2×10−6) is intronic to the lncRNA RP5-884C9.2. The third (rs7606353, P=1.9×10−6, Figure 4A) is a SNP 14 kilobases downstream of OTOS (otospiralin), a gene implicated in auditory function and survival of cochlear fibrocytes (33, 34). This variant is in near perfect linkage disequilibrium with rs74002277 in Europeans (CEU R2=0.96), a SNP in the 3’-UTR region of OTOS and which alters several transcription binding motifs (35). The minor allele (G, 4% frequency) increased CisIT risk (OR=4.20, 95% CI: 2.3–7.5). Since there has been prior evidence that OTOS protects cochlear fibrocytes against cisplatin cytotoxicity (36), we hypothesized that variants associated with higher OTOS expression lowered CisIT risk. In GTEx, OTOS was significantly expressed only in pituitary and thyroid tissues (Figure S4). We found that OTOS eQTLs were significantly enriched in GWAS independently from rs7606353 (P=0.018). One haplotype block containing six thyroid/pituitary eQTLs was driving this enrichment (Table S2, Figure S4). The minor allele (A) of the most significant variant within this block (rs10190781, 1.5% frequency) was associated with higher CisIT risk (OR=3.42, 95% CI: 1.5–9.5, P=0.007, Figure 4B) and lower OTOS expression (Figure 4C), suggesting that higher OTOS expression is protective. Tinnitus risk increased with the number of risk alleles in either locus (OR=3.77, 95% CI: 2.3–6.2, P=9.5 × 10−8, Figure 4D).
Figure 4). GWAS of Cisplatin-Induced Tinnitus Reveals Genetic Loci Near OTOS gene.
A. LocusZoom plot of the third most significant GWAS signal near two adjacent genes: OTOS and MYEOV2. Each point represents a SNP. The x-axis indicates chromosomal position. The left y-axis shows –log10(p-value) of association with CisIT and the right y-axis and blue line indicate the recombination rate in centimorgans/megabase (cM/MB). The color of each variant indicates the linkage disequilibrium R2 with the top signal in the region, rs7606353 (purple). B. Bar plot of CisIT frequency by genotype of OTOS eQTL, rs10190781. The minor allele (G) increases CisIT risk (OR = 3.42, P = 0.007). Error bars represent the binomial 95% CIs. C. Boxplot of OTOS expression in thyroid by rs10190781 genotype indicates that the minor allele is associated with lower expression of OTOS. Data were obtained from the GTEx Portal on 04/28/18 D. Bar plot of number of risk alleles for both rs7606353 and rs10190781 shows additive allele effects (OR = 3.77, 95% CI: 2.3–6.2], P=9.5 × 10−8. E. Scatter plot of cisplatin resistance as a function of normalized OTOS expression. Cisplatin resistance, measured as the area under the cisplatin dose-response curve, for all central nervous system tumor lines (19 glioma and 4 neuroblastoma lines) was extracted from CancerRX and normalized OTOS expressions were downloaded from the Cancer Cell Line Encyclopedia. Correlation was assessed non-parametrically using Spearman’s Rank method (Rho = 0.46, P = 0.03).
Functional Analysis
We tested the association between OTOS expression and cellular sensitivity to cisplatin (as measured by area under the dose response curve) in central nervous system (CNS) tumor lines (30, 31) and found a significant positive correlation (Spearman Rho=0.46, P=0.03; R2 = 0.25, P = 0.01, Figure 4E), further supporting a protective OTOS function against cisplatin-induced damage. To examine the specificity of this correlation, we compared the sensitivity of CNS tumor cell lines to eight antineoplastic agents (5-fluorouracil (5-FU), bleomycin, bortezomib, cisplatin, cytarabine, docetaxel, etoposide, and vinblastine) based on OTOS expression levels (Table S3). We also examined the relationship between cisplatin sensitivity and OTOS expression in seven cancer cell line types (aerodigestive, breast, CNS, digestive system, lung, skin, and urogenital system; Table S4). Of the cytotoxic drugs tested, only cisplatin sensitivity demonstrated a statistically significant correlation with OTOS expression in CNS lines. Further, cisplatin sensitivity was associated with OTOS expression only in CNS tumor lines and not cell lines of other tissue origins. These data suggest a CNS tumor-specific association between OTOS expression and cisplatin sensitivity.
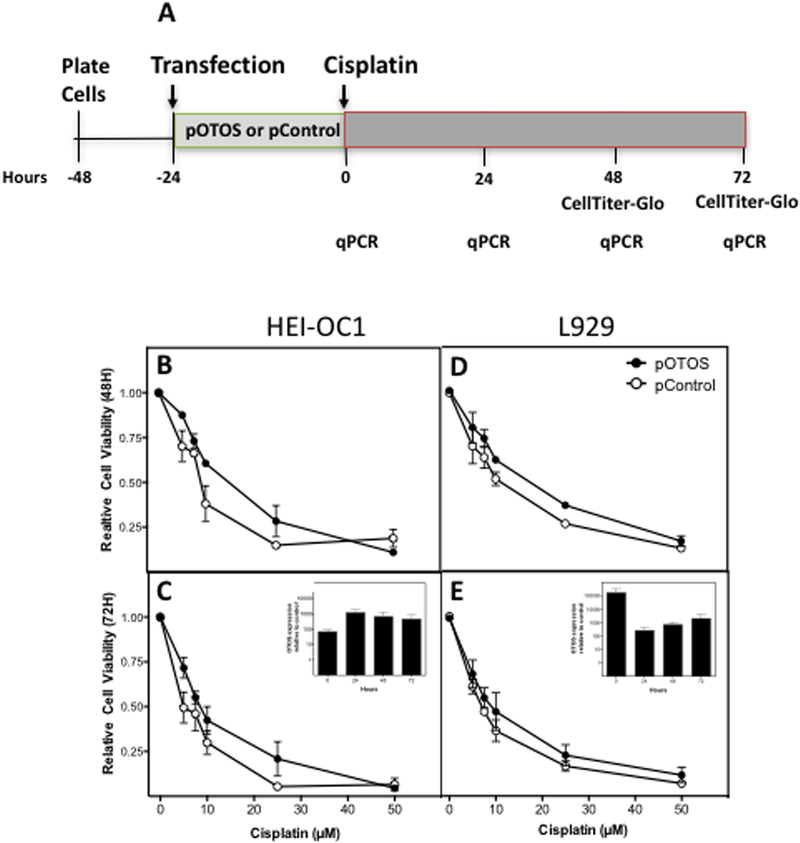
To functionally validate the role of OTOS gene expression in cellular sensitivity to cisplatin, we overexpressed OTOS in two cell lines, HEI-OC1 (a mouse auditory cell line) and L929 (a mouse fibroblast cell line), and compared gene expression levels and cellular proliferation following cisplatin treatment in OTOS-transfected cells and plasmid-transfected control cells (Figure 5A). Overexpression of human OTOS in HEI-OC1 resulted in increased cell viability following cisplatin treatment at 5, 7.5, 10, and 25 μM concentrations for 48 hours (P=0.004; Figure 5B) and 72 hours (P=0.008; Figure 5C). Figure S5 illustrates the effect of cisplatin on HEI-OC1 cells transfected with human OTOS and plasmid control. Furthermore, the cisplatin IC50 value of HEI-OC1 cells transfected with human OTOS (18.68 μM, 95% CI: 13.95–25.01 μM) was 1.8-fold higher than cells transfected with the control plasmid (10.66 μM, 95% CI: 6.04–18.82 μM) at 48 hours. Overexpression of OTOS was confirmed via qPCR, with human OTOS expression increasing 69-fold at the time of drug treatment, 24 hours following pOTOS transfection (Figure 5C inset). Similarly, overexpression of OTOS in L929 fibroblasts also markedly reduced cellular sensitivity to cisplatin following treatment for 48 hours (P=0.006; Figure 5D) and 72 hours (P=0.03; Figure 5E) concomitant with increased expression of OTOS (Figure 5E inset). The cisplatin IC50 value in OTOS-transfected cells (16.30 μM, 95% CI: 13.25–20.04 μM) was 1.5-fold higher than cells transfected with the control plasmid (10.96 μM, 95% CI: 8.82–13.61 μM) at 48 hours.
Figure 5). Effects of OTOS Overexpression on Cisplatin Sensitivity in HEI-OC1 Mouse Cochlear and L929 mouse fibroblast cell lines.
A. Experimental scheme of transfection and cisplatin treatment, followed by cell viability and qPCR analysis. Cells were transfected with human OTOS (pOTOS) or a control plasmid (pControl) for 24 hours prior to cisplatin treatment. B. Cell viability of HEI-OC1 cells transfected with either pOTOS (closed circles) or the control plasmid (pControl; open circles) following treatment with increasing concentrations of cisplatin for 48 hours (P=0.004) or C. 72 hours (P=0.008). Inset: Human OTOS mRNA expression in HEI-OC1 cells corresponding to 0, 24, 48, and 72 hours after cisplatin treatment (24, 48, 72, and 96 hours after transfection) depicted as pOTOS relative to pControl. D. Cell viability of L929 fibroblasts transfected with either pOTOS (closed circles) or the control plasmid (pControl; open circles) following treatment with increasing concentrations of cisplatin for 48 hours (P=0.006) or E. 72 hours (P=0.03). Inset: Human OTOS mRNA expression in L929 fibroblasts corresponding to 0, 24, 48, and 72 hours after cisplatin treatment (24, 48, 72, and 96 hours after transfection) depicted as pOTOS relative to pControl. Data shown are from 3 independent experiments, with each concentration or time point within an experiment evaluated in duplicate or triplicate. Error bars reflect S.E.M. Statistical significance was determined using 2-way ANOVA analysis with Sidak correction for multiple comparisons.
Pathway Analysis
We mapped SNPs to genes by proximity and performed pathway enrichment with Gene Ontology terms. We identified 134 coding genes with P<5×10−4 (Table S5). Ten pathways were significantly over-represented (Table S6), including nervous system process (q = 0.005), potassium ion transmembrane transport (q = 0.007), and sensory perception of sound (q = 0.005). Potassium transport most strongly implicated KCNQ1 (rs2283200, P = 8.9 × 10−6), which maintains high potassium concentrations in the endolymph of the inner ear (37, 38).
DISCUSSION
To our knowledge, this is the first investigation to consider the effects of clinical, genetic, and lifestyle factors on CisIT risk. Here 25.8% and 15.2% of cisplatin-treated TCS reported mild and moderate/severe tinnitus at a median of 5 years after treatment. Survivors treated with 100–400 mg/m2 cisplatin appeared to have similar CisIT risk, but those given >400 mg/m2 were at 2.6-fold increased risk, suggesting a non-linear threshold-dose response. Follow-up time was not associated with CisIT, suggesting irreversibility, but longitudinal data are needed to support this observation. Significant associations with tinnitus were observed for hearing loss, vertigo, sensory neuropathy, poorer overall health, and use of psychotropic medications, as well as for hypertension, noise exposure, and chronic smoking. GWAS revealed independent signals implicating OTOS that had relatively large effect sizes (ORs of 3.5–4.5) and were relatively rare (minor allele frequencies 1–5%). We verified protective effects of OTOS through eQTL analysis and functional validation in two cell lines, demonstrating that decreased OTOS expression increases susceptibility to CisIT. Whether thyroid/pituitary eQTLs co-regulate expression in cochlear fibrocytes is unknown, and human cochlear transcriptomic databases are necessary for more precise interpretations. However, we provide further evidence for OTOS’s protective functions in silico using central nervous system cell line data, and in vitro by overexpressing human OTOS in a mouse auditory cell line routinely used for in vitro drug ototoxicity screening (39) and a mouse fibroblast cell line. Pathway analysis implicated potassium transport genes, including the cochlear potassium transporter KCNQ1(37, 38).
Treatment-Related Risk Factors
Despite adequate statistical power, significant differences in tinnitus risk between TCS given 3 vs. 4 cycles of cisplatin-based chemotherapy were not evident. Brydøy et al. previously showed that TCS treated with >4 cycles of cisplatin-based chemotherapy had a 2.21-fold increased risk of tinnitus compared with those given 1–4 cycles (40), consistent with our findings. Bokemeyer et al. showed a dose-dependent increased risk for ototoxicity after cisplatin-based chemotherapy, but did not specifically assess tinnitus or compare by dosage group (41).
Modifiable Risk Factors
An approximate 2-fold association between hypertension and CisIT was apparent among our TCS. The relationship between hypertension and tinnitus in the general population is well-established, with a recent meta-analysis estimating a pooled OR of 1.37 (P<0.05) (42). It has been hypothesized that hypertension causes damage to either the cochlear microcirculation and/or the stria vascularis, which supplies blood to the organ of Corti (43). This could explain the observed association.
Multiple studies have identified a relationship between tobacco use and tinnitus in the general population (44–46) and we found a significantly increased risk of tinnitus in survivors with >15 years of tobacco use. It is possible that long-term tobacco use may reduce peripheral blood flow, including to the inner ear microcirculation. The role of tobacco use in CisIT was examined by Brydøy et al., but significant associations were not apparent for either current or former smokers compared with never smokers (40), similar to our results.
Noise exposure is a known risk factor for tinnitus (47), but its role in the development of the hearing disorder in other cisplatin-treated cohorts has not been evaluated to our knowledge. Although Bokemeyer et al. reported an association between a history of noise exposure and cisplatin-induced ototoxicity, this term included both tinnitus and hearing loss (41).
Sensory Neuropathy
In our cohort, tinnitus was positively associated with symptoms of sensory neuropathy. This could suggest that inherent susceptibility to cisplatin-induced neurophysiological damage may partially underlie various neuro-otological symptoms, possibly involving pharmacokinetic pathways, intrinsic cisplatin sensitivity, reduced regenerative capacity, or altered neural plasticity. Another possible explanation is that of patient perception. Self-reported tinnitus, hearing loss, and neuropathy in cancer survivors have been linked to the Impact of Events Scale-Revised (IES-R) hyperarousal scores (13), suggesting that differences in perception and appraisal could partially explain our findings. One study showed that audiometric thresholds correlated with tinnitus awareness time and loudness, but that tinnitus-induced annoyance was associated with proneness to exhibit anxious responses (48, 49). These investigations highlight how both cochlear and central nervous mechanisms contribute to tinnitus.
Tinnitus Pathophysiology
While single-variant analysis did not identify significant genetic signals, we found that cumulatively, additive SNP effects explain a large portion of variance in CisIT, further supporting the hypothesis that CisIT is a polygenic trait with complex genetic underpinnings (4). Several mechanisms of tinnitus have been proposed, but no consensus on its etiology has been reached. One model postulates that disruptions to the endocochlear potential result in inhibitory-excitatory imbalances affecting spontaneous cochlear activity and lead to tinnitus (50). Inner-ear fibrocytes supply potassium ions to strial cells which pump them into the endolymph to maintain the endocochlear potential, the “cochlear battery” driving auditory transduction (51). The link between endocochlear potential and spontaneous auditory nerve activity has been established (52). Our GWAS points to the potential importance of this hearing mechanism in CisIT.
OTOS mRNA and protein expression localize to spiral limbus and spiral ligament fibrocytes (33), and overexpression protects primary spiral ligament fibrocytes against cisplatin cytotoxicity (36). In support of the protective effect of OTOS against cisplatin sensitivity, our in silico analysis indicated that cisplatin sensitivity is positively associated with increased OTOS expression in CNS tumor cell lines, a rough approximation of cell types that would be affected by cisplatin-induced neurotoxicity. While CNS tumor cell lines are not an ideal proxy for assessing toxicity to inner ear cells, we further validated the protective effects of OTOS by demonstrating that its overexpression in vitro reduces cisplatin sensitivity in two cell lines (HEI-OC1 and L929), with HEI-OC1 more accurately reflecting inner ear cells that are adversely affected by cisplatin.
In support of our findings, two prior studies have elucidated the importance of OTOS expression in mammalian hearing. Inducible knockdown of OTOS in guinea pigs caused severe degeneration of the organ of Corti and deafness, along with damage to fibrocytes (33). Interestingly, although Otos −/− mice had damaged fibrocytes, they did not exhibit histological abnormalities of sensory hair cells, and experienced only moderate deafness (34). Delprat et al. attributed this difference to long-term adaptation to congenital Otos absence (33). Therefore, it is plausible that inducible knockdown, modeling key aspects of direct auditory insults such as cisplatin administration, will acutely alter the ionic composition of the endolymph, disrupting hair cell metabolism and survival.
Breglio et al. recently showed a reduction of endocochlear potential following cisplatin administration in mice, and preferential accumulation of cisplatin in the stria vascularis (53). We linked potassium transport to CisIT in our pathway analysis, which most strongly implicated KCNQ1, an important cochlear ion channel. This voltage-gated channel localizes to marginal cells of the stria vascularis where it maintains high K+ concentrations in the endolymph (37). Mutations in KCNQ1 result in deafness and long QT syndrome (54).
Taken together, these findings point to the function of supporting cells in the cochlear lateral wall in cisplatin ototoxicity and hair cell survival. Impaired maintenance of the endolymph causes hair cell death and permanent hearing loss (55), which may be partially mediated by a reduced endocochlear potential. However, prior research has largely focused directly on cisplatin’s cytotoxic mechanisms in hair cells (56). Our new findings, together with those in the literature, suggest that novel strategies aimed at protecting supporting cells in the cochlear lateral wall, perhaps by stimulating OTOS expression, may prove beneficial. Very little is known about the protein product of the gene, its functions, and its interactions. Better understanding of the functional protein and its cellular localization and interactions is warranted. A potential approach would be to use a gene expression-based high throughput screen to identify small molecules or siRNA capable of markedly increasing OTOS expression without perturbing the viability of auditory cells, such as HEI-OC1. The identification of compounds or siRNAs resulting in increased OTOS expression could be evaluated for their effect on protecting cells from cisplatin damage. Importantly, the utility of this approach depends on the modulation not impacting the antineoplastic activity of cisplatin.
It remains unclear whether the effects of OTOS on tinnitus occur directly or are mediated by effects on hearing loss. However, if OTOS protects against tinnitus indirectly by preventing hearing loss, it remains a viable candidate for cisplatin otoprotection. It is also unclear whether the actions of OTOS are uniquely pertinent to cisplatin or generalizable to other ototoxic insults like noise, although evidence of direct protection against cisplatin cytotoxicity is presented here and in the literature (36). Future studies should elucidate the cellular functions and interactions of otospiralin to assess its viability as a target for cisplatin otoprotection.
CONCLUSIONS
Strengths of our study include the collection of detailed treatment data, homogeneous cisplatin-based chemotherapy, long-term follow-up, quantitative hearing evaluations, the use of validated instruments to assess neurotoxic symptoms, and the novel implementation of genome-wide scans to nominate cellular mechanisms and targets. Our results point to cochlear mechanisms that have not yet been widely explored in cisplatin ototoxicity, and may provide novel opportunities for targeted otoprotection.
An intrinsic limitation of all cross-sectional studies is the inability to make causal inferences. Further, questionnaires with more detailed information on the experience of tinnitus, including baseline measurements pre-chemotherapy, would provide more conclusive evidence with regard to associations noted here. However, given the young median age of patients at treatment, it is unlikely that tinnitus was pre-existing. It is important to note that the results of our translational research could potentially impact millions of patients worldwide annually diagnosed with cisplatin-treated cancers who are at risk for CisIT. Our findings may also inform tinnitus research in non-platinum-treated patients. In view of our results, health care providers can improve management of cancer survivors experiencing CisIT by recommending monitoring of blood pressure, encouragement of smoking cessation, and avoidance of additional ototoxic insults such as noise exposure.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Cisplatin is one of the most commonly prescribed chemotherapeutics worldwide, but elicits several debilitating off-target toxicities, including cisplatin-induced tinnitus (CisIT; a perceived ringing/buzzing noise with no external sound present). CisIT significantly affects perceived quality of life, but its etiology remains poorly understood. We found that cumulative cisplatin doses exceeding 400 mg/m2 to markedly increase CisIT susceptibility, and that survivors with tinnitus were more likely to experience hearing loss, vertigo, and problems hearing in a crowd, as well as report poorer health and greater reliance on psychotropic medication. Through a genome-wide association study, we identified genetic variants in OTOS, a gene vital for the maintenance of normal hearing, to be associated with CisIT, and further demonstrated that increased OTOS expression was associated with reduced cisplatin sensitivity in silico and in vitro. These data provide novel mechanistic insights into CisIT susceptibility, and may inform clinicians of potential risk factors and comorbidities.
Acknowledgements
The HEI-OC1 cell line was a generous gift from Dr. Federico Kalinec, House Ear Institute, Los Angeles, CA. The L929 cell line was a generous gift from Dr. Wendy Stock at the University of Chicago. This work was supported by the National Institutes of Health Genetic Susceptibility and Biomarkers of Platinum‐related Toxicities grant (R01 CA157823), Pharmacogenomics Research Network (PGRN)-RIKEN Global Alliance (U19 GM061390). The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by the NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 04/28/18. RH acknowledges support of The Josh Carrick Charity and the Institute of Cancer Research and Royal Marsden NIHR Biomedical Research Centre.
Abbreviations:
- CCLE
Cancer Cell Line Encyclopedia
- CIPN20
Chemotherapy Induced Peripheral Neuropathy 20 item questionnaire
- CisIT
cisplatin-inducted tinnitus
- eQTL
expression quantitative trait loci
- GCT
germ cell tumor
- GWAS
genome-wide association study
- SCIN
Scale for Chemotherapy-Induced Neurotoxicity
- SNP
single nucleotide polymorphism
- TCS
testicular cancer survivors
- QoL
quality of life
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, Severity, Exposures, and Treatment Patterns of Tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959–65. doi: 10.1001/jamaoto.2016.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weidt S, Delsignore A, Meyer M, Rufer M, Peter N, Drabe N, Kleinjung T. Which tinnitus-related characteristics affect current health-related quality of life and depression? A cross-sectional cohort study. Psychiatry Res. 2016;237:114–21. doi: 10.1016/j.psychres.2016.01.065. [DOI] [PubMed] [Google Scholar]
- 3.Friberg E, Jansson C, Mittendorfer-Rutz E, Rosenhall U, Alexanderson K. Sickness absence due to otoaudiological diagnoses and risk of disability pension: a nationwide Swedish prospective cohort study. PLoS One. 2012;7(1):e29966. doi: 10.1371/journal.pone.0029966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vona B, Nanda I, Shehata-Dieler W, Haaf T. Genetics of Tinnitus: Still in its Infancy. Front Neurosci. 2017;11:236 Epub 2017/05/24. doi: 10.3389/fnins.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920–30. doi: 10.1016/S1474-4422(13)70160-1. [DOI] [PubMed] [Google Scholar]
- 6.Han BI, Lee HW, Kim TY, Lim JS, Shin KS. Tinnitus: characteristics, causes, mechanisms, and treatments. J Clin Neurol. 2009;5(1):11–9. doi: 10.3988/jcn.2009.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cianfrone G, Pentangelo D, Cianfrone F, Mazzei F, Turchetta R, Orlando MP, Altissimi G. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: a reasoned and updated guide. Eur Rev Med Pharmacol Sci. 2011;15(6):601–36. Epub 2011/07/30. [PubMed] [Google Scholar]
- 8.Rybak LP, Mukherjea D, Jajoo S, Ramkumar V. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med. 2009;219(3):177–86. Epub 2009/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisina RD, Wheeler HE, Fossa SD, Kerns SL, Fung C, Sesso HD, Monahan PO, Feldman DR, Hamilton R, Vaughn DJ, Beard CJ, Budnick A, Johnson EM, Ardeshir-Rouhani-Fard S, Einhorn LH, Lipshultz SE, Dolan ME, Travis LB. Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer. J Clin Oncol. 2016;34(23):2712–20. doi: 10.1200/JCO.2016.66.8822. online at www.jco.org. Author contributions are found at the end of this article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4(6):889–901. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- 11.Ruggiero A, Trombatore G, Triarico S, Arena R, Ferrara P, Scalzone M, Pierri F, Riccardi R. Platinum compounds in children with cancer: toxicity and clinical management. Anticancer Drugs. 2013;24(10):1007–19. doi: 10.1097/CAD.0b013e3283650bda. [DOI] [PubMed] [Google Scholar]
- 12.Miaskowski C, Paul SM, Mastick J, Schumacher M, Conley YP, Smoot B, Abrams G, Kober KM, Cheung S, Henderson-Sabes J, Chesney M, Mazor M, Wallhagen M, Levine JD. Hearing loss and tinnitus in survivors with chemotherapy-induced neuropathy. Eur J Oncol Nurs. 2018;32:1–11. doi: 10.1016/j.ejon.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miaskowski C, Paul SM, Mastick J, Abrams G, Topp K, Smoot B, Kober KM, Chesney M, Mazor M, Mausisa G, Schumacher M, Conley YP, Sabes JH, Cheung S, Wallhagen M, Levine JD. Associations Between Perceived Stress and Chemotherapy-Induced Peripheral Neuropathy and Otoxicity in Adult Cancer Survivors. J Pain Symptom Manage. 2018. doi: 10.1016/j.jpainsymman.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lanteri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R, Group EQoL. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–9. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Fung C, Sesso HD, Williams AM, Kerns SL, Monahan P, Abu Zaid M, Feldman DR, Hamilton RJ, Vaughn DJ, Beard CJ, Kollmannsberger CK, Cook R, Althouse S, Ardeshir-Rouhani-Fard S, Lipshultz SE, Einhorn LH, Fossa SD, Travis LB, Platinum Study G. Multi-Institutional Assessment of Adverse Health Outcomes Among North American Testicular Cancer Survivors After Modern Cisplatin-Based Chemotherapy. J Clin Oncol. 2017;35(11):1211–22. doi: 10.1200/JCO.2016.70.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler HE, Gamazon ER, Frisina RD, Perez-Cervantes C, El Charif O, Mapes B, Fossa SD, Feldman DR, Hamilton RJ, Vaughn DJ, Beard CJ, Fung C, Kollmannsberger C, Kim J, Mushiroda T, Kubo M, Ardeshir-Rouhani-Fard S, Einhorn LH, Cox NJ, Dolan ME, Travis LB. Variants in WFS1 and Other Mendelian Deafness Genes Are Associated with Cisplatin-Associated Ototoxicity. Clin Cancer Res. 2017;23(13):3325–33. doi: 10.1158/1078-0432.CCR-16-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolan ME, El Charif O, Wheeler HE, Gamazon ER, Ardeshir-Rouhani-Fard S, Monahan P, Feldman DR, Hamilton RJ, Vaughn DJ, Beard CJ, Fung C, Kim J, Fossa SD, Hertz DL, Mushiroda T, Kubo M, Einhorn LH, Cox NJ, Travis LB, Platinum Study G. Clinical and Genome-Wide Analysis of Cisplatin-Induced Peripheral Neuropathy in Survivors of Adult-Onset Cancer. Clin Cancer Res. 2017;23(19):5757–68. doi: 10.1158/1078-0432.CCR-16-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, Schlessinger D, Stambolian D, Loh PR, Iacono WG, Swaroop A, Scott LJ, Cucca F, Kronenberg F, Boehnke M, Abecasis GR, Fuchsberger C. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284–7. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loh PR, Danecek P, Palamara PF, Fuchsberger C, Y AR, H KF, Schoenherr S, Forer L, McCarthy S, Abecasis GR, Durbin R, A LP. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet. 2016;48(11):1443–8. doi: 10.1038/ng.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, Kang HM, Fuchsberger C, Danecek P, Sharp K, Luo Y, Sidore C, Kwong A, Timpson N, Koskinen S, Vrieze S, Scott LJ, Zhang H, Mahajan A, Veldink J, Peters U, Pato C, van Duijn CM, Gillies CE, Gandin I, Mezzavilla M, Gilly A, Cocca M, Traglia M, Angius A, Barrett JC, Boomsma D, Branham K, Breen G, Brummett CM, Busonero F, Campbell H, Chan A, Chen S, Chew E, Collins FS, Corbin LJ, Smith GD, Dedoussis G, Dorr M, Farmaki AE, Ferrucci L, Forer L, Fraser RM, Gabriel S, Levy S, Groop L, Harrison T, Hattersley A, Holmen OL, Hveem K, Kretzler M, Lee JC, McGue M, Meitinger T, Melzer D, Min JL, Mohlke KL, Vincent JB, Nauck M, Nickerson D, Palotie A, Pato M, Pirastu N, McInnis M, Richards JB, Sala C, Salomaa V, Schlessinger D, Schoenherr S, Slagboom PE, Small K, Spector T, Stambolian D, Tuke M, Tuomilehto J, Van den Berg LH, Van Rheenen W, Volker U, Wijmenga C, Toniolo D, Zeggini E, Gasparini P, Sampson MG, Wilson JF, Frayling T, de Bakker PI, Swertz MA, McCarroll S, Kooperberg C, Dekker A, Altshuler D, Willer C, Iacono W, Ripatti S, Soranzo N, Walter K, Swaroop A, Cucca F, Anderson CA, Myers RM, Boehnke M, McCarthy MI, Durbin R, Haplotype Reference C. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48(10):1279–83. doi: 10.1038/ng.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case-control association studies. Nat Protoc. 2010;5(9):1564–73. doi: 10.1038/nprot.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45(6):580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole W, Gibbs DL, Shmulevich I, Bernard B, Knijnenburg TA. Combining dependent P-values with an empirical adaptation of Brown’s method. Bioinformatics. 2016;32(17):i430–i6. doi: 10.1093/bioinformatics/btw438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Gene Ontology C Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 2017;45(D1):D331–D8. doi: 10.1093/nar/gkw1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimand J, Arak T, Adler P, Kolberg L, Reisberg S, Peterson H, Vilo J. g:Profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 2016;44(W1):W83–9. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, Reddy A, Liu M, Murray L, Berger MF, Monahan JE, Morais P, Meltzer J, Korejwa A, Jane-Valbuena J, Mapa FA, Thibault J, Bric-Furlong E, Raman P, Shipway A, Engels IH, Cheng J, Yu GK, Yu J, Aspesi P Jr., de Silva M, Jagtap K, Jones MD, Wang L, Hatton C, Palescandolo E, Gupta S, Mahan S, Sougnez, Onofrio, Liefeld, MacConaill, Winckler, Reich, Li N, Mesirov JP, Gabriel SB, Getz G, Ardlie K, Chan V, Myer VE, Weber BL, Porter J, Warmuth M, Finan P, Harris JL, Meyerson M, Golub TR, Morrissey MP, Sellers WR, Schlegel R, Garraway LA. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–7. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W, Soares J, Greninger P, Edelman EJ, Lightfoot H, Forbes S, Bindal N, Beare D, Smith JA, Thompson IR, Ramaswamy S, Futreal PA, Haber DA, Stratton MR, Benes C, McDermott U, Garnett MJ. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41(Database issue):D955–61. doi: 10.1093/nar/gks1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.H W. Ggplot2 Elegant Graphics for Data Analysis. New York, NY: Springer Science; 2009. [Google Scholar]
- 33.Delprat B, Boulanger A, Wang J, Beaudoin V, Guitton MJ, Venteo S, Dechesne CJ, Pujol R, Lavigne-Rebillard M, Puel JL, Hamel CP. Downregulation of otospiralin, a novel inner ear protein, causes hair cell degeneration and deafness. J Neurosci. 2002;22(5):1718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delprat B, Ruel J, Guitton MJ, Hamard G, Lenoir M, Pujol R, Puel JL, Brabet P, Hamel CP. Deafness and cochlear fibrocyte alterations in mice deficient for the inner ear protein otospiralin. Mol Cell Biol. 2005;25(2):847–53. doi: 10.1128/MCB.25.2.847-853.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–4. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuo XL, Wang Y, Zhuo WL, Zhang YS, Wei YJ, Zhang XY. Adenoviral-mediated up-regulation of Otos, a novel specific cochlear gene, decreases cisplatin-induced apoptosis of cultured spiral ligament fibrocytes via MAPK/mitochondrial pathway. Toxicology. 2008;248(1):33–8. doi: 10.1016/j.tox.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Chang Q, Wang J, Li Q, Kim Y, Zhou B, Wang Y, Li H, Lin X. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol Med. 2015;7(8):1077–86. doi: 10.15252/emmm.201404929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wangemann P K+ cycling and the endocochlear potential. Hear Res. 2002;165(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 39.Kalinec G, Thein P, Park C, Kalinec F. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear Res. 2016;335:105–17. Epub 2016/03/02. doi: 10.1016/j.heares.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 40.Brydoy M, Oldenburg J, Klepp O, Bremnes RM, Wist EA, Wentzel-Larsen T, Hauge ER, Dahl O, Fossa SD. Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst. 2009;101(24):1682–95. doi: 10.1093/jnci/djp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA, Kanz L. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77(8):1355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang P, Ma W, Zheng Y, Yang H, Lin H. A Systematic Review and Meta-Analysis on the Association between Hypertension and Tinnitus. Int J Hypertens. 2015;2015:583493. doi: 10.1155/2015/583493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang S, Cai Q, Bard J, Jamison J, Wang J, Yang W, Hu BH. Variation analysis of transcriptome changes reveals cochlear genes and their associated functions in cochlear susceptibility to acoustic overstimulation. Hear Res. 2015;330(Pt A):78–89. doi: 10.1016/j.heares.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711–8. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Nondahl DM, Cruickshanks KJ, Huang GH, Klein BE, Klein R, Nieto FJ, Tweed TS. Tinnitus and its risk factors in the Beaver Dam offspring study. Int J Audiol. 2011;50(5):313–20. doi: 10.3109/14992027.2010.551220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nondahl DM, Cruickshanks KJ, Wiley TL, Klein BE, Klein R, Chappell R, Tweed TS. The ten-year incidence of tinnitus among older adults. Int J Audiol. 2010;49(8):580–5. doi: 10.3109/14992021003753508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baguley D, McFerran D, Hall D. Tinnitus. Lancet. 2013;382(9904):1600–7. doi: 10.1016/S0140-6736(13)60142-7. [DOI] [PubMed] [Google Scholar]
- 48.Moon KR, Park S, Jung Y, Lee A, Lee JH. Effects of Anxiety Sensitivity and Hearing Loss on Tinnitus Symptom Severity. Psychiatry Investig. 2018;15(1):34–40. doi: 10.4306/pi.2018.15.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24(1):1–8. [DOI] [PubMed] [Google Scholar]
- 50.Norena AJ. Revisiting the cochlear and central mechanisms of tinnitus and therapeutic approaches. Audiol Neurootol. 2015;20 Suppl 1:53–9. doi: 10.1159/000380749. [DOI] [PubMed] [Google Scholar]
- 51.Garcia Berrocal JR, Mendez-Benegassi I, Marti C, Ramirez Camacho R. [Intervention of spiral ligament fibrocytes in the metabolic regulation of the inner ear]. Acta Otorrinolaringol Esp. 2008;59(10):494–9. [PubMed] [Google Scholar]
- 52.Sewell WF. The relation between the endocochlear potential and spontaneous activity in auditory nerve fibres of the cat. J Physiol. 1984;347:685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, Hall MD, Amable L, Cunningham LL. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. 2017;8(1):1654. doi: 10.1038/s41467-017-01837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vyas B, Puri RD, Namboodiri N, Nair M, Sharma D, Movva S, Saxena R, Bohora S, Aggarwal N, Vora A, Kumar J, Singh T, Verma IC. KCNQ1 mutations associated with Jervell and Lange-Nielsen syndrome and autosomal recessive Romano-Ward syndrome in India-expanding the spectrum of long QT syndrome type 1. Am J Med Genet A. 2016;170(6):1510–9. doi: 10.1002/ajmg.a.37636. [DOI] [PubMed] [Google Scholar]
- 55.Liu H, Li Y, Chen L, Zhang Q, Pan N, Nichols DH, Zhang WJ, Fritzsch B, He DZ. Organ of Corti and Stria Vascularis: Is there an Interdependence for Survival? PLoS One. 2016;11(12):e0168953. doi: 10.1371/journal.pone.0168953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheth S, Mukherjea D, Rybak LP, Ramkumar V. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front Cell Neurosci. 2017;11:338. doi: 10.3389/fncel.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.