Abstract
Background:
South Africa has among the highest incidence of multidrug-resistant tuberculosis (MDR-TB) and more than 70% of patients are HIV co-infected. MDR-TB treatment is associated with frequent adverse events (AEs). Although guidelines recommend concurrent treatment of MDR-TB and HIV, safety data on concurrent therapy are limited.
Methods:
We conducted a prospective observational study of MDR-TB patients with and without HIV-coinfection in South Africa between 2011 and 2015. Participants received standardized MDR-TB and HIV regimens. Participants were followed monthly for the duration of MDR-TB therapy and screened for clinical and laboratory AEs. Audiometry was performed monthly during the intensive phase; color discrimination testing was performed every two months.
Results:
We enrolled 150 HIV-infected and 56 HIV-uninfected participants. Nearly all experienced at least one clinical (93%) or laboratory (96%) AE. The most common clinical AEs were peripheral neuropathy (50%) and difficulty sleeping (48%); the most common laboratory AEs were hypokalemia (47%) and decreased creatinine clearance (46%). Among 19 clinical and lab AEs examined, there were no differences by HIV status, except for diarrhea (27% HIV-infected vs 13% HIV-uninfected, p=0.03). Hearing loss was experienced by 72% of participants (8% severe loss). Fourteen percent experienced color discrimination loss (4% severe loss). There were no differences in frequency or severity of hearing or vision loss by HIV status.
Conclusion:
AEs were common but not more frequent or severe among MDR-TB/HIV co-infected participants receiving concurrent ART. Given the favorable treatment outcomes associated with concurrent treatment, ART initiation should not be delayed in MDR-TB patients with HIV-coinfection.
Keywords: multidrug-resistant tuberculosis (MDR-TB), HIV, adverse events, side effects, toxicity
INTRODUCTION
Tuberculosis (TB) is the leading infectious cause of death worldwide.1 The rise of multidrug resistance (defined as resistance to at least isoniazid and rifampicin) is undermining recent successes in global control of TB. The World Health Organization (WHO) estimates that there are approximately 460,000 new cases of multidrug-resistant (MDR) TB each year and the disease is associated with worse treatment outcomes than drug-susceptible TB.1 Globally, 54-61% of patients with MDR-TB achieve treatment success 2,3, and outcomes are substantially worse in the setting of HIV co-infection.4–6
Treatment for MDR-TB requires the use of second-line medications which are more expensive and less effective than treatment for drug-susceptible TB; therapy traditionally lasts 9-24 months (vs. 6 months for drug-susceptible TB)7 and is associated with substantial toxicity. Second-line TB medications cause frequent and debilitating side effects, such as psychosis, permanent hearing loss, hypothyroidism, and renal failure.8–10 Reports from diverse settings have shown that the majority of patients treated for MDR-TB experience at least one adverse event (AE) during treatment.10–13 Patients with HIV co-infection have a potential risk of additive toxicities from concurrent antiretroviral therapy (ART) and MDR-TB treatment, including neuropsychiatric effects, nephrotoxicity, and peripheral neuropathy. We recently showed that MDR-TB/HIV co-infected patients who are treated with concurrent therapy have favorable treatment outcomes and improved survival.14 Although it is recommended that ART be initiated as soon as possible in all MDR-TB/HIV co-infected patients,7 the safety and tolerability of concurrent treatment has not been rigorously examined. Nearly all previous studies of MDR-TB treatment-associated AEs were retrospective and, thus, subject to methodologic biases such as underreporting of certain AEs, milder AEs, or key covariates if AEs were not measured and captured systematically.6,8,15 In addition, most of these studies were conducted in HIV-uninfected patients or in HIV-infected patients who were not receiving ART.8,15 Because patients who choose to discontinue therapy prematurely often cite treatment-associated AEs, understanding and managing AEs in co-infected patients may improve treatment adherence and outcomes.16,17
South Africa has among the highest incidence of TB (567 incident cases/100,000 population in 2017) and the highest number of persons living with HIV worldwide.1 There are approximately 20,000 cases of MDR-TB in South Africa each year, and more than 70% are HIV co-infected. We prospectively evaluated the incidence of AEs in a cohort of patients treated concurrently for MDR-TB and HIV in South Africa. Our objective was to compare the frequency, severity, and timing of AEs among MDR-TB/HIV co-infected patients with those of MDR-TB patients without HIV infection.
METHODS
Study Design, Setting, and Population
The Survival and HIV Outcomes in MDR-TB (SHOUT MDR-TB) study was a prospective observational cohort study conducted in KwaZulu-Natal province, South Africa between 2011 and 2015.14 KwaZulu-Natal is the epicenter of the drug-resistant TB and HIV co-epidemics; in 2017 the MDR-TB incidence was 22 cases per 100,000 population, and >70% of MDR-TB patients are HIV co-infected.1 We recruited adult HIV-infected and HIV-uninfected patients with culture-confirmed MDR-TB who were referred to three drug-resistant TB specialist hospitals that serve urban, peri-urban, and rural areas of the province. Participants were enrolled at the time of MDR-TB treatment initiation and followed every four weeks for the duration of therapy and every three months after the completion of treatment.
MDR-TB Treatment Regimen
During the intensive phase, all participants were treated with a standardized MDR-TB regimen in accordance with South African National TB guidelines: kanamycin (15 mg/kg, max 1g daily), moxifloxacin (400 mg daily), ethionamide (15-20 mg/kg, max 750mg daily), terizidone (15-20 mg/kg, max 750mg daily), ethambutol (15-20 mg/kg, max 1200mg daily), and pyrazinamide (20-30 mg/kg, max 2000 mg daily). Kanamycin was given intramuscularly for at least 6 months, or 4 months after culture-conversion, whichever was longer; after kanamycin was discontinued, oral medications were continued for an additional 12-18 months. All HIV co-infected participants were offered ART irrespective of CD4 count. The first-line ART regimen in 2011-2012 included efavirenz, stavudine, and lamivudine; most patients transitioned from stavudine to tenofovir disoproxil fumarate in 2013. Management of individual AEs was at the discretion of the treatment provider, including dose adjustments or medication changes.
AE Data Collection
Clinical and Laboratory Adverse Events
Clinical and laboratory AEs were recorded at baseline and at each follow-up visit (every 4 weeks) throughout MDR-TB treatment. Participants were interviewed using a structured AE questionnaire. Complete blood count and serum biochemistry tests were performed at each visit and thyroid stimulating hormone (TSH) was assessed every 6 months. Any symptoms or lab abnormalities present at baseline were considered pre-existing unless they changed in severity at a subsequent visit. Severity of AEs was categorized using the NIH Division of AIDS (DAIDS) Toxicity Table.18 An absolute TSH value above the upper limit of normal was considered abnormal and any value >8 mIU/L was considered grade 3. Serious adverse events (SAEs) were AEs which resulted in death, hospitalization, congenital abnormality, persistent disability, or required interventions to prevent permanent disability.
Hearing Loss
Pure tone audiometry was performed at enrolment (baseline), at each visit through week 36, and then again at week 52. Participants with at least two audiology reports were considered for analysis; if a true baseline audiology result was missing, the earliest audiology results were considered the patient’s baseline. The DAIDS toxicity table has no existing criteria for hearing loss, and other grading schemes were developed for other medications (e.g., cisplatin) which produce different patterns of ototoxicity from aminoglycosides. Aminoglycoside-induced hearing loss generally begins at higher frequencies which may not be noticed by participants and is often underreported.19,20 We therefore developed a novel set of audiometry grading criteria for hearing loss severity by modifying the current Common Terminology Criteria for Adverse Events (CTCAE)21 classification system to more appropriately detect the earlier, high-frequency hearing loss seen in aminoglycosides.22 These novel grading criteria are provided in the Appendix (Table S1).
Ophthalmologic Toxicity
Vision testing was used to detect red-green and blue-yellow color defects. Testing was performed at baseline and weeks 8, 16, 24, 52, and 104 using Hardy, Rand, and Rittler (HRR) test pseudoisochromatic plates.23,24 We adapted the original HRR testing procedure and severity scoring system as follows: participants were shown 6 cards to identify colored shapes that range in size and lightness. Loss of color discrimination was defined as identifying fewer than 5 of 6 plates correctly on any test.25 Sustained loss was defined as scoring below 5 plates on two or more tests, and extended loss as scoring below 5 plates on three or more tests. Severe loss was defined as scoring below 3 of 6 plates on at least one test.
Outcomes
The primary outcomes of interest were development of any clinical, laboratory, hearing, or vision AEs during MDR-TB treatment, in HIV co-infected participants compared to those in HIV-negative participants. Clinical, laboratory, and vision AEs were considered recurring events. AEs present at baseline were considered pre-existing and assumed not to have developed during treatment unless either the AE resolved and then restarted at a later time point during treatment or the AE worsened in severity. An AE occurring in consecutive visits was considered a single event unless it worsened in severity. Non-consecutive episodes of an AE were considered repeating events. Hearing loss is generally irreversible and was considered a non-recurring event, though it could worsen in severity.20 We attempted to determine the cause of death in all cases by reviewing available hospital records. Medical records were also reviewed to determine the cause and hospital course for all participants experiencing an SAE requiring hospitalization.
Statistical Methods
Frequencies and proportions were used to describe the development of new AEs during treatment, stratified by participants’ HIV status and baseline CD4 count. We tested for differences among groups using Chi-squared and Fisher’s exact tests, as appropriate. We also tested for an association between the presence of serious AEs and treatment outcome.
Time-to-Event Analysis
Classic time-to-event analyses were not appropriate in this setting due to competing risks. We used Gray’s method to estimate the cumulative incidence function (CIF) for AEs in the presence of a competing event;26 we used Fine and Gray’s method to model the rate of individual AEs in the presence of competing events,27 stratifying by participants’ HIV status and baseline CD4 count. Analogous to proportional hazards regression, the Fine and Gray method models the relative sub-distribution hazard function as a multiplicative function of covariates and log-relative sub-distribution hazard ratios (sHR).28 We considered death as competing risks for AEs, as it would preclude the AE of interest (or reporting of the event). Development of other AEs were not considered competing risks as a patient may have multiple AEs concurrently.
Ethical Approval
This study was approved by the institutional review boards at Emory University, Albert Einstein College of Medicine, and the University of KwaZulu-Natal, and by the KwaZulu-Natal Department of Health and U.S. Centers for Disease Control and Prevention. All participants provided signed informed consent.
RESULTS
Patient Population
Of the 206 enrolled participants, 150 were HIV-infected and 56 were HIV-uninfected (Table 1). The median age was 33 years (Interquartile Range [IQR]: 26-41) and the median duration of MDR-TB treatment was 23 months (IQR: 18-24). More HIV-infected participants were female (70% vs. 46%; p<0.01) and had a history of previous TB treatment (71% vs. 46%; p<0.01). Among HIV-infected participants, the median CD4 cell count was 215 (IQR: 114-378).
Table 1:
Patient demographic and clinical characteristics at enrolment
| Total (n=206) | HIV-Infected (n=150) | HIV-Uninfected (n=56) | P-Value | |
|---|---|---|---|---|
| Median age, years (IQR) | 33 (26-41) | 34 (28-40) | 27 (21-48) | 0.91 |
| Female | 131 (64) | 105 (70) | 26 (46) | <0.01 |
| Weight (Baseline), kg (IQR) | 55 (48-62) | 54 (47-61) | 56 (50-63) | 0.74 |
| Race, n (%) | 0.07 | |||
| Black | 204 (99) | 150 (100) | 54 (96) | |
| Indian | 1 (0.5) | 0 | 1 (2) | |
| White | 1 (0.5) | 0 | 1 (2) | |
| Previous treatment for TB, n (%) | 133 (65) | 107 (71) | 26 (46) | <0.01 |
| Outcome of most recent TB episode, n (%) | 0.29 | |||
| Cure/Complete | 94 (71) | 78 (73) | 16 (62) | |
| Failure | 28 (21) | 19 (18) | 9 (35) | |
| Interruption/Loss to follow-up | 8 (6) | 7 (7) | 1 (4) | |
| Unknown | 3 (2) | 3 (3) | 0 (0) | |
| Smoking, n (%) | 47 (23) | 35 (23) | 12 (21) | 0.77 |
| Alcohol use, n (%) | 63 (31) | 49 (33) | 14 (25) | 0.29 |
| Receiving Antiretroviral therapy (ART) at MDR-TB treatment initiation, n (%) | -- | 121 (81) | N/A | |
| Duration on ART at MDR-TB treatment initiation, median months (IQR) | -- | 9 (3-30) | N/A | |
| CD4 count at MDR-TB treatment initiation, median cells/mm3 (IQR) (n=144) | -- | 215 (114-378) | N/A | |
| Viral load <150 copies/ml at MDR-TB treatment initiation (n=86) | -- | 52 (60) | N/A | |
| Sputum smear status positive (n=154)a | 76 (49) | 57 (49) | 19 (51) | 0.78 |
| Baseline chest radiograph (n=204) | ||||
| Cavitary disease | 95 (47) | 71 (47) | 24 (44) | 0.72 |
| Bilateral disease | 122 (60) | 81 (54) | 41 (76) | <0.01 |
Sputum smear was available for 117 HIV-infected and 37 HIV-uninfected patients; N/A: Not Applicable; Bold indicates statistical significance.
Clinical and Laboratory Adverse Events
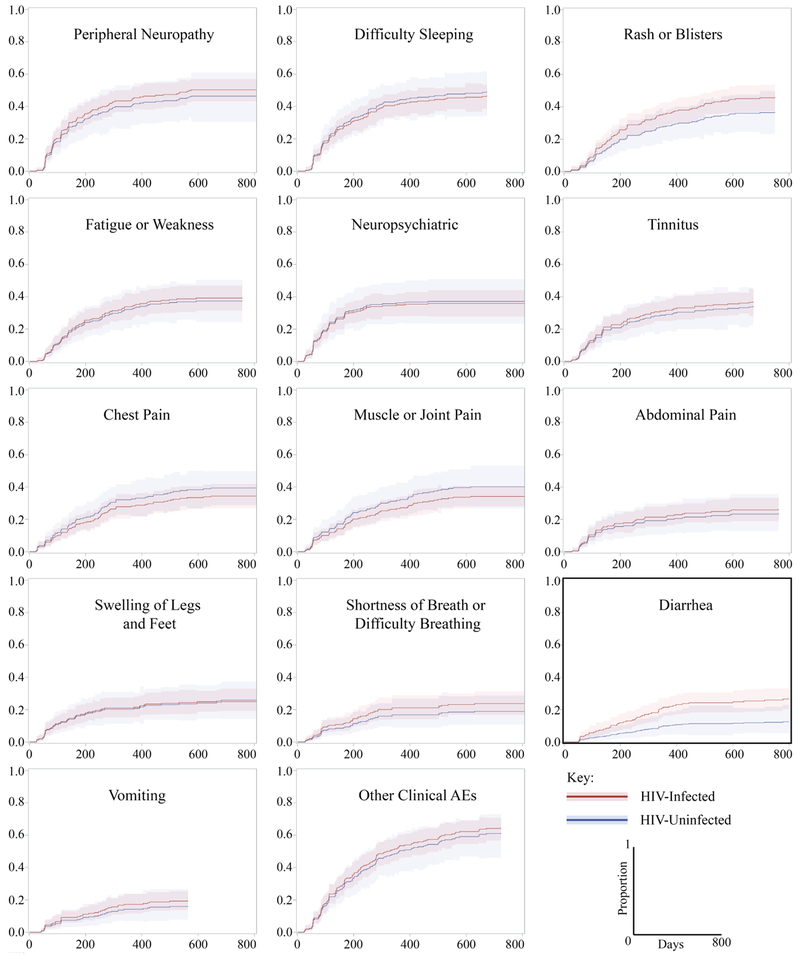
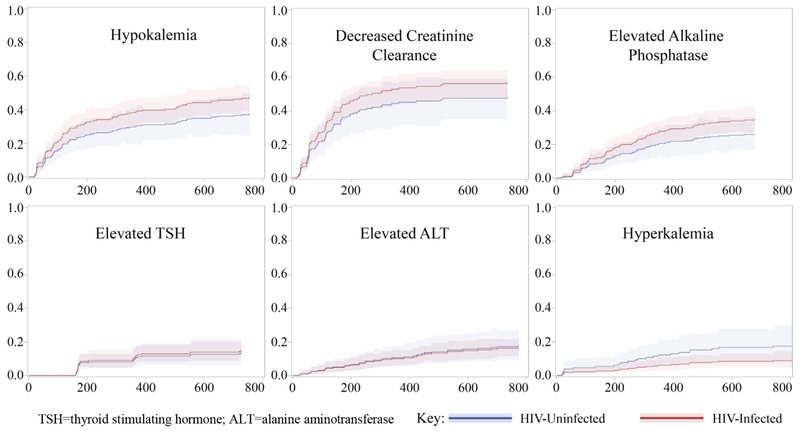
Nearly all participants experienced at least one clinical or laboratory AE (93% and 96%, respectively; Table 2), with a median of 8 (IQR: 4-15) clinical AEs and 4 (IQR: 2-9) laboratory AEs per participant. The most common clinical AEs were peripheral neuropathy (50%) and difficulty sleeping (48%); the most common laboratory AEs (of any grade) were decreased creatinine clearance (46%) and hypokalemia (47%). The median time to participants’ first clinical or laboratory AE ranged from 64 to 373 days. Importantly, AEs occurred throughout the duration of MDR-TB treatment and were not restricted to the intensive phase (Figures 1 and 2). AE-specific CIF curves provide additional insight for comparing the time-to-onset between individual AEs. For example, although a similar proportion of participants experienced neuropsychiatric symptoms and muscle/joint pain, the CIF curves show that neuropsychiatric AEs occurred more often in the intensive phase of treatment and then plateaued, while muscle/joint pain continued to occur as a new AE throughout treatment (Figure 1). Similarly, peripheral neuropathy, tinnitus, and decreases in creatinine clearance tended to occur early in therapy while diarrhea, rash, and elevations in ALT occurred throughout treatment.
Table 2:
Frequency of newly developed adverse event during MDR-TB treatment, by HIV status, n (%)
| All patients | HIV-Infected | HIV-Uninfected | p-value | |
|---|---|---|---|---|
| Clinical AEs | ||||
| Individuals reporting at least one AE | 192 (93) | 139 (93) | 53 (95) | 0.62 |
| Peripheral neuropathy | 103 (50) | 78 (52) | 25 (45) | 0.35 |
| Difficulty sleeping | 98 (48) | 71 (47) | 27 (48) | 0.91 |
| Rash or Blisters | 90 (44) | 69 (46) | 21 (38) | 0.27 |
| Fatigue or weakness | 81 (39) | 60 (40) | 21 (38) | 0.74 |
| Neuropsychiatrica | 76 (37) | 56 (37) | 20 (36) | 0.83 |
| Tinnitus | 75 (36) | 56 (37) | 19 (34) | 0.65 |
| Chest pain | 75 (36) | 52 (35) | 23 (41) | 0.39 |
| Muscle or joint pain | 75 (36) | 52 (35) | 23 (41) | 0.40 |
| Abdominal pain | 53 (26) | 40 (27) | 13 (23) | 0.61 |
| Swelling of legs and feet | 54 (26) | 40 (27) | 14 (25) | 0.81 |
| Shortness of breath/Difficulty breathing | 48 (23) | 37 (25) | 11 (20) | 0.44 |
| Diarrhea | 48 (23) | 41 (27) | 7 (13) | 0.03 |
| Vomiting | 39 (19) | 30 (20) | 9 (16) | 0.52 |
| Other Clinical Adverse Events | 132 (64) | 98 (65) | 34 (61) | 0.54 |
| Laboratory AEs | ||||
| Individuals having at least one AEb | 198 (96) | 144 (96) | 54 (96) | 0.94 |
| Hypokalamiab | 96 (47) | 74 (50) | 22 (39) | 0.17 |
| Decreased Creatinine Clearancec | 95 (46) | 71 (48) | 24 (43) | 0.56 |
| Elevated Alkaline Phosphatasec | 90 (44) | 69 (47) | 21 (38) | 0.24 |
| Elevated TSHd | 37 (24) | 28 (26) | 9 (20) | 0.37 |
| Elevated ALTb | 48 (23) | 38 (26) | 10 (18) | 0.25 |
| Hyperkalemiac | 26 (13) | 16 (11) | 10 (18) | 0.18 |
| Hearing Losse | ||||
| Any hearing loss | 125 (72) | 90 (72) | 35 (73) | 0.90 |
| Mild/moderate loss | 111 (64) | 78 (62) | 33 (69) | 0.43 |
| Severe loss | 14 (8) | 12 (10) | 2 (4) | 0.24 |
| Vision AEsf | ||||
| Any color discrimination loss | 17 (9) | 13 (10) | 4 (7) | 0.61 |
| Sustained loss | 5 (3) | 3 (2) | 2 (4) | 0.58 |
| Extended loss | 1 (1) | 1 (1) | 0 (0) | 0.71 |
| Severe loss | 6 (3) | 5 (4) | 1 (2) | 0.44 |
Bold indicates statistical significance; TSH=thyroid stimulating hormone;
Neuropsychiatric is defined as patients experiencing mental confusion, depression, hearing voices, hallucinations, or convulsions/seizures; Full cohort unless noted:
n=204 (n=56 HIV-uninfected, n=148 HIV-infected);
Calculated using Cockroft-Gault formula, n=205 (n=56 HIV-uninfected, n=149 HIV-infected);
n=152 (n=46 HIV-uninfected, n=106 HIV-infected);
n=173 (n=48 HIV-uninfected, n=125 HIV-infected)
n=187 (n=54 HIV-uninfected, n=133 HIV-infected; TSH=thyroid stimulating hormone; ALT=alanine aminotransferase
Figure 1:
Cumulative incidence function (CIF) curves of time to first clinical adverse event, by HIV status. Bold outline indicates statistical significance.
aNeuropsychiatric is defined as patients experiencing mental confusion, depression, hearing voices, hallucinations, or convulsions/seizures
Figure 2:
Cumulative incidence function (CIF) curves of time to first laboratory adverse event, by HIV status.
TSH=thyroid stimulating hormone; ALT=alanine aminotransferase
Eighty-three participants (40%) experienced at least one grade 3 or 4 laboratory AE, the majority of which were decreases in creatinine clearance. When comparing the cumulative incidence of the 19 clinical and laboratory AEs between HIV-infected and HIV-uninfected participants, a significant difference was found for only one AE (Table 2). HIV-infected participants were 2.4 (CI95%: 1.1-5.4) times as likely to experience diarrhea. There were no statistically significant differences in the frequency of severe laboratory AEs overall between HIV-infected and HIV-uninfected participants, but HIV-uninfected participants experienced more severe elevations of ALT (7.1% vs. 1.3%, p=0.048) (Appendix Table S2).
Hearing Loss
One-hundred and seventy-three participants (84%) had at least two audiology test results suitable for analysis, of which 129 (75%) had a true baseline result. Of participants included in analysis, 125 (72%) experienced hearing loss of any grade and 14 (8%) experienced severe loss (Table 2). There were no statistically significant differences in the risk of hearing loss or severity between HIV-infected and HIV-uninfected participants. For some participants, hearing loss occurred as early as the first month after treatment, while others developed loss after several months of therapy. The median time to hearing loss while on MDR-TB treatment was 109 days (IQR 61-171) and there was no significant difference by HIV status (HIV-infected participants: 106 days vs 141 days for HIV-uninfected participants; p=0.15).
Color Discrimination Loss
One-hundred and eighty-seven participants (91%) had vision testing results; nine (5%) participants were excluded due to pre-existing color discrimination loss, six (3%) participants had no testing results, and four (2%) had only one testing result. Among participants with testing results, 17 (9%) experienced loss of any grade, five (4%) experienced sustained loss, one (<1%) experienced extended loss, and 6 (3%) experienced severe loss (Table 2). There was no statistically significant difference in the risk of color discrimination loss or severity of loss between HIV-infected and HIV-uninfected participants. The median time to loss while on MDR-TB treatment was 124 days for HIV-infected participants (IQR: 107-196) and 82 days (IQR: 56-231) for HIV-uninfected participants (p=0.47).
Deaths and Hospitalizations
A total of 42 participants (20%) experienced at least one serious clinical adverse event (SAE), of which 24 were participant deaths. Of the 24 deaths, 14 were determined to be the result of either MDR-TB or HIV disease progression (often in the setting of known medication non-adherence). Among the remaining 10 deaths, a likely cause was identified for 4 cases (renal failure [n=3]; abdominal wall abscess [n=1]); the three deaths from renal failure were possibly related to either TB or HIV treatment. A cause of death could not be identified for the remaining 6 participants either because of insufficient documentation (n=4) or unavailable records (n=2). Two deaths occurred in the one year following completion of MDR-TB treatment after the participants had been deemed cured; one of these occurred after a recurrence of TB disease, while the cause of the other death could not be determined.
Twenty-four clinical SAEs occurred in 20 participants and resulted in hospitalization but not patient death (Table 3). Of these 20 participants, 16 were HIV-infected and 4 were HIV-uninfected (p=0.6). These SAEs represented a wide variety of conditions, including: miscarriage (n=3), lower respiratory tract infection (n=2), hypokalemia requiring intravenous repletion (n=2); MDR-TB treatment failure (n=2), psychosis (n=2), and rash (n=1) (Table 3). Of these 24 SAEs, 14 (58%) were classified as possibly due to TB or HIV medication toxicity. Ten participants experienced more than one clinical SAE (range 2-5). Notably, there was no difference in treatment outcome among those who experienced a clinical SAE (not including deaths), a severe laboratory AE, severe hearing loss, or severe/sustained loss of color discrimination.
Table 3:
Frequency of serious clinical adverse events requiring hospitalization, by HIV status (n, %)
| HIV-infected | HIV-uninfected | Total | |
|---|---|---|---|
| Reason for hospitalization | |||
| Lower respiratory tract Infection | 2 (1.3) | 1 (2.0) | 3 (1.5) |
| MDR-TB treatment failure | 4 (2.7) | 0 (0.0) | 4 (2.0) |
| Miscarriage | 2 (1.3) | 0 (0.0) | 2 (1.0) |
| Anemia | 2 (1.3) | 0 (0.0) | 2 (1.0) |
| Hypokalemia | 2 (1.3) | 0 (0.0) | 2 (1.0) |
| Psychosis | 1 (0.7) | 1 (2.0) | 2 (1.0) |
| Rash | 1 (0.7) | 0 (0.0) | 1 (0.5) |
| Pelvic inflammatory disease | 1 (0.7) | 0 (0.0) | 1 (0.5) |
| Ovarian mass | 0 (0.0) | 1 (2.0) | 1 (0.5) |
| Gastroenteritis | 1 (0.7) | 0 (0.0) | 1 (0.5) |
| Acute kidney injury | 1 (0.7) | 0 (0.0) | 1 (0.5) |
| Cor pulmonale | 1 (0.7) | 0 (0.0) | 1 (0.5) |
| Transient ischemic attack | 1 (0.7) | 0 (0.0) | 1 (0.5) |
| Drug-induced liver injury | 0 (0.0) | 1 (2.0) | 1 (0.5) |
| Cellulitis/abscess | 0 (0.0) | 1 (2.0) | 1 (0.5) |
AEs and CD4 Count
We investigated differences in the incidence of AEs by baseline CD4 count (≤100 CD4 cells/mm3, 101-200 cells/mm3, and >200 cells/mm3) compared to HIV-uninfected individuals. Individuals in the lowest CD4 category were more than twice as likely to experience hypokalemia and three times more likely to report diarrhea when compared to HIV-uninfected participants (Table 4). Individuals in the highest CD4 category demonstrated no difference in any clinical, vision, or hearing AEs compared to HIV-uninfected individuals. CIF curves by CD4 strata are shown in the Appendix (Figures S1 and S2).
Table 4:
Multivariable sub-distribution hazard ratios (sHR) of adverse events, by CD4 count and HIV statusa
| sHR (95% CI)b | sHR (95% CI) | |||
|---|---|---|---|---|
| CD4 ≤100 (n=31) | CD4 101-200 (n=34) | CD4 >200 (n=79) | All HIV-Infected Patients (n=150) | |
| Clinical AEs | ||||
| Presence of at least one AE | 0.9 (0.6, 1.4) | 1.1 (0.7, 1.8) | 1.0 (0.7, 1.4) | 1.0 (0.8, 1.4) |
| Peripheral neuropathy | 1.1 (0.6, 2.0) | 1.1 (0.6, 2.1) | 1.3 (0.8, 2.3) | 1.2 (0.7, 2.0) |
| Difficulty sleeping | 0.9 (0.5, 1.6) | 1.0 (0.5, 1.9) | 0.9 (0.5, 1.5) | 0.9 (0.6, 1.5) |
| Rash or Blisters | 1.7 (0.9, 3.0) | 1.2 (0.6, 2.4) | 1.2 (0.7, 2.2) | 1.3 (0.8, 2.2) |
| Fatigue or weakness | 0.7 (0.4, 1.5) | 1.4 (0.7, 2.7) | 0.9 (0.5, 1.7) | 1.0 (0.6, 1.6) |
| Neuropsychiatricc | 0.8 (0.4, 1.6) | 0.8 (0.4, 1.6) | 1.1 (0.6, 2.0) | 0.9 (0.6, 1.6) |
| Tinnitus | 1.1 (0.5, 2.2) | 1.1 (0.5, 2.1) | 1.0 (0.5, 1.9) | 1.1 (0.6, 1.8) |
| Chest pain | 0.9 (0.4, 1.9) | 1.1 (0.6, 2.2) | 0.8 (0.4, 1.4) | 0.9 (0.5, 1.4) |
| Muscle or joint pain | 1.6 (0.8, 3.1) | 0.8 (0.4, 1.8) | 0.7 (0.4, 1.3) | 0.9 (0.5, 1.5) |
| Abdominal pain | 1.0 (0.4, 2.2) | 1.3 (0.6, 3.0) | 1.0 (0.5, 2.1) | 1.1 (0.6, 2.0) |
| Swelling of legs and feet | 1.2 (0.5, 2.7) | 1.6 (0.7, 3.3) | 0.8 (0.4, 1.6) | 1.1 (0.6, 1.9) |
| Shortness of breath | 1.1 (0.4, 3.0) | 2.2 (0.8, 5.6) | 1.5 (0.7, 3.4) | 1.5 (0.7, 3.1) |
| Diarrhea | 3.3 (1.3, 8.8) | 1.8 (0.6, 5.3) | 2.6 (1.0, 6.4) | 2.6 (1.1, 6.0) |
| Vomiting | 1.4 (0.5, 3.8) | 2.2 (0.9, 5.4) | 1.0 (0.4, 2.4) | 1.4 (0.6, 2.8) |
| Other Clinical Adverse Events | 1.4 (0.8, 2.4) | 0.9 (0.5, 1.6) | 0.9 (0.5, 1.4) | 1.0 (0.6, 1.5) |
| Laboratory AEs | ||||
| Presence of at least one AE | 2.2 (1.5, 3.2) | 1.4 (0.9, 2.2) | 1.4 (1.0, 2.1) | 1.6 (1.1, 2.0) |
| Hypokalemia | 1.8 (1.1, 3.4) | 1.1 (0.6, 2.2) | 1.0 (0.5, 1.8) | 1.2 (0.7, 1.9) |
| Decreased Creatinine Clearance | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.2) | 1.0 (0.9, 1.1) | 1.1 (0.9, 1.2) |
| Elevated Alkaline Phosphatase | 1.3 (0.6, 2.9) | 2.4 (1.1, 5.3) | 1.3 (0.7, 2.6) | 1.6 (0.9, 2.9) |
| Elevated TSH | 1.4 (0.4, 4.1) | 2.0 (0.8, 5.2) | 1.2 (0.5, 3.1) | 1.4 (0.6, 3.0) |
| Elevated ALT | 0.8 (0.2, 2.8) | 0.9 (0.3, 2.6) | 0.9 (0.4, 2.1) | 0.8 (0.4, 1.9) |
| Hyperkalemia | 0.6 (0.2, 2.1) | 0.5 (0.1, 1.8) | 0.7 (0.2, 1.7) | 0.5 (0.2, 1.2) |
| Vision AEs | ||||
| Any color discrimination loss | 1.6 (0.5, 5.4) | 2.3 (0.7, 7.4) | 1.4 (0.5, 4.2) | 1.8 (0.7, 4.7) |
| Sustained loss | 2.7 (0.3, 22.9) | -- | -- | 1.5 (0.2, 10.6) |
| Extended loss | -- | -- | -- | -- |
| Severe loss | 1.7 (0.1, 20.1) | 1.7 (0.2, 18.9) | 1.5 (0.2, 11.5) | 2.0 (0.3, 11.8) |
| Hearing Loss | ||||
| Any hearing loss | 0.9 (0.5, 1.7) | 1.2 (0.7, 2.1) | 1.0 (0.7, 1.6) | 1.1 (0.7, 1.6) |
| Mild/moderate loss | 0.9 (0.5, 1.7) | 1.3 (0.7, 2.3) | 1.1 (0.7, 1.7) | 1.1 (0.7, 1.7) |
| Severe loss | 3.8 (0.7, 20.7) | 0.8 (0.1, 8.5) | 1.9 (0.4, 9.7) | 2.4 (0.5, 10.8) |
HIV-uninfected group is the reference for all categories;
CD4 category based on CD4 measured at baseline: n=200, 6 HIV-infected participants missing CD4 status at enrolment.
Neuropsychiatric is defined as patients experiencing mental confusion, depression, hearing voices, hallucinations, or convulsions/seizures.
Bolded numbers indicate statistical significance (α=0.05). TSH=thyroid stimulating hormone; ALT=alanine aminotransferase
DISCUSSION
To our knowledge, this is the first prospective study to rigorously examine patients with and without HIV for the development of AEs while on MDR-TB treatment. Overall, the frequency and severity of AEs did not substantially differ between HIV-infected participants on ART and HIV-uninfected participants, suggesting that concomitant treatment for MDR-TB and HIV should not be delayed in co-infected individuals. Among the clinical and laboratory AEs for which we found meaningful differences; the burden was largely among those with the most severe immune suppression. This suggests that these differences in AEs are probably not a result of medical therapy but may instead be due to direct effects of HIV or opportunistic infection. This underscores the need for urgent ART in patients with very low CD4 counts and, potentially, the need more intensive monitoring throughout the duration of MDR-TB treatment.
In this cohort we saw a very high overall risk of developing a clinical or laboratory AE (93% and 96%, respectively) while on MDR-TB treatment. The literature on total AE incidence among MDR-TB patients varies widely, from 18% to 100%, depending on the population, treatment regimen, country, and HIV prevalence.15 Our findings are consistent with other studies from South Africa,29,30 and reiterate previous findings that a large proportion of new AEs develop within the first six months of MDR-TB therapy, especially those associated with the injectable agent.10,31–33 Importantly, however, we also found that each AE developed throughout the entirety of treatment, regardless of HIV status, and that each AE had a different pattern of timing and development (Figures 1 and 2). Taken together, these findings demonstrate a clear need for clinicians to closely monitor individual AE development throughout therapy. Of particular note was the high incidence of peripheral neuropathy in both study groups. The etiology of this neuropathy is unknown but was likely multifactorial and heterogeneous. None of the study participants received linezolid, but many HIV-infected participants did receive stavudine. In addition, several second-line TB medications can cause neuropathy, as can HIV, itself, and diabetes. With new guidelines recommending that all MDR-TB patients receive linezolid,34 this AE is likely to become even more common.
Irreversible hearing loss from aminoglycosides is one of the most devastating AEs associated with MDR-TB treatment. A recent review of the literature shows a wide range of patients experiencing hearing loss, from 2.6% to 61.5%, although the authors note that this is likely an underestimate, as audiometry is typically not performed in asymptomatic patients.35 In our study, audiology exams were performed regularly, regardless of symptoms. Although we found no difference in hearing loss by HIV status, we found that a large proportion of patients (72%) developed hearing loss of any grade, and approximately one in twelve patients developed severe hearing loss. This is likely a conservative estimate of the true severity of hearing loss in this cohort because for those without true baseline audiology (25%), our analysis was based on relative loss from the patient’s first reading to subsequent readings while on treatment. These results are consistent with other studies that routinely tested for hearing loss regardless of symptoms and suggest that hearing loss is more common than typically reported.35,36 Our study also found that loss can occur within the first month of therapy, regardless of HIV status, indicating that hearing loss in MDR-TB patients is still of great concern even among patients eligible for short-course regimens (which include an injectable agent for the first 4-6 months). Active audiology testing should be performed at least monthly in all patients receiving aminoglycosides.
Deterioration of visual function in patients being treated for TB is typically attributed to ethambutol and has generally been considered rare.37 A 2013 systematic review of ethambutol-related visual impairment found 54 cases of color blindness out of 2091 patients (2.6%) pooled from 13 studies of drug-susceptible TB.38 However, ethambutol-induced vision loss is closely linked with dose,39 and whereas drug-susceptible TB patients typically receive only two months of ethambutol, MDR-TB patients routinely receive significantly longer durations (9-24 months). To our knowledge, this is the first study to report color vision loss among MDR-TB patients. In our cohort, approximately one in eleven patients (9%) developed color blindness of any grade while on MDR-TB treatment, a third of whom developed severe color blindness. There was no meaningful difference between HIV-infected and HIV-uninfected participants. These data suggest that loss of color discrimination among MDR-TB patients may be more common than previously assumed and raise newfound concerns regarding the long-term use of ethambutol. Routine vision testing should be implemented for all MDR-TB patients receiving ethambutol.
This study has several important limitations. First, clinical AEs were primarily identified by a questionnaire during clinic visits. Although the survey instrument was standardized and translated into participants’ primary language, a specific medical definition of each clinical AE was not given and thus may have introduced a degree of subjectivity in the patient’s response in some AEs. Second, all participants were on multidrug TB regimens and, if applicable, ART; thus, it was difficult to ascribe specific AEs to individual drugs. Similarly, it was unclear whether reported symptoms were drug-related AEs, a new disease process, or opportunistic infection. Third, we used a novel grading system that assesses hearing loss patterns more specific to aminoglycosides, but this prevents a true comparison of these results with others published in the literature using common grading systems. Lastly, we assessed loss of color discrimination as a proxy for optic neuritis but did not conduct further vision testing with an ophthalmologist. Future studies should validate our novel grading system for severity, as well as examine the potential long-term clinical implications of these findings, given how frequently loss of color discrimination was seen.
MDR-TB treatment is challenging and complex for both patients and providers. Despite increasing availability of newer medications (e.g. bedaquiline, delamanid) and new guidelines recommending all-oral regimens,34 many older, toxic drugs will remain core components of treatment regimens, especially in patients with more extensive resistance (e.g., XDR-TB) and as resistance to the newer drugs becomes more prevalent. We found that although AEs are frequent, patients with MDR-TB and HIV coinfection can be treated for both diseases concurrently. Our study findings reiterate that AEs are not exceptional events but remain a routine feature of MDR-TB therapy among both HIV-infected and HIV-uninfected patients. Providers and patients must navigate MDR-TB treatment by educating patients and family members at the start of therapy, actively monitoring for AEs throughout treatment, and aggressively treating AEs whenever possible. In addition to quantitative assessments, future research should provide further detail on the patient experience regarding AEs during MDR-TB treatment, which may provide more nuance and a level of depth not achieved by clinical and quantitative assessments. Supporting patients in this way will help to ensure high levels of medication adherence and successful MDR-TB and HIV outcomes.
Supplementary Material
Acknowledgements:
We are grateful to the study team at the University of KwaZulu-Natal for their tireless efforts in data collection, record abstraction, participant recruitment, and interviews. We would like to especially thank Lucretia Peterson at the University of Cape Town for her expertise and assistance in developing the audiometry grading criteria for hearing loss. We thank the study participants and their families who consented to participate in this study.
Funding Statement: This study was funded by the US National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH): R01AI087465 and R01AI089349 (both to NRG). It was also supported in part by NIH/NIAID grants: R01AI114304 (to JCMB), K24AI114444 (to NRG), Emory Center for AIDS Research (CFAR) P30AI050409, Einstein-Rockefeller-CUNY CFAR P30AI124414, Emory TB Research Unit (TBRU) U19AI111211, Einstein/Montefiore Institute for Clinical and Translational Research (ICTR) UL1TR001073 and the Georgia Clinical and Translational Science Alliance UL1TR002378.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official position of the U.S. National Institutes of Health (NIH) or the U.S. Centers of Disease Control and Prevention (CDC).
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report. Document No. WHO/CDS/TB/2018.20 Geneva: 2018. [Google Scholar]
- 2.Ahuja SD, Ashkin D, Avendano M, et al. Multidrug Resistant Pulmonary Tuberculosis Treatment Regimens and Patient Outcomes: An Individual Patient Data Meta-analysis of 9,153 Patients. PLOS Med 2012;9(8):e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborative Group for the Meta-Analysis of Individual Patient Data in MDRTB treatment, Ahmad N, Ahuja SD, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isaakidis P, Casas EC, Das M, Tseretopoulou X, Ntzani EE, Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. Int J Tuberc Lung Dis 2015;19(8):969–978. [DOI] [PubMed] [Google Scholar]
- 5.Heysell SK, Thomas TA, Gandhi NR, et al. Blood cultures for the diagnosis of multidrug-resistant and extensively drug-resistant tuberculosis among HIV-infected patients from rural South Africa: a cross-sectional study. BMC infectious diseases. 2010;10:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brust JC, Gandhi NR, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis 2010;14(4):413–419. [PMC free article] [PubMed] [Google Scholar]
- 7.Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38(3):516–528. [DOI] [PubMed] [Google Scholar]
- 8.Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis 2004;8(11):1382–1384. [PubMed] [Google Scholar]
- 9.Satti H, Mafukidze A, Jooste PL, McLaughlin MM, Farmer PE, Seung KJ. High rate of hypothyroidism among patients treated for multidrug-resistant tuberculosis in Lesotho. Int J Tuberc Lung Dis 2012;16(4):468–472. [DOI] [PubMed] [Google Scholar]
- 10.Torun T, Gungor G, Ozmen I, et al. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005;9(12):1373–1377. [PubMed] [Google Scholar]
- 11.Bloss E, Kuksa L, Holtz TH, et al. Adverse events related to multidrug-resistant tuberculosis treatment, Latvia, 2000–2004. Int J Tuberc Lung Dis 2010;14(3):275–281. [PubMed] [Google Scholar]
- 12.El-Din MAT, Halim HAA- E, El-Tantawy AM. Adverse reactions among patients being treated for multi-drug resistant tuberculosis in Egypt from July 2006 to January 2009. Egypt J Chest Dis Tuberc 2015;64(3):657–664. [Google Scholar]
- 13.Reuter A, Tisile P, von Delft D, et al. The devil we know: is the use of injectable agents for the treatment of MDR-TB justified? Int J Tuberc Lung Dis 2017;21(11):1114–1126. [DOI] [PubMed] [Google Scholar]
- 14.Brust JCM, Shah NS, Mlisana K, et al. Improved Survival and Cure Rates With Concurrent Treatment for Multidrug-Resistant Tuberculosis–Human Immunodeficiency Virus Coinfection in South Africa. Clin Infect Dis 2018;66(8):1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S, Zhang Y, Sun F, et al. Adverse Events Associated With the Treatment of Multidrug-Resistant Tuberculosis: A Systematic Review and Meta-analysis. Am J Ther 2016;23(2):e521–530. [DOI] [PubMed] [Google Scholar]
- 16.Shringarpure KS, Isaakidis P, Sagili KD, Baxi RK, Das M, Daftary A. “When Treatment Is More Challenging than the Disease”: A Qualitative Study of MDR-TB Patient Retention. PLoS One. 2016;11(3):e0150849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deshmukh RD, Dhande DJ, Sachdeva KS, et al. Patient and Provider Reported Reasons for Lost to Follow Up in MDRTB Treatment: A Qualitative Study from a Drug Resistant TB Centre in India. PLoS One. 2015;10(8):e0135802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events (Version 2.0). US Department of Health and Human Services, National Institute of Allergy and Infectious Diseases; https://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf Published 2014. Accessed. [Google Scholar]
- 19.Schacht J, Talaska AE, Rybak LP. Cisplatin and Aminoglycoside Antibiotics: Hearing Loss and Its Prevention. Anatomical record (Hoboken, NJ : 2007). 2012;295(11):1837–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fausti SA, Henry JA, Schaffer HI, Olson DJ, Frey RH, McDonald WJ. High-frequency audiometric monitoring for early detection of aminoglycoside ototoxicity. J Infect Dis 1992;165(6):1026–1032. [DOI] [PubMed] [Google Scholar]
- 21.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. United States Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, 2009. (available at https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf; accessed March 26, 2019). [Google Scholar]
- 22.Brust JCM, Shah NS, Mthiyane T, et al. Novel grading scheme to determine severity of hearing loss in patients treated for MDR-TB. Programs and Abstacts of the American Thoracic Society International Conference May 16-21, 2014, San Diego, CA (Abstract #2562). 2014. [Google Scholar]
- 23.Hardy LH, Rand G, Rittler MC. H–R–R Polychromatic Plates*. J Opt Soc Am 1954;44(7):509–523. [Google Scholar]
- 24.Dain SJ. Colorimetric analysis of four editions of the Hardy-Rand-Rittler pseudoisochromatic tests. Vis Neurosci 2004;21(3):437–443. [DOI] [PubMed] [Google Scholar]
- 25.Thiadens AA, Hoyng CB, Polling JR, Bernaerts-Biskop R, van den Born LI, Klaver CC. Accuracy of four commonly used color vision tests in the identification of cone disorders. Ophthalmic Epidemiol 2013;20(2):114–121. [DOI] [PubMed] [Google Scholar]
- 26.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat 1988;16(3):1141–1154. [Google Scholar]
- 27.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999;94(446):496–509. [Google Scholar]
- 28.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017;36(27):4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shean K, Streicher E, Pieterson E, et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One. 2013;8(5):e63057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brust JC, Shah NS, van der Merwe TL, et al. Adverse events in an integrated home-based treatment program for MDR-TB and HIV in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr 2013;62(4):436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnippel K, Berhanu RH, Black A, et al. Severe adverse events during second-line tuberculosis treatment in the context of high HIV Co-infection in South Africa: a retrospective cohort study. BMC Infect Dis 2016;16(1):593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin SS, Pasechnikov AD, Gelmanova IY, et al. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis 2007;11(12):1314–1320. [PubMed] [Google Scholar]
- 33.Van der Walt M, Lancaster J, Odendaal R, Davis JG, Shean K, Farley J. Serious treatment related adverse drug reactions amongst anti-retroviral naive MDR-TB patients. PLoS One. 2013;8(4):e58817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. WHO treatment guidelines for multidrug- and rifampicin-resistant tuberculosis: 2018 update (pre-final text). Document no WHO/CDS/TB/201815 Geneva: 2018. [Google Scholar]
- 35.Seddon JA, Godfrey-Faussett P, Jacobs K, Ebrahim A, Hesseling AC, Schaaf HS. Hearing loss in patients on treatment for drug-resistant tuberculosis. Eur Respir J. 2012;40(5):1277. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy B, O’Connor B, Korn B, Gibbons N, O’Connor T, Keane J. Multi-drug resistant tuberculosis: experiences of two tertiary referral centres. Ir Med J. 2011;104(6):182–185. [PubMed] [Google Scholar]
- 37.Kandel H, Adhikari P, Shrestha GS, Ruokonen E- L, Shah DN. Visual Function in Patients on Ethambutol Therapy for Tuberculosis. J Ocul Pharmacol Ther 2012;28(2):174–178. [DOI] [PubMed] [Google Scholar]
- 38.Ezer N, Benedetti A, Darvish-Zargar M, Menzies D. Incidence of ethambutol-related visual impairment during treatment of active tuberculosis [Review article]. Int J Tuberc Lung Dis. 2013;17(4):447–455. [DOI] [PubMed] [Google Scholar]
- 39.Leibold JE. The Ocular Toxicity of Ethambutol and its Relation to Dose. Ann N Y Acad Sci 1966;135(2):904–909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.