Abstract
Background:
Findings regarding brain circuitry abnormalities in suicide attempters (SAs) converge across bipolar disorder (BD) and major depressive disorder (MDD), the most common disorders observed in suicides. These abnormalities appear to be present during adolescence/young adulthood when suicide rates increase steeply, and suicide is a leading cause of death in this age group. Identification of brain circuitry common to adolescent/young adult SAs with BD and MDD is important for generating widely effective early prevention strategies. We examined brain circuitry in SAs in adolescents/young adults across these two disorders.
Methods:
Eighty-three participants (ages 14-25 years), 46 with BD (21 SAs) and 37 with MDD (19 SAs), underwent structural and diffusion-weighted magnetic resonance scanning. Whole-brain analyses compared grey matter (GM) volume and white matter (WM) fractional anisotropy (FA) between SAs and non-suicide attempters (NSAs) across and within BD and MDD (p<0.005).
Results:
Across and within BD and MDD, SAs showed differences compared to NSAs in ventral prefrontal cortex (PFC) GM volume and fronto-limbic (including uncinate fasciculus (UF)) WM FA. Exploratory analyses showed additional within-disorder differences for BD SAs in dorsolateral PFC (dlPFC) and hippocampus GM volume and UF FA, and for MDD SAs dorsomedial and dlPFC GM and dorsal frontal WM. However, there was no significant interaction between suicide attempt status and diagnosis.
Limitations:
Modest sample size.
Conclusions:
Common fronto-limbic grey and white matter alterations in adolescent/young adult SAs are potential targets for suicide prevention strategies across mood disorders. Preliminary findings of disorder-specific regional findings could suggest diagnostic-specific optimal targets may exist.
Keywords: Magnetic Resonance Imaging, Diffusion Tensor Imaging, Suicide, Adolescent, Young Adult, Frontal Lobe
Introduction
Bipolar disorder (BD) and major depressive disorder (MDD) are common mood disorders that have lifetime prevalences of 2.4% and 13.2% respectively (Hasin et al., 2005; Merikangas et al., 2011). Both BD and MDD can cause a great deal of suffering, be debilitating and pose major risk for suicide (Angst et al., 1999; Costa Lda et al., 2015). It has been estimated that 56% of individuals with BD and 15% with MDD attempt suicide (Abreu et al., 2009; Kessler and Bromet, 2013). Suicide thoughts and behavior often emerge during adolescence or young adulthood. Globally, suicide has been identified as the second most common cause of death in adolescents and young adults (Hawton et al., 2012) and the majority of adolescents and young adults who die by suicide have BD or MDD (Zametkin et al., 2001). Identification of common brain alterations across mood disorders that underlie risk for suicide attempts during adolescence and young adulthood could provide early prevention targets that may be most broadly effective. Brain alterations identified to differ for suicide attempters (SAs) with different disorders could be targets that are more beneficial for individuals with a specific disorder.
The pathophysiology underlying suicidal behavior in BD and MDD remains unknown. However, convergent evidence implicates impairments in the fronto-limbic brain circuitry that subserves emotion regulation and impulse control (Cox Lippard et al., 2014; Du et al., 2017; Johnston et al., 2017; Monkul et al., 2007). This is supported by studies showing both grey matter (GM) and white matter (WM) alterations in this circuitry in SAs (Cox Lippard et al., 2014). Although these studies have primarily been performed within one diagnosis and with differing single imaging modalities, similar findings suggest commonalities across SAs with BD and MDD, although some studies suggest there may be some distinctions between SAs with differing disorders (Cox Lippard et al., 2014).
Structural magnetic resonance imaging (sMRI) studies in adults with BD or with MDD showed that SAs, compared to non-suicide attempters (NSAs), have decreased frontal GM volume, with a convergence of findings particularly in ventral PFC, an important brain region in the fronto-limbic circuitry subserving emotion regulation (Benedetti et al., 2011; Cox Lippard et al., 2014; Hwang et al., 2010; Monkul et al., 2007; van Heeringen et al., 2011). Other brain regions in the fronto-limbic circuitry have also shown GM reductions in adult SAs with BD and with MDD. For instance, reductions in GM volume were seen in dorsolateral PFC (dlPFC) and medial PFC (mPFC) in adult SAs with BD and with MDD (Benedetti et al., 2011; Hwang et al., 2010; Wagner et al., 2011; Wagner et al., 2012). In addition, GM volume decreases were observed in the hippocampus in SAs with both BD and MDD (Johnston et al., 2017; Wagner et al., 2011). However, some findings have been reported in one disorder. For example, decreases in GM volume in the thalamus were reported in MDD in SAs compared to NSAs (Benedetti et al., 2011).
SMRI studies also support other WM alterations in adult SAs, with early studies showing increased WM hyperintensities in both BD and MDD SAs (Ehrlich et al., 2005; Pompili et al., 2008; Serafini et al., 2011). More recently, diffusion tensor imaging (DTI) studies have shown diminished structural integrity of WM that provides fronto-limbic connections in SAs with BD or with MDD. For instance, decreased fractional anisotropy (FA) (a measure of white matter structural integrity) has been shown within ventral frontal WM in SAs with BD and MDD, including within the region of the uncinate fasciculus (UF), which carries major ventral PFC-amygdala connections, with an association to impulsivity in BD (Carballedo et al., 2012; Mahon et al., 2012). In MDD, SAs showed similar WM FA reductions, as well as reductions in dorsomedial frontal regions. For example, lower FA values in ventral frontal and medial frontal (including dorsomedial) regions were observed in the SAs with MDD (Jia, 2013; Olvet et al., 2014), who also showed altered WM in frontal cortex–basal ganglia connections (Jia, 2013; Jia et al., 2010). Decreases in WM volume in the external capsule have also to date been reported only in SAs with MDD (Hwang et al., 2010).
Few neuroimaging studies have focused on suicide behaviors in adolescents and young adults. In a study of adolescent and young adult SAs with BD (Johnston et al., 2017), GM volume reductions were observed in ventral PFC and hippocampus; WM FA reductions were observed in WM that carries connections from ventral PFC, including in the region of the UF. In adolescent and young adult SAs with MDD, GM decreases were observed in a rostral PFC region (Fradkin et al., 2017) that overlapped a region of dysfunction previously observed in adolescent and young adult SAs with BD (Johnston et al., 2017).
Here, we studied adolescents and young adults both with BD and with MDD, with both sMRI and DTI methods, to compare GM and WM between SAs with NSAs across and within each disorder. We aimed to identify brain circuitry features common across BD and MDD SAs and to study potential features distinct to SAs within each disorder. We tested the hypothesis that SAs across BD and MDD would show common decreases in GM volume and WM FA in the frontotemporal regions of fronto-limbic circuitry, and explored for potential differences in patterns for SAs within each diagnosis.
Methods
Subjects
Forty-six adolescents/young adults with BD and 37 with MDD (ages 14-25 years; 85% female) participated in the study. Twenty-one (45.7%) of BD and 19 (52.8%) of MDD participants who had at least one suicide attempt comprised the SA group. Written informed consent was obtained from participants (≥18 years), and written assent and parent/guardian permission were obtained from minors (<18 years), in accordance with the Yale University institutional review board. To confirm the presence or absence of DSM-IV axis I diagnoses and mood state at scanning, the Structured Clinical Interview for DSM-IV was used for participants ≥18 years of age, and the Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al., 1997) was used for participants <18 years. No subjects had major medical or neurological disorders including a history of loss of consciousness for more than 5 minutes, except two BD subjects with treated hypothyroidism. Mood disorder history in first degree relatives (FDRs) was assessed using the Family History Screen for Epidemiological Studies (Lish et al., 1995). In the BD SA group, 5 (23.8%) FDRs had BD and 7 (33.3%) MDD; in the BD NSA group, 9 (36%) FDRs had BD and 9 (36%) had MDD. In the MDD SA group 6 (31.6%) FDRs had BD and 5 (26.3%) had MDD; in the MDD NSA group, 4 (22.2%) FDRs had BD and 6 (33.3%) MDD. Forty-five participants (56%) reported taking psychotropic medication at the time of scanning, 19 (23.5%) had a lifetime comorbid substance abuse or dependence disorder (no subject met criteria for a substance use disorder within five months of study), 55 (67.9%) were euthymic, 20 (24.7%) were depressed and 16 (19.8%) were in a mixed mood state, and 20 (24.7%) had lifetime psychosis (9 of the 21 BD SAs (42.9%), 9 of the 25 BD NSAs (36%) and 2 of the 19 MDD SAs (10.5%)). Nineteen of the 46 (41.3%) BD participants (11 of the 21 BD SAs (52%) and 8 of the 25 BD NSAs (32%)) had rapid cycling (see Table 1 for further participant details).
TABLE 1.
Demographic and Clinical Characteristic of Subjects with Bipolar Disorder and Major Depressive Disorder.
| Bipolar Disorder with Attempts (N =21) |
Bipolar Disorder without Attempts (N=25) |
Major Depressive Disorder with Attempts (N=19) |
Major Depressive Disorder without Attempts (N=18) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 20.3 | 3 | 20.2 | 3.4 | 18.7 | 3 | 18.9 | 2.7 |
| N | % | N | % | N | % | N | % | |
| Female | 18 | 86 | 21 | 84 | 16 | 84 | 15 | 83 |
| Unmedicated | 8 | 38.1 | 11 | 44 | 5 | 26.3 | 12 | 70.6 |
| Medications | ||||||||
| Lithium Carbonate | 3 | 14.3 | 3 | 12 | - | - | - | - |
| Anticonvulsants | 6 | 28.6 | 8 | 32 | 4 | 21.1 | - | - |
| Antipsychotics | 6 | 28.6 | 13 | 52 | 7 | 36.8 | 3 | 16.7 |
| Antidepressants | 4 | 19 | 3 | 12 | 12 | 63.2 | 3 | 16.7 |
| Stimulants | 8 | 38.1 | 4 | 16 | 5 | 26.3 | 1 | 5.6 |
| Benzodiazepines | 5 | 23.8 | 2 | 8 | 3 | 15.8 | - | - |
| Levothyroxine | 1 | 4.8 | 1 | 4 | - | - | - | - |
| Adrenergic agonists | 1 | 4.8 | 3 | 12 | 3 | 15.8 | - | - |
| Non-benzodiazepine Hypnotic | - | - | 1 | 4 | - | - | - | - |
| Substance Use Disorder Comorbidity | ||||||||
| Alcohol Abuse | 1 | 4.8 | 3 | 12 | - | - | 1 | 5.6 |
| Alcohol Dependence | 1 | 4.8 | - | - | - | - | - | - |
| Cannabis Abuse | 2 | 9.5 | 2 | 8 | - | - | 1 | 5.6 |
| Cannabis Dependence | 2 | 9.5 | 2 | 8 | - | - | - | - |
| Cocaine Abuse | 1 | 4.8 | - | - | - | - | - | - |
| Cocaine Dependence | - | - | 2 | 8 | - | - | - | - |
| Polysubstance Dependence | - | - | 1 | 4 | - | - | - | - |
| Other Lifetime Psychiatric Comorbidity | ||||||||
| Panic Disorder | 4 | 19 | 2 | 8 | 1 | 5.3 | 3 | 16.7 |
| Post Traumatic Stress Disorder | 1 | 4.8 | 1 | 4 | 2 | 10.5 | - | - |
| Social Phobia | 1 | 4.8 | 1 | 4 | - | - | - | - |
| Specific Phobia | 1 | 4.8 | - | - | - | - | - | - |
| Obsessive Compulsive | 1 | 4.8 | - | - | - | - | - | - |
| Anorexia Nervosa | 1 | 4.8 | 1 | 4 | 1 | 5.3 | 1 | 5.6 |
| Bulimia Nervosa | - | - | 2 | 8 | - | - | - | - |
| Attention Deficit/Hyperactivity* | 1 | 4.8 | 5 | 20 | - | - | - | - |
Assessed only in individuals <18 years of age, N (% adolescents).
Suicide attempts (defined as self-injurious acts committed with at least some intent to die) were assessed using the Columbia Suicide History Form (Oquendo et al., 2004). Medical lethality of attempts were assessed using the Beck Medical Lethality Scale (for both “maximum lethality” and “most recent lethality”) (Beck et al., 1975). Suicidal ideation severity was assessed with the Beck Scale for Suicide Ideation (for both “most severe ideation” and “most recent ideation”) (Beck et al., 1979) and past intent to die was assessed using the Suicide Intent Scale (for both “most lethal attempt” and “most recent lethal attempt”) (Beck et al., 1975). Additionally, Beck Hopelessness Scale (Beck et al., 1974) and Barratt Impulsiveness Scale, Version 11 (Patton et al., 1995) were used to assess for hopelessness and impulsivity respectively.
MRI Acquisition
SMRI and DTI data were acquired using a single 3-Tesla Siemens Trio MR scanner (Siemens, Erlangen, Germany). Sagittal sMRI images were obtained with a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence (TR = 1,500ms, TE=2.83ms, matrix=256×256, field of view = 256×256mm2, 160 1-mm slices without gap and two averages). DTI data was obtained with 32 noncolinear directions (b=1,000 seconds/mm2) and an acquisition without diffusion weighting b=0 was acquired with alignment of the anterior commissure-posterior commissure plane (TR=7,400ms, TE=115ms, matrix= 128×128, field of view=256×256mm2, 40 3-mm slices without gap).
SMRI Processing
SPM 8 (http://www.fil.ion.ucl.ac.uk/spm) was used to process images. The segmentation function was used for bias correction, spatial normalization, and segmentation of the original structural images in the same model. A modulation step was used in normalization to ensure the overall tissue amount was not altered. An 8-mm full width at half maximum isotropic kernel was applied to smooth GM images (Wang et al., 2011).
DTI Processing
Diffusion-weighted data were processed with interpolation to 1.5-mm isotropic voxels and denoised by a three-dimensional isotropic sigma 2-mm full width at half maximum Gaussian kernel. Diffusion eigenvectors and corresponding eigenvalues (lambda 1, lambda 2, and lambda 3) were subsequently acquired after diagonalizing the diffusion-weighted data. FA was calculated and the whole-brain FA maps were normalized with SPM 8 to Montreal Neurological Institute (MNI) space using a tissue probability map of WM template resampled to 2×2×2mm3 during normalization and spatially smoothed at 10-mm full width at half maximum (Wang et al., 2009).
Statistical Analyses
Potential between-group differences, across the four subgroups (SAs with BD, SAs with MDD, NSAs with BD, and NSAs with MDD), in age and gender were assessed using one-way analysis of variance (ANOVA) and chi-square tests respectively. Within each diagnostic group (BD or MDD), two-sample t-tests and chi-square tests were used to assess potential differences between the SAs and the NSAs in medication status (on or off), lifetime comorbid substance abuse or dependence (yes or no), mood state (euthymic, depressed, mixed), psychosis, rapid cycling, medical lethality of attempts, suicide ideation severity, suicide intent, hopelessness and impulsivity.
Imaging Analyses
For primary hypothesis testing, separately for GM and FA, two-by-two full factorial models were built using SPM 8 to examine the main effect of attempt status (SA, NSA), effects of attempt status within each diagnosis, and interactions between attempt status and diagnosis, with age as a co-variate. For main effect and within diagnostic comparisons for attempt status, results in GM volume in the hypothesized frontal circuitry subserving emotion and impulse control, including PFC and mesial temporal regions, and in FA in WM carrying connections between these regions, including the UF, were considered significant at p<0.005 (uncorrected) and with a spatial extent of 20 contiguous voxels. Similar analyses were performed excluding subjects with MDD with a FDR with BD. For exploratory analyses for interactions between attempt status and diagnosis, and for brain regions outside of a priori hypothesized regions, findings were considered significant with p<0.05 family-wise error (FWE)-corrected and an extent threshold of 10 voxels (Emsell et al., 2013).
Mean GM volume and WM FA, calculated from extracted values in clusters showing significant differences between groups, were assessed post hoc to determine the nature of group differences and to compare in exploratory analyses among clinical factors. For the latter, two-sample t-tests were used to test for differences in medication status, lifetime alcohol or other substance use disorder, psychosis, or rapid cycling, and by one-way ANOVA for mood state. Pearson correlations were used to assess potential associations between GM volume and FA with suicide-related symptoms and behaviors (maximum lethality of attempts, most recent lethality of attempts, maximum intent, intent for most recent attempt, most severe ideation, most recent ideation, current hopelessness and impulsivity). Correlational analyses were only performed in the SAs for lethality and intent measures. For the other suicide-related symptoms/behaviors correlational analyses were performed for all subjects, with partial correlations estimated after controlling for group (SA, NSA) using ANCOVA. Given the exploratory nature of analyses, correlations were considered significant at p<0.05, uncorrected.
Results
Distribution of age and gender were similar between groups (see Table 1). As expected, subjects with suicide attempts had greater suicide ideation (most severe) (t= 7.49, df=73, p<0.001). Differences were not observed in suicide ideation severity (most severe) between the SAs with BD compared to the SAs with MDD (p=0.69). No between group differences (overall SAs vs. NSAs and SAs vs. NSAs within each diagnosis) were observed for medication status, lifetime comorbid substance use disorders, mood state, psychosis, rapid cycling, hopelessness and impulsivity. No between group differences (SAs with BD vs. SAs with MDD) were found for (most recent) suicide ideation, medical lethality of attempts and suicide intent (see Table 2).
TABLE 2.
Suicide Scales of Subjects with Bipolar Disorder and Major Depressive Disorder.
| Bipolar Disorder with Attempts (N =21) |
Bipolar Disorder without Attempts (N=25) |
Major Depressive Disorder with Attempts (N=19) |
Major Depressive Disorder without Attempts (N=18) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Beck Scale for Maximum Lethality of Suicide Attempts* | 2.4 | 1.8 | - | - | 2.3 | 2.1 | - | - |
| Beck Scale for Most Recent Lethality of Suicide Attempts* | 2.3 | 1.7 | - | - | 2.1 | 2 | - | - |
| Beck Scale for Suicide Ideation (Most Severe Ideation) | 17.3 | 9.7 | 6 | 8.3 | 18.5 | 8.8 | 0.43 | 1.6 |
| Beck Scale for Suicide Ideation (Most Recent Ideation)* | 5.7 | 9.6 | - | - | 7.6 | 9.6 | - | - |
| Beck Scale for Suicide Intent Scale (Most Lethal Attempt)* | 11.3 | 5.4 | - | - | 12.7 | 7.1 | - | - |
| Suicide Intent Scale (Most Recent Lethal Attempt)* | 10.2 | 1.7 | - | - | 9.6 | 7.8 | - | - |
| Beck Hopelessness Scale | 7.2 | 5.8 | 5.6 | 4.4 | 5.8 | 7 | 4.1 | 3.6 |
| Barratt Impulsiveness Scale-11 Total | 73.5 | 13.8 | 69.3 | 10.6 | 66.2 | 9.7 | 73.6 | 13.8 |
Assessed only in individuals with suicide attempts.
Main Effects of Suicide Attempt Status Within Imaging Modalities
sMRI
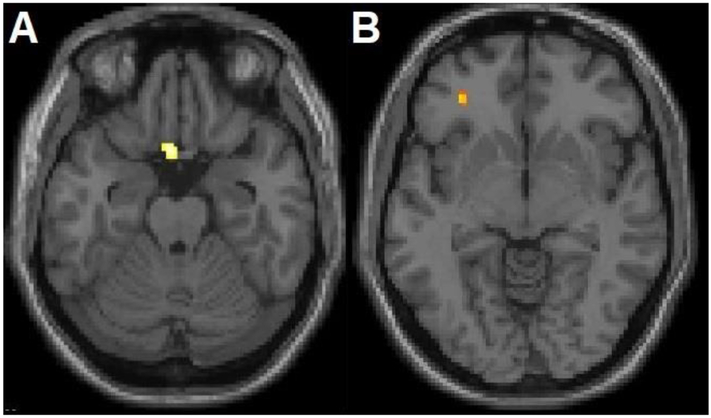
Within a priori hypothesized regions, a significant main effect of suicide attempt status in GM volume was observed in two clusters in the left ventral PFC (Brodmann area [BA] 11, Montreal Neurological Institute (MNI) space coordinates: x=−4mm, y=14mm, z=−24mm, cluster=73 voxels and BA47, x=−32mm, y=40mm, z=−8mm, cluster=22 voxels), hereafter to be called the “main ventral PFC regions” (Figure 1). The extracted values in the BA11 region revealed the effect was driven by GM volume decreases in SAs compared to the NSAs across diagnostic groups. Similar decreases were observed within BD and MDD diagnoses. Higher GM volume in region BA47 was observed among MDD SAs compared to each of the other 3 subgroups (MDD NSAs, BD SAs, BD NSAs). GM volume in these regions did not show significant associations with the clinical and suicide-related factors explored. No significant differences in GM volume were observed outside of hypothesized regions.
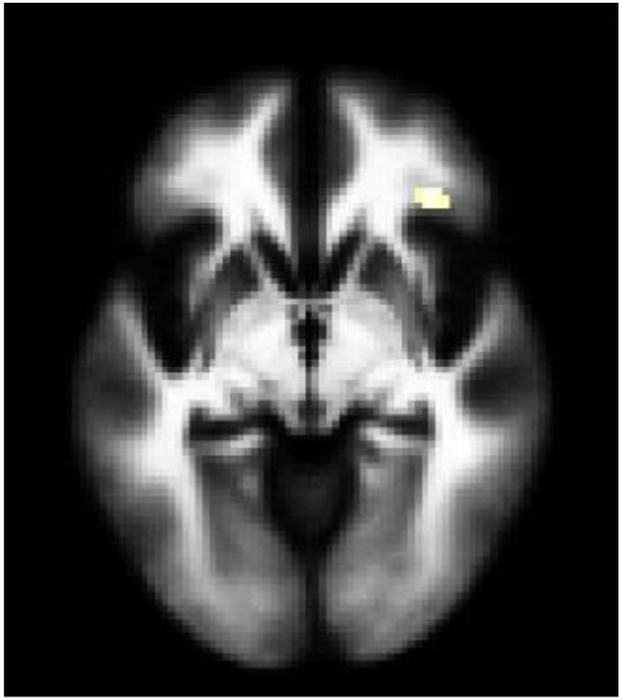
Figure 1. Grey Matter Volume Differences between Adolescents and Young Adults with Bipolar Disorder and Major Depressive Disorder with History of Suicide Attempts, Compared to Without Suicide Attempts.
The structural magnetic resonance T1 axial-oblique images display the left ventral prefrontal cortex regions where grey matter volume differed significantly between attempters and non-attempters (p<0.005 uncorrected and spatial extent of 20 contiguous voxels), including A) the region driven by lower grey matter volume in suicide attempters compared to non-suicide attempters across and within each disorder and B) the region driven by higher grey volume in the attempters with major depressive disorder compared to each of the other 3 subgroups (non-suicide attempters with major depressive disorder, suicide attempters with bipolar disorder, non-suicide attempters with bipolar disorder). The right side of the images is the right side of the brain.
DTI
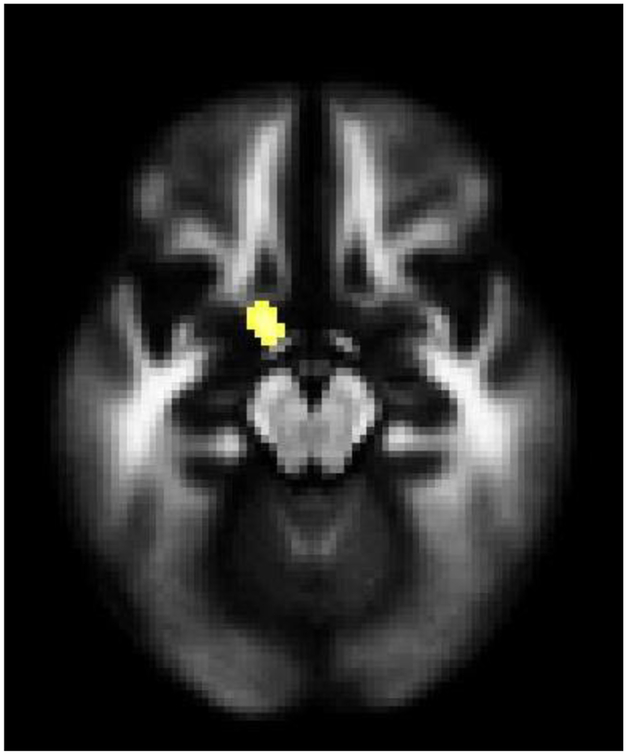
Within a priori hypothesized regions, a significant main effect of suicide attempt status in FA was demonstrated in a left frontotemporal WM region that included the region of the UF (Mori et al., 2008) (x=−12mm, y=2mm, z=−16mm, cluster=34 voxels) (Figure 2), hereafter to be called the “main UF region”, owing to lower WM FA in SAs compared to NSAs. Similar decreases were observed within BD and MDD diagnoses. Among SAs with BD, lower FA values in the main UF region were associated with greater suicide ideation (most recent ideation) (r=−0.53, p=0.02). No significant differences in WM FA were observed outside of hypothesized regions.
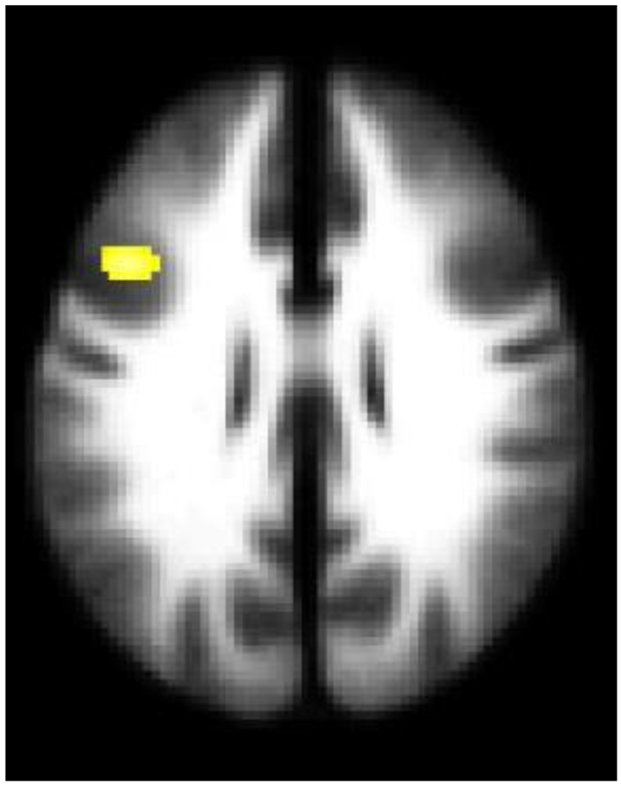
Figure 2. White Matter Fractional Anisotropy Differences between Adolescents and Young Adults with Bipolar Disorder and Major Depressive Disorder with History of Suicide Attempts, Compared to Without Suicide Attempts.
The axial-oblique image displays the left uncinate fasciculus region where white matter fractional anisotropy was significantly lower in attempters than non-attempters across and within each disorder (p<0.005 uncorrected and spatial extent of 20 contiguous voxels). The right side of the axial-oblique image is the right side of the brain.
Effects of Suicide Attempt Status within Diagnosis
sMRI
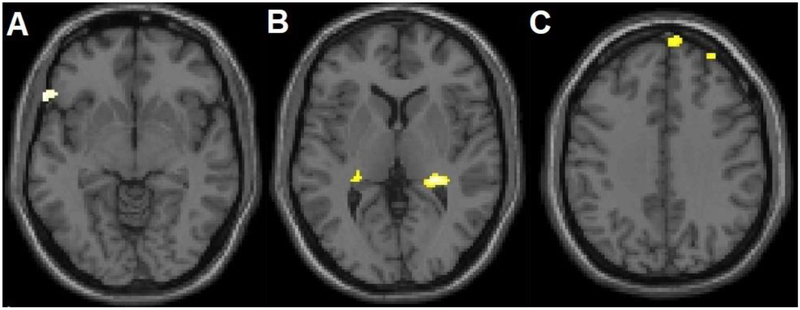
Within BD subjects, within a priori hypothesized regions, SAs showed significant GM volume reductions in the main left ventral PFC region (BA11, x=10, y=56, z=−20, cluster=50 voxels) compared to NSAs, as well as in lateral left ventral PFC (BA47, x=−60mm, y=24mm, z=−6mm, cluster=47 voxels), the right dlPFC and dorsomedial PFC (BA9, x=32mm, y=54mm, z=38mm, cluster=34 voxels; x=8mm, y=62mm, z=34mm, cluster=41 voxels), and bilateral hippocampus regions (x=26mm, y=−34mm, z=4mm, cluster=263 voxels; x=−26mm, y=−32mm, z=4mm, cluster=29 voxels) (Figure 3). No significant associations with clinical factors were observed. The SA group did not show any areas of greater GM volume in hypothesized regions. No significant differences in GM volume were observed outside of hypothesized regions.
Figure 3. Additional Grey Matter Volume Differences between Adolescents and Young Adults with Bipolar Disorder with History of Suicide Attempts, Compared to Without Suicide Attempts.
The structural magnetic resonance T1 axial-oblique images display additional regions where grey matter volume was significantly lower in attempters than non-attempters only within the bipolar disorder group, including A) left ventral lateral prefrontal cortex, B) bilateral hippocampus and C) right dorsomedial and dorsolateral prefrontal cortex (p<0.005 uncorrected and spatial extent of 20 contiguous voxels). The right side of the axial-oblique images is the right side of the brain.
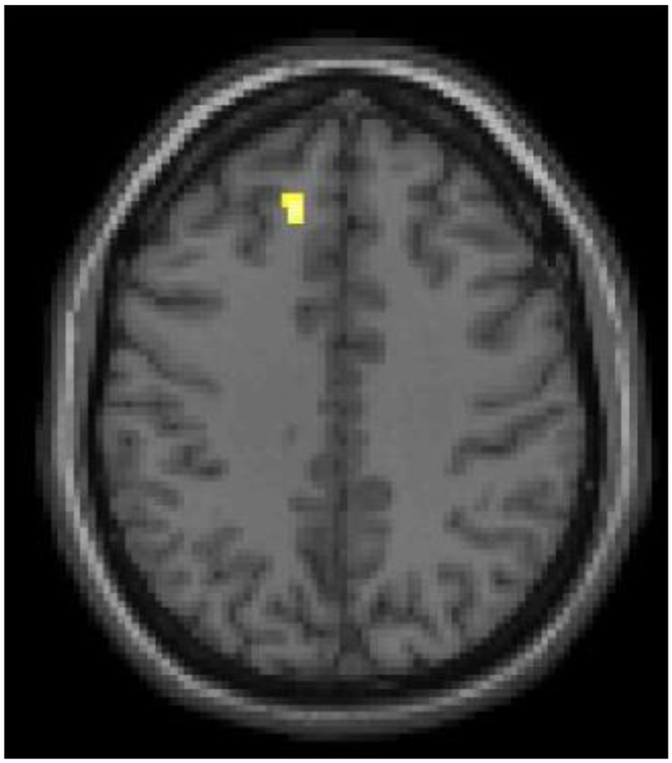
Within MDD, within a priori hypothesized regions, SAs showed significant GM volume reductions in the main left ventral PFC BA11 region (x=−4mm, y=14mm, z=−24mm, cluster=50 voxels), as well as in left dlPFC (BA9, x=−12mm, y=34mm, z=38mm, cluster=26 voxels) (Figure 4). Significantly increased GM volume was observed in the main left vlPFC BA47 region (x=−32mm, y=40mm, z=−8mm, cluster=34 voxels). No significant associations with clinical factors were observed. No significant differences in GM volume were observed outside of hypothesized regions.
Figure 4. Additional Grey Matter Volume Differences between Adolescents and Young Adults with Major Depressive Disorder with History of Suicide Attempts, Compared to Without Suicide Attempts.
The structural magnetic resonance T1 axial-oblique image displays the additional region where grey matter volume was significantly lower in attempters than non-attempters across only within the major depressive disorder group in left dorsal lateral prefrontal cortex region (p<0.005 uncorrected and spatial extent of 20 contiguous voxels). The right side of the image is the right side of the brain.
DTI
Within BD, within a priori hypothesized regions, SAs showed trend-level WM FA reductions, at threshold p=0.01, in the main left UF region (x=−24mm, y=8mm, z=−14mm, cluster=20 voxels), as well as in a right ventral frontal area that also included the UF (Mori et al., 2008) (x=36mm, y=32mm, z=6mm, cluster=35 voxels) (Figure 5). No significant associations with clinical factors were observed. The SA group did not show any areas of greater WM FA in hypothesized regions. No significant differences in WM FA were observed outside of hypothesized regions.
Figure 5. Additional White Matter Integrity Fractional Anisotropy Differences between Adolescents and Young Adults with Bipolar Disorder with History of Suicide Attempts, Compared to Without Suicide Attempts.
The axial-oblique image displays the additional region where white matter fractional anisotropy was significantly lower in attempters than non-attempters only within the bipolar disorder group in a right ventral lateral prefrontal cortex region (p<0.005 uncorrected and spatial extent of 20 contiguous voxels). The right side of the image is the right side of the brain.
Within MDD, within a priori hypothesized regions, SAs showed significant WM FA reductions in the left UF region (x=34mm, y=−12mm, z=−16mm, cluster=34 voxels), as well as in a left dorsal frontal region (x=−46mm, y=16mm, z=32mm, cluster=44 voxels) (Figure 6). No significant associations with clinical factors were observed. The SA group did not show any areas of greater WM FA in hypothesized regions. No significant differences in WM FA were observed outside of hypothesized regions.
Figure 6. White Matter Integrity Fractional Anisotropy Differences between Adolescents and Young Adults with Major Depressive Disorder with History of Suicide Attempts, Compared to Without Suicide Attempts.
The axial-oblique image displays the additional region where white matter fractional anisotropy was significantly lower in attempters than non-attempters across only within the major depressive disorder group in a left dorsal lateral prefrontal cortex region (p<0.005 uncorrected and spatial extent of 20 contiguous voxels). The right side of the image is the right side of the brain.
Interaction Effects Between Suicide Attempts and Diagnostic Groups
No significant interaction effects between suicide attempts and diagnostic groups in GM volume and WM FA were observed.
Analyses Excluding MDD Subjects with FDRs with BD
The main effects and effects within the MDD group of suicide status remained although at the p-threshold of 0.01. The interaction analyses with this sample did not reveal additional findings.
Discussion
We investigated GM volume and WM FA in adolescents and young adult SAs, compared to NSAs, with BD and MDD. Main effects of suicide attempt status were observed in GM volume in left ventral PFC regions (BAs11/47) and WM FA in a left ventral frontal region that included the UF. The main left BA11 GM and left UF WM group differences were decreases in the SAs, compared to the NSAs, that were consistent across diagnoses. Main effects of GM in the left BA47 of increases in the SAs, compared to the NSAs, were driven by the MDD SA group. Within BD, decreases in the SAs compared to the NSAs were observed within BD in an additional left ventrolateral PFC region (BA47), as well as in right dlPFC and dorsomedial PFC (BA9) and bilateral hippocampus GM volume and right ventral frontal WM FA. In the SAs with BD, there was a negative association between WM FA in the main left UF region and most recent suicide ideation. Within MDD, additional decreases in the SAs compared to the NSAs were in left dlPFC (BA9) GMV volume and dorsal frontal WM FA. No significant interaction effects between suicide attempts and diagnostic groups in GM volume and WM FA were observed.
We observed reductions in the GM volume in the ventral PFC, particular within medial orbitofrontal cortex (OFC) (BA11), in SAs across mood disorders and within each diagnosis. This region of OFC plays an essential role in emotion regulation processes implicated in the emotion symptoms of both BD and MDD (Blond and Blumberg, 2011; Monkul et al., 2007; Morgan et al., 1993). The finding is consistent with previous studies of suicide behavior in BD or in MDD that showed GM volume reduction in this region in SAs (Benedetti et al., 2011; Monkul et al., 2007). This suggests that impairments in emotion regulation circuitry may contribute to suicide behavior commonly to BD and MDD. We also observed commonly reduced ventral frontotemporal WM FA in an area of the UF in SAs across diagnoses. This WM provides major connections between the OFC and the amygdala, including reciprocal inhibitory connections critical to adaptive emotion regulation processes implicated in mood disorders (Cox Lippard et al., 2014; Olson et al., 2015; Thiebaut de Schotten et al., 2012). Lower FA in the UF region was associated with higher most recent suicide ideation in the BD group, suggesting that decreases in structural integrity in the WM in this region may contribute to suicidal ideation particularly within BD. Further research with larger samples is needed for more definitively investigate the association with suicide ideation across the disorder. This could be very important as, to date, there has been rare study of ideation, although suicide attempts following ideation have been demonstrated as the most significant predictor of further suicide attempts and eventual suicide (Ballard et al., 2016; Malhi et al., 2018; Thompson, 2010).
Though within diagnosis analyses were exploratory, they suggest that there may be some distinctions in the brain circuitry of suicide attempters with BD or MDD. In BD, the SAs compared to the NSAs showed decreases in a greater extent of the ventral PFC including the left BA11, as well as the left vlPFC (BA47) (in an area more ventral than the difference in the MDD SAs), and in WM FA bilaterally in UF regions. The vlPFC has critical roles in emotion regulation, such as in the adaptive regulation of emotions and associated behaviors during rapidly changing reinforcement contingencies (Aron et al., 2004; Kringelbach and Rolls, 2004; Rolls, 2004). The preliminary evidence here for more extensive and bilateral WM findings suggest that SAs with BD may also have greater ventral PFC connectivity abnormalities. In addition to structural connectivity, lower functional connectivity from the amygdala to the ventral PFC was observed in adolescent and young adult SAs with BD (Johnston et al., 2017). Pathology in the vlPFC has long been implicated in completed suicide, with postmortem studies showing alterations in serotonergic receptor systems in persons who died by suicide (Arango et al., 1995). Together, these findings suggest that ventral PFC and its connections may be important in risk for suicide, particularly in BD.
The SAs with BD also showed reduced GM volume in dlPFC and dorsomedial PFC (BA9). These regions are important in executive functions including top-down regulation of goal-direct behaviors, control of impulses, the cognitive flexibility to generate and weigh alternative strategies (Bechara and Van Der Linden, 2005; Kim and Lee, 2011; Miller and Cohen, 2001), and the allocation of attentional resources to mental states especially for emotional information (Walter et al., 2009). The findings are consistent with a previous study that showed GM volume reduction in the medial PFC associated with suicide behavior in BD (Ding et al., 2015). We speculate that the ventral frontotemporal regions may be more involved in contributions of emotion dysregulation to painful emotions that generate suicide ideation and intent, while alterations in the more dorsal PFC regions may be important in the constricted decision making and loss of behavioral control that can occur in the transition to performing suicide behavior (Benedetti et al., 2011; Ding et al., 2015; Miller and Cohen, 2001). Bilateral hippocampus GM was also reduced in BD SAs. The hippocampus has connections to the PFC regions and has roles in the above processes, including memory processes shown to be impaired in suicide attempters (Richard-Devantoy et al., 2015).
Within MDD, GM volume differences in the SAs in the left vlPFC (BA47) were increases. Increases in GM volume found in the ventral PFC in MDD have previously been associated with antidepressant medication (Benedetti et al., 2011). We did not observe associations between GM volume and antidepressant medication in this sample; however, power was limited. In MDD, the SAs showed GM volume reductions in the left dlPFC, an area where we and others have found evidence for trait abnormalities in MDD (Kerestes et al., 2012), lower GM volume was found in adult SAs with MDD (Hwang et al., 2010) and dysfunction was associated with lethality of attempts in individuals with MDD (Oquendo et al., 2003). Decreases in dlPFC GM were observed in SAs with BD and MDD; however, the hemispheric laterality of the findings differed in the two disorders. It is possible this reflects the greater right hemisphere abnormalities associated with manic and left with depressive symptoms (Blond and Blumberg, 2011; Blumberg et al., 2003) and may relate to differing characteristics of attempters between the disorders. Within MDD, reductions in WM FA were also observed in the area of the left dlPFC in the SAs compared to the NSAs. Further study of the regional localization and hemispheric laterality of differences in the PFC and its connections in SAs with BD or MDD warrants further study.
Study limitations include the modest sample size. Although we did not detect significant effects of gender, medication status, substance comorbidity, mood state or psychosis and suicide-related factors (except for most severe suicide ideation), the number of subjects in the subgroups for factors assessed was small, limiting the statistical power. There were not enough subjects to perform meaningful analyses for subtypes of medication and psychiatric comorbidities other than prior substance use disorder. The study included MDD subjects with FDRs with BD, which might have contributed to the findings of common effects across diagnoses. Although analyses excluding those subjects showed similar findings, as many of the subjects have not passed through the age of risk for developing BD, it is possible that more MDD subjects will develop BD and the findings would have limited applicability to MDD. We speculate that the observed GM and WM alterations in SAs with mood disorders in emotion regulation circuitry may suggest that emotion dysregulation and its underlying circuitry might increase suicide risk. However, as the SA subjects had already made an attempt, it is possible that the circuitry differences were results of the SAs. Longitudinal studies of individuals who are followed for subsequent SAs are needed to establish the relationship to risk. A significant association between the SA-related brain circuitry and impulsiveness as observed previously (Mahon et al., 2012) was not detected in this study, possibly owing to factors such as the sample size and that trait impulsiveness was assessed at scanning which may differ from impulsiveness closer to the time of the attempt. However, impulsive versus planned suicide attempts have been suggested to be different phenotypes (Oquendo, 2015) and it is possible that this study included a higher proportion of more planful attempters. Although age was included as a covariate, developmental brain changes could still affect results. Our results were reported with a primary threshold of p<0.005, in order to avoid false negative findings. However, caution should be taken when interpreting these results, considering them as preliminary with further study warranted.
In conclusion, this study demonstrated GM and WM alterations in fronto-limbic circuitry that subserves emotion regulation common to adolescent and young adult suicide attempters with both BD and MDD, suggesting emotion regulation and its underlying circuitry as potential targets for early prevention of suicide broadly across mood disorders. Preliminary evidence from this study also suggests that there may be some different patterns associated with SAs in each disorder in the extent of the ventral frontotemporal and additional dorsal frontal and hippocampus abnormalities. Further study is needed with larger samples for more definitive investigation of SAs across BD and MDD, and other disorders to assess generalizability and specificity, as well as with longitudinal designs to investigate the development of the fronto-limbic system features, their role in the transition form suicidal ideation to behavior and risk for completed suicide. However, this study suggests that ventral frontotemporal GM and WM alterations may be potential biomarkers for suicide prevention, particularly in adolescents and young adults with mood disorders.
Highlights.
Mood disorders, bipolar and major depressive, are major suicide risk factors.
Adolescent/young adult attempters with mood disorders have common brain circuitry.
Brain alterations common to attempters are potential targets to prevent suicide.
The brain circuitry associated with suicide attempts subserves emotion regulation.
Both grey and white matter brain alterations were associated with suicide attempts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu LN, Lafer B, Baca-Garcia E, Oquendo MA, 2009. Suicidal ideation and suicide attempts in bipolar disorder type I: an update for the clinician. Rev Bras Psiquiatr 31, 271–280. [DOI] [PubMed] [Google Scholar]
- Angst J, Angst F, Stassen HH, 1999. Suicide risk in patients with major depressive disorder. J Clin Psychiatry 60 Suppl 2, 57–62; discussion 75-56, 113-116. [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ, 1995. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res 688, 121–133. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA, 2004. Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8, 170–177. [DOI] [PubMed] [Google Scholar]
- Ballard ED, Vande Voort JL, Luckenbaugh DA, Machado-Vieira R, Tohen M, Zarate CA, 2016. Acute risk factors for suicide attempts and death: prospective findings from the STEP-BD study. Bipolar Disord 18, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Van Der Linden M, 2005. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol 18, 734–739. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beck R, Kovacs M, 1975. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. Am J Psychiatry 132, 285–287. [DOI] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A, 1979. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol 47, 343–352. [DOI] [PubMed] [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L, 1974. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol 42, 861–865. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Radaelli D, Poletti S, Locatelli C, Falini A, Colombo C, Smeraldi E, 2011. Opposite effects of suicidality and lithium on gray matter volumes in bipolar depression. J Affect Disord 135, 139–147. [DOI] [PubMed] [Google Scholar]
- Blond BN, Blumberg HP, 2011. Functional neuroimaging research in bipolar disorder. Curr Top Behav Neurosci 5, 227–245. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS, 2003. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry 60, 601–609. [DOI] [PubMed] [Google Scholar]
- Carballedo A, Amico F, Ugwu I, Fagan AJ, Fahey C, Morris D, Meaney JF, Leemans A, Frodl T, 2012. Reduced fractional anisotropy in the uncinate fasciculus in patients with major depression carrying the met-allele of the Val66Met brain-derived neurotrophic factor genotype. Am J Med Genet B Neuropsychiatr Genet 159B, 537–548. [DOI] [PubMed] [Google Scholar]
- Costa Lda S, Alencar AP, Nascimento Neto PJ, dos Santos Mdo S, da Silva CG, Pinheiro Sde F, Silveira RT, Bianco BA, Pinheiro RF Jr., de Lima MA, Reis AO, Rolim Neto ML, 2015. Risk factors for suicide in bipolar disorder: a systematic review. J Affect Disord 170, 237–254. [DOI] [PubMed] [Google Scholar]
- Cox Lippard ET, Johnston JA, Blumberg HP, 2014. Neurobiological risk factors for suicide: insights from brain imaging. Am J Prev Med 47, S152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Lawrence N, Olie E, Cyprien F, le Bars E, Bonafe A, Phillips ML, Courtet P, Jollant F, 2015. Prefrontal cortex markers of suicidal vulnerability in mood disorders: a model-based structural neuroimaging study with a translational perspective. Transl Psychiatry 5, e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Zeng J, Liu H, Tang D, Meng H, Li Y, Fu Y, 2017. Fronto-limbic disconnection in depressed patients with suicidal ideation: A resting-state functional connectivity study. J Affect Disord 215, 213–217. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Breeze JL, Hesdorffer DC, Noam GG, Hong X, Alban RL, Davis SE, Renshaw PF, 2005. White matter hyperintensities and their association with suicidality in depressed young adults. J Affect Disord 86, 281–287. [DOI] [PubMed] [Google Scholar]
- Emsell L, Langan C, Van Hecke W, Barker GJ, Leemans A, Sunaert S, McCarthy P, Nolan R, Cannon DM, McDonald C, 2013. White matter differences in euthymic bipolar I disorder: a combined magnetic resonance imaging and diffusion tensor imaging voxel-based study. Bipolar Disord 15, 365–376. [DOI] [PubMed] [Google Scholar]
- Fradkin Y, Khadka S, Bessette KL, Stevens MC, 2017. The relationship of impulsivity and cortical thickness in depressed and non-depressed adolescents. Brain Imaging Behav 11, 1515–1525. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF, 2005. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 62, 1097–1106. [DOI] [PubMed] [Google Scholar]
- Hawton K, Bergen H, Waters K, Ness J, Cooper J, Steeg S, Kapur N, 2012. Epidemiology and nature of self-harm in children and adolescents: findings from the multicentre study of self-harm in England. Eur Child Adolesc Psychiatry 21, 369–377. [DOI] [PubMed] [Google Scholar]
- Hwang JP, Lee TW, Tsai SJ, Chen TJ, Yang CH, Lirng JF, Tsai CF, 2010. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol 23, 171–184. [DOI] [PubMed] [Google Scholar]
- Jia Z, Huang X, Wu Q, Zhang T, Lui S, Zhang J, Amatya N, Kuang W, Chan RC, Kemp GJ, Mechelli A, Gong Q, 2010. High-field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am J Psychiatry 167, 1381–1390. [DOI] [PubMed] [Google Scholar]
- Jia Z, Wang Z, Huang X, et al. , 2013. Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. Psychiatry Neurosci 38 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JAY, Wang F, Liu J, Blond BN, Wallace A, Liu J, Spencer L, Cox Lippard ET, Purves KL, Landeros-Weisenberger A, Hermes E, Pittman B, Zhang S, King R, Martin A, Oquendo MA, Blumberg HP, 2017. Multimodal Neuroimaging of Frontolimbic Structure and Function Associated With Suicide Attempts in Adolescents and Young Adults With Bipolar Disorder. Am J Psychiatry 174, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Bhagwagar Z, Nathan PJ, Meda SA, Ladouceur CD, Maloney K, Matuskey D, Ruf B, Saricicek A, Wang F, Pearlson GD, Phillips ML, Blumberg HP, 2012. Prefrontal cortical response to emotional faces in individuals with major depressive disorder in remission. Psychiatry Res 202, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Bromet EJ, 2013. The epidemiology of depression across cultures. Annu Rev Public Health 34, 119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee D, 2011. Prefrontal cortex and impulsive decision making. Biol Psychiatry 69, 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET, 2004. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 72, 341–372. [DOI] [PubMed] [Google Scholar]
- Lish JD, Weissman MM, Adams PB, Hoven CW, Bird H, 1995. Family psychiatric screening instruments for epidemiologic studies: pilot testing and validation. Psychiatry Res 57, 169–180. [DOI] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Wu J, Ardekani BA, Szeszko PR, 2012. Relationship between suicidality and impulsivity in bipolar I disorder: a diffusion tensor imaging study. Bipolar Disord 14, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Outhred T, Das P, Morris G, Hamilton A, Mannie Z, 2018. Modeling suicide in bipolar disorders. Bipolar Disord. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z, 2011. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry 68, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD, 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, Sassi RB, Mallinger AG, Keshavan MS, Soares JC, 2007. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry 12, 360–366. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE, 1993. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett 163, 109–113. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J, 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40, 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Von Der Heide RJ, Alm KH, Vyas G, 2015. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev Cogn Neurosci 14, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Peruzzo D, Thapa-Chhetry B, Sublette ME, Sullivan GM, Oquendo MA, Mann JJ, Parsey RV, 2014. A diffusion tensor imaging study of suicide attempters. J Psychiatr Res 51, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, 2015. Impulsive versus planned suicide attempts: different phenotypes? J Clin Psychiatry 76, 293–294. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Lizardi D, Greenwald S, Weissman MM, Mann JJ, 2004. Rates of lifetime suicide attempt and rates of lifetime major depression in different ethnic groups in the United States. Acta Psychiatr Scand 110, 446–451. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Placidi GP, Malone KM, Campbell C, Keilp J, Brodsky B, Kegeles LS, Cooper TB, Parsey RV, van Heertum RL, Mann JJ, 2003. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry 60, 14–22. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Pompili M, Innamorati M, Mann JJ, Oquendo MA, Lester D, Del Casale A, Serafini G, Rigucci S, Romano A, Tamburello A, Manfredi G, De Pisa E, Ehrlich S, Giupponi G, Amore M, Tatarelli R, Girardi P, 2008. Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry 32, 1501–1507. [DOI] [PubMed] [Google Scholar]
- Richard-Devantoy S, Berlim MT, Jollant F, 2015. Suicidal behaviour and memory: A systematic review and meta-analysis. World J Biol Psychiatry 16, 544–566. [DOI] [PubMed] [Google Scholar]
- Rolls ET, 2004. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. Anat Rec A Discov Mol Cell Evol Biol 281, 1212–1225. [DOI] [PubMed] [Google Scholar]
- Serafini G, Pompili M, Innamorati M, Fusar-Poli P, Akiskal HS, Rihmer Z, Lester D, Romano A, de Oliveira IR, Strusi L, Ferracuti S, Girardi P, Tatarelli R, 2011. Affective temperamental profiles are associated with white matter hyperintensity and suicidal risk in patients with mood disorders. J Affect Disord 129, 47–55. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M, 2012. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48, 82–96. [DOI] [PubMed] [Google Scholar]
- Thompson AH, 2010. The suicidal process and self-esteem. Crisis 31, 311–316. [DOI] [PubMed] [Google Scholar]
- van Heeringen C, Bijttebier S, Godfrin K, 2011. Suicidal brains: a review of functional and structural brain studies in association with suicidal behaviour. Neurosci Biobehav Rev 35, 688–698. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlosser RG, 2011. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? Neuroimage 54, 1607–1614. [DOI] [PubMed] [Google Scholar]
- Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlosser RG, 2012. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J Psychiatr Res 46, 1449–1455. [DOI] [PubMed] [Google Scholar]
- Walter M, Matthia C, Wiebking C, Rotte M, Tempelmann C, Bogerts B, Heinze HJ, Northoff G, 2009. Preceding attention and the dorsomedial prefrontal cortex: process specificity versus domain dependence. Hum Brain Mapp 30, 312–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, Blumberg HP, 2009. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 66, 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, Womer FY, Edmiston EE, Chepenik LG, Chen R, Spencer L, Blumberg HP, 2011. Olfactocentric paralimbic cortex morphology in adolescents with bipolar disorder. Brain 134, 2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zametkin AJ, Alter MR, Yemini T, 2001. Suicide in teenagers: assessment, management, and prevention. JAMA 286, 3120–3125. [DOI] [PubMed] [Google Scholar]