Abstract
Since the discovery of the first trypsinogen mutation in families with hereditary pancreatitis, the field of pancreatic genetics has made rapid progress. The identification of mutations in genes involved in the digestive protease/antiprotease pathway has lent additional support to the notion that pancreatitis is a disease of autodigestion. Clinical and experimental observations provided compelling evidence that premature, intrapancreatic activation of digestive proteases is critical in pancreatitis onset. Disease course and severity, however, are mostly governed by inflammatory cells that drive local and systemic immune responses. Here we review the genetics, cell biology and immunology of pancreatitis with a focus on protease activation pathways and other early events.
Keywords: trypsinogen, pancreatitis, genetics, inflammation, cell death
INTRODUCTION:
Pancreatitis is the leading cause for GI-disease related hospital admissions and it is associated with considerable morbidity, mortality and socioeconomic burden1. Recent years shed light on the pathophysiology of pancreatitis opening up new avenues for causal treatment. In this review article, we dissect the complexity of premature protease activation and its effect on local and systemic inflammation in pancreatitis.
GENETICS OF PANCREATITIS
Acute pancreatitis (AP), recurrent acute pancreatitis (RAP) and chronic pancreatitis (CP) form a disease continuum2. The progression of a sentinel attack of AP to RAP and eventually to CP is often driven by chronic alcohol consumption or genetic risk factors. Genetic risk for RAP and CP overlaps, while genetic studies in AP are difficult to interpret in the absence of adequate follow-up that can exclude RAP and CP cases.
The majority of the pancreatitis risk genes codes for digestive proteases, a trypsin inhibitor or other proteins highly expressed in the pancreas. Functional studies classified the various mutations and other genetic alterations into pathological pathways driving pancreatitis onset and progression. Here we discuss the trypsin-dependent, misfolding-dependent and ductal pathways of pancreatitis risk.
THE TRYPSIN-DEPENDENT PATHWAY OF GENETIC RISK IN CP
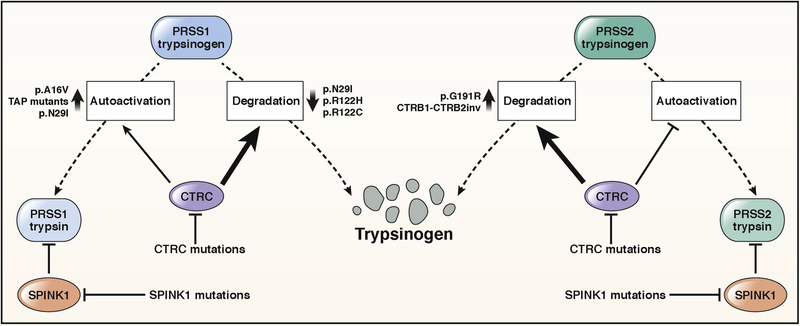
Pancreatic acinar cells secrete digestive proteases in inactive precursor forms that are flushed from the ductal system in a sodium bicarbonate-rich fluid. Trypsinogen, the precursor to trypsin, becomes activated by the serine protease enteropeptidase in the duodenum3. Trypsin activates chymotrypsinogens, proelastases and procarboxypeptidase B1 (CPB1) while activation of procarboxypeptidases A1 (CPA1) and A2 (CPA2) requires the concerted action of trypsin and chymotrypsin C (CTRC)4. Trypsinogen can be also activated by trypsin, and this process is called autoactivation3. Premature, intra-pancreatic activation of trypsinogen may occur via autoactivation or may be catalyzed by the lysosomal cysteine protease cathepsin B. Protective mechanisms that prevent trypsinogen activation in the pancreas include trypsin inhibition by the serine protease inhibitor Kazal type 1 (SPINK1) and trypsinogen degradation by CTRC and cathepsin L5–7. Although the principal action of CTRC is to promote trypsinogen degradation, it also enhances trypsinogen activation by processing the trypsinogen activation peptide to a shorter form, which is more sensitive to trypsin-mediated activation7–9 (Figure 1). As discussed below, certain trypsinogen mutations can hijack this mechanism and thereby stimulate trypsinogen activation to a pathological extent. Human genetic studies strongly support trypsinogen autoactivation and CTRC-dependent trypsinogen degradation as key mechanisms determining intrapancreatic trypsin activity whereas similarly compelling genetic evidence for the role of cathepsins B and L has been lacking.10.
Figure 1.
Genetic risk factors associated with the trypsin-dependent pathological pathway. See text for details.
PRSS1 mutations.
Mutations in human cationic trypsinogen cause autosomal dominant hereditary pancreatitis with incomplete penetrance or act as risk factors in sporadic CP7,11. Around 90% of PRSS1-mutation positive HP families carry the p.N29I, p.R122C, or p.R122H mutation in the heterozygous state. Mechanistically, the p.R122C and p.R122H mutations prevent CTRC-mediated trypsinogen degradation9. The p.N29I mutation has multiple distinct effects on trypsinogen biochemistry, the combination of which markedly increases trypsinogen autoactivation. These effects include an increase in N-terminal processing, decreased CTRC-dependent degradation and a slightly increased propensity for autoactivation9. The p.A16V variant, sensitizes the activation peptide of trypsinogen to CTRC-mediated processing, which, in turn, enhances autoactivation8,9. Pathological trypsin levels generated by mutation p.A16V are lower than those seen with the p.R122H variant, which explains the reduced penetrance of the p.A16V variant. More recently, mutation p.P17T was found to exhibit characteristics that were similar to those of p.A16V12. Rare mutations affecting the activation peptide of cationic trypsinogen (p.D19A, p.D21A, p.D22G, p.K23R and p.K23_I24insIDK) robustly stimulate autoactivation independently of CTRC13–15. Cell culture experiments indicate that these activation peptide mutants are secreted poorly due to intracellular activation and degradation, which can lead to cellular stress and consequent acinar cell death16. Taken together, PRSS1 mutations stimulate activation of cationic trypsinogen by reducing CTRC-dependent trypsinogen degradation, increasing CTRC-mediated processing of the activation peptide or directly stimulating autoactivation. GWAS studies identified a commonly occurring haplotype in the PRSS1-PRSS2 locus that slightly decreases CP risk (OR 1.5) with a more pronounced effect in alcoholic CP17–19. A variant (c.−204C>A) that lies in the promoter region of PRSS1 and reduces trypsinogen expression appears to be responsible for this small protective effect20.
SPINK1 mutations.
The association between the most common p.N34S SPINK1 variant and CP was first described by a candidate gene study in 200021. A meta-analysis reported a carrier frequency of 9.7% in CP patients and 1% in controls with an average odds ratio (OR) of 11, making the p.N34S the clinically most significant risk factor for CP22. When considering European populations only, p.N34S increases CP risk by about 10-fold23. Although several studies attempted to identify the functional effect of p.N34S and its associated haplotype, the molecular mechanism underlying CP risk remains unclear. Neither p.N34S nor any of the four linked intronic variants affect trypsin inhibitory function or cellular expression of SPINK124–27. Interestingly, in pancreatic cancer cell lines carrying the heterozygous p.N34S variant reduced expression of the mutant allele was observed in comparison to the wild-type allele28. The authors suggested that the c.−4141G>T variant or a hitherto unknown variant located in the 5’ region of the gene may be responsible for the reduced expression of the p.N34S allele. The second most frequently reported SPINK1 haplotype in CP contains the c.−215G>A promoter variant and the c.194+2T>C variant in intron 321,29. This haplotype was observed more frequently in East Asia than in Europe7. Functional studies revealed that the c.194+2T>C variant causes skipping of exon 3, which results in diminished SPINK1 expression27,30,31. However, the c.−215G>A variant increases promoter activity, which might mitigate the effect of the c.194+2T>C mutation and allow for some residual SPINK1 expression even in homozygous carriers32,33. Finally, a large number of rare or private alterations in SPINK1 have been found in CP, which cause loss of SPINK1 function by various mechanisms7.
Protective anionic trypsinogen (PRSS2) variant.
Although PRSS1 and PRSS2 share 90% identity at the amino acid level and PRSS2 rapidly autoactivates, no pathogenic PRSS2 variants were identified in HP or sporadic CP34,35. The absence of PRSS2 mutations in CP may be due to the more effective CTRC-mediated degradation of anionic trypsinogen, which would prevent intra-pancreatic activation of the enzyme even if it were mutated36. However, a protective variant p.G191R with a ~3–6-fold effect and circa 5% population frequency was discovered35,37. The mutation introduces a new trypsin cleavage site into anionic trypsinogen, which increases autocatalytic proteolysis and inactivation35.
CTRC mutations.
Direct DNA sequencing of the CTRC gene in patients with nonalcoholic CP revealed heterozygous mutations in 4% of patients that increased CP risk by 5-fold on average38,39. The mutations cause loss of CTRC function by various mechanisms, which include defective secretion due to misfolding, resistance to trypsin-mediated activation, catalytic deficiency or increased degradation by trypsin40,41. Considering the clinically significant variants, p.A73T exhibits a severe secretion defect, p.K247_R254del is inactive and prone to degradation, p.R254W is degraded by trypsin and p.V235I has partially reduced activity40. Subsequent studies reported a frequent p.G60= variant found in about 30% of CP patients42–45. The heterozygous p.G60= increases the risk of CP by 2.5-fold, while the homozygous state by 10-fold43,45. The variant is associated with reduced CTRC mRNA expression (GTEx Portal), possibly due to altered pre-mRNA splicing.
CTRB1-CTRB2 locus inversion.
A recent European GWAS study identified a large inversion at the CTRB1/CTRB2 locus that modestly (OR 1.35) modifies the risk for alcoholic and nonalcoholic CP19. The inversion changes the expression ratio of the CTRB1 and CTRB2 chymotrypsin isoforms in such a manner that protective trypsinogen degradation is increased and CP risk is reduced. In China the reported population frequency of the inverted (major) allele is 99.6%, thus the allele is virtually fixed and does not contribute to CP risk46. A mouse model with genetic deletion of the major mouse chymotrypsin CTRB1 exhibited increased intra-acinar trypsin activation and more severe pancreatitis induced by the secretagogue caerulein47. These observations provided the first in vivo proof for the protective role of chymotrypsin-mediated trypsinogen degradation against pancreatitis.
THE MISFOLDING-DEPENDENT PATHWAY OF GENETIC RISK IN CP
More recently, an alternative pathomechanism seemingly unrelated to premature intra-pancreatic trypsinogen activation has been identified, in which mutation-induced misfolding and consequent endoplasmic reticulum (ER) stress lead to acinar cell damage and pancreatitis48.
Misfolding-associated PRSS1 mutations.
In 2009 it was demonstrated that a subset of PRSS1 variants cause reduced secretion, intracellular retention and elevated ER stress markers, as judged by in vitro cell culture experiments49. These PRSS1 mutations occur rarely and are mostly associated with sporadic disease (e.g., p.C139F, p.C139S, p.G208A), but were also found in HP families with incomplete penetrance (p.L104P, p.R116C)48. Variant p.G208A is prevalent in East Asia (4% of CP cases) and was detected in Europe only in a single case so far50,51.
Misfolding-associated CPA1 mutations.
A candidate gene study in 2013 revealed that mutations in the CPA1 gene are associated with CP (OR ~25), especially with early-onset disease (OR~80)52. The vast majority of pathogenic CPA1 variants occur with low frequency and are mostly found in sporadic CP. The p.S282P variant was described in two HP families53. Pathogenic CPA1 variants cause proenzyme misfolding resulting in a secretion defect, intracellular retention and ER stress52,53. In contrast to CPA1, variants of CPB1 and CPA2 are not associated with CP54. Interestingly, ER stress-inducing CPA1 and CPB1 variants were overrepresented in pancreatic cancer patients without a clinical history of RAP or CP55. Most of these variants caused premature truncation and did not overlap with those found in CP. A mouse model for the misfolding-dependent pathway was described recently. This study demonstrates that CPA1 N256K knock-in mice harboring the most frequent p.N256K human CPA1 mutation develop spontaneous and progressive CP and exhibit signs of ER stress in their pancreas56.
Misfolding-associated CEL mutations and CEL-HYB allele.
Single-nucleotide deletions in the last exon of the CEL gene encoding carboxyl ester lipase cause maturity-onset diabetes of the young type 8 (MODY8)57. The deletions alter the reading frame of the C-terminal variable number tandem repeat (VNTR) sequence resulting in CEL proteins with unnatural extensions that are prone to aggregation58,59. The exocrine dysfunction in MODY8 is in all likelihood caused by misfolding-induced ER stress and consequent acinar cell loss. A hybrid CEL allele (CEL-HYB1) formed between CEL and its neighboring pseudogene CELP was found about 5-fold overrepresented in idiopathic CP versus the average population frequency of 0.5–1%60. In cell culture experiments, the hybrid protein was secreted poorly due to intracellular retention, suggesting that the CEL-HYB1 variant may increase CP risk via the misfolding-dependent pathway. A second hybrid CEL allele (CEL-HYB2) that does not associate with CP was described in Asian populations61. Interestingly, the CEL protein carries blood group antigens and a GWAS study in 2015 indicated that fucosyltransferase 2 (FUT2) non-secretor status and blood group B are risk factors for CP62. Although other studies in ethnically mixed cohorts failed to replicate this association63,64 with the exception of azathioprine-induced pancreatitis in IBD patients65 it is still interesting to speculate that the observed effects may have been due to changes in CEL folding or trafficking.
THE DUCTAL PATHWAY OF GENETIC RISK IN CP
CFTR variants.
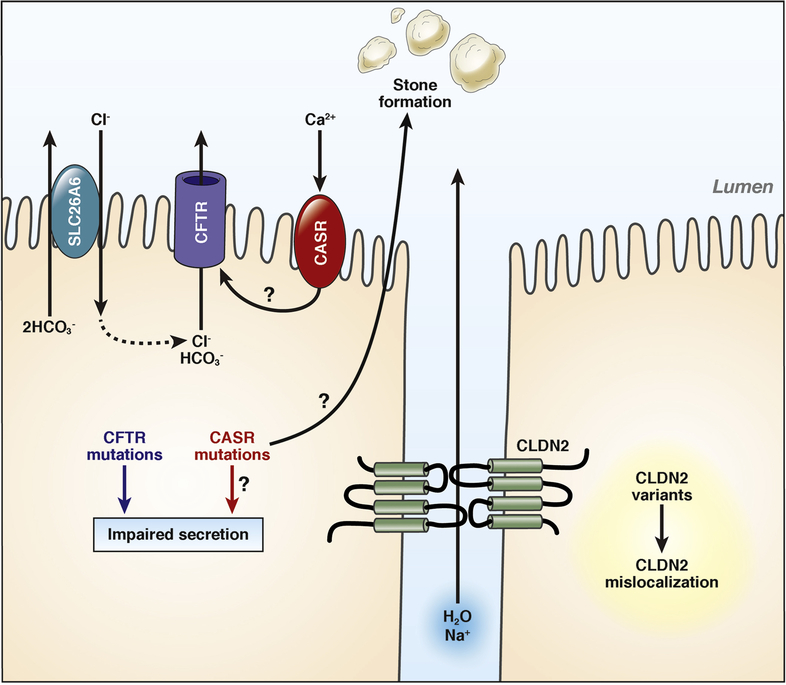
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cyclic AMP-regulated chloride/bicarbonate channel localized to the apical plasma membrane of epithelial cells66 (Figure 2). CFTR mutations disrupt channel activity or affect membrane levels and are associated with various phenotypes, ranging from asymptomatic state to multi-organ symptoms leading to the diagnosis of cystic fibrosis (CF) in homozygous carriers of severe mutations. Observations that heterozygous and compound heterozygous CFTR mutations are associated with CP were reported by two papers in 199867,68. In the first analysis of the entire CFTR coding region, the frequency of abnormal CFTR alleles in CP patients was 18.6% in comparison to 9.2% in controls69. More recent, large cohort analyses corroborate the pathogenic role of CFTR variants in CP although the effect and frequency of CFTR variants was less pronounced than reported previously39,70,71. Heterozygous carrier status of the severe p.F508del mutation confers a small risk for CP with an OR of 2.5, whereas the mild p.R117H mutation increases risk by about 4-fold. Compound heterozygous state for one severe and one mild CFTR allele represents strong risk for CP and may be considered causative70. The role of common polymorphic CFTR alleles (e.g. T5, TG12) and the non-CF-causing, so-called bicarbonate-defective CFTR variants in CP remains controversial as the preponderance of data does not support their association with CP66. Unlike CFTR, variants in the solute-linked carrier 26 member 6 anion transporter (SLC26A6) do not alter the genetic risk in CP72.
Figure 2.
Genetic risk factors associated with the ductal pathological pathway. See text for details.
CLDN2 variants.
GWAS studies of CP identified several SNPs in the CLDN2-MORC4 locus to be associated with CP risk17,19. The OR was about 2 and the effect was more pronounced in alcoholic CP. Within this locus, CLDN2 seems to be the clinically relevant risk gene, as it is expressed in pancreatic ducts at low levels as a tight junction protein. It was proposed that CLDN2-MORC4 variants might cause CLDN2 mislocalization. Additional work is required to clarify the mechanism of action of this risk locus and to confirm whether assignment to the ductal pathway is appropriate (Figure 2).
CASR variants.
The calcium-sensing receptor (CASR) regulates calcium homeostasis through parathyroid hormone secretion and renal tubular calcium reabsorption. Functional CASR is also expressed in the pancreas, including ductal cells where CASR may respond to high calcium concentrations in the juice by increasing ductal fluid secretion, thereby preventing stone formation and pancreatitis73 (Figure 2). A US population based study failed to demonstrate the previously anticipated association between CASR variants and the SPINK1 p.N34S haplotype, but reported the p.R990G variant to increase CP risk, especially in subjects with moderate or heavy alcohol consumption74. More recently, a French study found overrepresentation of rare CASR coding variants in idiopathic CP and significant association of the p.A986S variant, but only in the homozygous state, with CP75. However, the previously reported association with the p.R990G variant was not observed in this cohort. Taken together, current evidence does not support a clear role for CASR variants in CP pathogenesis.
In summary, human genetic data indicate that premature activation or mifolding of pancreatic proteases play a central role in the onset of pancreatitis and progression to chronic pancreatitis (see suppl. table 1).
THE ROLE OF PROTEASES IN THE PATHOPHYSIOLOGY AND CELL BIOLOGY OF PANCREATITIS
While genetic evidence for the involvement of the protease/antiprotease balance in the pathogenesis of pancreatitis dates back only two decades and mainly focusses on chronic pancreatitis, pathophysiological and biochemical investigations have implicated this system for over a century. Due to lack of adequate animal models and the inability to keep isolated pancreatic acinar cells in culture for long periods of time, experimental studies has focussed primarily on acute pancreatitis. The relative importance of the pathways discussed below might change with respect to etiology. It is our general understanding that these mechanisms are also relevant to chronic pancreatitis, although experimental evidence is mostly lacking.
Autodigestion by pancreatic proteases.
The pathophysiological concept of autodigestion was first developed by the Austrian pathologist Hans Chiari in Prague more than 120 years ago. He claimed that pancreatitis was caused and driven by the glands own digestive properties76. Ever since the pathomechanism of premature activation of pancreatic enzymes and its contribution to disease severity and progression has captured the attention of many pancreatologists. Bialek and colleagues first showed, that protease activation during pancreatitis begins in the exocrine pancreas77 and Saluja et al. reported that it begins in a membrane-confined vesicular compartment and parallels acinar cell damage78. Although the fact that activation of digestive proteases is an early event during acute pancreatitis is widely accepted, the question where and through what mechanism this process is initiated and whether it plays a role in chronicity remains under debate.
Protease activation during pancreatitis – clues from mechanistic studies
Role of calcium for intracellular protease activation.
States of hypercalcemia such as primary hyperparathyroidism are a risk factor for the development of acute pancreatitis in humans and rats79–81. Intracellular calcium concentrations and compartmental distribution in acinar cells are tightly regulated as calcium serves as a second messenger for the physiological release of digestive enzymes in response to vagal nerve stimulation or humoral activation82,83. Upon intraperitoneal treatment of rats with supramaximal doses of caerulein, an analogue of cholecystokinin84, a time-dependent disruption of the physiological oscillating intracellular calcium signal was observed in rat acini using the Ca2+-sensitive dye fura-285. Employing for the first time fluorescent trypsin substrates that allow the subcellular imaging localization and quantification of protease activation86, Krüger et al. could demonstrate that a specific extended plateau release of calcium at the apical pole of acinar cells is necessary for premature trypsin activation to occur, which is different from the calcium oscillations required for enzyme secretion87. These findings where confirmed by others who also demonstrated that acetylcholine- or CCK-induced intracellular protease activation was associated with formation of cytoplasmic vacuoles in acinar cells that resemble those overserved in experimental pancreatitis in vivo88 and that inhibition of calcium release also inhibits the formation of these vesicles89. Acinar cells can replace calcium for magnesium in its stores and when this is done not only the premature activation of proteases in vitro and in vivo but also the severity of pancreatitis is significantly reduced90. Two ongoing clinical trials (one in acute and one in chronic pancreatitis) have now been based on this observation. Orabi et al. took the concept of calcium- dependent protease activation further by showing that acute pancreatitis induced by supramaximal doses of the muscarinic agonist carbachol can be abrogated by inhibition of the intracellular calcium channel ryanodine receptor, which is located at the basolateral membrane of acinar cells. Therefore, the importance of spatial distribution of calcium to the apical pole might be a specific feature of the caerulein model. Interestingly, carbachol-induced protease activation is more severe if cells are pre-treated with ethanol91. Calcium signaling may also play a role in other forms of experimental pancreatitis, like bile acid-induced pancreatitis92 as well as pressure induced pancreatitis/experimental post-ERCP pancreatitis93. Experimental pancreatitis can be ameliorated by modulating calcium release from intracellular stores or influx through the plasma membrane via pharmacological inhibition of inositol-tri-phosphate-receptor (IP3R, predominantly types 2 and 3) signaling94,95 or calcium release-activated calcium modulator 1 (ORAI1)96,97. The calcium-dependent protease activation also heavily depends on calcineurin, a calcium-activated phosphatase, and its downstream signaling via the transcription factor NFAT. Inhibition of calcineurin by inhibitors or in mice lacking calcineurin-subunits causes reduced intracellular protease activity in either secretagogue or bile acid induced pancreatitis, without affecting vesicular transport98,99.
Mechanisms of protease activation.
Three major concepts have been investigated over the past decades: autoactivation, spatial redistribution and fusion of zymogen granules with other organelles and failure of protective mechanisms.
Autoactivation of trypsin.
There is strong evidence from human genetic studies, indicating that autoactivation of trypsinogen100 causes chronic pancreatitis in affected humans (chapter 1.). In contrast, trypsinogen autoactivation is unimportant in experimental caerulein induced pancreatitis. It could be shown that inhibition of active trypsin by the reversible chemical inhibitor S124 could prevent trypsin activity in experimental caerulein-induced acute pancreatitis, but had no effect on the generation of the trypsin-activation-peptide (TAP) or active trypsin after wash-out of S124, thus indicating that hydrolysis of trypsinogen to trypsin appeared in a trypsin-independent fashion101.
Activation by lysosomal proteases.
Inhibition of the lysosomal hydrolase cathepsin B by the cysteine protease inhibitor E-64d, leads to a significant decrease in trypsin activity and TAP-formation, indicating that the trypsin activation in response to caerulein depends on cathepsin B101–103. Similarly, in cathepsin B knock-out mice, the trypsin-activation is significantly inhibited after caerulein administration104. However, active cathepsin B (but inactive105) and trypsinogen under physiological conditions are not located in the same cell organelles (zymogen granules for exocytosis vs. lysosomes for degradation of content of endosomes and autophagosomes). In 1998 the group of Michael Steer showed in subcellular fractions of caerulein-treated acini a co-localization of cathepsin B and digestive enzymes, including trypsin and TAP, in heavy fractions78 containing zymogen granules and lysosomes early in the disease course and later a shift of trypsin and cathepsin B activity to the cytosol106. This confirmed earlier findings from in vivo studies that indicated a fusion of zymogen containing vacuoles with lysosomes in secretagogue-, duct-obstruction- or diet-induced acute pancreatitis107–112. Intracellular activation of proteases other than trypsin, like chymotrypsin and carboxypeptidase B, also depend on non-physiological co-localization with other cell components, but are independent of cathepsin B113. A misorting in the exocrine machinery as well as secretion blockage and re-uptake of previously secreted proteases via endocytosis have been described under experimental conditions. The fact that an acidic pH, as found in lysosomes, enhances secretagogue-dependent zymogen activation supports the fusion hypothesis114. A very recent study reports that CCK or ethanol treatment depletes acinar cells of syntaxin 2, a key regulator of apical exocytosis, thus leading to increased basolateral exocytosis and formation of autolysosomes mediated by syntaxin 3 and 4, in which trypsinogen activation takes place115. Inhibition of syntaxin-4-mediated basolateral exocytosis in experimental pancreatitis decreases disease severity116. Another concept introduces endocytic vacuoles (EVs) as the site of intracellular trypsinogen activation. These occur under physiological and pathophysiological conditions following compound exocytosis of zymogen granules117. EV formation is calcium dependent118. Their content is acidic and calcium rich and after supramaximal CCK- or taurocholate-stimulation, trypsin activity within these post-exocytic structures can be visualized using fluorescent dyes119. During pancreatitis, EVs are larger than normal; tend to fuse with the plasma membrane or even rupture, discharging active trypsin into the cytosol or extracellular space. This instability is thought to be caused by disruption of otherwise protective actin filaments surrounding the EV120. Rupture of EVs is however, independent of trypsin or cathepsin B activity. Secretory blockage may contribute to these events. In experimental pancreatitis vesicle-associated membrane protein-8 (VAMP-8) mediated secretion is impaired, due to a loss of early endosomal proteins, resulting in retention of trypsinogen and transformation to active trypsin in a cathepsin-dependent manner. Knock-out of VAMP-8 protects from pancreatitis and restoration of early endosomal trafficking decreases severity of pancreatitis121,122. Vesicular trafficking is regulated in a calcium dependent manner123,124.
Loss of trypsin-inhibitors.
Little is known about the role of failing protective mechanisms for protease activation in the early phase of pancreatitis. The most potent cellular trypsin-inhibitor is the before mentioned SPINK1. Although SPINK1 mutations are among the most common genetic risk-factors for the development of recurrent acute and chronic pancreatitis, so far none of the described mutations seemed to impair the SPINK-function, and therefore do not explain the increased risk for pancreatitis (see chapter 1). In mice, carrying a heterozygous SPINK3-deletion, a significant reduction of functional SPINK does not lead to development of spontaneous pancreatitis or more severe disease after supramaximal caerulein administration when compared to wild type controls125. The fact that mice with a homozygous SPINK3-deletion suffer from pancreatic atrophy and that this phenotype can be rescued by transgenic expression of rat PSTI-1125, points towards a role of trypsin-inhibitors during pancreatic development, but does not explain its role in pancreatitis.
In conclusion, the current cumulative evidence suggests a cathepsin B-dependent mechanism of protease activation in experimental pancreatitis.
Cell death cause or consequence of protease activation?
Premature intracellular protease activation in acinar cells leads to cell injury. The type of cell death, be it necrosis, apoptosis, autophagy, necroptosis or pyroptosis determines disease severity126. Necrosis is understood as an unregulated response to damage. In animal models of acute pancreatitis approximately 1 to 5% of acinar cells undergo apoptosis and severity of pancreatitis is inversely correlated to the rate of apoptosis127. Macroautophagy is a multistep, lysosomal driven, adaptive process by which cells degrade cytoplasmic organelles and long-lived protein128. Pancreatitis presents with impaired autophagic flux evident by vacuole accumulation129. Currently it is debated, whether impaired autophagy stimulates cell death through accumulation of damaged mitochondria mediating an inflammatory response via a ROS-dependent mechanism as in LAMP2130 or Atg7-knock-out animals131 or whether autophagy prevents an inflammatory response as in Atg5 knock-out mice132,133. The regulated process of necrosis is termed necroptosis and triggered by TNF, TRAIL, FasL, type 1 interferon and TLRs all of which are released in the early phase of acute pancreatitis in response to protease activation134. RIP-1 (receptor-interacting protein) and RIP-3 form a phosphorylated complex the necrosome and phosphorylate MLKL (mixed lineage kinase domain-like) resulting in membrane rupture. 40% of cells undergo necroptosis in pancreatitis and RIP3 deletion or treatment with necrostatin ameliorates pancreatitis135,136. Necroptosis releases DAMPs and those will activate the NLRP3 pathway resulting in pyroptosis137. Pyroptosis is an innate immune sensing mechanism with poorly understood upstream signaling. Nevertheless, some interesting inhibitors are known such as lactate, beta-hydroxybutyrate and aspartate. The term pyroptosis describes activation of the inflammasome via NLRP3. The inflammasome is a macroscopic cytosolic protein complex which proteolytically cleaves IL1β pro-IL18 and releases HMGB1. NLRP3 activation requires lysosomal rupture and cathepsin release, calcium influx and mitochondria derived ROS production and thus is closely linked to pancreatitis138,139. However, NLRP3 expression is restricted to innate immune cells140.
Cell death pathways in pancreatitis intersect. Caspase 3 activation cannot only induce apoptosis but pyroptosis. Necroptosis can shift to pyroptosis via caspase 8 activation and necroptosis activates pyroptosis141. As of today, we have not understood why some of our patients deteriorate and develop a necrotizing course of pancreatitis. A shift of regulated cell death from apoptosis to pyroptosis as well as stimulation of necroptosis might explain this observation. Inhibition of pyroptosis e.g. by using Ringer’s lactate for volume resuscitation142 or inhibition of necroptosis by necrostatin143 might well be a way forward in the treatment of pancreatitis.
An interesting question is whether cell death is a result of premature protease activation or a consequence of inflammation. The answer is ambiguous: Saluja and co-workers show a direct effect on lysosomal stability mediated by active trypsin and lysosomal rupture leads to the release of cathepsins into the cytosol causing dose dependently apoptosis or necrosis106. Our own group showed that inhibition of protease activation, especially trypsin by specific inhibitors results in a decreased rate of apoptosis, but did not affect necrosis144. Thus, cell death is a result of intracellular protease activation, but this has only been shown for isolated acinar cells mimicking the early phase of pancreatitis. Taking into account pyroptosis and necroptosis inflammation is the origin and consequence of cell death in pancreatitis (figure 3).
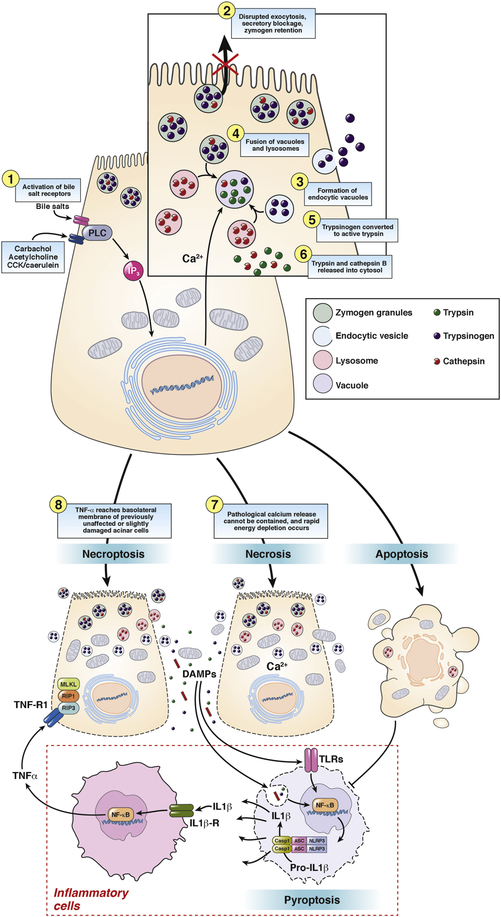
Figure 3.
Trypsinogen activation and cell death in pancreatic acinar cells. Intracellular trypsinogen activation is an early event at the onset of acute experimental pancreatitis. Supramaximal secretagogue-receptor stimulation or activation of bile salt receptors leads to an un-physiological peak-plateau calcium signal. This results in disrupted exocytosis of zymogen granules, secretory blockage, zymogen retention and formation of endocytic vacuoles, which contain trypsin and trypsinogen, taken up from the extracellular space. Those vacuoles co-localize and fuse with lysosomes containing cathepsin B, which in turn transforms trypsinogen into active trypsin. Due to increasing instability endocytic vacuoles often rupture, releasing trypsin and cathepsin B into the cytosol. Active trypsin is thought to induce mainly apoptosis, a silent form of cell death, which suppresses inflammation. In contrast, if the pathological calcium release cannot be contained, rapid energy depletion occurs and cells undergo necrosis during which the plasma membrane becomes leaky and cellular components e.g. DNA or mitochondria reach the extracellular space. Those will be recognized by leukocytes, which will be activated via the inflammasome signaling pathway. IL1β- and TNFα-release as well as pyroptosis occur. If TNFα reaches the basolateral membrane of previously unaffected or slightly damaged acinar cells it can induce another form of programmed cell death, called necroptosis.
Protease activity and disease severity
This raises the question whether intracellular protease activation of trypsin as it is linked to cell death can also mediate systemic disease severity. The notion that trypsin activation is linked to disease severity is supported by the correlation of TAP urine levels to severity in patients with acute pancreatitis145. However, several studies question the role of trypsin for severity of pancreatitis. Expression of mutant human trypsin bearing the hereditary pancreatitis mutation R122H in mice leads to slightly more severe caerulein pancreatitis146, but, when compared to mice expressing normal human trypsinogen, there is no increase in disease severity. Moreover, the effect seems to be not solely dependent on trypsin, since mice with human trypsin mutations (R122H or N29I) show lower trypsin activity after caerulein hyperstimulation. This might in part be explained by a higher rate of acinar cell apoptosis even in untreated animals transgenic for human trypsinogen147. A similar effect was seen in the PACE-tryp(on) mice, which conditionally express an endogenously activated trypsinogen within pancreatic acinar cells. Those mice will develop acute pancreatitis in a trypsin activity dependent way, which can lead to organ dysfunction and mortality, but they also show a pronounced caspase-3 activation with consecutive apoptotic loss of acinar cells and replacement by fatty tissue148. Similarly, the group of Bar-Sagi described a transgenic mouse, where the human mutation R122H was inserted in the murine PRSS1 gene. Those mice develop spontaneous pancreatitis and show a more pronounced inflammatory infiltrate as well as cellular damage in response to caerulein149. The fact that the predominant way of cell death determines the overall severity of experimental pancreatitis in mice has been demonstrated in animals deleted for cathepsin L. Cathepsin L degrades trypsinogen into an inactive elongated TAP, but cathepsin L deficiency leads to a milder form a of caerulein induced pancreatitis, which is linked to a shift from necrosis to apoptosis6.
The most convincing data, questioning the role of trypsin for disease severity was generated by using a mouse strain lacking trypsinogen 7 (T7 ko), the murine counterpart of human cationic trypsinogen. Dawra et al showed that a significant reduction in trypsin and chymotrypsin activity after caerulein hyperstimulation in these mice had no effect on disease severity in terms of cell death, local or systemic inflammation in an acute and chronic pancreatitis model, which they claim is mediated by a NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) dependent mechanism150,151. This confirms previous findings generated in the PACE-tryp(on) model, where NFκB -activation was independent of increased intracellular trypsin activity in vitro152.
THE ROLE OF SYSTEMIC INFLAMMATION IN PANCREATITIS
NFκB activation - initial step of inflammation.
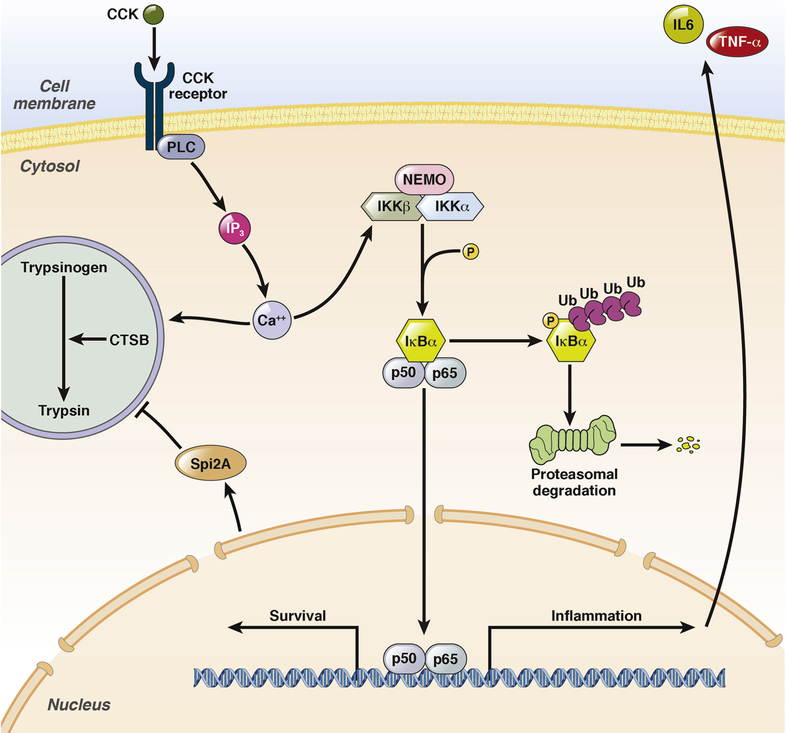
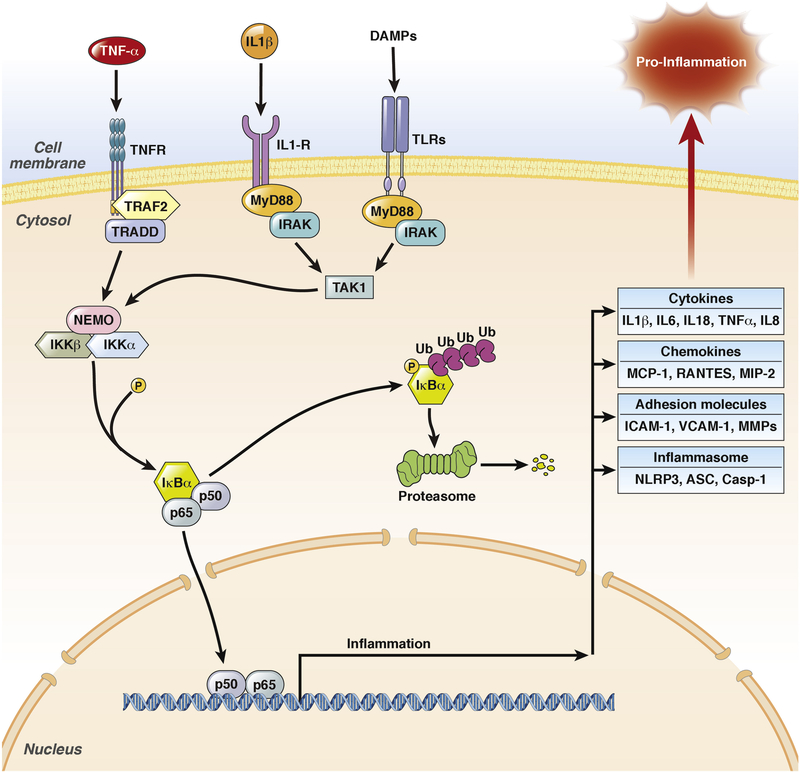
The activation of NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) is an early event during pancreatitis and occurs within the first minutes after onset of the disease153,154. One main function of NFκB is the transcriptional regulation of the immune response155. The principle pathway of NFκB signal transduction is depicted in figure 4. The fact that NFκB is already present in the cytoplasm explains its rapid activation after induction of pancreatitis153,154.
Figure 4:
NFκB pathway in pancreatic acinar cells. The early activation of NFκB follows the same time pattern as trypsinogen activation. Both are induced by cytoplasmatic Ca2+ influx, but NFκB did not depend on trypsinogen activation. The phosphorylation of IκBα, followed by proteasomal degradation and the nuclear translocation of NFκB (p65/p50) occurs in parallel to protease activation. NFκB as transcription factor acts in two directions; first the transcriptions of pro-inflammatory genes like IL6 or TNFα to initiate the immune response and second the transcription of pro-survival genes. Therefore NFκB can directly influence protease activity to protect cells by the up-regulation of Spi2A, a serine protease inhibitor.
Intraacinar protease activation and NFκB activation are early cellular events during pancreatitis153,154, which have been suggested to occur independent from each other151, but follow a similar kinetic. Trypsinogen activation depends on intracellular Ca2+ signaling87 and NFκB activation can also be induced by protein kinase C and Ca2+, which could be the reason for parallel kinetics156. The deletion of T7 trypsinogen or cathepsin B, which both result in greatly reduced protease activation104,144,151, do not influence NFκB activation during caerulein-induced pancreatitis151. This can be regarded as evidence that protease activation does not directly lead to NFκB activation in acinar cells while NFκB activation can still interact with protease activity: the transcriptional regulation of Spi2A (serine protease inhibitor 2a) is mediated by NFκB and is able to inhibit trypsin activity in a mouse model of acute pancreatitis (figure 4)157. These results suggest a more complex role of NFκB during disease progression which is not restricted to pro-inflammation158. Surprisingly, the pancreas-specific deletion of Rel-A(p65) resulted in increased systemic inflammation and pancreatic necrosis159. In contrast, the pancreas-specific deletion of IκBα results in nuclear translocation of Rel-A and ameliorated pancreatitis157. The same phenotype could be observed in MyD88 deficient mice, which develop a more severe disease phenotype compared to controls160. MyD88 is a central adaptor for the TLR/IL1-receptor signaling pathway which induces NFκB activation. These results ultimately suggest a protective role of NFκB activation in pancreatic acinar cells. Other studies attributed a more critical role to NFκB regarding disease severity. Mice which constitutively over-express active IKKβ under a pancreas-specific promoter, showed chronic infiltration of immune cells, associated with an increased disease severity after caerulein stimulation161,162. However, the infiltration of immune cells alone, or the constitutive activity of NFκB in acinar cells, did not result in acute pancreatitis, organ damage and necrotic or apoptotic cell death162; an additional pathophysiological stimulus was still needed. The increased severity of pancreatitis in IKKβ over-expressing mice can be explained by the presence of leukocytes within the pancreas, which become rapidly activated after disease induction and do not need to infiltrate the pancreas. The pancreas-specific deletion of IKKα, another IκBα phosphorylating kinase, caused spontaneous pancreatitis in mice163, but this process appeared to be independent of NFκB. IKKα also regulates autophagic flux which is essential for pancreas homeostasis164. These data demonstrate the complexity of the NFκB network which often hampers the interpretation of results. Taken together the constitutive activation of NFκB leads to a chronic infiltration of immune cells, but pancreatitis only develops after induction by an external stimulus161,162. The presence of immune cells within the pancreas is required but insufficient for pancreatitis to develop and these cells need to be activated in order to contribute to disease severity. On the other hand, data from pancreas-specific RelA deleted mice show that NFκB activation in acinar cells is not essential for the recruitment of immune cells to the side of damage159. Quite to the contrary, the absence of p65 in acinar cells result in greater disease severity. While all the mentioned studies investigated the role of NFκB in acinar cells NFκB activation may play a much greater role in infiltrating immune cells, which directly regulate the immune response.
Another master switch in the transcriptional machinery of acinar cells is AP1 (activator protein 1). AP1 is implicated in multiple transcriptional networks within the acinar cells regulating pancreatic development, differentiation, cell death and inflammation. Mice heterozygous for the orphan nuclear receptor NR5A2 develop an acinar-cell-autonomous AP1 dependent pre-inflammatory state, which, on a transcriptome level, mimics that of early acute pancreatitis165.
It seems however, that the extent of NFκB and AP1 activity greatly differs with respect to the cause of pancreatitis. In caerulein models, an activation of both transcription factors is described and submaximal CCK stimulation induces acinar cell dedifferentiation and proliferation via the MAPK/c-Jun/AP-1 pathway probably as part of a pancreatic regeneration program166,167. In contrast, the metabolites occurring in ethanol-induced experimental pancreatitis can positively and negatively regulate NFκB and AP1 depending on the predominance of oxidative or non-oxidative alcohol metabolism in the pancreas168.
The role of infiltrating immune cells.
The infiltration of immune cells start within minutes after the onset of disease and plays a crucial role for the severity and prognosis of pancreatitis169–172. Cells of the innate immune system like neutrophil granulocytes and monocytes/macrophages represent the majority of infiltrating cells. NFκB plays a crucial role for the activation of leukocytes and is a central mediator of the innate and adaptive immune system173. Pancreatitis is primarily a sterile inflammation, so pathogen-associated molecular patterns (PAMPs) play no role in the activation of immune cells during the early phase of disease. In pancreatitis, the activation of immune cells is thus mediated by cytokines or damage-associated molecular patterns (DAMPs) that arise from acinar cell necrosis. Acinar cells release various cytokines and chemokines in response to CCK stimulation and NFκB activation such as TNFα174, IL6 or MCP-1175. DAMPs and cytokines result in the nuclear translocation of p65/p50 within infiltrating immune cells, which enhances the cytokine storm via the secretion of pro-inflammatory mediators175 (figure 5). DAMPs can act in the same way as PAMPs via Toll-like receptors (TLRs)176, or specific receptors like P2RX7 which uses extracellular ATP as a ligand. Acinar cells, which undergo necrotic cell death, release a multitude of different DAMPs, like free DNA177, histones or free ATP138 which can act as immune activators. Finally, activated immune cells increase pancreatic damage and contribute to systemic inflammation169,175,178. Several studies have focused on different populations of immune cells and their role during acute pancreatitis.
Figure 5:
NFκB activation in inflammatory cells. There are multiple pathways how NFκB could be activated within leukocytes; here are the major pathways which play a role during acute pancreatitis. Cytokines like IL1β or TNFα as well as DAMP signals acting via Toll-like receptors can induce the translocation of p65/p50 into the nucleus. In leukocytes the majority of inflammatory mediators are under the control of NFκB: cytokines, chemokines, adhesion molecules and components of the inflammasome pathway. Leukocyte mediated NFκB activation enhances the immune response in a very prominent manner and therefore has a different role in pancreatitis compared to NFκB activation within acinar cell.
Neutrophil granulocytes are often used as reference marker for pancreatic inflammation via measurements of myeloperoxidase (MPO) activity in tissue and reflects the amount of infiltrating neutrophils. One major function of neutrophils is removing pathogens by the release of proteases, antimicrobial peptides and reactive oxygen species (ROS). During pancreatitis neutrophils are the major source of ROS production, they can induce oxidative damage on acinar cells and enhance trypsinogen activation178. The release of proteases like PMN-elastase contributes to tissue destruction and acinar cell dissociation179. These data indicate that neutrophils have a direct effect on disease severity. This was confirmed by the depletion of neutrophils using anti-neutrophils serum, which resulted in reduced pancreatic damage and protease activation169,178. Recent studies investigated the role of neutrophil extracellular trap formation (NETs) in the context of pancreatitis. NETs are extracellular networks consisting of neutrophil DNA and used to bind pathogens180. This suicide mechanism of neutrophils is a last line of defense against bacterial infections. NET formation is induced via TLR-4 activation, and the activation of NADPH-oxidase which lead to the oxidation of peptidylarginine-deiminase-4 (PAD4)181,182. TLR4 is not only responsible for the detection of pathogens but also DAMPs can activate the TLR-signaling pathway176. Merza et.al. could show that NET formation enhances the immune response during severe acute pancreatitis and is accountable for trypsinogen activation183. Treatment with DNAses prevents NET formation and reduces disease severity183. Beside bacterial infections, also crystals can induce NET formation184. Another group has shown that NET formation is a critical step in bile stone development and plays an important role for ductal obstruction contributing to onset and severity of pancreatitis185,186. Neutrophil infiltration during acute pancreatitis is an unspecific reaction of the immune system, which enhances local damage by formation of NETs and the release of ROS, or activates digestive enzymes.
Monocytes/macrophages belong to the cells of the innate immune system. In contrast to neutrophil granulocytes macrophages are characterized by a high plasticity. Classical activated macrophages (M1) act in a pro-inflammatory manner and secrete high amounts of IL6, TNFα, IL12 and IL1β, an increased expression of inducible nitric oxide synthases (iNOS) results in the release of NO187. Alternatively activated macrophages (M2) are associated with wound healing, tissue regeneration and fibrogenesis and act in an anti-inflammatory manner via the release of IL10 or TGFβ. They are characterized by reduced iNOS- and increased Arginase-1 (Arg1) expression187. Macrophages are phagocytosing cells which remove tissue debris, necrotic and apoptotic cells. During acute pancreatitis, macrophage infiltration correlates to a greater extent with pancreatic damage and necrosis than the number of infiltrating neutrophils175. The reason for that is that macrophages are required for the removal of necrosis and thus mitigate pancreatic damage. Phagocytosing macrophages could be observed in different models of acute and chronic pancreatitis130,164,169,175. In contrast to apoptosis, necrosis is a pro-inflammatory cell death because it entails the release of multiple DAMPs which induce an M1 polarization of macrophages188. Therefore acinar cell apoptosis is suggested to be protective against hyperinflammation and decreases disease severity189. M1 macrophages release high amounts of TNFα which have a direct effect on pancreatic acinar cells174. Two independent groups could show that TNFα secreted from infiltrating monocytes is responsible for pancreatic damage and digestive protease activation169,190. Depletion of macrophages by clodronate containing liposomes decreases disease severity and protected mice from caerulein-induced pancreatitis169,191. TNFα acts on cells via cell death receptors and is necessary to induce necroptotic cell death via the RIP1/RIP3 pathway135, which has been suggested to be the major cell death pathway during acute pancreatitis136. Therefore infiltrating macrophages are responsible for induction of necroptosis as well as for the clearance of necrotic areas within the damaged pancreas175.
Besides TNFα, macrophages also produce high amounts of IL1β a pro-inflammatory cytokine associated with the acute disease phase. In contrast to other cytokines IL1β needs to be processed. During maturation pro-IL1β and pro-IL18 undergo activation by the caspase-1/inflammasome complex and are released by the gasdermin D pore complex from the cytosol to the extracellular space192. In consequence to this process the cell undergoes pyroptotic cell death192,193. During pancreatitis the activation of the inflammasome complex and the release of IL1β contribute critically to disease severity138,139,175,177. Inflammasome complex formation and pyroptotic cell death is mainly known from macrophages and not present in acinar cells175. Inflammasome activation is a complex mechanism requiring two signals for activation: the first signal induces the transcriptional up-regulation of inflammasome components by NFκB and the second signal induces the oligomerization of the inflammasome complex and the activation of pro-caspase-1. Major inducer of the inflammasome pathway is the TLR/MyD88 cascade. The second signal, which is necessary for inflammasome complex formation, can be a high potassium influx, TNFα, or the release of cathepsins from phagosomes into the cytosol194. Phagocytosis of zymogens by macrophages results in a co-localization of trypsinogen and cathepsin B in non-acinar cell phagolysosomes and results activation of trypsinogen175 and macrophage activation. The rupture of these trypsin and cathepsin B containing vesicles leads to a cytosolic redistribution of cathepsins which acts as a second signal of inflammasome activation and indirectly links trypsinogen activation to the NFκB pathway via IL1β release. IL1β acts as activator for other immune cells, the IL1 receptor being directly linked to the MyD88 NFκB pathway. The importance of IL1β during pancreatitis could nicely be shown by a transgenic mouse model of pancreas-specific, IL1β over-expressing mice which develop chronic pancreatitis including complete loss of pancreatic function within weeks after birth195. Therefore M1 macrophages contribute prominently to the systemic immune response syndrome (SIRS) which is associated with multi-organ dysfunction syndrome (MODS) and increased mortality170. In contrast to M1 macrophages, the M2 phenotype is associated with organ regeneration and fibrosis development196–198.
In conclusion, early protease activation as well as NFκB activation are essential characteristics of pancreatitis, both events occur in parallel during disease manifestation and strongly influence each other. Recent prove that not only the activation of proteases and NFκB play a critical role, but also the type of cell, in which their activation takes place is of importance. Pancreatitis is not a disease of acinar cells alone.
Supplementary Material
Funding:
PePPP center of excellence MV ESF/14-BM-A55-0045/16; ESF MV V-630-S-150-2012/132/133); DFG-CRC 1321.-P14.; DFG SE 2702/2-1. National Institutes of Health (NIH) grants R01 DK058088 and R01 DK117809 (to MST).
Footnotes
Conflict of interest: none declared
This manuscript is dedicated to the memory of Walter Halangk, PhD, to honour his work as a pioneer unraveling the pathophysiology of pancreatitis.
References
- 1.Peery AF, Crockett SD, Murphy CC, et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinderknecht H. Activation of pancreatic zymogens. Normal activation, premature intrapancreatic activation, protective mechanisms against inappropriate activation. Dig Dis Sci 1986;31:314–321. [DOI] [PubMed] [Google Scholar]
- 4.Szmola R, Bence M, Carpentieri A, et al. Chymotrypsin C is a co-activator of human pancreatic procarboxypeptidases A1 and A2. J Biol Chem 2011;286:1819–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szmola R, Sahin-Tóth M. Chymotrypsin C (caldecrin) promotes degradation of human cationic trypsin: identity with Rinderknecht’s enzyme Y. Proc Natl Acad Sci U S A 2007;104:11227–11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wartmann T, Mayerle J, Kähne T, et al. Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology 2010;138:726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegyi E, Sahin-Tóth M. Genetic Risk in Chronic Pancreatitis: The Trypsin-Dependent Pathway. Dig Dis Sci 2017;62:1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemoda Z, Sahin-Tóth M. Chymotrypsin C (caldecrin) stimulates autoactivation of human cationic trypsinogen. J Biol Chem 2006;281:11879–11886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabó A, Sahin-Tóth M. Increased activation of hereditary pancreatitis-associated human cationic trypsinogen mutants in presence of chymotrypsin C. J Biol Chem 2012;287:20701–20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss FU, Behn C-O, Simon P, et al. Cathepsin B gene polymorphism Val26 is not associated with idiopathic chronic pancreatitis in European patients. Gut 2007;56:1322–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Németh BC, Sahin-Tóth M. Human cationic trypsinogen (PRSS1) variants and chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 2014;306:G466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Németh BC, Szücs Á, Hegyi P, et al. Novel PRSS1 Mutation p.P17T Validates Pathogenic Relevance of CTRC-Mediated Processing of the Trypsinogen Activation Peptide in Chronic Pancreatitis. Am J Gastroenterol 2017;112:1896–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J-M, Kukor Z, Le Maréchal C, et al. Evolution of trypsinogen activation peptides. Mol Biol Evol 2003;20:1767–1777. [DOI] [PubMed] [Google Scholar]
- 14.Geisz A, Hegyi P, Sahin-Tóth M. Robust autoactivation, chymotrypsin C independence and diminished secretion define a subset of hereditary pancreatitis-associated cationic trypsinogen mutants. FEBS J 2013;280:2888–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joergensen MT, Geisz A, Brusgaard K, et al. Intragenic duplication: a novel mutational mechanism in hereditary pancreatitis. Pancreas 2011;40:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kereszturi E, Sahin-Tóth M. Intracellular autoactivation of human cationic trypsinogen mutants causes reduced trypsinogen secretion and acinar cell death. J Biol Chem 2009;284:33392–33399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet 2012;44:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derikx MH, Kovacs P, Scholz M, et al. Polymorphisms at PRSS1-PRSS2 and CLDN2-MORC4 loci associate with alcoholic and non-alcoholic chronic pancreatitis in a European replication study. Gut 2015;64:1426–1433. [DOI] [PubMed] [Google Scholar]
- 19.Rosendahl J, Kirsten H, Hegyi E, et al. Genome-wide association study identifies inversion in the CTRB1-CTRB2 locus to modify risk for alcoholic and non-alcoholic chronic pancreatitis. Gut 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulling A, Sato M, Masson E, et al. Identification of a functional PRSS1 promoter variant in linkage disequilibrium with the chronic pancreatitis-protecting rs10273639. Gut 2015;64:1837–1838. [DOI] [PubMed] [Google Scholar]
- 21.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet 2000;25:213–216. [DOI] [PubMed] [Google Scholar]
- 22.Aoun E, Chang C-CH, Greer JB, et al. Pathways to injury in chronic pancreatitis: decoding the role of the high-risk SPINK1 N34S haplotype using meta-analysis. PloS One 2008;3:e2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Leo M, Bianco M, Zuppardo RA, et al. Meta-analysis of the impact of SPINK1 p.N34S gene variation in Caucasic patients with chronic pancreatitis. An update. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver 2017;49:847–853. [DOI] [PubMed] [Google Scholar]
- 24.Kuwata K, Hirota M, Shimizu H, et al. Functional analysis of recombinant pancreatic secretory trypsin inhibitor protein with amino-acid substitution. J Gastroenterol 2002;37:928–934. [DOI] [PubMed] [Google Scholar]
- 25.Király O, Wartmann T, Sahin-Tóth M. Missense mutations in pancreatic secretory trypsin inhibitor (SPINK1) cause intracellular retention and degradation. Gut 2007;56:1433–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulling A, Le Maréchal C, Trouvé P, et al. Functional analysis of pancreatitis-associated missense mutations in the pancreatic secretory trypsin inhibitor (SPINK1) gene. Eur J Hum Genet EJHG 2007;15:936–942. [DOI] [PubMed] [Google Scholar]
- 27.Kereszturi E, Király O, Sahin-Tóth M. Minigene analysis of intronic variants in common SPINK1 haplotypes associated with chronic pancreatitis. Gut 2009;58:545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kereszturi É, Sahin-Tóth M. Pancreatic Cancer Cell Lines Heterozygous for the SPINK1 p.N34S Haplotype Exhibit Diminished Expression of the Variant Allele. Pancreas 2017;46:e54–e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfützer RH, Barmada MM, Brunskill AP, et al. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology 2000;119:615–623. [DOI] [PubMed] [Google Scholar]
- 30.Kume K, Masamune A, Kikuta K, et al. [−215G>A; IVS3+2T>C] mutation in the SPINK1 gene causes exon 3 skipping and loss of the trypsin binding site. Gut 2006;55:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou W-B, Boulling A, Masson E, et al. Clarifying the clinical relevance of SPINK1 intronic variants in chronic pancreatitis. Gut 2016;65:884–886. [DOI] [PubMed] [Google Scholar]
- 32.Boulling A, Witt H, Chandak GR, et al. Assessing the pathological relevance of SPINK1 promoter variants. Eur J Hum Genet EJHG 2011;19:1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derikx MHM, Geisz A, Kereszturi É, et al. Functional significance of SPINK1 promoter variants in chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 2015;308:G779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kukor Z, Tóth M, Sahin-Tóth M. Human anionic trypsinogen: properties of autocatalytic activation and degradation and implications in pancreatic diseases. Eur J Biochem 2003;270:2047–2058. [DOI] [PubMed] [Google Scholar]
- 35.Witt H, Sahin-Tóth M, Landt O, et al. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat Genet 2006;38:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jancsó Z, Sahin-Tóth M. Tighter Control by Chymotrypsin C (CTRC) Explains Lack of Association between Human Anionic Trypsinogen and Hereditary Pancreatitis. J Biol Chem 2016;291:12897–12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kume K, Masamune A, Takagi Y, et al. A loss-of-function p.G191R variant in the anionic trypsinogen (PRSS2) gene in Japanese patients with pancreatic disorders. Gut 2009;58:820–824. [DOI] [PubMed] [Google Scholar]
- 38.Rosendahl J, Witt H, Szmola R, et al. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet 2008;40:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosendahl J, Landt O, Bernadova J, et al. CFTR, SPINK1, CTRC and PRSS1 variants in chronic pancreatitis: is the role of mutated CFTR overestimated? Gut 2013;62:582–592. [DOI] [PubMed] [Google Scholar]
- 40.Beer S, Zhou J, Szabó A, et al. Comprehensive functional analysis of chymotrypsin C (CTRC) variants reveals distinct loss-of-function mechanisms associated with pancreatitis risk. Gut 2013;62:1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szabó A, Ludwig M, Hegyi E, et al. Mesotrypsin Signature Mutation in a Chymotrypsin C (CTRC) Variant Associated with Chronic Pancreatitis. J Biol Chem 2015;290:17282–17292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masson E, Chen J-M, Scotet V, et al. Association of rare chymotrypsinogen C (CTRC) gene variations in patients with idiopathic chronic pancreatitis. Hum Genet 2008;123:83–91. [DOI] [PubMed] [Google Scholar]
- 43.Paliwal S, Bhaskar S, Mani KR, et al. Comprehensive screening of chymotrypsin C (CTRC) gene in tropical calcific pancreatitis identifies novel variants. Gut 2013;62:1602–1606. [DOI] [PubMed] [Google Scholar]
- 44.LaRusch J, Lozano-Leon A, Stello K, et al. The Common Chymotrypsinogen C (CTRC) Variant G60G (C.180T) Increases Risk of Chronic Pancreatitis But Not Recurrent Acute Pancreatitis in a North American Population. Clin Transl Gastroenterol 2015;6:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grabarczyk AM, Oracz G, Wertheim-Tysarowska K, et al. Chymotrypsinogen C Genetic Variants, Including c.180TT, Are Strongly Associated With Chronic Pancreatitis in Pediatric Patients. J Pediatr Gastroenterol Nutr 2017;65:652–657. [DOI] [PubMed] [Google Scholar]
- 46.Tang X-Y, Zou W-B, Masson E, et al. The CTRB1-CTRB2 risk allele for chronic pancreatitis discovered in European populations does not contribute to disease risk variation in the Chinese population due to near allele fixation. Gut 2018;67:1368–1369. [DOI] [PubMed] [Google Scholar]
- 47.Jancsó Z, Hegyi E, Sahin-Tóth M. Chymotrypsin Reduces the Severity of Secretagogue-induced Pancreatitis in Mice. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahin-Tóth M. Genetic risk in chronic pancreatitis: the misfolding-dependent pathway. Curr Opin Gastroenterol 2017;33:390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kereszturi E, Szmola R, Kukor Z, et al. Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: a novel disease mechanism. Hum Mutat 2009;30:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masamune A, Nakano E, Kume K, et al. PRSS1 c.623G>C (p.G208A) variant is associated with pancreatitis in Japan. Gut 2014;63:366. [DOI] [PubMed] [Google Scholar]
- 51.Hegyi E, Cierna I, Vavrova L, et al. Chronic pancreatitis associated with the p.G208A variant of PRSS1 gene in a European patient. JOP J Pancreas 2014;15:49–52. [DOI] [PubMed] [Google Scholar]
- 52.Witt H, Beer S, Rosendahl J, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nat Genet 2013;45:1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kujko AA, Berki DM, Oracz G, et al. A novel p.Ser282Pro CPA1 variant is associated with autosomal dominant hereditary pancreatitis. Gut 2017;66:1728–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakano E, Geisz A, Masamune A, et al. Variants in pancreatic carboxypeptidase genes CPA2 and CPB1 are not associated with chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 2015;309:G688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamura K, Yu J, Hata T, et al. Mutations in the pancreatic secretory enzymes CPA1 and CPB1 are associated with pancreatic cancer. Proc Natl Acad Sci U S A 2018;115:4767–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hegyi E, Sahin-Tóth M. Human CPA1 mutation causes digestive enzyme misfolding and chronic pancreatitis in mice. Gut 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raeder H, Johansson S, Holm PI, et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet 2006;38:54–62. [DOI] [PubMed] [Google Scholar]
- 58.Johansson BB, Torsvik J, Bjørkhaug L, et al. Diabetes and pancreatic exocrine dysfunction due to mutations in the carboxyl ester lipase gene-maturity onset diabetes of the young (CEL-MODY): a protein misfolding disease. J Biol Chem 2011;286:34593–34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao X, Jones G, Sevilla WA, et al. A Carboxyl Ester Lipase (CEL) Mutant Causes Chronic Pancreatitis by Forming Intracellular Aggregates That Activate Apoptosis. J Biol Chem 2016;291:23224–23236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fjeld K, Weiss FU, Lasher D, et al. A recombined allele of the lipase gene CEL and its pseudogene CELP confers susceptibility to chronic pancreatitis. Nat Genet 2015;47:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zou W-B, Boulling A, Masamune A, et al. No Association Between CEL-HYB Hybrid Allele and Chronic Pancreatitis in Asian Populations. Gastroenterology 2016;150:1558–1560.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss FU, Schurmann C, Guenther A, et al. Fucosyltransferase 2 (FUT2) non-secretor status and blood group B are associated with elevated serum lipase activity in asymptomatic subjects, and an increased risk for chronic pancreatitis: a genetic association study. Gut 2015;64:646–656. [DOI] [PubMed] [Google Scholar]
- 63.Kirsten H, Scholz M, Kovacs P, et al. Genetic variants of lipase activity in chronic pancreatitis. Gut 2016;65:184–185. [DOI] [PubMed] [Google Scholar]
- 64.Greer JB, LaRusch J, Brand RE, et al. ABO blood group and chronic pancreatitis risk in the NAPS2 cohort. Pancreas 2011;40:1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teich N, Bokemeyer B, Mohl W, et al. Blood group B is associated with azathioprine-induced acute pancreatitis in patients with IBD. Gut 2017;66:1531–1532. [DOI] [PubMed] [Google Scholar]
- 66.Hegyi P, Wilschanski M, Muallem S, et al. CFTR: A New Horizon in the Pathomechanism and Treatment of Pancreatitis. Rev Physiol Biochem Pharmacol 2016;170:37–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharer N, Schwarz M, Malone G, et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med 1998;339:645–652. [DOI] [PubMed] [Google Scholar]
- 68.Cohn JA, Friedman KJ, Noone PG, et al. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med 1998;339:653–658. [DOI] [PubMed] [Google Scholar]
- 69.Weiss FU, Simon P, Bogdanova N, et al. Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic chronic pancreatitis and controls. Gut 2005;54:1456–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masson E, Chen J-M, Audrézet M-P, et al. A conservative assessment of the major genetic causes of idiopathic chronic pancreatitis: data from a comprehensive analysis of PRSS1, SPINK1, CTRC and CFTR genes in 253 young French patients. PloS One 2013;8:e73522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.LaRusch J, Jung J, General IJ, et al. Mechanisms of CFTR functional variants that impair regulated bicarbonate permeation and increase risk for pancreatitis but not for cystic fibrosis. PLoS Genet 2014;10:e1004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balázs A, Ruffert C, Hegyi E, et al. Genetic analysis of the bicarbonate secreting anion exchanger SLC26A6 in chronic pancreatitis. Pancreatol Off J Int Assoc Pancreatol IAP Al 2015;15:508–513. [DOI] [PubMed] [Google Scholar]
- 73.Rácz GZ, Kittel A, Riccardi D, et al. Extracellular calcium sensing receptor in human pancreatic cells. Gut 2002;51:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muddana V, Lamb J, Greer J-B, et al. Association between calcium sensing receptor gene polymorphisms and chronic pancreatitis in a US population: role of serine protease inhibitor Kazal 1type and alcohol. World J Gastroenterol 2008;14:4486–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masson E, Chen J-M, Férec C. Overrepresentation of Rare CASR Coding Variants in a Sample of Young French Patients With Idiopathic Chronic Pancreatitis. Pancreas 2015;44:996–998. [DOI] [PubMed] [Google Scholar]
- 76.Chiari H. Über die Selbstverdauung des menschlichen Pankreas. Z Für Heilkd 1896:69–96. [Google Scholar]
- 77.Bialek R, Willemer S, Arnold R, et al. Evidence of intracellular activation of serine proteases in acute cerulein-induced pancreatitis in rats. Scand J Gastroenterol 1991;26:190–196. [DOI] [PubMed] [Google Scholar]
- 78.Hofbauer B, Saluja AK, Lerch MM, et al. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol 1998;275:G352–362. [DOI] [PubMed] [Google Scholar]
- 79.Fernández-del Castillo C, Harringer W, Warshaw AL, et al. Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N Engl J Med 1991;325:382–387. [DOI] [PubMed] [Google Scholar]
- 80.Mithöfer K, Fernández-del Castillo C, Frick TW, et al. Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology 1995;109:239–246. [DOI] [PubMed] [Google Scholar]
- 81.Bai HX, Giefer M, Patel M, et al. The association of primary hyperparathyroidism with pancreatitis. J Clin Gastroenterol 2012;46:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kasai H, Augustine GJ. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature 1990;348:735–738. [DOI] [PubMed] [Google Scholar]
- 83.Gerasimenko JV, Gerasimenko OV, Petersen OH. The role of Ca2+ in the pathophysiology of pancreatitis. J Physiol 2014;592:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol 1977;373:97–117. [DOI] [PubMed] [Google Scholar]
- 85.Ward JB, Sutton R, Jenkins SA, et al. Progressive disruption of acinar cell calcium signaling is an early feature of cerulein-induced pancreatitis in mice. Gastroenterology 1996;111:481–491. [DOI] [PubMed] [Google Scholar]
- 86.Krüger B, Lerch MM, Tessenow W. Direct detection of premature protease activation in living pancreatic acinar cells. Lab Investig J Tech Methods Pathol 1998;78:763–764. [PubMed] [Google Scholar]
- 87.Krüger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol 2000;157:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raraty M, Ward J, Erdemli G, et al. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci U S A 2000;97:13126–13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mooren FC, Hlouschek V, Finkes T, et al. Early changes in pancreatic acinar cell calcium signaling after pancreatic duct obstruction. J Biol Chem 2003;278:9361–9369. [DOI] [PubMed] [Google Scholar]
- 90.Schick V, Scheiber JA, Mooren FC, et al. Effect of magnesium supplementation and depletion on the onset and course of acute experimental pancreatitis. Gut 2014;63:1469–1480. [DOI] [PubMed] [Google Scholar]
- 91.Orabi AI, Shah AU, Muili K, et al. Ethanol enhances carbachol-induced protease activation and accelerates Ca2+ waves in isolated rat pancreatic acini. J Biol Chem 2011;286:14090–14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muili KA, Wang D, Orabi AI, et al. Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin. J Biol Chem 2013;288:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romac JM-J, Shahid RA, Swain SM, et al. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat Commun 2018;9:1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gerasimenko JV, Lur G, Sherwood MW, et al. Pancreatic protease activation by alcohol metabolite depends on Ca2+ release via acid store IP3 receptors. Proc Natl Acad Sci U S A 2009;106:10758–10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang W, Cane MC, Mukherjee R, et al. Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1,4,5-trisphosphate receptor-mediated Ca2+ release. Gut 2017;66:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lur G, Sherwood MW, Ebisui E, et al. InsP₃receptors and Orai channels in pancreatic acinar cells: co-localization and its consequences. Biochem J 2011;436:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wen L, Voronina S, Javed MA, et al. Inhibitors of ORAI1 Prevent Cytosolic Calcium-Associated Injury of Human Pancreatic Acinar Cells and Acute Pancreatitis in 3 Mouse Models. Gastroenterology 2015;149:481–492.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Husain SZ, Grant WM, Gorelick FS, et al. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol 2007;292:G1594–1599. [DOI] [PubMed] [Google Scholar]
- 99.Muili KA, Ahmad M, Orabi AI, et al. Pharmacological and genetic inhibition of calcineurin protects against carbachol-induced pathological zymogen activation and acinar cell injury. Am J Physiol Gastrointest Liver Physiol 2012;302:G898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kassell B, Kay J. Zymogens of proteolytic enzymes. Science 1973;180:1022–1027. [DOI] [PubMed] [Google Scholar]
- 101.Halangk W, Krüger B, Ruthenbürger M, et al. Trypsin activity is not involved in premature, intrapancreatic trypsinogen activation. Am J Physiol Gastrointest Liver Physiol 2002;282:G367–374. [DOI] [PubMed] [Google Scholar]
- 102.Saluja AK, Donovan EA, Yamanaka K, et al. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology 1997;113:304–310. [DOI] [PubMed] [Google Scholar]
- 103.Van Acker GJD, Saluja AK, Bhagat L, et al. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am J Physiol Gastrointest Liver Physiol 2002;283:G794–800. [DOI] [PubMed] [Google Scholar]
- 104.Halangk W, Lerch MM, Brandt-Nedelev B, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest 2000;106:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kukor Z, Mayerle J, Krüger B, et al. Presence of cathepsin B in the human pancreatic secretory pathway and its role in trypsinogen activation during hereditary pancreatitis. J Biol Chem 2002;277:21389–21396. [DOI] [PubMed] [Google Scholar]
- 106.Talukdar R, Sareen A, Zhu H, et al. Release of Cathepsin B in Cytosol Causes Cell Death in Acute Pancreatitis. Gastroenterology 2016;151:747–758.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koike H, Steer ML, Meldolesi J. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am J Physiol 1982;242:G297–307. [DOI] [PubMed] [Google Scholar]
- 108.Saito I, Hashimoto S, Saluja A, et al. Intracellular transport of pancreatic zymogens during caerulein supramaximal stimulation. Am J Physiol 1987;253:G517–526. [DOI] [PubMed] [Google Scholar]
- 109.Saluja A, Hashimoto S, Saluja M, et al. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol 1987;253:G508–516. [DOI] [PubMed] [Google Scholar]
- 110.Saluja A, Saluja M, Villa A, et al. Pancreatic duct obstruction in rabbits causes digestive zymogen and lysosomal enzyme colocalization. J Clin Invest 1989;84:1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hirano T, Saluja A, Ramarao P, et al. Apical secretion of lysosomal enzymes in rabbit pancreas occurs via a secretagogue regulated pathway and is increased after pancreatic duct obstruction. J Clin Invest 1991;87:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lerch MM, Saluja AK, Rünzi M, et al. Luminal endocytosis and intracellular targeting by acinar cells during early biliary pancreatitis in the opossum. J Clin Invest 1995;95:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thrower EC, Diaz de Villalvilla APE, Kolodecik TR, et al. Zymogen activation in a reconstituted pancreatic acinar cell system. Am J Physiol Gastrointest Liver Physiol 2006;290:G894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhoomagoud M, Jung T, Atladottir J, et al. Reducing extracellular pH sensitizes the acinar cell to secretagogue-induced pancreatitis responses in rats. Gastroenterology 2009;137:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dolai S, Liang T, Orabi AI, et al. Pancreatitis-Induced Depletion of Syntaxin 2 Promotes Autophagy and Increases Basolateral Exocytosis. Gastroenterology 2018;154:1805–1821.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dolai S, Liang T, Orabi AI, et al. Depletion of the membrane-fusion regulator Munc18c attenuates caerulein hyperstimulation-induced pancreatitis. J Biol Chem 2018;293:2510–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Behrendorff N, Dolai S, Hong W, et al. Vesicle-associated membrane protein 8 (VAMP8) is a SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) selectively required for sequential granule-to-granule fusion. J Biol Chem 2011;286:29627–29634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Voronina S, Collier D, Chvanov M, et al. The role of Ca2+ influx in endocytic vacuole formation in pancreatic acinar cells. Biochem J 2015;465:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sherwood MW, Prior IA, Voronina SG, et al. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci U S A 2007;104:5674–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chvanov M, De Faveri F, Moore D, et al. Intracellular rupture, exocytosis and actin interaction of endocytic vacuoles in pancreatic acinar cells: initiating events in acute pancreatitis. J Physiol 2018;596:2547–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Messenger SW, Thomas DD, Cooley MM, et al. Early to Late Endosome Trafficking Controls Secretion and Zymogen Activation in Rodent and Human Pancreatic Acinar Cells. Cell Mol Gastroenterol Hepatol 2015;1:695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Messenger SW, Jones EK, Holthaus CL, et al. Acute acinar pancreatitis blocks vesicle-associated membrane protein 8 (VAMP8)-dependent secretion, resulting in intracellular trypsin accumulation. J Biol Chem 2017;292:7828–7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomas DDH, Martin CL, Weng N, et al. Tumor protein D52 expression and Ca2+-dependent phosphorylation modulates lysosomal membrane protein trafficking to the plasma membrane. Am J Physiol Cell Physiol 2010;298:C725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Messenger SW, Falkowski MA, Groblewski GE. Ca2+-regulated secretory granule exocytosis in pancreatic and parotid acinar cells. Cell Calcium 2014;55:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Romac JM-J, Ohmuraya M, Bittner C, et al. Transgenic expression of pancreatic secretory trypsin inhibitor-1 rescues SPINK3-deficient mice and restores a normal pancreatic phenotype. Am J Physiol Gastrointest Liver Physiol 2010;298:G518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sendler M, Mayerle J, Lerch MM. Necrosis, Apoptosis, Necroptosis, Pyroptosis: It Matters How Acinar Cells Die During Pancreatitis. Cell Mol Gastroenterol Hepatol 2016;2:407–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gukovskaya AS, Gukovsky I. Which way to die: the regulation of acinar cell death in pancreatitis by mitochondria, calcium, and reactive oxygen species. Gastroenterology 2011;140:1876–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gukovskaya AS, Gukovsky I, Algül H, et al. Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology 2017;153:1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest 2009;119:3340–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mareninova OA, Sendler M, Malla SR, et al. Lysosome associated membrane proteins maintain pancreatic acinar cell homeostasis: LAMP-2 deficient mice develop pancreatitis. Cell Mol Gastroenterol Hepatol 2015;1:678–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Iwahashi K, Hikita H, Makino Y, et al. Autophagy impairment in pancreatic acinar cells causes zymogen granule accumulation and pancreatitis. Biochem Biophys Res Commun 2018. [DOI] [PubMed] [Google Scholar]
- 132.Hashimoto D, Ohmuraya M, Hirota M, et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol 2008;181:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gukovsky I, Gukovskaya AS. Impaired autophagy triggers chronic pancreatitis: lessons from pancreas-specific atg5 knockout mice. Gastroenterology 2015;148:501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.He S, Huang S, Shen Z. Biomarkers for the detection of necroptosis. Cell Mol Life Sci CMLS 2016;73:2177–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009;137:1100–1111. [DOI] [PubMed] [Google Scholar]
- 136.Louhimo JM, Steer ML, Perides G. Necroptosis Is an Important Severity Determinant and Potential Therapeutic Target in Experimental Severe Pancreatitis. CMGH Cell Mol Gastroenterol Hepatol 2016;2:519–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Malik A, Kanneganti T-D. Inflammasome activation and assembly at a glance. J Cell Sci 2017;130:3955–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 2011;141:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoque R, Farooq A, Ghani A, et al. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 2014;146:1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hoque R, Mehal WZ. Inflammasomes in pancreatic physiology and disease. Am J Physiol Gastrointest Liver Physiol 2015;308:G643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vince JE, Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci CMLS 2016;73:2349–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Iqbal U, Anwar H, Scribani M. Ringer’s lactate versus normal saline in acute pancreatitis: A systematic review and meta-analysis. J Dig Dis 2018;19:335–341. [DOI] [PubMed] [Google Scholar]
- 143.Louhimo J, Steer ML, Perides G. Necroptosis Is an Important Severity Determinant and Potential Therapeutic Target in Experimental Severe Pancreatitis. Cell Mol Gastroenterol Hepatol 2016;2:519–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sendler M, Maertin S, John D, et al. Cathepsin B Activity Initiates Apoptosis via Digestive Protease Activation in Pancreatic Acinar Cells and Experimental Pancreatitis. J Biol Chem 2016;291:14717–14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Neoptolemos JP, Kemppainen EA, Mayer JM, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet Lond Engl 2000;355:1955–1960. [DOI] [PubMed] [Google Scholar]
- 146.Selig L, Sack U, Gaiser S, et al. Characterisation of a transgenic mouse expressing R122H human cationic trypsinogen. BMC Gastroenterol 2006;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Athwal T, Huang W, Mukherjee R, et al. Expression of human cationic trypsinogen (PRSS1) in murine acinar cells promotes pancreatitis and apoptotic cell death. Cell Death Dis 2014;5:e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gaiser S, Daniluk J, Liu Y, et al. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut 2011;60:1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Archer H, Jura N, Keller J, et al. A mouse model of hereditary pancreatitis generated by transgenic expression of R122H trypsinogen. Gastroenterology 2006;131:1844–1855. [DOI] [PubMed] [Google Scholar]
- 150.Dawra R, Sah RP, Dudeja V, et al. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology 2011;141:2210–2217.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sah RP, Dudeja V, Dawra RK, et al. Cerulein-induced chronic pancreatitis does not require intra-acinar activation of trypsinogen in mice. Gastroenterology 2013;144:1076–1085.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ji B, Gaiser S, Chen X, et al. Intracellular trypsin induces pancreatic acinar cell death but not NF-kappaB activation. J Biol Chem 2009;284:17488–17498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gukovsky I, Gukovskaya AS, Blinman TA, et al. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol 1998;275:G1402–1414. [DOI] [PubMed] [Google Scholar]
- 154.Steinle AU, Weidenbach H, Wagner M, et al. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology 1999;116:420–430. [DOI] [PubMed] [Google Scholar]
- 155.Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 1996;14:649–683. [DOI] [PubMed] [Google Scholar]
- 156.Han B, Logsdon CD. CCK stimulates mob-1 expression and NF-kappaB activation via protein kinase C and intracellular Ca(2+). Am J Physiol Cell Physiol 2000;278:C344–351. [DOI] [PubMed] [Google Scholar]
- 157.Neuhöfer P, Liang S, Einwächter H, et al. Deletion of IκBα activates RelA to reduce acute pancreatitis in mice through up-regulation of Spi2A. Gastroenterology 2013;144:192–201. [DOI] [PubMed] [Google Scholar]
- 158.Rakonczay Z, Hegyi P, Takács T, et al. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut 2008;57:259–267. [DOI] [PubMed] [Google Scholar]
- 159.Algül H, Treiber M, Lesina M, et al. Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J Clin Invest 2007;117:1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koike Y, Kanai T, Saeki K, et al. MyD88-dependent interleukin-10 production from regulatory CD11b+Gr-1(high) cells suppresses development of acute cerulein pancreatitis in mice. Immunol Lett 2012;148:172–177. [DOI] [PubMed] [Google Scholar]
- 161.Baumann B, Wagner M, Aleksic T, et al. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J Clin Invest 2007;117:1502–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aleksic T, Baumann B, Wagner M, et al. Cellular immune reaction in the pancreas is induced by constitutively active IkappaB kinase-2. Gut 2007;56:227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li N, Wu X, Holzer RG, et al. Loss of acinar cell IKKα triggers spontaneous pancreatitis in mice. J Clin Invest 2013;123:2231–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Diakopoulos KN, Lesina M, Wörmann S, et al. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology 2015;148:626–638.e17. [DOI] [PubMed] [Google Scholar]
- 165.Cobo I, Martinelli P, Flández M, et al. Transcriptional regulation by NR5A2 links differentiation and inflammation in the pancreas. Nature 2018;554:533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guo L, Sans MD, Hou Y, et al. c-Jun/AP-1 is required for CCK-induced pancreatic acinar cell dedifferentiation and DNA synthesis in vitro. Am J Physiol Gastrointest Liver Physiol 2012;302:G1381–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Koh Y-H, Tamizhselvi R, Bhatia M. Extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase, through nuclear factor-kappaB and activator protein-1, contribute to caerulein-induced expression of substance P and neurokinin-1 receptors in pancreatic acinar cells. J Pharmacol Exp Ther 2010;332:940–948. [DOI] [PubMed] [Google Scholar]
- 168.Gukovskaya AS, Mouria M, Gukovsky I, et al. Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology 2002;122:106–118. [DOI] [PubMed] [Google Scholar]
- 169.Sendler M, Dummer A, Weiss FU, et al. Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut 2013;62:430–439. [DOI] [PubMed] [Google Scholar]
- 170.Johnson CD. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut 2004;53:1340–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gukovsky I, Li N, Todoric J, et al. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1199–1209.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Habtezion A. Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol 2015;31:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu T, Zhang L, Joo D, et al. NF-κB signaling in inflammation. Signal Transduct Target Ther 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gukovskaya AS, Gukovsky I, Zaninovic V, et al. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest 1997;100:1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sendler M, Weiss F-U, Golchert J, et al. Cathepsin B-Mediated Activation of Trypsinogen in Endocytosing Macrophages Increases Severity of Pancreatitis in Mice. Gastroenterology 2018;154:704–718.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhao Q, Wei Y, Pandol SJ, et al. STING Signaling Promotes Inflammation in Experimental Acute Pancreatitis. Gastroenterology 2018;154:1822–1835.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gukovskaya AS, Vaquero E, Zaninovic V, et al. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology 2002;122:974–984. [DOI] [PubMed] [Google Scholar]
- 179.Mayerle J, Schnekenburger J, Krüger B, et al. Extracellular cleavage of E-cadherin by leukocyte elastase during acute experimental pancreatitis in rats. Gastroenterology 2005;129:1251–1267. [DOI] [PubMed] [Google Scholar]
- 180.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 181.Kirchner T, Möller S, Klinger M, et al. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators Inflamm 2012;2012:849136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Leshner M, Wang S, Lewis C, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol 2012;3:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Merza M, Hartman H, Rahman M, et al. Neutrophil Extracellular Traps Induce Trypsin Activation, Inflammation, and Tissue Damage in Mice With Severe Acute Pancreatitis. Gastroenterology 2015;149:1920–1931.e8. [DOI] [PubMed] [Google Scholar]
- 184.Schorn C, Janko C, Latzko M, et al. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front Immunol 2012;3:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Leppkes M, Maueröder C, Hirth S, et al. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat Commun 2016;7:10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schauer C, Janko C, Munoz LE, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 2014;20:511–517. [DOI] [PubMed] [Google Scholar]
- 187.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964. [DOI] [PubMed] [Google Scholar]
- 188.Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol 2002;2:787–795. [DOI] [PubMed] [Google Scholar]
- 189.Mareninova OA, Sung K-F, Hong P, et al. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem 2006;281:3370–3381. [DOI] [PubMed] [Google Scholar]
- 190.Perides G, Weiss ER, Michael ES, et al. TNF-alpha-dependent regulation of acute pancreatitis severity by Ly-6C(hi) monocytes in mice. J Biol Chem 2011;286:13327–13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Saeki K, Kanai T, Nakano M, et al. CCL2-induced migration and SOCS3-mediated activation of macrophages are involved in cerulein-induced pancreatitis in mice. Gastroenterology 2012;142:1010–1020.e9. [DOI] [PubMed] [Google Scholar]
- 192.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 2006;8:1812–1825. [DOI] [PubMed] [Google Scholar]
- 193.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009;7:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guo H, Callaway JB, Ting JP-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015;21:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Marrache F, Tu SP, Bhagat G, et al. Overexpression of interleukin-1beta in the murine pancreas results in chronic pancreatitis. Gastroenterology 2008;135:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xue J, Sharma V, Hsieh MH, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun 2015;6:7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Criscimanna A, Coudriet GM, Gittes GK, et al. Activated macrophages create lineage-specific microenvironments for pancreatic acinar- and β-cell regeneration in mice. Gastroenterology 2014;147:1106–1118.e11. [DOI] [PubMed] [Google Scholar]
- 198.Sendler M, Beyer G, Mahajan UM, et al. Complement Component 5 Mediates Development of Fibrosis, via Activation of Stellate Cells, in 2 Mouse Models of Chronic Pancreatitis. Gastroenterology 2015;149:765–776.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.