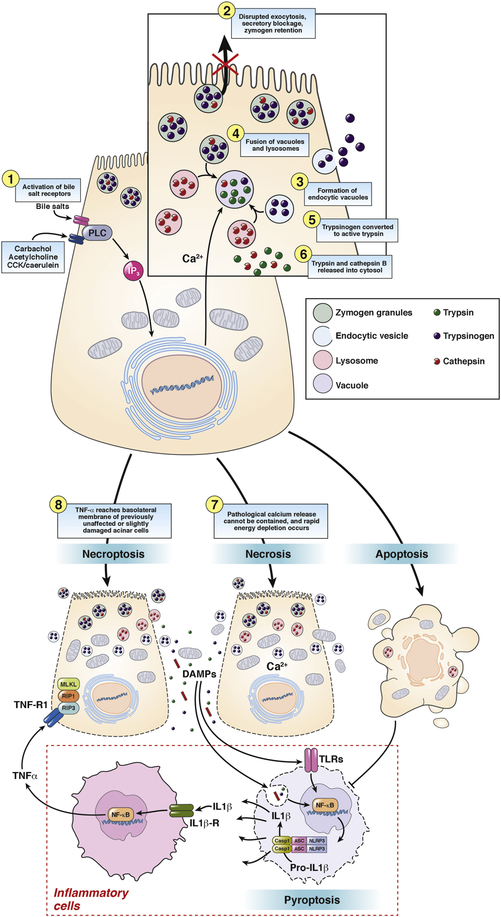
Figure 3.
Trypsinogen activation and cell death in pancreatic acinar cells. Intracellular trypsinogen activation is an early event at the onset of acute experimental pancreatitis. Supramaximal secretagogue-receptor stimulation or activation of bile salt receptors leads to an un-physiological peak-plateau calcium signal. This results in disrupted exocytosis of zymogen granules, secretory blockage, zymogen retention and formation of endocytic vacuoles, which contain trypsin and trypsinogen, taken up from the extracellular space. Those vacuoles co-localize and fuse with lysosomes containing cathepsin B, which in turn transforms trypsinogen into active trypsin. Due to increasing instability endocytic vacuoles often rupture, releasing trypsin and cathepsin B into the cytosol. Active trypsin is thought to induce mainly apoptosis, a silent form of cell death, which suppresses inflammation. In contrast, if the pathological calcium release cannot be contained, rapid energy depletion occurs and cells undergo necrosis during which the plasma membrane becomes leaky and cellular components e.g. DNA or mitochondria reach the extracellular space. Those will be recognized by leukocytes, which will be activated via the inflammasome signaling pathway. IL1β- and TNFα-release as well as pyroptosis occur. If TNFα reaches the basolateral membrane of previously unaffected or slightly damaged acinar cells it can induce another form of programmed cell death, called necroptosis.