Abstract
Epidermal stem cells residing in the skin play an essential role in epidermal regeneration. When skin is injured, the stem cells are first activated to proliferate, and subsequently the progeny migrate and differentiate to regenerate the epidermis. Here, we demonstrate that the vitamin D receptor (VDR) is essential for these processes to occur. The requirement for VDR on epidermal stem cell function was revealed in conditional VDR knockout mice, in which VDR was deleted from stem cells and progeny, and mice were maintained on a low calcium diet. First, self-renewal and niche formation of epidermal stem cells were impaired. Wound-induced activation of epidermal stem cells was blunted associated with a reduction of β-catenin signaling. Second, wound induced migration of stem cells and progeny was impaired as shown by lineage tracing and delayed migration of VDR silenced cells. Epidermal differentiation of progeny was impaired at the wounding site associated with reduced E-cadherin expression. Deletion of VDR also changed stem cell fate blunting hair development, increasing sebaceous glands, and altering expression and location of epidermal markers. These results suggest that VDR is required for self-renewal, migration, and differentiation of epidermal stem cells and progeny during cutaneous wound healing.
INTRODUCTION
The mammalian epidermis provides a protective barrier that retains essential body fluids. At the interface between the external environment and the body, skin is constantly subjected to physical trauma and must be primed to repair wounds in response to injury. Epidermal stem cells (SCs) residing in the skin play essential roles for maintenance (Mascre et al., 2012) and regeneration of epidermis during wound repair (Lim et al., 2013; Plikus et al., 2012). During embryonic development, the skin epithelia originate from ectoderm and differentiate into the interfollicular epidermis (IFE), sebaceous gland (SG), and hair follicle (HF). After birth, adult SCs residing in different locations are responsible for the regeneration of IFE, SG, and HF. The putative epidermal stem cells, which reside in the basal layer of IFE (eSCs), are regulated, at least in part, by β-catenin signaling to maintain IFE (Lim et al., 2013). SCs residing in the bulge region of HF (bSCs) marked by CD34 expression are responsible for HFs (Blanpain and Fuchs, 2006). When the skin is injured, eSCs (Mascre et al., 2012; Plikus et al., 2012) and bSCs (Ito et al., 2005) are first to be activated to proliferate. Subsequently, the progeny migrate and differentiate to re-epithelize the wounds to regenerate the intact epidermis. A number of signaling pathways, including β-catenin and calcium, control the epidermal SCs and progeny during regeneration processes in wound repair (Blanpain and Fuchs, 2006).
The vitamin D receptor (VDR) and its ligand of 1,25(OH)2D3 are well-known regulators of epidermal proliferation and differentiation (Bikle, 2011). The epidermis is the major source of vitamin D for the body, produced from 7-dehydrocholesterol under the influence of UVB, and keratinocytes are capable of metabolizing vitamin D to its active ligand 1,25(OH)2D3 (Bikle, 2011). However, the role of VDR and its ligand in epidermal SCs has received less attention, especially in the context of epidermal regeneration during wound repair. The role of VDR in epidermal SCs is mainly studied in the context of hair regulation, as alopecia is a striking phenotype in mice lacking VDR (Li et al., 1997). The global VDR knockout (KO) mice demonstrated no change in bSCs (Palmer et al., 2008). A gradual decrease in bSCs was reported after hair defects, although CD34+ niche formation was not affected (Cianferotti et al., 2007). Moreover, it was reported by the same group that wound healing in the global VDR KO mouse was due to altered dermal fibroblast function, but not epidermal repair (Luderer et al., 2013).
However, we found a role for VDR in the keratinocyte during wound repair (Oda et al., 2015). When we generated conditional VDR knockout (cKO) mice in which VDR is deleted from Krt14-expressing epidermal SCs and progeny, wound closure was delayed and expression of β-catenin target genes decreased. Our results indicated that VDR exerts its function through regulation of β-catenin signaling in the skin, which plays an important role in maintenance of epidermal SCs, such as CD34+bSCs (Choi et al., 2013; Lien et al., 2014) and eSCs (Lim et al., 2013). The VDR directly binds to β-catenin in the AF2 domain and drives target gene transcription (Palmer et al., 2008; Shah et al., 2006). Delayed wound closure was significant in cKO mice compared to Cre-negative control (CON) mice, when both mice were maintained on a low-calcium diet. However, such a diet had no impact on CON mice with intact VDR (Oda et al., 2015). Here, we examined the role of VDR in epidermal wound repair under the experimental condition of lower dietary calcium. We tested the hypothesis that the defect in wound repair in mice lacking VDR in epidermal cells lies in the inability of epidermal SCs to be activated to proliferate, or their progeny to migrate and differentiate to re-epithelialize the wound and regenerate epidermis. In particular, we explored the hypothesis that VDR with adequate dietary calcium regulates epidermal SC activation through the interaction with β-catenin signaling and epidermal differentiation via adherens junction signaling.
RESULTS AND DISCUSSION
VDR is expressed in epidermal SCs, and its deletion decreases gene expression of SC markers
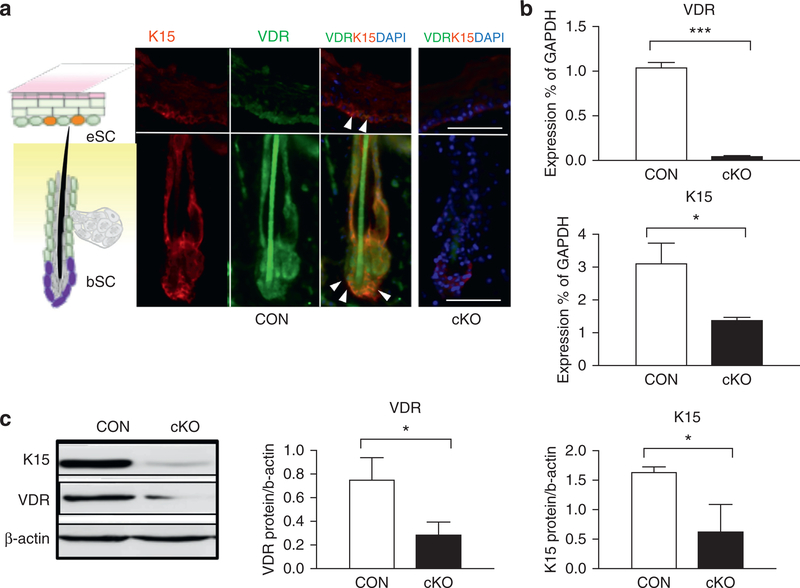
Previously, we generated VDR cKO mice, in which VDR is removed from KRT14-expressing epidermal SCs and progeny by using the Cre-loxP strategy (Oda et al., 2015). Homozygous floxed VDR mice with the KRT14-driven Cre transgene (VDR cKO) were compared to CON littermates that have floxed VDR alleles but no Cre. VDR was expressed in the bSCs and IFE that are marked by the stem marker KRT15, but efficiently removed in IFE and HF in cKO at 3 months (Figure 1a). Deletion of VDR was further confirmed by mRNA analysis (Figure 1b,3months), Western analysis (Figure 1c, Supplementary Figure S1b online), and PCR in both neonatal (postnatal days1–3) and adult stages (3 months) (Supplementary Figure S1a). Expression of KRT15 was also substantially reduced in VDR cKO (Figure 1a), as shown by mRNA analyses (Figure 1b) and protein analysis (Figure 1c, Supplementary Figure S1b) with statistical significance. Both cKO and CON mice were maintained on a low-calcium diet after weaning, in which serum calcium levels did not change, and 25(OH)D3 levels increased slightly (Supplementary Figure S2a, S2b online).
Figure 1. Deletion of VDR decreases gene expression in epidermal stem cells.
(a) Immunostaining of VDR (green), Krt15 (red), and overlay of both (yellow with white triangles) of CON and VDR cKO skin. The schematic diagram (left) shows the location of bSCs and eSCs in the skin. Scale bar = 50 μm. (b, c) Expression of VDR and K15 in neonatal skin (postnatal days 1–3) of VDR cKO and CON mice was detected by quantitative PCR (b) and Western blotting (c) (*P < 0.05; ***P < 0.001). bSC, hair follicle bulge stem cell; cKO, conditional knockout; CON, control; eSC, interfollicular epidermal stem cell; VDR, vitamin D receptor.
We then investigated the impact of VDR deletion on gene expression profiles. Keratinocytes were isolated from VDR cKO and CON skin at 3 months (n = 3), as described (Nowak and Fuchs, 2009), and high-purity RNA was isolated. The gene expression profiles were analyzed using an Illumina beads-based microarray (Illumina, San Diego, CA), including 25,600 annotated transcripts and 19,100 genes as described (Yoshizaki et al., 2014) (data are available in GSE68727 under super-series GSE68729). These analyses revealed that the deletion of VDR significantly down-regulated the expression of epidermal SC markers, which were previously identified for bSCs (Lien et al., 2011) and eSCs (Lim et al., 2013) (Supplementary Figure S1c). Expression of Krt15 decreased in microarray analyses (fold change: –1.39 shown by heat map in Supplementary Figure S1c), which was consistent with decreases in mRNA and protein expression in cKO.
Deletion of VDR impairs self-renewal of epidermal SCs in both the eSC and bSC niches
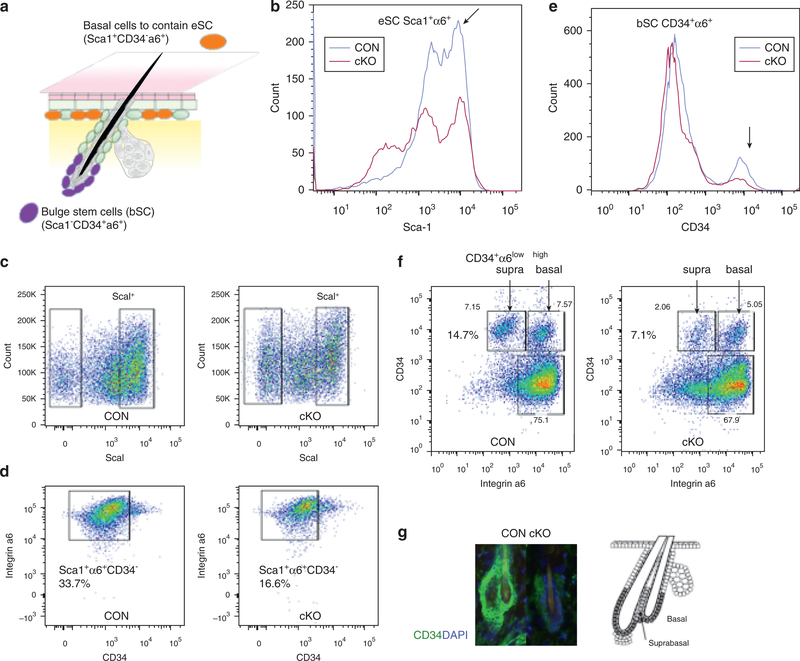
We then conducted cell sorting to address whether the marked decreases in gene expression of SC markers is due to a decrease in the number of epidermal SCs residing in the skin. Keratinocytes were isolated from the epidermis of VDR cKO and CON skin (n = 3), in which both cKO and CON mice were maintained under low dietary calcium, in which the level of serum calcium did not change (Supplementary Figure S3b online). They were incubated with antibodies against Sca1, CD34, and integrin α6, and separated by FACS analysis, as described (Adam et al.,2015). The IFE basal cells containing eSCs express Sca1, and HF bSCs were marked by CD34 (Figure 2a). The doublepeaksofSca1+populations(arrow) decreased in cKO to generate a Sca1 weak population (Figure 2b). The percent of basal cells (Sca1+integrin α6+CD34−) per total cells was decreased in VDR cKO compared to CON by 51.8% ± 2.8% (e.g., from 33.7% to 16.6% in the sample shown) (n = 3, P < 0.05) (Figure 2c, 2d). In addition, the CD34+ peak (arrow) representing bSCs was lower in cKO (Figure 2e). The number of cells in the two subpopulations together of basal bSCs (Sca1− integrin α6highCD34+) and suprabasal bSCs (Sca1− integrin α6lowCD34+) per total cells also decreased by 53.1% ± 4.8% (e.g., from 14.7% to 7.1% in sample shown) (n = 3, P < 0.05) with the greater loss in the suprabasal bSCs (e.g.,from7.15%to 2.86% in sample shown)(n=3,P<0.05) in cKO (Figure 2f), as is well illustrated in the reduced number of CD34+ cells in the cKO in the immunofluorescence results (Figure 2g). These data demonstrated that VDR deletion impaired self-renewal for bSCs and eSCs and niche formation for bSCs.
Figure 2. VDR deficiency decreased self-renewal potentials for epidermal SC.
FACS analyses for basal cells containing eSCs (Sca1+α6+CD34−) and bSCs (Sca1−α6+CD34+) in VDR cKO and CON are shown. (a) The scheme to show the location and expression markers for bSCs and eSCs. (b, e) Representative histograms for Sca1 (b) and (e) for CD34 to compare CON (blue) and cKO (red) are shown. (c, d) The sorting profiles and number of basal cells containing eSCs (Sca1+α6+CD34-). (f) The profiles and number for suprabasal bSCs (Sca1−α6lowCD34+) and basal bSCs (Sca1+α6highCD34−). (g) Immunostaining of CD34 (CD34 green, DAPI blue) on HFs (3-month) with a diagram to show locations of basal and suprabasal bSCs. These results were reproduced in two experiments and representative data are shown. α6, integrin α6; bSC, hair follicle bulge stem cell; cKO, conditional knockout; CON, control; eSC, interfollicular epidermal stem cell; VDR, vitamin D receptor.
Deletion of VDR blunts injury-induced proliferation of epidermal SCs and prevents activation of β-catenin signaling
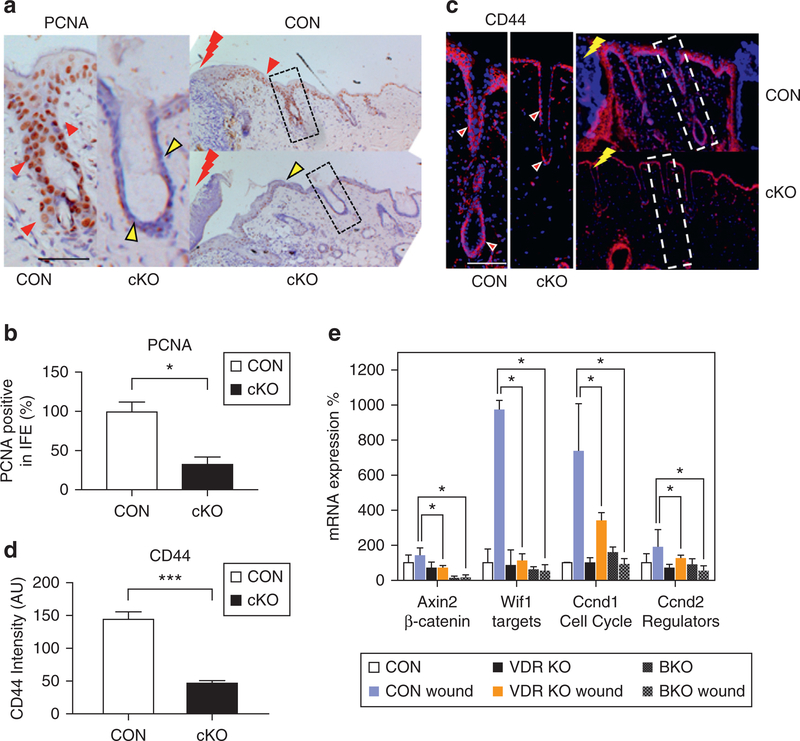
We then determined the impact of deleting VDR on the ability of SCs to respond to wounding to activate their proliferation. A full-thickness 3-mm skin biopsy was obtained from the back at 3 months (telogen skin) of cKO and CON mice. After 3 days, wounded skin and non-wounded CON skin were harvested, and wound-induced activation of SCs was evaluated by measuring cell proliferation using proliferating cell nuclear antigen. In CON skin, HFs were enlarged near the wound margin (shown by red bolt), where the number of proliferating cell nuclear antigene–positive proliferating cells markedly increased in both the HF and IFE (Figure 3a, red triangles, Supplementary Figure S3a, S3b). However, cKO HFs remained abnormal, and proliferating cell nuclear antigene–positive cells were considerably fewer in both HF (dotted box) and IFE (Figure 3a, yellow triangles, Supplementary Figure S3a, S3b), in which results were quantitated by counting the number of proliferating cell nuclear antigene–positive cells in the epidermis and HF at the wound margin (Figure 3b). Pathway analysis (Ingenuity Pathway Analysis; Qiagen, Hilden, GA) on microarray data generated a mechanistic hypothesis that VDR deletion causes defects through inhibition of β-catenin signaling, as shown by a heat map (Supplementary Figure S3c) and pathway model (Supplementary Figure S4 online). The β-catenin target gene of CD44 was specifically stimulated in epidermal cells in both IFE and HF at the wounding edge of CON (Figure 3c), but was lower in both HF (dotted boxes) and IFE in cKO wounds (Figure 3c, 3d). The decrease of Cd44 was restricted to epithelial tissues at the wounding edge, but was not observed in non-wounded normal skin (quantitative PCR) or in normal cells (microarray, Supplementary Figure S3c). Quantitative PCR demonstrated that wounding increased the expression of β-catenin target genes in CON, but their increased expression was blunted in the VDR cKO (Figure 3e) as shown previously (Oda et al., 2015). However, in this case, we compared the quantitative PCR results with comparable wound biopsies from Krt14-specific β-catenin knockout mice, which, as expected, showed little expression of these genes at baseline or after wounding (Figure 3e). These results demonstrate that VDR is essential for injury-induced activation of SCs and the stimulation of β-catenin signaling essential for SC activation.
Figure 3. VDR deletion blunts injury-induced proliferation of epidermal SCs and activation of β-catenin signaling.
(a) Cell proliferation evaluated by PCNA staining in CON and VDR cKO mice 3 days after wounding (brown signal and blue counterstaining; scale bar = 50 μm). (b) PCNA immunostaining quantification by Bioquant (*P < 0.05). (c) The β-catenin target gene CD44 expression in wounding edge after 3 days (CD44 red; DAPI blue; bar = 50 μm). (d) CD44 immunostaining quantification by Bioquant (***P < 0.001). (e) The mRNA levels for β-catenin targets (Axin2, Wif1) and cell cycle regulators (Ccnd1, Ccnd2) in the wounded skin from VDR cKO and from BKO wounded skin evaluated by quantitative PCR (*P < 0.05; n = 3). BKO, β-catenin knockout mice; cKO, conditional knockout CON, control; eSC, interfollicular epidermal stem cell; PCNA, proliferating cell nuclear antigen; VDR, vitamin D receptor.
Deletion of VDR impaired epidermal SC-driven regeneration during wound re-epithelialization
We then determined the role of VDR in regulating the ability of SCs and progeny to re-epithelialize the wound. First, we conducted lineage-tracing experiments to examine SC fate as described (Alcolea and Jones, 2014; Mascre et al., 2012). Mice lacking VDR were generated by mating tamoxifen-inducible Krt14CreERT2 mice expressing Rosa TdTomato (TdT) (red fluorescence) with floxed VDR mice (cKO). They were compared to CON mice with Krt14CreERT2 and RosaTdT, but lacking floxed VDR. The scheme in Figure 4a demonstrates the strategy. A single injection of low-dose tamoxifen was given to label some of the basal cells, including eSCs, to facilitate the ability to follow individual eSCs. After labeling, a full-thickness skin biopsy (2 mm) was taken from the plantar epidermis, where only IFE eSCs contribute to wound re-epithelialization because HF is not present in plantar epidermis. As expected, only a few basal cells are labeled in both CON and VDR cKO 1 day after tamoxifen injection in the biopsy samples, as shown by strongly fluorescent cells (Figure 4b, white triangles). Weak fluorescence in all of the basal cells is not considered as a labeling. After the wounds were completely healed (30 days), labeled eSCs proliferate from the adjacent skin into the wound to regenerate the epidermis, as strongly labeled cells are found in stable columns of basal and differentiated suprabasal cells. In contrast, cKO skin did not show clonal expansion (Figure 4b, cKO and Supplementary Figure S5 online), indicating an inability of SCs to regenerate epidermis. However, cKO closed the wounds, probably through unlabeled transient amplifying cells that may compensate SC defects. The results were quantitated by counting the number of red cells, including weak background cells, per epidermis on multiple sections and normalized by CON (Figure 4c, n = 4, P < 0.05). We also observed decreased ability of SCs to differentiate the epidermis in cKO as shown by decreased FLG expression in suprabasal layer of cKO epidermis compared to CON epidermis (Figure 4b, right panels). Similar results were obtained in back skin–wounding model (3-mm biopsy) (Supplementary Figure S5).
Figure 4. VDR ablation impairs SC-driven epidermal regeneration during wound re-epithelialization.
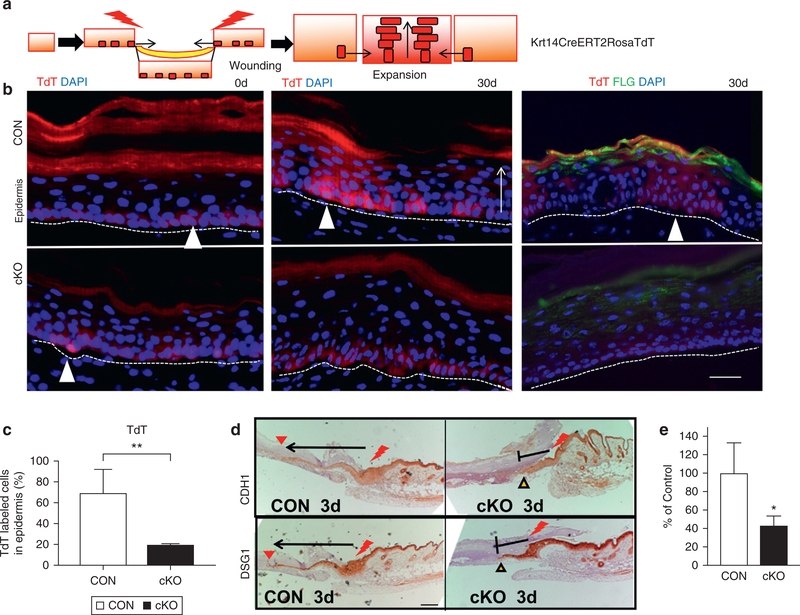
(a) The strategy for lineage tracing using Krt14CreERT2RosaTdT. (b) Representative images of initial skin biopsy (0 days) (left panels) and after 30 days of wound healing (middle panels), TdT-labeled SCs migrated to form clones in CON (white triangles, upper panels), but not in cKO (bottom panels). Differentiation was verified by FLG (right two panels) (TdT red; FLG green; DAPI blue). Scale bar = 25 μm (c) Quantification of the TdT-labeled cells in the epidermis by Bioquant (**P < 0.05). (d) Migration of epithelial tongues at 3 days, in which wounding edge is shown by red bolt, and the extent of the epithelial tongue is shown by arrows. Scale bar = 100 μm. (e) Quantitated migration at 3 days (n = 3, P < 0.05). cKO, conditional knockout CON, control; SC, stem cell; TdT, TdTomato; VDR, vitamin D receptor.
The migration of progeny also was examined in back skin–wounding model. The migration of epithelial tongues was shown by epithelial-specific proteins of E-cadherin (CDH1) and desmoglein 1 (DSG1). At an early stage of wound healing (3 days after wounding on a 3-mm biopsy in back skin), epithelia migrated from the original wound site (Figure 4d, red bolt) and extended (arrows) to reach the middle of the wounds (red triangles) in CON (Figure 4d). In contrast, the length of the epithelial tongue was markedly shorter in the cKO (Figure 4d stop signs with yellow triangles), as shown by quantitated data in Figure 4e (n = 3, P < 0.05). The migration delay was observed only at the early stage (3 days), and not at the late remodeling stage (5 days).
Deletion of VDR impaired epidermal remodeling by reducing E-cadherin and epidermal differentiation
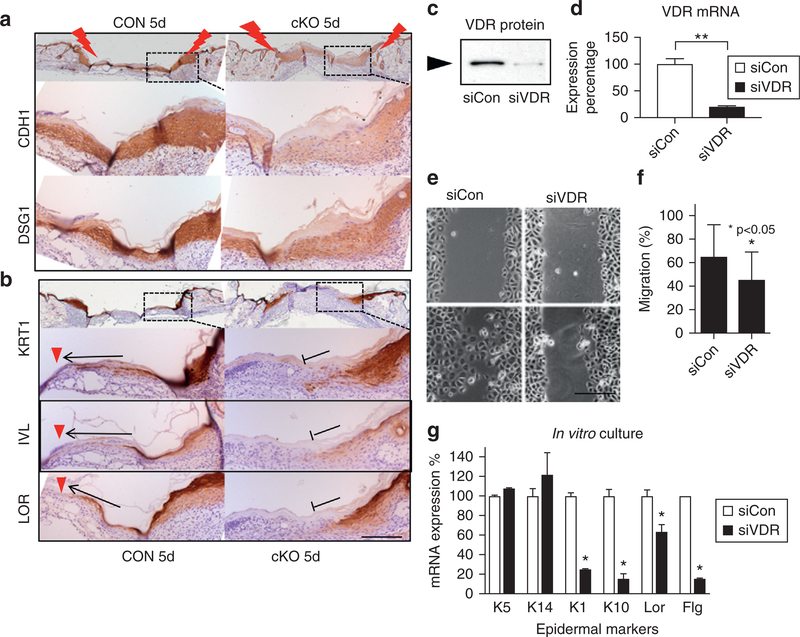
Ingenuity Pathway Analysis of microarray data also showed that VDR deletion decreased epithelial adherens junction signaling, as shown by a heat map (Supplementary Figure S6a online) and pathway model (Supplementary Figure S6b). We have previously shown that E-cadherin–mediated adherens junction signaling is essential for epidermal differentiation in a VDR-dependent manner (Bikle et al., 2012). Therefore, we determined the impact of deletion of VDR on E-cadherin and on the epidermal differentiation following re-epithelialization. Both epithelial tongues were merged to restore the epidermal layers in both CON and cKO wounds by 5 days after wounding (Figure 5a). The expression levels of E-cadherin (CDH1), a component of adherens junction to mediate epidermal differentiation, decreased in cKO wounds compared to CON (Figure 5a). The epithelial specific desmosomal component of DSG1 was likewise decreased in cKO. The early IFE marker of KRT1, was detected in the migrating epithelia covering the wound (arrow) through to the center of the wounds (red triangles) in CON (Figure 5b), demonstrating that epithelia are differentiating while the cells are migrating to close the wounds. However, KRT1 expression did not extend across the wound and remained disorganized in a patchy pattern in the shortened epithelial tongues of cKO wound (Figure 5b, KRT1, cKO stop sign). Similarly, impaired differentiation was shown by the blunted extension of the intermediate marker of Involucrin and the late marker of differentiation, Loricrin (Figure 5b). The decreased expression of CDH1 was observed only in newly regenerated epidermis covering the wounds (5 days), but not in surrounding non-wounded normal skin (Figure 5a) or keratinocytes isolated from normal skin (heat map in Supplementary Figure S6a).
Figure 5. VDR ablation prevents differentiation of progeny.
At late stage of wound healing (5 days after wounding), epithelial markers (a) and differentiation markers (b) are shown at the edge of CON and cKO wounds (brown with blue counterstaining). Arrows and red triangles show extension of differentiated cells in CON, and stop signs show their defects in cKO. Scale bar = 100 μm. (c–g) VDR was silenced in cultured keratinocytes (siVDR). Blocking efficiency of VDR by Western (c) and quantitative PCR (d) (**P < 0.01) is shown. (e) Images for cell migration by the scratch assay (scale bar = 25 μm) and (f) their quantitation (*P < 0.05, n = 12). (g) mRNA expression of differentiation markers (quantitative PCR, 1.2 mM calcium, 3-day culture) (n = 3, P < 0.05). cKO, conditional knockout CON, control; si, small interfering; VDR, vitamin D receptor.
To confirm whether the reduction in re-epithelialization in the cKO at 3 days is due to decreased migration and differentiation, we cultured human keratinocytes, in which VDR expression was blocked by transfection of small interfering (si) RNA (siVDR). The efficiency of VDR silencing in keratinocytes was confirmed by Western blotting (Figure 5c) and quantitative PCR (Figure 5d). After cells reached confluence, they were switched to supplement-free media to block further proliferation and with low-calcium medium (0.07 mM) to prevent differentiation. The monolayer culture was scratched with a pipette tip. After 16 hours, the cultures were photographed and the empty spaces remaining after cell migration were quantitated. Fewer siVDR cells migrated into the scratched area compared to control cells (siCON) as shown by representative images and quantitation (Figure 5e, 5f). Cells were switched to high-calcium media (1.2 mM) to induce epidermal differentiation. After 3 days, the expression levels of epidermal differentiation markers (Krt1, Krt10, Lor, Flg) were lower in siVDR cells compared to control cells (siCon), although basal keratins (Krt14, Krt5) did not change (Figure 5g). These data demonstrate that VDR regulates E-cadherin–mediated epidermal differentiation, as well as keratinocyte migration, each essential for wound re-epithelialization.
VDR ablation alters epidermal stem cell fate for hair, epidermis, and sebaceous glands
As the keratinocyte culture system represents only interfollicular epidermis, we evaluated an impact of deletion of VDR on different epidermal lineages in vivo. Microarray data also demonstrated that VDR deletion in vivo down-regulated hair differentiation genes, but up-regulated genes involved with epidermal and sebocyte differentiation (Supplementary Figure S7c online). The mRNA levels for representative differentiation markers for IFE (Flg) and SG (Scd1) were higher in the normal and wounded skin of VDR cKO and (Figure 6a) and in β-catenin null skin (same samples shown in Figure 3e). The expression of HF markers of Krt31 and Dlx3 were low to nearly undetectable levels in VDR cKO and β-catenin KO skin (Figure 6a). Consistent with mRNA data, the protein expression levels of hair keratin KRT71 was lower and that of FLG was higher in VDR cKO in both whole skin and epidermis (Figure 6b, Supplementary Figure S7a, S7b). The size of sebocytes and the SG was enlarged especially near the edge of the wound in VDR cKO (Figure 6c) (260% ± 55%, n = 3, P < 0.05) as observed in β-catenin KO skin (Lien et al., 2014). The expression of the FLG in interfollicular epidermis was similar in cKO. In contrast, FLG was specifically increased in the utricles in VDR cKO (Figure 6d, red arrows), indicating that HF keratinocytes are converted to an epidermal phenotype. These results indicate that VDR regulates cell fate commitment of epidermal SCs, potentially through regulation of β-catenin signaling.
Figure 6. VDR ablation alters cell fate of epidermal SC for hair, epidermis, and sebocytes.
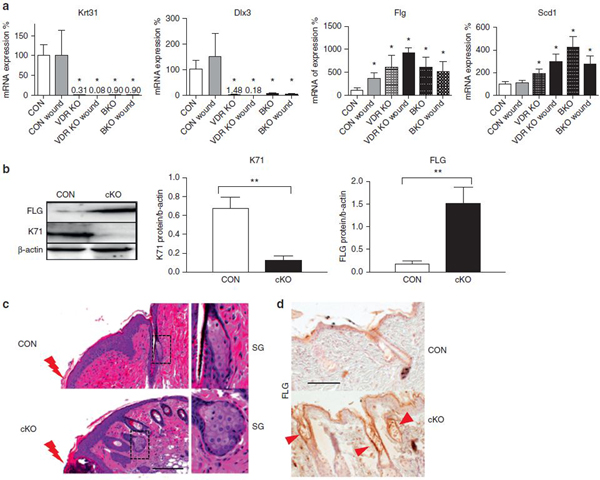
(a) The mRNA levels for markers for hair (Krt31, Dlx3), IFE (Flg), and SG (Scd1) were evaluated in control and wounded skin (3 days after wound) from VDR cKO and BKO (3 months) by quantitative PCR (*P < 0.05). (b) Levels of hair keratin KRT71 and epidermal marker of FLG in epidermis of CON and VDR cKO mice by Western blotting (**P < 0.01). (c) The enlarged sebocytes and SG at the wound edge (red bolt) are shown in hematoxylin and eosin–stained skin of CON and VDR cKO. Scale bar = 100 μm. (d) Increased level of FLG in cKO skin compared to CON. FLG expression in utricles are shown by red arrows. Scale bar = 100 μm. BKO, β-catenin knockout mice; CON, control; IFE, interfollicular epidermis; SC, stem cell; SG, sebaceous gland; VDR, vitamin D receptor.
We propose that VDR regulates wound re-epithelialization in different locations and different stages of the healing processes. First, VDR may be essential for self-renewal of the epidermal SCs residing in the bulge or epidermis, that are essential for injury induced activation through regulation of β-catenin signaling. VDR activated by its ligand, 1,25(OH)2D3 is translocated to the nucleus where it facilitates the transcription of β-catenin target genes involved with the regulation of self-renewal and activation of epidermal SCs through interaction with β-catenin and other transcription factors to regulate the genes involved in activation of epidermal SCs. Second, VDR may play an important role in later stages of wound healing, regulating the migration of the progeny to the wounds, and their subsequent differentiation to regenerate the epidermis.
VDR may also direct a lineage commitment of epidermal SCs. VDR ablation blunts hair fate by inducing SG fate and altering the regions of expression of genes associate with IFE fate. The epidermal marker of FLG was detected in abnormal HF (utricles) of VDR cKO skin (Figure 6e), suggesting that the bSCs and outer root sheath epithelium are converted to an epidermal fate, an observation consistent to β-catenin knockout mice and to an earlier study with the global VDR KO mice. These observations and the loss of β-catenin signaling in the VDR KO suggest that VDR may regulate SC fate through β-catenin signaling. In contrast, VDR supports epidermal differentiation of the regenerating epidermis as IFE markers of KRT1/Involucrin/Loricrin and FLG were reduced in cKO wounds (Figure 4b and 5c). VDR likely regulates differentiation through calcium and E-cadherin–mediated adherens junction signaling, in which adequate dietary calcium is required to maintain adherens junction formation, potentially accounting for the synergistic effects of a low-calcium diet in the VDR cKO epidermis (Bikle, 2011).
Our studies support a clinical role for adequate dietary calcium and vitamin D in cutaneous wound healing. A significant percentage of the population is vitamin D–deficient (Kojima et al., 2013), and vitamin D deficiency is associated with poor wound healing (Burkiewicz et al., 2012). Our study exploring the mechanisms by which VDR and calcium signaling regulate wound healing should further the development of methods to expedite wound healing.
MATERIALS AND METHODS
Animals
We used mice for floxed VDR, floxed β-catenin, Rosa tomato (TdT), Krt14-Cre, Krt14-CreERT2. Details for these mice are described in Supplementary Material online. All experiments were approved by the Institutional Animal Care and Ethics Committee at the San Francisco VA Medical Center.
FACS analysis
A single-cell suspension of freshly isolated primary keratinocytes was washed by FACS buffer (phosphate buffered saline containing 2% chelated fetal bovine serum) and incubated with the following antibodies: integrin α6-phycoerythrin (1:100 or 200; eBiosciences, San Diego, CA), CD34-eFluoro660 (1:100; eBiosciences) and Sca1-peridinin chlorophyll binding protein-Cy5.5 (1:200; eBiosciences) for 30 minutes on ice. The cells were washed with FACS buffer. Cell viability was assessed by 7-aminoactinomycin D labeling. FACS analysis was carried out using FACSAria sorters (BD Biosciences, San Jose, CA) running FACSDiva software (BD Biosciences), and analyzed using the FlowJo program (FlowJo, Ashland, OR).
Lineage tracing
The red fluorescent reporter gene Rosa TdT was introduced into mice expressing tamoxifen-inducible Krt14-CreERT2 with (cKO) or without (controls) the floxed VDR gene. First, the triple transgenic mice expressing floxed VDR and Krt14-CreERT2 and RosaTdT were prepared. The VDR gene was deleted and TdT expression was activated by intraperitoneal injection of tamoxifen (Sigma, St. Louis, MO) dissolved in corn oil at postnatal day 21. Details for clonal fate mapping are described in the Supplementary Material. Other methods are described in the Supplementary Material.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Menendez and W. Mayer for their assistance in maintaining the mice. We are also grateful to Rochelle Elp and Min Zhang for technical assistance. This work was supported by the National Institutes of Health grant R01 AR050023 (DDB), Department of Defense grant CA110338 (DDB), Veterans Affairs Merit I01 BX003814–01 (DDB) and the Chinese National Natural Science Foundation of China (81573075, 81301360 to LH) and the Science Foundation of Tianjin University (2013KY06 to LH).
Abbreviations:
- bSC
bulge stem cell
- cKO
conditional knockout
- CON
control
- eSC
interfollicular epidermal stem cell
- HF
hair follicle
- IFE
interfollicular epidermis
- SC
stem cell
- SG
sebaceous gland
- si
small interfering
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2018.04.033.
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Adam RC, Yang H, Rockowitz S, Larsen SB, Nikolova M, Oristian DS, et al. Pioneer factors govern super-enhancer dynamics in stem cell plasticity and lineage choice. Nature 2015;521:366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcolea MP, Jones PH. Lineage analysis of epidermal stem cells. Cold Spring Harbor Perspect Med 2014;4:a015206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol 2011;347:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Xie Z, Tu CL. Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab 2012;7:461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 2006;22:339–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkiewicz CJ, Guadagnin FA, Skare TL, do Nascimento MM, Servin SC, de Souza GD. Vitamin D and skin repair: a prospective, double-blind and placebo controlled study in the healing of leg ulcers. Rev Col Bras Cir 2012;39:401–7. [DOI] [PubMed] [Google Scholar]
- Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, et al. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 2013;13: 720–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci U S A 2007;104:9428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 2005;11:1351–4. [DOI] [PubMed] [Google Scholar]
- Kojima G, Tamai A, Masaki K, Gatchell G, Epure J, China C, et al. Prevalence of vitamin D deficiency and association with functional status in newly admitted male veteran nursing home residents. J Am Geriatr Soc 2013;61: 1953–7. [DOI] [PubMed] [Google Scholar]
- Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A 1997;94:9831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Guo X, Polak L, Lawton LN, Young RA, Zheng D, et al. Genome-wide maps of histone modifications unwind in vivo chromatin states of the hair follicle lineage. Cell Stem Cell 2011;9:219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Polak L, Lin M, Lay K, Zheng D, Fuchs E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat Cell Biol 2014;16:179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, et al. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 2013;342:1226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luderer HF, Nazarian RM, Zhu ED, Demay MB. Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-beta signaling during the inflammatory response to cutaneous injury. Endocrinology 2013;154:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012;489:257–62. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Fuchs E. Isolation and culture of epithelial stem cells. Methods Mol Biol 2009;482:215–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol 2015;164:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS One 2008;3:e1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol 2012;23:946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell 2006;21:799–809. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K, Hu L, Nguyen T, Sakai K, He B, Fong C, et al. Ablation of coactivator Med1 switches the cell fate of dental epithelia to that generating hair. PLoS One 2014;9:e99991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.