Purpose:
We examined patients presenting in a tertiary eye hospital in Nepal, focusing on information relevant to screening and management programs for vitreo-retinal (VR) disease.
Design:
Retrospective, cross-sectional study.
Methods:
We reviewed all patients presenting for the first time to the VR-clinic over 1 year. We quantified patient demography, symptoms and duration, systemic diseases, ophthalmological examinations, diagnostic investigations, and final diagnoses.
Results:
Of the 1905 cases, 1148 were male (60.3%). The 25th percentile of ages was 29 and 38 years for male and female, respectively; thus, female presented later (P < 0.0001). Hypertension was the commonest systemic disease (40.8%), followed by diabetes (32.5%). Age-related macular degeneration (AMD) and diabetic retinopathy (DR) affected 447 eyes (11.8%) and 416 eyes (10.9%), respectively. Male and female AMD and DR patients did not differ in age or disease duration. Similarly, age or disease duration for DR did not correlate with severity. Asymmetry of disease severity between eyes with AMD and DR was largest in patients with 1 normal eye. Presenting acuity was asymmetric between eyes (P < 0.0001) with people more often reporting once their right eyes had acuity of 6/18 or worse.
Conclusions:
The screening of blood pressure and glucose levels combined with fundus photography could prevent many from progressing to life-changing visual impairment and blindness. Later reporting by females began at childbearing age; therefore, education and ocular screening could be usefully coupled in reproductive health programs. Clubbing VR disease screening with other established health programs like diabetes control program, hypertension clinics, school health program, and so on, would provide economical and sustainable approach.
Keywords: AMD, diabetic retinopathy, retinal diseases, laterality, sex differences
Vitreo-retinal (VR) diseases are common causes of visual impairment and blindness. Population-based studies have reported the overall prevalence of VR disorders to be 8.56% (range 10.4%–21.02%) among the population aged 40 years and older.1,2 The 1981 Nepal Blindness Survey reported VR disorders as the third leading cause of bilateral blindness, second only to cataract and its complications.3 A more recent Nepalese population-based study reported VR disorders to be the second commonest cause of bilateral blindness, second only to cataract, and the most common cause among pseudophakics.4
A population-based study in Bhutan reported that 22.1% of visual impairment and blindness was due to VR pathologies among the population aged 50 years or older.5 A similar survey in Bangladesh reported VR diseases as the second leading cause of bilateral blindness accounting for 13.3%,6 and in India 17.1% among persons aged 30 years or older.7 By contrast, a Nigerian study reported a prevalence of 8.1%, with AMD, DR, retinal vein occlusion (RVO), and retinal detachment (RD) as the most common retinal diseases.8 An Ethiopian study reported RD as the commonest cause of both bilateral (59.4%) and unilateral (41.2%) blindness.9 The Tehran study reported VR prevalence of 8.56% with acquired retinopathies and peripheral lesions as the most common retinal diseases.2
In developed countries, AMD affects nearly 10% of those older than 65 years, and 25% of those older than 75 years,10 including Australia.11 In the United States, >8 million people have intermediate AMD and nearly 2 million have advanced AMD.12 In the UK, the prevalence of late AMD was 2.4% among the population aged 50 years and older, 4.8% for 65 years or older, and 12.2% for 80 years or older.13
DR often affects adults of working age.14 The American National Health and Nutritional Examination Survey 2005–2008 reported that 28.5% of diabetic patients had some degree of DR, and 4.4% had vision-threatening DR.15 In 2012, global prevalence was 34.6% for any DR, 6.96% for proliferative DR (PDR), 6.81% for diabetic macular edema, and 10.2% for vision-threatening DR.16 India and China are confronting a growing epidemic of diabetes and DR.17–19
The current study focuses on the features of VR diseases presenting to a tertiary eye care center in Nepal. Surprisingly the results indicate that analysis of the disease laterality is sometimes required. The study provides clear recommendations for improving care that are applicable outside Nepal, and the work provides a baseline for planned comparison studies.
METHODS
Setting
This study was conducted at the Tilganga Institute of Ophthalmology (TIO), Kathmandu, Nepal. The TIO is a tertiary eye care center with subspeciality clinics providing services for ophthalmological patients from all over Nepal, India, and Bhutan. All subspeciality clinics in the TIO, including the VR clinic, run from morning till afternoon, and an extended paying clinic operates in the evening. Despite this availability of extended facilities, 1843 cases (96.7%) presented to the routine public VR clinic. Only 36 cases (1.9%) presented to the paying clinic, whereas 26 cases (1.4%) reported to the emergency department.
Study Design and Ethics
The study has been approved by the TIO-Institutional Review Committee (TIO-IRC) vide letter number: Ref: 10/2018. The need for consent was waived by TIO-IRC because the retrograde study collected de-identified data from patient files.
Study Population
This study covers all cases presenting to the VR clinic for the first time over 1 year. Patients who presented for repeat or follow-up visits were not included. The study ran from January 1, 2010 to December 31, 2010. A key objective of this study is to provide a baseline reference for planned follow-up studies that will use similar analyses. A further benefit of recording this hospital-based study is that there are population-based studies of disease prevalence from both Nepal4,20 and Bhutan5 from 2009/2010 with which this study can be compared, adding extra value for both the region and similar countries.
Data Collection and Analysis
Detailed demographic information, presenting complaints, treatment history, and associated systemic diseases were noted. Best corrected visual acuity (BCVA), refractive status, Goldmann applanation tonometry, findings of slit-lamp examination, 90D biomicroscopy, fundoscopy, and diagnostic investigations performed were recorded. Diagnoses were noted separately for right and left eyes. Data collection was supervised by the head of the VR unit to ensure high quality and uniform practice. Hypertensive retinopathy was graded according to Keith-Wagener-Barker classification system,21 and DR was classified as per the modified Airlie House and Abbreviated Early Treatment Diabetic Retinopathy Study (ETDRS) classification of DR.22
The data were analyzed using SPSS (version 20.0, IBM, New York, NY) and MATLAB (2016b, The MathWorks, Natick, MA). Comparisons of the expected and observed frequency of sex or eye-wise effects were done using Chi-squared tests. To compare the 10th, 25th, 50th, 75th, and 90th percentiles of the distributions of Figure 1B for male and female, we applied a sampling-with-replacement-based bootstrap analysis23 to estimate the population means and the standard errors (SE) of the means of those percentiles. We employed 10,000 bootstrap cycles to insure the estimated means and SE converged to within 2 decimal places on 5 independent cross-validations. We then applied t tests employing the estimated means and SE.
FIGURE 1.
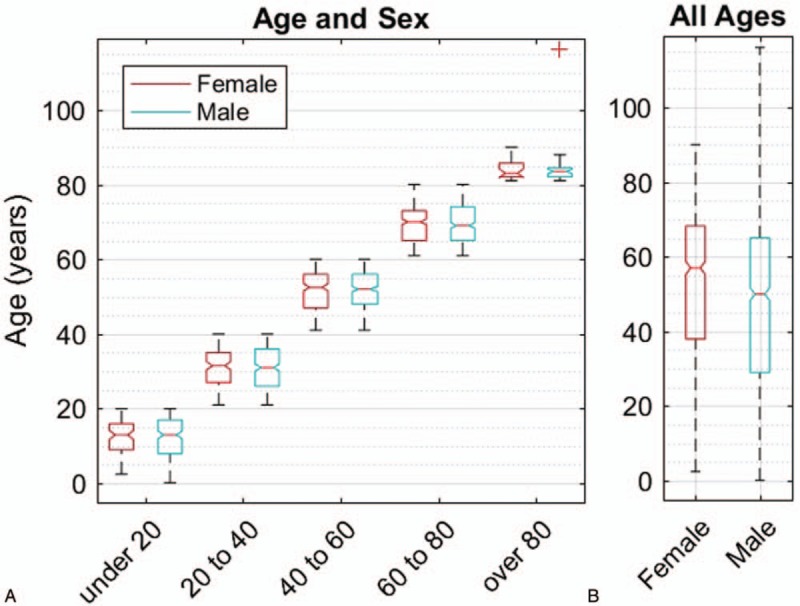
Age and sex boxplot. A, Presenting ages of the 1905 patients in cohorts of 20 years. The boxes indicate 25th, 50th, and 75th percentile of the age distributions. The red + represents a 116-year-old male outlier. B. The overall distribution of ages. A bootstrap analysis showed that the 10th, 25th, 50th, and 75th percentiles for female were all significantly older than for male (Text).
RESULTS
We will first give an overview of the general presentation of the 1905 cases. We will then present the ocular disease data as: the nonretinal diseases involving posterior segment other than retina, and the main analysis of the retinal diseases. Background data are presented in 5 Supplementary tables.
General Presentation
The 1905 new cases presenting to the VR clinic were split as: 1148 male (60.3%) and 757 female (39.7%). Demographic data are shown in Table 1. Figure 1A gives a breakdown of the presenting ages in 20-year cohorts for each sex. Figure 1B shows that, for the whole cohort. The 25th percentile was 29 years for male and 38 years for female. A bootstrap analysis revealed that the 10th, 25th, 50th and 75th percentiles were all significantly older for female than male by 3.95, 9.28, 7.25, and 3.08 years (ie, median is 5.6 years). The 10th percentile difference was significant at P < 0.003, and the others at P = 0.0001. It is noted that the 25th percentiles for the males were lower in the 20-year cohorts of Figure 1A as well.
TABLE 1.
Demographic Characteristics of Patients
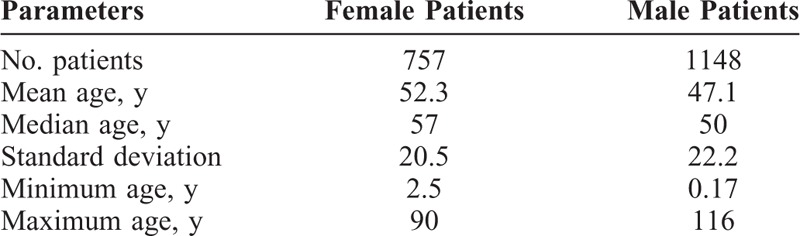
A total of 802 cases (42.1%) were from the Kathmandu valley, 1038 cases (54.5%) were from Nepal outside the Kathmandu valley, and 65 cases (3.4%) were from other countries. A total of 1574 cases (82.6%) reported to the hospital as primary cases, whereas 331 cases (17.4%) were referred from other hospitals or health care centers.
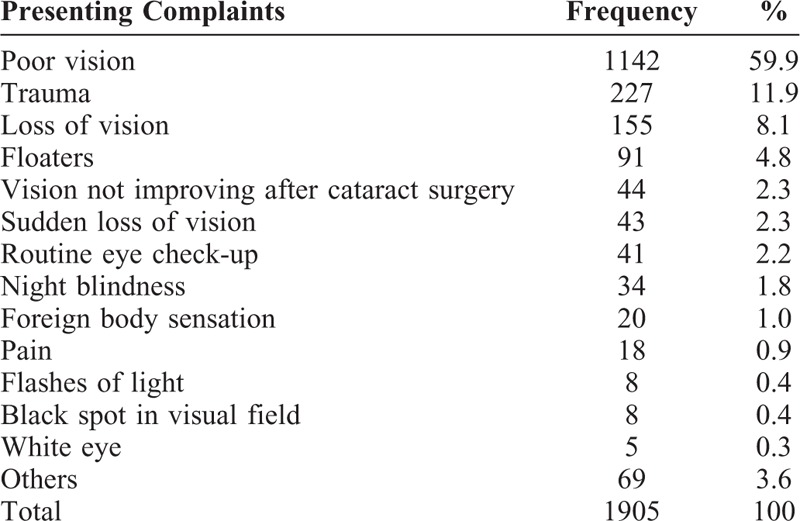
The commonest presenting complaint was poor vision accounting for 1142 cases (59.9%), followed by trauma in 227 (11.9%), loss of vision in 155 (8.1%), floaters in 91 (4.8%), and vision not improving after cataract surgery in 44 (2.3%). All presenting complaints are summarized in Table 2. A total of 565 cases (30.5%) presented within a month of the onset of their symptoms, whereas 500 (27.0%) presented only after 12 months. In 53 cases, we could not affirm the time (Supplemental Digital Content 1, Table S1).
TABLE 2.
Presenting Symptoms
Hypertension was the commonest systemic disease associated in 250 cases (40.8%), followed by diabetes in 199 cases (32.5%) and combined diabetes and hypertension in 124 cases (20.2%) (Fig. 2). The other systemic diseases found in 40 cases (6.5%) are summarized in Supplemental Digital Content 1, Table S2. The duration of systemic disease association was found to be <5 years in 42.8% of cases, 5 to 10 years in 33.2%, and >10 years in 24.0%.
FIGURE 2.
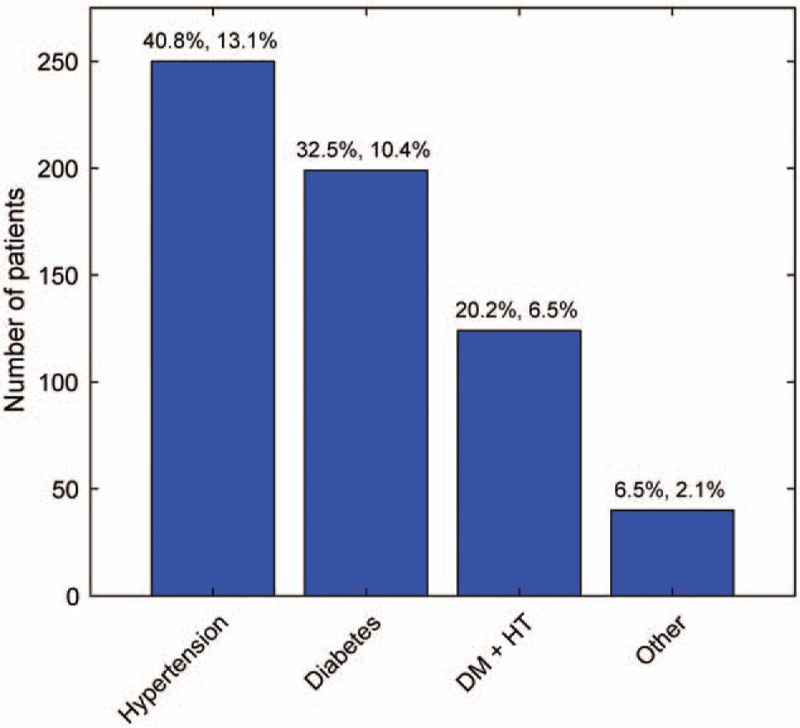
Systemic disease prevalence. The breakdown of the commonest systemic diseases found in 613 (32.2%) of the 1905 patients. The values at the top of the columns give the percentage of the systemic disease patients (left), and the percentage of all patients (right). DM indicates diabetes mellitus; HT, hypertension.
We quantified interventions done at other centers before patients reported to our clinic, including surgical, laser, or ophthalmic injections. These cases included cataract surgery in 55 (2.9%), VR surgery in 27 (1.5%), retinal laser in 27 (1.5%), and primary repair of corneoscleral tear or eyelid lacerations in 20 (1.1%). All previous interventions are summarized in Table 2.
Hematological testing was the most commonly diagnostic test performed in 584 cases (23.1%), followed by optical coherence tomography in 516 (20.4%), B-scan in 436 (17.2%), fundus fluorescence angiography in 89 (3.5%), and visual fields in 39 (1.5%). Diagnostic investigation was not indicated or not done in 687 cases (27.1%). Supplemental Digital Content 1, Table S4, summarizes the diagnostic procedures.
BCVA was 6/18 or better in the right eye in 660 cases (34.8%), and in left eyes in 1025 cases (54.1%). In 751 cases (39.4%) right eyes, and 355 cases (18.6%) left eyes, the BCVA was 6/18 to 6/60. Figure 3 illustrates how surprisingly asymmetric these values were (P < 0.0001, see legend). These findings prompted us to explore our data to see whether any other such asymmetries occurred. Cases with BCVA <6/60 or no perception of light (NPL) were more common on the left, but not significantly so. NPL was found in 37 (1.8%) right eyes and 44 (2.3%) left eyes.
FIGURE 3.
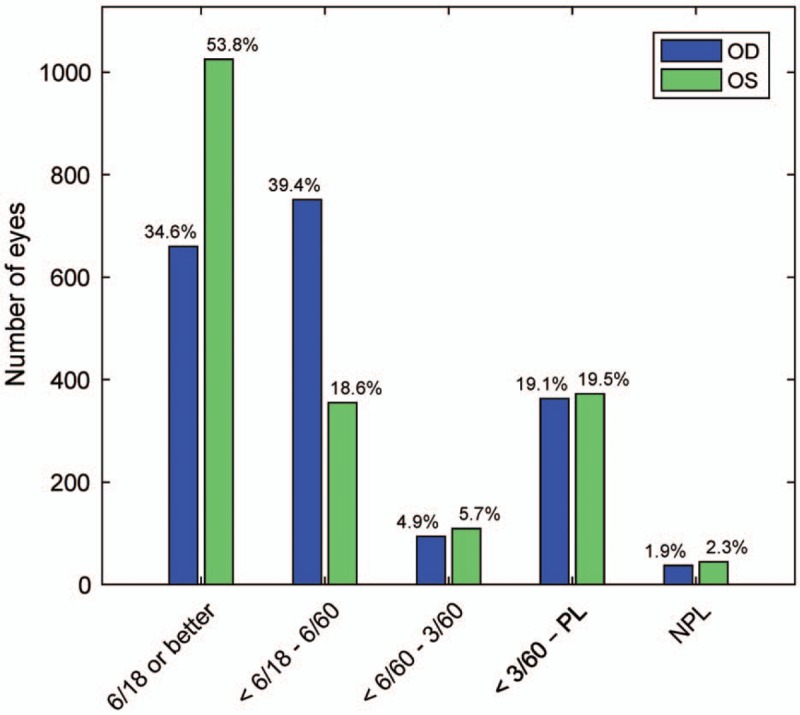
Presenting BCVA. The presenting BCVAs provided significant asymmetries between eyes (P < 0.0001, Bonferroni corrected) in the 2 groups with acuities of 6/60 or better (chi-square = 158 for “6/18 or better”, and 283 for “6/18 to 6/60”). BCVA indicates best corrected visual acuity; NPL, no perception of light; PL, perception of light.
Nonretinal Diseases
Before detailing the VR diseases, we quickly summarize the other issues discovered. Posterior vitreous detachment was the commonest nonretinal disease with 79 cases (35 OS). There were 51 cases of endophthalmitis (24 OS): 33 (64.7%) were post-traumatic, 13 (25.5%) postoperative, and 5 (9.8%) endogenous in origin. The other diseases involving posterior segment other than the retina are enumerated in Supplemental Digital Content 1, Table S5.
Most of the trauma-related conditions involved the left eyes, but this bias did not reach statistical significance. When pooled together, atrophic bulbi, phthisis, and absolute eyes were more common in left eyes: 16 (0.8%) compared with only 8 right eyes (0.4%, P = 0.05, chi-square). Dislocated cataract was similar in left eyes: 14 (0.8%) compared with 10 right eyes (0.5%, P = 0.25, chi-square). Dislocated intraocular lens into the vitreous cavity was also seen in 7 left eyes (0.4%) and 3 right eyes (0.2%, P = 0.10, chi-square). Retained intraocular foreign body involved 7 left and 4 right eyes (P = 0.15, chi-square). Open- and close-globe injuries affected both eyes equally. The fundus was not visible in 6 left eyes (0.3%), and 7 right eyes (0.4%).
Retinal Diseases
The commonest retinal disease was AMD, affecting 223 right eyes (11.7%) and 224 left eyes (11.8%), followed by DR involving 208 left, and 208 right eyes (10.9%). RD was the third commonest, affecting 112 right eyes (5.9%) and 126 left eyes (6.6%), of which 71% were rhegmatogenous, 17.2% exudative, and 11.8% tractional RD. There were 150 cases of branch RVO (BRVO), 80 in right and 70 in left eyes. Myopic degeneration affected 63 right and 54 left eyes, and retinitis pigmentosa (RP) was seen in 56 right and 57 left eyes. Other retinal diseases diagnosed were hypertensive retinopathy in total of 87 eyes, central serous chorioretinopathy in 77 eyes, central RVO in 65 eyes, macular scar in 53 eyes, macular hole in 55 eyes, retinal vasculitis in 46 eyes, and others detailed in Table 3. 94.8% of full-thickness macular hole and 84.6% lamellar macular hole were unilateral. The fundus was found to be normal in 623 left eyes (32.7%) and 593 right eyes (31.1%). The types of AMD and the severity of DR are shown in Table 4.
TABLE 3.
Diseases Involving Retina
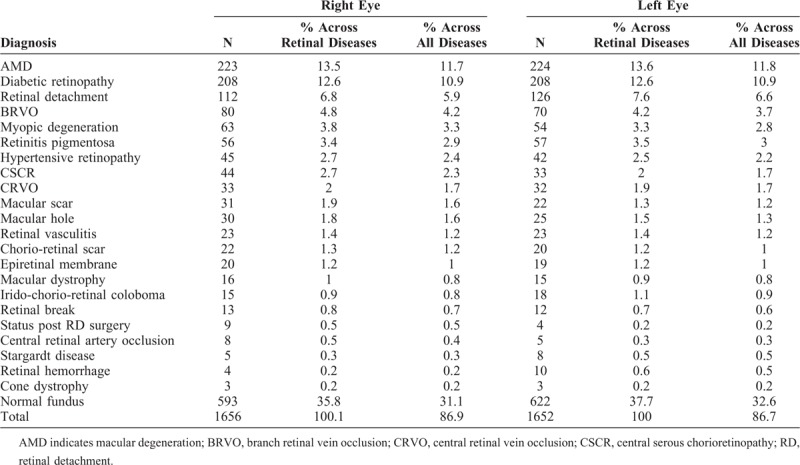
TABLE 4.
Types of AMD and Severity of DR
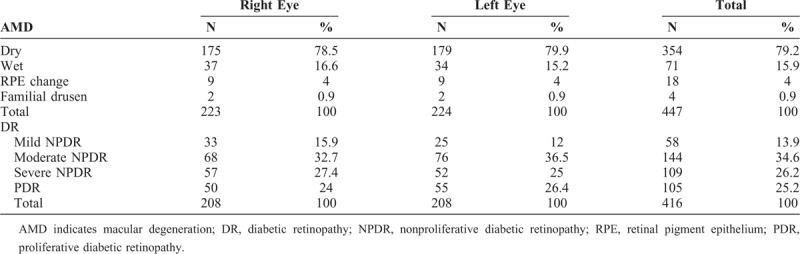
Among the AMD patients, dry AMD was the commonest type (79.2%), followed by wet AMD (15.9%), retinal pigment epithelium (RPE) change (4%), and familial drusen (0.9%). In contrast to the results of all patients (Fig. 1), there was no statistical difference in the number of male (122) and female (100) AMD patients. The male and female AMD patients also did not differ significantly in age: male 71.0 ± 9.48 years, female 68.8 ± 10.3 years (mean ± SD years). Among these patients, 81.7% of the AMD was bilateral and 18.3% unilateral. As might be expected, patients with unilateral disease were significantly younger than those with bilateral disease: 67.0 ± 13.1 cf. 70.7 ± 8.93 years (P = 0.031, t test).
In part because of the higher rate of smoking by male in Nepal (32.8% among male cf. 15.8% among female),24 we decided to examine wet AMD eyes relative to fellow eyes. 2 patients had familial bilateral drusen, and for this analysis they were classed as having dry AMD. 4 patients had nonvisible fundus (mainly due to cataract) and were eliminated from the analysis. The age of male whose worst eye had wet AMD was not different from female: 66.0 ± 15.3 versus 65.8 ± 13.7 years. There were 40 males whose worst eye had wet AMD, versus 15 females, which was marginally significant (P = 0.063, t test, correcting for the relative abundance of males in the study population). Interestingly, patients whose worst eye had dry AMD were older than those with a worst eye having wet AMD [69.5 ± 14.8 versus 65.9 ± 10.5 years (P = 0.045)]. Overall, there was a suggestion that males develop wet AMD earlier, and in relatively greater numbers compared with females. This might have been an effect of smoking, but further investigation is needed.
We also examined severity and laterality by scoring normal fundus to wet AMD on a scale of 1 to 3 (normal, dry, wet) and then examining the absolute value of the difference in scores between eyes. 6 patients had bilateral wet AMD OU. For the 186 patients whose best eye had dry AMD, only 30 had a worst eye with wet AMD. Patients whose best eye was normal were even more heterogeneous. Of those 36 patients, 17 had a fellow eye that was dry, and 19 had a fellow eye that was wet. Thus, the pattern of progression seemed to follow relative heterogeneity earlier, progressing to more bilateral disease later, rather than simple bilateral disease at each stage.
Among DR eyes, moderate nonproliferative DR (NPDR) was the commonest (34.6%), followed by severe NPDR (26.2%), proliferative diabetic retinopathy (PDR) (25.2%), and mild NPDR (13.9%). A total of 230 eyes had clinically significant macular edema (CSME): 50.9% involving left eyes and 49.1% right eyes. Like the AMD patients, the DR group contained more males (123) than females (91), but this was not significant. Their ages also did not differ at 58.7 ± 9.91 years for males, and 58.3 ± 11.21 years for females. Their durations of diabetes did not differ at 11.5 ± 6.79 and 10.4 ± 6.96 years, respectively. 9 patients had a nonvisible fundus in 1 eye and were removed from further analysis. We examined the laterality of DR in the remaining 205 patients.
We scored the 5 DR diagnostic-categories/severities from normal fundus to PDR as 1 to 5. As with AMD, the pattern of progression seemed to follow relative heterogeneity earlier, progressing to more bilateral disease later. As with AMD, we then binned subjects according to the diagnosis in their least affected eye. To quantify the degree of laterality, we took the absolute value of the difference of these severity steps in each pair of eyes. A box plot of the results is shown in Figure 4A. Basically, as with AMD, eyes tended to become more similar as severity of the best eye increased. As shown in Figure 4B, severity of DR was not correlated with duration of disease.
FIGURE 4.
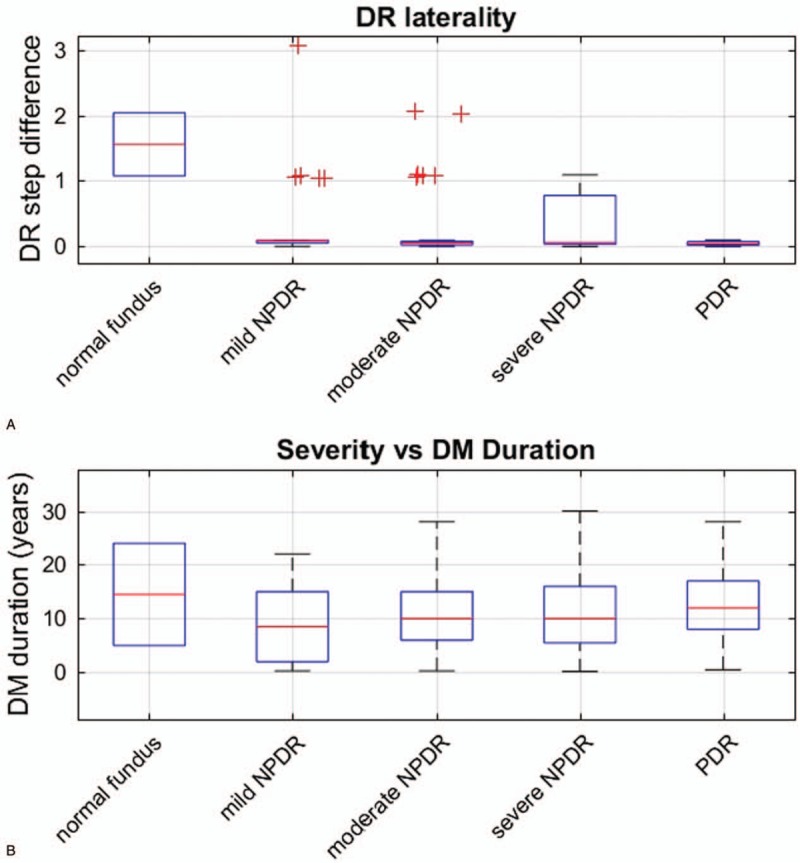
DR laterality and DM duration. Laterality and duration disease grouped by the 205 DR patients’ better eye (abscissa). A, The absolute value of the difference between DR-severity steps in the 2 eyes when the severity levels (normal to PDR) are scored as 1 to 5. Generally the DR became more bilaterally symmetric. A small amount of uniformly distributed noise (0–0.03) was added to the data to make the outliers more distinct (red+). B, Diabetes duration as a function of the best eye. Duration did not seem to strongly determine severity. DM indicates diabetes mellitus; DR, diabetic retinopathy; NPDR, nonproliferative DR; PDR, proliferative diabetic retinopathy.
DISCUSSION
The visual handicap experienced by individuals suffering from unilateral eye disease, such as macular hole, is reported to be strongly influenced by ocular dominance.25 Ocular-dominance and handedness are associated, with about 65% of right-handers, and 43% of left-handers, being right eye-dominant.26 Some of our data (Fig. 3) suggested that eye dominance played a role in patients reporting to the hospital, with the predominantly right-handed subjects only reporting that their right eye had BCVA worse than 6/18 once. This type of asymmetry has been reported before.25 AMD and DR were very homogenous with respect to age and sex; however, when the best eye was normal, the range of severity in the fellow eye was surprisingly broad (eg, Fig. 4). Taking together the results might mean that if their nondominant eye is affected, then patients tended not to notice their sight threatening disease, indicating an excellent argument for more screening. Given the subjects only reporting (Fig. 3), we subsequently analyzed other data by eye (Tables 3 and 4, and Supplemental Digital Content 1, Table S4) to elucidate any eye-wise biases; however, few were found.
The preponderance of males in the total group was 60.3%, and for subgroup of AMD and DR patients combined it was 56.5%, marginally more than for females (P = 0.052). This agrees with the findings of some hospital-based studies on VR diseases,9,27 but differs from a Nepalese population-based study from the same era, in which only 45.5% were male,20 and 1 in India with only 45% male.1 Our results could be due to women being reserved due to social norms and therefore not coming forward for medical check-ups. Here the 25th percentile of ages reporting was nearly 10 years higher for female (P < 0.0001, t test 9.27 years; 95% confidence level (CL) 6.05 and 12.5 years). The mean and median ages of male and female (49 years and 54 years, respectively) tally well with other studies of VR diseases.1,9 Reported ages ranged from 2 months to 116 years.
Only 41 cases (2.2%) presented for routine check-up. Chronic retinal diseases like AMD, DR, RD, RVO, RP, and so on, ranked high in the diagnostic list, but only 2.2% of cases were found to present for routine check-up, indicating that there is a need to emphasize patient education and counseling about the importance of regular check-ups and follow-ups.
A total of 565 cases (30.5%) presented within a month, 507 cases (27.1%) between 1 to 6 months, 280 cases (15.1%) between 6 to 12 months, and 500 cases (27.0%) presented after 12 months from the onset of the symptoms. The delayed presentation time might be because of illiteracy and ignorance (the overall literacy rate of Nepal for population aged 5 years and above was 65.9% in 2011, 75.1% for male and 57.4% for female).28 Remote areas and associated poor accessibility to modern medical services encourage people initially to rely on traditional methods of healing.
Systemic disease association was not found in 1292 of the 1905 cases. As shown in Fig. 2 (and Supplemental Digital Content 1, Table S2), among the 613 remaining cases, the commonest associated diseases were hypertension (40.8%), diabetes (32.5%), and both (20.2%). This agrees with the Tehran eye study which reported hypertension (21.14%), followed by diabetes (15.99%).2 By contrast, a Nigerian study found diabetes to be more common (14.6%) than hypertension (13.2%).27 Other systemic diseases were found in 40 of our cases (6.5%, Supplemental Digital Content 1, Table S2). The duration of systemic disease association was <5 years in 210 cases (42.8%), 5 to 10 years in 163 cases (33.2%), and >10 years in 118 cases (24.0%).
The commonest VR disease was AMD affecting 11.8%, followed by DR at 10.9%; matching another Nepalese study which reported that AMD was the commonest VR disease at 28.3%, followed by DR at 17.9%.20 This does not match with a Nigerian hospital study, which reported DR (24.9%) as the commonest VR disease, followed by hypertensive retinopathy (13.3%) and AMD (10.7%).27 In our case, 208 of 324 (64.2%) confirmed diabetic cases had some form of DR, which did not tally with population-based studies (10.5%).1 In our study, 117 left eyes and 113 right eyes of 323 patients had CSME but a population-based study in Nepal found it in only 2 of 305 diabetic cases.20 This disparity could be explained by the early onset of macular edema causing a high percentage of CSME patients presenting to the TIO.
RD was the third commonest VR disease in our study, affecting 112 right eyes (5.9%) and 126 left eyes (6.6%), whereas a Nepalese population-based study reported population prevalence of 0.10%.20 A hospital-based study in Ethiopia reported RD as the second commonest VR disease at 24.5%.9 Of 65 cases of full-thickness macular hole in our study, 33 cases (50.8%) affected right eyes, whereas others have reported 48% of right eyes affected.25 In our study, 94.8% of the full-thickness macular hole and 84.6% of the lamellar macular hole were unilateral, which are similarly reported in other studies, and are basically the consequence of factors affecting the macula locally.25 When such unilateral macular diseases affect the dominant eyes of the individuals, they cause greater functional visual impairment.25
In eastern Asia, pathological myopia is a major issue found in 80% to 90% of school-leavers, and 10% to 20% of those completing secondary school,29 and contributes to RD numbers. The prevalence of myopia varies from 0.8% to 53.4%, depending on geographical area, age, occupation, and ethnicity.30–32 In Nepal, Sherpa children had a prevalence of 2.9% as compared with 21.7% for Tibetan children.33 A myriad of myopic complications like atrophic retinal holes and RD, choroidal neovascular membranes, degeneration, cataract, and glaucoma, cause visual loss warranting attention.34 Thapa et al20 reported the population prevalence of macular hole as 0.20% in Nepal. Asymptomatic macular holes occur at a prevalence of 6.26% among high myopes with >−20 diopters.35
Limitations
19 patients were excluded due to incomplete data. AMD was not classified as per the Age-Related Eye Disease Study (AREDS) classification system. Only the final diagnoses, but not ocular comorbidities, have been considered for analysis, which might have altered the reported disease patterns. The ocular dominance and handedness were not recorded in the case records of the study population, but based on the literature reviewed and cited above, we speculated that if only the nondominant eye was affected, this might cause the patients to present late. In future studies it would be valuable to record the dominant eye. Similarly, the reviewed medical records of the diabetic patients under study did not differentiate the type of diabetes, which we will consider in follow-up studies.
CONCLUSIONS
We found that without screening programs, patients often fail to notice developing visual impairment until the disease progresses to advanced stage, especially in their nondominant eye. In Nepal, and perhaps in similar countries, females report later for care than men. That was true from childbearing age, so education and screening could be usefully coupled for reproductive health programs. This study indicates that low-cost screening and management programs for retinal disease could be of immense value in developing countries. The high prevalence of hypertension and diabetes among retinal disease patients suggest that a simple screening of blood pressure and glucose levels combined with fundus photography could prevent many from progressing to life-changing visual impairment and blindness. We recommend that coordinating with other established health programs would provide economical and sustainable screening methods such as diabetes control program for screening DR, hypertension clinics for screening hypertensive retinopathy, reproductive health program for VR diseases affecting female of childbearing age, comprehensive school health program for pathological myopia and hereditary diseases among school children, and counseling and rehabilitation centers for counseling people affected with permanent visual impairment or blindness, and other hereditary diseases.
Supplementary Material
Footnotes
Funding: The study did not receive any funding.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Nirmalan PK, Katz J, Robin AL, et al. Prevalence of vitreoretinal disorders in a rural population of southern India: the Aravind Comprehensive Eye Study. Arch Ophthalmol 2004; 122:581–586. [DOI] [PubMed] [Google Scholar]
- 2.Hatef E, Fotouhi A, Hashemi H, et al. Prevalence of retinal diseases and their pattern in Tehran: the Tehran eye study. Retina 2008; 28:755–762. [DOI] [PubMed] [Google Scholar]
- 3. Brilliant GE, Pokhrel RP, Grasset NC, et al. The Epidemiology of blindness in Nepal: report of the 1981 Nepal Blindness survey Nepal; 1981. [Google Scholar]
- 4.Thapa SS, Berg RV, Khanal S, et al. Prevalence of visual impairment, cataract surgery and awareness of cataract and glaucoma in Bhaktapur district of Nepal: The Bhaktapur Glaucoma Study. BMC Ophthalmology 2011; 11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepcha NT, Chettri CK, Getshen K, et al. Rapid assessment of avoidable blindness in Bhutan. Ophthal Epidemiol 2013; 20:212–219. [DOI] [PubMed] [Google Scholar]
- 6.Wadud Z, Kuper H, Polack S, et al. Rapid assessment of avoidable blindness and needs assessment of cataract surgical services in Satkhira District, Bangladesh. Br J Ophthalmol 2006; 90:1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dandona L, Dandona R, Naduvilath TJ, et al. Is current eye-care-policy focus almost exclusively on cataract adequate to deal with blindness in India? Lancet 1998; 351:1312–1316. [DOI] [PubMed] [Google Scholar]
- 8.Nwosu SN. Prevalence and pattern of retinal diseases at the Guinness Eye Hospital, Onitsha, Nigeria. Ophthal Epidemiol 2000; 7:41–48. [PubMed] [Google Scholar]
- 9.Teshome T, Melaku S, Bayu S. Pattern of retinal diseases at a teaching eye department, Addis Ababa, Ethiopia. Ethiop Med J 2004; 42:185–193. [PubMed] [Google Scholar]
- 10.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology 2001; 108:697–704. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell P, Bressler N, Doan QV, et al. Estimated Cases of Blindness and Visual Impairment from Neovascular Age-Related Macular Degeneration Avoided in Australia by Ranibizumab Treatment. PLoS One 2014; 9:e101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratnapriya R, Chew EY. Age-related macular degeneration—clinical review and genetics update. Clin Genet 2013; 84:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen CG, Jarrar Z, Wormald R, et al. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol 2012; 96:752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol 2012; 60:428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010; 304:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang FH, Liang YB, Zhang F, et al. Prevalence of diabetic retinopathy in rural China: the Handan Eye Study. Ophthalmology 2009; 116:461–467. [DOI] [PubMed] [Google Scholar]
- 18.Gadkari SS, Maskati QB, Nayak BK. Prevalence of diabetic retinopathy in India: The All India Ophthalmological Society Diabetic Retinopathy Eye Screening Study 2014. Indian J Ophthalmol 2016; 64:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raman R, Ganesan S, Pal SS, et al. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study III (SN-DREAMS III), report no 2. BMJ Open Diabetes Res Care 2014; 2: e000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thapa SS, Thapa R, Paudyal I, et al. Prevalence and pattern of vitreo-retinal diseases in Nepal: the Bhaktapur glaucoma study. BMC Ophthalmol 2013; 13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downie LE, Hodgson LA, Dsylva C, et al. Hypertensive retinopathy: comparing the Keith-Wagener-Barker to a simplified classification. J Hypertens 2013; 31:960–965. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes 2013; 4:290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Efron B. Bootstrap methods: another look at the jackknife. Ann Statist 1979; 1:1–26. [Google Scholar]
- 24.Sreeramareddy CT, Ramakrishnareddy N, Harsha Kumar H, et al. Prevalence, distribution and correlates of tobacco smoking and chewing in Nepal: a secondary data analysis of Nepal Demographic and Health Survey-2006. Subst Abuse Treat 2011; 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waheed K, Laidlaw DAH. Disease laterality, eye dominance, and visual handicap in patients with unilateral full thickness macular holes. Br J Ophthalmol 2003; 87:626–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McManus IC, Porac C, Bryden MP, et al. Eye-dominance, writing hand, and throwing hand. Laterality 1999; 4:173–192. [DOI] [PubMed] [Google Scholar]
- 27.Eze BI, Uche JN, Shiweobi JO. The burden and spectrum of vitreo-retinal diseases among ophthalmic outpatients in a resource-deficient tertiary eye care setting in South-Eastern Nigeria. Middle East Afr J Ophthalmol 2010; 17:246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. NPHC. National Population and Housing Census 2011 (National Report). In: Commission, N.P., editor. Kathmandu, Nepal 2012. [Google Scholar]
- 29.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet 2012; 379:1739–1748. [DOI] [PubMed] [Google Scholar]
- 30.Saw SM, Katz J, Schein OD, et al. Epidemiology of myopia. Epidemiol Rev 1996; 18:175–187. [DOI] [PubMed] [Google Scholar]
- 31.Nepal BP, Koirala S, Adhikary S, et al. Ocular morbidity in schoolchildren in Kathmandu. Br J Ophthalmol 2003; 87:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adhikari S, Nepal BP, Shrestha JK, et al. Magnitude and determinants of refractive error among school children of two districts of Kathmandu, Nepal. Oman J Ophthalmol 2013; 6:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garner LF, Owens H, Kinnear RF, et al. Prevalence of myopia in Sherpa and Tibetan children in Nepal. Optom Vis Sci 1999; 76:282–285. [DOI] [PubMed] [Google Scholar]
- 34.Tano Y. Pathologic myopia: where are we now? Am J Ophthalmol 2002; 134:645–660. [DOI] [PubMed] [Google Scholar]
- 35.Coppé AM, Ripandelli G, Parisi V, et al. Prevalence of asymptomatic macular holes in highly myopic eyes. Ophthalmology 2005; 112:2103–2109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


