Abstract
Background
A poor fat‐soluble micronutrient (FMN) and a high oxidative stress status are associated with frailty. Our aim was to determine the cross‐sectional association of FMNs and oxidative stress biomarkers [protein carbonyls (PrCarb) and 3‐nitrotyrosine] with the frailty status in participants older than 65 years.
Methods
Plasma levels of vitamins A (retinol), D3, E (α‐tocopherol and γ‐tocopherol) and carotenoids (α‐carotene and β‐carotene, lycopene, lutein/zeaxanthin, and β‐cryptoxanthin), PrCarb, and 3‐nitrotyrosine were measured in 1450 individuals of the FRAILOMIC initiative. Participants were classified into robust, pre‐frail, and frail using Fried's frailty criteria. Associations between biomarkers and frailty status were assessed by general linear and logistic regression models, both adjusted for cohort, season of blood sampling, gender, age, height, weight, and smoking.
Results
Robust participants had significantly higher vitamin D3 and lutein/zeaxanthin concentrations than pre‐frail and frail subjects; had significantly higher γ‐tocopherol, α‐carotene, β‐carotene, lycopene, and β‐cryptoxanthin concentrations than frail subjects, and had significantly lower PrCarb concentrations than frail participants in multivariate linear models. Frail subjects were more likely to be in the lowest than in the highest tertile for vitamin D3 (adjusted odds ratio: 2.15; 95% confidence interval: 1.42–3.26), α‐tocopherol (2.12; 1.39–3.24), α‐carotene (1.69; 1.00–2.88), β‐carotene (1.84; 1.13–2.99), lycopene (1.94; 1.24–3.05), lutein/zeaxanthin (3.60; 2.34–5.53), and β‐cryptoxanthin (3.02; 1.95–4.69) and were more likely to be in the highest than in the lowest tertile for PrCarb (2.86; 1.82–4.49) than robust subjects in multivariate regression models.
Conclusions
Our study indicates that both low FMN and high PrCarb concentrations are associated with pre‐frailty and frailty.
Keywords: Fat‐soluble micronutrients, Carotenoids, Frail, Protein carbonyls, 3‐Nitrotyrosine
Introduction
Frailty, a geriatric syndrome caused by an age‐related dynamic process affecting multiple physiological systems1, 2, is associated with a higher risk for falls, hospitalization, disability, and death3 and its prevalence increases with age and is more common in women.3, 4, 5, 6 Age‐associated oxidative stress (OS) and impairments in redox homeostasis as well as impairments in muscle structure, function, and performance are key factors in the development of frailty.7, 8, 9 Fortunately, frailty might be reversed by exercise and decelerated by nutritional interventions.10
Higher fruit and vegetable consumption and higher adherence to a Mediterranean diet were associated with a lower risk for frailty in older individuals.11, 12, 13 Additionally, a suboptimal vitamin and carotenoid intake and/or status as well as a micronutrient pattern low in vitamins A and E were associated with a higher prevalence and risk for frailty.14, 15, 16, 17 Furthermore, a suboptimal vitamin D (VD) status was shown to be related with low physical activity, weakness, and slowness, which are main constituents of the frailty syndrome,18, 19 with reduced muscle mass and poor physical performance in frail subjects20 and with a higher prevalence and incidence of frailty.19, 21 Moreover, VD is linked to redox homeostasis as shown in both VD deficient rat muscles and VD‐treated murine myoblast C2C12 cells.22
Some fat‐soluble micronutrients (FMN) can counteract OS, which is associated with several age‐related diseases and the aging process itself. OS can be monitored by biomarkers such as protein carbonyls (PrCarb) and 3‐nitrotyrosine (3‐NT),23, 24, 25 and some OS biomarkers have been shown to be elevated in frail subjects.26 Both higher OS and lower antioxidant parameters are associated with frailty.27
To the best of our knowledge, no study explored the association of frailty with FMN and OS biomarkers simultaneously in a large cohort so far. We therefore investigated the cross‐sectional relationship of plasma vitamins A, D3, E, carotenoids, PrCarb, and 3‐NT with frailty status (robust, pre‐frail, and frail) of participants aged >65 years in the European FRAILOMIC initiative.
Materials and methods
Study population and cohorts
In this study, we investigated 1450 individuals out of 1636 participants from the FRAILOMIC database by excluding subjects with missing values for frailty status (n = 114) and VD3 (n = 74). The FRAILOMIC initiative aims to identify and validate classical and ‘omics'‐based biomarkers that predict the risk of frailty, detect frailty, and assess the progression of frailty.28 Participants of the FRAILOMIC initiative come from four population‐based European cohorts of older adults: the Bordeaux sample of the Three‐City Study (France),29 the Aging Multidisciplinary Investigation cohort (Gironde, France),30 the Toledo Study for Healthy Aging (TSHA, Toledo, Spain),31 and the Invecchiare in Chianti (InCHIANTI) study (Chianti geographical area, Tuscany, Italy).32 These cohorts were described more in detail elsewhere.14 Study protocols of all cohorts were approved by Ethical Committees according to the principles of the Declaration of Helsinki and all participants signed a written consent. Three‐City Study, Aging Multidisciplinary Investigation, TSHA, and InCHIANTI were approved by the Ethical Committee of the University Hospital of Kremlin‐Bicêtre,29 Ethics Committee of the University Hospital of Bordeaux,30 Clinical Research Ethics Committee of the University Hospital of Toledo,31 and Ethical Committee of the Italian National Research Council on Aging,32 respectively.
Frailty classification
Participants were classified into robust, pre‐frail, and frail using criteria by Fried et al.3 The harmonization of criteria across cohorts was described in detail elsewhere.14, 31 Briefly, participants exhibiting ≥3 of the five following criteria were considered as frail: slowness, low energy expenditure, shrinking, weakness, and self‐reported exhaustion; while those exhibiting 1 to 2 of these criteria were considered as pre‐frail.
Participant characteristics
Participants' information included gender, age (years), weight (kg), height (cm), body mass index (BMI; kg/m2), smoking (current smoker), and global cognitive performance (Mini‐Mental State Examination). The assessment of characteristics is described elsewhere.14, 29, 30, 31, 32
Biomarker analyses
All analyses were carried out at the Department of Nutritional Toxicology (University of Jena, Germany) between 2010 and 2013.
Vitamin D3 in plasma samples was measured by the high‐performance liquid chromatography (HPLC) method described by Pilleron et al.14 VD3 was detected in all samples; in contrary, only 20/1430 samples (1.4%) revealed values above the limit of detection for VD2. Therefore, only VD3 is described and included in the statistical analyses. Analysis of retinol, α‐tocopherol and γ‐tocopherol, α‐carotene and β‐carotene, lycopene, lutein/zeaxanthin, and β‐cryptoxanthin in plasma samples was performed by the HPLC method described by Stuetz et al.33 and Weber et al.34 PrCarbs and protein bound 3‐NT in plasma samples were measured by non‐commercial in‐house ELISA methods as described by Weber et al.34, 35
Statistical analyses
Demographic characteristics are described using means ± standard deviation for continuous variables (age, weight, height, BMI, and Mini‐Mental State Examination) and frequencies (%) for categorical variables (gender, frailty status, and smoking). Differences in characteristics between frailty groups and between cohorts were assessed by general linear models (GLMs; continuous variables) and Pearson's χ2 test (categorical variables). When necessary, biomarker concentrations were transformed to achieve normal distribution using logarithmic (LN) transformation and are described by geometric means with 95% confidence intervals. Differences of biomarkers between frailty groups were assessed by simple (frailty status as only factor) and adjusted (covariates included cohort, season of blood sampling, gender, age, height, weight, and smoking) GLMs. Additionally, simple [odds ratio (OR); frailty status as only factor] and multiple adjusted [adjusted OR (AOR); covariates included cohort, season of blood sampling, gender, age, height, weight, and smoking] logistic regression analysis using tertiles (Supporting Information, Table S1) of FMN and OS biomarkers were applied: ORs and AORs of the lowest tertile and the median tertile vs. the highest tertiles for FMN and, vice versa for OS markers, in pre‐frail and frail compared with control groups were calculated. For a clearer presentation of the results, ORs and AORs are not shown for median vs. highest tertiles for FMN, and vice versa for OS biomarkers. Statistically significant differences were considered to be present at P < 0.05. All statistical analyses were carried out using SPSS software (SPSS Inc., Chicago, IL, USA; Version 20.0.0). Figures were prepared by using SPSS Version 20.0.0 and Microsoft Office Power Point 2007.
Results
Study characteristics for both the total sample and the three frailty groups are shown in Table 1. In our study, 41.7% and 22.1% of the participants were pre‐frail and frail, respectively, and 65.7% of the frail participants were women. Frail participants (81.4 ± 6.3 years) were significantly older than pre‐frail (78.0 ± 6.0 years) and robust participants (74.6 ± 5.9 years), and also significantly lighter (P = 0.012) and shorter (P < 0.001) than robust participants, while BMI was not associated with frailty status. Additionally, characteristics and biomarker concentrations were different between the individual FRAILOMIC cohorts (Supporting Information, Tables S2 and S3).
Table 1.
Study characteristics by frailty groups
| Total | Robust | Pre‐frail | Frail | P | |
|---|---|---|---|---|---|
| N, % (n) | 100 (1450) | 36.1 (524) | 41.7 (605) | 22.1 (321) | — |
| Females, % (n) | 55.9 (811) | 48.1 (252) | 57.5 (348) | 65.7 (211) | <0.001 # |
| Age, years | 77.5 ± 6.5 | 74.6 ± 5.9a | 78.0 ± 6.0b | 81.4 ± 6.3c | <0.001 |
| Weight, kg | 70.6 ± 13.7 | 72.0 ± 12.0a | 69.9 ± 14.0b | 69.7 ± 15.5b | 0.012 |
| Height, cm | 160.0 ± 9.6 | 161.5 ± 9.5a | 160.0 ± 9.5b | 157.6 ± 9.6c | <0.001 |
| BMI, kg/m2 | 27.6 ± 4.6 | 27.6 ± 3.9 | 27.3 ± 4.6 | 28.0 ± 5.6 | 0.066 |
| Smoker, % (n) | 5.0 (73) | 5.6 (29) | 5.6 (34) | 3.1 (10) | 0.209 # |
| MMSE, points | 25.6 ± 4.1 | 26.5 ± 3.1a | 26.1 ± 3.3a | 22.9 ± 5.7b | <0.001 |
All results reported as means ± standard deviation or % (n). BMI, body mass index; MMSE, Mini‐Mental State Examination. Superscript letters indicate statistical significant differences between frailty groups by unadjusted GLM.
Differences between frailty groups determined by Pearson's χ2 test. P < 0.05.
Fat‐soluble micronutrient and OS concentrations differed significantly between the frailty groups (Table 2 and Figure 1). Significantly higher VD3 (Figure 1A) and lutein/zeaxanthin (Figure 1B) concentrations were observed in robust participants compared with pre‐frail and frail participants, and in pre‐frail compared with frail participants (all P < 0.01), in simple GLMs. Furthermore, robust and pre‐frail participants had higher γ‐tocopherol, α‐carotene, β‐carotene, lycopene, and β‐cryptoxanthin (Figure 1C) concentrations than frail subjects (all P ≤ 0.02); pre‐frail participants had higher α‐tocopherol concentrations than frail subjects (P = 0.002); and robust subjects had significantly lower PrCarb concentrations than frail and pre‐frail participants (P < 0.001; Figure 1D). In multivariate analyses, β‐cryptoxanthin was higher in robust than in pre‐frail and frail participants and in pre‐frail compared with frail participants. Furthermore, VD3 and β‐carotene concentrations were higher in robust than in pre‐frail and frail participants (all P ≤ 0.001); lycopene and lutein/zeaxanthin concentrations were higher in robust and pre‐frail participants compared with frail subjects (all P ≤ 0.02); α‐tocopherol concentrations were higher in pre‐frail participants than in frail subjects (P = 0.013); and lower PrCarb concentrations were found in robust compared with frail and pre‐frail participants (P = 0.006). No association was observed between retinol or 3‐NT and frailty status.
Table 2.
Plasma concentrations of biomarkers by frailty groups
| Biomarker | FRAILOMIC | Robust | Pre‐frail | Frail | P |
|---|---|---|---|---|---|
| Vitamin D3, nmol/L | 42.0 (40.8–43.2) | 47.2 (45.0–49.5)a | 40.9 (39.1–42.7)b | 36.6 (34.4–38.9)c | <0.001 |
| adjusted | 46.6 (44.0–9.5)a | 39.5 (37.2–41.8)b | 37.5 (34.9–40.2)b | <0.001 | |
| Retinol, μmol/L | 1.88 (1.85–1.91) | 1.87 (1.82–0.91) | 1.90 (1.86–1.94) | 1.87 (1.82–1.93) | 0.577 |
| adjusted | 1.84 (1.78–0.90) | 1.87 (1.81–1.93) | 1.86 (1.79–1.93) | 0.757 | |
| α‐Tocopherol, μmol/L | 29.4 (29.0–29.9) | 29.2 (28.5–9.9)a,b | 30.3 (29.6–30.9)a | 28.3 (27.4–29.2)b | 0.002 |
| adjusted | 28.8 (27.9–0.7)a,b | 29.4 (28.5–30.3)a | 27.2 (26.1–28.3)b | 0.013 | |
| γ‐Tocopherol, μmol/L | 1.19 (1.16–1.22) | 1.21 (1.16–1.26)a | 1.22 (1.18–1.27)a | 1.10 (1.04–1.16)b | 0.005 |
| adjusted | 1.18 (1.12–1.25) | 1.17 (1.11–1.24) | 1.12 (1.05–1.19) | 0.405 | |
| α‐Carotene, μmol/L | 0.12 (0.12–0.13) | 0.13 (0.12–0.14)a | 0.12 (0.12–0.14)a | 0.11 (0.10–0.12)b | 0.020 |
| adjusted | 0.12 (0.11–0.13)a | 0.10 (0.10–0.11)a,b | 0.10 (0.09–0.11)b | 0.018 | |
| β‐Carotene, μmol/L | 0.41 (0.40–0.43) | 0.44 (0.41–0.47)a | 0.43 (0.40–0.46)a | 0.35 (0.32–0.39)b | <0.001 |
| adjusted | 0.42 (0.39–0.45)a | 0.36 (0.34–0.39)b | 0.34 (0.31–0.37)b | 0.001 | |
| Lycopene, μmol/L | 0.36 (0.34–0.37) | 0.40 (0.37–0.42)a | 0.36 (0.34–0.39)a | 0.29 (0.26–0.31)b | <0.001 |
| adjusted | 0.35 (0.32–0.38)a | 0.33 (0.30–0.35)a | 0.28 (0.25–0.30)b | 0.002 | |
| Lutein/zeaxanthin, μmol/L | 0.33 (0.32–0.34) | 0.36 (0.35–0.38)a | 0.33 (0.32–0.34)b | 0.27 (0.25–0.28)c | <0.001 |
| adjusted | 0.34 (0.32–0.36)a | 0.32 (0.30–0.33)a | 0.27 (0.25–0.29)b | <0.001 | |
| β‐Cryptoxanthin, μmol/L | 0.20 (0.19–0.21) | 0.22 (0.21–0.24)a | 0.21 (0.20–0.23)a | 0.16 (0.14–0.18)b | <0.001 |
| adjusted | 0.21 (0.20–0.23)a | 0.17 (0.16–0.19)b | 0.14 (0.13–0.16)c | <0.001 | |
| Protein carbonyls, nmol/mg | 0.31 (0.30–0.31) | 0.26 (0.25–0.27)a | 0.30 (0.29–0.32)b | 0.31 (0.30–0.33)b | <0.001 |
| adjusted | 0.26 (0.25–0.27)a | 0.27 (0.25–0.28)a | 0.30 (0.28–0.32)b | 0.006 | |
| 3‐Nitrotyrosine, pmol/mg | 7.22 (6.94–7.51) | 7.64 (7.15–8.16) | 6.98 (6.56–7.42) | 7.01 (6.45–7.63) | 0.103 |
| adjusted | 7.49 (6.90–8.14) | 7.93 (7.32–8.60) | 7.13 (6.45–7.88) | 0.202 |
All results reported as geometric means (95% confidence interval). Superscript letters indicate statistical significant differences between frailty groups by GLM (unadjusted; adjusted for cohort, season of blood sampling, gender, age, height, weight, and smoking status); unadjusted model: n = 1448, except PrCarb (n = 1446), 3‐NT (n = 1441), and VD3 (n = 1450); adjusted model: n = 1448, except PrCarb (n = 1429), 3‐NT (n = 1441), and VD3 (n = 1450). P < 0.05.
Figure 1.
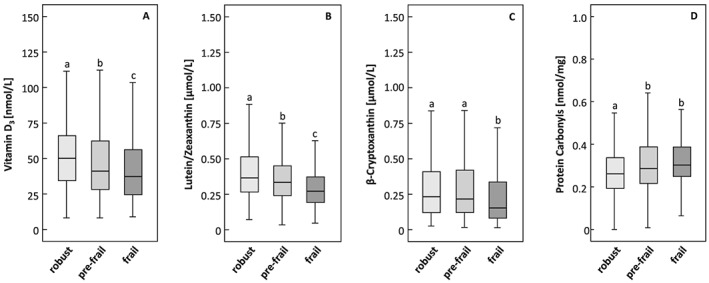
Plasma concentrations of (A) vitamin D 3 , (B) lutein/zeaxanthin, (C) β‐cryptoxanthin, and (D) protein carbonyls by frailty groups. Superscript letters indicate statistical significant differences between frailty groups by unadjusted GLM; P < 0.05.
Results from GLMs were confirmed by logistic regression analyses (Table 3). Pre‐frail and frail participants were more likely to be in the lowest than in the highest tertile for VD3 and lutein/zeaxanthin than robust participants. Frail participants were more likely to be in the lowest than in the highest tertile for α‐tocopherol, γ‐tocopherol, α‐carotene, β‐carotene, lycopene, and β‐cryptoxanthin than robust participants. Subsequently, multivariable adjustment showed that pre‐frail and frail compared with robust participants were more likely to be in the lowest than in the highest tertiles for VD3 (AOR: 1.98; 95% confidence interval: 1.42–2.76 and 2.15; 1.42–3.26), β‐carotene (1.56; 1.06–2.28 and 1.84; 1.13–2.99), and especially for lutein/zeaxanthin (1.38; 1.00–1.91 and 3.60; 2.34–5.53) and β‐cryptoxanthin (1.70; 1.20–2.41 and 3.02; 1.95–4.69). Furthermore, frail participants were more likely to be in the lowest than in the highest tertile for α‐tocopherol (2.12; 1.39–3.24), α‐carotene (1.69; 1.00–2.88), and lycopene (1.94; 1.24–3.05) than robust participants. In contrast, for OS markers, ORs and AORs to be in the highest than in the lowest tertiles were calculated. Pre‐frail and frail participants were associated with a higher likelihood to be in the highest than in the lowest tertile for PrCarb. For frail participants, this association was confirmed in the adjusted logistic regression model (2.86; 1.82–4.49). No associations were found between retinol and 3‐NT tertiles and frailty status in both logistic regression models.
Table 3.
Odds ratios of pre‐frail and frail participants (robust participants as reference) related to biomarker tertiles
| Pre‐frailty | Frailty | |
|---|---|---|
| Vitamin D3 | 1.96 (1.46–2.63) * | 2.83 (1.99–4.01) * |
| adjusted | 1.98 (1.42–2.76) * | 2.15 (1.42–3.26) * |
| Retinol | 0.88 (0.66–1.17) | 0.98 (0.70–1.37) |
| adjusted | 0.93 (0.67–1.28) | 0.82 (0.55–1.22) |
| α‐Tocopherol | 0.82 (0.62–1.09) | 1.54 (1.09–2.19) * |
| adjusted | 1.12 (0.81–1.54) | 2.12 (1.39–3.24) * |
| γ‐Tocopherol | 0.95 (0.72–1.27) | 1.62 (1.15–2.28)* |
| adjusted | 1.04 (0.76–1.42) | 1.46 (0.98–2.19) |
| α‐Carotene | 0.96 (0.72–1.28) | 1.48 (1.05–2.07) * |
| adjusted | 1.40 (0.92–2.13) | 1.69 (1.00–2.88) |
| β‐Carotene | 1.10 (0.82–1.46) | 1.75 (1.24–2.47) * |
| adjusted | 1.56 (1.06–2.28) * | 1.84 (1.13–2.99) * |
| Lycopene | 1.28 (0.96–1.71) | 2.36 (1.66–3.36) * |
| adjusted | 1.18 (0.83–1.68) | 1.94 (1.24–3.05) * |
| Lutein/zeaxanthin | 1.58 (1.18–2.12) * | 4.09 (2.84–5.87) * |
| adjusted | 1.38 (1.00–1.91) * | 3.60 (2.34–5.53) * |
| β‐Cryptoxanthin | 1.11 (0.83–1.49) | 2.09 (1.48–2.93) * |
| adjusted | 1.70 (1.20–2.41) * | 3.02 (1.95–4.69) * |
| Protein carbonyls | 1.83 (1.37–2.44) * | 2.90 (2.00–4.21) * |
| adjusted | 1.21 (0.87–1.68) | 2.86 (1.82–4.49) * |
| 3‐Nitrotyrosine | 0.79 (0.59–1.05) | 0.72 (0.52–1.02) |
| adjusted | 1.31 (0.94–1.82) | 0.90 (0.59–1.36) |
All results reported as odds ratios (95% confidence interval). Multivariate logistic regression models (unadjusted; adjusted for cohort, season of blood sampling, gender, age, height, weight, and smoking status); T3 was the reference for vitamin D3, retinol, α‐tocopherol, γ‐tocopherol, α‐carotene, β‐carotene, β‐cryptoxanthin, lycopene, and lutein/zeaxanthin, and T1, was the reference for PrCarb and 3‐NT; unadjusted model: n = 1448, except PrCarb (n = 1445), 3‐NT (n = 1441), and VD3 (n = 1450); adjusted model: n = 1431, except PrCarb (n = 1432), 3‐NT (n = 1428), and VD3 (n = 1433).
P < 0.05.
Discussion
In our study, we aimed to determine cross‐sectional associations of FMNs and OS biomarkers simultaneously with the frailty status of participants older than 65 years, and we demonstrated both differences in concentrations between robust, pre‐frail, and frail individuals and associations of FMN and PrCarb with pre‐frailty and frailty.
The prevalences of pre‐frailty (41.7%) and frailty (22.1%) are in accordance to previously shown prevalences of 42.3% and 17.0%, respectively, in community‐dwelling individuals from 10 European countries.4 Our findings confirm previously published associations of frailty with age and female gender.4, 5, 6
A higher risk for frailty has previously been related to a low intake of micronutrients and possibly resulting lower micronutrient status. Low intakes of vitamin E or VD led to higher ORs to be frail than non‐frail, after adjusting for several confounders, in 802 subjects (>65 years) from the InCHIANTI cohort.15 Women (>65 years) in the lowest quartile of serum carotenoids had a higher risk of becoming frail during a 3 year follow‐up period in the Women's Health and Aging Study I (WHAS I).17 Lower VD, retinol, α‐carotene, β‐carotene, lycopene, lutein/zeaxanthin, and β‐cryptoxanthin plasma concentrations were found in frail compared with non‐frail women of the WHAS I and WHAS II, and the ORs of being frail were significantly higher for women in the lowest quartile compared with the top three quartiles for total carotenoids, α‐tocopherol, and VD.16 The strongest association was found for β‐carotene, lutein/zeaxanthin, and total carotenoids, after adjusting for age, sociodemographic status, smoking, and BMI.16 Using FRAILOMIC initiative data, Pilleron et al. observed a significant relationship between a circulating micronutrient pattern, which is low in vitamins A and E, and high in carotenoid concentrations, and a higher prevalence of frailty.14 However, all these studies did not make the distinction between frail and pre‐frail, considering pre‐frail as robust.
The lack of association between retinol and frailty status, found in our study, was previously reported in participants of the FRAILOMIC initiative and the InCHIANTI cohort.14, 15 This might be due to the homeostatically regulated retinol metabolism, and furthermore, circulating retinol is considered a poor relevant marker of vitamin A status.36
Beside low micronutrient concentrations possibly leading to frailty, VD itself may play a role in the pathogenesis of frailty. Vitamin D, beside its endocrine/indirect effects on muscle function, can act directly on skeletal muscle via the VD receptor, contributing to a normal muscle structure and metabolism.37 A low VD status was related to a reduced muscle mass and an impaired physical performance in 127 pre‐frail and frail Dutch participants (>65 years).20 Vogt et al. observed that participants (>65 years) having baseline VD levels <37.5 nmol/L compared with ≥75 nmol/L were more likely to become pre‐frail (2.4; 1.17–5.03) and pre‐frail/frail combined (2.5; 1.23–5.22), after a 3 year follow‐up.19 Our findings of an association between low VD3 and pre‐frailty and frailty prevalence differ from those found by Pilleron et al.14 who observed no association between VD3 and frailty status in the same sample cohort. This was possibly due to the fact that Pilleron et al. used different cut‐offs for VD3 and included the pre‐frail participants in the robust group. In our analyses, tertiles of VD3 were chosen to be able to compare groups with similar sample size. The lowest VD3 tertile (<33.7 nmol/L) in our study meets the definition of a deficient or even severely deficient VD3 status, depending on the references used.38
Low FMN concentrations might be due to a low intake of fruits, vegetables, nuts, seeds, and oils, which could potentially originate from a decreased ability for older persons to go shopping or prepare meals themselves. A low VD status may occur due to less exposure to sunlight and less physical activity, which might be a result of frailty itself. The reason for low concentrations of some carotenoids may lie in their food source. Elderly persons may experience physiological changes in the gastrointestinal tract leading to reflux, heartburn, or constipation that may result in a decreased intake of fruits and vegetables. Our observations might just reflect a low intake of these fruits and vegetables; unfortunately, we are not able to adjust our models for dietary intake.
Inadequate micronutrient intake and plasma concentrations may result in higher OS leading to an impaired muscle function and performance and contributing to frailty. A low dietary intake of a combination of vitamins A, E, B6, and B12, folate, selenium, and zinc led to a lower oxidative capacity and reduced muscle function and physical activity in aged male C57/BL6J mice.39 In contrast, a high intake of fruits and vegetables was previously associated with low biomarkers of OS in 296 healthy, middle‐aged men40 and a lower risk of frailty in older individuals (>60 years).13 In a previous analysis of the TSHA cohort, higher PrCarb concentrations were observed in frail compared with non‐frail individuals but no association was observed with age.26 In contrast, higher plasma PrCarb were previously observed in older adults (61–85 years) compared with young subjects (21–40 years)24 supported by an age‐dependent increase in PrCarb levels in 80 healthy persons (18–85 years).25 In our study, there was a significant positive correlation between age and PrCarb (r = 0.196; P < 0.001; not shown) but also a negative correlation between age and 3‐NT (r = −0.225; P < 0.001; not shown) in robust participants. In frail subjects, no correlations between age and OS markers were found. In addition, an association between higher OS levels and frailty was reported,27 but only one study on frailty used PrCarb as OS marker, and therefore, no comparisons with other studies are possible. However, PrCarb was two‐fold lower and 3‐NT was two‐fold higher compared with subjects from a general population in the MARK‐AGE study.41 The FMN concentrations on the other hand were comparable with those found in the MARK‐AGE study, except for lycopene that was two‐fold higher in MARK‐AGE.33
Due to the cross‐sectional design of our study, we cannot conclude whether frailty leads to a low micronutrients/high PrCarb status or if a low micronutrients/high PrCarb status leads to frailty. Furthermore, data regarding socio‐economic status and income were not available and, therefore, are missing in the multivariate‐adjusted models. Strengths of our study are the large sample size including frailty classification into robust, pre‐frail, and frail and the harmonized frailty criteria used in all four cohorts. Especially the possibility to include the pre‐frail group is a novel feature in a study with such a large sample size. Additionally, participants from different European countries were analyzed; thus, our study results reflect a broad range of the society and different lifestyles. Furthermore, the broad spectrum of parameters, including nutritional biomarkers, antioxidants, and OS biomarkers, are a unique feature of this study. The high‐quality blood analyses were performed by the same trained persons in one laboratory, thus limiting variability related to operators, methods, and analytical instruments.
From our study, we conclude that both low concentrations of several single FMN (VD3, β‐carotene, lutein/zeaxanthin, and β‐cryptoxanthin) and high concentrations of PrCarb are associated with pre‐frailty and frailty in four European cohorts of adults aged 65 years and older. Thus, we suggest that following a diet rich in FMN, subsequently leading to higher micronutrient and lower OS concentrations, may support the prevention of frailty. Further large‐scale longitudinal and intervention studies are needed to investigate the role of FMN on the frailty risk and the potential mediating effect of OS.
Conflict of interest
C.F. received fees for conferences from Danone Research and Nutricia not related to the present work. No further conflicts of interest are declared.
Supporting information
Table S1 Tertiles of fat‐soluble micronutrients and oxidative stress markers
Table S2 Study characteristics by individual FRAILOMIC cohorts
Table S3 Plasma concentrations of biomarkers by the FRAILOMIC cohorts
Acknowledgements
This work was supported by the European Union's Seventh Framework Programme (FP7‐HEALTH; grant number 305483, FRAILOMIC Project).
The 3‐C Study is conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Médicale (INSERM), Victor Segalen–Bordeaux2 University, and Sanofi‐Synthélabo. Fondation pour la Recherche Médicale funded the preparation and beginning of the study. The 3‐C Study is sponsored by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, Conseils Régionaux of Aquitaine and Bourgogne, Fondation de France, Ministry of Research‐INSERM Program Cohortes et collections de données biologiques, Fondation Plan Alzheimer (FCS 2009‐2012), and Caisse Nationale pour la Solidarité et l'Autonomie (CNSA) and ‘Programme Longévité et vieillissement' (COGICARE 07‐LVIE 003 01). The AMI project was funded by AGRICA (CAMARCA, CRCCA, CCPMA PREVOYANCE, CPCEA, and AGRI PREVOYANCE), la Mutualité Sociale Agricole (MSA) de Gironde, and la Caisse Centrale de la Mutualité Sociale Agricole (CCMSA). The InCHIANTI study baseline (1998–2000) was supported as a ‘targeted project' (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the Follow‐up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1‐AG‐1‐1 and N.1‐AG‐1‐2111); the Follow‐ups 2 and 3 (2004–2010) were financed by the U.S. National Institute on Aging (Contract: N01‐AG‐5‐0002) and supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland. The TSHA cohort was funded by grants PI07/90637, PI10/01532, and CB16/10/00456 from the Instituto de Salud Carlos III (Ministerio de Economía y Competitividad, Spain), 03031‐00 from the Instituto de Ciencias de la Salud de Castilla la Mancha, and PI2010/020 from FISCAM.5.
The authors certify that they comply with the Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.42
Kochlik B., Stuetz W., Pérès K., Pilleron S., Féart C., García García F. J., Bandinelli S., Gomez‐Cabrero D., Rodriguez‐Mañas L., Grune T., and Weber D. (2019) Associations of fat‐soluble micronutrients and redox biomarkers with frailty status in the FRAILOMIC initiative, Journal of Cachexia, Sarcopenia and Muscle, 10, 1339–1346. 10.1002/jcsm.12479.
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodriguez‐Mañas L, Fried LP. Frailty in the clinical scenario. The Lancet 2015;385:e7–e9. [DOI] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 4. Santos‐Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle‐aged and older community‐dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci 2009;64:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trevisan C, Veronese N, Maggi S, Baggio G, Toffanello ED, Zambon S, et al. Factors influencing transitions between frailty states in elderly adults: the Progetto Veneto Anziani Longitudinal Study. J Am Geriatr Soc 2017;65:179–184. [DOI] [PubMed] [Google Scholar]
- 6. Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci 2010;65:377–381. [DOI] [PubMed] [Google Scholar]
- 7. Angulo J, El Assar M, Rodriguez‐Manas L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol Aspects Med 2016;50:1–32. [DOI] [PubMed] [Google Scholar]
- 8. Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle 2018;9:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sakellariou GK, Lightfoot AP, Earl KE, Stofanko M, McDonagh B. Redox homeostasis and age‐related deficits in neuromuscular integrity and function. J Cachexia Sarcopenia Muscle 2017;8:881–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keevil VL, Romero‐Ortuno R. Ageing well: a review of sarcopenia and frailty. Proc Nutr Soc 2015;74:337–347. [DOI] [PubMed] [Google Scholar]
- 11. Rahi B, Ajana S, Tabue‐Teguo M, Dartigues JF, Peres K, Feart C. High adherence to a Mediterranean diet and lower risk of frailty among French older adults community‐dwellers: results from the Three‐City‐Bordeaux Study. Clin Nutr 2018;37:1293–1298. [DOI] [PubMed] [Google Scholar]
- 12. Bollwein J, Diekmann R, Kaiser MJ, Bauer JM, Uter W, Sieber CC, et al. Dietary quality is related to frailty in community‐dwelling older adults. J Gerontol A Biol Sci Med Sci 2013;68:483–489. [DOI] [PubMed] [Google Scholar]
- 13. Garcia‐Esquinas E, Rahi B, Peres K, Colpo M, Dartigues JF, Bandinelli S, et al. Consumption of fruit and vegetables and risk of frailty: a dose‐response analysis of 3 prospective cohorts of community‐dwelling older adults. Am J Clin Nutr 2016;104:132–142. [DOI] [PubMed] [Google Scholar]
- 14. Pilleron S, Weber D, Peres K, Colpo M, Gomez‐Cabrero D, Stuetz W, et al. Patterns of circulating fat‐soluble vitamins and carotenoids and risk of frailty in four European cohorts of older adults. Eur J Nutr 2018;1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bartali B, Frongillo EA, Bandinelli S, Lauretani F, Semba RD, Fried LP, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci 2006;61:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michelon E, Blaum C, Semba RD, Xue QL, Ricks MO, Fried LP. Vitamin and carotenoid status in older women: associations with the frailty syndrome. J Gerontol A Biol Sci Med Sci 2006;61:600–607. [DOI] [PubMed] [Google Scholar]
- 17. Semba RD, Bartali B, Zhou J, Blaum C, Ko CW, Fried LP. Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci 2006;61:594–599. [DOI] [PubMed] [Google Scholar]
- 18. Demircioglu DT. The association of vitamin D levels and the frailty phenotype among non‐geriatric dialysis patients: a cross‐sectional study. Clinics (Sao Paulo) 2018;73:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vogt S, Decke S, de Las Heras Gala T, Linkohr B, Koenig W, Ladwig KH, et al. Prospective association of vitamin D with frailty status and all‐cause mortality in older adults: results from the KORA‐Age Study. Prev Med 2015;73:40–46. [DOI] [PubMed] [Google Scholar]
- 20. Tieland M, Brouwer‐Brolsma EM, Nienaber‐Rousseau C, van Loon LJ, De Groot LC. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur J Clin Nutr 2013;67:1050–1055. [DOI] [PubMed] [Google Scholar]
- 21. Wong YY, McCaul KA, Yeap BB, Hankey GJ, Flicker L. Low vitamin D status is an independent predictor of increased frailty and all‐cause mortality in older men: the Health in Men Study. J Clin Endocrinol Metab 2013;98:3821–3828. [DOI] [PubMed] [Google Scholar]
- 22. Bhat M, Ismail A. Vitamin D treatment protects against and reverses oxidative stress induced muscle proteolysis. J Steroid Biochem Mol Biol 2015;152:171–179. [DOI] [PubMed] [Google Scholar]
- 23. Gil L, Siems W, Mazurek B, Gross J, Schroeder P, Voss P, et al. Age‐associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res 2006;40:495–505. [DOI] [PubMed] [Google Scholar]
- 24. Mutlu‐Turkoglu U, Ilhan E, Oztezcan S, Kuru A, Aykac‐Toker G, Uysal M. Age‐related increases in plasma malondialdehyde and protein carbonyl levels and lymphocyte DNA damage in elderly subjects. Clin Biochem 2003;36:397–400. [DOI] [PubMed] [Google Scholar]
- 25. Pandey KB, Mehdi MM, Maurya PK, Rizvi SI. Plasma protein oxidation and its correlation with antioxidant potential during human aging. Dis Markers 2010;29:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ingles M, Gambini J, Carnicero JA, Garcia‐Garcia FJ, Rodriguez‐Manas L, Olaso‐Gonzalez G, et al. Oxidative stress is related to frailty, not to age or sex, in a geriatric population: lipid and protein oxidation as biomarkers of frailty. J Am Geriatr Soc 2014;62:1324–1328. [DOI] [PubMed] [Google Scholar]
- 27. Saum KU, Dieffenbach AK, Jansen EH, Schottker B, Holleczek B, Hauer K, et al. Association between oxidative stress and frailty in an elderly German population: results from the ESTHER cohort study. Gerontology 2015;61:407–415. [DOI] [PubMed] [Google Scholar]
- 28. Erusalimsky JD, Grillari J, Grune T, Jansen‐Duerr P, Lippi G, Sinclair AJ, et al. In search of ‘omics'‐based biomarkers to predict risk of frailty and its consequences in older individuals: the FRAILOMIC initiative. Gerontology 2016;62:182–190. [DOI] [PubMed] [Google Scholar]
- 29. 3C Study Group . Vascular factors and risk of dementia: design of the Three‐City Study and baseline characteristics of the study population. Neuroepidemiology 2003;22:316–325. [DOI] [PubMed] [Google Scholar]
- 30. Peres K, Matharan F, Allard M, Amieva H, Baldi I, Barberger‐Gateau P, et al. Health and aging in elderly farmers: the AMI cohort. BMC Public Health 2012;12:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia‐Garcia FJ, Gutierrez Avila G, Alfaro‐Acha A, Andres MA, Aparicio ME, Aparicio SH, et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo Study for Healthy Aging. J Nutr Health Aging 2011;15:852–856. [DOI] [PubMed] [Google Scholar]
- 32. Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 2000;48:1618–1625. [DOI] [PubMed] [Google Scholar]
- 33. Stuetz W, Weber D, Dolle ME, Jansen E, Grubeck‐Loebenstein B, Fiegl S, et al. Plasma carotenoids, tocopherols, and retinol in the age‐stratified (35‐74 years) general population: a cross‐sectional study in six European countries. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weber D, Stuetz W, Bernhard W, Franz A, Raith M, Grune T, et al. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur J Clin Nutr 2014;68:215–222. [DOI] [PubMed] [Google Scholar]
- 35. Weber D, Kneschke N, Grimm S, Bergheim I, Breusing N, Grune T. Rapid and sensitive determination of protein‐nitrotyrosine by ELISA: application to human plasma. Free Radic Res 2012;46:276–285. [DOI] [PubMed] [Google Scholar]
- 36. Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr 2011;94:658S–665S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molina P, Carrero JJ, Bover J, Chauveau P, Mazzaferro S, Torres PU, et al. Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. J Cachexia Sarcopenia Muscle 2017;8:686–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol 2017;13:466–479. [DOI] [PubMed] [Google Scholar]
- 39. van Dijk M, Dijk FJ, Hartog A, van Norren K, Verlaan S, van Helvoort A, et al. Reduced dietary intake of micronutrients with antioxidant properties negatively impacts muscle health in aged mice. J Cachexia Sarcopenia Muscle 2018;9:146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cocate PG, Natali AJ, Oliveira A, Longo GZ, Alfenas Rde C, do Carmo PM, et al. Fruit and vegetable intake and related nutrients are associated with oxidative stress markers in middle‐aged men. Nutrition 2014;30:660–665. [DOI] [PubMed] [Google Scholar]
- 41. Weber D, Stuetz W, Toussaint O, Debacq‐Chainiaux F, Dolle MET, Jansen E, et al. Associations between specific redox biomarkers and age in a large European cohort: the MARK‐AGE project. Oxid Med Cell Longev 2017;2017:1401452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Tertiles of fat‐soluble micronutrients and oxidative stress markers
Table S2 Study characteristics by individual FRAILOMIC cohorts
Table S3 Plasma concentrations of biomarkers by the FRAILOMIC cohorts


