Abstract
Background
Exercise protocols applied during hospitalization can prevent functional and cognitive decline in older adults. The purpose of this study was to examine the individual response of acutely hospitalized patients to usual care and to physical exercise on functional capacity, muscle strength, and cognitive function and to assess the relationship with mortality at 1 year post‐discharge.
Methods
In a single‐blind randomized clinical trial, 370 hospitalized patients [56.5% women; mean age (standard deviation) 87.3 (4.9) years] were allocated to an exercise intervention group (IG, n = 185) or a control group (CG, n = 185). The participants were older adults aged 75 years or older in an acute care unit in a tertiary public hospital in Navarra, Spain. The usual care group received habitual hospital care, which included physical rehabilitation when needed. The in‐hospital intervention included individualized multicomponent exercise training programme performed during 5–7 consecutive days (two sessions/day). Functional capacity was assessed with the Short Physical Performance Battery (SPPB) test and the Gait Velocity Test (GVT). Handgrip strength and cognitive function were also measured at admission and discharge. Patients in both groups were categorized as responders (Rs), non‐responders (NRs), and adverse responders (ARs) based on the individual response to each treatment during hospitalization.
Results
The prevalence of Rs was higher and the prevalence of NRs and ARs was lower in the intervention group than in the control group for functional capacity (SPPB IG: Rs 85.3%, NRs 8.7%, ARs 6.0% vs. CG: Rs 37.9%, NRs 28.8%, ARs 33.3% and GVT IG: Rs 51.2%, NRs 47.3, ARs 1.6% vs. CG: Rs 18.0%, NRs 67.7%, ARs 14.3%), muscle strength (IG: Rs 62.3%, NRs 26.5%, ARs 11.3% vs. CG: Rs 20.0%, NRs 38.0%, ARs 42.0%), and cognition (IG: Rs 41.5%, NRs 57.1%, ARs 1.4% vs. CG: Rs 13.8%, NRs 76.6%, ARs 9.7%) (all P < 0.001). The ARs for the GVT in the control group and the ARs for the SPPB in the intervention group had a significantly higher rate of mortality than the NRs and Rs in the equivalent groups (0.01 and 0.03, respectively) at follow‐up.
Conclusions
Older patients performing an individualized exercise intervention presented higher prevalence of Rs and a lower prevalence of NRs and ARs for functional capacity, muscle strength, and cognitive function than those who were treated with usual care during acute hospitalization. An adverse response on functional capacity in older patients to physical exercise or usual care during hospitalization was associated with mortality at 1 year post‐discharge.
Keywords: Multicomponent exercise programme, Frailty, Iatrogenic Nosocomial Disability
Introduction
Adequate hospital care for older adults (≥75 years) with acute medical disorders is an important clinical issue in our aging societies.1, 2, 3, 4 In this context, acute illness requiring hospitalization is a sentinel event in older adults, which can lead to functional decline and frequently, long‐term disability.5, 6, 7 Loss of functional capacity is strongly associated with caregiver burden, higher resource use, institutionalization, and death.8, 9, 10, 11 Accordingly, this is a challenge that health care professionals and policy makers should prioritize given the expectations of further growth of the elderly population.12
Health care systems remain poorly adapted to meet the needs of older patients with frailty, disability, multimorbidity, and polypharmacy,13 and low in‐hospital mobility is directly related to functional impairment at discharge and even more so at follow‐up.14, 15 However, a recent randomized clinical trial (RCT) showed no significant benefit of an in‐hospital mobility programme and a behavioural strategy to encourage mobility in older patients' ability to perform activities of daily living after acute hospitalization.16 In this context, tailored exercise interventions can play a key role in preventing functional decline and cognitive impairment in acutely hospitalized patients of advanced age (including octogenarians and nonagenarians).12, 17
Despite the frequent reports of ‘average' exercise‐related benefits, there is, nevertheless, a wide inter‐individual variability in the response to exercise training.18 Under the same exercise conditions, some subjects, termed responders (Rs), achieve benefits after intervention, whereas others, termed non‐responders (NRs; unchanged response) and adverse responders (ARs; worsened response), do not.19, 20 To the best of our knowledge, the inter‐individual analysis of exercise training effects has not been previously investigated in acutely hospitalized older adults. In addition, it remains unclear if the response influences in mortality following discharge.
The main aim of the present study was to assess the prevalence of these categories (as indicated by functional, strength, and cognitive variables) under usual care or an individualized multicomponent exercise intervention applied in an Acute Care of the Elderly (ACE) unit. We also sought to examine the relationship between the aforementioned categories of each group with mortality at 1 year post‐discharge and the relevance of functional status at admission in the prognosis during hospitalization in acutely hospitalized older adults.
Methods
Design
The study is a secondary analysis of an RCT (NCT02300896)12 ; 17 conducted in the ACE unit of the Department of Geriatrics in a tertiary public hospital (Complejo Hospitalario de Navarra, Spain). This department has 35 allocated beds, and its staff is composed of eight geriatricians (distributed in the ACE unit, orthogeriatrics, and outpatient consultations). Admissions in the ACE unit derive mainly from the Accident and Emergency Department, with heart failure, pulmonary, and infectious diseases being the main causes of admissions.
Acutely hospitalized patients who met inclusion criteria were randomly assigned to the intervention or control (usual care) group within the first 48 h of admission. Usual care was offered to patient by the geriatricians and consists of standard physiotherapy focused on walking exercises for restoring the functionality conditioned by potentially reversible pathologies. A formal exercise prescription was not provided at study entry, and patients were instructed to continue with the current activity practices through the duration of the study. The study followed the principles of the Declaration of Helsinki and was approved by the institutional Clinical Research Ethics Committee. All patients or their legal representatives provided written consent.
Participants and randomization
A trained research assistant conducted a screening interview to determine whether potentially eligible patients met the following inclusion criteria: age ≥ 75 years, Barthel Index score ≥ 60 points, able to ambulate (with/without assistance), and able to communicate and collaborate with the research team. Exclusion criteria included expected length of stay <6 days, very severe cognitive decline (i.e. Global Deterioration Scale score = 7), terminal illness, uncontrolled arrhythmias, acute pulmonary embolism and myocardial infarction, or extremity bone fracture in the past 3 months.
After the baseline assessment was performed, participants were randomly assigned following a 1:1 ratio, without restrictions (http://www.randomizer.org). Assessment staffs were blinded to the main study design and group allocation. Participants were explicitly informed and reminded not to discuss their randomization assignment with the assessment staff.
Intervention
The usual care group received habitual hospital care, which included physical rehabilitation when needed. For the intervention group, exercise training was programmed in two daily sessions (morning and evening) of 20 min duration over 5–7 consecutive days (including weekends) supervised by a qualified fitness specialist. Adherence to the exercise intervention programme was documented in a daily register. A session was considered completed when ≥90% of the programmed exercises were successfully performed.
Each session was performed in a room equipped ad hoc in the ACE unit. Exercises were adapted from the ‘Vivifrail' multicomponent physical exercise programme to prevent weakness and falls.21 Morning sessions included individualized supervised progressive resistance, balance, and walking training exercises. The resistance exercises were tailored to the individual's functional capacity using variable resistance training machines (Matrix, Johnson Health Tech, Ibérica, S.L., Torrejón de Ardoz, Spain, and Exercycle S.L., BHGroup, Vitoria, Spain) aiming at two to three sets of eight to 10 repetitions with a load equivalent to 30–60% of the estimated one‐repetition maximum (1RM). Participants performed three exercises involving mainly lower limb muscles (squats rising from a chair, leg press, and bilateral knee extension) and one involving the upper body musculature (seated bench ‘chest' press). They were instructed to perform the exercises at a high speed to optimize muscle power output, and care was taken to ensure proper exercise execution. Balance and gait retraining exercises gradually progressed in difficulty and included the following: semi‐tandem foot standing, line walking, stepping practice, walking with small obstacles, proprioceptive exercises on unstable surfaces (foam pads sequence), altering the base of support, and weight transfer from one leg to the other. The evening session consisted of functional unsupervised exercises using light‐loads (0.5–1 kg anklets and handgrip ball), such as knee extension/flexion, hip abduction, and daily walking in the corridor of the ACE unit with a duration based on the clinical physical exercise guide ‘Vivifrail'.21
When the clinician in charge of the patient considered that the haemodynamic situation was acceptable and the patient could collaborate, the following endpoints were assessed, and the intervention was started. Endpoints were also assessed on the day of discharge.
Measures and endpoints
Measures of functional performance
The Short Physical Performance Battery (SPPB) and 6 m Gait Velocity Test (GVT) were used to assess functional capacity. The SPPB includes usual walking speed over 4 m, a balance test, and the Five Times Sit to Stand Test, with the sum of the three individual categorical scores yielding the final SPPB score [range points: 0 (worst) to 12 (best)].22 For the GVT, the participants were instructed to walk at their self‐selected usual pace on a smooth, horizontal walkway.
Handgrip strength
Isometric handgrip strength was measured in the dominant hand with a handheld dynamometer (T.K.K. 5401 Grip‐D, Japan). Patients were placed in a sitting position in a chair, with an elbow complete extension, and were asked to squeeze the handle as forcefully as possible for 3 s. After this, two valid trials followed, and the highest value was used as the data point.
Cognitive function
Changes in cognitive function were assessed using the Mini‐Mental State Examination (MMSE)23 [30‐point questionnaire; scale of 0 (worst) to 30 (best)].
Classification of responders, non‐responders, and adverse responders
The inter‐individual variability of the patients in the response to usual care in the control group and exercise training in the intervention group was used to categorize them as Rs, NRs, or ARs using the clinical meaningful change of each variable: 1 point for the SPPB test,24 1 kg for the handgrip test,25 0.1 m/s for the GVT,26 and 3 points for the MMSE test.27 Considering the SPPB, a similar categorization was performed in the main analysis of the RCT.12 Patients were categorized as Rs for an endpoint if there was an improvement equal or higher than the clinical meaningful change at discharge compared with the admission score; patients were considered NRs if they obtained an improved/worsened score at discharge less than the meaningful clinical value; and ARs were those older adults who scored a worse punctuation equal or higher than the clinical meaningful change at discharge in comparison with the admission value.
Statistical analysis
Standard statistical methods were used to calculate the mean and standard deviation. Statistical normality was tested using both statistical (Kolmogorov–Smirnov test) and graphical (normal probability plots) procedures. We used Student's t‐test or the Mann–Whitney U‐test and χ2 or Fisher's test to analyse significant differences between the intervention and control groups for continuous and categorical variables at baseline, respectively. The χ2 test was used for assessing differences in the prevalence of Rs, NRs, and ARs in each endpoint between groups. Differences in mortality at 1 year post‐discharge between categories in each group were also assessed using the χ2 test. One‐way analysis of variance was used to test differences in functional endpoints (SPPB and GVT) at baseline between categories in the control and intervention groups. The Bonferroni post hoc test was applied to establish differences between categories in each group. Data were analysed using SPSS‐IBM (Software, v.21.0 SPSS Inc., Chicago, IL, USA), and a P value <0.05 was considered statistically significant.
Results
The study flow diagram is shown in Figure 1 . No significant differences were found between groups at baseline for demographic and clinical characteristics for study endpoints (Table 1). A total of 370 patients were included in the analysis (209 women, 56.5%) with a mean age 87.3 (4.9) years (range 75–101 years), and 130 patients (35.1%) were nonagenarians. The median length of hospital stay was 8 days in both groups (interquartile range, 4). The mean number of intervention days for each patient was 5.3 ± 0.5 days, and most training days were consecutive (97%). The number of completed morning and evening sessions per patient averaged 5 ± 1 and 4 ± 1, respectively. Mean adherence to the intervention was 97 ± 8% for the morning sessions (i.e. 806 successfully completed sessions of 841 total possible sessions) and 85 ± 30% in the evening sessions (574 of 688). No adverse effects or falls associated with the prescribed exercises were recorded, and no patient had to interrupt the intervention or had their hospital stay modified because of it. The mortality rate at 1 year post‐discharge was 20.3% (42 patients in the control group and 33 patients in the intervention group).
Figure 1.
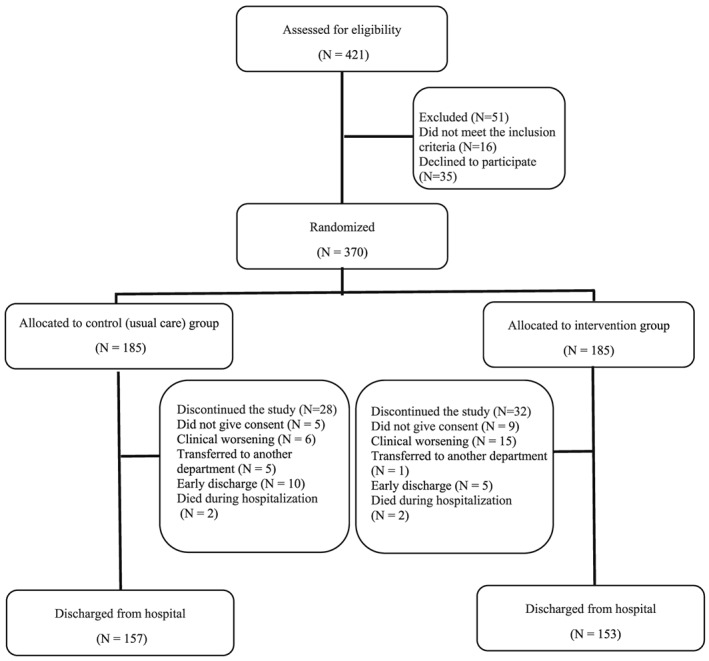
Study flow diagram.
Table 1.
Baseline characteristics of the participants
| Variable | Control group (n = 185) | Intervention group (n = 185) |
|---|---|---|
| Demographic data | ||
| Age, years | 87.1 (5.2) | 87.6 (4.6) |
| Women, N (%) | 109 (59%) | 100 (54%) |
| Body mass index, kg/m2 | 26.9 (4.9) | 27.1 (4.4) |
| Clinical data | ||
| Barthel Index, score | 83 (17) | 84 (17) |
| CIRS (median, IQR), score | 12 (5) | 13 (5) |
| MNA (median, IQR), score | 24 (4) | 24 (4) |
| 1RM leg press, kg | 62 (31) | 57 (25) |
| 1RM chest press, kg | 25 (12) | 24 (11) |
| 1RM knee extension, kg | 41 (14) | 39 (13) |
| GDS, score | 3.6 (2.9) | 4.0 (2.4) |
| QoL (EQ‐VAS), score | 60 (21) | 58 (22) |
| Delirium (CAM, %) | 12% | 17% |
| Endpoint measures | ||
| SPPB scale, score | 4.7 (2.7) | 4.4 (2.5) |
| 6 m GVT, s | 16.1 (8.8) | 16.2 (13.1) |
| Handgrip, kg | 17 (8) | 17 (6) |
| MMSE, score | 23 (4) | 22 (5) |
| Admission reason, N (%) | ||
| Cardiovascular | 67 (36) | 65 (35) |
| Infectious | 33 (18) | 33 (18) |
| Pulmonary | 20 (11) | 28 (15) |
| Gastrointestinal | 17 (9) | 20 (11) |
| Neurological | 9 (5) | 9 (5) |
| Other | 39 (21) | 30 (16) |
1RM, one‐repetition maximum; CAM, Confussion Assessment Method; CIRS, Cumulative Illness Rating Scale; GDS, Yesavage Geriatric Depression Scale; GVT, Gait Velocity Test; IQR, interquartile range; MNA: Mini‐nutritional Assessment; MMSE: Mini‐Mental State Evaluation; QoL, quality of life; EQ‐VAS, visual analogue scale of the EuroQol questionnaire (EQ‐5D); SPPB: Short Physical Performance Battery.
Data are mean (SD) unless otherwise stated. No statistically significant differences were found between groups (all P > 0.05).
The results of the prevalence of Rs, NRs, and ARs to usual care and individualized exercise training programme are shown in Figure 2 . Significant differences were found between groups in the prevalence of Rs, NRs, and ARs in all the endpoints examined (all P < 0.001). Considering the functional endpoints, 33.3% of acutely hospitalized older adults in the control group were ARs, 28.8% were NRs, and 37.9% were Rs for the SPPB in the control group, and 6.0% were ARs, 8.7% NRs, and 85.3% Rs in the intervention group. For the GVT, 14.3% were ARs, 67.7% NRs, and 18.0% Rs in the control group, and 1.6% were ARs, 47.3% NRs, and 51.2% Rs in the intervention group. Regarding the handgrip strength, 42.0% were ARs, 38.0% NRs, and 20.0% Rs in the control group, and 11.3% were ARs, 26.5% NRs, and 62.3% Rs in the intervention group. For the cognitive function test, 9.7% of the patients in the control group were ARs, 76.6% NRs, and 13.8% Rs, whereas 1.4% were ARs, 57.1% NRs, and 41.5% Rs in the exercise training group.
Figure 2.
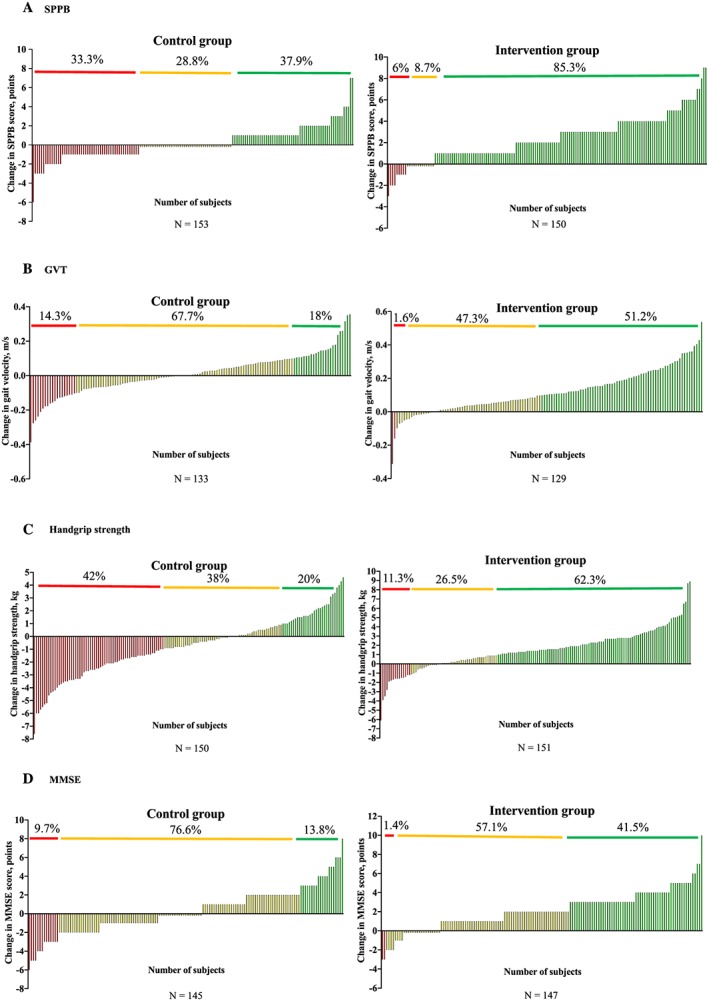
Responders (green line), non‐responders (yellow line), and adverse responders (red line) on functional (A and B), muscle strength (C), and cognitive (D) endpoints. GVT, Gait Velocity Test; MMSE, Mini‐Mental State Examination; SPPB, Short Physical Performance Battery.
Functional, maximal strength, and cognitive changes of all the patients of both groups are shown in Figure 3 based on the response obtained for the functional endpoints (SPPB and GVT, see Figure 2 ).
Figure 3.
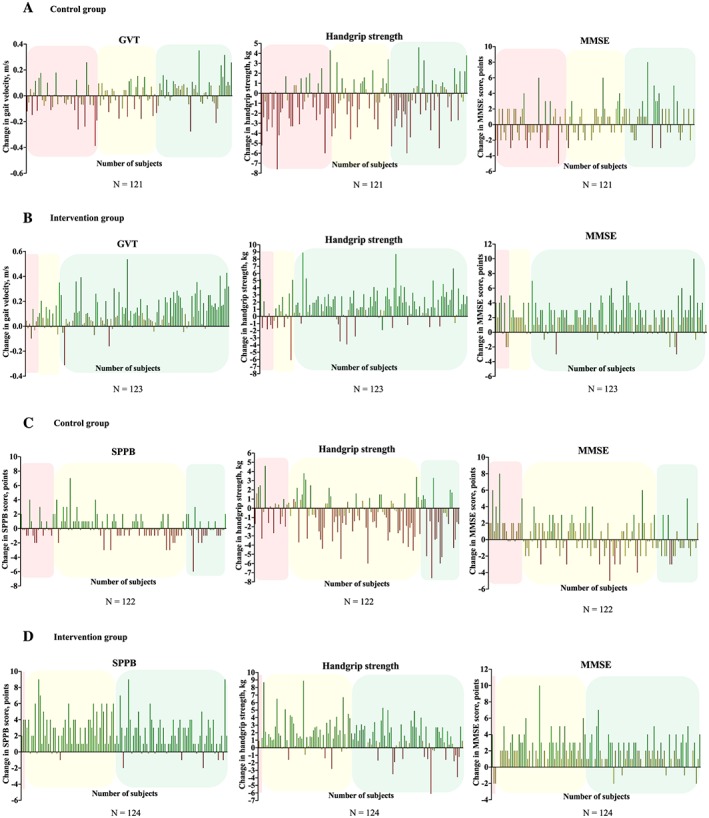
Responders (green line), non‐responders (yellow line), and adverse responders (red line) on functional, muscle strength, and cognitive endpoints based on the SPPB response (A and B) and GVT response (C and D). SPPB response (see Figure 2 A) and GVT response (see Figure 2 b) is represented with green colour (responders), yellow colour (non‐responders), and red colour (adverse responders). GVT, Gait Velocity Test; MMSE, Mini‐Mental State Examination; SPPB, Short Physical Performance Battery.
The secondary analysis showed that patients with an adverse response on the functional endpoints was associated with mortality at 1 year post‐discharge in both control and intervention groups (Table 2). Significant differences were found between categories for the SPPB in the intervention group (0.01) and for the GVT in the control group (0.03).
Table 2.
Mortality rate at 1 year post‐discharge
| Endpoints | Control group | Intervention group |
|---|---|---|
| SPPB | ||
| Adverse responders | 25.5 (95% CI 13.1, 37.9) | 62.5 (95% CI 19.2, 105.8)* |
| Non‐responders | 27.3 (95% CI 13.6, 41.0) | 23.1 (95% CI 0, 49.6) |
| Responders | 24.1 (95% CI 12.8, 35.5) | 18.0 (95% CI 11.2, 24.7) |
| GVT | ||
| Adverse responders | 36.8 (95% CI 13.0, 60.7)* | 0 |
| Non‐responders | 22.2 (95% CI 13.5, 31.0) | 27.9 (95% CI 16.3, 39.4) |
| Responders | 4.2 (95% CI 0, 12.8) | 13.6 (95% CI 5.1, 22.1) |
| Handgrip strength | ||
| Adverse responders | 33.3 (95% CI 21.4, 45.3) | 17.6 (95% CI 0, 37.9) |
| Non‐responders | 19.3 (95% CI 7.4, 27.7) | 17.5 (95% CI 5.2, 29.8) |
| Responders | 26.7 (95% CI 7.3, 39.4) | 22.3 (95% CI 13.8, 30.9) |
| MMSE | ||
| Adverse responders | 35.7 (95% CI 7.0, 64.4) | 0 |
| Non‐responders | 27 (95% CI 18.6, 35.4) | 20.2 (95% CI 11.5, 29.0) |
| Responders | 20 (95% CI 0.8, 39.2) | 19.7 (95% CI 9.4, 29.9) |
CI, confidence interval; GVT, Gait Velocity Test; MMSE, Mini‐Mental State Examination; SPPB, Short Physical Performance Battery.
Data are presented as % (95% CI).
P < 0.05.
We also observed significant differences between categories for the SPPB score at admission in the intervention group (ARs = 3.6 ± 1.2 points, NRs = 4.4 ± 3.4 points, Rs = 4.5 ± 2.5 points; 0.01) and for the GVT in the control group (ARs = 0.59 ± 0.2 m/s, NRs = 0.46 ± 0.2 m/s, Rs = 0.38 ± 0.2 m/s; P < 0.01).
Discussion
Our study shows that acutely hospitalized older adults performing an individualized exercise intervention presented a higher prevalence of Rs and a lower prevalence of NRs and ARs for functional capacity, muscle strength, and cognitive function compared with patients receiving usual care. An adverse response on functional capacity in older patients treated with physical exercise or usual care during hospitalization was associated with mortality at 1 year post‐discharge. Moreover, the functional status presented at admission seems to play a key role in the trajectory of patients during hospital stay and even more so at follow‐up. To the best of our knowledge, this is the first study to analyse the inter‐individual variability in response to physical exercise and usual care in this population.
Acute illness requiring hospitalization is often a crucial event for many older adults,7 and functional decline is one of the negative short‐term consequences of bed rest during hospitalization.28 However, recent evidence has demonstrated that specific in‐hospital exercises could provide significant benefits over usual care and could help to reverse the functional decline associated with acute hospitalization in older adults.12 Although beneficial effects of exercise intervention on functional capacity are well established, frequent reports based of ‘average' exercise‐related changes do not represent the wide individual variability in response to exercise.18 The present inter‐individual analysis study may be a first step to a greater precision in each individual, in‐hospital treatments. We found a higher prevalence of Rs in the exercise training group compared with usual care group for both functional endpoints. Thus, tailored multicomponent exercise training appears to be an effective therapy for improving functional capacity in acutely hospitalized older adults. In addition, we observed a higher prevalence of Rs and a lower prevalence of NRs and ARs for handgrip strength and cognitive function in the intervention group than in the control group. We believe that these findings are important because muscle mass and neuromuscular function tend to decrease during hospital stay in older adults, with muscle strength and mass strongly associated with disability, morbidity, and cardiometabolic disease‐related mortality.29 Moreover, prolonged bed rest increases the risk of developing cognitive impairment and dementia in older adults.30
We also explored whether the response rate for functional capacity was accompanied by similar changes for muscle strength and cognition. Our findings indicate a considerable heterogeneity of response for handgrip strength and cognitive function after usual care or physical exercise. Therefore, response rate for functional capacity could not predict similar changes in other clinical characteristics, such as muscle strength and cognition.
Changes in functional status during hospitalization play an important role in the life trajectory of older adults after discharge. In agreement with previous studies,8, 11 our findings show that functional decline (i.e. ARs for the GVT) during hospitalization is associated with a higher rate of mortality at 1 year post‐discharge compared with NRs and Rs. In the intervention group, those patients who experienced loss of functional capacity after the exercise training programme (i.e. ARs for the SPPB) also showed a higher rate of mortality at follow‐up in comparison with other categories. Our results support the importance of measuring functional status in hospitalized older patients,11 a useful vital sign that should be assessed by hospital clinicians.28
Finally, functional status at admission contains crucial information about prognosis of different interventions in acutely hospitalized older people. Our data suggest that those older adults with higher gait velocity at admission had worse response to usual care and, consequently, major vulnerability to iatrogenic nosocomial disability than those with less functional reserve at baseline. A greater window of worsening during hospitalization could be a possible explanation for the major functional decline. Our findings also showed differences in responses to exercise training based on the functional capacity presented at baseline. Older adults who experienced a worsened response in the intervention group had less functional reserve at admission (SPPB score < 4 points) compared with NRs and Rs. It means that patients at worst functional status at admission have a greater possibility to be an adverse responder to the exercise intervention. Taken together with the aforementioned association between adverse responsiveness to exercise and mortality, older adults with poor scores in the SPPB at admission are also at major risk of mortality after discharge.
Overall, our study is in line with the long trajectory of research supporting the relevance of patients' baseline function as a useful benchmark and goal for discharge and follow‐up outcomes.28
Our study has some limitations, including patients' difficulty in completing all the measurements at both hospital admission and discharge. Another possible limitation was that only old patients with relatively good functional capacity at pre‐admission (i.e. Barthel Index score ≥ 60 points) were included in the study; thus, the results may not be generalizable to the entire hospitalized elderly population. Also, we did not collect functional data prior to the acute illness and functional decline in acutely hospitalized older people frequently occurs before admission.28
Our study, nevertheless, has several strengths. An innovative exercise intervention of few days (i.e. 5 ± 1 and 4 ± 1 morning and evening sessions, respectively) was performed with older adults in acute settings. Also, patients with multiple co‐morbidities and mild dementia/cognitive impairment were included in the study (routinely excluded from exercise studies). The prevalence of Rs was higher for functional capacity, muscle strength, and cognitive function in the exercise training group compared with the usual care group, indicating that the physical exercise programme was effective to reverse functional decline and cognitive impairment associated with hospitalization in older adults. Both functional capacity endpoints (SPPB and GVT) measured in the study for monitoring functional trajectory of patients were associated with mortality at 1 year post‐discharge. Finally, we identified clinical differences between categories at admission in both exercise and usual care groups.
Conclusions
Older patients performing an individualized exercise intervention showed a higher prevalence of Rs and a lower prevalence of NRs and ARs for functional capacity, muscle strength, and cognitive function than those who were treated with usual care during acute hospitalization. An adverse response on functional capacity in older medical patients to physical exercise or usual care during hospitalization was associated with mortality at 1 year post‐discharge. Moreover, the functional status presented at admission seems to be a cornerstone in the trajectory of patients during hospital stay and even more so at follow‐up. These findings support the need for a shift from the traditional disease‐focused approach in hospital ACE to one that recognizes functional status as a clinical vital sign.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
The study protocol was developed by M.L.S.A., A.C.H., N.M.V., and M.I. Data acquisition and statistical analysis were done by M.L.S.A., F.Z.F., N.M.V., and M.I. Finally, M.L.S.A., F.Z.F., N.M.V., E.L.D., R.R.R., and M.I. prepared the manuscript and revised it critically for intellectual content.
Acknowledgements
This study was funded by a Gobierno de Navarra project Resolución grant 2186/2014 and acknowledged with the ‘Beca Ortiz de Landazuri' as the best research clinical project in 2014, as well as by a research grant PI17/01814 of the Ministerio de Economía, Industria y Competitividad (ISCIII, FEDER). We thank Fundación Miguel Servet (Navarrabiomed) for its support during the implementation of the trial, as well as Fundación Caja Navarra and Fundación la Caixa. Finally, we thank our patients and their families for their confidence in the research team. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.31
Sáez de Asteasu M. L., Martínez‐Velilla N., Zambom‐Ferraresi F., Casas‐Herrero Á., Cadore E. L., Ramirez‐Velez R., and Izquierdo M. (2019) Inter‐individual variability in response to exercise intervention or usual care in hospitalized older adults, Journal of Cachexia, Sarcopenia and Muscle, 10, 1266–1275. 10.1002/jcsm.12481.
Trial Registration: http://ClinicalTrials.gov Identifier: NCT02300896.
References
- 1. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rechel B, Grundy E, Robine JM, Cylus J, Mackenbach JP, Knai C, et al. Ageing in the European Union. Lancet 2013;381:1312–1322. [DOI] [PubMed] [Google Scholar]
- 3. WHO . World report on ageing and health. 2015. Available at: http://www.who.int/ageing/events/world-report-2015-launch/en/ (Accesed 13 Jul. 2018)
- 4. Spillman BC, Lubitz J. The effect of longevity on spending for acute and long‐term care. N Engl J Med 2000;342:1409–1415. [DOI] [PubMed] [Google Scholar]
- 5. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA 2011;306:1782–1793. [DOI] [PubMed] [Google Scholar]
- 6. Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA 2004;292:2115–2124. [DOI] [PubMed] [Google Scholar]
- 7. Gill TM, Gahbauer EA, Han L, Allore HG. The role of intervening hospital admissions on trajectories of disability in the last year of life: prospective cohort study of older people. BMJ 2015;350:h2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Covinsky KE, Justice AC, Rosenthal GE, Palmer RM, Landefeld CS. Measuring prognosis and case mix in hospitalized elders. The importance of functional status. J Gen Intern Med 1997;12:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Covinsky KE, Wu AW, Landefeld CS, Connors AF, Phillips RS, Tsevat J, et al. Health status versus quality of life in older patients: does the distinction matter? Am J Med 1999;106:435–440. [DOI] [PubMed] [Google Scholar]
- 10. Fortinsky RH, Covinsky KE, Palmer RM, Landefeld CS. Effects of functional status changes before and during hospitalization on nursing home admission of older adults. J Gerontol A Biol Sci Med Sci 1999;54:M521–M526. [DOI] [PubMed] [Google Scholar]
- 11. Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA 1998;279:1187–1193. [DOI] [PubMed] [Google Scholar]
- 12. Martinez‐Velilla N, Casas‐Herrero A, Zambom‐Ferraresi F, Sáez de Asteasu ML, Lucia A, Galbete A, et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: a randomized clinical trial. JAMA Intern Med 2019;179:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez‐Velilla N, Herrero AC, Cadore EL, Saez de Asteasu ML, Izquierdo M. Iatrogenic nosocomial disability diagnosis and prevention. J Am Med Dir Assoc 2016;17:762–764. [DOI] [PubMed] [Google Scholar]
- 14. Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc 2004;52:1263–1270. [DOI] [PubMed] [Google Scholar]
- 15. Zisberg A, Shadmi E, Sinoff G, Gur‐Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc 2011;59:266–273. [DOI] [PubMed] [Google Scholar]
- 16. Brown CJ, Foley KT, Lowman JD Jr, MacLennan PA, Razjouyan J, Najafi B, et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial. JAMA Intern Med 2016;176:921–927. [DOI] [PubMed] [Google Scholar]
- 17. Martinez‐Velilla N, Casas‐Herrero A, Zambom‐Ferraresi F, Suárez N, Alonso‐Renedo J, Contín KC, et al. Functional and cognitive impairment prevention through early physical activity for geriatric hospitalized patients: study protocol for a randomized controlled trial. BMC Geriatr 2015;15:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Astorino TA, Schubert MM. Individual responses to completion of short‐term and chronic interval training: a retrospective study. PLoS ONE 2014;9:e97638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alvarez C, Ramirez‐Campillo R, Ramirez‐Velez R, Izquierdo M. Effects and prevalence of nonresponders after 12 weeks of high‐intensity interval or resistance training in women with insulin resistance: a randomized trial. J Appl Physiol (1985) 2017;122:985–996. [DOI] [PubMed] [Google Scholar]
- 20. Bouchard C, Blair SN, Church TS, Earnest CP, Hagberg JM, Häkkinen K, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS ONE 2012;7:e37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Izquierdo M, Casas‐Herrero A, Zambom‐Ferraresi F, Martínez‐Velilla N, Alonso‐Bouzón C, Rodríguez‐Mañas L. Multicomponent physical exercise program VIVIFRAIL. 2017. Retrieved from http://www.vivifrail.com/images/recursos/VIVIFRAIL-ENG-Interactivo.pdf
- 22. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 24. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743–749. [DOI] [PubMed] [Google Scholar]
- 25. Rijk JM, Roos PR, Deckx L, van den Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta‐analysis. Geriatr Gerontol Int 2016;16:5–20. [DOI] [PubMed] [Google Scholar]
- 26. Veronese N, Stubbs B, Volpato S, Zuliani G, Maggi S, Cesari M, et al. Association between gait speed with mortality, cardiovascular disease and cancer: a systematic review and meta‐analysis of prospective cohort studies. J Am Med Dir Assoc 2018;19:981–988. [DOI] [PubMed] [Google Scholar]
- 27. Clark CM, Sheppard L, Fillenbaum GG, Galasko D, Morris JC, Koss E, et al. Variability in annual Mini‐Mental State Examination score in patients with probable Alzheimer disease: a clinical perspective of data from the Consortium to Establish a Registry for Alzheimer's Disease. Arch Neurol 1999;56:857–862. [DOI] [PubMed] [Google Scholar]
- 28. Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc 2003;51:451–458. [DOI] [PubMed] [Google Scholar]
- 29. Artero EG, Lee DC, Lavie CJ, España‐Romero V, Sui X, Church TS, et al. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev 2012;32:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA 2010;303:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


