Abstract
Background
Successful strategies to halt or reverse sarcopenia require a basic understanding of the factors that cause muscle loss with age. Acute periods of muscle loss in older individuals have an incomplete recovery of muscle mass and strength, thus accelerating sarcopenic progression. The purpose of the current study was to further understand the mechanisms underlying the failure of old animals to completely recover muscle mass and function after a period of hindlimb unloading.
Methods
Hindlimb unloading was used to induce muscle atrophy in Fischer 344–Brown Norway (F344BN F1) rats at 24, 28, and 30 months of age. Rats were hindlimb unloaded for 14 days and then reloaded at 24 months (Reloaded 24), 28 months (Reloaded 28), and 24 and 28 months (Reloaded 24/28) of age. Isometric torque was determined at 24 months of age (24 months), at 28 months of age (28 months), immediately after 14 days of reloading, and at 30 months of age (30 months). During control or reloaded conditions, rats were labelled with deuterium oxide (D2O) to determine rates of muscle protein synthesis and RNA synthesis.
Results
After 14 days of reloading, in vivo isometric torque returned to baseline in Reloaded 24, but not Reloaded 28 and Reloaded 24/28. Despite the failure of Reloaded 28 and Reloaded 24/28 to regain peak force, all groups were equally depressed in peak force generation at 30 months. Increased age did not decrease muscle protein synthesis rates, and in fact, increased resting rates of protein synthesis were measured in the myofibrillar fraction (Fractional synthesis rate (FSR): %/day) of the plantaris (24 months: 2.53 ± 0.17; 30 months: 3.29 ± 0.17), and in the myofibrillar (24 months: 2.29 ± 0.07; 30 months: 3.34 ± 0.11), collagen (24 months: 1.11 ± 0.07; 30 months: 1.55 ± 0.14), and mitochondrial (24 months: 2.38 ± 0.16; 30 months: 3.20 ± 0.10) fractions of the tibialis anterior (TA). All muscles increased myofibrillar protein synthesis (%/day) in Reloaded 24 (soleus: 3.36 ± 0.11, 5.23 ± 0.19; plantaris: 2.53 ± 0.17, 3.66 ± 0.07; TA: 2.29 ± 0.14, 3.15 ± 0.12); however, in Reloaded 28, only the soleus had myofibrillar protein synthesis rates (%/day) >28 months (28 months: 3.80 ± 0.10; Reloaded 28: 4.86 ± 0.19). Across the muscles, rates of protein synthesis were correlated with RNA synthesis (all muscles combined, R 2 = 0.807, P < 0.0001).
Conclusions
These data add to the growing body of literature that indicate that changes with age, including following disuse atrophy, differ by muscle. In addition, our findings lead to additional questions of the underlying mechanisms by which some muscles are maintained with age while others are not.
Keywords: Skeletal muscle, Stable isotopes, Atrophy, Aging, Regrowth, Protein synthesis
Introduction
The Centers for Disease Control and Prevention now recognizes sarcopenia as an independently reportable medical condition. This recognition as a syndrome gives hope for increased rigor in assessment and diagnosis,1 which may provide insight into the discrepant measures of prevalence.2, 3, 4 However, even with an understanding of the full scope of the incidence of disease, implementation of effective strategies to prevent or reverse the progression of sarcopenia are limited for the immediate future owing to an incomplete understanding of the mechanisms underlying the condition. Therefore, implementation of successful strategies to halt or reverse sarcopenia still require a basic understanding of the factors that give rise to muscle loss with age.
Muscle loss is characterized by slow and gradual loss over time, as well as acute periods of accelerated loss, such as during inactivity or bed rest.5 Acute periods of muscle loss in older individuals are often met with an incomplete recovery of muscle mass and strength, thus accelerating the gradual sarcopenic progression.6, 7, 8 This inability to completely regain muscle mass and strength is common in both human8 and animal studies.6, 7 Therefore, understanding the mechanisms of impaired regrowth in animal models may help direct treatment strategies for sarcopenia in humans.
In a previous study from our group, 29‐month‐old male Fischer 344–Brown Norway (F344BN F1) rats that had their hindlimbs unloaded via tail suspension (HU) for 14 days and subsequently reloaded did not recover muscle mass or function within 14 days, whereas adult 9‐month‐old rats recovered both mass and strength.7 These studies are in agreement with previous reports of impaired regrowth in aging rat models that implement unloading or immobilization at 29–32 months of age.6, 9, 10, 11 Another important finding from this study was that there were muscle‐dependent differences in the amount of atrophy and ability to recover muscle mass and strength. As a previous study showed, there was a greater loss of mass during HU in extensor muscles as compared with flexor muscles.12 The degree of atrophy with unloading was not greater in the older animals compared with the adult animals.7 Therefore, the inability to fully recover muscle mass in older animals is predominantly determined by impaired mechanisms of regrowth, and differences exist across muscles. Muscle growth impairment occurs in the functional overload model at 18 months of age; however, the age at which impairment occurs during regrowth following atrophy is still not known.9
When assessing protein turnover, there are limitations with using markers of protein synthesis instead of direct measures because there are extensive post‐transcriptional control mechanisms.13, 14 In addition, commonly employed short‐term assessments of protein synthesis are limited by the timing of the assessment. The brief duration of the label administration period fails to integrate all aspects of feeding, resting, and/or other physical activity and physiological stresses that characterize free‐living conditions. Moreover, we have demonstrated that short‐duration labelling methods bias synthesis rates to abundant proteins or rapidly turning over proteins.15 To circumvent these issues, we have used deuterium oxide (D2O) in a variety of models and interventions to measure long‐term protein synthesis.16, 17, 18, 19, 20 Measurements of protein synthesis made with D2O are unaffected by the immediate physiological state of the animal at the time the samples are taken but rather integrate rates over the entire labelling period. Therefore, for a period of reloading, one can assess the cumulative protein synthesis of the entire reloading period.
The use of long‐term labelling with D2O has facilitated the exploration of sub‐fractions of protein synthesis and mechanisms that lead to changes in protein turnover. Previous studies indicate that protein synthesis increases during the reloading phase in older rats as indicated by markers of translation initiation,6 markers of ribosomal biogenesis,7 and short‐term labelling of protein synthesis using the puromycin technique.7 However, not all studies are in agreement.11 If it is true that protein synthesis increases in older animals during reloading, but muscle mass is still lost, there must be an increase in protein breakdown, increases in protein synthesis of non‐myofibrillar proteins, or problems with protein assembly. Therefore, a further investigation using direct measures of the synthesis of protein sub‐fractions and anabolic responses such as ribosomal biogenesis over the entire reloaded period is still needed.
The goal of this study was to understand muscle‐specific and age‐specific synthetic responses in skeletal muscle during a period of regrowth after atrophy. To do so, we used direct measures of protein synthesis during a 14 day period of regrowth to examine cumulative protein synthesis in three different muscles. In addition, we performed experiments in 24‐ and 28‐month‐old rats to better determine the age at which impairments in muscle regrowth occur. Finally, we examined sub‐fractions of proteins to better understand myofibrillar and collagen protein synthesis, and mitochondrial and ribosomal biogenesis. Our overall hypothesis was that synthetic responses after a period of disuse atrophy would differ by muscle and with increasing age.
Materials and methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of California, Davis. Thirty‐six male Fischer 344–Brown Norway F1 hybrid (F344BN F1) rats were obtained from the National Institute of Aging at 24 months of age. After acclimating in their cages for one week, the rats were randomly assigned to one of five groups (refer to ‘Experimental Design' section). For this study, we chose to study the soleus, plantaris, and tibialis anterior (TA) because of their variable response to reloading in aged rats and their variability physiological function: the soleus and plantaris represent ankle extensor muscles, while the TA represents an ankle flexor. Further, these muscles vary in their fibre‐type distribution. Our previous21 (and unpublished) data indicate the following fibre‐type distribution in adult rats (9 months of age): soleus = 95% Type I and 5% Type IIa; plantaris = 9% Type I, 26% Type IIa, 11% Type IIb, 44% Type IIx, and 10% hybrid; and TA = 3% Type I, 21% Type IIa, 43% Type IIb, 23% Type IIx, and 10% hybrid. In addition, our previous data show that all three muscles atrophy in response to hindlimb unloading after 14 days, although to varying degrees: soleus, 38%; PL, 26%; and TA, 20%.
Experimental design
In this 6 month study, we used hindlimb unloading to induce muscle atrophy in rats at various times to determine how age and the number of atrophy events impact muscle recovery, force production, and muscle protein and RNA synthesis. The rats were divided into five groups starting at 24 months of age (Figure 1). For the first group, 24‐month‐old rats (24 months) were given deuterium oxide (D2O) for 14 days and then euthanized (n = 4). This group was used as the control for all subsequent groups. In the second group, a baseline muscle force measurement (refer to succeeding discussion) was taken at 24 months, and then the rats underwent hindlimb unloading for 14 days (n = 8). Following the unloading period, another force measurement was taken, and the rats were returned to normal cages. Three of the rats were then given D2O for 14 days (Reloaded 24). After 14 days, the three rats on D2O were sacrificed, while force was measured in the remaining five rats. The remaining rats (n = 4 due to loss of one rat at 29 months of age) were aged to 30 months, upon which a final force measurement was taken, and then the animals were euthanized. In the third group, a baseline muscle force measurement was taken at 24 months of age, and then the rats were aged to 28 months (n = 8). At 28 months, a force measurement was taken, and then three of the rats were given D2O for 14 days (28 months). After 14 days, the three rats on D2O were euthanized, while the five remaining rats were aged to 30 months. At 30 months, force was measured in the five remaining rats, and then the animals were sacrificed. In the fourth group, a baseline muscle force measurement was taken at 24 months of age, and then the rats were aged to 28 months (n = 8). At 28 months, force was measured, and then these rats underwent hindlimb unloading for 14 days. At the end of the unloading period, force was measured, and the rats were returned to normal cages. Three of the rats were then given D2O for 14 days (Reloaded 28). After 14 days, the three rats on D2O were sacrificed, while force was measured in the remaining five rats. The five remaining rats were aged to 30 months, upon which a final force measurement was taken, and then the animals were euthanized. For the final group, a baseline force measurement was taken at 24 months of age, and then the rats underwent hindlimb unloading for 14 days (n = 8). Following the unloading period, another force measurement was taken, and the rats were returned to normal cages. After 14 days, muscle force was measured, and then the rats were aged to 28 months. At 28 months, force was measured, and then these rats underwent a second bout of hindlimb unloading for 14 days. At the end of the second unloading period, force was measured, and the rats were returned to normal cages. Three of the rats were then given D2O for 14 days (Reloaded 24/28). After 14 days, the three rats on D2O were sacrificed, while force was measured in the remaining five rats. The five remaining rats were aged to 30 months, upon which a final force measurement was taken, and then the animals were euthanized.
Figure 1.
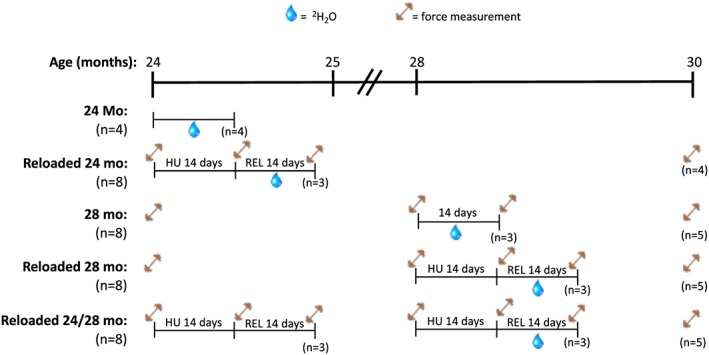
Study design of the five different sets of F344BN rats that were studied from 24 to 30 months of age. The number of rats for each group and outcome is noted. The water drop indicates the period of D2O loading. The barbell symbol depicts when force–frequency measurements were made.
Hindlimb unloading
For the rats assigned to groups Reloaded 24, Reloaded 28, and Reloaded 24/28, tail suspension was used to unload the hindlimbs as previously described.7, 22 The rats had access to food and water ad libitum during the 14 day unloading period. On Day 15, the rats were released from the tail suspension, individually housed, and allowed unrestricted cage activity.
Force measurements
Beginning at 24 months of age, six isometric contractions were used to determine the baseline in vivo isometric torque–frequency profile of the ankle plantar flexor muscles (gastrocnemius, plantaris, and soleus) as previously described.7 Briefly, while anaesthetized with isoflurane, the left hindfoot of each rat was secured to a footplate attached to an Aurora Scientific 300B servomotor, and the plantar flexor muscles were stimulated by two needle electrodes inserted proximally to the peroneal nerve. Torque was measured at stimulation frequencies of 20, 40, 60, 80, 100, and 125 Hz. Each contraction was 200 ms in duration with 45 s of rest between contractions. Data acquisition and analysis were completed using Dynamic Muscle Control and Dynamic Muscle Analysis software (Aurora Scientific). Torque was not measured in the rats that received D2O.
Deuterium oxide labelling protocol
Rats were labelled as previously described.23, 24 Briefly, to initiate labelling, the rats we administered a bolus dose of isotonic deuterium oxide (D2O, 99%) equivalent to 5% of the body water pool. For the remainder of the 14 day labelling period, the rats were allowed free access to drinking water enriched 8% with D2O. At the end of 14 days, rats were euthanized after an overnight fast, and tissue was collected as described subsequently.
Tissue collection
At each sacrificial time point, the rats were weighed and anaesthetized with isoflurane, and then the TA, plantaris, and soleus muscles were excised from both hindlimbs. The left TA, plantaris, and soleus muscles were weighed and frozen in liquid nitrogen. The muscles from the right side were weighed, pinned at resting length, and flash frozen in liquid nitrogen‐cooled isopentane. Blood was collected from each animal by cardiac puncture. Following centrifugation of each blood sample for 10 min at 2000 g at 4°C, the serum was transferred into a 1.5 mL microcentrifuge tube and flash frozen in liquid nitrogen.
Isotope analysis
For analysis of protein, tissues were fractionated according to our previously published procedures,23, 24 including collagen25 and RNA.26 For protein, skeletal muscle tissue was homogenized 1:10 in isolation buffer (100 mM KCl, 40 mM Tris HCl, 10 mM Tris base, 5 mM MgCl2, 1 mM EDTA, and 1 mM ATP, pH = 7.5) with phosphatase and protease inhibitors (HALT, Thermo Scientific, Rockford, IL, USA) using a bead homogenizer (Next Advance Inc., Averill Park, NY, USA). After homogenization, subcellular fractions were isolated via differential centrifugation as previously described.23, 24, 25 Once protein pellets were isolated and purified, 250 μL of 1 M NaOH was added, and pellets were incubated for 15 min at 50°C while slowly being mixed. Protein was hydrolysed by incubation for 24 h at 120°C in 6 N HCl. The pentafluorobenzyl‐N,N‐di (pentafluorobenzyl) derivative of alanine was analysed on an Agilent 7890A GC (Agilent, Santa Clara, USA) coupled to an Agilent 5975C MS (Agilent, Santa Clara, USA) as previously described.23, 24
RNA isolation was performed according to our previously published procedures.26 Approximately 15–25 mg of skeletal muscle was homogenized in 800 μL of TRIzol (Thermo Fisher, Rockford, IL, USA) using a bead blender. The homogenate was centrifuged at 12 000 g for 10 min at 4°C. The resulting supernatant was removed, and 160 μL of chloroform was added. The mixture was shaken vigorously and then centrifuged at 12 000 g for 15 min at 4°C. The upper aqueous layer was isolated, mixed with 400 μL of isopropanol, and then left to incubate at room temperature for 10 min. After incubation, the mixture was centrifuged for 10 min at 4°C to pellet RNA. The RNA pellet was isolated, rinsed with 800 μL of 75% ethanol, and resuspended in 50 μL of molecular biology‐grade H2O. The isolated RNA was hydrolysed overnight at 37°C with nuclease S1 and potato acid phosphatase. Hydrolysates were reacted with pentafluorobenzyl hydroxylamine and acetic acid and then acetylated with acetic anhydride and 1‐methylimidazole. Dichloromethane extracts were dried, resuspended in ethyl acetate, and analysed on an Agilent 7890A GC coupled to an Agilent 5975C MS. For gas chromatography–mass spectrometry analysis, we used a DB‐17 column and negative chemical ionization, with helium as carrier and methane as the reagent gas. The fractional molar isotope abundances at m/z 212 (M0) and 213 (M1) of the pentafluorobenzyl triacetyl derivative of purine ribose were quantified using ChemStation software. All analyses were corrected for abundance with an unenriched pentafluorobenzyl triacetyl purine ribose derivative standard.
To determine body water enrichment, 125 μL of plasma was placed into the inner well of o‐ring screw cap and inverted on heating block overnight; 2 μL of 10 M NaOH and 20 μL of acetone were added to all samples and to 20 μL 0–20% D2O standards and then capped immediately. Samples were vortexed at low speed and left at room temperature overnight. Extraction was performed by the addition of 200 μL hexane. The organic layer was transferred through anhydrous Na2SO4 into GC vials and analysed via Electron impact (EI) mode.
The newly synthesized fraction (f) of proteins was calculated from the enrichment of alanine bound in muscle proteins over the entire labelling period, divided by the true precursor enrichment (p), using plasma D2O enrichment with Mass Isotopomer Distribution Analysis (MIDA) adjustment.27 Similarly, RNA synthesis (~85% of total RNA exists as ribosomal RNA) was determined by deuterium incorporation into purine ribose of RNA as previously published,26 with MIDA adjustment of the equilibration of the enrichment of the body water pool with purine ribose.
Statistical analyses
Results are presented as mean ± standard error of measure. For the force–frequency curve, we determined differences in peak force between groups by a one‐way analysis of variance (ANOVA) with Tukey's correction for multiple comparisons. For muscle mass, we first evaluated the effect of muscle and age by a two‐way ANOVA. When there was a significant effect of age, we used Tukey's correction for multiple comparisons to determine what muscles had a significant effect of age. For the effect of age on synthetic rates, we used a two‐way (age and muscle) ANOVA. When there was a significant effect of age, we used Bonferroni's correction for multiple comparisons to determine what muscles had a significant effect of age. To examine synthetic responses to reloading, we used 24 and 28 month values as control conditions and performed a one‐way ANOVA with Bonferroni's correction for the following planned comparisons: 24 months vs. Reloaded 24, 28 months vs. Reloaded 28, 28 months vs. Reloaded 24/28, and Reloaded 28 vs. Reloaded 24/28. Results were considered significant when P < 0.05.
Results
Peak force and muscle mass
In Figure 2A, we demonstrate that with normal aging, soleus mass was maintained from 24 to 30 months; however, there was a significant decrease in plantaris mass at 30 months and TA mass by 28 months. Peak isometric ankle extensor torque was also decreased by 30 months (Figure 2B). When the hindlimb was unloaded and reloaded for a 14 day period, peak isometric force significantly decreased in all groups (Figure S1). After 14 days of reloading, in vivo torque returned to baseline in the Reloaded 24 group (Figure 2C), but not the Reloaded 28 and Reloaded 24/28 groups (Figure 2D and E). Interestingly, despite the failure of Reloaded 28 and Reloaded 24/28 to regain peak force, all groups, including the groups that were not unloaded, were equally depressed in peak force generation at 30 months of age (Figure 2F). This loss by 30 months was reflected in muscle mass as well (Figure 2G). With one exception, at 30 months of age, all groups had equal soleus mass and lower plantaris and TA mass than at 24 months of age, independent of weight bearing or unloading conditions.
Figure 2.
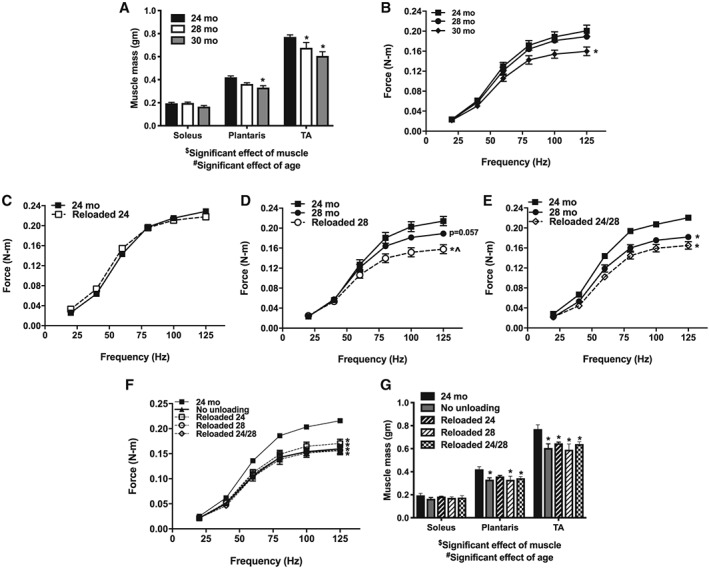
The effect of age from 24 to 30 months on muscle mass (grams) of the soleus, plantaris, and TA (A; n = 3–6). Force (N–m) frequency curve at 24, 28, and 30 months of age (B; n = 6–9). The effect of age and a 14 day reloading period after disuse atrophy on force at 24 months (C; n = 5–9), 28 months (D; n = 5–9), and two periods of unloading at 24 and 28 months (E; n = 5–9). Force frequency curve at 30 months of age as an effect of normal aging, or with periods of unloading and reloading (F; n = 5–32). Muscle mass at 30 months of age in the soleus, plantaris, and TA with and without periods of unloading (G; n = 4–6). $Significant effect of muscle (P < 0.05). #Significant effect of age (P < 0.05). *P < 0.05 compared with 24 months. ^P < 0.05 compared with 28 months. Significant differences in force frequency curves were based on peak force.
Rates of synthesis
In Figure 3, we show the effect of aging among three muscles without the influence of hindlimb unloading. We present these data because we felt it was important to appreciate the effect of age alone prior to interpreting the reloading responses. We assessed myofibrillar protein synthesis (Figure 3A), RNA synthesis (ribosomal biogenesis, Figure 3B), collagen protein synthesis (Figure 3C), and mitochondrial protein synthesis (mitochondrial biogenesis, Figure 3D) in all three muscles. In all cases, there was a significant main effect of age in that age increased synthesis rates from 24 to 28 months. Like force and mass, these synthetic rates differed by muscle. The soleus was remarkably stable in synthetic rates between 24 and 28 months, although there were trends for increased rates at 28 months in the myofibrillar and mitochondrial protein fractions. The plantaris and TA had greater rates of myofibrillar protein synthesis at 28 months compared with 24 months. The TA also had significantly greater rates of synthesis at 28 months for RNA, collagen, and mitochondria.
Figure 3.
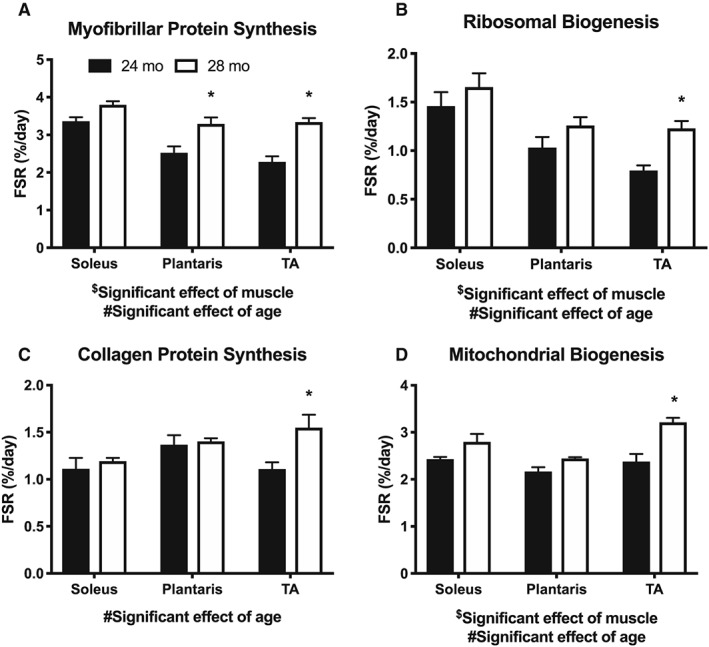
The effect of aging, without disuse atrophy, on synthesis rates (%/day) of (A) myofibrillar proteins, (B) ribosomal RNA, (C) collagen protein, and (D) mitochondrial proteins in the soleus, plantaris, and TA (n = 3–4). $Significant effect of muscle (P < 0.05). #Significant effect of age (P < 0.05). *P < 0.05 compared with 24 months.
When we explored the differences in synthetic rates in the soleus with reloading, we found that both Reloaded 24 and Reloaded 28 had greater myofibrillar protein synthesis rates than had 24 and 28 months, respectively (Figure 4A). These increases were similar for ribosomal biogenesis (Figure 4B), but not collagen protein synthesis (Figure 4C). Mitochondrial protein synthesis of Reloaded 24 was >24 months, but Reloaded 28 was not >28 months (Figure 4D). It appears that the soleus maintained anabolic responses to a reloading stimulus with aging.
Figure 4.
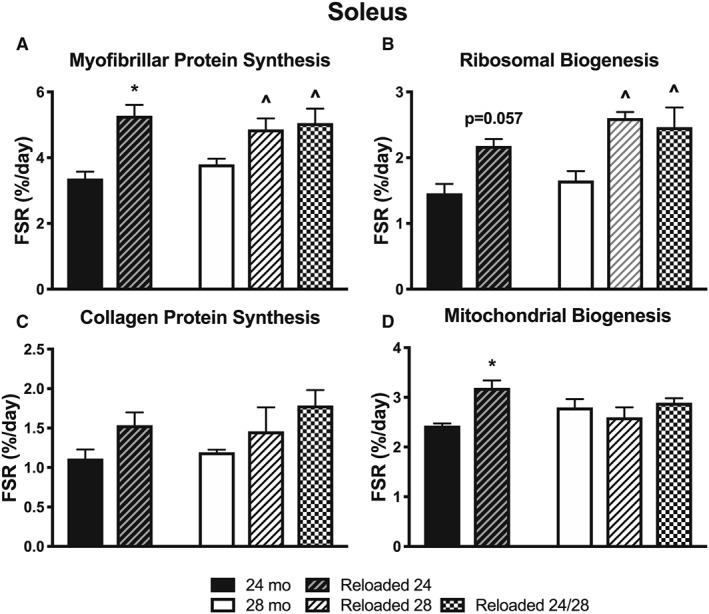
The effect of aging and reloading after a period of disuse atrophy in the soleus on synthesis rates (%/day) of (A) myofibrillar proteins, (B) ribosomal RNA, (C) collagen protein, and (D) mitochondrial proteins (n = 3–4). *P < 0.05 compared with 24 months. ^P < 0.05 compared with 28 months.
For the plantaris, myofibrillar protein synthesis for Reloaded 24 was >24 months, but these differences were not noted at 28 months (Figure 5A). As was shown in Figure 3A, plantaris myofibrillar protein synthesis increased at 28 months compared with 24 months in the absence of unloading/reloading. Therefore, the lack of increase in Reloaded 28 is because the 28 mo synthesis rate is already increased compared to 24 mo. For ribosomal biogenesis, there was a trend (P < 0.10) for an increase in Reloaded 24 compared with 24 months (Figure 5B), and a trend for an increase in collagen synthesis when unloaded and reloaded twice (Figure 5C). Finally, there was a significantly greater mitochondrial protein synthesis rate with Reloaded 24 compared with 24 months (Figure 5D), which was not apparent when Reloaded 28 was compared with 28 months.
Figure 5.
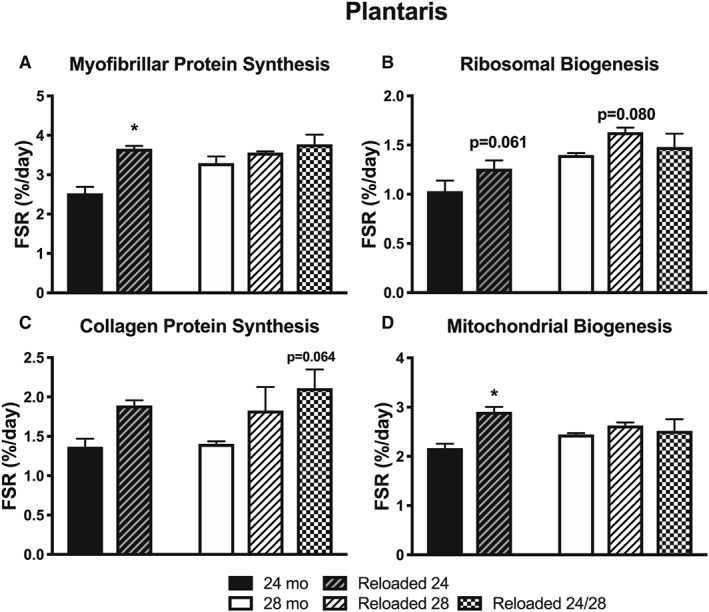
The effect of aging and reloading after a period of disuse atrophy in the plantaris on synthesis rates (%/day) of (A) myofibrillar proteins, (B) ribosomal RNA, (C) collagen protein, and (D) mitochondrial proteins (n = 3–4). *P < 0.05 compared with 24 months.
For the TA, there were significantly greater synthesis rates in all outcomes for Reloaded 24 compared with 24 months (Figure 6A–D). There were no differences in any parameter at 28 months. However, as shown in Figure 3, all 28 month values were increased in the absence of unloading and reloading than were 24 month values.
Figure 6.
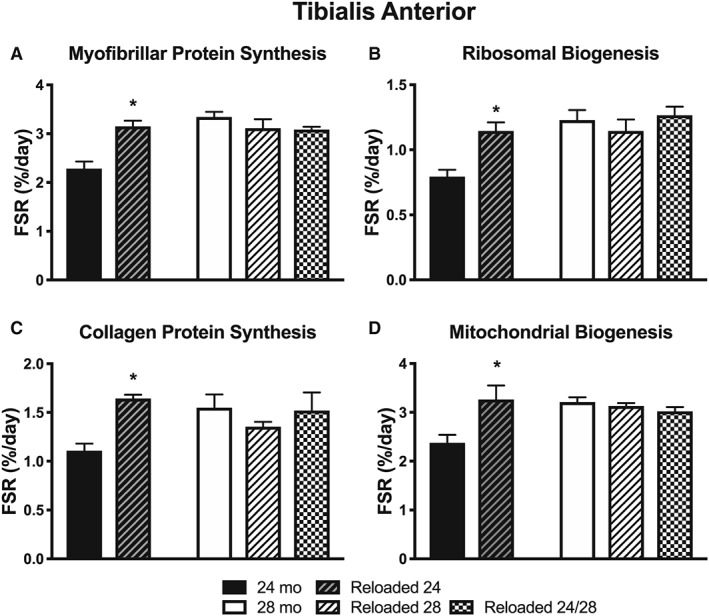
The effect of aging and reloading after a period of disuse atrophy in the TA on synthesis rates (%/day) of (A) myofibrillar proteins, (B) ribosomal RNA, (C) collagen protein, and (D) mitochondrial proteins (n = 3–4). *P < 0.05 compared with 24 months.
Finally, we explored if the rates of myofibrillar protein synthesis were related to ribosomal biogenesis. When we explored individual muscles (Figure 7A, soleus; B, plantaris; C, TA), or all muscles together (Figure 7D), there was a strong and significant correlation between ribosomal biogenesis and myofibrillar protein synthesis during the reloading period.
Figure 7.
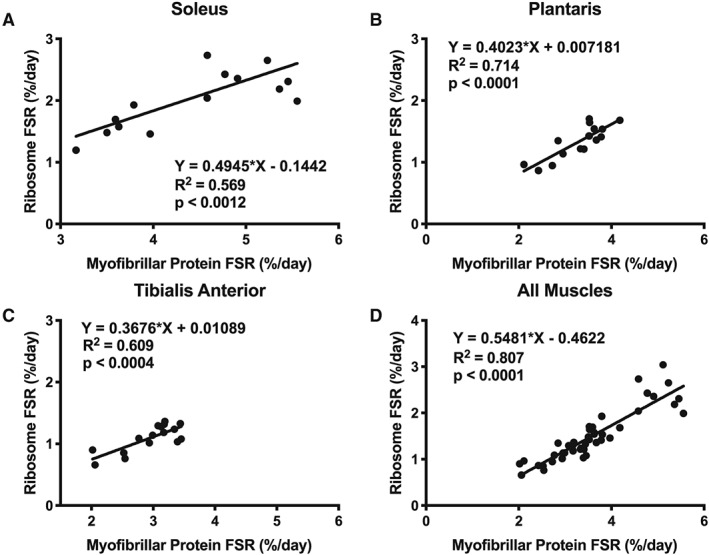
Correlations between myofibrillar protein synthesis (%/day) and ribosomal RNA synthesis (%/day), a measure of ribosomal biogenesis in (A) soleus, (B) plantaris, (C) TA, and (D) all muscles combined.
Discussion
The goal of this study was to understand muscle‐specific and age‐specific synthetic responses in skeletal muscle during a period of regrowth after atrophy. We found that contrary to prevailing thought, protein synthesis is not decreased and in many cases is increased at older age. Further, we found that there is a marked difference in anabolic responses after disuse atrophy in rat skeletal muscle between 24 and 28 months of age. Despite these differences, however, by 30 months of age, muscle mass and peak force were equal whether or not there was a previous period of unloading. From our study design, we were able to directly compare synthesis rates of multiple tissue fractions between three muscles and found strikingly different anabolic responses between the soleus, TA, and plantaris. Finally, there was a strong correlation between myofibrillar protein synthesis and ribosomal biogenesis during reloading, adding evidence that the ability to increase protein synthesis is dependent on ribosomal biogenesis. In total, these data highlight the need to consider long‐term changes in muscle mass and function as well as careful attention to muscle type and age as covariates.
Changes in mass and force differ by muscle
In previous studies, we and others have shown that old age does not worsen disuse atrophy but does slow down, or even prevent, recovery.6, 7, 21 In the current study, we again demonstrate that aging impairs the recovery of peak muscle force generation after a period of disuse atrophy. When unloading occurred at 24 months of age, muscle peak force generation was completely restored after 14 days of reloading; however, this was not the case when unloaded at 28 months. In the current study, we aged animals to 30 months of age to determine how these periods of disuse affected force generation later in life. Surprisingly, no matter if unloading occurred or not, or at what age the unloading occurred, all groups had equally impaired peak force production at 30 months compared with 24 months of age. This insight allows further refinement of current thought about the effect of age on the loss of muscle function after disuse. The data show that disuse atrophy that occurs in older age decreases force production at an earlier age because of a failure to completely recover from the period of atrophy. However, one or more transient periods of rapid loss did not progressively worsen the trajectory of sarcopenia because all groups were equally impaired at 30 months. In this study, the disuse atrophy events occurred during a period when the loss of muscle mass and strength was occurring at a relatively slow rate, i.e. prior to 30 months of age. It is possible that disuse atrophy that occurs after the age of 30 months could lead to accelerated sarcopenia.
The changes in peak force production with age were similar to changes in muscle mass. With normal aging, in the absence of unloading, there were differences in the degree of muscle loss between muscles. The degree of loss corresponded to the fibre‐type composition of the muscle: the soleus, which is predominantly Type I fibres, maintained muscle mass from 24 to 30 months; whereas, the TA, which is predominantly Type II, significantly decreased muscle mass by 28 months. Like peak force production, by 30 months of age, there were equal losses in mass of the plantaris and TA regardless of whether there was a previous period of unloading. On the other hand, the soleus maintained its mass through 30 months even with multiple periods of disuse. This maintenance of mass in the soleus is despite the greater degree of atrophy that occurs in the soleus during hindlimb unloading, compared with the plantaris and TA.7, 21 It is well established that there is a preferential atrophy of Type II muscle fibres with age.28 Our previous data show that in mixed muscles like the TA, Type IIb fibers atrophy more than other fiber types in response to unloading7,21 and are also the most resistant to recovery upon reloading in older rats (unpublished data). From the current data, it appears that fibre type influences the response to periods of disuse atrophy and reloading and again illustrates the importance of considering muscle‐specific responses to aging.7, 21
Age increases muscle protein synthesis
Because of the progressive loss of muscle mass with age, it is thought that there is an impairment of muscle protein synthesis with age,29 which tips net protein balance to a loss over time. Most of the data collected in humans have used short‐term stable isotope labelling methods. Using mathematical modelling, we have demonstrated that these short‐term measurements can bias measurements of fractional synthesis rates to abundant proteins or rapidly turning over proteins.15 On the other hand, the use of D2O as a tracer allows for long‐term measurements that capture the various anabolic states during free‐living conditions, as well as the integrated responses of different protein pools. A recent report used 6 weeks of labelling with D2O and showed no difference in quadriceps FSR in young (mean age = 23 years) vs. older (mean age = 69 years) men.30 This study had an exercise trained and untrained limb, which could affect FSR in the untrained limb31 but, at a minimum, did not show a decrease in muscle protein synthesis with aging.
In the current study, we examined three different hindlimb muscles that varied in their fibre‐type composition and primary function. When examining myofibrillar protein synthesis, there was a significant effect of age across all three muscles. In two of the muscles, the plantaris and TA, there was an increase in myofibrillar protein synthesis from 24 to 28 months of age. These findings are similar to a previous study using short‐term labelling methods in Sprague–Dawley rats that demonstrated an increase in gastrocnemius protein synthesis rates from 21 to 24 months of age, but no further increase at 27 months of age.32 Further, a study using a perfused muscle model and short‐term labelling procedures (25 min) in F344BN rats showed an increase in resting protein synthesis of gastrocnemius from 4 to 32 months of age but a decrease in resting soleus protein synthesis from young (4 months) to middle age (12 months).33 Our data add to these previous two studies and emphasize that it is not correct to broadly state that muscle protein synthesis decreases with age. Our data also demonstrate that there was a significant effect of age on ribosomal biogenesis, indicating that the synthesis of machinery to make new proteins is also increased with age.
In our current study, we likewise demonstrate that there was a significant effect of age on mitochondrial protein synthesis (biogenesis), with the TA muscle showing greater rates of synthesis at 28 months compared with 24 months and the soleus muscle and plantaris showing no decrease with age. These results contradict the commonly held notion that mitochondrial biogenesis decreases with aging.34, 35, 36 However, a close examination of the literature demonstrates that very few studies have actually directly measured mitochondrial protein synthesis rates but instead have relied on indirect measures or protein content. We have discussed the limitations of these approaches previously.14 Although not definitive by themselves, our data strongly indicate that there is a need for a more rigorous examination of the effect of age on skeletal muscle mitochondrial biogenesis.
In addition to the effect of age, we were able to examine the main effect of muscle on protein synthesis rates. We did not perform post hoc analyses on the differences between muscles, but there was a significant effect of muscle for both myofibrillar and mitochondrial proteins synthesis. From our analyses, it appears that the soleus had the highest rates of myofibrillar protein synthesis. Therefore, although the soleus is highly oxidative and is not known to hypertrophy to great extent, it maintains a high rate of myofibrillar protein turnover. It is likely that this high rate of turnover is because of the chronically active nature of the soleus. These data illustrate the importance of considering both fibre type and muscle activity when making conclusions about skeletal muscle protein turnover.
In summary, it is clear that skeletal muscle protein synthesis increases with age in F344BN F1 rats, although this increase varies by muscle and protein fraction. In two of the muscles, the plantaris and TA, these increases are during a time when there is a decrease in muscle mass. This finding is similar to the study of Kimball et al., which showed that increased myofibrillar protein synthesis coincided with loss of muscle mass.32 In our study, muscle protein breakdown must have also accelerated from 24 to 30 months; and the plantaris and TA must have done so to a degree that was greater than the increase in muscle protein synthesis. These data contribute interesting insight into the ongoing inquiry about what processes that ultimately lead to the loss of muscle mass with aging.37 Our data indicate that with normal aging, there is no impairment of protein synthesis, although it is not possible to evaluate if the proteins are processed in a manner that is as effective as at younger ages. Further, additional information is needed about the specific proteins that are being synthesized during the reloading periods and with advancing age. Over the time frame (24–30 months) examined in this study, there are changes in fibre‐type composition and the appearance of fibre‐type grouping, suggesting that denervation and reinnervation processes are occurring. We38 and others39 have reported that protein synthesis increases during denervation, which could account for some of the increased protein synthesis we observed with aging.
Anabolic responses after a period of disuse atrophy differ by muscle
There has been extensive debate about the contributions of protein synthesis and breakdown to muscle loss during disuse atrophy.40, 41 The majority of these discussions have been focused on the period of disuse where both synthesis and breakdown likely contribute to the loss of muscle mass.42 A recent study from our group showed that the degree of atrophy during the disuse period differed by muscle but did not differ between young and old.21 However, although the period of disuse did not change the degree of muscle loss, the ability to regain mass was impaired in older muscle.21 In this latter study, the surface sensing of translation (SUnSET) method was used to measure protein synthesis and indicated that there was no difference in protein synthesis between adult and old rats during the recovery phase. However, there are notable limitations to the SUnSET method including the brevity of measurement and narrow range of proteins measured.15 Therefore, we used D2O to re‐examine muscle‐specific protein synthesis rates during the period of reloading.
In the soleus muscle, there was a markedly greater myofibrillar protein synthesis in Reloaded 24 and Reloaded 28 compared with 24 and 28 month controls, respectively. These greater rates of myofibrillar protein synthesis were matched by greater ribosomal biogenesis during the reloaded period. Our previous studies showed that of the muscles studied, the soleus had the greatest decrease in muscle mass after unloading.21 However, the soleus retained anabolic capacity after unloading, at both 24 and 28 months, and retained muscle mass at 30 months despite periods of unloading. The responses to reloading are markedly different in the soleus compared with the TA. The TA had greater myofibrillar and mitochondrial protein synthesis and greater ribosomal biogenesis during Reloaded 24, but not during Reloaded 28. However, an important distinction is that the values at 28 months in the TA were increased than at 24 months, whereas in the soleus, there was no increase or just a trend for increased synthesis rates at 28 months. In other words, the lack of increase in the reloaded period for the TA was largely because the 28 month value was already elevated because of age. The plantaris muscle responses were intermediate between the soleus and TA.
Our 30 month measurements of mass show that the soleus maintains muscle mass, despite unloading periods, whereas the plantaris and TA did not. However, the period of unloading did not increase the magnitude of loss by 30 months for the plantaris and TA. The soleus retained an ability to increase protein synthesis during reloading periods, but the TA did not. However, the baseline rates of protein synthesis were already elevated in the TA at 28 months, which may have been a maximum rate as indicated by no further increases during reloading. We can therefore speculate that with normal aging, there is a high rate of protein turnover, both synthesis and breakdown, in the soleus owing to its chronic use. When chronic activity is removed, as with unloading, there is a large loss of muscle mass. However, the soleus retains an ability to respond to the reloading and the reinitiating of chronic activity. It is likely, but speculative at this point, that the high oxidative capacity of the soleus facilitates the ability to maintain the energetically costly high rates of turnover and thus the flexibility to remodel as needed. In addition, it is possible that the greater number of myonuclei per area (i.e. decreased myonuclear domain) in the soleus compared with muscles with a greater proportion of fast‐twitch fibres43 facilitates ribosomal biogenesis and growth. In contrast, the TA has predominantly Type II fibres and loses mass with aging. The loss of mass is despite a higher rate of protein synthesis with older age. Further, during reloading, the TA fails to increase protein synthesis rates above rates that are already increased from aging. In this case, the decreased oxidative capacity of the TA may restrict anabolic capacity. In addition, our previous study suggests that the TA may experience some denervation or neuromuscular junction injury during the early reloading period, which could impair recovery of mass and strength.7, 21 It is also possible that some fibres, especially the IIb fibres in the TA and other mixed muscles such as the plantaris, are not recruited during the low‐intensity cage activity and, thus, are not adequately reloaded to initiate a growth response.
The role of ribosomal biogenesis with aging
Recently, the ability to increase the synthesis of ribosomes, ribosomal biogenesis, was identified as an important mechanism to increase protein synthesis. For example, in a study of resistance training in older subjects, a retrospective analysis of responders to non‐responders identified that the ability or inability to respond to resistance training corresponded with ribosome biogenesis.44 In a study of mechanical overload (synergist ablation) in young and old mice, blunted regrowth was explained by a failure to increase ribosomal biogenesis.45 However, both of these studies used measures of content and markers of ribosomal biogenesis, which can obscure biogenic responses. Recently, a study that examined both interval training in rats and resistance training in humans showed that myofibrillar protein synthesis was correlated to ribosomal biogenesis.46 Importantly, this study simultaneously made direct measures of ribosomal biogenesis and myofibrillar protein synthesis using D2O. In the current study, we used our previously described method to directly measure ribosomal biogenesis26 simultaneously with protein synthesis to show that ribosomal biogenesis is not impaired with age and, in the case of the soleus, not impaired with aging and reloading. In the TA, similar to protein synthesis, ribosomal biogenesis was elevated at 28 months compared with 24 months so that further increases were not apparent with Reloaded 28. In each muscle type, and when all muscles were combined, myofibrillar protein synthesis correlated with ribosomal biogenesis. Therefore, our data support that ribosomal biogenesis may be a key mechanism for anabolic responses. However, our data do not support the conclusion that ribosomal biogenesis is impaired with aging because some muscles have higher basal rates of ribosomal biogenesis with aging whereas others can increase ribosomal biogenesis with reloading even at older age.
Two periods of unloading were not worse than one
In one of our groups, we unloaded and reloaded at both 24 and 28 months of age to determine if there is a progressive decrease in the ability to respond to disuse atrophy because of repeated exposures. Almost uniformly, there were no differences between unloading once or unloading twice in any of the responses measured. Across muscles, the maintenance of muscle mass or loss of mass and force was equal at 30 months despite one or two periods of unloading. However, there was one notable trend in the plantaris muscle that deserves comment. When unloaded two times, there was a trend (0.064) for an increase in collagen protein synthesis that was not apparent with Reloaded 24 or Reloaded 28. These data are consistent with a recent report that compared one or two periods of unloading and reloading in young rats that showed no differences in any outcome except for an elevation of collagen content when unloaded twice47. Future studies should examine the potential role of multiple bouts of disuse atrophy on muscle fibrosis.
Limitations and future directions
There are a couple of limitations to the current study. First, the force data were longitudinal in the same rats, but the isotope data were collected on a subset from each group. This limitation was unavoidable in order to collect tissues at the appropriate time points. Second, the number of rats in some subsets is low (n = 3–4). For our isotope measurements, this was not a problem given the low variability of the measurement. However, we were not confident in muscle mass after the period of reloading and therefore did not report those values. Our previous study7, 9 as well as others6 gave us confidence that mass was regained at 24 months, but not at 28 months. The finding of increased synthesis rates with aging deserves further examination. Although we fractionated the tissue into protein pools, there still could be abundant proteins that are driving the increases in synthesis rates whereas the synthesis of other proteins is decreased. It would be valuable to have a direct measure of protein breakdown to confirm that increased synthesis rates are matched by breakdown, or if there is an alternative post‐translational deficiency (e.g. protein assembly). Further, a time course approach, as we have performed previously,15, 16, 48 would allow us to determine what proportion of the protein pool continues to turnover vs. becoming resistant to turnover. Finally, additional studies are needed to further dissect the influence of muscle activation patterns, fibre types, and oxidative capacity on the maintenance of muscle mass with age and the anabolic response after disuse atrophy.
Conclusions
This study adds novel insight into skeletal muscle protein turnover with age and in a period of reloading after disuse atrophy. We found that contrary to prevailing thought, muscle protein synthesis does not decrease with age and is actually increased in some protein fractions at older age. In addition, at 30 months, muscle mass was maintained in the soleus, but not in the plantaris and TA whether or not there were periods of unloading. These changes in mass were matched by a decrease in force production. Protein synthesis during a period of reloading was able to increase in the soleus at older age, but not in the plantaris and TA. However, the rates of protein synthesis in the plantaris and TA were already elevated by aging alone. Finally, there was a strong correlation between myofibrillar protein synthesis and ribosomal biogenesis during reloading, adding evidence that the ability to increase protein synthesis is dependent on ribosomal biogenesis. These data add to the growing body of literature that changes with age, including following disuse atrophy, differ by muscle. In addition, they lead to additional questions of the underlying mechanisms by which some muscles during the aging process are maintained and others are not.
Conflict of interest
S.C.B. is on the scientific advisory board for Emmyon. All other authors declare that they do not have a conflict of interest.
Supporting information
Figure S1. The effect of unloading and age on force (N‐m) frequency curves when unloaded at a) 24, b) 28, and c) 24 and 28 mo of age (n = 8‐9). Unloaded 24 and Unloaded 28 were analyzed by a paired t‐test. Unloaded 24/28 were analyzed by a one‐way ANOVA with Tukey's adjustment. *p < 0.05 compared to 24 mo, ^p < 0.05 compared to 28 mo.
Acknowledgements
This work was funded by Veterans Affairs RR&D Merit Grant 1I01RX000673‐01A1 (S.C.B.). The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle 49.
Miller B. F., Baehr L. M., Musci R. V., Reid J. J., Peelor F. F. III, Hamilton K. L., and Bodine S. C. (2019) Muscle‐specific changes in protein synthesis with aging and reloading after disuse atrophy, Journal of Cachexia, Sarcopenia and Muscle, 10, 1195–1209. 10.1002/jcsm.12470.
References
- 1. Falcon LJ, Harris‐Love MO. Sarcopenia and the new ICD‐10‐CM code: screening, staging, and diagnosis considerations. Fed Pract 2017;34:24–32. [PMC free article] [PubMed] [Google Scholar]
- 2. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 3. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
- 4. Batsis JA, Mackenzie TA, Barre LK, Lopez‐Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 2014;68:1001–1007. [DOI] [PubMed] [Google Scholar]
- 5. English KL, Paddon‐Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 2010;13:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White JR, Confides AL, Moore‐Reed S, Hoch JM, Dupont‐Versteegden EE. Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways. Exp Gerontol 2015;64:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baehr LM, West DWD, Marcotte G, Marshall AG, De Sousa LG, Baar K, et al. Age‐related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 2016;8:127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, et al. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol 2009;107:1172–1180. [DOI] [PubMed] [Google Scholar]
- 9. Hwee DT, Bodine SC. Age‐related deficit in load‐induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci 2009;64:618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zarzhevsky N, Carmeli E, Fuchs D, Coleman R, Stein H, Reznick AZ. Recovery of muscles of old rats after hindlimb immobilisation by external fixation is impaired compared with those of young rats. Exp Gerontol 2001;36:125–140. [DOI] [PubMed] [Google Scholar]
- 11. Morris RT, Spangenburg EE, Booth FW. Responsiveness of cell signaling pathways during the failed 15‐day regrowth of aged skeletal muscle. J Appl Physiol 2004;96:398–404. [DOI] [PubMed] [Google Scholar]
- 12. Deschenes MR, Britt AA, Chandler WC. A comparison of the effects of unloading in young adult and aged skeletal muscle. Med Sci Sports Exerc 2001;33:1477–1483. [DOI] [PubMed] [Google Scholar]
- 13. Miller BF, Konopka AR, Hamilton KL. The rigorous study of exercise adaptations: why mRNA might not be enough. J Appl Physiol 2016;121:594–596. [DOI] [PubMed] [Google Scholar]
- 14. Miller BF, Hamilton KL. A perspective on the determination of mitochondrial biogenesis. Am J Physiol Endocrinol Metab 2012;302:E496–E499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller BF, Wolff CA, Peelor FF, Shipman PD, Hamilton KL. Modeling the contribution of individual proteins to mixed skeletal muscle protein synthetic rates over increasing periods of label incorporation. J Appl Physiol 2015;118:655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruns DR, Ehrlicher SE, Khademi S, Biela LM, Peelor FF, Miller BF, et al. Differential effects of vitamin C or protandim on skeletal muscle adaptation to exercise. J Appl Physiol 2018;125:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Konopka AR, Laurin JL, Musci RV, Wolff CA, Reid JJ, Biela LM, et al. Influence of Nrf2 activators on subcellular skeletal muscle protein and DNA synthesis rates after 6 weeks of milk protein feeding in older adults. Geroscience 2017;21:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller BF, Ehrlicher SE, Drake JC, Peelor FF, Biela LM, Pratt‐Phillips S, et al. Assessment of protein synthesis in highly aerobic canine species at the onset and during exercise training. J Appl Physiol 2015;118:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long‐term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J 2011;25:3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, et al. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 2014;28:2705–2714. [DOI] [PubMed] [Google Scholar]
- 21. Baehr LM, West DWD, Marshall AG, Marcotte GR, Baar K, Bodine SC. Muscle‐specific and age‐related changes in protein synthesis and protein degradation in response to hindlimb unloading in rats. J Appl Physiol 2017;122:1336–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomason DB, Herrick RE, Surdyka D, Baldwin KM. Time course of soleus muscle myosin expression during hindlimb suspension and recovery. J Appl Physiol 1987;63:130–137. [DOI] [PubMed] [Google Scholar]
- 23. Estrada AL, Hudson WM, Kim PY, Stewart CM, Peelor FF, Wei Y, et al. Short‐term changes in diet composition do not affect in vivo hepatic protein synthesis in rats. Am J Physiol Endocrinol Metab 2018;314:E241–E250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newsom SA, Miller BF, Hamilton KL, Ehrlicher SE, Stierwalt HD, Robinson MM. Long‐term rates of mitochondrial protein synthesis are increased in mouse skeletal muscle with high fat feeding regardless of insulin sensitizing treatment. Am J Physiol Endocrinol Metab 2017;313:E552–E562, ajpendo.00144.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller BF, Olesen JL, Hansen M, Døssing S, Crameri RM, Welling RJ, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 2005;567:1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mathis AD, Naylor BC, Carson RH, Evans E, Harwell J, Knecht J, et al. Mechanisms of in vivo ribosome maintenance change in response to nutrient signals. Mol Cell Proteomics 2017;16:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Busch R, Kim Y‐K, Neese RA, Schade‐Serin V, Collins M, Awada M, et al. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta 2006;1760:730–744. [DOI] [PubMed] [Google Scholar]
- 28. Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15‐ to 83‐year‐old men. J Neurol Sci 1988;84:275–294. [DOI] [PubMed] [Google Scholar]
- 29. Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy‐chain and sarcoplasmic protein in humans. Am J Physiol 1997;273:E790–E800. [DOI] [PubMed] [Google Scholar]
- 30. Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, et al. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age‐related anabolic resistance to exercise in humans. J Physiol 2016;594:7399–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller BF, Hamilton KL, Majeed ZR, Abshire SM, Confides AL, Hayek AM, et al. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non‐massaged hindlimb. J Physiol 2018;596:83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kimball SR. Assessment of biomarkers of protein anabolism in skeletal muscle during the life span of the rat: sarcopenia despite elevated protein synthesis. Am J Physiol Endocrinol Metab 2004;287:E772–E780. [DOI] [PubMed] [Google Scholar]
- 33. Fluckey JD, Vary TC, Jefferson LS, Evans WJ, Farrell PA. Insulin stimulation of protein synthesis in rat skeletal muscle following resistance exercise is maintained with advancing age. J Gerontol: Series A 1996;51:B323–B330. [DOI] [PubMed] [Google Scholar]
- 34. Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, et al. Aging‐associated reductions in AMP‐activated protein kinase activity and mitochondrial biogenesis. Cell Metab 2007;5:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A 1996;93:15364–15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seo DY, Lee SR, Kim N, Ko KS, Rhee BD, Han J. Age‐related changes in skeletal muscle mitochondria: the role of exercise. Integr Med Res 2016;5:182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burd NA, Wall BT, van Loon LJC. The curious case of anabolic resistance: old wives' tales or new fables? J Appl Physiol 2012;112:1233–1235. [DOI] [PubMed] [Google Scholar]
- 38. Gomes AV, Waddell DS, Siu R, Stein M, Dewey S, Furlow JD, et al. Upregulation of proteasome activity in muscle RING finger 1‐null mice following denervation. FASEB J 2012;26:2986–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Argadine HM, Mantilla CB, Zhan W‐Z, Sieck GC. Intracellular signaling pathways regulating net protein balance following diaphragm muscle denervation. AJP: Cell Physiology 2011;300:C318–C327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reid MB, Judge AR, Bodine SC. CrossTalk opposing view: the dominant mechanism causing disuse muscle atrophy is proteolysis. J Physiol 2014;592:5345–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Phillips SM, McGlory C. CrossTalk proposal: the dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J Physiol 2014;592:5341–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab 2016;311:E594–E604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bruusgaard JC, Liestøl K, Gundersen K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle‐aged, and old mice. J Appl Physiol 2006;100:2024–2030. [DOI] [PubMed] [Google Scholar]
- 44. Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training‐induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 2016;310:E652–E661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ. Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol 2015;119:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brook MS, Wilkinson DJ, Mitchell WK, Lund JL, Phillips BE, Szewczyk NJ, et al. A novel D2O tracer method to quantify RNA turnover as a biomarker of de novo ribosomal biogenesis, in vitro, in animal models, and in human skeletal muscle. Am J Physiol Endocrinol Metab 2017;313:E681–E689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shimkus KL, Shirazi‐Fard Y, Wiggs MP, Ullah ST, Pohlenz C, Gatlin DM, et al. Responses of skeletal muscle size and anabolism are reproducible with multiple periods of unloading/reloading. J Appl Physiol 2018;95:2185. [DOI] [PubMed] [Google Scholar]
- 48. Pettit AP, Jonsson WO, Bargoud AR, Mirek ET, Peelor FF, Wang Y, et al. Dietary methionine restriction regulates liver protein synthesis and gene expression independently of eukaryotic initiation factor 2 phosphorylation in mice. J Nutr 2017;147:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The effect of unloading and age on force (N‐m) frequency curves when unloaded at a) 24, b) 28, and c) 24 and 28 mo of age (n = 8‐9). Unloaded 24 and Unloaded 28 were analyzed by a paired t‐test. Unloaded 24/28 were analyzed by a one‐way ANOVA with Tukey's adjustment. *p < 0.05 compared to 24 mo, ^p < 0.05 compared to 28 mo.


