Abstract
Background
Studies show that low skeletal muscle index (SMI) and low skeletal muscle density (SMD) are negative prognostic factors and associated with more toxicity from systemic therapy in cancer patients. However, muscle depletion can be caused by a range of diseases, and many cancer patients have significant co‐morbidity. The aim of this study was to investigate whether there were associations between co‐morbidity and muscle measures in patients with advanced non‐small cell lung cancer.
Methods
Patients in a Phase III trial comparing two chemotherapy regimens in advanced non‐small cell lung cancer were analysed (n = 436). Co‐morbidity was assessed using the Cumulative Illness Rating Scale for Geriatrics (CIRS‐G), which rates co‐morbidity from 0 to 4 on 14 different organ scales. Severe co‐morbidity was defined as having any grades 3 and 4 CIRS‐G score. Muscle measures were assessed from baseline computed tomography slides at the L3 level using the SliceOMatic software.
Results
Complete data were available for 263 patients (60%). Median age was 66, 57.0% were men, 78.7% had performance status 0–1, 25.9% Stage IIIB, 11.4% appetite loss, 92.4% were current/former smokers, 22.8% were underweight, 43.7% had normal weight, 26.6% were overweight, and 6.8% obese. The median total CIRS‐G score was 7 (range: 0–16), and 48.2% had severe co‐morbidity. Mean SMI was 44.7 cm2/m2 (range: 27–71), and the mean SMD was 37.3 Hounsfield units (HU) (range: 16–60). When comparing patients with and without severe co‐morbidity, there were no significant differences in median SMI (44.5 vs. 44.1 cm2/m2; 0.70), but patients with severe co‐morbidity had a significantly lower median SMD (36 HU vs. 39 HU; 0.001), mainly due to a significant difference in SMD between those with severe heart disease and those without (32.5 vs. 37.9 HU; 0.002). Linear regression analyses confirmed the association between severe co‐morbidity and SMD both in the simple analysis (0.001) and the multiple analysis (0.037) adjusting for baseline characteristics. Stage of disease, gender, and body mass index (BMI) were significantly associated with SMI in both the simple and multiple analyses. Age and BMI were significantly associated with SMD in the simple analysis; and age, gender, and BMI were significantly associated in the multiple analysis.
Conclusions
There were no significant differences in SMI between patients with and patients without severe co‐morbidity, but patients with severe co‐morbidity had lower SMD than other patients, mainly due to severe heart disease. Co‐morbidity might be a confounder in studies of the clinical role of SMD in cancer patients.
Keywords: Co‐morbidity, Muscle wasting, Skeletal muscle index, Skeletal muscle density, Metastatic
Introduction
Studies of body composition assessed by analyses of computed tomography (CT) images suggest that muscle depletion is a negative prognostic factor for survival in advanced cancer including non‐small cell lung cancer (NSCLC)1, 2, 3, 4 and is associated with severe toxicity from systemic cancer therapy.5, 6, 7, 8, 9 Similar associations have been observed for patients with low skeletal muscle density (SMD),3, 10, 11, 12, 13, 14 which is believed to reflect fat infiltration and reduced muscle quality.15 It appears that both muscle depletion and low muscle density are secondary to malignant diseases and are linked to cancer cachexia, although the exact pathophysiology is not completely understood.16
Other conditions, such as heart, vascular, lung and muscle diseases, and diabetes, are also associated with muscle wasting.17, 18, 19, 20, 21, 22, 23 Many cancer patients have severe co‐morbidity, and several studies have shown that co‐morbidity is an independent negative prognostic factor.24, 25, 26, 27, 28, 29 Studies of patients with colorectal cancer have demonstrated that patients with co‐morbidity had lower skeletal muscle mass than other patients,30, 31 possibly indicating that co‐morbidity should be adjusted for in studies of the clinical importance of muscle measures in cancer patients.
Lung cancer patients have a relatively high median age at the time of diagnosis (approximately 71 years)32 and appears to have more co‐morbidity than other cancer patients, probably due to older age and because most lung cancer patients have a history of tobacco smoking.26, 27, 33
We have previously investigated the associations between co‐morbidity and treatment outcomes in patients participating in a randomized Phase III trial of first‐line chemotherapy in advanced NSCLC34 and found that patients with severe co‐morbidity had similar survival as other patients but experienced more severe toxicity.35 This cohort was also included in our previous studies of the prognostic and predictive role of muscle measures in advanced NSCLC, in which we found that low SMD was a negative prognostic factor and that patients with a low SMI experienced more haematologic toxicity.13, 36 In the present study, we have combined the data from these studies and aim to investigate whether there were any associations between severe co‐morbidity and skeletal muscle measures among advanced NSCLC patients.
Materials and methods
Approvals
The study was approved by the Regional Committee for Medical and Health Research Ethics in South‐East of Norway and was conducted according to the Declaration of Helsinki and its later amendments.
Patients
Patients enrolled in a randomized Phase III study comparing pemetrexed plus carboplatin with gemcitabine plus carboplatin as a first‐line therapy in advanced NSCLC were analysed.34 The main end points were patient reported health‐related quality of life, overall survival, and toxicity. Eligible patients gave written informed consent, had Stage IIIB or stage IV NSCLC, World Health Organization performance status 0–2, and adequate bone marrow, kidney, and liver function for chemotherapy. All other co‐morbidities were allowed. Patients who were ≥75 years had a 25% dose reduction from the first course.
Assessment of co‐morbidity
Co‐morbidity was measured at baseline using the Cumulative Illness Rating Scale for Geriatrics (CIRS‐G). This index contains 14 scales that each represents different organ systems. The severity of disorders on each scale is graded from 0 to 4. ‘0' indicates no problem, ‘1' a current mild problem or past significant problem, ‘2' a moderate disability or morbidity requiring ‘first line' therapy, ‘3' a severe/constant significant disability or an ‘uncontrollable' chronic problem, and ‘4' an extremely severe/immediate treatment required/end organ failure/severe impairment in function.
Two researchers, both oncologists, independently assessed co‐morbidity for each patient from the hospital medical records according to the CIRS‐G manual.37 Any differences in scores were discussed, and the two physicians agreed on a final score. The total score (i.e. sum of the scores on all scales) and the numbers of grades 3 and 4 scores were calculated for each patient.
Classification of co‐morbidity
There are no established cut‐off values for the definition of ‘severe' co‐morbidity when using the CIRS‐G. In the present study, the prevalence of grade 4 conditions was low (9%), and when scoring co‐morbidity, we found it difficult to accurately distinguish between grade 3 and grade 4 severity. As in our previous study of co‐morbidity, we therefore defined ‘severe co‐morbidity' as the presence of ≥1 CIRS‐G score 3 or 4.35
Body mass index, muscle measures and appetite loss
Body mass index (BMI) (weight/height2) was categorized as underweight (<20.0 for patients <70 years and <22 for patients ≥70 years), normal (20.0/22.0–24.9), overweight (25.0–29.9), and obese (≥30.0).38 The muscle measures were assessed from CT scans of the thorax and upper abdomen taken within 4 weeks before chemotherapy commenced. The CT scans were analysed using the SliceOMatic software (v.4.3 Tomovision, Montreal Canada) by three observers blinded for patient data. The total cross‐sectional area of skeletal muscle (cm2) was quantified from images at the L3 level, which is strongly correlated to the whole body skeletal muscle mass. Well‐established thresholds of Hounsfield units (HU) in the range of –29 to +150 HU were used for demarcation of muscle tissue.15 The total cross‐sectional skeletal muscle area (cm2) was divided by height (m2) and expressed as skeletal muscle index (SMI) (cm2/m2). SMD, expressed in HU, was reported for the entire muscle area at the L3 level.
Appetite loss was reported by the patients on the baseline quality of life questionnaire (the EORTC QLQ‐C30).39 Patients reporting ‘not at all' were defined as having no appetite loss, while patients reporting ‘a little', ‘quite a bit', and ‘very much' were defined as having appetite loss.
Statistical considerations
Skeletal muscle index and SMD were first compared between patients with and patients without severe co‐morbidity using the Student's t‐test. Because not all co‐morbidities registered by the CIRS‐G are known to cause muscle depletion, we performed subgroup analyses to investigate whether patients with the three most commonly observed severe co‐morbidities known to be associated with muscle depletion (i.e. respiratory,22 heart,18 and vascular disease17, 20) had lower SMI or SMD than the remaining patients in our cohort. To assess the independent impact of overall severe co‐morbidity on SMI and SMD, simple and multiple linear regression analyses controlling for baseline patient characteristics and stage of disease were performed. The significance level was defined as P < 0.05. The statistical analyses were performed using the SPSS v25 software.
Results
Patients
From May 2005 until June 2006, 436 patients were enrolled in the Phase III trial. Co‐morbidity data were missing in 23 patients, and CT slides were missing or not analysable in 160. Thus, 263 patients (60%) were analysed in the present study (Figure 1).
Figure 1.
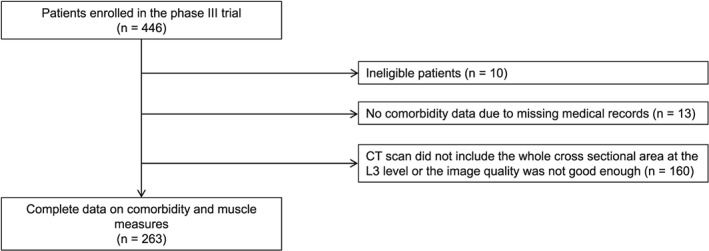
Patient selection. CT, computed tomography.
Baseline characteristics for all patients are shown in Table 1. Median age was 66 years, 20.9% were ≥ 75 years, 57.0% were men, 78.7% had performance status 0–1, 25.9% had Stage IIIB, 50.2% received pemetrexed/carboplatin, 11.4% reported appetite loss at baseline, 92.4% were former or current smokers, and the mean BMI was 24 (range: 14–36). According to BMI, 22.8% were underweight and 6.8% were obese. The baseline characteristics were comparable between patients included and patients excluded in the present study (data not shown).
Table 1.
Baseline patient characteristics
| All patients (n = 263) | Severe co‐morbidity (n = 127) | No severe co‐morbidity (n = 136) | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age, years | Mean (range) | 65.5 (37–90) | 67.7 (48–85) | 63.4 (37–90) | |||
| ≥70 years | 96 | 36.5 | 55 | 43.3 | 41 | 30.1 | |
| Gender | Male | 150 | 57.0 | 79 | 62.2 | 71 | 52.2 |
| Female | 113 | 43.0 | 48 | 37.8 | 65 | 47.8 | |
| Stage of disease | IIIB | 68 | 25.9 | 40 | 31.5 | 28 | 20.6 |
| IV | 195 | 74.1 | 87 | 68.5 | 108 | 79.4 | |
| Performance status | 0–1 | 208 | 78.7 | 99 | 78.0 | 109 | 19.9 |
| 2 | 55 | 21.3 | 28 | 22.0 | 27 | 80.1 | |
| Treatment | Pemetrexed/carboplatin | 132 | 50.2 | 57 | 44.9 | 75 | 55.1 |
| Gemcitabin/carboplatin | 131 | 49.8 | 70 | 55.1 | 61 | 44.9 | |
| Appetite loss | Yes | 30 | 11.4 | 14 | 11.0 | 16 | 11.8 |
| No | 229 | 87.1 | 112 | 88.2 | 117 | 86.0 | |
| Unknown | 4 | 1.5 | 1 | 0.8 | 3 | 2.2 | |
| Body mass index | Underweight (<20.0 for patients <70 years and <22 for patients ≥70 years) | 60 | 22.8 | 29 | 22.8 | 31 | 22.8 |
| Normal weight (20.0/22.0 to 24.9) | 115 | 43.7 | 53 | 41.7 | 62 | 45.6 | |
| Overweight (25.0 to 29.9) | 70 | 26.6 | 35 | 27.6 | 35 | 25.7 | |
| Obesity (≥30) | 18 | 6.8 | 10 | 7.9 | 8 | 5.9 | |
| Smoking history | Never smoker | 17 | 6.5 | 6 | 4.7 | 11 | 8.1 |
| Former or current smoker | 243 | 92.4 | 120 | 94.4 | 123 | 90.4 | |
| Unknown | 3 | 1.1 | 1 | 0.8 | 2 | 1.4 | |
Co‐morbidity
The distribution of the total CIRS‐scores is shown in Figure 2. The median total CIRS‐G score was 7 (range 0–16), 2% had no co‐morbidity, 5% had no CIRS‐G scores > grade 1, 48% had severe co‐morbidity (one or more grades 3 and 4 CIRS‐G scores), and 11% had two or more grades 3 and 4 CIRS‐G scores. Most grades 3 and 4 CIRS‐G scores were registered on the respiratory (26%), heart (10%), and vascular (7%) scales (Figure 2).
Figure 2.
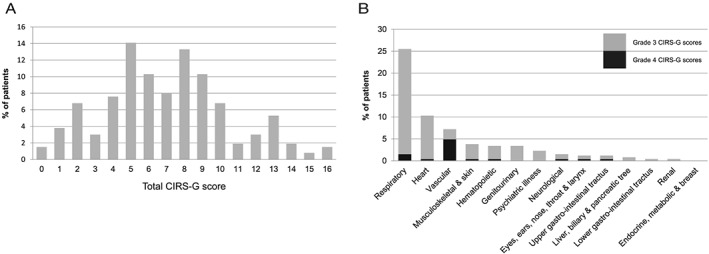
Co‐morbidity scores. (A) Distribution of the total CIRS‐G scores (sum of the scores on all 14 organ scales). (B) Distribution of severe co‐morbidity (grades 3 and 4 CIRS‐G scores). CIRS‐G, Cumulative Illness Rating Scale for Geriatrics.
Muscle measures
Overall, the mean SMI was 44.7 cm2/m2 (range: 26.9–70.7) and was higher in men than in women (48.6 vs. 39.6 cm2/m2; P < 0.001). The mean SMD was 37.3 HU (range: 15.6–60.4) and was similar for men and women (37.1 vs. 37.6 HU; 0.58).
When comparing patients with and without severe co‐morbidity, there were no significant differences in the median SMI in the overall population (44.5 vs. 45.0 cm2/m2; 0.66), in men (48.5 vs. 48.7 cm2/m2; 0.85) or women (39.1 vs. 39.9 cm2/m2; 0.47), and there were no significant differences in SMI between those with and those without severe heart disease (47.5 vs. 44.4 cm2/m2; 0.065), those with and without severe respiratory disease (44.4 vs. 44.8 cm2/m2; 0.68), or those with and without severe vascular disease (46.0 vs. 44.6 cm2/m2; 0.50)—neither in the overall population or among men or women (Table 2).
Table 2.
Muscle measures in subgroups defined by overall and the most common severe co‐morbidities
| L3 SMI | P | SMD | P | ||
|---|---|---|---|---|---|
| cm2/m2 ± SD | HU ± SD | ||||
| Overall study population | All patients (n = 263) | 44.7 ± 8.1 | <0.001 | 37.3 ± 8.3 | 0.582 |
| Men (n = 150) | 48.6 ± 7.5 | 37.3 ± 8.3 | |||
| Women (n = 113) | 39.6 ± 5.7 | 37.6 ± 9.1 | |||
| All patients | No severe co‐morbidity (n = 136) | 44.5 ± 7.7 | 0.663 | 38.9 ± 8.5 | 0.001 |
| Severe co‐morbidity (n = 127) | 45.0 ± 8.5 | 35.6 ± 7.9 | |||
| Men | No severe co‐morbidity (n = 71) | 48.7 ± 6.9 | 0.850 | 38.7 ± 8.1 | 0.013 |
| Severe co‐morbidity (n = 79) | 48.5 ± 8.0 | 35.6 ± 7.2 | |||
| Women | No severe co‐morbidity (n = 65) | 39.9 ± 5.9 | 0.467 | 39.1 ± 8.9 | 0.045 |
| Severe co‐morbidity (n = 48) | 39.1 ± 5.5 | 35.6 ± 9.1 | |||
| All patients | No severe heart disease (n = 236) | 44.4 ± 8.0 | 0.065 | 37.9 ± 8.3 | 0.002 |
| Severe heart disease (n = 27) | 47.5 ± 8.5 | 32.5 ± 7.8 | |||
| Men | No severe heart disease (n = 128) | 48.5 ± 7.4 | 0.748 | 37.8 ± 7.6 | 0.009 |
| Severe heart disease (n = 22) | 49.1 ± 8.4 | 32.8 ± 7.7 | |||
| Women | No severe heart disease (n = 108) | 39.5 ± 5.7 | 0.777 | 37.9 ± 9.1 | 0.111 |
| Severe heart disease (n = 5) | 40.3 ± 5.1 | 31.3 ± 9.1 | |||
| All patients | No severe respiratory disease (n = 196) | 44.8 ± 8.0 | 0.677 | 37.7 ± 8.3 | 0.166 |
| Severe respiratory disease (n = 67) | 44.4 ± 8.6 | 36.1 ± 8.5 | |||
| Men | No severe respiratory disease (n = 110) | 48.8 ± 7.3 | 0.605 | 37.7 ± 7.9 | 0.119 |
| Severe respiratory disease (n = 40) | 48.1 ± 8.2 | 35.4 ± 7.3 | |||
| Women | No severe respiratory disease (n = 86) | 39.8 ± 5.7 | 0.464 | 37.8 ± 8.9 | 0.692 |
| Severe respiratory disease (n = 27) | 38.9 ± 5.8 | 37.0 ± 10.1 | |||
| All patients | No severe vascular disease (n = 244) | 44.6 ± 8.1 | 0.500 | 37.4 ± 8.5 | 0.372 |
| Severe vascular disease (n = 18) | 46.0 ± 9.4 | 35.6 ± 7.3 | |||
| Men | No severe vascular disease (n = 135) | 48.6 ± 7.5 | 0.814 | 37.1 ± 7.9 | 0.606 |
| Severe vascular disease (n = 14) | 49.1 ± 8.0 | 37.1 ± 7.9 | |||
| Women | No severe vascular disease (n = 109) | 39.7 ± 5.7 | 0.109 | 37.8 ± 9.1 | 0.439 |
| Severe vascular disease (n = 4) | 35.1 ± 4.5 | 34.1 ± 10.9 | |||
The patients with severe co‐morbidity did, however, have a significantly lower median SMD (35.6 vs. 38.9 HU; 0.001) both in the overall population, among men (35.6 vs. 38.7 HU; 0.013) and among women (35.6 vs. 29.1 HU; 0.045). Subgroup analyses revealed that the main reason was a significant difference in SMD between patients with and patients without severe heart disease (32.5 vs. 37.9 HU; 0.002). There were no significant differences in SMD between those with and those without severe respiratory disease (36.1 vs. 37.7 HU; 0.17), or those with and without severe vascular disease (37.4 vs. 35.6 HU; 0.37).
Simple linear regression analyses showed that stage of disease (0.021), gender (P < 0.001), and BMI (P < 0.001) but not severe co‐morbidity (0.663) were significantly associated with SMI and that age (P < 0.001), BMI (P < 0.001), and severe co‐morbidity (0.001) were significantly associated with SMD. Linear multiple regression analyses showed that age (0.028), stage of disease (0.011), gender (P < 0.001), and BMI (P < 0.001) but not severe co‐morbidity (0.68) were significantly associated with SMI, whereas age (P < 0.001), BMI (P < 0.001), and severe co‐morbidity (0.037) were significantly associated with SMD (Table 3).
Table 3.
Linear regression analyses of the associations between baseline patient characteristics and muscle measures
| SMI | SMD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simple | Multiple | Simple | Multiple | |||||||||
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| Severe co‐morbidity | 0.44 | −1.54 to 2.42 | 0.663 | −0.33 | −1.91 to 1.25 | 0.678 | −3.32 | −5.31 to −1.32 | 0.001 | −1.91 | −3.71 to −0.12 | 0.037 |
| Age | 0.001 | −0.099 to 0.102 | 0.977 | −0.09 | −0.18 to 0.01 | 0.028 | −0.40 | −0.49 to −0.31 | <0.001 | −0.39 | −0.48 to −0.30 | <0.001 |
| Stage | −2.63 | −4.87 to −0.40 | 0.021 | −2.30 | −4.07 to −0.53 | 0.011 | −0.21 | −2.53 to 2.12 | 0.861 | −1.10 | −3.11 to 0.91 | 0.282 |
| Gender | −0.04 | −10.71 to −7.38 | <0.001 | −8.67 | −10.32 to −7.02 | <0.001 | 0.58 | −1.48 to 2.63 | 0.582 | −1.91 | −3.78 to −0.04 | 0.045 |
| PS | −0.77 | −3.19 to 1.66 | 0.536 | 0.23 | −1.68 to 2.14 | 0.813 | −0.93 | −3.43 to 1.57 | 0.465 | −1.79 | −3.96 to 0.37 | 0.104 |
| BMI | 0.75 | 0.53–0.97 | <0.001 | 0.54 | 0.34–0.73 | <0.001 | −0.57 | −0.81 to −0.34 | <0.001 | −0.60 | −0.82 to −0.38 | <0.001 |
| Appetite loss | −2.62 | −5.73 to 0.50 | 0.099 | −0.53 | −3.02 to 1.95 | 0.672 | −1.14 | −4.35 to 2.06 | 0.483 | 0.90 | −1.92 to 3.73 | 0.530 |
| Smoking history | 0.36 | −3.58 to 4.30 | 0.857 | −1.20 | −4.46 to 2.07 | 0.472 | 2.01 | −2.13 to 6.15 | 0.340 | −0.96 | −4.67 to 2.75 | 0.610 |
CI, confidence interval; SMD, skeletal muscle density.
Discussion
In this study of advanced NSCLC patients, we found that patients with severe co‐morbidity had significantly lower SMD, that is, poorer muscle quality, than other patients, both in the overall population and among men and women, mainly due to a lower SMD among patients with heart disease. There were no significant differences in skeletal muscle index (SMI), that is, muscle mass, between those with severe co‐morbidity and the remaining study population, but there were significant associations between BMI and both SMI and SMD.
To the best of our knowledge, only one former study has reported results related to muscle measures and co‐morbidity in NSCLC patients. Kim et al. aimed at investigating whether there were any associations between loss of muscle mass and histologic subtypes of NSCLC. No such association was found in the cohort of 778 patients with varying stages of disease, but in contrast to the present findings, they reported that loss of muscle mass was significantly associated with a high co‐morbidity score. The relationship between SMD and co‐morbidity was not investigated.40 In colorectal cancer, a few more studies have been reported. Two studies investigated co‐morbidity in relation to loss of muscle mass and a decline in muscle density, respectively, and both reported significant associations.12, 31 The results of the most recent and largest study are fully consistent with ours. Xiao et al. addressed 3051 patients with non‐metastatic colorectal cancer and found that co‐morbidity was more common among patients with a low SMD compared with those with a normal SMD. There was no difference between patients with a low and those with a normal SMI. Furthermore, subgroup analyses revealed that heart disease was significantly associated with a low SMD, which was also the case in our study. Additionally, they reported significant associations with peripheral vascular disease, diabetes, and renal failure. These results could not be confirmed in our study as renal failure was an exclusion criteria, only one patient had diabetes, and in accordance with the CIRS‐G, ‘vascular disease' did not exclusively include peripheral vascular disease but also conditions such as hypertension and thromboembolism.37
As demonstrated, present and former findings regarding muscle measures and co‐morbidity in cancer patients are not entirely consistent. One possible explanation is the differences in the choice of co‐morbidity measure. No former study has used the CIRS‐G but rather assessed co‐morbidity by counting ICD codes31 or used the Charlson Comorbidity Index.12, 30, 40 Furthermore, differences in diagnoses and stage of disease may affect the results, as may also possible differences in the distribution of co‐morbidities, smoking habits, and obesity. Overall, however, there are clear indications that muscle wasting in cancer is associated with pre‐existing diseases, and according to our study and the larger study by Xiao et al.,30 loss of muscle density might be the most important factor related to subgroups of co‐morbidities. How the latter may be explained is still a question as the pathophysiological mechanisms of muscle wasting in various malignant and non‐malignant diseases are not fully understood. It has been speculated that different findings between muscle abnormalities may be due to a more pronounced decrease in SMD than that of SMI loss under certain chronic disorders.30 In this respect, a major limitation of the present and all former studies is the lack of longitudinal, repeated muscle measurements. Thus, whether muscle wasting, and in particular loss of density, has already occurred in patients with pre‐existing co‐morbidities, or if the cancer disease interacts to initiate or accelerate the process, cannot yet be decided. To answer these questions, further research is needed.
The differences in SMD between patients with and patients without severe co‐morbidity and between patients with and patients without severe heart disease were statistically significant. The clinical relevance of the observed differences of 3.3–5.4 HU is, however, not established. But in a former study on a larger sample of advanced NSCLC patients, which included the present cohort, we found a significant association between SMD and survival, and a Cox regression analysis showed that an incremental increase in SMD of 1 HU was associated with a 2% decrease in the risk of death,13 corresponding to a risk reduction of death of 6.5–10.4% for the aforementioned differences in SMD observed in the present study. Further studies are, however, required to establish the clinical relevance of differences in SMD not only for survival but also for physical function. Including tests of physical performance such as handgrip strength in studies of cancer patients receiving chemotherapy is feasible41 and might improve our understanding of the implications of differences in SMD and help define thresholds for abnormal SMD that are generally applicable. Studies show that the SMD distribution and also thresholds for survival differences differ between patient cohorts, age groups, and regions.1, 3, 13, 42 One factor that may contribute is the variation in the BMI distribution. As observed in our and other studies, there are significant associations between BMI and both SMI and SMD.43 Thus, studies of large cohorts of both healthy individuals and patients with different ethnicities, age, and BMI using standardized CT protocols and preferably physical functional tests are probably needed in order to establish more generally applicable thresholds for abnormal SMD.
A limitation of our study is the lack of information about protocols for the CT scans. It has been shown that the thickness of CT slides, use of contrast media, and tube voltage might influence the SMI and SMD values.43, 44 The body composition analyses were not pre‐planned, and the study protocol did not comprise recommendations for how the CT scans should be performed in order to optimize assessment of the muscle measures, although most of the patients did receive contrast injections according to Norwegian recommendations for diagnostic CT scans. Nevertheless, there might be variations in all three variables that might have influenced our measurements of SMI and SMD. Whether this explains the somewhat different results between our study and some of the other studies of co‐morbidity and muscle measures is not possible to assess because details about CT protocols are seldom provided.12, 30, 40
The strengths of the present study are the use of otherwise well‐established methods for the analyses of the CT slides3 and the widely accepted attenuation ranges for demarcation of the muscle area on the CT slides.15 The patients' characteristics including the distributions of co‐morbidity, SMI and SMD, and overall survival in our study cohort are similar to other studies of advanced NSCLC, except that fewer patients were obese.3, 13, 24, 28, 29, 33, 45, 46 Thus, we consider our cohort representative for advanced NSCLC patients receiving palliative chemotherapy. For co‐morbidity assessment, we used the CIRS‐G, which has a good inter‐rater and test–retest reliability, has been used in several cancer studies,25 and has been found to provide independent prognostic information, also predicting toxicity, in stages I–IV NSCLC.26, 27, 35 In contrast to Charlson Comorbidity Index, however, the CIRS‐G accounts for all coexisting conditions. If, as suggested by Xiao et al.,30 some co‐morbid diseases may be more important than others in relation to muscle wasting, significant associations might be obscured by the comprehensiveness of the CIRS‐G.
In conclusion, our data indicate that co‐morbidity is associated with muscle wasting in terms of loss of muscle density in a cohort of cancer patients in which muscle depletion is frequent and often attributed to the malignancy. Thus, co‐morbidity may be a confounder in studies of the clinical impact of SMD in cancer patients. Further studies are, however, needed in order to assess to what extent, for which co‐morbidities this is relevant, and to decide how such co‐morbidities should be adjusted for. Finally, the significant associations between BMI and both SMI and SMD suggest that also BMI might be a confounder in studies of muscle measures in cancer patients.
Conflict of interest
None of the authors have any disclosures.
Acknowledgements
We want to thank Rachel Murphy (PhD) and Nina Esfandiari (BSc) both at the Department of Oncology, University of Alberta, Canada, for their participation in the body composition analyses, and Stein H. Sundstrøm (MD, PhD) at the Cancer Clinic, St. Olav's Hospital, Trondheim University Hospital, Trondheim, Norway, for participating in the co‐morbidity assessment. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.47
Grønberg B. H., Valan C. D., Halvorsen T., Sjøblom B., and Jordhøy M. S. (2019) Associations between severe co‐morbidity and muscle measures in advanced non‐small cell lung cancer patients, Journal of Cachexia, Sarcopenia and Muscle, 10, 1347–1355. 10.1002/jcsm.12469.
References
- 1. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131–140. [DOI] [PubMed] [Google Scholar]
- 2. Kazemi‐Bajestani SM, Mazurak VC, Baracos V. Computed tomography‐defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 2016;54:2–10. [DOI] [PubMed] [Google Scholar]
- 3. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 4. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 5. Sjoblom B, Gronberg BH, Benth JS, Baracos VE, Flotten O, Hjermstad MJ, et al. Low muscle mass is associated with chemotherapy‐induced haematological toxicity in advanced non‐small cell lung cancer. Lung Cancer 2015;90:85–91. [DOI] [PubMed] [Google Scholar]
- 6. Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 2014;66:583–589. [DOI] [PubMed] [Google Scholar]
- 7. Cousin S, Hollebecque A, Koscielny S, Mir O, Varga A, Baracos VE, et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Invest New Drugs 2014;32:382–387. [DOI] [PubMed] [Google Scholar]
- 8. Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5‐fluorouracil‐based chemotherapy toxicity. Clin Cancer Res 2007;13:3264–3268. [DOI] [PubMed] [Google Scholar]
- 9. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 10. Antoun S, Lanoy E, Iacovelli R, Albiges‐Sauvin L, Loriot Y, Merad‐Taoufik M, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013;119:3377–3384. [DOI] [PubMed] [Google Scholar]
- 11. Miller BS, Ignatoski KM, Daignault S, Lindland C, Doherty M, Gauger PG, et al. Worsening central sarcopenia and increasing intra‐abdominal fat correlate with decreased survival in patients with adrenocortical carcinoma. World J Surg 2012;36:1509–1516. [DOI] [PubMed] [Google Scholar]
- 12. Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 2011;18:3579–3585. [DOI] [PubMed] [Google Scholar]
- 13. Sjoblom B, Gronberg BH, Wentzel‐Larsen T, Baracos VE, Hjermstad MJ, Aass N, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non‐small cell lung cancer. Clin Nutr 2016;35:1386–1393. [DOI] [PubMed] [Google Scholar]
- 14. Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr 2016;35:1103–1109. [DOI] [PubMed] [Google Scholar]
- 15. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fearon KCH. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer 2008;44:1124–1132. [DOI] [PubMed] [Google Scholar]
- 17. Addison O, Prior SJ, Kundi R, Serra MC, Katzel LI, Gardner AW, et al. Sarcopenia in peripheral arterial disease: prevalence and effect on functional status. Arch Phys Med Rehabil 2018;99:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Heart Fail 2017;4:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, et al. excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI Study. J Am Geriatr Soc 2004;52:405–410. [DOI] [PubMed] [Google Scholar]
- 21. Johansen KL, Lee C. Body composition in chronic kidney disease. Curr Opin Nephrol Hypertens 2015;24:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones SE, Maddocks M, Kon SSC, Canavan JL, Nolan CM, Clark AL, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015;70:213–218. [DOI] [PubMed] [Google Scholar]
- 23. Limpawattana P, Inthasuwan P, Putraveephong S, Boonsawat W, Theerakulpisut D, Sawanyawisuth K. Sarcopenia in chronic obstructive pulmonary disease: A study of prevalence and associated factors in the Southeast Asian population. Chron Respir Dis 2018;15:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asmis TR, Ding K, Seymour L, Shepherd FA, Leighl NB, Winton TL, et al. Age and comorbidity as independent prognostic factors in the treatment of non‐small‐cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol 2008;26:54–59. [DOI] [PubMed] [Google Scholar]
- 25. Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol 2000;35:181–200. [DOI] [PubMed] [Google Scholar]
- 26. Firat S, Bousamra M, Gore E, Byhardt RW. Comorbidity and KPS are independent prognostic factors in stage I non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2002;52:1047–1057. [DOI] [PubMed] [Google Scholar]
- 27. Firat S, Byhardt RW, Gore E. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non‐small‐cell lung cancer: an institutional analysis of patients treated on four RTOG studies. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2002;54:357–364. [DOI] [PubMed] [Google Scholar]
- 28. Jacot W, Colinet B, Bertrand D, Lacombe S, Bozonnat MC, Daures JP, et al. Quality of life and comorbidity score as prognostic determinants in non‐small‐cell lung cancer patients. Ann Oncol 2008;19:1458–1464. [DOI] [PubMed] [Google Scholar]
- 29. Read WL, Tierney RM, Page NC, Costas I, Govindan R, Spitznagel EL, et al. Differential prognostic impact of comorbidity. J Clin Oncol 2004;22:3099–3103. [DOI] [PubMed] [Google Scholar]
- 30. Xiao JJ, Caan BJ, Weltzien E, Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Associations of pre‐existing co‐morbidities with skeletal muscle mass and radiodensity in patients with non‐metastatic colorectal cancer. J Cachexia Sarcopeni 2018;9:654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brustugun OT, Gronberg BH, Fjellbirkeland L, Helbekkmo N, Aanerud M, Grimsrud TK, et al. Substantial nation‐wide improvement in lung cancer relative survival in Norway from 2000 to 2016. Lung Cancer 2018;122:138–145. [DOI] [PubMed] [Google Scholar]
- 33. Janssen‐Heijnen ML, Schipper RM, Razenberg PP, Crommelin MA, Coebergh JW. Prevalence of co‐morbidity in lung cancer patients and its relationship with treatment: a population‐based study. Lung Cancer 1998;21:105–113. [DOI] [PubMed] [Google Scholar]
- 34. Gronberg BH, Bremnes RM, Flotten O, Amundsen T, Brunsvig PF, Hjelde HH, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first‐line chemotherapy in advanced non‐small‐cell lung cancer. J Clin Oncol 2009;27:3217–3224. [DOI] [PubMed] [Google Scholar]
- 35. Gronberg BH, Sundstrom S, Kaasa S, Bremnes RM, Flotten O, Amundsen T, et al. Influence of comorbidity on survival, toxicity and health‐related quality of life in patients with advanced non‐small‐cell lung cancer receiving platinum‐doublet chemotherapy. Eur J Cancer 2010;46:2225–2234. [DOI] [PubMed] [Google Scholar]
- 36. Sjoblom B, Benth JS, Gronberg BH, Baracos VE, Sawyer MB, Flotten O, et al. Drug dose per kilogram lean body mass predicts hematologic toxicity from carboplatin‐doublet chemotherapy in advanced non‐small‐cell lung cancer. Clin Lung Cancer 2017;18:e129–e136. [DOI] [PubMed] [Google Scholar]
- 37. Miller MD, Towers A. A manual of guidelines for scoring the Cumulative Illness Rating Scale for Geriatrics (CIRS‐G). 1991. http://www.anq.ch/fileadmin/redaktion/deutsch/20121211_CIRSG_Manual_E.pdf.
- 38. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1–9. [DOI] [PubMed] [Google Scholar]
- 39. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 40. Kim CR, Kim EY, Kim YS, Ahn HK, Kim KW, Jeong YM, et al. Histologic subtypes are not associated with the presence of sarcopenia in lung cancer. PLoS ONE 2018;13:e0194626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ordan MA, Mazza C, Barbe C, Perrier M, Botsen D, Renard Y, et al. Feasibility of systematic handgrip strength testing in digestive cancer patients treated with chemotherapy: The FIGHTDIGO study. Cancer 2018;124:1501–1506. [DOI] [PubMed] [Google Scholar]
- 42. van der Werf A, Langius JAE, de van der Schueren MAE, Nurmohamed SA, van der Pant K, Blauwhoff‐Buskermolen S, et al. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr 2018;72:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van der Werf A, Dekker IM, Meijerink MR, Wierdsma NJ, de van der Schueren MAE, Langius JAE. Skeletal muscle analyses: agreement between non‐contrast and contrast CT scan measurements of skeletal muscle area and mean muscle attenuation. Clin Physiol Funct Imaging 2018;38:366–372. [DOI] [PubMed] [Google Scholar]
- 44. Morsbach F, Zhang YH, Martin L, Lindqvist C, Brismar T. Body composition evaluation with computed tomography: contrast media and slice thickness cause methodological errors. Nutrition 2019;59:50–55. [DOI] [PubMed] [Google Scholar]
- 45. Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non‐small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr 2010;91:1133s–1137s. [DOI] [PubMed] [Google Scholar]
- 46. Maione P, Perrone F, Gallo C, Manzione L, Piantedosi F, Barbera S, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non‐small‐cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol 2005;23:6865–6872. [DOI] [PubMed] [Google Scholar]
- 47. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


