Abstract
Background
Despite no international consensus on the diagnostic criteria for sarcopenia, low lean mass, muscle strength, and physical function are important risk factors for disability, frailty, and mortality in older individuals, as well as in a wide range of patients with muscle loss. Here, we provide a population‐based reference material of total and regional lean body mass, muscle strength/power parameters, and physical function in a healthy cohort of Danish men and women across the lifespan.
Methods
Volunteers aged 20–93 years from the Copenhagen City Heart Study were invited to establish a Danish reference material (Copenhagen Sarcopenia Study) on lean mass characteristics [appendicular lean mass (ALM), iDXA, GE Lunar], muscle function [handgrip strength (HGS), Jamar dynamometer and leg extension power (LEP), Nottingham Power Rig], and physical function [30 s sit‐to‐stand test (STS), 10‐m maximal and habitual gait speed (GS)].
Results
A total of 1305 participants [729 women (age: 56.4 ± 18.9 years, height: 1.66 ± 0.01 m, body mass index: 24.6 ± 4.3 kg/m2 and 576 men, age: 57.0 ± 17.5 years, height: 1.80 ± 0.07 m, body mass index: 26.0 ± 3.9 kg/m2] completed all measurements and were included in the present analysis. Lean mass characteristics (TLM, ALM, and ALM/h2) decreased with increasing age in both men and women (P < 0.001). Men demonstrated larger absolute and relative total ALM and higher HGS and LEP compared with women at all age intervals (P < 0.001). HGS and LEP decreased progressively with age in both men and women (P < 0.01); 30 s STS performance, habitual GS, and maximal GS decreased at an accellerated rate of decline with increasing age in both men and women (P < 0.001). Habitual GS was reduced in men and women aged ≥70 years, while maximal GS was reduced from the age of ≥60 years compared with young adults (P < 0.001). Regardless of sex, 30 s STS was reduced from the age of ≥50 years compared with the young reference group (P < 0.001)
Conclusions
While the power‐based measurements (LEP and 30 s STS) started to decline already at age +50 years, less power‐based parameters (GS and HGS) and lean mass characteristics (TLM, ALM, and ALM/h2) remained unaltered until after the age of +70 years. Notably, the cut‐off thresholds derived in the present study differed from earlier reference data, which underlines the importance of obtaining updated and local reference materials.
Keywords: Sarcopenia, Body composition, DXA, Lean mass, Handgrip strength, Leg power
Introduction
Sarcopenia was initially defined as a multifactorial syndrome characterized by the slow and progressive loss of muscle mass associated with aging in the absence of any underlying disease or condition.1, 2 Since the first proposed definition in 1989, the field of sarcopenia research has evolved substantially with a number of consensus reports published in 2010,3 2011,4 2014,5 2014,6 and recently an updated definition of sarcopenia was proposed by the European Working Group on Sarcopenia in Older People.7 During the last three decades, the field has moved from focusing primarily on assessment of muscle mass to integrating muscle strength and physical function as part of a more comprehensive definition on sarcopenia, with muscle strength [handgrip strength (HGS)] replacing muscle mass as the primary assessment parameter.7 These initiatives have contributed to shed light on the clinical significance of sarcopenia, with a major milestone being reached in 2016 when sarcopenia was formally recognized as a separate condition of muscle disease (ICD‐10‐MC diagnosis code).8 Yet there is still no international consensus of a common operational definition, which is hindering implementation of the sarcopenia diagnose and effective treatment options in the clinical field.
In contrast to the slow and progressive loss of muscle mass associated with aging,9, 10 loss of lean mass often occurs at an accelerated rate secondary to acute and chronic disease states such as cancer, infections, chronic organ failure, immobilization, and disability11, 12, 13 collectively termed as secondary sarcopenia.7
Skeletal muscle mass can be assessed using computed tomography, magnetic resonance imaging, dual‐energy X‐ray absorptiometry (DXA), or estimated by bioelectrical impedance analysis.14, 15 Although computed tomography and magnetic resonance imaging are considered the gold standard methods for the quantification of skeletal muscle mass, both methods are expensive and time consuming and therefore primarily suited for research.15 For the clinical implementation of lean mass assessment in patients, DXA is recommended due to its high reliability, low cost, and low radiation doses.14, 15 However, population‐specific reference data for lean mass estimates based on DXA scanning remain limited, especially for the European population. Classical reference data on age‐related trajectories in lean mass have been derived in New Mexico2 and from American population studies16 with the use of an older generation of DXA equipment.17 However, the resolution and quality of DXA scanners and analysis software have been markedly improved over the last decade, enabling a more precise evaluation of the biological variations in body composition and lean mass across the lifespan.18 More recently, reference data have emerged from Australian,19, 20 Mexican,21 and American22 population studies using advanced scanners (combination of GE Lunar Prodigy and iDXA), whereas European based population data remain more scarce.23, 24
In order to gain increased knowledge about the age‐specific changes in the domains of muscle dysfunction (muscle mass, muscle strength, and physical function) included in the recent sarcopenia definitions,3, 4, 7 assessments of HGS, maximal horizontal gait speed (GS), and sit‐to‐stand (STS) performance were combined with the assessment of appendicular lean mass (ALM) in the present study. In addition, maximal leg extension muscle power (LEP) was recorded25 as muscle power declines more rapidly with age than muscle strength and therefore is considered a sensitive predictor of frailty, fall risk, and mortality in older individuals.26 Further, LEP appears to provide a sensitive outcome measure to evaluate individual responses to interventional treatments (nutritional, pharmaceutical, and exercise based) aimed at improving muscle mass, power, and strength in order to enhance functional capacity in aging adults.27, 28 Lastly, LEP assessments have relevance also in younger populations, where the assessment of maximal GS and HGS may be of limited prognostic value due to systematic ceiling effects.
The aim of the present study, therefore, was to establish reference data on lean mass, maximal muscle strength/power [HGS and leg extension power (LEP)], and functional capacity (walking speed and STS test) in a Danish cohort of healthy male and female adults aged 20–93 years to establish reference data across the full adult lifespan as well as for establishing specific reference values for successive 10 year age intervals, spanning from young to old age.
Subjects and methods
Study cohort
The Copenhagen Sarcopenia Study is a population‐based cross‐sectional study conducted at Copenhagen University Hospital Rigshospitalet, Glostrup, from December 2013 to June 2016. Participants were recruited through the Copenhagen City Heart Study, a prospective cardiovascular population study comprising a random sample of more than 24 000 men and women aged 20 to 101 years, drawn from the Copenhagen Population Register as of 1 January 1976. More detailed information on the Copenhagen City Heart Study has been described elsewhere.29 Specifically, a subpopulation of 3000 men and women aged 20–93 years were invited from this cohort to participate in the present study. Apart from the oldest participants (+80 years), all participants had to provide for their own transportation to the hospital and were characterized by living independently and being apparently healthy. Exclusion criteria were (i) pregnancy, (ii) acute medical illness, (iii) surgery within the last 3 months, (iv) ongoing medication known to affect body composition, and (v) history of compromised ambulation or prolonged immobilization. All participants gave their written informed consent, and all investigations were performed in accordance with the Declaration of Helsinki II and approved by the Ethical Committee of Copenhagen (H‐3‐2013‐124).
Physical measurements
All measurements were carried out by three designated and trained biotechnicians. Height (m) was assessed without shoes to the nearest 0.1 cm. Weight (kg) was measured wearing light clothing (hospital shirt) to the nearest 0.1 kg and subsequently body mass index (BMI) was calculated (kg/m2).
Muscle function
Handgrip strength
Handgrip strength was measured in three successive trials separated by 45 s pause using a Jamar dynamometer (Sammons Preston Rolyan, Chicago, Illinois, USA). Subjects were seated in the upright position with the arm along the side, bent at 90° in the elbow with the arm supported by a horizontal surface. The width of the dynamometer handle was adjusted to fit the size of the hand, and the best of the three trials was used for each arm.30 High inter‐rater and test–retest reliabilities have previously been demonstrated for this apparatus and procedure.30
Leg extension power
Maximal LEP was assessed using a Nottingham Leg Extension Power Rig (Medical Engineering Unit, University of Nottingham Medical School, Nottingham, UK).25, 31 Subjects were seated in the power rig chair, and the seat was adjusted to allow a knee angle of 15° with the footplate being fully pushed down. Subjects were instructed to push down the footplate connected to a flywheel as hard and fast as possible. The maximum speed of the flywheel was used to calculate the average power produced by the lower limb extensor muscles. Subjects were familiarized with the test procedure in two warm‐up trials followed by at least five trials with 30 s rest for each leg that were repeated until the subject did not improve LEP in two successive trials. Subjects were carefully instructed to keep their hands across the chest and to not move the upper body while pushing. Verbal encouragement was given to ensure maximal performance, and an on‐line visual feedback of the power curve was provided on a PC screen after each trial.
Functional capacity
Gait speed
Gait speed was measured over a 10 m straight walking course.32, 33 From a standing position, the participants were asked to walk at their maximal safe walking pace, without running and continue further than 10 m to avoid stopping or deceleration before reaching the 10 m mark.32, 34 No verbal encouragement was given during the test. The time was measured with a stopwatch to the nearest 0.1 s. Maximal GS was computed as the 10‐m distance divided by the elapsed time (m/s). Subsequently, habitual GS was estimated in subjects <65 years old based on normative data on the ratio between habitual and maximal GS in people aged 20–60 years,32 whereas habitual GS for people aged 65 years and older was calculated by means of a conversion equation previously published for this specific age group.33
Sit‐to‐stand performance
Sit‐to‐stand performance was assessed as the number of times a person was able to rise and sit from a standardized chair within 30 s (30 s STS).35, 36 The participant was seated in the middle of a standardized chair (with no arm rest, seat height 45 cm) back straight and arms crossed against the chest. At the signal ‘go', the participant was instructed to rise to a full stand (body erect and straight) and then immediately return back to the initial seated position. Participants were encouraged to complete as many full stands as possible within 30 s and were carefully instructed and monitored to fully sit between each stand. The total number of stands within 30 s was measured.35 Incorrect executed stands were not counted.
Dual‐energy X‐ray absorptiometry
Whole body DXA scans were performed using an iDXA fan beam densitometer (GE Lunar, Madison, Wisconsin, USA). The same scanner was used for all body scans and was carried out by one of three designated and trained technicians. Analyses of all exams were performed using Encore software version 16.0. Lean soft tissue assessed by DXA is composed of all fat‐free mass components except for mineral content. ALM was defined as the sum of lean soft tissue from the arms and legs. Relative ALM was acquired by normalizing ALM to height2 as previously suggested to account for allometric differences in body size.2 However, both absolute and normalized parameters are reported in the present study because differential age‐related changes in lean mass and body height may be caused by different mechanisms, which affects the estimated loss of muscle mass observed with aging.15
Statistical analysis
Data are presented as group mean ± standard deviation (SD) unless otherwise stated. All analyses were performed separately for women and men. Sex‐specific young reference groups comprising all participants aged between 20 and 39 years were formed, and cut‐off thresholds were obtained based on normative data obtained in this age group (T scores of –2.0 and –1.0, indicating the number of SDs below the young adult reference mean).2 One‐way ANOVA was used to compare obtained outcome variables between age groups (40–49, 50–59, 60–69, 70–79, and ≥80 years) and the young reference groups (i.e. 20–39 years). Statistical differences between women and men were evaluated using Student's t‐test for independent samples. In addition, the relationship between age and measured variables was assessed by regression analyses. Least square linear and quadratic regression models were compared based on the coefficient of determination (R 2) in order to determine the most appropriate regression model. For the analysis of variance, residuals were checked for normal distribution by visual inspection. Finally, contingency tables were used to calculate the proportion and numbers (for each 10 year age group) of subjects allocated within each T score category (T score below –2.0, T score between –2.0 and –1.0, and T score above –1.0) for each investigated outcome variables. Statistical analyses were performed using SPSS v20 (SPSS Inc., Chicago, Illinois, USA), and the level of significance was set at α = 0.05 using two‐tailed testing.
Results
A total of 1365 persons (767 women and 598 men) aged 20–93 years volunteered to participate in the study of which 1305 (729 women and 576 men) completed all the measurements and were selected for further analysis (Table 1). Anthropometric characteristics of the study cohort (n = 1305) are listed in Table 1.
Table 1.
Characteristics of study participants displayed as mean ± standard deviation
| Men (n = 576) | Women (n = 729) | |
|---|---|---|
| Age (years) | 57.01 ± 17.48 | 56.39 ±18.94 |
| Weight (kg) | 83.83 ± 13.35 | 67.57 ±11.73 |
| Height (m) | 1.80 ± 0.07 | 1.66 ±0.07 |
| Body mass index (kg/m2) | 25.99 ± 3.86 | 24.64 ±4.31 |
Main characteristics of the young female and male reference groups are reported in Table 2. Compared with women, men were taller, heavier, and had a larger BMI (all P < 0.001). Men also had larger absolute and relative total ALM and higher levels of HGS and LEP compared with women in all age groups (P < 0.001). Finally, men demonstrated a faster maximal GS compared with women (P < 0.001), while no sex differences were observed regarding the number of repetitions performed in the 30 s STS test (P < 0.05).
Table 2.
Young adult (20–39 years) reference data and cut‐points equivalent to T scores of –1.0 and –2.0
| Men (n = 110) | Women (n = 172) | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | T score = –1.0 | T score = –2.0 | Mean ± SD | T score = –1.0 | T score = –2.0 | |
| Age (years) | 30.04 ± 5.15 | 29.93 ± 5.22 | ||||
| Weight (kg) | 82.99 ± 12.39 | 64.35 ± 9.67 | ||||
| Height (m) | 1.83 ± 0.07 | 1.68 ± 0.07 | ||||
| BMI (kg/m2) | 24.77 ± 3.41 | 22.71 ± 3.14 | ||||
| TLM (kg) | 60.71 ± 6.97 | 53.74 | 46.77 | 42.26 ± 5.28 | 36.98 | 31.70 |
| ALM (kg) | 29.03 ± 3.89 | 25.14 | 21.25 | 18.76 ± 2.77 | 15.99 | 13.22 |
| Relative TLM (kg/m2) | 18.12 ± 1.82 | 16.30 | 14.58 | 14.88 ± 1.37 | 13.51 | 12.14 |
| Relative ALM (kg/m2) | 8.66 ± 1.03 | 7.63 | 6.60 | 6.61 ± 0.79 | 5.82 | 5.03 |
| HG strength (kg) | 52.99 ± 8.44 | 44.55 | 36.11 | 34.83 ± 7.33 | 27.50 | 20.17 |
| LEP (W) | 384.69 ± 78.61 | 306.08 | 227.47 | 232.33 ± 61.34 | 170.99 | 109.65 |
| Habitual GS (m/s) | 1.84 ± 0.25 | 1.59 | 1.34 | 1.63 ± 0.26 | 1.37 | 1.11 |
| Maximal GS (m/s) | 2.81 ± 0.42 | 2.39 | 1.97 | 2.57 ± 0.42 | 2.15 | 1.73 |
| 30 s STS test (n) | 27.26 ± 5.55 | 21.71 | 16.16 | 27.24 ± 6.07 | 21.17 | 15.10 |
ALM, appendicular lean mass; BMI, body mass index; GS, gait speed; HG, handgrip; LEP, leg extensor power; SD, standard deviation; STS, sit‐to‐stand; TLM, total lean mass.
T score = –1.0 corresponds to 1 SD below the young adult reference mean; T score = –2.0 corresponds to 2 SD below the young adult reference mean.
Lean mass
Both total and ALM decreased with age in men (Figures 1A and 2A) and women (Figures 1B and 2B) (P < 0.001). Decreased levels of total and ALM were found in the 60 to 69 year, 70 to 79 year, and ≥80 year age groups compared with the young reference group (each P < 0.01) for men and among women aged 70 to 79 year and ≥80 year (both P < 0.05) (Table 3). Men showed larger total and ALM values compared with women at all age groups (P < 0.001). When lean mass measures were normalized to height squared (Figures 3 and 4), decreased levels of relative total and ALM were observed in men aged 70 to 79 year and ≥80 year compared with the young reference group (P < 0.001) (Table 4). In women, relative ALM decreased compared with the young reference group in individuals aged 80 years and older (P < 0.001), but no differences were observed in terms of relative total lean mass (TLM) (P > 0.05). Men demonstrated larger relative total and ALM than women for all age groups (P < 0.001).
Figure 1.
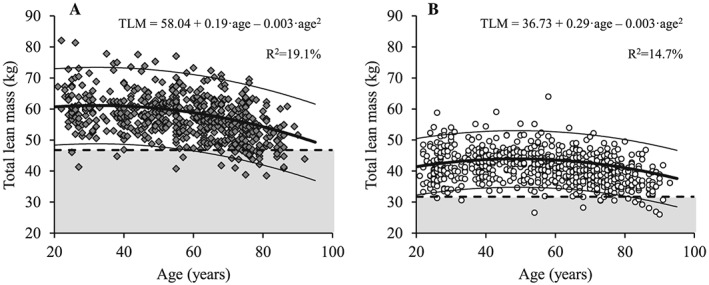
The association between age and total lean mass for men (A; dark grey diamonds) and women (B; white circles). Regression line (wide solid line) and 95% prediction interval (narrow solid line), T score equal to –2.0 (dashed line), data more than 2 SD below the young adult reference mean (shaded area), regression equations and adjusted R 2 values are shown. TLM, total lean mass.
Figure 2.
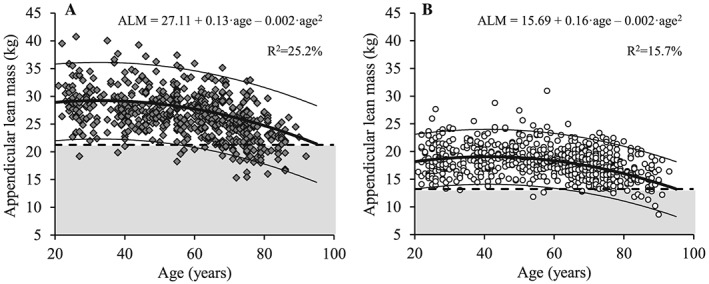
The association between age and appendicular lean mass for men (A; dark grey diamonds) and women (B; white circles). Regression line (wide solid line) and 95% prediction interval (narrow solid line), T score equal to –2.0 (dashed line), data more than 2 SD below the young adult reference mean (shaded area), regression equations and adjusted R 2 values are shown. ALM, appendicular lean mass.
Table 3.
Absolute total and appendicular lean mass for men and women by 10 year age groups and for the full age range (20–93 years, displayed as mean ± standard deviation)
| Age group | Men | Women | ||||
|---|---|---|---|---|---|---|
| n | TLM (kg) | ALM (kg) | n | TLM (kg) | ALM (kg) | |
| 20–29 | 59 | 61.33 ± 7.26 | 29.26 ± 3.85 | 98 | 41.74 ± 5.32 | 18.57 ± 2.79 |
| 30–39 | 51 | 60.00 ± 6.63 | 28.77 ± 3.97 | 74 | 42.97 ± 5.16 | 19.01 ± 2.73 |
| 40–49 | 83 | 59.50 ± 6.31 | 28.02 ± 3.67 | 96 | 43.29 ± 4.27 | 19.30 ± 2.36 |
| 50–59 | 96 | 58.71 ± 6.23 | 27.61 ± 3.48 | 109 | 42.63 ± 5.29 | 19.03 ± 2.87 |
| 60–69 | 118 | 57.64 ± 6.21* | 26.82 ± 3.49* | 130 | 40.80 ± 4.10 | 17.96 ± 2.20 |
| 70–79 | 127 | 53.36 ± 6.09* | 23.97 ± 3.38* | 151 | 39.36 ± 4.25* | 17.18 ± 2.42* |
| ≥80 | 42 | 51.10 ± 5.61* | 22.63 ± 2.98* | 71 | 36.92 ± 4.59* | 15.66 ± 2.51* |
| All | 576 | 57.23 ± 7.00 | 26.61 ± 4.08 | 729 | 41.07 ± 5.05 | 18.10 ± 2.76 |
ALM, appendicular lean mass; TLM, total lean mass.
Statistically significant differences compared with the young adult (20–39 years) reference data (P < 0.05).
Figure 3.
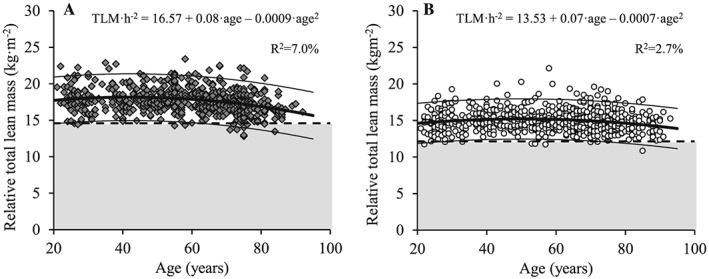
The association between age and relative total lean mass for men (A; dark grey diamonds) and women (B; white circles). Regression line (wide solid line) and 95% prediction interval (narrow solid line), T score equal to –2.0 (dashed line), data more than 2 SD below the young adult reference mean (shaded area), regression equations and adjusted R 2 values are shown. TLM/h2, relative total lean mass (normalized to height squared).
Figure 4.
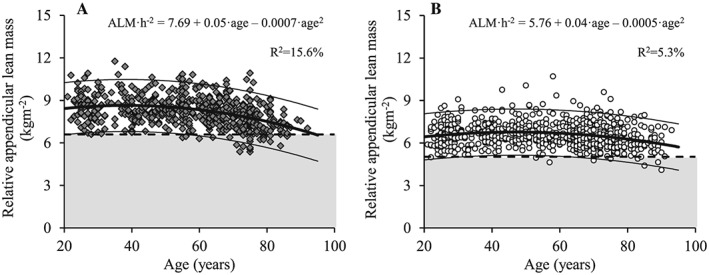
The association between age and relative appendicular lean mass for men (A; dark grey diamonds) and women (B; white circles). Regression line (wide solid line) and 95% prediction interval (narrow solid line), T score equal to –2.0 (dashed line), data more than 2 SD below the young adult reference mean (shaded area), regression equations and adjusted R 2 values are shown. ALM/h2, relative appendicular lean mass (normalized to height squared).
Table 4.
Relative total and appendicular lean mass for men and women by 10 year age groups and for the full age range (20–93 years, displayed as mean ± standard deviation)
| Age group | Men | Women | ||||
|---|---|---|---|---|---|---|
| n | Relative TLM (kg/m2) | Relative ALM (kg/m2) | n | Relative TLM (kg/m2) | Relative ALM (kg/m2) | |
| 20–29 | 59 | 18.00 ± 1.84 | 8.58 ± 0.97 | 98 | 14.72 ± 1.33 | 6.55 ± 0.77 |
| 30–39 | 51 | 18.26 ± 1.80 | 8.76 ± 1.11 | 74 | 15.10 ± 1.41 | 6.69 ± 0.82 |
| 40–49 | 83 | 17.85 ± 1.46 | 8.40 ± 0.86 | 96 | 15.31 ± 1.28 | 6.82 ± 0.77 |
| 50–59 | 96 | 18.22 ± 1.75 | 8.56 ± 1.00 | 109 | 15.12 ± 1.61 | 6.75 ± 0.94 |
| 60–69 | 118 | 17.92 ± 1.53 | 8.33 ± 0.87 | 130 | 15.01 ± 1.33 | 6.61 ± 0.75 |
| 70–79 | 127 | 17.08 ± 1.66* | 7.67 ± 0.96* | 151 | 14.78 ± 1.47 | 6.45 ± 0.87 |
| ≥80 | 42 | 16.70 ± 1.36* | 7.39 ± 0.74* | 71 | 14.41 ± 1.35 | 6.11 ± 0.80* |
| All | 576 | 17.72 ± 1.70 | 8.23 ± 1.02 | 729 | 14.93 ± 1.43 | 6.58 ± 0.84 |
ALM, appendicular lean mass; TLM, total lean mass.
Statistically significant differences compared with the young adult (20–39 years) reference data (P < 0.05).
Muscle strength and power
Compared with women, men showed HGS and LEP for all age groups (P < 0.001), but regardless of gender, both HGS and LEP decreased progressively with age (Figures 5 and 6) (P < 0.001). More specifically, HGS decreased in men aged 60 to 69 years, 70 to 79 years, and ≥80 years (P < 0.001) and in women aged 50 to 59 years, 60 ot 69 years, 70 to 79 years, and ≥80 years compared with young reference values (P < 0.01) (Table 5). Likewise, LEP decreased in both men and women aged 50 to 59 years, 60 to 69 years, 70 to 79 years, and ≥80 years compared with the young reference group (P < 0.001).
Figure 5.
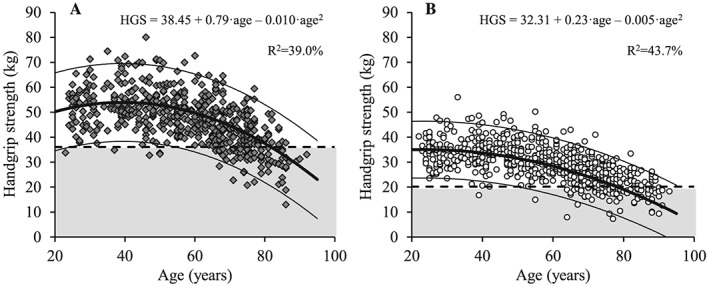
The association between age and handgrip strength for men (A; dark grey diamonds) and women (B; white circles). Regression line (wide solid line) and 95% prediction interval (narrow solid line), T score equal to –2.0 (dashed line), data more than 2 SD below the young adult reference mean (shaded area), regression equations and adjusted R 2 values are shown. HGS, handgrip strength.
Figure 6.
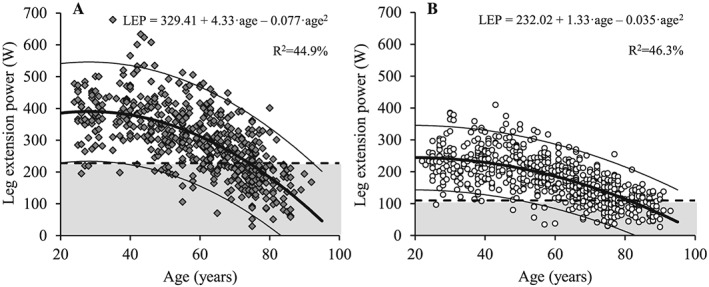
The association between age and leg extension power for men (A; dark grey diamonds) and women (B; white circles). Regression line (wide solid line) and 95% prediction interval (narrow solid line), T score equal to –2.0 (dashed line), data more than 2 SD below the young adult reference mean (shaded area), regression equations and adjusted R 2 values are shown. LEP, leg extension power.
Table 5.
Handgrip strength and leg extension power for men and women by 10 year age groups and for the full age range (20–93 years, displayed as mean ± standard deviation)
| Age group | Men | Women | ||||
|---|---|---|---|---|---|---|
| n | Handgrip strength (kg) | Leg extension power (W) | n | Handgrip strength (kg) | Leg extension power (W) | |
| 20–29 | 38 | 51.59 ± 7.68 | 375.15 ± 78.40 | 60 | 34.88 ± 8.11 | 228.73 ± 49.16 |
| 30–39 | 51 | 54.11 ± 8.87 | 391.37 ± 78.84 | 73 | 34.79 ± 6.67 | 234.46 ± 67.73 |
| 40–49 | 83 | 53.39 ± 8.95 | 385.00 ± 95.88 | 97 | 34.39 ± 5.63 | 232.74 ± 59.30 |
| 50–59 | 95 | 50.26 ± 7.73 | 322.70 ± 83.36* | 108 | 31.83 ± 5.99* | 200.12 ± 55.85* |
| 60–69 | 116 | 47.54 ± 8.31* | 297.19 ± 82.10* | 128 | 28.09 ± 5.81* | 165.37 ± 51.49* |
| 70–79 | 125 | 39.99 ± 7.56* | 221.11 ± 71.27* | 148 | 23.90 ± 5.09* | 129.46 ± 43.97* |
| ≥80 | 43 | 33.73 ± 7.96* | 164.88 ± 72.26* | 71 | 20.30 ± 4.62* | 97.79 ± 36.73* |
| All | 551 | 46.98 ± 10.22 | 299.90 ± 107.56 | 685 | 29.17 ± 7.75 | 176.72 ± 70.61 |
Statistically significant differences compared with the young adult (20–39 years) reference data (P < 0.05).
Functional capacity
Habitual and maximal GS both decreased with increasing age in men (Figures 7A and 8A) and women (Figures 7B and 8B) (P < 0.001). Compared with young reference values, habitual GS was reduced in both men and women aged 70 to 79 years and ≥80 years, while maximal GS was reduced in age groups 60 to 69 years, 70 to 79 years, and ≥80 years compared with young adults (Table 6) (P < 0.001). In addition, men showed elevated maximal and habitual GS compared with women for all age groups (P < 0.05).
Figure 7.
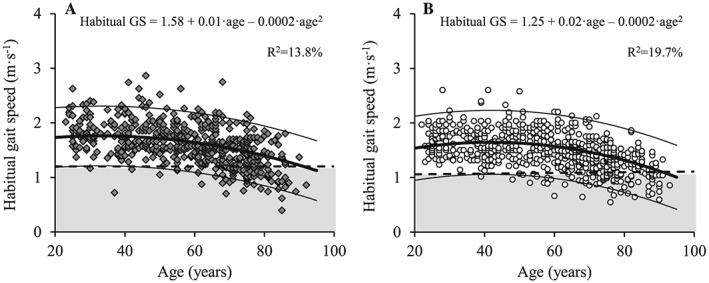
The association between age and habitual gait speed for men (A; dark grey diamonds) and women (B; white circles). Regression line (wide solid line) and 95% prediction interval (narrow solid line), T score equal to –2.0 (dashed line), data more than 2 SD below the young adult reference mean (shaded area), regression equations and adjusted R 2 values are shown. GS, gait speed.
Figure 8.
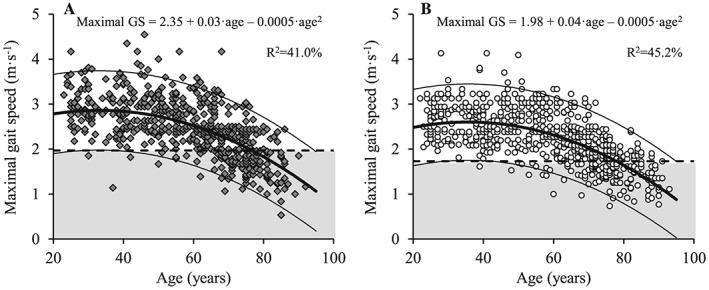
The association between age and maximal gait speed for men (A; dark grey diamonds) and women (B; white circles). Regression line (wide solid line) and 95% prediction interval (narrow solid line), T score equal to –2.0 (dashed line), data more than 2 SD below the young adult reference mean (shaded area), regression equations and adjusted R 2 values are shown. GS, gait speed.
Table 6.
Maximal and habitual gait speed for men and women by 10 year age groups and for the full age range (20–93 years, displayed as mean ± standard deviation)
| Age group | Men | Women | ||||
|---|---|---|---|---|---|---|
| n | Habitual gait speed (m/s) | Maximal gait speed (m/s) | n | Habitual gait speed (m/s) | Maximal gait speed (m/s) | |
| 20–29 | 40 | 1.84 ± 0.25 | 2.92 ± 0.40 | 60 | 1.63 ± 0.26 | 2.58 ± 0.42 |
| 30–39 | 51 | 1.72 ± 0.26 | 2.72 ± 0.42 | 74 | 1.61 ± 0.26 | 2.55 ± 0.42 |
| 40–49 | 83 | 1.76 ± 0.31 | 2.79 ± 0.49 | 96 | 1.57 ± 0.28 | 2.49 ± 0.44 |
| 50–59 | 96 | 1.68 ± 0.28 | 2.63 ± 0.44 | 109 | 1.57 ± 0.32 | 2.49 ± 0.51 |
| 60–69 | 118 | 1.66 ± 0.35 | 2.43 ± 0.50* | 130 | 1.54 ± 0.34 | 2.19 ± 0.49* |
| 70–79 | 127 | 1.54 ± 0.29* | 2.00 ± 0.42* | 150 | 1.37 ± 0.27* | 1.76 ± 0.39* |
| ≥80 | 42 | 1.30 ± 0.34* | 1.65 ± 0.49* | 72 | 1.15 ± 0.23* | 1.44 ± 0.34* |
| All | 557 | 1.64 ± 0.33 | 2.42 ± 0.59 | 691 | 1.49 ± 0.32 | 2.18 ± 0.59 |
Statistically significant differences compared with the young adult (20–39 years) reference data (P < 0.05).
Thirty second STS performance declined with increasing age in both men and women (P < 0.01) (Figure 9). In addition, men and women aged 50 to 59 years, 60 to 69 years, 70 to 79 years, and ≥80 years demonstrated impaired 30 s STS performance compared with their young reference counterparts (P < 0.001) (Table 7). Differences in 30 s STS values between men and women were only observed for the age group 70 to 80 years, with men performing a higher number of stands than women (P < 0.05).
Figure 9.
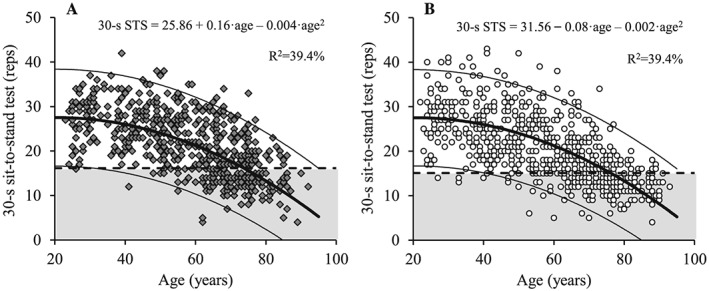
The association between age and 30 s sit‐to‐stand performance for men (A; dark grey diamonds) and women (B; white circles). Regression line (wide solid line) and 95% prediction interval (narrow solid line), T score equal to –2.0 (dashed line), data more than 2 SD below the young adult reference mean (shaded area), regression equations and adjusted R 2 values are shown. STS, sit‐to‐stand.
Table 7.
Thirty second sit‐to‐stand performance for men and women by 10 year age groups and for the full age range (20–93 years, displayed as mean ± standard deviation)
| Age group | Men | Women | ||
|---|---|---|---|---|
| n | 30 s sit‐to‐stand test (reps) | n | 30 s sit‐to‐stand test (reps) | |
| 20–29 | 35 | 26.07 ± 5.34 | 43 | 27.09 ± 5.70 |
| 30–39 | 50 | 28.20 ± 5.58 | 72 | 27.36 ± 6.37 |
| 40–49 | 76 | 26.22 ± 4.98 | 92 | 24.60 ± 6.30 |
| 50–59 | 95 | 23.30 ± 6.05* | 108 | 22.18 ± 6.47* |
| 60–69 | 118 | 19.44 ± 6.39* | 128 | 18.57 ± 5.94* |
| 70–79 | 125 | 16.45 ± 5.00* | 148 | 15.03 ± 4.43* |
| ≥80 | 42 | 13.55 ± 4.37* | 72 | 12.87 ± 3.04* |
| All | 541 | 21.31 ± 7.16 | 664 | 20.30 ± 7.46 |
Statistically significant differences compared with the young adult (20–39 years) reference data (P < 0.05).
Discussion
Skeletal muscle function is vital for locomotion, bone health, neuromuscular function, and metabolism and serves as an important protein reserve in catabolic conditions.11, 37 Consequently, low muscle mass represents an independent and substantial risk factor for frailty, morbidity, falls, fractures, and mortality in old age as well as for a wide range of acute and chronic diseases.11, 37, 38 Yet, despite solid evidence of the detrimental effects of sarcopenia, no universally accepted definition appears to exist, and consequently, the prevalence of sarcopenia reported in the literature varies considerably depending on the definitions used and the specific populations studied.39 Moreover, the introduction of physical performance (i.e. functional capacity) and muscle strength as integral parts of the sarcopenia concept underlines the need for establishing reference materials that combine muscle mass characteristics with an evaluation of muscle strength and functional capacity.6 The present study is the first to report the combination of total and regional lean mass (DXA), muscle strength and power (HGS and LEP), and functional capacity (GS and STS performance) in men and women across the adult lifespan. Notably, the present population‐based healthy cohort aged 20–93 years provides the first European reference values for total and regional lean mass measures obtained in young, middle‐aged, and old adults.
When the term sarcopenia was introduced by Irwin Rosenberg in 1989, it was referring to low skeletal muscle mass,1 which was operationalized by Baumgartner as values measured by DXA 2 SD below the normal mean of a young reference population.2 Although a wide range of assessment techniques can be used to measure or estimate lean body mass, DXA is considered the clinical gold standard based on the feasibility, high validity, accuracy, low radiation exposure, and price.14, 15 Still, it is noteworthy that the two dominant DXA manufacturers (Hologic and GE Healthcare) when validated against criterion four‐compartment models40, 41 have been shown to produce different body composition results, stressing the importance of obtaining common reference data using both scanner types.42
In line with earlier findings, the present study demonstrate greater ALM in men compared with women for all age groups (cf. Figure 1).19, 20, 22 Moreover, a non‐linear decline in TLM and ALM was observed with increasing age in both sexes, also in agreement with previous reports.19, 20, 22 More specifically, ALM decreased 22.7% and 18.9% throughout the lifespan in men and women, respectively, whereas the decrease in ALM/h2 was 15.6% in men and 10.4% in women, respectively.
Notably, the sarcopenia cut‐off threshold for ALM/h2 derived in the present study (men 6.60 kg/m2 and women 5.03 kg/m2, Table 2) was lower compared with those reported in classical studies from New Mexico and USA,2, 16 while more similar to newer ALM/h2 reference data obtained both in Australia and in the USA.19, 20, 22 The difference may partly be explained by a lower BMI in the present population (men 25.99 kg/m2 and women 24.64 kg/m2, cf. Table 2) compared with the data from the USA2, 16 and Australia.19, 20 Regardless, a general trend seems to exist towards lower cut‐off values in more recent studies compared with earlier reference data,2, 16 which may partly be explained by a lower spatial resolution in the older scanner types leading to a systematic overestimation of lean mass.43 This calls for updated reference values not only in Europe but also in other parts of the world.
In parallel with the observed decline in lean mass, progressively non‐linear reductions in HGS and LEP were observed with increasing age in both men and women (cf. Figures 5 and 6). Moreover, men showed greater muscle strength and power (HGS and LEP) than women at all age intervals examined. Compared with the cut‐off values for HGS suggested by the European Working Group on Sarcopenia in Older People (EWGSOP) in 20103 (men: 30 kg; women: 20 kg), 20187 (men: 27 kg; women: 16 kg), and by the International Working Group on Sarcopenia in 20114 (men: 26 kg; women: 16 kg), the present values were considerably higher for men (36.1 kg) and women (20.2 kg) based on the most recent recommendations.4, 7 The recent cut‐off values for HGS suggested by the EWGSOP are based on normative data from the 12 British studies46 and values 2.5 SD below the sex‐specific young reference group were used. Notably, the cut‐off values based on 2 SD below the young reference group would have been 32 kg for men and 19 kg for women, which is very similar to the threshold values in the present study and the findings from a previous Danish cohort aged 19–72 years.31
In the present study, the cut‐off threshold for LEP was 227.5 and 109.7 W for men and women, respectively. To our best knowledge, no previous cut‐off values have been reported based on assessment in large cohorts. However, LEP has been assessed in a subgroup of subjects in the aforementioned Danish cohort and, notably, the present cut‐off values were 10–20% higher for both sexes in all age groups,31 which is supporting the present cohort being representative for healthy individuals.
Similar to HGS and LEP, a significant decrease in GS with aging was observed in both men and women. However, differences in both magnitude and timing of onset were observed. Thus, habitual GS decreased from the age of 70 and above in both men and women, whereas maximal GS showed an earlier deflection point by decreasing from the age of 60 years in men and women compared with young adults (cf. Table 6). In addition, both GS measures were elevated in men compared with women for all age groups. Although most GS test methods have excellent inter‐rater and test–retest reliabilities, there is no consensus regarding the optimal measurement protocol including walking distance, instructed pace, and start mode.44 Notably, maximal GS has been associated with skeletal muscle mass and self‐rated health in older individuals,45 indicating that walking speed at maximal pace is an important parameter to show early changes in health and functional performance.45 By contrast, habitual GS may be influenced by the desire of the person being tested to demonstrate a ‘normal' physical function.
In line with maximal GS and LEP, 30 s STS performance was found to decline from the age of 50 years in men and women compared with young individuals (cf. Table 7). However, in contrast to all other measurements, STS performance did not differ between men and women, apart from the age group of 70–79 years. Yet, despite that both HGS and STS started to decline from the fifth decade, the 30 s STS outcome variable was considerable more senstive to aging. Thus, compared with the young reference population, 14.0% men and 12.3% women (Table 8) demonstrated reduced HGS, whereas 29.5% men and 31.8% women (Table 8) had a 30 s STS test below the present cut‐off values (women: 15 STS per 30 s, men: 16 STS per 30 s) supporting that the 30 s STS test represents a low cost, fast, and sensitive screening tool for sarcopenia and age‐related loss in functional capacity.36
Table 8.
Prevalence of subjects according to T score categories displayed by 10 year age group for men and women
| Age group | Men [n (%)] | Women [n (%)] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Less than –2.0 | –2.0 to –1.0 | Greater than –1.0 | Less than –2.0 | –2.0 to –1.0 | Greater than –1.0 | |||||||
| TLM | ||||||||||||
| 20–29 | 2 | (3.5) | 3 | (5.3) | 54 | (91.2) | 1 | (1.0) | 18 | (18.4) | 79 | (80.6) |
| 30–39 | 1 | (2.0) | 8 | (16.0) | 42 | (82.0) | 1 | (1.4) | 6 | (8.3) | 67 | (90.3) |
| 40–49 | 0 | (0.0) | 15 | (18.3) | 68 | (81.7) | 0 | (0.0) | 7 | (7.4) | 89 | (92.6) |
| 50–59 | 2 | (2.1) | 17 | (17.9) | 77 | (80.0) | 1 | (0.9) | 12 | (11.0) | 96 | (88.1) |
| 60–69 | 2 | (1.7) | 28 | (23.9) | 88 | (74.4) | 2 | (1.6) | 22 | (17.2) | 106 | (81.2) |
| 70–79 | 17 | (13.5) | 49 | (38.9) | 61 | (47.6) | 5 | (2.7) | 45 | (30.0) | 101 | (67.3) |
| ≥80 | 7 | (16.7) | 21 | (50.0) | 14 | (33.3) | 8 | (11.3) | 32 | (45.1) | 31 | (43.7) |
| All | 31 | (5.4) | 141 | (24.8) | 397 | (69.8) | 17 | (2.4) | 142 | (19.6) | 564 | (78.0) |
| ALM | ||||||||||||
| 20–29 | 1 | (1.7) | 5 | (8.5) | 53 | (89.8) | 0 | (0.0) | 16 | (16.3) | 82 | (83.7) |
| 30–39 | 1 | (2.0) | 8 | (15.7) | 42 | (82.4) | 1 | (1.4) | 10 | (13.5) | 63 | (85.1) |
| 40–49 | 1 | (1.2) | 17 | (20.5) | 65 | (78.3) | 0 | (0.0) | 8 | (8.3) | 88 | (91.7) |
| 50–59 | 3 | (3.1) | 19 | (19.8) | 74 | (77.1) | 2 | (1.8) | 7 | (6.4) | 100 | (91.7) |
| 60–69 | 6 | (5.1) | 31 | (26.3) | 81 | (68.6) | 2 | (1.5) | 21 | (16.2) | 107 | (82.3) |
| 70–79 | 27 | (21.3) | 55 | (43.3) | 45 | (35.4) | 3 | (2.0) | 47 | (31.1) | 101 | (66.9) |
| ≥80 | 12 | (28.6) | 30 | (47.6) | 10 | (23.8) | 10 | (14.1) | 31 | (43.7) | 30 | (42.3) |
| All | 51 | (8.9) | 155 | (26.9) | 370 | (64.2) | 18 | (2.5) | 140 | (19.2) | 571 | (78.3) |
| Relative TLM | ||||||||||||
| 20–29 | 2 | (3.5) | 6 | (10.5) | 51 | (86.0) | 2 | (2.0) | 17 | (17.3) | 79 | (80.6) |
| 30–39 | 0 | (0.0) | 6 | (12.0) | 45 | (88.0) | 1 | (1.4) | 8 | (11.1) | 65 | (87.5) |
| 40–49 | 0 | (0.0) | 11 | (13.4) | 72 | (86.6) | 0 | (0.0) | 7 | (7.4) | 89 | (92.6) |
| 50–59 | 2 | (2.1) | 10 | (10.5) | 84 | (87.4) | 3 | (2.8) | 8 | (7.3) | 98 | (89.9) |
| 60–69 | 0 | (0.0) | 14 | (12.0) | 104 | (88.0) | 0 | (0.0) | 14 | (10.2) | 116 | (89.8) |
| 70–79 | 7 | (5.6) | 29 | (23.0) | 91 | (71.4) | 2 | (1.3) | 23 | (15.3) | 126 | (83.3) |
| ≥80 | 2 | (4.8) | 14 | (33.3) | 26 | (61.9) | 1 | (1.4) | 19 | (26.8) | 51 | (71.8) |
| All | 13 | (2.3) | 90 | (15.8) | 473 | (81.9) | 9 | (1.2) | 96 | (13.1) | 624 | (85.6) |
| Relative ALM | ||||||||||||
| 20–29 | 0 | (0.0) | 9 | (15.3) | 50 | (84.7) | 1 | (1.0) | 17 | (17.3) | 80 | (81.6) |
| 30–39 | 0 | (0.0) | 7 | (13.7) | 44 | (86.3) | 0 | (0.0) | 11 | (14.9) | 63 | (85.1) |
| 40–49 | 0 | (0.0) | 15 | (18.1) | 68 | (81.9) | 0 | (0.0) | 8 | (8.3) | 88 | (91.7) |
| 50–59 | 2 | (2.1) | 15 | (15.6) | 79 | (82.3) | 2 | (1.8) | 7 | (6.4) | 100 | (91.7) |
| 60–69 | 3 | (2.5) | 22 | (18.6) | 93 | (78.8) | 0 | (0.0) | 18 | (13.8) | 112 | (86.2) |
| 70–79 | 13 | (10.2) | 47 | (37.0) | 67 | (52.8) | 5 | (3.3) | 36 | (23.8) | 110 | (72.8) |
| ≥80 | 6 | (14.3) | 23 | (54.8) | 13 | (31.0) | 5 | (7.0) | 18 | (25.4) | 48 | (67.6) |
| All | 24 | (4.2) | 138 | (24.0) | 414 | (71.9) | 13 | (1.8) | 115 | (15.8) | 601 | (82.4) |
| Handgrip strength | ||||||||||||
| 20–29 | 1 | (2.6) | 6 | (15.8) | 31 | (81.6) | 0 | (0.0) | 8 | (13.3) | 52 | (86.7) |
| 30–39 | 1 | (2.0) | 6 | (11.8) | 44 | (86.3) | 1 | (1.4) | 6 | (8.2) | 66 | (90.4) |
| 40–49 | 1 | (1.2) | 10 | (12.0) | 72 | (86.7) | 0 | (0.0) | 11 | (11.3) | 86 | (88.7) |
| 50–59 | 2 | (2.1) | 20 | (21.1) | 73 | (76.8) | 1 | (0.9) | 27 | (25.0) | 80 | (74.1) |
| 60–69 | 8 | (6.9) | 34 | (29.3) | 74 | (63.8) | 12 | (9.4) | 45 | (35.2) | 71 | (55.5) |
| 70–79 | 38 | (30.4) | 52 | (41.6) | 35 | (28.0) | 33 | (22.3) | 78 | (52.7) | 37 | (25.0) |
| ≥80 | 26 | (60.5) | 14 | (32.6) | 3 | (7.0) | 37 | (52.1) | 31 | (43.7) | 3 | (4.2) |
| All | 77 | (14.0) | 142 | (25.8) | 332 | (60.3) | 84 | (12.3) | 206 | (30.1) | 395 | (57.7) |
| Leg extensor power | ||||||||||||
| 20–29 | 3 | (8.6) | 4 | (11.4) | 28 | (80.0) | 1 | (2.3) | 4 | (9.3) | 38 | (88.4) |
| 30–39 | 1 | (2.0) | 5 | (10.0) | 44 | (88.0) | 1 | (1.4) | 13 | (17.8) | 59 | (80.8) |
| 40–49 | 3 | (3.9) | 12 | (15.8) | 61 | (80.3) | 1 | (1.1) | 14 | (15.2) | 77 | (83.7) |
| 50–59 | 12 | (12.6) | 29 | (30.5) | 54 | (56.8) | 5 | (4.6) | 29 | (26.9) | 74 | (68.5) |
| 60–69 | 26 | (22.0) | 40 | (33.9) | 52 | (44.1) | 16 | (12.5) | 58 | (45.3) | 54 | (42.2) |
| 70–79 | 70 | (56.0) | 38 | (30.4) | 17 | (13.6) | 49 | (33.1) | 68 | (45.9) | 31 | (20.9) |
| ≥80 | 38 | (90.5) | 2 | (4.8) | 2 | (4.8) | 44 | (61.1) | 27 | (37.5) | 1 | (1.4) |
| All | 153 | (28.3) | 130 | (24.0) | 258 | (47.7) | 117 | (17.6) | 213 | (32.1) | 334 | (50.3) |
| Habitual gait speed | ||||||||||||
| 20–29 | 0 | (0.0) | 11 | (27.5) | 29 | (72.5) | 0 | (0.0) | 11 | (18.3) | 49 | (81.7) |
| 30–39 | 4 | (7.8) | 18 | (35.3) | 29 | (56.9) | 2 | (2.7) | 12 | (16.2) | 60 | (81.1) |
| 40–49 | 5 | (6.0) | 31 | (37.3) | 47 | (56.6) | 1 | (1.0) | 24 | (25.0) | 71 | (74.0) |
| 50–59 | 13 | (13.5) | 38 | (39.6) | 45 | (46.9) | 12 | (11.0) | 17 | (15.6) | 80 | (73.4) |
| 60–69 | 19 | (16.1) | 39 | (33.1) | 60 | (50.8) | 13 | (10.0) | 30 | (23.1) | 87 | (66.9) |
| 70–79 | 41 | (32.3) | 34 | (26.8) | 52 | (40.9) | 20 | (13.3) | 63 | (42.0) | 67 | (44.7) |
| ≥80 | 23 | (54.8) | 11 | (26.2) | 8 | (19.0) | 29 | (40.3) | 33 | (45.8) | 10 | (13.9) |
| All | 105 | (18.9) | 182 | (32.7) | 270 | (48.5) | 77 | (11.1) | 190 | (27.5) | 424 | (61.4) |
| Maximal gait speed | ||||||||||||
| 20–29 | 0 | (0.0) | 4 | (10.0) | 36 | (90.0) | 0 | (0.0) | 7 | (11.7) | 53 | (88.3) |
| 30–39 | 1 | (2.0) | 11 | (21.6) | 39 | (76.5) | 2 | (2.7) | 11 | (14.9) | 61 | (82.4) |
| 40–49 | 1 | (1.2) | 15 | (18.1) | 67 | (80.7) | 1 | (1.0) | 23 | (24.0) | 72 | (75.0) |
| 50–59 | 5 | (5.2) | 21 | (21.9) | 70 | (72.9) | 12 | (11.0) | 13 | (11.9) | 84 | (77.1) |
| 60–69 | 16 | (13.6) | 46 | (39.0) | 56 | (47.5) | 27 | (20.8) | 38 | (29.2) | 65 | (50.0) |
| 70–79 | 60 | (47.2) | 43 | (33.9) | 24 | (18.9) | 83 | (55.3) | 41 | (27.3) | 26 | (17.3) |
| ≥80 | 30 | (71.4) | 9 | (21.4) | 3 | (7.1) | 62 | (86.1) | 8 | (11.1) | 2 | (2.8) |
| All | 113 | (20.3) | 149 | (26.8) | 295 | (53.0) | 187 | (27.1) | 141 | (20.4) | 363 | (52.5) |
| 30 s STS test | ||||||||||||
| 20–29 | 1 | (2.5) | 10 | (25.0) | 29 | (72.5) | 1 | (1.7) | 8 | (13.8) | 49 | (84.5) |
| 30–39 | 0 | (0.0) | 6 | (11.8) | 45 | (88.2) | 2 | (2.7) | 10 | (13.5) | 62 | (83.8) |
| 40–49 | 2 | (2.4) | 12 | (14.5) | 69 | (83.1) | 5 | (5.2) | 28 | (29.2) | 63 | (65.6) |
| 50–59 | 16 | (16.7) | 22 | (22.9) | 58 | (60.4) | 19 | (17.4) | 34 | (31.2) | 56 | (51.4) |
| 60–69 | 44 | (37.3) | 31 | (26.3) | 43 | (36.4) | 42 | (32.8) | 52 | (40.6) | 34 | (26.6) |
| 70–79 | 71 | (56.8) | 31 | (24.8) | 23 | (18.4) | 93 | (62.4) | 43 | (28.9) | 13 | (8.7) |
| ≥80 | 29 | (72.5) | 10 | (25.0) | 1 | (2.5) | 56 | (78.9) | 15 | (21.1) | 0 | (0.0) |
| All | 163 | (29.5) | 122 | (22.1) | 268 | (48.5) | 218 | (31.8) | 190 | (27.7) | 277 | (40.4) |
ALM, appendicular lean mass; STS, sit‐to‐stand; TLM, total lean mass.
Less than –2.0, measure more than 2 SD below the young adult reference mean; –2.0 to –1.0, measure equal to or between 1 and 2 SD below the young adult reference mean; greater than –1.0, measure <1 SD below the young adult reference mean.
A major strength of the present study was that all measurements in a given participant were performed on the same day by one of three designated and trained technicians. Further, all assessments of lean mass were performed using the same iDXA scanner (GE Healthcare), which is rare compared with other reference reports.19, 20, 22 In relation to the updated EWGSOP recommendations, it would have been valuable to obtain data using the 5×STS test; however, we chose to measure the 30 s STS test as it seems more sensitive with low and high performance values (below and above 5 reps) as demonstrated by McAllister and coworkers.35 Another potential study limitation was that cut‐off values for habitual GS may have been overestimated or underestimated, because only maximal GS were directly measured. Lastly, similarly to previous reference materials,2, 16, 19, 20, 22 only limited information could be obtained regarding the persons who were invited but did not actively accept to participate in the present study. However, based on the obtained data, the present study population appears to represent a healthy Danish population, which, depending on the perspective, may be considered a limitation or a strength of the study. Thus, it may be argued that the present data might not be representative for the general Danish population. On the other hand, the present reference material based on an apparently healthy population may be usable to identify individuals at risk of low physical performance or low muscle mass independently of any co‐morbidities.
In conclusion, the present data obtained in 1305 healthy citizens located in greater Copenhagen revealed that power‐based measures of functional capacity (LEP and 30 s STS) started to decline already at age +50 years, whereas grip strength and habitual gait parameters (GS and HGS) and lean mass characteristics (TLM, ALM, and ALM/h2) remained unaltered until after the age of +70 years. Further, our data underline a strong need for establishing local (regional) reference data given that the lean mass and BMI cut‐off values were lower compared with previous reference data obtained in populations from New Mexico and the USA,2, 16 respectively, whereas cut‐off values for GS, HGS, and STS performance generally were higher compared with previous reports.7, 46
Perspectives
Despite aetiological differences,47 both primary and secondary sarcopenia are globally under‐recognized and negatively affecting millions of elderly people and patients, and being closely related to deteriorations in functional capacity, increased risk of frailty, and increased morbidity and mortality.3, 11, 37 In both conditions, there is a strong need for effective diagnostic tools to identify the different domains of muscle loss, for example, low muscle mass and/or parallel impairments in muscle strength and functional capacity. A distinction between the different domains of muscle dysfunction may facilitate the development of targeted individualized treatments with the purpose of increasing muscle mass, muscle strength, and/or physical function, respectively, as a result of individualized non‐pharmacological (exercise and/or nutrition based) and/or pharmacological interventions. Reference material as presented in the present study may help to individualize such targeted intervention efforts.
Conflict of interests
The authors declare that they have no conflict of interests and certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.48
Acknowledgements
Merethe Appleyard is warmly acknowledged for her help during the recruitment process. We also thank Nanna Freja Folkmann for her help during the study.
Suetta C., Haddock B., Alcazar J., Noerst T., Hansen O., Ludvig H., Kamper R. S., Schnohr P., Prescott E., Andersen L. L., Frandsen U., Aagaard P., Bulow J., Hovind P., and Simonsen L. (2019) The Copenhagen Sarcopenia Study: lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years, Journal of Cachexia, Sarcopenia and Muscle, 10, 1316–1329. 10.1002/jcsm.12477.
References
- 1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997. May;127:990S–991S, Review. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 3. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010. Jul;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014. Feb;15:95–101. [DOI] [PubMed] [Google Scholar]
- 6. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014. Nov;43:748–759, Epub 2014 Sep 21. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2018. Oct 12;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anker SD, Morley JE, von Haehling S. Welcome to the ICD‐10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016. Dec;7:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports 2010. Feb;20:49–64, Review. [DOI] [PubMed] [Google Scholar]
- 10. Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 2010;95:139–159, Epub 2010 Mar 2. [DOI] [PubMed] [Google Scholar]
- 11. Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci 2010. Nov;1211:25–36, Review. [DOI] [PubMed] [Google Scholar]
- 12. Welch C, Hassan‐Smith ZK, Greig CA, Lord JM, Jackson TA. Acute sarcopenia secondary to hospitalisation—an emerging condition affecting older adults. Aging Dis 2018. Feb 1;9:151–164, eCollection 2018 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ørtenblad N, et al. Effects of aging on muscle mechanical function and muscle fiber morphology during short‐term immobilization and subsequent retraining. J Appl Physiol 2010. Dec;109:1628–1634. [DOI] [PubMed] [Google Scholar]
- 14. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res 2016. Dec;28:1047–1060, Epub 2016 Jun 2. [DOI] [PubMed] [Google Scholar]
- 15. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018. Apr;9:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X‐ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009;4:e738:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faulkner KG, Roberts LA, McClung MR. Discrepancies in normative data between lunar and hologic DXA systems. Osteoporos Int 1996;6:432–436. [DOI] [PubMed] [Google Scholar]
- 18. Saarelainen J, Hakulinen M, Rikkonen T, Kroger H, Tuppurainen M, Koivumaa‐Honkanen H, et al. Cross‐calibration of GE Healthcare Lunar prodigy and iDXA dual‐energy X‐ray densitometers for bone mineral measurements. J Osteoporos 2016;2016:1424582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gould H, Brennan SL, Kotowicz MA, Nicholson GC, Pasco JA. Total and appendicular lean mass reference ranges for Australian men and women: the Geelong Osteoporosis Study. Calcif Tissue Int 2014. Apr;94:363–372. [DOI] [PubMed] [Google Scholar]
- 20. Yu S, Appleton S, Adams R, Chapman I, Wittert G, Visvanathan T, et al. The impact of low muscle mass definitions on the prevalence of sarcopenia in older Australians. Biomed Res Int 2014;2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clark P, Denova‐Gutiérrez E, Ambrosi R, Szulc P, Rivas‐Ruiz R, Salmerón J. Reference values of total lean mass, appendicular lean mass, and fat mass measured with dual‐energy X‐ray absorptiometry in a healthy Mexican population. Calcif Tissue Int 2016. Nov;99[5;99:462–471. [DOI] [PubMed] [Google Scholar]
- 22. Imboden MT, Swartz AM, Finch HW, Harber MP, Kaminsky LA. Reference standards for lean mass measures using GE dual energy X‐ray absorptiometry in Caucasian adults. PLoS ONE 2017. Apr 20;12[4;12:e0176161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tankó LB, Movsesyan L, Mouritzen U, Christiansen C, Svendsen OL. Appendicular lean tissue mass and the prevalence of sarcopenia among healthy women. Metabolism 2002. Jan;51:69–74. [DOI] [PubMed] [Google Scholar]
- 24. Coin A, Sergi G, Minicuci N, Giannini S, Barbiero E, Manzato E, et al. Fat‐free mass and fat mass reference values by dual‐energy X‐ray absorptiometry (DEXA) in a 20‐80 year‐old Italian population. Clin Nutr 2008. Feb;27:87–94. [DOI] [PubMed] [Google Scholar]
- 25. Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol 1990;60:385–390. [DOI] [PubMed] [Google Scholar]
- 26. Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65‐89 years. Age Ageing 1994. Sep;23:371–377. [DOI] [PubMed] [Google Scholar]
- 27. Skelton DA, Young A, Greig CA, Malbut KE. Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J Am Geriatr Soc 1995. Oct;43:1081–1087. [DOI] [PubMed] [Google Scholar]
- 28. Caserotti P, Aagaard P, Larsen JB, Puggaard L. Explosive heavy‐resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports 2008. Dec;18:773–782. [DOI] [PubMed] [Google Scholar]
- 29. Appleyard M, Hansen AT, Schnohr P, Jensen G, Nyboe J. The Copenhagen City Heart Study. Østerbro undersøgelsen. A book of tables with data from the first examination (1976–78) and a five year follow‐up (1981–83). Scand J Soc Med 1989;170:1–160. [PubMed] [Google Scholar]
- 30. Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011. Jul;40:423–429, Epub 2011 May 30. Review. [DOI] [PubMed] [Google Scholar]
- 31. Aadahl M, Beyer N, Linneberg A, Thuesen BH, Jørgensen T. Grip strength and lower limb extension power in 19‐72‐year‐old Danish men and women: the Health2006 study. BMJ Open 2011. Jan 1;1:e000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bohannon R. Comfortable and maximum walking speed of adults aged 20‐79 years: reference values and determinants. Age Ageing 1997;26:15–19. [DOI] [PubMed] [Google Scholar]
- 33. Sustakoski BS, Perera S, VanSwearingen JM, Studenski SA, Brach JS. The impact of testing protocol on recorded gait speed. Gait Posture 2015;41:329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suetta C, Magnusson SP, Rosted A, Aagaard P, Jakobsen AK, Larsen LH, et al. Resistance training in the early postoperative phase reduces hospitalization and leads to muscle hypertrophy in elderly hip surgery patients—a controlled, randomized study. J Am Geriatr Soc 2004. Dec;52:2016–2022. [DOI] [PubMed] [Google Scholar]
- 35. McAllister LS, Palombaro KM. Modified 30‐second sit‐to‐stand test: reliability and validity in older adults unable to complete traditional sit‐to‐stand testing. J Geriatr Phys Ther 2019. Feb 21;1, 10.1519/JPT.0000000000000227. [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36. Alcazar J, Losa‐Reyna J, Rodriguez‐Lopez C, Alfaro‐Acha A, Rodriguez‐Mañas L, Ara I, et al. The sit‐to‐stand muscle power test: an easy, inexpensive and portable procedure to assess muscle power in older people. Exp Gerontol 2018. Oct 2;112:38–43, Epub 2018 Sep 1. [DOI] [PubMed] [Google Scholar]
- 37. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass [sarcopenia] in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002. May;50:889–896. [DOI] [PubMed] [Google Scholar]
- 38. Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010. Apr;29:154–159, Epub 2010 Jan 8. [DOI] [PubMed] [Google Scholar]
- 39. Bijlsma AY, Meskers CG, Ling CH, Narici M, Kurrle SE, Cameron ID, et al. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle‐aged cohort. Age 2013;35:871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wells JC, Haroun D, Williams JE, Wilson C, Darch T, Viner RM, et al. Evaluation of DXA against the four‐component model of body composition in obese children and adolescents aged 5‐21 years. Int J Obes 2010. Apr;34:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Der Ploeg GE, Withers RT, Laforgia J. Percent body fat via DEXA: comparison with a four‐compartment model. J Appl Physiol (1985) 2003. Feb;94:499–506. [DOI] [PubMed] [Google Scholar]
- 42. Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL, et al. A multinational study to develop universal standardization of whole‐body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res 2012. Oct;27:2208–2216. [DOI] [PubMed] [Google Scholar]
- 43. Morrison SA, Petri RM, Hunter HL, Raju D, Gower B. Comparison of the lunar prodigy and iDXA dual‐energy X‐ray absorptiometers for assessing total and regional body composition. J Clin Densitom 2016. Jul‐Sep;19:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Graham JE, Ostir GV, Kuo YF, Fisher SR, Ottenbacher KJ. Relationship between test methodology and mean velocity in timed walk tests: a review. Arch Phys Med Rehabil 2008;89:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim H, Park I, Lee H, Lee O. The reliability and validity of gait speed with different walking pace and distances against general health, physical function, and chronic disease in aged adults. J Exerc Nutr Biochem 2016. Sep;20:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: normative data from twelve British studies. PLoS ONE 2014. Dec 4;9:e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hepple RT. Muscle atrophy is nost always sarcopenia. J Appl Physiol (1985) 2012. Aug 15;113:677–679. [DOI] [PubMed] [Google Scholar]
- 48. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


