Abstract
Background
It has long been recognized that vitamin D deficiency is associated with muscle weakness and falls. Vitamin D receptor (VDR) is present at very low levels in normal muscle. Whether vitamin D plays a direct role in muscle function is unknown and is a subject of hot debate. Myocyte‐specific deletion of VDR would provide a strategy to answer this question.
Methods
Myocyte‐specific vitamin D receptor (mVDR) null mice were generated by crossing human skeletal actin‐Cre mice with floxed VDR mice. The effects of gene deletion on the muscle phenotype were studied in terms of body tissue composition, muscle tissue histology, and gene expression by real‐time PCR.
Results
Unlike whole‐body VDR knockout mice, mVDR mice showed a normal body size. The mVDR showed a distinct muscle phenotype featuring reduced proportional lean mass (70% vs. 78% of lean mass), reduced voluntary wheel‐running distance (22% decrease, P = 0.009), reduced average running speed, and reduced grip strength (7–16% reduction depending on age at testing). With their decreased voluntary exercise, and decreased lean mass, mVDR have increased proportional fat mass at 20% compared with 13%.
Surprisingly, their muscle fibres showed slightly increased diameter, as well as the presence of angular fibres and central nuclei suggesting ongoing remodelling. There were, however, no clear changes in fibre type and there was no increase in muscle fibrosis. VDR is a transcriptional regulator, and changes in the expression of candidate genes was examined in RNA extracted from skeletal muscle. Alterations were seen in myogenic gene expression, and there was decreased expression of cell cycle genes cyclin D1, D2, and D3 and cyclin‐dependent kinases Cdk‐2 and Cdk‐4. Expression of calcium handling genes sarcoplasmic/endoplasmic reticulum calcium ATPases (SERCA) Serca2b and Serca3 was decreased and Calbindin mRNA was lower in mVDR muscle.
Conclusions
This study demonstrates that vitamin D signalling is needed for myocyte function. Despite the low level of VDR protein normally found muscle, deleting myocyte VDR had important effects on muscle size and strength. Maintenance of normal vitamin D signalling is a useful strategy to prevent loss of muscle function and size.
Keywords: Vitamin D, Vitamin D receptor, Muscle, Sarcopenia, Weakness
Introduction
Vitamin D plays a critical role in the regulation of calcium homeostasis, and skeletal muscle requires calcium for normal development and physiology. Vitamin D deficiency and inactivating mutations in the vitamin D receptor (VDR) are associated with muscle weakness in humans and in mouse models.1, 2, 3
The role of vitamin D and VDR in muscle is the subject of many reviews.2, 3, 4, 5 Despite this, it remains unclear whether vitamin D has direct effects in muscle. Vitamin D regulates absorption of dietary calcium and phosphate, renal calcium handling, and bone calcium homeostasis. Normal serum calcium and phosphate are needed for normal muscle function, complicating study of the role of vitamin D in muscle function.
We have previously described a mouse model of vitamin D deficiency with normal calcium and phosphate in which muscle weakness is present.1 In addition, mice with whole‐body deletion of VDR have decreased muscle mass and are weak, even if fed rescue diet from weaning to maintain normal serum calcium and phosphate.1 Unfortunately, the whole‐body VDR‐null mice have many potential confounding factors, including liver cirrhosis and poor bone health.4, 6 Despite this, these two models suggest that direct effects of vitamin D on muscle are plausible, as in both cases, calcium and phosphate did not differ from the controls.
The presence of VDR in muscle is controversial, particularly since the meticulous demonstration that many of the previously used antibodies for VDR demonstrate clear bands in the absence of VDR protein.7 However, a number of recent studies have demonstrated the presence of VDR in muscle with well‐validated antibodies for mouse7, 8 and human muscle.9 However, in uninjured normal muscle, the expression of VDR is very low, making its direct biological importance unclear.
We have shown that treatment of a cultured muscle cell line with 25‐hydroxyvitamin D or 1,25‐dihydroxyvitamin D caused important changes in the myotubes including increased myofibre diameter and changes in gene expression.
In order to examine the direct role of vitamin D in myocytes, mice with inactivation of VDR in myocytes (mVDR) were generated and compared with floxed littermate controls. Analysis of mVDR mice was performed including phenotype analysis of body composition, muscle tissue histology, and expression of putative candidate genes downstream of VDR signalling.
Methods
Mice
Mice with floxed VDR genes were created as previously described and were kindly supplied by Professor Geert Carmeliet.10 Floxed VDR mice were bred with mice expressing Cre‐recombinase under control of the human skeletal actin (HSA) promoter. HSA‐Cre is highly expressed in myocytes and is not expressed in cardiac myocytes.11 The resulting mice have deletion of VDR in skeletal myocytes. Confirmation of homozygosity for VDR‐flox was done every 4–6 generations. Mouse genotypes were classified based on presence or absence of Cre as mVDR and floxed control respectively as we have previously reported. Deletion efficacy was assessed by measuring expression of Vdr mRNA in whole muscles, as shown in Figure 1.
Figure 1.
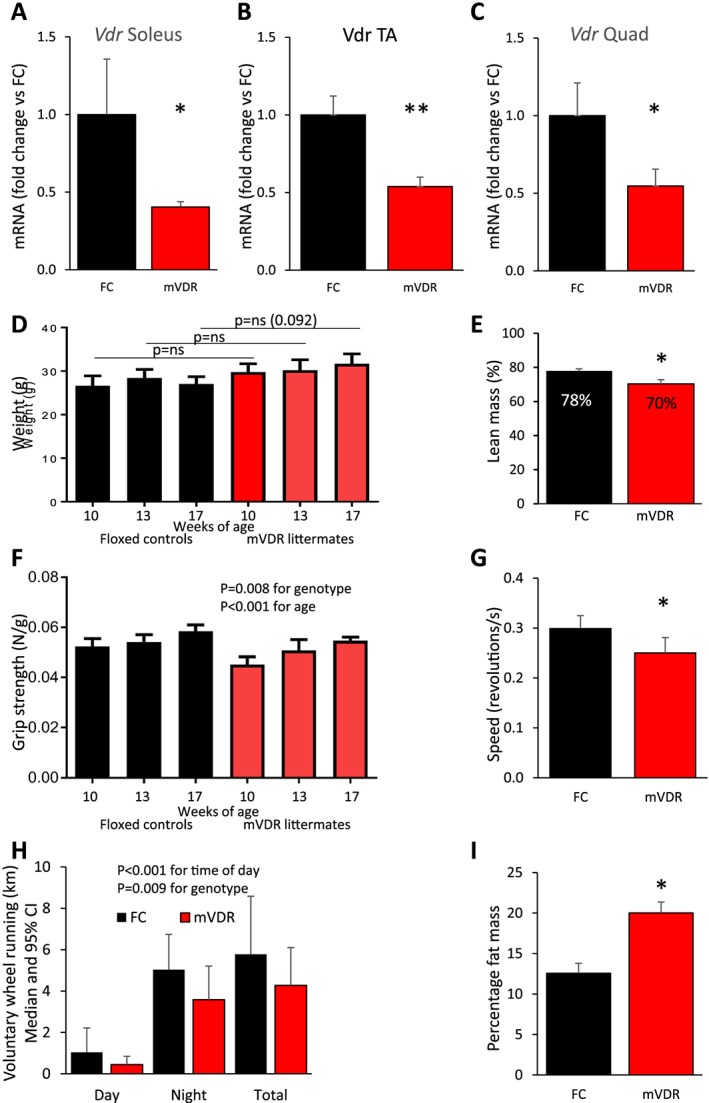
Phenotype of mVDR mice mRNA for Vdr in six mice per group at 13 weeks in (A) soleus muscle (B) tibialis anterior, and (C) quadriceps. (D) Mouse body weight (N = 6–16); (E) lean mass measured by DEXA at 13 weeks; N = 5–6; (F) grip strength (N = 6–16); (G) wheel‐running speed; and (H) running distance at 13 weeks of age (N = 7–8, median, and 95% CI). (I) Fat mass in mice at 13 weeks of age, measured by DEXA (N = 5–6). FC, floxed control; mVDR, myocytes vitamin D receptor null. * = P < 0.05, ** = P < 0.01.
Mice were bred at Australian Bioresources (Mossvale, Australia), and studies were conducted in the Biological Services Facility at the Westmead Institute for Medical Research. Male mice were used for all studies. Mice were fed a standard chow diet and food and water were freely available. The rooms have a standard 12 h light and 12 h dark cycle and the temperature is maintained at 22°C. Studies were approved by the WSLHD Animal Ethics Committee.
DEXA
Dual X‐ray absorptiometry was performed using a Lunar PIXImus small animal scanner (GE Lunar) to assess body composition as previously reported.12 During the scans, animals were kept anaesthetized using inhaled isoflurane.
Grip strength
Grip was measured using a grip strength metre from Columbus Instruments, Ohio, USA, as we have previously reported.1 Results are expressed as Newtons/gram of mouse weight. At the ages indicated in Figure 1, mice were lifted by the base of the tail and placed so that their front paws gripped the trapeze with their body horizontal. Each mouse was tested 15 times in sets of three with a short rest between each set.
Wheel running
Mice were placed in individual cages with running wheels (Promethion) that measured wheel use including distance travelled, speed, and proportion of time in the wheel. Mice were allowed to acclimatize to the cages for 24 h and then running distance was measured over the following 24 h. Running speed was measured in revolutions per second, and percentage of time running was measured each hour.
Tissue collection and histology
At study completion, mice were anaesthetized with a cocktail of ketamine and xylazine. Blood was collected by cardiac puncture and then the great vessels were severed while the mouse was still deeply anaesthetized. Muscles were dissected and weighed. Samples for RNA/western blotting were snap frozen in liquid nitrogen. Samples intended for histology were collected in OCT for frozen sections or in 10% paraformaldehyde for paraffin sections.
Haematoxylin and eosin (H&E), succinate dehydrogenase (SDH) and Sirius red stains were performed as previously reported13, 14 on paraffin sections. Fibre counting at the quadriceps midpoint used the whole muscle section, stained with H&E, and the whole quadriceps section was scanned for counting using a Nanozoomer.
Content of VDR protein in muscle was examined using paraffin‐embedded quadriceps sections from 13‐week‐old mice. Antigen retrieval was carried out using pH 9 buffer from Dako. Blocking was with Phosphate buffered saline (PBS) and 2% Bovine serum albumin (BSA). Primary antibody was the well‐validated D‐6 antibody (Santa Cruz, sc‐13133 at 1:50 dilution in PBS with 0.2% BSA).7, 8 Washes were carried out with the diluent (PBS with 0.2% BSA). Secondary antibody was Cy3 (Jackson ImmunoResearch, 711‐166‐152, used at 1:250 dilution). Pictures were captured with an Olympus VS120 slide scanner.
Apoptosis was assessed in muscle using cleaved caspase 3 staining as previously reported,13, 15 with the same Cy3 secondary antibody and images were acquired with the Olympus VS120 slide scanner.
Western blot
A total of 40 mg of snap‐frozen quadriceps muscle was homogenized in ice‐cold RIPA buffer [65 mM Tris·HCl, 150 mM NaCl, 5 mM EDTA, 1% NP‐40 (v/v), 0.5% sodium deoxycholate (w/v), 0.1% SDS (w/v), and 10% glycerol (v/v), (pH 7.4) containing 1 mg/L aprotinin, 1 mg/L leupeptin, 10 mmol/L NaF, 1 mmol/L Na3VO4, and 1 mmol/L PMSF]. Cleared lysates (50 μg per lane) were electrophoresed in 12% polyacrylamide gels and transferred to PVDF membranes (Thermo Scientific, #88518). Total OXPHOS Rodent WB Antibody Cocktail (Abcam, ab110413) was used to measure components of the mitochondrial oxidative phosphorylation pathway. Membranes were washed and probed with an appropriate horse‐radish peroxidase (HRP)‐conjugated secondary antibody (Cell Signaling), and proteins were visualized using Super Signal West Pico Chemiluminscent Substrate (Thermo) and a ChemiDoc Imaging System (Bio‐Rad). Densitometry was performed using ImageJ software (NIH freeware).
Fibre typing
Fibre typing was performed as previously described.16, 17, 18 Briefly, serial transverse 8 μm cryosections of mouse quadriceps muscle were cut onto slides and blocked with 2% BSA for 10 min, followed by further blocking with AffiniPure Fab fragment goat anti‐mouse IgG (Jackson ImmunoResearch Laboratories Inc.) for 1 h at room temperature to prevent cross reactivity with endogenous mouse antibodies. Sections were then incubated with primary antibodies to either slow myosin type I (MAB1628; Chemicon, 1:300), myosin heavy chain IIA (SC71; DSHB, University of Iowa, neat), or myosin heavy chain IIB (BF‐F3, DSHB, University of Iowa, USA, neat) overnight at 4°C. The next day, sections were washed three times with PBS, re‐blocked with 2% BSA for 10 min, and incubated with either Alexa555 goat anti‐mouse IgG (for MAB1628 and SC71, A21424, Molecular Probes, 1:300) or Alexa555 goat anti‐mouse IgM (for BF‐F3, A‐21426, Molecular Probes, 1:300) for 2 h at room temperature protected from light. Sections were then washed and fixed with 3% paraformaldehyde (PFA) for 10 min. To label membranes, sections were incubated with wheat germ agglutinin‐Alexa488 (ThermoFisher, 5 μg/mL in HBSS) for 10 min. Finally, sections were washed, incubated with DAPI (1 μg/Ml) for 5 min at room temperature, washed again, and mounted with coverslips using Immu‐Mount mounting reagent (Thermo Scientific). Images were captured on a Leica SP5 scanning confocal microscope with a 20×/0.7NA objective.
Real‐time PCR
Real‐time PCR was performed as previously described.12 RNA was extracted from muscle using Qiagen RNEasy kits. cDNA was synthesized using the Invitrogen kit, and real‐time PCR was performed using primers as shown in Table 1 and SybrGreen. Cyclophilin was used as the housekeeping gene, and it did not differ between groups (raw cross threshold (CT) values are shown in the Results section). The data are expressed as proportion of the value in floxed controls.13, 19, 20
Table 1.
Sequences of real‐time PCR primers used
| Genes | Forward primer | Reverse primer |
|---|---|---|
| Cyclophilin | 5′‐TGGACCAAACACAAACGGTTCC‐3′ | 5′‐ACATTGCGAGCAGATGGGGTAG‐3′ |
| Calbindin | 5′‐GCCAGCCAATAGAGTTGCTC‐3′ | 5′‐TTCCTCGCAGGACTTCAGTT‐3′ |
| Cdk2 | 5′‐AAATTCATGGATGCCTCTGC‐3′ | 5′‐ACAGGGACTCCAAAGGCTCT‐3′ |
| Cdk4 | 5′‐ACTCTGAAGCCGACCAGTTG‐3′ | 5′‐CCAGACTCCTCCATCTCTGG‐3′ |
| CyclinD1 | 5′‐AGTGCGTGCAGAAGGAGATT‐3′ | 5′‐CACAACTTCTCGGCAGTCAA‐3′ |
| CyclinD2 | 5′‐TCGATGATTGCAACTGGAAG‐3′ | 5′‐ATGCTGCTCTTGACGGAACT‐3′ |
| CyclinD3 | 5′‐CGCCCCTGACTATTGAGAAG‐3′ | 5′‐GTCTGGGCATGCTTTTTGAC‐3′ |
| Cyp24a1 | 5′‐CCCTTCTGCAAGAAAACTGC‐3′ | 5′‐CTCTTGAGGGCTCTGATTGG‐3′ |
| Mafbx | 5′‐CTCTGCTGTGAGTGCCACAT‐3′ | 5′‐CAATGAGCCTGGGTACCACT‐3′ |
| Murf1 | 5′‐TGGAAACGCTATGGAGAACC‐3′ | 5′‐AACGACCTCCAGACATGGAC‐3′ |
| Myf5 | 5′‐AGGAAAAGAAGCCCTGAAGC‐3′ | 5′‐GCAAAAAGAACAGGCAGAGG‐3′ |
| MyoD | 5′‐AGTGAATGAGGCCTTCGAGA‐3′ | 5′‐GCATCTGAGTCGCCACTGTA‐3′ |
| Myogenin | 5′‐CCTTGCTCAGCTCCCTCA‐3′ | 5′‐TGGGAGTTGCATTCACTGG‐3′ |
| Myostatin | 5′‐CTGTAACCTTCCCAGGACCA‐3′ | 5′‐TCTTTTGGGTGCGATAATCC‐3′ |
| p19 | 5′‐TCCATTGAAGAAGGGAGTGG‐3′ | 5′‐ACCGTTTAGATGGCTGTTGC‐3′ |
| p21 | 5′‐GCCTTAGCCCTCACTCTGTG‐3′ | 5′‐AGGGCCCTACCGTCCTACTA‐3′ |
| p27 | 5′‐CAGAATCATAAGCCCCTGGA‐3′ | 5′‐TCTGACGAGTCAGGCATTTG‐3′ |
| Serca2a | 5′‐GATCCTCTACGTGGAACCTTTG‐3′ | 5′‐GGTAGATGTGTTGCTAACAACG‐3′ |
| Serca2b | 5′‐GATCCTCTACGTGGAACCTTTG‐3′ | 5′‐CCACAGGGAGCAGGAAGAT‐3′ |
| Serca3 | 5′‐GCATTTTCTTATCCTCCTGGTG‐3′ | 5′‐TCTGCTCCCAGGATTTACTTC‐3′ |
| TgfB1 | 5′‐TTTGGAGCCTGGACACACAGTACA‐3′ | 5′‐TGTGTTGGTTGTAGAGGGCAAGGA‐3′ |
Statistics
Unless otherwise specified, unpaired Student's t‐tests were performed to assess significance. A P‐value of <0.05 was considered signficiant. Statistical analysis was performed with either Microsoft Excel (Student's t‐tests) or Prism Graphpad (version 6), which was used for all other tests. Unless otherwise described, data are presented as means ± standard error.
Results
Generation of myocyte‐specific vitamin D receptor mice
The mVDR strain was generated by crossing HSA‐Cre and Vdr flox/flox strains over two generations of breeding. The resultant mice were viable and showed no changes in gross morphology. Expression of Vdr mRNA was significantly decreased in the soleus, tibialis anterior, and quadriceps in mVDR mice compared with littermate controls (Figure 1A–C). Whole muscle contains a range of other cell types that would be expected to continue to express normal Vdr so a ~50% decrease is appropriate.
Phenotype and muscle function
Whole‐body VDR‐null mice have reduced body size. In contrast, the mVDR mice showed no decrease in body weight and at older ages showed a trend to increased weight (Figure 1D). Analysis of body composition by DEXA at 3 months of age (13 weeks) revealed a decreased percentage of lean mass, at 70.3% vs. 77.9%, as shown in Figure 1E.
On grip strength testing, mVDR mice had a lower grip strength at each of the ages tested from 10 to 17 weeks. The decrease was 7–16% in magnitude (Figure 1F). Mice were placed in cages with voluntary running wheels. Per cent of time running was not significantly different in mVDR mice (daytime 13.4% vs. 9.7% in mVDR and night‐time 28.2% vs. 25.4% in mVDR). However, running speed was decreased in mVDR mice (Figure 1G), and total running distance was decreased by 22% in mVDR mice (Figure 1H, P = 0.009). Fat mass was increased in mVDR mice at 20% of body weight compared with 13% in controls (Figure 1I).
At sacrifice, muscle wet weight was significantly decreased for mVDR quadriceps, tibialis anterior, extensor digitorum longus, and flexor digitorum longus (Figure 2A). Gastrocnemius showed a trend towards a decreased size, and other muscles were non‐significantly lighter in mVDR mice.
Figure 2.
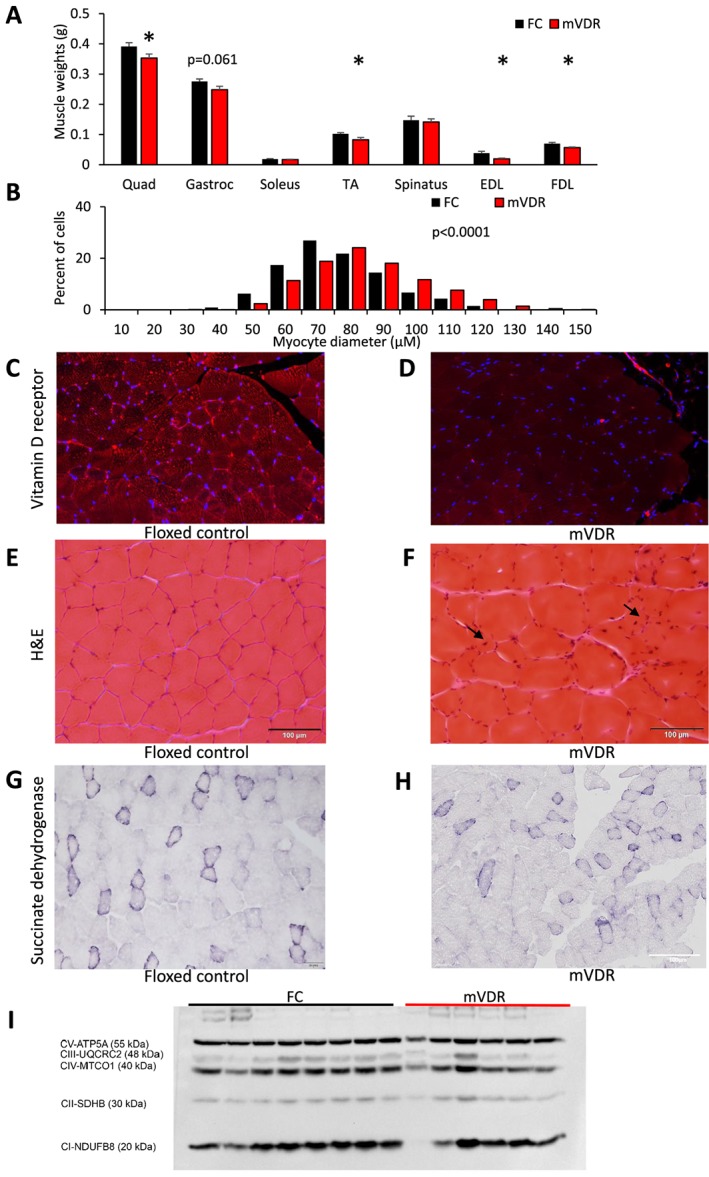
Muscle weights and fibre analysis (A) wet weight of muscles at sacrifice in 8–10 male mice at 13 weeks of age. EDL, extensor digitorum longus; FDL, flexor digitorum longus; gastroc, gastrocnemius; TA, tibialis anterior; Quad, quadriceps. (B) Myocyte diameter was measured in quadriceps in at least 900 fibres per genotype. (C) Vitamin D receptor immunostaining of quadriceps in floxed control and (D) mVDR mice. (E) Haematoxylin and eosin staining of quadriceps in floxed control and (F) mVDR mice. (G) Succinate dehydrogenase staining (SDH) of quadriceps in floxed control and (H) mVDR mice. (I) Western blotting of quadriceps muscle probed with ‘mitomix' antibody showing mitochondrial subunits as labelled. FC, floxed control; mVDR, myocytes vitamin D receptor null. * = P < 0.05.
Muscle fibre analysis
We have previously reported smaller myocyte diameter in mice with whole‐body VDR deletion.1 It was therefore surprising to find that myocytes were slightly larger in mVDR mice than in their control littermates (Figure 2B). There was low but detectable staining for VDR in floxed control muscle (quadriceps, Figure 2C), and less VDR staining in mVDR mice (Figure 2D). H&E staining is shown in Figure 2E (floxed control) and 2f (mVDR). As well as the increase in myofibre size, H&E staining of the mVDR muscle showed more cells with angular fibres and some centralized nuclei suggesting ongoing regeneration (black arrows). In combination with the smaller muscle weights, the increased muscle fibre size suggests fewer fibres per muscle, and this was seen on counting fibres; floxed controls had a median of 2525 fibres in a cross section at mid‐level of quadriceps compared with 1412 in mVDR mice (P < 0.05). The full cross section of the quadriceps was examined.
No clear differences in muscle metabolism was seen when fibre type was examined by SDH staining (Figure 2G and H). Similarly, western blotting did not indicate any significant differences in the mitochondrial subunits, including SDH which is complex II (Figure 2I). Formal fibre typing of quadriceps muscle was examined. Type 2B fibres were the most common in both genotypes, with similar, low proportions of other fibre types seen (Type 1, Type 2A, and Type 2X, Figure 3). This suggests that neither a shift in fibre type nor gross mitochondrial abnormalities are responsible for the defects in mass or muscle function in mVDR mice. We have previously reported increased fibrosis in liver of mice lacking VDR6 so we examined muscle using Sirius red staining. There were no obvious differences between floxed control and mVDR mice (Figure 3B). Again, despite overall larger fibres, there are more small, angular fibres present in the mVDR slides (black arrows).
Figure 3.
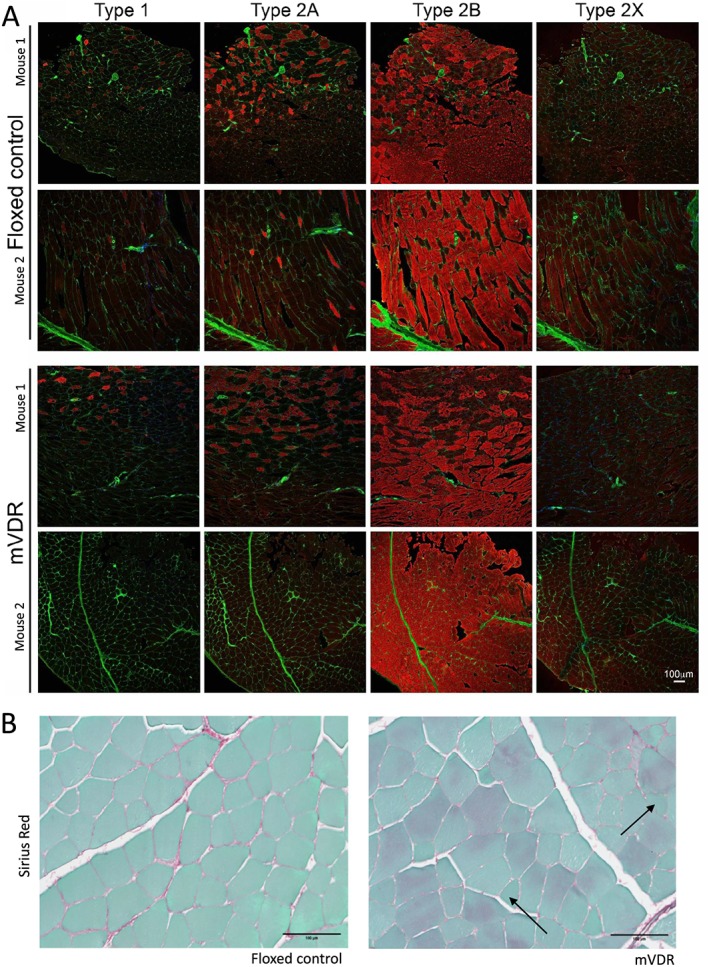
Fibre typing of floxed control and mVDR mice (A) representative images are shown for two mice per genotype. Red colour in each image is myosin, antibody of the type indicated at the top of the column. Green is wheat germ agglutinin. All images are taken at the same magnification (see scale bar at bottom right). (B) Representative images of Sirius red histology from floxed control and myocyte vitamin D receptor‐null (mVDR) mice. Collagen stains red.
Apoptosis is a potential cause of smaller muscles. We examined sections of quadriceps in floxed control and mVDR mice. In one mVDR mouse, there was a block of myocytes that had condensed cytoplasm that were strongly positive for cleaved caspase 3 (Figure 4A), indicating apoptosis. However, this was not seen in any other mVDR or any floxed control (Figure 4B) mice so does not seem to be an active process in the mice, at least at 13 weeks of age.
Figure 4.
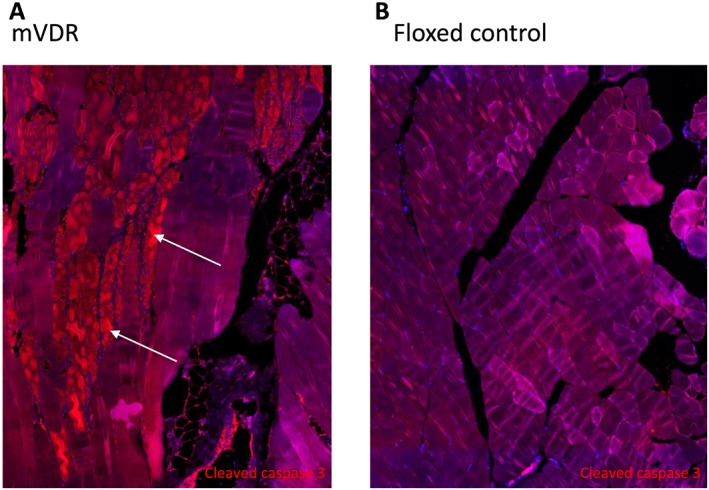
Cleaved caspase 3 staining in quadriceps from (A) mVDR and (B) floxed control mice at 13 weeks of age. Cleaved caspase 3 is in red. White arrows indicate apoptotic myofibres in the mVDR sample. mVDR, myocyte vitamin D receptor.
Gene expression changes
Vitamin D receptor is a transcription factor, and so we examined changes in gene expression by real‐time PCR. We focused on genes that we have previously reported to be altered in either whole‐body VDR deletion or vitamin D deficiency1, 8, 21 as the mVDR model allows examination of whether these changes in muscle are direct via myocyte VDR or indirect via other mechanisms in those whole‐body models.
Raw expression of the housekeeping gene Cyclophilin did not differ between floxed control and mVDR mice, with Cq values of 21.01 ± 0.10 vs. 20.85 ± 0.11 (P > 0.2). Results in the figures show gene expression corrected for Cyclophilin. Cyp24a1 is part of the vitamin D signalling pathway. Cyp24a1 expression was decreased in mVDR muscles (Figure 5).
Figure 5.
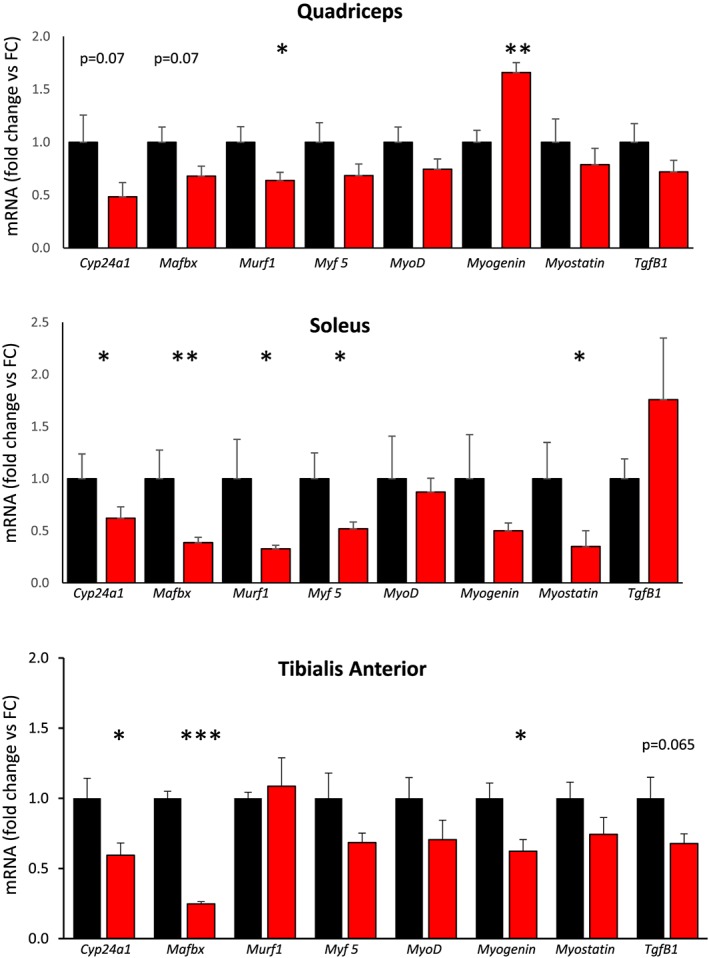
Real‐time PCR gene expression of myogenic genes and transforming growth factor β (Tgfβ1). FC, floxed control; mVDR, myocytes vitamin D receptor null. N = 6–12 per group.* = P < 0.05, ** = P < 0.01.
We then measured mRNA expression of a range of myogenic factors and transforming growth factor β1 (TgfB1), many of which are altered in muscle from whole‐body VDR knockout mice (Figure 5). There was decreased expression of Mafbx and Murf1. This is in contrast to the whole‐body VDR mice, which show increased expression. Decreases in Mafbx and Murf1 could contribute to the lesser reduction in muscle size in mVDR mice in comparison with whole‐body VDR knockout (VDRKO) mice. Another potential contributor to the smaller decrease in muscle mass in mVDR mice is the trend towards a decrease seen in Myostatin expression, which is increased in whole‐body VDRKO mice. This was statistically significant in soleus. Myostatin inhibits muscle hypertrophy and hyperplasia, so lower levels permit increased muscle growth.22
Several genes that are important in cell cycle progression showed decreased expression in quadriceps from mVDR mice.23, 24 These included cyclins D1, D2, and D3 and cyclin‐dependent kinases (Cdk)2 and 4 (Figure 6A). Cyclins D1, D2, and D3 and Cdk2 and Cdk4 all showed significantly decreased expression in soleus muscle (Figure 6B). Expression of the cell cycle inhibitors p19, p21, and p27 were all unchanged (Figure 6C).
Figure 6.
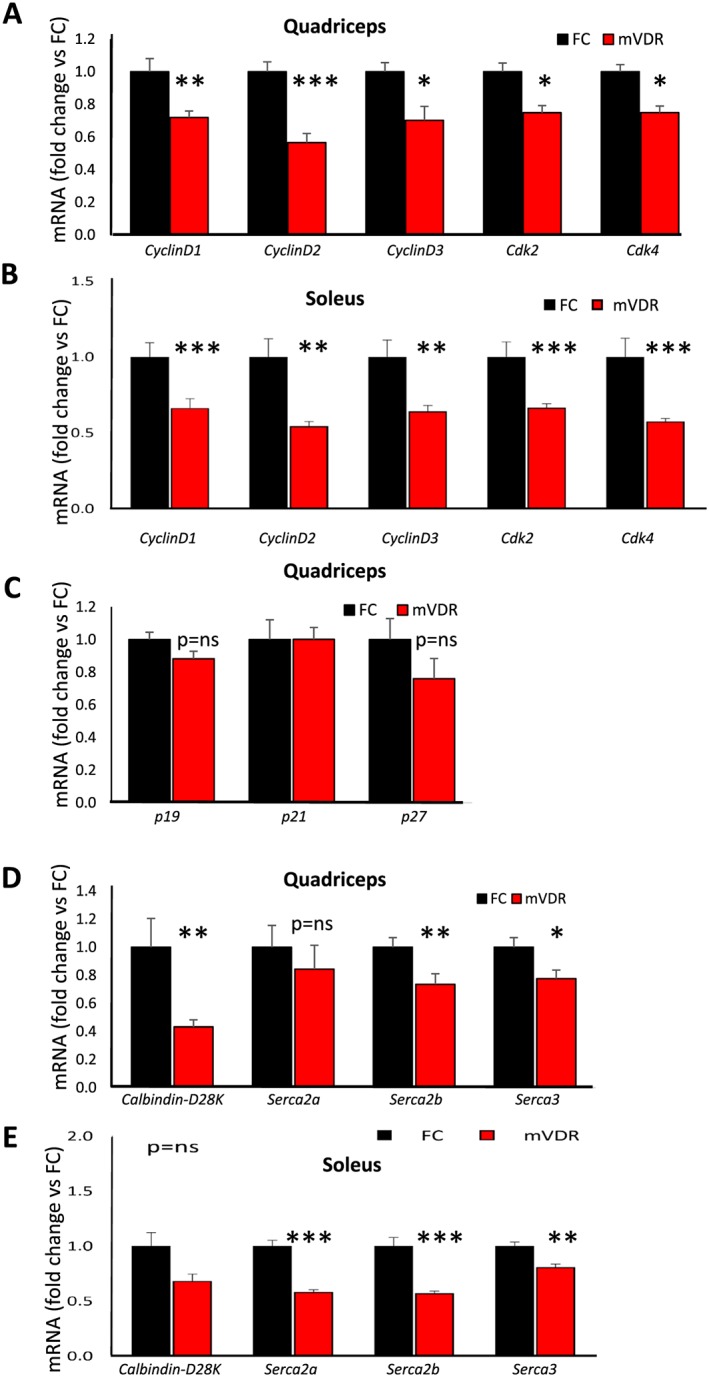
Real‐time PCR gene expression in quadriceps of cell cycle and calcium handling genes Cdk, cyclin‐dependent kinase; Serca, sarcoplasmic/endoplasmic reticulum calcium ATPase. FC, floxed control; mVDR, myocytes vitamin D receptor null. N = 6–12 per group. * = P < 0.05, ** = P < 0.01.
The function of mVDR muscle was impaired, and vitamin D in other tissues regulates a number of cellular calcium handling genes, so we assessed expression of intracellular calcium handling genes. There was no significant change in Serca2a (sarcoplasmic/endoplasmic reticulum calcium ATPase) in quadriceps. However, expression of Calbindin, Serca2b, and Serca3 were all lower in mVDR quadriceps (Figure 6D). In soleus, there were significant decreases in expression of each of the Sercas tested: Serca2a, Serca2b, and Serca3 (Figure 6E).
Conclusions
Loss of muscle mass and function, most commonly associated with age, is called sarcopenia. It results in greater risks of falls, fracture, disability, and death.25, 26, 27, 28 Vitamin D deficiency is associated with sarcopenia and lower muscle mass in most studies in older people.29, 30, 31, 32, 33, 34, 35, 36 However, a direct role in muscle has not been able to be shown in people.
The results presented in this study confirm that Vdr is expressed in myocytes in mice. We now demonstrate that myocyte VDR plays a significant role; deletion affects muscle function and gene expression. mVDR mice have significantly decreased grip strength, which persists to at least 4 months of age. It would be interesting to age these mice and determine whether they develop more severe changes with middle and older age. As well as decreased lean mass, mVDR mice had a significant increase in fat mass. There was a significant decrease in voluntary exercise in the mice, and a more than 20% decrease in voluntary exercise would be consistent with the increase in fat mass seen.
In people with colorectal cancer, physical activity measured by accelerometer correlated with serum vitamin D levels.37 This association was also seen in the NHANES study.38 While increased exercise is likely in many people to be associated with increased sun exposure, it is interesting to hypothesize that lower vitamin D may associate, as in mVDR mice, with lower voluntary exercise.
Interestingly, the effects of myocyte VDR deletion showed some important differences from those seen with whole‐body VDR deletion.1 The reduction in size of individual muscles in mVDR mice is significant and of clinically relevant magnitude, but it appears to be less than the effect seen in whole‐body VDR‐null mice. This suggests that VDR in other cells types may play a role in muscle development in early life and in individual myofibre size and that these may partially negate the atrophic effects of VDR deletion in myocytes. As there was only a 50% decrease in Vdr mRNA in whole muscle, there may also be a contribution of incomplete deletion to the milder phenotype.
Murf1 and Mafbx are ubiquitin ligases that regulate proteosomal degradation. In muscle, they are associated with myonuclear apoptosis and reduced fibre size.39, 40, 41 MAFbx additionally regulates protein synthesis and muscle regeneration via targeting of regulators including MyoD and eIF3F.41, 42 Their expression is increased in whole‐body VDR‐null mice, in which there is decreased fibre size. There was decreased expression of Murf1 in quadriceps and soleus, and significantly decreased expression of Mafbx in two muscles with a trend towards a decrease in the third (P = 0.07). Decreases in Murf1 and Mafbx in mVDR mice would support better maintenance of fibre size with inhibition of the ubiquitin–proteosome pathway. Interestingly, these were also decreased in human myotubes treated with vitamin D,43 suggesting the possibility that in mVDR mice, the decrease could be mediated by effects of another cell type with intact vitamin D signalling.
Another potential contributor to the smaller decrease in muscle mass in mVDR mice is Myostatin, which is increased in whole‐body VDRKO mice. Myostatin inhibits muscle hypertrophy and hyperplasia, so lower levels permit increased muscle growth.22 In contrast to whole‐body VDR‐null mice, myostatin was significantly decreased in soleus, and it is interesting to note that there was no decrease in weight of this muscle in mVDR mice.
Because the whole muscles are lighter, but individual fibres are not smaller, it follows that there must be fewer myofibres in mVDR mice, and this was confirmed in quadriceps by counting total fibres at the muscle midpoint for the whole muscle. The decreased expression of cyclin D genes 1–3 and decreased expression of Cdk2 and Cdk4 could also contribute to decreased myofibre number as they would decrease cell cycle progression. Decreased myofibre number and muscle mass would both contribute to decreased strength. Impaired muscle function was shown by two modalities: grip strength and running speed. These techniques test different muscles, and acute vs. more chronic effort; both were significantly affected.
As discussed earlier, calcium handling is important for normal muscle function44 and calcium handling in the whole body is regulated by vitamin D signalling.45, 46 We examined expression of intracellular calcium handling genes and found significant changes in expression of Serca2b and 3 in quadriceps. These sarcoplasmic/endoplasmic reticulum calcium ATPase proteins are calcium pumps in the ER and muscle sarcoplasmic reticulum membrane that help to concentrate calcium in the lumen of the ER.47 This permits muscle relaxation after contraction. Calbindin acts both as a calcium buffer and in some tissues as a calcium sensor. Its buffering function would lower the available cytosolic calcium and also help with normal muscle relaxation. It is known to be regulated by vitamin D signalling in other tissues including kidney.48 It is therefore possible that reduced grip strength in these mice was the cause not only of reduced muscle mass but also alterations in contraction–relaxation via alterations in the calcium handling apparatus.
We therefore report significant phenotypic changes in muscle following tissue‐specific deletion of VDR. VDR is present in muscle, deletion in myocytes impairs muscle function, muscle size is reduced, but myofibres are slightly larger. The larger myofibres contrast to the findings in whole‐body knockout VDR mice. This suggests that as well as the role for myocyte VDR in muscle size and function, vitamin D signalling in other tissues or cell types plays a role in initial muscle development.
The results suggest that maintenance of normal vitamin D signalling is important for preservation of muscle bulk and function. These findings also suggest that therapies targeting VDR could impact skeletal muscle mass and function and may present a novel strategy in addressing or preventing age‐related sarcopenia and other disorders of muscle function.
Conflict of interest
The authors declare that they have no relevant conflicts of interest.
Funding
This work was supported by a Sydney Medical School grant (University of Sydney) and a grant from the Staff Specialist Trust Fund (Westmead Hospital).
Acknowledgements
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017.49
Girgis C. M., Cha K. M., So B., Tsang M., Chen J., Houweling P. J., Schindeler A., Stokes R., Swarbrick M. M., Evesson F. J., Cooper S. T., and Gunton J. E. (2019) Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function, Journal of Cachexia, Sarcopenia and Muscle, 10, 1228–1240. 10.1002/jcsm.12460.
References
- 1. Girgis CM, Cha KM, Houweling PJ, Rao R, Mokbel N, Lin M, et al. Vitamin D receptor ablation and vitamin D deficiency result in reduced grip strength, altered muscle fibers, and increased myostatin in mice. Calcif Tissue Int 2015;97:602–610. [DOI] [PubMed] [Google Scholar]
- 2. Girgis CM, Clifton‐Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function and metabolism. Endocr Rev 2013;34:33–83. [DOI] [PubMed] [Google Scholar]
- 3. Girgis CM, Clifton‐Bligh RJ, Turner N, Lau SL, Gunton JE. Effects of vitamin D in skeletal muscle: falls, strength, athletic performance and insulin sensitivity. Clin Endocrinol (Oxf) 2014;80:169–181. [DOI] [PubMed] [Google Scholar]
- 4. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouillon R, Verstuyf A. Vitamin D, mitochondria, and muscle. J Clin Endocrinol Metab 2013;98:961–963. [DOI] [PubMed] [Google Scholar]
- 6. Ding N, Ruth TY, Subramaniam N, Sherman MH, Wilson C, Rao R, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 2013;153:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, DeLuca HF. Is the vitamin D receptor found in muscle? Endocrinology 2011;152:354–363. [DOI] [PubMed] [Google Scholar]
- 8. Girgis CM, Mokbel N, Minn Cha K, Houweling PJ, Abboud M, Fraser DR, et al. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25‐hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014;155:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pojednic RM, Ceglia L, Olsson K, Gustafsson T, Lichtenstein AH, Dawson‐Hughes B, et al. Effects of 1, 25‐dihydroxyvitamin D 3 and vitamin D 3 on the expression of the vitamin D receptor in human skeletal muscle cells. Calcif Tissue Int 2015;96:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, et al. Duodenal calcium absorption in vitamin D receptor–knockout mice: functional and molecular aspects. Proc Natl Acad Sci 2001;98:13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCarthy JJ, Srikuea R, Kirby TJ, Peterson CA, Esser KA. Inducible Cre transgenic mouse strain for skeletal muscle‐specific gene targeting. Skelet Muscle 2012;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lau SM, Lin S, Stokes RA, Cheng K, Baldock PA, Enriquez RF, et al. Synergistic effects of genetic beta cell dysfunction and maternal glucose intolerance on offspring metabolic phenotype in mice. Diabetologia 2011;54:910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stokes R, Cheng K, Swarbrick MM, Thomas H, Loudovaris T, Kay TW, et al. Transplantation sites for human and murine islets. Diabetologia 2017;60:1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Girgenrath S, Song K, Whittemore L‐A. Loss of myostatin expression alters fiber‐type distribution and expression of myosin heavy chain isoforms in slow‐ and fast‐type skeletal muscle. Muscle Nerve 2005;31:34–40. [DOI] [PubMed] [Google Scholar]
- 15. Stokes RA, Cheng K, Deters N, Lau SM, Hawthorne WJ, O'Connell PJ, et al. Hypoxia‐inducible factor 1α (HIF‐1α) potentiates β‐cell survival after islet transplantation of human and mouse islets. Cell Transplant 2013;22:253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hogarth MW, Garton FC, Houweling PJ, Tukiainen T, Lek M, Macarthur DG, et al. Analysis of the ACTN3 heterozygous genotype suggests that α‐actinin‐3 controls sarcomeric composition and muscle function in a dose‐dependent fashion. Hum Mol Genet 2015;25:866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hogarth MW, Houweling PJ, Thomas KC, Gordish‐Dressman H, Bello L, Vishwanathan V, et al. Evidence for ACTN3 as a genetic modifier of Duchenne muscular dystrophy. Nat Commun 2017;8:14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garton F, Seto JT, North KN, Yang N. Validation of an automated computational method for skeletal muscle fibre morphometry analysis. Neuromuscul Disord 2010;20:540–547. [DOI] [PubMed] [Google Scholar]
- 19. Lau SM, Cha KM, Karunatillake A, Stokes RA, Cheng K, McLean M, et al. Beta‐cell ARNT is required for normal glucose tolerance in murine pregnancy. PLoS ONE 2013;8:e77419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scott C, Cha K, Rao R, Liddle C, George J, Gunton JE. Hepatocyte‐specific deletion of ARNT (aryl hydrocarbon receptor nuclear translocator) results in altered fibrotic gene expression in the thioacetamide model of liver injury. PLoS ONE 2015;10:e0121650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Girgis CM, Clifton‐Bligh RJ, Mokbel N, Cheng K, Gunton JE. Vitamin D signaling regulates proliferation, differentiation and myotube size in C2C12 skeletal muscle cells. Endocrinology 2014;155:347–357. [DOI] [PubMed] [Google Scholar]
- 22. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self‐renewal. J Cell Biol 2003;162:1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Busk PK, Hinrichsen R, Bartkova J, Hansen AH, Christoffersen TE, Bartek J, et al. Cyclin D2 induces proliferation of cardiac myocytes and represses hypertrophy. Exp Cell Res 2005;304:149–161. [DOI] [PubMed] [Google Scholar]
- 24. Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, et al. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem 2003;278:8826–8836. [DOI] [PubMed] [Google Scholar]
- 25. Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 2006;41:1234–1238. [DOI] [PubMed] [Google Scholar]
- 26. Bunout D, de la Maza MP, Barrera G, Leiva L, Hirsch S. Association between sarcopenia and mortality in healthy older people. Australas J Ageing 2011;30:89–92. [DOI] [PubMed] [Google Scholar]
- 27. Filippin LI, Teixeira VN, da Silva MP, Miraglia F, da Silva FS. Sarcopenia: a predictor of mortality and the need for early diagnosis and intervention. Aging Clin Exp Res 2015;27(3):249–254. [DOI] [PubMed] [Google Scholar]
- 28. Girgis CM. Integrated therapies for osteoporosis and sarcopenia: from signaling pathways to clinical trials. Calcif Tissue Int 2015;96:243–255. [DOI] [PubMed] [Google Scholar]
- 29. Marantes I, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ 3rd, Amin S, et al. Is vitamin D a determinant of muscle mass and strength? J bone Miner Res off J Am Soc Bone Miner Res 2011;26:2860–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tieland M, Brouwer‐Brolsma EM, Nienaber‐Rousseau C, van Loon LJ, De Groot LC. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur J Clin Nutr 2013;67:1050–1055. [DOI] [PubMed] [Google Scholar]
- 31. Rousseau AF, Foidart‐Desalle M, Ledoux D, Remy C, Croisier JL, Damas P, et al. Effects of cholecalciferol supplementation and optimized calcium intakes on vitamin D status, muscle strength and bone health: a one‐year pilot randomized controlled trial in adults with severe burns. Burns 2015;41:317–325. [DOI] [PubMed] [Google Scholar]
- 32. Liu G, Lu L, Sun Q, Ye X, Sun L, Liu X, et al. Poor vitamin D status is prospectively associated with greater muscle mass loss in middle‐aged and elderly Chinese individuals. J Acad Nutr Diet 2014;114:1544–1551.e2. [DOI] [PubMed] [Google Scholar]
- 33. Sanders KM, Scott D, Ebeling PR. Vitamin D deficiency and its role in muscle‐bone interactions in the elderly. Curr Osteoporos Rep 2014;12:74–81. [DOI] [PubMed] [Google Scholar]
- 34. Lee SG, Lee YH, Kim KJ, Lee W, Kwon OH, Kim JH. Additive association of vitamin D insufficiency and sarcopenia with low femoral bone mineral density in noninstitutionalized elderly population: the Korea National Health and Nutrition Examination Surveys 2009‐2010. Osteoporos Int A J Established Result of Cooperation between Eur Found Osteoporos Natl Osteoporos Found USA 2013;24:2789–2799. [DOI] [PubMed] [Google Scholar]
- 35. Montero‐Odasso M, Duque G. Vitamin D in the aging musculoskeletal system: an authentic strength preserving hormone. Mol Aspects Med 2005;26:203–219. [DOI] [PubMed] [Google Scholar]
- 36. Dupuy C, Lauwers‐Cances V, van Kan GA, Gillette S, Schott AM, Beauchet O, et al. Dietary vitamin D intake and muscle mass in older women. Results from a cross‐sectional analysis of the EPIDOS study. J Nutr Health Aging 2013;17:119–124. [DOI] [PubMed] [Google Scholar]
- 37. Skender S, Bohm J, Schrotz‐King P, Chang‐Claude J, Siegel EM, Steindorf K, et al. Plasma 25‐hydroxyvitamin D(3) levels in colorectal cancer patients and associations with physical activity. Nutr Cancer 2017;69:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choi JE, Ainsworth BE. Associations of food consumption, serum vitamins and metabolic syndrome risk with physical activity level in middle‐aged adults: the National Health and Nutrition Examination Survey (NHANES) 2005‐2006. Public Health Nutr 2016;19:1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koyama S, Hata S, Witt CC, Ono Y, Lerche S, Ojima K, et al. Muscle RING‐finger protein‐1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol 2008;376:1224–1236. [DOI] [PubMed] [Google Scholar]
- 40. Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, et al. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone‐treated skeletal muscle. Cell Metab 2007;6:376–385. [DOI] [PubMed] [Google Scholar]
- 41. Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem 2005;280:2847–2856. [DOI] [PubMed] [Google Scholar]
- 42. Lagirand‐Cantaloube J, Cornille K, Csibi A, Batonnet‐Pichon S, Leibovitch MP, Leibovitch SA. Inhibition of atrogin‐1/MAFbx mediated MyoD proteolysis prevents skeletal muscle atrophy in vivo. PLoS ONE 2009;4:e4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hayakawa N, Fukumura J, Yasuno H, Fujimoto‐Ouchi K, Kitamura H. 1alpha,25(OH)2D3 downregulates gene expression levels of muscle ubiquitin ligases MAFbx and MuRF1 in human myotubes. Biomed Res 2015;36:71–80. [DOI] [PubMed] [Google Scholar]
- 44. Berchtold MW, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 2000;80:1215–1265. [DOI] [PubMed] [Google Scholar]
- 45. Schubert L, DeLuca HF. Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch Biochem Biophys 2010;500:157–161. [DOI] [PubMed] [Google Scholar]
- 46. Rodman JS, Baker T. Changes in the kinetics of muscle contraction in vitamin D‐depleted rats. Kidney Int 1978;13:189–193. [DOI] [PubMed] [Google Scholar]
- 47. Stammers AN, Susser SE, Hamm NC, Hlynsky MW, Kimber DE, Kehler DS, et al. The regulation of sarco (endo)plasmic reticulum calcium‐ATPases (SERCA). Can J Physiol Pharmacol 2015;93:843–854. [DOI] [PubMed] [Google Scholar]
- 48. Hemmingsen C. Regulation of renal calbindin‐D28K. Pharmacol Toxicol 2000;87:5–30. [PubMed] [Google Scholar]
- 49. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


