Abstract
STUDY QUESTION
What is good practice in ultrasound (US), and more specifically during the different stages of transvaginal oocyte retrieval, based on evidence in the literature and expert opinion on US practice in ART?
SUMMARY ANSWER
This document provides good practice recommendations covering technical aspects of US-guided transvaginal oocyte retrieval (oocyte pick up: OPU) formulated by a group of experts after considering the published data, and including the preparatory stage of OPU, the actual procedure and post-procedure care.
WHAT IS KNOWN ALREADY
US-guided transvaginal OPU is a widely performed procedure, but standards for best practice are not available.
STUDY DESIGN, SIZE, DURATION
A working group (WG) collaborated on writing recommendations on the practical aspects of transvaginal OPU. A literature search for evidence of the key aspects of the procedure was carried out. Selected papers (n = 190) relevant to the topic were analyzed by the WG.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The WG members considered the following key points in the papers: whether US practice standards were explained; to what extent the OPU technique was described and whether complications or incidents and how to prevent such events were reported. In the end, only 108 papers could be used to support the recommendations in this document, which focused on transvaginal OPU. Laparoscopic OPU, transabdominal OPU and OPU for IVM were outside the scope of the study.
MAIN RESULTS AND THE ROLE OF CHANCE
There was a scarcity of studies on the actual procedural OPU technique. The document presents general recommendations for transvaginal OPU, and specific recommendations for its different stages, including prior to, during and after the procedure. Most evidence focussed on comparing different equipment (needles) and on complications and risks, including the risk of infection. For these topics, the recommendations were largely based on the results of the studies. Recommendations are provided on equipment and materials, possible risks and complications, audit and training. One of the major research gaps was training and competence. This paper has also outlined a list of research priorities (including clarification on the value or full blood count, antibiotic prophylaxis and flushing, and the need for training and proficiency).
LIMITATIONS, REASONS FOR CAUTION
The recommendations of this paper were mostly based on clinical expertise, as at present, only a few clinical trials have focused on the oocyte retrieval techniques, and almost all available data are observational. In addition, studies focusing on OPU were heterogeneous with significant difference in techniques used, which made drafting conclusions and recommendations based on these studies even more challenging.
WIDER IMPLICATIONS OF THE FINDINGS
These recommendations complement previous guidelines on the management of good laboratory practice in ART. Some useful troubleshooting/checklist recommendations are given for easy implementation in clinical practice. These recommendations aim to contribute to the standardization of a rather common procedure that is still performed with great heterogeneity.
STUDY FUNDING/COMPETING INTEREST(S)
The meetings of the WG were funded by ESHRE. The other authors declare that they have no conflict of interest.
TRIAL REGISTRATION NUMBER
NA.
ESHRE Pages content is not externally peer reviewed. The manuscript has been approved by the Executive Committee of ESHRE.
Keywords: good practice, recommendations, ultrasound, oocyte retrieval, oocyte pick up, ART, guideline, needle, competence, quality
WHAT DOES THIS MEAN FOR PATIENTS?
Removal of eggs from the ovaries under US guidance (oocyte recovery) is a minor surgical procedure, which is commonly performed as a part of IVF, but there are few agreed standards for best practice. The current paper describes practical recommendations for oocyte recovery, so that clinicians can make sure that the procedure is done correctly and safely. Advice is provided on how best to provide care prior to, during and after the procedure, as well as on the most appropriate equipment and materials. The authors also consider possible risks, training needs and ways of checking the quality of care.
Introduction
The World Health Organization (WHO) estimated that 48.5 million couples worldwide are affected by infertility (Mascarenhas et al., 2012). During fertility treatment, women need an ultrasound (US) approach for both diagnostic and therapeutic procedures, which can be performed with either a transvaginal or a transabdominal approach (Lutz and Buscarini, 2013). Results of the images obtained during these scans are vital for the patient’s care, as they might impact on which treatment protocol the patient will follow and on the treatment outcome. This is why the operator’s findings and approach play an essential role in the treatment of the clinical problem.
During the early days of IVF, oocyte retrieval was systematically performed by laparoscopy. This required a surgical procedure, general anaesthesia and hospital admission. After the first reports on transvaginal oocyte retrieval in the early 1980s (Gleicher et al., 1983; Dellenbach et al., 1984; Schulman et al., 1985), oocyte retrieval is almost always performed transvaginally. The advantages of transvaginal oocyte retrieval, in comparison with the transabdominal or laparoscopic approach, include:
– better visualization and shorter distance of ovary from the transducer,
high recovery rate of good-quality oocytes with minimal discomfort for patients,
the use of local anaesthesia with sedation instead of general anaesthesia,
decreased risk of intestinal trauma,
it can be easily learned, especially by operators trained in US,
decreased costs for patients
and quick post-interventional recovery.
However, in some patients, transabdominal US facilitated access when the ovaries were transposed or enlarged above the pelvic brim. Transabdominal-guided oocyte retrieval continues to be used at some centres for rare patients who have ovaries inaccessible by transvaginal US.
Nowadays, transvaginal oocyte retrieval is a widely performed procedure, with a low complication rate (European IVF monitoring Consortium for the European Society of Human Reproduction and Embryology et al., 2017). In this paper, recommendations for different steps of transvaginal oocyte retrieval will be described. Laparoscopic oocyte retrieval, transabdominal oocyte retrieval and oocyte retrieval for IVM are outside the scope of this document.
The recommendations for good practice in this paper were based on evidence (where available) and experts’ opinions on US practice in ART. This paper is aimed to guide clinicians, especially in countries where there are no national guidelines, and it could have a significant impact on patients’ care and safety worldwide.
Materials and Methods
The current recommendations were written by a working group (WG) of experts on US, according to the methodology described in the manual for development of recommendations for good practice (Vermeulen et al., 2018).
The current document’s first draft was based on the results of the doctoral thesis of one of the authors (C.P.) (Panayotidis, 2017). C.P. conducted a systematic review of the literature and a Delphi survey of 15 experts reporting their opinions on current practice in US-guided oocyte retrieval. The Delphi method survey included 53 questions completed in three rounds and resulted in 32 standards of practice.
In addition to the results of the dissertation, a new literature search was conducted. Databases (PUBMED/Medline and the Cochrane Library) were searched from inception to 17 July 2018. Search terms focussed on US, oocyte retrieval/pick up, Doppler, sedation, anaesthesia, infection, antibiotics, hydrosalpinx and flushing, and included extended key words, commonly used synonyms and MESH terms. References were divided according to topic, and full texts were assessed (Supplementary Fig. S1). Where possible, references of papers providing indirect evidence or referrals to other guidelines were added, based on expert opinion.
The first draft of the paper, based on the dissertation, was presented and discussed by the WG during a teleconference in November 2017, after which WG members submitted their written comments and suggestions for improvement. A full-day consensus meeting was organized to discuss the paper further until consensus. The results of the literature search were included where relevant. The document was published on the ESHRE website for 4 weeks (between 25 March and 23 April 2019), and stakeholders were invited to submit their comments. After addressing all comments from the stakeholder review (report available on www.eshre.eu/guidelines), the document was finalized and approved by the ESHRE Executive Committee.
The paper outlines recommendations for good practice in oocyte retrieval, with addition of further basic information, checklists and troubleshooting in Figs 1–6. For ease of use of the recommendations, a list of abbreviations is provided in Table I.
Figure 1.

Basic principles of the OPU technique. OPU: oocyte pick up
Figure 6.

Recommendations for future research in OPU. FBC: full blood count.
Table I.
List of abbreviations used in the text
| 3D | Three-dimensional |
| AIUM | American Institute of Ultrasound in Medicine |
| ART | Assisted reproductive technologies |
| CRP | C-reactive protein |
| CT | Computed tomography |
| ECG | Electrocardiogram |
| FBC | Full blood count |
| fx | For example |
| GnRH | Gonadotrophin-releasing hormone |
| Hb | Haemoglobin level |
| hCG | Human chorionic gonadotrophin |
| HIV | Human immunodeficiency virus |
| IV | Intravenous |
| IVF | In vitro fertilization |
| IVM | In vitro maturation |
| LH | Luteinizing hormone |
| OHSS | Ovarian hyper-stimulation syndrome |
| OPU | Oocyte pick up |
| PACS | Picture archiving and communication system |
| PCOS | Polycystic ovary syndrome |
| PCSA | Patient controlled sedation/analgesia |
| PID | Pelvic inflammatory disease |
| RCOG | Royal College of Obstetricians and Gynaecologists |
| TVOR | Transvaginal oocyte retrieval |
| TV-US | Transvaginal ultrasound |
| US | Ultrasound |
| VA | Verbal anaesthesia |
| WG | Working group |
| WHO | World Health Organization |
Recommendations
OPU is defined as follows: OPU is an US-guided technique in which oocytes are aspirated using a needle connected to a suction pump.
There are different terms and abbreviations used in clinical practice and research to describe the collection of oocytes in ART, including (transvaginal) oocyte retrieval, egg retrieval, oocyte collection and follicle aspiration. The WG suggests for further use of the term ‘OPU’, to increase consistency, facilitate literature searches and further assess best clinical practice in performing OPU.
The current paper outlines recommendations for good practice in OPU and is sub-divided into the following sections:
– Prior to OPU
Equipment and consumables
OPU preparation
OPU procedure
Post-procedure care
Associated pathologies and cautions during OPU
Complications and risks
Future developments
Training and competence
Quality assurance and performance.
Some general aspects of the OPU technique are outlined in Fig. 1.
Prior to OPU
Pelvic US
– An US evaluation should be performed before starting an ART treatment: to decide the ovarian stimulation protocol; to determine whether there is any anatomical abnormality or a malposition of the ovaries (Grimbizis et al., 2016) and to assess ovarian placement and ovarian/follicular accessibility after previous surgery (gynaecological surgery for myomas, endometriomas, adhesions). A basic diagnostic US examination also allows for the detection of recent lesions, such as endometrial abnormalities or ovarian cysts, in a timely manner. In addition, such transvaginal diagnostic US is of value to visualize not only the ovaries, but also the uterus and to check for potential difficulties during OPU. The accessibility of the ovaries and follicles and any potential complications or difficulties of the OPU should be clearly documented in the patient case notes, for the team to be prepared and for the patient to be counselled accordingly.
Pre-OPU 3D US and Doppler investigation are considered helpful for the operator to become familiar with the anatomy of the patient and to prevent (vascular) complications.
The time frame to perform the US is at the discretion of the clinician. The American Institute of Ultrasound in Medicine (AIUM) guidelines suggest a comprehensive sonographic evaluation of the pelvis within 4–6 months from the start of ovarian stimulation (American Institute of Ultrasound in Medicine, 2017). This time frame should be shortened in cases of significant conditions (endometriosis, surgery, specific symptoms). The WG recommends a baseline US closer to the OPU to highlight any difficulties or reconfirm previous findings, for example, shortly before starting the ovarian stimulation with gonadotrophins.
Vaginal infection screening
– Screening for vaginal infection (by taking a vaginal sample for bacteriological examination) should be performed during diagnostic work-up and can be required based on local guidelines and regulations. However, the incidence of vaginal infections after OPU is low, and several aspects of vaginal screening and treatment remain unclear in asymptomatic patients without a history of pelvic infections, including the relevance of the screening tests, the implications of treatment before OPU and the impact on future pregnancy (Amso, 1995; Matorras et al., 2018).
In women with symptoms of infection, it is recommended to perform specific testing for cervical and vaginal infections and take appropriate actions.
Patient medical history
– As OPU is performed under sedation or anaesthesia, a full blood count (FBC) and any additional test can be ordered, depending on local regulations regarding pre-operative management. There is no evidence suggesting value for an FBC or any additional test before OPU with regard to preventing complications.
Taking accurate patient history before OPU is essential to highlight potential comorbidities and take actions to prevent any possible associated complications. Patients should at least be asked about the use of medications—more specifically the use of blood thinning agents (aspirin and others), relevant previous surgeries and any relevant disease or deficit of coagulation factors.
Information provision and informed consent
– Recent or confirmation of (written) informed consent for treatment should be obtained according to local regulations.
Verbal and written information should be provided to patients, according to local templates, explaining the procedure, the risks and their incidence. Counselling should be provided regarding additional risks associated with specific diagnostic or incidental findings.
Equipment and consumables
During the OPU, the operator should be equipped as required by European standards and local regulations.
The following equipment for OPU should be available on a sterile operation table: sterile small gauzes and a disposable or reusable speculum for cervical examination and to visualize any bleeding site. Furthermore, a test tube warmer and heating block should be available (at 37°C), and culture medium for flushing should be prepared and ready at 37°C. Additional equipment and consumables that might be used during OPU should also be available in the procedure room, such as ovary clamps, sponge holder, vaginal surgery equipment, including (absorbable) sutures. Resuscitation equipment, reversal anaesthetic drugs, a prepared kit for anaphylactic shock treatment and oxygen should also be available in (the near proximity of) the procedure room. All equipment, materials and consumables used should be compliant with the European standards.
US system and transducer
– The US system should be fit for OPU with a high-frequency transvaginal US transducer, which offers the best quality in real-time imaging of the field of view.
The US system should have the ability to adjust the field of view depth and zoom; adjust the focal zone to the region of interest (except where image processing techniques have dispensed with this feature); adjust the acoustic power, colour and power Doppler capabilities; display the mechanical and thermal indices on screen; display the needle guide superimposed on the field of view; and print or save images/cine loops in the system’s hard drive or a central picture archiving and communication system include image gain adjustment controls.
The software of the system should be up to date, the system should be calibrated regularly and it should be serviced according to the manufacturer’s instructions and any local institutional requirements. To ensure safety, the system should be replaced in a timely manner, as recommended by the manufacturer.
Some manufacturers have introduced software that automatically counts and calculates the mean diameter and volume of follicles. Caution should be taken in correlating these new parameters with oocyte maturity in comparison with conventional mean follicular diameter.
The transvaginal transducer, or probe, should have a frequency range of 5–8 MHz and an abdominal transducer with a frequency range of 2–6 MHz, or their contemporaneous equivalents at the time of purchase.
An appropriate protocol for transducer disinfection should be established, in accordance with manufacturer’s instructions. OPU operators and assistants should be familiar with the disinfection technique and keep a detailed documentation of the disinfection procedure.
The transducer should be designed for easy application of a specific sterile cover, incorporating a good-quality sonographic gel on the tip of the transducer.
An appropriate transducer cover should be used, powder-free and compatible with the US device, as this may affect the quality of the image. A latex-free cover should be used in case of latex allergy.
The use of lubrication (on the outside of the cover) does not offer any improvement for the quality of the image and should be avoided as there is a hypothetical gametotoxic and embryotoxic effect. When needed, sterile water or culture media can act as lubricant and conductor of US waves.
Needle
– A single-lumen 17- or 18-gauge needle is the most commonly used one for OPU. However, needles of different sizes and shapes with different flexibility exist. It seems that needles with a smaller diameter provoke less discomfort to the patient (Aziz et al., 1993; Awonuga et al., 1996).
Needles with edging are recommended. The operator should be able to see the needle edge tip and know how to recognize it during the US investigation.
Translucent tubing should be attached to the needle to enable the operator to see the content and the colour of the fluid aspirated.
The needle guide should ideally be disposable and attached at the tip and the bottom of the transducer.
Double-lumen needles, or variations of them, can also be used. With these needles, oocyte collection media is infused into the follicle at the same time as the follicular fluid is being aspirated.
The operator should be familiar with the design and the sharpness of the needle.
The needle patency and aspiration ability should be checked before insertion into the guide and prior to OPU (Fig. 2).
Figure 2.
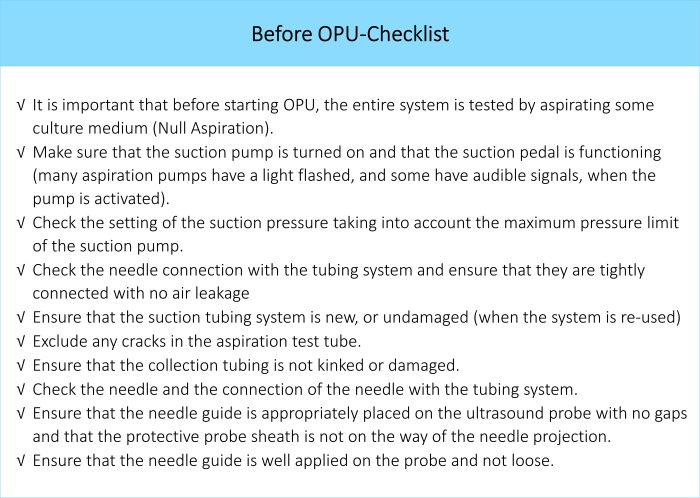
Before OPU checklist
Suction pump and pressure
– The suction pump creates a negative pressure for aspirating follicles. At present, there is no conclusion on the optimal aspiration pressure level, and a variety of pressures between 100 and 200 mmHg are used, often based on manufacturer’s instructions (Panayotidis, 2017; American Institute of Ultrasound in Medicine, 2017). In a single study, higher pressures (140 mmHg) were not associated with damage to the oocytes (Kumaran et al., 2015).
The suction pump should be validated prior to use, and a spare suction pump should be available in case one does not work.
A stable pressure should be maintained during the procedure, as changes can induce turbulence. Changes in pressure can be acceptable in cases of needle blockage.
Further research is needed, and an auditable record of the needle and pressure characteristics correlated with the oocyte yield would be helpful.
OPU preparation
The team performing the OPU should consist of at least the operator and one assistant or nurse. It is recommended that at least one person in the room is trained in advanced life support (Hinkelbein et al., 2018).
The identity of the patient should be checked, and the WHO surgical safety checklist (time out) applied (World Alliance for Patient Safety, 2008).
Ovarian stimulation
It is important that OPU is performed according to a precise timing. Most authors recommend a 36-h interval between medical triggering and OPU, but intervals between 34 and 38 h have been applied (Weiss et al., 2014).
– Immediately before OPU, patients should be specifically asked about the timing of hCG or GnRH agonist injection. In case it is not clear whether the patient has had hCG administered, an hCG immunology urine test or serum beta-hCG test should be performed. Serum beta-hCG levels below 23 mUI/ml suggest inadequate hCG administration (Matorras et al., 2012). The WG does not suggest routine hCG testing for all patients before OPU.
In agonist trigger cycles, the baseline serum level of LH should be measured on the day of the trigger. If no oocytes are found during OPU, LH levels can be checked and compared with baseline LH levels. If LH levels are below 0.5 mIU/ml, the trigger should be repeated with recombinant hCG instead of GnRH agonists (Meyer et al., 2015).
In patients undergoing OPU under general anaesthesia and/or undergoing a natural cycle where there was the development of only a few follicles and/or when there are concerns of premature ovulation, transvaginal US should be performed before starting the procedure.
Patient position and preparation for the procedure
– The patient must fast, 6 h for food and 2 h for clear fluids (see also section sedation).
A peripheral intravenous line should be established, with Ringer’s lactate solution or physiological saline solution at the minimum speed required in order to keep the vein open. Correction of blood glucose levels and any other infusions should be performed according to local protocols.
The patient must empty her bladder immediately prior to OPU. An empty bladder improves the image quality during transvaginal examination as it decreases the posterior enhancement (US artefact), whereas a full bladder can distort the anatomy of the uterus and ovary and may increase the risk of injuries.
Patient positioning during OPU needs to be comfortable for both patient and operator.
Gynaecological positioning in the semi-lithotomy or lithotomy position can facilitate the OPU manoeuvre. The position of the patient may need to be adapted to patient mobility.
The operator can be seated or standing during the OPU procedure.
Speculum examination
– Speculum vaginal and cervical examination should be performed before OPU to check normal anatomy and exclude any new leucorrhoea, polyp or other conditions that could interfere with the procedure.
Disinfection
– Cleansing of the vagina/cervix should be done prior to OPU to minimize bacterial vaginal/cervical contamination. Vaginal cleansing is commonly done with (warmed) normal saline (Ludwig et al., 2006; Tobler et al., 2014). Other vaginal preparations are used (e.g. 0.5% chlorhexidine solution, povidone iodine or culture medium), but there is no evidence on safety or superiority to normal saline. Furthermore, these agents may act on the cell membrane and not be safe for oocytes (Mangram et al., 1999). Povidone–iodine preparations have been shown to be toxic to murine oocytes/embryos (Hershlag et al., 2003).
Further studies are needed on the safety of antiseptic methods, and how different agents may influence reproductive outcomes and post-OPU complications (Tsai et al., 2005; Funabiki et al., 2014).
Sedation
Sedation is categorized as a ‘continuum’ (Apfelbaum et al., 2018) (Supplementary Table SI available at Human Reproduction Open online).
For ambulatory procedures, conscious sedation is preferable for the patients as their recovery times are shorter in comparison with general anaesthesia (Piroli et al., 2012). Therefore, OPU under conscious sedation is usually a suitable option for patients and operators alike (Kwan et al., 2018).
Patient selection is an important consideration. Although conscious sedation is a well-tolerated option for most patients, in some circumstances, deep sedation could be preferable such as in cases of:
– extreme anxiety,
associated pathologies that can complicate OPU, making the procedure longer or painful such as in extensive endometriosis, pelvic adhesions, ovaries with many follicles or ovaries that are difficult to access,
patients with associated personal, psychological or social conditions (medical fertility preservation, oocyte donors, transgenders)
and issues with conscious sedation in previous attempts.
On the other hand, OPU could be performed more easily under conscious sedation in:
– Obese patients, where intra-abdominal pressure and abdominal respiration can occur. This could make stimulated ovaries move up and down, which would then complicate OPU.
Patients where the deep sedation induced hiccups, which produces ovarian movement, which can jeopardize OPU. However, hiccups can rarely be foreseen before OPU.
An escalation policy must be in place where an anaesthetist is available for advice. The team can then decide whether sedation or a general anaesthetic should be considered in difficult cases, or if the patient requests it. The risk benefit must be discussed and taken into consideration, including the hypothetical effect of the anaesthetic drugs on the oocyte quality, airway difficulties, airway reflex loss for up to 4 h post-procedure due to a supra-glottic mask airway devices or intubation and longer recovery times. For these reasons, all patients undergoing OPU fast as before a general anaesthetic, 6 h for food and 2 h for clear fluids (Checketts, 2016).
For a general anaesthetic to take place, a full anaesthetic team for safety of the patient (an anaesthetist and operation department personnel or an anaesthetic nurse) needs to be present during OPU, and the procedure needs to be carried out in an appropriate setting, which may affect timing of procedures and cost and cause delay in treatment (Youn et al., 2015).
Overall, evidence does not support one particular method or technique over another (Kwan et al., 2018). Different options should be discussed with the patients; patient preference (including cultural preferences) should be considered, as well as patient selection. Important consideration must be given to the risk versus benefit with sedation and general anaesthesia. Further studies on complication rates related to the OPU performance under sedation versus general anaesthesia are needed. Specific recommendations for good practice for sedation in assisted conception were recently published by the British Fertility Society (Acharya et al., 2019). Further information on different types of sedation is provided in Table II.
Table II.
Further information on different types of sedation
| Conscious sedation |
|---|
| During conscious sedation, the patient should be able to communicate with personnel and be able to follow orders, for example ‘Breathe deeply’. All respiratory and cardiovascular parameters should remain intact. Conscious sedation involves the following options: Normal conscious sedation (not with an anaesthetist or sedationist with anaesthetic skills) may require: • Midazolam 1 mg/ml to give no more than 7 mg in divided doses with Fentanyl (2 ml, 100 mg diluted with 8 ml of normal saline to make a dilution of 10 mcg/ml). When giving this combination Fentanyl must be given first as the synergy increases the potency of Midazolam by 8×. • If needed supplementary doses of 20 mcg Fentanyl can be given during the procedure up to a maximum of 100 mcg (not exceeding 1 mcg/kg). Anaesthetic conscious sedation (with an anaesthetist or sedationist with anaesthetic skills) may require: • Propofol 1% 18 ml combined with Alfentanil 1 mg (2 ml) to provide a dilution of 50 mcg/ml. • Midazolam is not advised due to synergism with Propofol causes the potency of Propofol to increase by over 50% (Short and Chui, 1991) In patient-controlled sedation/analgesia, using Propofol with Alfentanil provides an acceptable and effective alternative to bolus administration (Roseveare et al., 1998). |
| Local anaesthesia |
| A para-cervical block can be applied in addition to sedation, as pain relief during the OPU. It appears to be superior when compared with sedation alone (Kwan et al., 2018). A local anaesthetic agent is usually deposited in four locations around the cervix in the vaginal mucosa. In total 100 mg lidocaine (10 ml of 1% lidocaine, xylocaine 10 mg/ml) is injected at two (3 and 9 o’clock) or four points around the cervix. Other authors employ two para-cervical locations (without further sedation or analgesia) with good results (Rolland et al., 2017; Kwan et al., 2018). Satisfaction with the procedure was higher when the blocks were used during a general anaesthesia and post-operative pain was also lower (Rolland et al., 2017). |
| Other forms of anxiolysis |
| – VA by the sedationist is a very important part of any OPU that is performed with conscious sedation and/or local anaesthetics (Gange and Baum, 2017). VA is a conversational distraction associated with measures to ensure a calming environment, thereby reducing pain, anxiety, and stress. Good VA begins with clear preoperative communication. It is important to set patient expectations at the time of scheduling; the role of the ‘verbal anaesthetist’ is to begin to set the tone with calming conversation while taking the patient into the room. The environment can be made more relaxing with darkened lights, music in the background and care taken to ensure that the room temperature is made comfortable (21–23°C) (Yeo et al., 2013; Zhang et al., 2014; Cho and Choi, 2016). It is commonly used in in-office procedures of many disciplines but is poorly described in the literature. – Hypnosis is another form of anxiolytic, which can be used to achieve better patient satisfaction, fewer complications and less drug usage (Faymonville et al., 1995). |
VA, verbal anaesthesia.
Monitoring: Non-invasive blood pressure and pulse oximetry must be used when drugs have been administered intravenously; electrocardiogram and CO2 monitoring are developmental standards for conscious sedation but are minimal monitoring standards for deep sedation and general anaesthesia (Checketts, 2016).
Post-operative analgesia: Most studies have reported very little difference in effectiveness of analgesia administered before starting the procedure versus immediately after starting the OPU. The most favourable post-operative pain management is that of a multi-modal peri-operative approach, using intra-operative analgesia with opiates and local anaesthetic block with oral/IV/per rectal medication (Vadivelu et al., 2014). Post-operative analgesia can consist of the following:
– Oral paracetamol 1 g + codeine 60 mg is shown to be more effective than paracetamol alone (Zhang and Li Wan Po, 1996).
Non-steroidal anti-inflammatory drugs can be given (if no contraindications) about 1–1.5 h prior to the procedure.
Per rectal diclofenac (100 mg) can be given post-procedure, followed by IV paracetamol (15 mg/kg) in the recovery. Whether given orally or rectally, this has the same post-operative analgesic effect, although the rectal dose has a faster onset of action.
Sperm collection
It is not exceptional that the semen sample cannot be obtained on the day of OPU. Sperm collection at home or storing a previously obtained sperm sample as a back-up is advisable. If a back-up sample is not available, oocyte cryopreservation, or further options for sperm retrieval (administration of sildenafil, or surgical sperm retrieval) can be considered. In any case, patients should be counselled on the possibility of such complications and informed consent should be obtained before starting the OPU procedure.
Antibiotic prophylaxis
Patients with a history of endometriosis, pelvic inflammatory disease (PID), pelvic adhesions, dermoids or previous pelvic surgery can be considered at high risk for pelvic infection. In these patients, administration of antibiotics is recommended shortly before or during OPU (according to local protocols).
There is no evidence for the use of antibiotic prophylaxis in low-risk patients, and this can be decided according to local protocols and regulations, taking into account generic antibiotic resistance (Aslam et al., 2018) and the lack of studies on the effect on the uterine environment.
Further evidence (studies or observational/audit data) should be collected on infection rates and their association with antibiotic administration.
Change to transmyometrial or laparoscopic oocyte retrieval
In exceptional cases, transmyometrial or laparoscopic oocyte retrieval may be required, usually due to abnormal ovarian placement or tubal adhesions. For transmyometrial OPU, there were no significant differences in oocyte recovery rates, implantation rates and pregnancy rates compared to transvaginal OPU (Davis and Ginsburg, 2004; Roman-Rodriguez et al., 2015). An anaesthesiologist may be present during the procedure, depending on local protocol.
OPU procedure
Setting and image optimization
– The surgical theatre/procedure room for OPU should be of reasonable size and in semi-darkness, as this allows a better visualization of the US images and the hypothetical adverse effect of the light into the oocytes is precluded. The preferred temperature is about 22–23°C and, if necessary, a warming blanket and socks can be used by the patient. Hypothermia has been associated with increased perception of pain.
During OPU, the US field and anatomical orientation are set from the top or from the lower part of the monitor. The representation of the transducer from the lower part of the monitor may help the controlled manipulation of the transducer. The initial structures are seen exactly at the beginning of the transducer as seen in the monitor, and the tactile sensation during the scanning and needle manipulation is more realistically represented. This point is very important as the anatomy is better visualized, which is of crucial importance for safety of the procedure. The laterality is again a parameter that can be set depending on the operator’s preference.
Before starting the surgical procedure, the pelvis should be systematically scanned to assess the anatomy and check for incidental findings. Special attention should be focused on identification of big iliac vessels to avoid incorrect interpretation as a follicle. The needle guide track should be on the screen. It is relevant to first have a panoramic view of the ovary and then have a closer look. A larger view field is preferable during the OPU—magnify until the whole ovary occupies 75% of the field (depending on the dynamics of the ovary)—to ensure visualization of the intrapelvic part of the needle during the OPU.
In case of doubt, Doppler study is advised for recognition of the vascular structures [positioned in the line between transducer (vaginal wall) and ovary] and to reduce the risk of haemorrhagic complications (Risquez and Confino, 2010). Using Doppler imaging during the OPU procedure may be seen as an additional complex study to perform, switching from bi-dimensional to Doppler image, and it is not recommended to do this during the OPU. However, Doppler study can be useful to detect vascular areas in case of doubt in 2D-US imaging before OPU. It could differentiate the hypo-echogenic areas that look alike, such as superficial follicles versus iliac or para-ovarian vessels (position, content, fluid movement). Further research is needed to determine whether this modality of imaging needs to be applied routinely before starting OPU in order to further decrease the risks of an accidental vascular trauma.
Information regarding the peri-follicular Doppler vascularization, before OPU, could be used for academic purposes (for instance oocyte quality) (Bhal et al., 1999).
Adaptation of the US frequency with other image adjustments should be considered in real time to improve the clarity of the image and facilitate the accurate visualization of the needle. The US frequency used for OPU varies between 5 and 7 MHz to obtain sufficient resolution and depth of penetration. Additional filters and image adjustment set-ups can be activated to improve the image.
Recording the OPU procedure can be a useful audit tool for quality control of the US image, learning and teaching as well as to explore factors related to complications. Video recording can be used in prevention of future complications and improvement of OPU techniques.
Technique
– The transvaginal ultrasonographic transducer must be gently applied well into the vaginal wall in order to position the ovary just adjacent to vaginal fornices. There should be no space between the vaginal transducer and ovarian cortex, thus avoiding any bowel loop within the trajectory of the needle. To stabilize the ovary in one place, external abdominal pressure or supra-pubic pressure can be applied (with the help of an assistant) towards the vaginal fornix of the patient by the site (right or left) where OPU is performed. This can move the ovarian follicles closer to the vaginal wall, thereby avoiding multiple ovarian punctures. Push the puncture needle through the needle guide to the vaginal top and gently puncture the vaginal wall until just below the ovary, then puncture the nearest follicle in one movement.
The operator needs to be familiar with the tactile resistance when the follicle/ovary is penetrated by the needle and able to manipulate the transducer, which produces the US image. A fingertip handle on the distal end of the needle can facilitate the puncture with good clinical touch. In case of resistance or hard-to-reach follicle(s), one should pull back the needle in one movement.
Techniques for tracking the needle (edging) should be used. The edging of the needle should always be visible.
Follicle curetting involves gently and rapidly rotating the needle in a clockwise and counter-clockwise fashion inside the follicle after complete aspiration of the follicular fluid (Yao and Schust, 2002). It has been suggested (in a single retrospective study) that follicle curetting during OPU could increase the number of recovered oocytes as well as the number of mature oocytes, without damaging the oocyte, and prevent adhesion of the granulosa cell layer to the needle lumen, which could block it during aspiration (Dahl et al., 2009). More studies are needed to confirm the clinical value and safety of this technique.
Vacuum suction should be used just before the follicle penetration. The pressure of the pump suction must be calibrated to 100–220 mmHg (according to manufacturer’s instructions) just before starting, and it should be kept constant during the procedure. The pedal for the pump can be controlled by the operator or by an assistant. Collapse of the follicle should be visualized when aspirating in order not to lose oocytes. If the collapse of the follicle cannot be seen, the oocyte can remain in the follicular cavity.
The needle should be gently withdrawn without negative suction pressure to avoid sudden forward flow of follicular fluid towards the collection tube (Horne et al., 1996). The movement of the hand holding the transducer should be minimal when the needle is in the ovary. Lateral movements cause more pain and can result in increased intra-ovarian bleeding.
Small follicles (<10 mm) can be left un-punctured (to avoid collection of immature eggs), unless there is a high risk of ovarian hyper-stimulation syndrome (OHSS).
Experts prefer to start the OPU from the ovary nearest to the vaginal probe rather than the ovary with the largest follicles or complex appearances, because in hyper-stimulated ovaries, sometimes the length of needle cannot reach the length of ovary, and the procedure can be dangerous if near vascular structures. The laterality, whether to prefer the right versus the left ovary, is based on operator’s preferences rather than on anatomical considerations.
One should use both planes, longitudinal and transverse, while performing the OPU in order to be sure about the anatomy and boundaries of the ovarian cortex.
It is preferred to maintain the needle within the ovary—avoiding repetitive punctures or ovarian penetrations—during the OPU as this could reduce the risk of complications, mainly ovarian surface bleeding. Multiple punctures should be avoided as much as possible.
It is recommended to access as many follicles as is safely possible through the same ovarian cortex puncture.
Manipulation of the OPU needle should be gentle and steady, avoiding abrupt movements.
Flushing
– Follicular flushing has been proposed to increase the number of retrieved oocytes. Closed flushing (i.e. every follicle is rinsed three to four times, and tubes are passed on to the laboratory when all follicles are punctured) has been recommended for patients with >6 follicles, and open flushing (i.e. with direct communication between the laboratory staff and the operator, the follicle is rinsed until an oocyte is detected in the laboratory, or until no cell material is detected) for those with ≤6 follicles. However, studies on flushing performed failed to show any benefit (Georgiou et al., 2018). The results of this technique should be audited regularly. Flushing needs to be performed with a double-lumen needle to reduce damage to the oocyte.
Oocyte recovery
– The follicular fluid should be collected in preheated test tubes held in a heating block calibrated at 37°C.
Embryology laboratory staff should inform the medical doctor of oocytes and granulosa cells during the OPU procedure in order to differentiate empty follicle syndrome and wrong timing of hCG injection.
In case of suspected premature ovulation, peritoneal fluid can be aspirated at the end of OPU in search of additional oocytes that could have been ovulated or fallen into the peritoneal cavity during the procedure.
End of procedure
– At the end of the procedure, the ovary should be checked to see whether all follicles were punctured and to detect any internal bleeding.
Speculum examination should be performed to check for vaginal bleeding.
If abdominal bleeding is suspected, transabdominal US should be performed before moving the patient.
Post-OPU vaginal compression with a swab may enhance haemostasis and stop potential vaginal bleeding, which may otherwise be a disturbing finding for the couple after OPU. With prolonged bleeding, packing can be applied (No, 2016). A pad can also be used after vaginal compression for monitoring of vaginal bleeding. If necessary, a haemostatic suture is placed.
Post-OPU analgesia (paracetamol, ibuprofen) should be considered, especially in cases where more than 10 oocytes are retrieved and in patients with endometriosis.
-
The proportion of OPUs without obtaining an oocyte is usually 1–2% (Ben-Shlomo et al., 1991; Traina et al., 1993; Matorras et al., 2012). This lack of oocyte recovery is much more common in women with few adequately sized follicles (Zreik et al., 2000). It has been reported that in 5–20% of dominant adequately sized follicles, no oocytes are retrieved (Nargund et al., 2001; Coskun et al., 2010; Coskun et al., 2011). Furthermore, poor responders may have an increased rate of impaired folliculogenesis and oocytes may have lower quality (Matorras et al., 2014) (Fig. 3).
– hCG determination is mandatory for patients with no oocyte retrieval in hCG-triggered cycles, to assess the correct hCG administration. No oocyte retrieval with high hCG levels could indicate an ectopic pregnancy (Bringer-Deutsch et al., 2010).
In cases where OPU failed to recover oocytes and the administration of the medication was adequate, performing a (rescue) IUI is associated with very low pregnancy rates (<7%) and should not be carried out (Matorras et al., 2014).
Figure 3.

Troubleshooting during OPU
Post-procedure care
– After the procedure, patients should remain in bed at the centre for recovery (about 2 h, or less if the procedure was performed with local anaesthetics only). General status, abdominal distension, blood pressure and heart rate should be monitored by a nurse (Fig. 4).
In cases of significant pain or abdominal distension, a blood analysis and/or an US scan should be performed before discharge to check for potential intra-abdominal bleeding.
Patients should be able to eat, drink and pass urine before discharge. Furthermore, it is important to check awareness, orientation and respiratory rate. A written information leaflet about post-care procedures, complications and a 24-h emergency number should be provided.
In patients with haematoma, bleeding or infection after the OPU, antibiotic coverage is recommended.
Procedures should be in place when severe complications occur, including hospital admission arrangements, specialist responsibility and continued care.
Figure 4.

After OPU checklist.
Associated pathologies and cautions during OPU
– Standard management of hydrosalpinx should be removal or clipping before OPU (Song et al., 2017). When a hydrosalpinx is only discovered during OPU, the first option should be oocyte or embryo cryopreservation. The benefit of aspiration on the day of OPU needs further study (Hammadieh et al., 2008; Fouda et al., 2015; Zhou et al., 2016).
Patients with potential infectious risk (HIV, hepatitis) should be managed in an isolated circuit or in specialized centres to avoid cross-contamination.
In women with endometriosis, OPU can be challenging and it may affect the individual operator’s or centre’s performance rate (Kasapoglu et al., 2018). Endometriomas should not be aspirated. During OPU, the puncture of endometriomas should be avoided to prevent contamination of the follicular aspirate and reduce the risk of intra-abdominal infection. However, piercing the endometrioma is often the only way to avoid losing an important number of oocytes. Dermoid cysts should not be punctured during OPU, since this could increase the risk of PID and peritonitis. In patients with an endometrioma or teratoma, the risk of PID is increased, even if they have not been punctured (Moini et al., 2005; Benaglia et al., 2008; Villette et al., 2016; Kasapoglu et al., 2018). These patients should be counselled preoperatively and consent obtained appropriately.
If an endometrioma or a haemorrhagic follicle is inadvertently punctured, the needle should be immediately withdrawn and flushed with media, and the collecting tube should be changed.
In patients with borderline ovarian tumours, it is unclear whether ART procedures are associated with an increased risk of recurrence (Denschlag et al., 2010).
Increased risk for bleeding has been suggested in lean women, and in women with polycystic ovary syndrome (Liberty et al., 2010; Zhen et al., 2010).
Complications and risks
ESHRE IVF monitoring (EIM) data collection reports data on complications from OPU. In the latest data available, including 776 556 cycles, complications from OPU were reported in 1328 cycles (0.17%), including 919 bleeding (0.11% of cycles), 108 infections (0.013%) and 301 (0.038%) other complications. There were three maternal deaths reported as complications of ART, but none were related to the OPU procedure (De Geyter et al., 2018).
Large observational studies in oocyte donors undergoing OPU have reported low incidences of complications (0.42% in Bodri et al. (2008), 0.7% in Maxwell et al. (2008)). The most common reported complications were OHSS (although a complication of ovarian stimulation rather than OPU) and intra-abdominal bleeding. Large observational studies have also been conducted (Table III). Overall, the incidence and severity of the reported complications are low (Ludwig et al., 2006; Aragona et al., 2011; Siristatidis et al., 2013; Ozaltin et al., 2018).
Table III.
Complications observed during OPU in patients undergoing ART
| (Levi-Setti et al., 2018) | (Ozaltin et al., 2018) | (Siristatidis et al., 2013) | (Aragona et al., 2011) | (Ludwig et al., 2006) | |
|---|---|---|---|---|---|
| Number of OPUs | 23 827 | 1031 | 524 | 7098* | 1058 |
| Overall incidence of complications | 0.4% | 0.72% | |||
| Related to sedation, anaesthesia | 14 (0.06%) | 0 | 2 (0.36%) | 0 | |
| Vaginal bleeding | 2 (0.01%) | 32 (3.1%) | 98 (18.08%) | 29 (2.8%) | |
| Intra-abdominal/intra-peritoneal bleeding | 54 (0.23%) | 0 | 2 (0.36%) | 4 (0.06%) | 0 |
| Injury of pelvic structures | 2 (0.01%) | 0 | 1 (0.1%) | ||
| Pelvic abscess | 2 (0.19%) | 0 | |||
| Ovarian abscess | 2 (0.03%) | ||||
| Pelvic infections | 10 (0.04%) | 8 (0.77%) | 0 | 0 | |
| Severe pain (requiring hospitalization) | 14 (0.06%) | 1 (0.09%) | 0 | 7 (0.7%) | |
| OHSS (all) | 47 (4.55%) | 17 (3.24%) | 28 (2.7%) |
OPU: oocyte pick up; OHSS: ovarian hyper-stimulation syndrome.
*Only intra-peritoneal bleeding and pelvic abscess were reported.
Associated conditions may increase the risk of complications, but very little information is available, and over- and under-reporting have been suggested (Villette et al., 2016). Recommendations regarding associated conditions during OPU have been addressed above.
Apart from data collection and observational studies (Ludwig et al., 2006, Aragona et al., 2011, Siristatidis et al., 2013, Ozaltin et al., 2018), most reports on serious complications during and after OPU have been published in case reports (Table IV). Reported complications include bleeding, infection, urinary tract injury and pseudoaneurysm.
– Infection: an infection can originate from the vaginal puncture during the OPU procedure where there is a contamination from vaginal bacteria into the intra-peritoneal space (Kelada and Ghani, 2007). The presence of pre-existent latent pelvic infection or pelvic endometriosis or teratoma may be another contributing factor. In some difficult cases, puncture of hydrosalpinx or an accidental puncture of an attached bowel loop during the procedure may occur, which may lead to severe septicaemia (Amso, 1995).
Bleeding: the quantity of blood loss following OPU is clinically unremarkable in most women. In a prospective study of 150 consecutive OPUs, the estimated median blood loss was 72 ml (interquartile range 8–162 ml) (Ragni et al., 2009). None of the recruited women was found to have signs of haemoperitoneum (Ragni et al., 2009). Haemoperitoneum after OPU has been defined as a haemoglobin reduction of >2 g/day, an increase in the pelvic-free fluid of >200 ml or a calculated blood loss of >500 ml.
After para-cervical block, a transient leg paresis may develop, which usually disappears after 2–4 h.
The IVF centre should have proactively established policies with respect to how to provide patient resuscitation and access to surgical theatre in case of internal bleeding or other organ injury in a haemodynamically unstable patient (safety standard).
OPU video recording may help to identify reasons for complications and how to improve the OPU technique with respect to US settings for clear imaging and types of manoeuvres during oocyte aspiration.
Table IV.
Serious complications of OPU reported in case reports (published between 1998 and 2018)
| Complication | Case report | Clinical signs/symptoms (day of OPU) | Clinical signs/symptoms (post-OPU) | Clinical signs/symptoms (during pregnancy) | Management | |
|---|---|---|---|---|---|---|
| Bleeding | Intra-abdominal bleeding | (Mashiach et al., 2013) | None |
OPU + 2 days:
- Severe abdominal and shoulder pain - Abdominal bloating Tenesmus |
Exploratory laparoscopy—The vessel was successfully coagulated | |
| None |
OPU + 3 days:
- Lower abdominal pain - Dyspnoea with stable Hb concentration (10.43–10.95 g/dl). OPU + 4 days: - Pale and tachycardiac, with a drop in Hb level (8.84 g/dl) that continued (8.66 g/dl) despite blood transfusion |
Laparoscopy—the tear was successfully coagulated with an accurate haemostasis | ||||
| (Kart et al., 2011) | None |
OPU + 10 days:
- Severe abdominal pain - Vomiting - Vaginal bleeding for 3 days |
Transfusion with 2 units of fresh-frozen plasma and packed red blood cell Percutaneous transcatheter pelvic angiography + immediate bilateral uterine artery embolization |
|||
| Massive retroperitoneal bleeding | (Azem et al., 2000) |
OPU + 10 h:
- Severe lower abdominal pain - Vomiting - Tenesmus |
OPU + 10 days: | Laparotomy—retroperitoneal haematoma evacuated and drained Recurrence of symptoms after 10 days— treated with IV antibiotics |
||
| Haemoperitoneum | (Chatrian et al., 2012) |
OPU + 3 h:
- Abdominal pain - Blood pressure: normal - Pulse rate: 70 beats per minute. - No fever - Abdomen rebound defence - Haemoglobin level (Hb): 99 g/l - Haematocrit: 29% |
Emergency laparoscopy (7 h post-TVOR) The only way to stop the bleeding was by using an absorbable fibrinogen and thrombin sealant sponge, which was applied around the ovary During laparoscopy three pints of packed red blood were administered |
|||
| Pseudoaneurysm | Pelvic pseudoaneurysm | (Pappin and Plant, 2006) |
12 weeks gestation:
- Painless vaginal bleeding |
Angiography demonstrated the aneurysm to originate from anterior branches of the left internal iliac artery close to the lower uterus and cervix. Drainage was via a leash of vessels both locally and across the midline to the right internal iliac circulation. Selective embolization was performed with coils and intra-arterial thrombin | ||
| Pseudoaneurysm of the internal iliac artery | (Bozdag et al., 2004) |
29 weeks gestation:
- No symptoms during a follow-up visit, a unilocular, anechoic mass with a diameter of 40 mm was noted on the left upper side of the uterus. The Doppler examination was consistent with a (pseudo)aneurysm |
After delivery; the pseudoaneurysm of the left inferior pudendal artery was completely embolized with 1 mL (50%) of N-butyl-2-cyanoacrylate | |||
| Haemorrhage from a pseudoaneurysm of the obturator artery | (Bolster et al., 2014) |
OPU + 4 days:
- life threatening haemorrhagic shock |
Surgical laparotomy followed by CT and selective angiography. The haemorrhage was successfully managed endovascularly with a vessel preserving covered stent | |||
| Infection | Pelvic abscess | (den Boon et al., 1999) |
End of 2nd trimester:
Rupture of bilateral ovarian abscesses |
Emergency laparotomy was necessary because of an acute abdomen Severe maternal and neonatal morbidity, preterm birth and neonatal death |
||
| (Patounakis et al., 2012) |
Gestation of 11 weeks + 2 days:
- left lower quadrant abdominal pain. Serial pelvic US showed growth of the mass from 13.2 to 15 cm over 3 days and a viable twin pregnancy (Streptococcus anginosus) |
Left salpingo-oophorectomy for resection of the mass. Complete spontaneous pregnancy loss by vaginal delivery of both foetuses on post-operative day 1 |
||||
| (Asemota et al., 2013) |
OPU + 6 days:
Actinomycosis pelvic abscess
- Urinary retention - Pelvic pain - Fever |
6 days of intravenous antibiotics CT-guided drainage of the pelvic abscesses |
||||
| Infection | Tubo-ovarian abscess | (Han et al., 2015) |
Gestation of 31 weeks and 2 days
- Lower abdominal pain for 8 h |
Emergent exploratory laparotomy and Caesarean section to terminate gestation. +IV antibiotics | ||
| (Kim et al., 2013) |
7th week of gestation:
- Intermittent right lower abdominal pain. US: 1 foetus appropriate for gestational age and growth of the mass (10.6 × 7.4 cm) 14 weeks: - Right abdominal pain |
Laparoscopy. The abscess was encapsulated within the ovary and there was no pus within the pelvis. IV cefotiam (1 g every 12 h for 10 days) and metronidazole (500 mg every 8 h for 5 days) Spontaneous delivery at 37 weeks and 3 days of gestation without any complications |
||||
| (Romero et al., 2013) |
OPU + 1 month:
- 8 cm pelvic abscess |
Surgical drainage | ||||
|
OPU + 2 months:
- 9 cm pelvic abscess |
IV antibiotics (did not resolve) + surgical drainage | |||||
|
OPU + 3 weeks:
- 9 cm pelvic abscess |
IV antibiotic treatment (favourably response) + surgical drainage and right adnexectomy | |||||
| (Yalcinkaya et al., 2011) | Early pelvic infection | Broad spectrum antibiotics TV-US-guided drainage was performed, posterior colpotomy and T-drain replacement into the cul-de-sac. (OPU + 9 days) Pregnancy follow-up uncomplicated |
||||
| (Van Hoecke, 2013) | Bacteraemia due to actinomyces urogenitalis. Bacteraemia was secondary to a tubo-ovarian abscess | |||||
| (Kelada and Ghani, 2007) |
OPU + 16 days:
- Left iliac fossa pain for 5 days. - Diarrhoea - Vomiting 3 times - Fresh vaginal bleeding. Bilateral ovarian abscesses (staphylococci) |
Laparotomy, a large amount of pus was drained on incising the capsule of each ovary. The peritoneal cavity was washed with normal saline. Two drains were placed through the abdominal wall in the pouch of Douglas IV Gentamicin and Clindamycin were continued post-operatively |
||||
| (Sharpe et al., 2006) |
30 weeks gestation:
- Low-grade fever |
Broad-spectrum antibiotics Abscess was drained percutaneously after Caesarean delivery of twins |
||||
| (Matsunaga et al., 2003) |
16 weeks gestation:
- Fever - Lower abdominal pain 20 weeks gestation: readmitted - Fever - Lower abdominal pain - Small amount of bloody discharge |
Treatment with IV antibiotics Left salpingo-oophorectomy after delivery Delivered at 22 weeks of gestation |
||||
| (Varras et al., 2003) | - Abdominal pain, fever and leukocytosis | |||||
| Infection | Pelvic infection (gram-positive cocci arranged in chains similar to group A b-haemolytic streptococci.) | (El-Toukhy and Hanna, 2006) |
OPU + 1 day:
- Tiredness - Nausea - Lower - Abdominal pain. - Tachycardic, normotensive and afebrile - Mild abdominal distension and tenderness - Cervical motion tenderness |
IV hydration with physiological saline solution and human albumin 4.5% infusion for suspected OHSS. IV antibiotics |
||
| Spondylodiscitis | (Debusscher et al., 2005) |
OPU + 1 day:
- Increasing pelvic and sacroiliac pain OPU + 2 days: - Unbearable pain - Tenderness in lumbosacral area without neurological implications - CRP of 14.2 mg/dl Later - Chills and fever |
IV antibiotics Surgery; the lumbosacral joint was carefully débrided and filled up with a tricortical iliac crest graft. Oral antibiotics continued for 8 months |
|||
| Infectious spondylitis (Staphylococcus aureus) | (Kim et al., 2015) |
OPU + 14 weeks:
- Lower back pain over the past 3 weeks. Lumbar spine magnetic resonance imaging showed infectious spondylitis |
Intravenous cefazolin was continued for 6 weeks Delivery healthy baby |
|||
| Pyometra (vancomycin-resistant enterococci) | (Nikkhah-Abyaneh et al., 2010) |
OPU + 4 weeks:
- High fever, chills - No gynaecologic symptoms OPU + 6 weeks: - Unrelenting fever - Abdominal pain |
Antibiotics After recurrence of symptoms: hysterectomy, (showing autolyzed endometrium, sub-serosal and intramural abscess) |
|||
| Vertebral osteomyelitis | (Almog et al., 2000) |
OPU + 0 h:
- Low back pain |
OPU + 1 week:
- Fever OPU + 2 weeks: - Elevated erythrocyte sedimentation rate |
Treated with antibiotics | ||
| Urinary tract injury | Ureteric/ureteral injury | (Choudhary et al., 2017) |
OPU + 0 h:
- Ureteric injury identified immediately during post-procedure US |
A double-J catheter was inserted under cystoscopic guidance. (in the same sitting) | ||
| (Catanzarite et al., 2015) |
OPU + 4 h:
- Gross haematuria. Cystoscopy, laparoscopy, and retrograde pyelography revealed bleeding from the left ureter, no intra-abdominal bleeding, and a patent left urinary collecting system |
The ureteral bleeding was successfully managed with placement of a ureteral stent | ||||
| (Vilos et al., 2015) |
OPU + 0 h:
- Ureteric injury identified immediately |
Treated with ureteral stents with full resolution. During a subsequent IVF cycle, stenting allowed better visualization, resulting in an uneventful retrieval and subsequent pregnancy |
||||
| (Burnik Papler et al., 2015) |
OPU + 1 day:
- Abdominal pain OPU + 4 days: - Massive haematuria OPU + 6 days: - Reappearing haematuria No signs of renal dysfunction or urinary leakage into retroperitoneal space |
Monopolar coagulation with wire electrode and insertion of a double-J-stent during operative cystoscopy | ||||
| (Grynberg et al., 2011) |
OPU + 1 day:
- Acute pelvic pain Later - Recurrence of the pelvic pain with radiation to the right lumbar region |
Cystoscopy with uncomplicated right ureteral stent placement | ||||
| (Fiori et al., 2006) |
OPU + 2 h:
- Severe abdominal pain - Dysuria - Mild tachycardia - No vaginal bleeding - No vesical globe |
OPU + 1 day:
- Fever (38.4°C) - Nausea - Vomiting - Urinary urgency - Bladder tenesmus Acute-onset uro-retroperitoneum |
Intravenous antibacterial therapy Cystoscopy and right ureteral stenting |
|||
| Urinary tract injury | Bladder injury with haematuria and urinary retention | (Modder et al., 2006) |
OPU + 8 h:
- Urinary retention - Supra-pubic pain |
Foley catheter, intravenous fluid bolus, bladder irrigation, and computed tomography with post-void films that showed a blood clot in the bladder | ||
| Acute ureteral obstruction | (Miller et al., 2002) |
OPU + 7 h:
- Right lower quadrant and right flank pain with nausea and emesis - Normal temperature and blood pressure with mild tachycardia |
Cystoscopy and right ureteroscopy with ureteral stent placement | |||
| Ureterovaginal fistula | (Spencer et al., 2017) |
OPU + 0 h:
- Severe abdominal pain - Vaginal leakage |
Placement of the left ureteral stent The IVF cycle was converted to a freeze-all cycle |
|||
| (Mongiu et al., 2009) |
OPU + 2 days:
- Fever - Worsening episodes of cramping right lower quadrant abdominal pain Embryo transfer + 2 days: - Vaginal leakage of fluid (slowly increasing) OPU + 21 days: - Continuing fluid leakage (urine) - No fever or pain |
A percutaneous nephrostomy tube was placed using US guidance, and the fistula was allowed to close secondarily | ||||
| (von Eye et al., 2006) |
OPU + 0 h:
- Right lower abdominal pain with irradiation to the supra-pubic area - Vaginal discharge |
A double-J catheter was inserted under general anaesthesia. | ||||
| Other | Acute psychiatric episode | (Hwang et al., 2002) |
OPU + 0 h:
- Acute psychiatric episode (Tachycardia, tachypnoea, transient hypertension and limb rigidity, alterations to stupor and posture) OPU + 9 h: - Unresponsive to stimuli |
OPU + 1 day:
- Aphasia - Wishful thinking of having delivered a baby OPU + 3 days: - Memory loss |
Supportive psychotherapy | |
| Acute portal vein thrombosis | (Mmbaga et al., 2012) |
OPU + XX days:
- Worsening, right upper quadrant pain |
Therapeutic anticoagulation | |||
| Other | Anaphylactic shock | (Iikura et al., 2002) |
End of OPU: anaphylactic shock - Decrease in blood pressure (<50 mm Hg) - Tachycardia (pulse rate 150 beats/min) - Systemic urticarial reactions - Abdominal pain |
Treatment, including epinephrine | ||
|
Middle of an OPU:
- Anaphylactic shock |
Treatment, including epinephrine | |||||
| Pelvic tuberculosis | (Annamraju et al., 2003) | - No change in her bowel or bladder function - Regular periods - No fever, cough, weight loss, or loss of appetite Painless left lower abdominal mass, growing slowly during a 3-month period |
Drainage of the ovarian abscess and biopsy | |||
| Peri-umbilical haematoma (Cullen’s sign) | (Bentov et al., 2006) | - |
OPU + 3 days:
- Urinary tract infection Physical examination revealed a non-tender bluish discoloration around umbilicus |
IV cefuroxime and metronidazole | ||
| - |
OPU + 1 week:
- Abdominal pain Abdominal inspection revealed a peri-umbilical haematoma with dark red-blue color |
Laparoscopy: bilateral ovarian torsion was found and detorsion was performed + aspiration of a few large corpora lutea | ||||
| Severe bradycardia and bradypnoea | (Ayestaran et al., 2000) |
OPU + 85 min:
- Severe bradycardia and bradypnoea |
Emergency application of a pacemaker | |||
| Intra-abdominal needle rupture | (Sõritsa et al., 2017) | Nonz | CT-scan to locate the broken needle and laparoscopy to remove it |
CT, computerized tomography; US, ultrasound.
All severe complications should be registered according to local requirements. It is recommended that more details on severe complications are gathered from the EIM data collection.
Future developments
– Doppler studies can be useful to detect vascular areas in case of doubt in 2D-US imaging. Doppler could differentiate hypo-echogenic areas that look alike, such as superficial follicles versus iliac or para-ovarian vessels (position, content, fluid movement). Further research is needed to determine whether this modality of imaging needs to be applied routinely during OPU.
Artificial intelligence, based on US features, patient profile and biochemical metrics information, can be used as a predictor of how much the follicle grows in the next few days. Artificial intelligence here can be very useful when predicting the growth of poor responders’ follicles, and this can be a direction for future research. Another application could be to classify the follicles during the process of growth and use this information in OPU and embryo selection for transfer.
Training and competence
OPU should be performed by doctors competently trained in reproductive medicine. In some European countries, fertility specialist or nurses can be trained to perform OPU. This depends on local regulations and on clinical practice.
There are currently no generally accepted minimal requirements for OPU training; the Royal College of Obstetricians and Gynaecologists (RCOG) sub-specialty curriculum does not contain any specific minimum number of OPUs to be performed (RCOG, 2015), nor does the recent AIUM Practice Parameter for Ultrasound Examinations in Reproductive Medicine and Infertility (American Institute of Ultrasound in Medicine, 2017).
– For safety reasons, and wherever feasible, the simulator could be the initial part of a structured training for novices who want to perform OPU, enabling them to acquire basic skills and to reach a predefined level of performance in a safe and controlled environment, before applying the procedure to real patients (Soave et al., 2019).
Adequate training in OPU includes basic training in IVF US, and at least 30 OPU procedures should be performed under supervision to reach the minimum criteria for competency (but this can vary depending on the type of training, background and progress of trainees) and at least 50 OPUs should be performed independently before the acquirement of the qualification.
Proficiency has been defined as retrieving ≥80% of the oocytes compared with a senior operator performing OPU in the contralateral ovary (Dessolle et al., 2014) (see also Fig. 5).
Maintaining skills is essential, aiming for at least 50 OPUs per year. If this cannot be achieved, additional simulator training can be helpful.
To reach an expert level in OPU, at least 250 OPUs should be performed, based on a large retrospective analysis showing an association of the expertise of the operator with the risk of complications, and significantly fewer complications if the operator has performed at least 250 OPUs (Levi-Setti et al., 2018).
Figure 5.
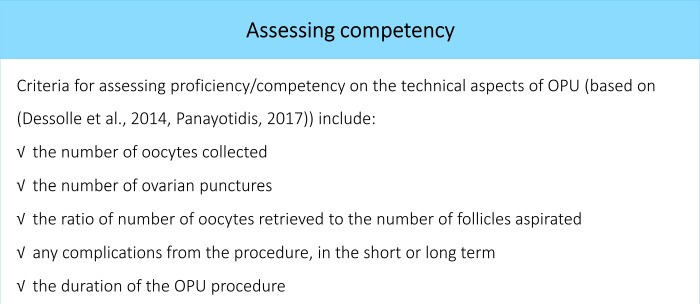
Assessing competency
Quality assurance and performance
Good practice suggests keeping clear and readable documentation regarding the OPU description with images and results (fx how many oocytes obtained, difficulties during the procedure, information for future audit or research, information about the equipment). Forms should be available for admission and discharge, and for reporting on the procedure.
Quality assurance in OPU performance should analyze key clinical performance indicators, and it should be undertaken at least once per year in the IVF centre. A list of key performance indicators for ART, including US-guided OPU, will be published shortly.
The entire OPU procedure should be audited at least once a year. This can be carried out by external auditors or quality managers, or by members of the same team observing each other performing the procedure (Human Fertilisation and Embryology Authority, 2017).
Discussion
This paper provides good practice recommendations for US-guided OPU. A literature search for evidence of the key aspects of the procedure revealed that there was a scarcity of studies on the actual procedural OPU technique. Selected papers (n = 190) relevant to the topic were analyzed by the WG. The WG members considered the following key points in the papers: whether US practice standards were explained; to what extent the OPU technique was described and whether complications or incidents and how to prevent such events were reported. In the end, only 108 papers could be used to support the recommendations in this document.
Most evidence focussed on comparing different equipment (needles) and on complications and risks, including the risk of infection. For these topics, the recommendations were largely based on the results of the studies. Evidence for the other aspects was limited, and these recommendations were based mainly on expert opinion, considering whatever (indirect) evidence was available (Fig. 6).
One of the major research gaps was training and competence. Training and OPU proficiency appeared to be less specific and new more objective ways of evaluating performance should be used in future. Newer technologies, such as simulation training, could help to improve and standardize future training.
The current recommendations were aimed to support clinics in assessing their OPU procedures and to update them to the highest standards of patient care. In addition, this paper has outlined a list of research priorities, which if conducted in future might support confirmation or changes to the current recommendations.
Supplementary Material
Acknowledgements
The WG would like to thank Dr Jouni Ahonen, Dr Orhan Binici and Dr Tahsin Zatman for their helpful expert input on the sedation section. The WG would also like to thank all contributors to the stakeholder review.
Authors’ roles
ADA proposed the topic, composed the group of experts and chaired the WG. C.P. provided substantial contributions to conception and design. N.V. performed the literature search and contributed to the coordination of the WG. All other authors contributed equally in drafting the article or revising it critically for important intellectual content. All authors approved of the final version to be published.
Funding
European Society of Human Reproduction and Embryology to WG.
Conflict of interest
Dr Amso reports: My wife and I have shares in commercial businesses, one of which is in the medical field, Intelligent Ultrasound plc, UK. The other authors declare that they have no conflict of interests.
References
- Acharya U, Elkington N, Manning L, Thorp-Jones D, Tavener G. Recommendations for good practice for sedation in assisted conception. Hum Fertil 2019;1–9. [DOI] [PubMed] [Google Scholar]
- Almog B, Rimon E, Yovel I, Bar-Am A, Amit A, Azem F. Vertebral osteomyelitis: a rare complication of transvaginal ultrasound-guided oocyte retrieval. Fertil Steril 2000;73:1250–1252. [DOI] [PubMed] [Google Scholar]
- American Institute of Ultrasound in Medicine AIUM Practice Parameter for Ultrasound Examinations in Reproductive Medicine and Infertility, 2017.
- Amso NN. Potential health hazards of assisted reproduction. Problems facing the clinician. Hum Reprod 1995;10:1628–1630. [DOI] [PubMed] [Google Scholar]
- Annamraju H, Ganapathy R, Webb B. Pelvic tuberculosis reactivated by in vitro fertilization egg collection? Fertil Steril 2003;2008:e2001–e2003. [DOI] [PubMed] [Google Scholar]
- Apfelbaum J, Gross J, Connis R, Arnold D, Coté C, Dutton R, Tung A. Practice guidelines for moderate procedural sedation and analgesia 2018: a report by the American Society of Anesthesiologists Task Force on moderate procedural sedation and analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American dental association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology 2018;128:437–479. [DOI] [PubMed] [Google Scholar]
- Aragona C, Mohamed MA, Espinola MS, Linari A, Pecorini F, Micara G, Sbracia M. Clinical complications after transvaginal oocyte retrieval in 7,098 IVF cycles. Fertil Steril 2011;95:293–294. [DOI] [PubMed] [Google Scholar]
- Asemota OA, Girda E, Duenas O, Neal-Perry G, Pollack SE. Actinomycosis pelvic abscess after in vitro fertilization. Fertil Steril 2013;100:408–411. [DOI] [PubMed] [Google Scholar]
- Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisar MA, Alvi RF, Aslam MA, Qamar MU et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist 2018;11:1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awonuga A, Waterstone J, Oyesanya O, Curson R, Nargund G, Parsons J. A prospective randomized study comparing needles of different diameters for transvaginal ultrasound-directed follicle aspiration. Fertil Steril 1996;65:109–113. [DOI] [PubMed] [Google Scholar]
- Ayestaran C, Matorras R, Gomez S, Arce D, Rodriguez-Escudero F. Severe bradycardia and bradypnea following vaginal oocyte retrieval: a possible toxic effect of paracervical mepivacaine. Eur J Obstet Gynecol Reprod Biol 2000;91:71–73. [DOI] [PubMed] [Google Scholar]
- Azem F, Wolf Y, Botchan A, Amit A, Lessing JB, Kluger Y. Massive retroperitoneal bleeding: a complication of transvaginal ultrasonography-guided oocyte retrieval for in vitro fertilization-embryo transfer. Fertil Steril 2000;74:405–406. [DOI] [PubMed] [Google Scholar]
- Aziz N, Biljan MM, Taylor CT, Manasse PR, Kingsland CR. Effect of aspirating needle calibre on outcome of in-vitro fertilization. Hum Reprod 1993;8:1098–1000. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo I, Schiff E, Levran D, Ben-Rafael Z, Mashiach S, Dor J. Failure of oocyte retrieval during in vitro fertilization: a sporadic event rather than a syndrome. Fertil Steril 1991;55:324–327. [DOI] [PubMed] [Google Scholar]
- Benaglia L, Somigliana E, Iemmello R, Colpi E, Nicolosi AE, Ragni G. Endometrioma and oocyte retrieval-induced pelvic abscess: a clinical concern or an exceptional complication? Fertil Steril 2008;89:1263–1266. [DOI] [PubMed] [Google Scholar]
- Bentov Y, Levitas E, Silberstein T, Potashnik G. Cullen's sign following ultrasound-guided transvaginal oocyte retrieval. Fertil Steril 2006;85:227. [DOI] [PubMed] [Google Scholar]
- Bhal PS, Pugh ND, Chui DK, Gregory L, Walker SM, Shaw RW. The use of transvaginal power Doppler ultrasonography to evaluate the relationship between perifollicular vascularity and outcome in in-vitro fertilization treatment cycles. Hum Reprod 1999;14:939–945. [DOI] [PubMed] [Google Scholar]
- Bodri D, Guillen JJ, Polo A, Trullenque M, Esteve C, Coll O. Complications related to ovarian stimulation and oocyte retrieval in 4052 oocyte donor cycles. Reprod Biomed Online 2008;17:237–243. [DOI] [PubMed] [Google Scholar]
- Bolster F, Mocanu E, Geoghegan T, Lawler L. Transvaginal oocyte retrieval complicated by life-threatening obturator artery haemorrhage and managed by a vessel-preserving technique. Ulster Med J 2014;83:146–148. [PMC free article] [PubMed] [Google Scholar]
- Bozdag G, Basaran A, Cil B, Esinler I, Yarali H. An oocyte pick-up procedure complicated with pseudoaneurysm of the internal iliac artery. Fertil Steril 2004;2008:e2011–e2003. [DOI] [PubMed] [Google Scholar]
- Bringer-Deutsch S, Mayenga JM, Grefenstette I, Grzegorczyk V, Kulski O, Belaisch-Allart J. In vitro fertilization: beware of oocyte retrieval without oocyte! Gynecol Obstet Fertil 2010;38:690–692. [DOI] [PubMed] [Google Scholar]
- Burnik Papler T, Vrtacnik Bokal E, Salamun V, Galic D, Smrkolj T, Jancar N. Ureteral injury with delayed massive hematuria after transvaginal ultrasound-guided oocyte retrieval. Case Rep Obstet Gynecol 2015;2015:760805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzarite T, Bernardi LA, Confino E, Kenton K. Ureteral trauma during transvaginal ultrasound-guided oocyte retrieval: a case report. Female Pelvic Med Reconstr Surg 2015;21:e44–e45. [DOI] [PubMed] [Google Scholar]
- Chatrian A, Vidal C, Equy V, Hoffmann P, Sergent F. Hemoperitoneum presenting with the use of a topical hemostatic agent in oocyte retrieval: a case report. J Med Case Rep 2012;6:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checketts MR. AAGBI recommendations for standards of monitoring during anaesthesia and recovery 2015. Anaesthesia 2016;71:470–471. [DOI] [PubMed] [Google Scholar]
- Cho SW, Choi HJ. Effect of music on reducing anxiety for patients undergoing transrectal ultrasound-guided prostate biopsies: randomized prospective trial. Urol J 2016;13:2612–2614. [PubMed] [Google Scholar]
- Choudhary RA, Bhise NM, Mehendale AV, Ganla KN. Ureteric injury during transvaginal oocyte retrieval (TVOR) and review of literature. J Hum Reprod Sci 2017;10:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun D, Gunaydin B, Tas A, Inan G, Celebi H, Kaya K. A comparison of three different target-controlled remifentanil infusion rates during target-controlled propofol infusion for oocyte retrieval. Clinics 2011;66:811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun S, Madan S, Bukhari I, Al-Hassan S, Al-Rejjal R, Awartani K. Poor prognosis in cycles following "genuine" empty follicle syndrome. Eur J Obstet Gynecol Reprod Biol 2010;150:157–159. [DOI] [PubMed] [Google Scholar]
- Dahl SK, Cannon S, Aubuchon M, Williams DB, Robins JC, Thomas MA. Follicle curetting at the time of oocyte retrieval increases the oocyte yield. J Assist Reprod Genet 2009;26:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LB, Ginsburg ES. Transmyometrial oocyte retrieval and pregnancy rates. Fertil Steril 2004;81:320–322. [DOI] [PubMed] [Google Scholar]
- De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens V et al. ART in Europe, 2014: results generated from European registries by ESHRE: the European IVF-monitoring consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod 2018;33:1586–1601. [DOI] [PubMed] [Google Scholar]
- Debusscher F, Troussel S, Van Innis F, Holemans X. Spondylodiscitis after transvaginal oocyte retrieval for in vitro fertilisation. Acta Orthop Belg 2005;71:249–251. [PubMed] [Google Scholar]
- Dellenbach P, Nisand I, Moreau L, Feger B, Plumere C, Gerlinger P, Brun B, Rumpler Y. Transvaginal, sonographically controlled ovarian follicle puncture for egg retrieval. Lancet 1984;1:1467. [DOI] [PubMed] [Google Scholar]
- den Boon J, Kimmel CE, Nagel HT, van Roosmalen J. Pelvic abscess in the second half of pregnancy after oocyte retrieval for in-vitro fertilization: case report. Hum Reprod 1999;14:2402–2403. [DOI] [PubMed] [Google Scholar]
- Denschlag D, von Wolff M, Amant F, Kesic V, Reed N, Schneider A, Rodolakis A. Clinical recommendation on fertility preservation in borderline ovarian neoplasm: ovarian stimulation and oocyte retrieval after conservative surgery. Gynecol Obstet Investig 2010;70:160–165. [DOI] [PubMed] [Google Scholar]
- Dessolle L, Leperlier F, Biau DJ, Freour T, Barriere P. Proficiency in oocyte retrieval assessed by the learning curve cumulative summation test. Reprod Biomed Online 2014;29:187–192. [DOI] [PubMed] [Google Scholar]
- El-Toukhy T, Hanna L. Pelvic infection after oocyte retrieval: a preventable complication or an inevitable risk? J Obstet Gynaecol 2006;26:701–703. [DOI] [PubMed] [Google Scholar]
- Enien WM, el Sahwy S, Harris CP, Seif MW, Elstein M. Human chorionic gonadotrophin and steroid concentrations in follicular fluid: the relationship to oocyte maturity and fertilization rates in stimulated and natural in-vitro fertilization cycles. Hum Reprod 1995;10:2840–2844. [DOI] [PubMed] [Google Scholar]
- European IVF monitoring Consortium for the European Society of Human Reproduction and Embryology, Calhaz-Jorge C, De C, Kupka MS, de J, Erb K, Mocanu E, Motrenko T, Scaravelli G, Wyns C et al. Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE†. Hum Reprod 2017;32:1957–1973. [DOI] [PubMed] [Google Scholar]
- Faymonville ME, Fissette J, Mambourg PH, Roediger L, Joris J, Lamy M. Hypnosis as adjunct therapy in conscious sedation for plastic surgery. Reg Anesth 1995;20:145–151. [PubMed] [Google Scholar]
- Fiori O, Cornet D, Darai E, Antoine JM, Bazot M. Uro-retroperitoneum after ultrasound-guided transvaginal follicle puncture in an oocyte donor: a case report. Hum Reprod 2006;21:2969–2971. [DOI] [PubMed] [Google Scholar]
- Fouda UM, Sayed AM, Abdelmoty HI, Elsetohy KA. Ultrasound guided aspiration of hydrosalpinx fluid versus salpingectomy in the management of patients with ultrasound visible hydrosalpinx undergoing IVF-ET: a randomized controlled trial. BMC Womens Health 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki M, Taguchi S, Hayashi T, Tada Y, Kitaya K, Iwaki Y, Karita M, Nakamura Y. Vaginal preparation with povidone iodine disinfection and saline douching as a safe and effective method in prevention of oocyte pickup-associated pelvic inflammation without spoiling the reproductive outcome: evidence from a large cohort study. Clin Exp Obstet Gynecol 2014;41:689–690. [PubMed] [Google Scholar]
- Gange SN, Baum NH. Verbal anesthesia: how it’s used in urologic procedures In: Gonzalez Christopher M. (ed). Urology Times, 2017
- Georgiou EX, Melo P, Brown J, Granne IE. Follicular flushing during oocyte retrieval in assisted reproductive techniques. Cochrane Database Syst Rev 2018;4:CD004634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Friberg J, Fullan N, Giglia RV, Mayden K, Kesky T, Siegel I. EGG retrieval for in vitro fertilisation by sonographically controlled vaginal culdocentesis. Lancet 1983;2:508–509. [DOI] [PubMed] [Google Scholar]
- Green-top Guideline No 52 Prevention and management of postpartum haemorrhage. BJOG 2016;124:e106–e149. [DOI] [PubMed] [Google Scholar]
- Grimbizis GF, Di Spiezio Sardo A, Saravelos SH, Gordts S, Exacoustos C, Van Schoubroeck D, Bermejo C, Amso NN, Nargund G, Timmerman D et al. The Thessaloniki ESHRE/ESGE consensus on diagnosis of female genital anomalies. Hum Reprod 2016; 31:2–7. Simultaneous publication in Gynecol Surg. 2016;2013:2011–2016. [DOI] [PubMed] [Google Scholar]
- Grynberg M, Berwanger AL, Toledano M, Frydman R, Deffieux X, Fanchin R. Ureteral injury after transvaginal ultrasound-guided oocyte retrieval: a complication of in vitro fertilization-embryo transfer that may lurk undetected in women presenting with severe ovarian hyperstimulation syndrome. Fertil Steril 2011;96:869–871. [DOI] [PubMed] [Google Scholar]
- Hammadieh N, Coomarasamy A, Ola B, Papaioannou S, Afnan M, Sharif K. Ultrasound-guided hydrosalpinx aspiration during oocyte collection improves pregnancy outcome in IVF: a randomized controlled trial. Hum Reprod 2008;23:1113–1117. [DOI] [PubMed] [Google Scholar]
- Han C, Wang C, Liu XJ, Geng N, Wang YM, Fan AP, Yuan BB, Xue FX. In vitro fertilization complicated by rupture of tubo-ovarian abscess during pregnancy. Taiwan J Obstet Gynecol 2015;54:612–616. [DOI] [PubMed] [Google Scholar]
- Hershlag A, Feng HL, Scholl GS. Betadine (povidone-iodine) is toxic to murine embryogenesis. Fertil Steril 2003;79:1249–1250. [DOI] [PubMed] [Google Scholar]
- Hinkelbein J, Lamperti M, Akeson J, Santos J, Costa J, De Robertis E, Longrois D, Novak-Jankovic V, Petrini F, Struys M et al. European Society of Anaesthesiology and European Board of Anaesthesiology guidelines for procedural sedation and analgesia in adults. Eur J Anaesthesiol 2018;35:6–24. [DOI] [PubMed] [Google Scholar]
- Horne R, Bishop CJ, Reeves G, Wood C, Kovacs GT. Aspiration of oocytes for in-vitro fertilization. Hum Reprod Update 1996;2:77–85. [DOI] [PubMed] [Google Scholar]
- Human Fertilisation and Embryology Authority, London, UK. Code of Practice, 2017
- Hwang JL, Kuo MC, Hsieh BC, Chang CH, Jou LC, Chen WH, Wu GJ. An acute psychiatric episode following transvaginal oocyte retrieval. Hum Reprod 2002;17:1124–1126. [DOI] [PubMed] [Google Scholar]
- Iikura M, Yamaguchi M, Hirai K, Suenaga A, Fujiwara T, Fujii T, Taketani Y, Yamamoto K. Case report: streptomycin-induced anaphylactic shock during oocyte retrieval procedures for in vitro fertilization. J Allergy Clin Immunol 2002;109:571–572. [DOI] [PubMed] [Google Scholar]
- Kart C, Guven S, Aran T, Dinc H. Life-threatening intraabdominal bleeding after oocyte retrieval successfully managed with angiographic embolization. Fertil Steril 2011;96:e99–e102. [DOI] [PubMed] [Google Scholar]
- Kasapoglu I, Turk P, Dayan A, Uncu G. Does the presence of endometriosis cause a challenge for transvaginal oocyte retrieval? A comparison between patients with and without endometriosis. J Turk Ger Gynecol Assoc 2018;19:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelada E, Ghani R. Bilateral ovarian abscesses following transvaginal oocyte retrieval for IVF: a case report and review of literature. J Assist Reprod Genet 2007;24:143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, Yun NR, Kim DM, Kim SA. Successful delivery following Staphylococcus aureus bacteremia after in vitro fertilization and embryo transfer. Chonnam Med J 2015;51:47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Lee WS, Yoon TK, Han JE. Term delivery following tuboovarian abscess after in vitro fertilization and embryo transfer. Am J Obstet Gynecol 2013;208:e3–e6. [DOI] [PubMed] [Google Scholar]
- Kumaran A, Narayan PK, Pai PJ, Ramachandran A, Mathews B, Adiga SK. Oocyte retrieval at 140-mmHg negative aspiration pressure: a promising alternative to flushing and aspiration in assisted reproduction in women with low ovarian reserve. J Hum Reprod Sci 2015;8:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan I, Wang R, Pearce E, Bhattacharya S. Pain relief for women undergoing oocyte retrieval for assisted reproduction. Cochrane Database Syst Rev 2018;5:CD004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Setti PE, Cirillo F, Scolaro V, Morenghi E, Heilbron F, Girardello D, Zannoni E, Patrizio P. Appraisal of clinical complications after 23,827 oocyte retrievals in a large assisted reproductive technology program. Fertil Steril 2018;109:1038–1043e1031. [DOI] [PubMed] [Google Scholar]
- Liberty G, Hyman JH, Eldar-Geva T, Latinsky B, Gal M, Margalioth EJ. Ovarian hemorrhage after transvaginal ultrasonographically guided oocyte aspiration: a potentially catastrophic and not so rare complication among lean patients with polycystic ovary syndrome. Fertil Steril 2010;93:874–879. [DOI] [PubMed] [Google Scholar]
- Ludwig AK, Glawatz M, Griesinger G, Diedrich K, Ludwig M. Perioperative and post-operative complications of transvaginal ultrasound-guided oocyte retrieval: prospective study of >1000 oocyte retrievals. Hum Reprod 2006;21:3235–3240. [DOI] [PubMed] [Google Scholar]
- Lutz H, Buscarini E. Manual of Diagnostic Ultrasound. World Health Organization, Geneva, 2013 [Google Scholar]
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, Committee HICPA. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 1999;20:247–280. [DOI] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiach R, Stockheim D, Zolti M, Orvieto R. Delayed intra-abdominal bleeding following trans-vaginal ultrasonography guided oocyte retrieval for in vitro fertilization in patients at risk for thrombo-embolic events under anticoagulant therapy. F1000Res 2013;2:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matorras R, Aparicio V, Corcostegui B, Prieto B, Mendoza R, Ramon O, Gomez-Picado O, Exposito A. Failure of intrauterine insemination as rescue treatment in low responders with adequate HCG timing with no oocytes retrieved. Reprod Biomed Online 2014;29:634–639. [DOI] [PubMed] [Google Scholar]
- Matorras R, Meabe A, Mendoza R, Prieto B, Ramon O, Mugica J, Aspichueta F, Exposito A. Human chorionic gonadotropin (hCG) plasma levels at oocyte retrieval and IVF outcomes. J Assist Reprod Genet 2012;29:1067–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matorras R, Rubio K, Iglesias M, Vara I, Exposito A. Risk of pelvic inflammatory disease after intrauterine insemination: a systematic review. Reprod Biomed Online 2018;36:164–171. [DOI] [PubMed] [Google Scholar]
- Matsunaga Y, Fukushima K, Nozaki M, Nakanami N, Kawano Y, Shigematsu T, Satoh S, Nakano H. A case of pregnancy complicated by the development of a tubo-ovarian abscess following in vitro fertilization and embryo transfer. Am J Perinatol 2003;20:277–282. [DOI] [PubMed] [Google Scholar]
- Maxwell KN, Cholst IN, Rosenwaks Z. The incidence of both serious and minor complications in young women undergoing oocyte donation. Fertil Steril 2008;90:2165–2171. [DOI] [PubMed] [Google Scholar]
- Meyer L, Murphy LA, Gumer A, Reichman DE, Rosenwaks Z, Cholst IN. Risk factors for a suboptimal response to gonadotropin-releasing hormone agonist trigger during in vitro fertilization cycles. Fertil Steril 2015;104:637–642. [DOI] [PubMed] [Google Scholar]
- Miller PB, Price T, Nichols JE Jr, Hill L. Acute ureteral obstruction following transvaginal oocyte retrieval for IVF. Hum Reprod 2002;17:137–138. [DOI] [PubMed] [Google Scholar]
- Mmbaga N, Torrealday S, McCarthy S, Rackow BW. Acute portal vein thrombosis complicating in vitro fertilization. Fertil Steril 2012;98:1470–1473. [DOI] [PubMed] [Google Scholar]
- Modder J, Kettel LM, Sakamoto K. Hematuria and clot retention after transvaginal oocyte aspiration: a case report. Fertil Steril 2006;86:e721–e722. [DOI] [PubMed] [Google Scholar]
- Moini A, Riazi K, Amid V, Ashrafi M, Tehraninejad E, Madani T, Owj M. Endometriosis may contribute to oocyte retrieval-induced pelvic inflammatory disease: report of eight cases. J Assist Reprod Genet 2005;22:307–309. [DOI] [PubMed] [Google Scholar]
- Mongiu AK, Helfand BT, Kielb SJ. Ureterovaginal fistula formation after oocyte retrieval. Urology 2009;73:e441–e443. [DOI] [PubMed] [Google Scholar]
- Nargund G, Waterstone J, Bland J, Philips Z, Parsons J, Campbell S. Cumulative conception and live birth rates in natural (unstimulated) IVF cycles. Hum Reprod 2001;16:259–262. [DOI] [PubMed] [Google Scholar]
- Nikkhah-Abyaneh Z, Khulpateea N, Aslam MF. Pyometra after ovum retrieval for in vitro fertilization resulting in hysterectomy. Fertil Steril 2010;93:e261–e262. [DOI] [PubMed] [Google Scholar]
- Ozaltin S, Kumbasar S, Savan K. Evaluation of complications developing during and after transvaginal ultrasound—guided oocyte retrieval. Ginekol Pol 2018;89:1–6. [DOI] [PubMed] [Google Scholar]
- Panayotidis C. Interventional ultrasound: standardisation of oocyte retrieval in assisted reproduction treatments. Dissertation. Postgraduate Medical School University of Cardiff. UK: Cardiff University; 2017. https://www.researchgate.net/publication/327142563_Interventional_Ultrasound_Standardisation_of_Oocyte_Retrieval_in_Assisted_Reproduction_Treatments/related. [Google Scholar]
- Pappin C, Plant G. A pelvic pseudoaneurysm (a rare complication of oocyte retrieval for IVF) treated by arterial embolization. Hum Fertil (Camb) 2006;9:153–155. [DOI] [PubMed] [Google Scholar]
- Patounakis G, Krauss K, Nicholas SS, Baxter JK, Rosenblum NG, Berghella V. Development of pelvic abscess during pregnancy following transvaginal oocyte retrieval and in vitro fertilization. Eur J Obstet Gynecol Reprod Biol 2012;164:116–117. [DOI] [PubMed] [Google Scholar]
- Piroli A, Marci R, Marinangeli F, Paladini A, Di G, Giovanni Artini P, Caserta D, Tatone C. Comparison of different anaesthetic methodologies for sedation during in vitro fertilization procedures: effects on patient physiology and oocyte competence. Gynecol Endocrinol 2012;28:796–799. [DOI] [PubMed] [Google Scholar]
- Ragni G, Scarduelli C, Calanna G, Santi G, Benaglia L, Somigliana E. Blood loss during transvaginal oocyte retrieval. Gynecol Obstet Investig 2009;67:32–35. [DOI] [PubMed] [Google Scholar]
- RCOG Reproductive Medicine Curriculum and Logbook in 2015. https://www.rcog.org.uk/en/careers-training/specialty-training-curriculum/subspecialty-training/reproductive-medicine-subspecialty-training/#post (31 May 2019, date last accessed).
- Risquez F, Confino E. Can Doppler ultrasound-guided oocyte retrieval improve IVF safety? Reprod Biomed Online 2010;21:444–445. [DOI] [PubMed] [Google Scholar]
- Rolland L, Perrin J, Villes V, Pellegrin V, Boubli L, Courbiere B. IVF oocyte retrieval: prospective evaluation of the type of anesthesia on live birth rate, pain, and patient satisfaction. J Assist Reprod Genet 2017;34:1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Rodriguez CF, Weissbrot E, Hsu CD, Wong A, Siefert C, Sung L. Comparing transabdominal and transvaginal ultrasound-guided follicular aspiration: a risk assessment formula. Taiwan J Obstet Gynecol 2015;54:693–699. [DOI] [PubMed] [Google Scholar]
- Romero B, Aibar L, Martinez Navarro L, Fontes J, Calderon MA, Mozas J. Pelvic abscess after oocyte retrieval in women with endometriosis: a case series. Iran J Reprod Med 2013;11:677–680. [PMC free article] [PubMed] [Google Scholar]
- Roseveare C, Seavell C, Patel P, Criswell J, Kimble J, Jones C, Shepherd H. Patient-controlled sedation and analgesia, using propofol and alfentanil, during colonoscopy: a prospective randomized controlled trial. Endoscopy 1998;30:768–773. [DOI] [PubMed] [Google Scholar]
- Schulman JD, Dorfmann A, Jones S, Joyce B, Hanser J. Outpatient in vitro fertilization using transvaginal oocyte retrieval and local anesthesia. N Engl J Med 1985;312:1639. [PubMed] [Google Scholar]
- Sharpe K, Karovitch AJ, Claman P, Suh KN. Transvaginal oocyte retrieval for in vitro fertilization complicated by ovarian abscess during pregnancy. Fertil Steril 2006;86:e211–e213. [DOI] [PubMed] [Google Scholar]
- Short TG, Chui PT. Propofol and midazolam act synergistically in combination. Br J Anaesth 1991;67:539–545. [DOI] [PubMed] [Google Scholar]
- Siristatidis CS, Vrachnis N, Creatsa M, Maheshwari A, Bhattacharya S. In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database Syst Rev 2013. 10, CD006606. doi: 10.1002/14651858.CD006606.pub3. [DOI] [PubMed] [Google Scholar]
- Soave I, D’Angelo A, Piva I, Marci R. A pilot study on oocyte retrieval simulator: a new tool for training? J Med Syst 2019;43:202. [DOI] [PubMed] [Google Scholar]
- Song XM, Jiang H, Zhang WX, Zhou Y, Ni F, Wang XM. Ultrasound sclerotherapy pretreatment could obtain a similar effect to surgical intervention on improving the outcomes of in vitro fertilization for patients with hydrosalpinx. J Obstet Gynaecol Res 2017;43:122–127. [DOI] [PubMed] [Google Scholar]
- Sõritsa D, Sõritsa A, Peters M, Salumets A. A case report of broken needle during complicated oocyte retrieval In: 7th International IVI Congress|Reproductive Medicine & Beyond; May 11th to 13th 2017; Bilbao, Spain, 2017
- Spencer ES, Hoff HS, Steiner AZ, Coward RM. Immediate ureterovaginal fistula following oocyte retrieval: a case and systematic review of the literature. Urol Ann 2017;9:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler KJ, Zhao Y, Weissman A, Majumdar A, Leong M, Shoham Z. Worldwide survey of IVF practices: trigger, retrieval and embryo transfer techniques. Arch Gynecol Obstet 2014;290:561–568. [DOI] [PubMed] [Google Scholar]
- Traina V, Boyer P, Piccinni O, Costantino G, D'Amato G, D'Addario V, Cho YS. Failed oocyte retrieval in in-vitro fertilization with documented positive serum beta-human chorionic gonadotrophin (HCG) concentration on day HCG+1. Hum Reprod 1993;8:1854–1855. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Lin MY, Chen SH, Chung MT, Loo TC, Huang KF, Lin LY. Vaginal disinfection with povidone iodine immediately before oocyte retrieval is effective in preventing pelvic abscess formation without compromising the outcome of IVF-ET. J Assist Reprod Genet 2005;22:173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadivelu N,Mitra S, Schermer E, Kodumudi V, Kaye AD, Urman RD. Preventive analgesia for postoperative pain control: a broader concept. Local Reg Anesth 2014;7:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoecke F, Beuckelaers E, Lissens P, Boudewijns M. Actinomyces urogenitalis bacteremia and tubo-ovarian abscess after an in vitro fertilization (IVF) procedure. J Clin Microbiol 2013;51:4252–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varras M, Polyzos D, Tsikini A, Antypa E, Apessou D, Tsouroulas M. Ruptured tubo-ovarian abscess as a complication of IVF treatment: clinical, ultrasonographic and histopathologic findings. A case report. Clin Exp Obstet Gynecol 2003;30:164–168. [PubMed] [Google Scholar]
- Vermeulen N, Le Clef N, D'Angelo A, Veleva Z, Tilleman K. Manual for the Development of Recommendations for Good Practice, 2018. [DOI] [PubMed]
- Villette C, Bourret A, Santulli P, Gayet V, Chapron C, de Ziegler D. Risks of tubo-ovarian abscess in cases of endometrioma and assisted reproductive technologies are both under- and overreported. Fertil Steril 2016;106:410–415. [DOI] [PubMed] [Google Scholar]
- Vilos AG, Feyles V, Vilos GA, Oraif A, Abdul-Jabbar H, Power N. Ureteric injury during transvaginal ultrasound guided oocyte retrieval. J Obstet Gynaecol Can 2015;37:52–55. [DOI] [PubMed] [Google Scholar]
- von Eye CH, Moretto M, D'Avila AM, Berger M. Immediate ureterovaginal fistula secondary to oocyte retrieval--a case report. Fertil Steril 2006;2008:e2001–e2003. [DOI] [PubMed] [Google Scholar]
- Weiss A, Neril R, Geslevich J, Lavee M, Beck-Fruchter R, Golan J, Shalev E. Lag time from ovulation trigger to oocyte aspiration and oocyte maturity in assisted reproductive technology cycles: a retrospective study. Fertil Steril 2014;102:419–423. [DOI] [PubMed] [Google Scholar]
- World Alliance for Patient Safety WHO Surgical Safety Checklist, 2008.
- Yalcinkaya TM, Erman-Akar M, Jennell J. Term delivery following transvaginal drainage of bilateral ovarian abscesses after oocyte retrieval: a case report. J Reprod Med 2011;56:87–90. [PubMed] [Google Scholar]
- Yao M, Schust D. Infertility In: Berek JS. (ed). Novak’s Gynecology. Philadelphia, PA: Lippincott Company, 2002 [Google Scholar]
- Yeo JK, Cho DY, Oh MM, Park SS, Park MG. Listening to music during cystoscopy decreases anxiety, pain, and dissatisfaction in patients: a pilot randomized controlled trial. J Endourol 2013;27:459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn AM, Ko YK, Kim YH. Anesthesia and sedation outside of the operating room. Korean J Anesthesiol 2015;68:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WY, Li Wan Po A. Analgesic efficacy of paracetamol and its combination with codeine and caffeine in surgical pain--a meta-analysis. J Clin Pharm Ther 1996;21:261–282. [DOI] [PubMed] [Google Scholar]
- Zhang ZS, Wang XL, Xu CL, Zhang C, Cao Z, Xu WD, Wei RC, Sun YH. Music reduces panic: an initial study of listening to preferred music improves male patient discomfort and anxiety during flexible cystoscopy. J Endourol 2014;28:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Qiao J, Ma C, Fan Y, Liu P. Intraperitoneal bleeding following transvaginal oocyte retrieval. Int J Gynaecol Obstet 2010;108:31–34. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang H, Zhang WX, Ni F, Wang XM, Song XM. Ultrasound-guided aspiration of hydrosalpinx occurring during controlled ovarian hyperstimulation could improve clinical outcome of in vitro fertilization-embryo transfer. J Obstet Gynaecol Res 2016;42:960–965. [DOI] [PubMed] [Google Scholar]
- Zreik TG, Garcia-Velasco JA, Vergara TM, Arici A, Olive D, Jones EE. Empty follicle syndrome: evidence for recurrence. Hum Reprod 2000;15:999–1002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


