Abstract
Memory retrieval involves the interaction between external sensory or internally generated cues and stored memory traces (or engrams) in a process termed ‘ecphory’. While ecphory has been examined in human cognitive neuroscience research, its neurobiological foundation is less understood. To the extent that ecphory involves ‘reawakening’ of engrams, leveraging recently developed technologies that can identify and manipulate engrams in rodents provides a fertile avenue for examining retrieval at the level of neuronal ensembles. Here we evaluate emerging neuroscientific research of this type, using cognitive theory as a guiding principle to organize and interpret initial findings. Our Review highlights the critical interaction between engrams and retrieval cues (environmental or artificial) for memory accessibility and retrieval success. These findings also highlight the intimate relationship between the mechanisms important in forming engrams and those important in their recovery, as captured in the cognitive notion of ‘encoding specificity’. Finally, we identify several questions that currently remain unanswered.
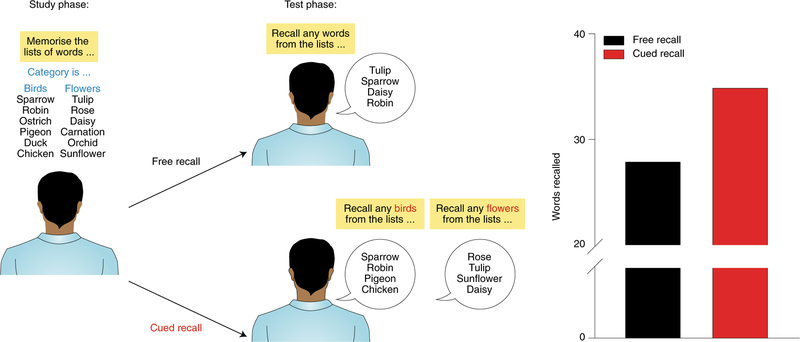
In 1966, Tulving and Pearlstone1 reported a highly influential finding that profoundly altered the direction of subsequent research on memory in ways that few papers do. Up until this point, almost all experimental research on human memory was concerned with learning or forgetting. The prevalent perspective at the time considered failure in memory performance as the outcome of two possible scenarios. Failure might indicate either that information had not been learned or that it had been learned but subsequently forgotten. However, Tulving and Pearlstone’s work suggested a third possibility. Memory failure could also reflect a problem in retrieval. Specifically, they demonstrated that the same memory could be retrieved successfully with some retrieval cues, but not others (Fig. 1).
Fig. 1 |. Tulving and Pearlstone’s experiment on retrieval failure1.
Subjects were presented with a series of words. These words were drawn from multiple categories (for example, types of birds, flowers, etc.). In the test phase, subjects were asked to recall as many words as they could from the list (free recall) or from the specific categories (cued recall). The cued recall group performed considerably better than the free recall group across categories, indicating that retrieval cues present at the time of recall determine engram accessibility and subsequent success at remembering.
From this work, Tulving developed an important conceptual distinction between availability versus accessibility of information in memory. According to this view2,3, some forms of memory failure reflect a lack of availability of pertinent information (i.e., permanent loss), whereas other forms of memory failure reflect temporary problems in accessibility. Phenomenologically, this relates to the common ‘tip of the tongue’ experience, in which one might struggle to recall a familiar name or place while having the strong impression that the information is present. Indeed, often this information subsequently comes to mind. Cues available at retrieval represent perhaps the most critical factor that determines memory accessibility and corresponding success at remembering.
In making this distinction between memory availability versus accessibility, Tulving also recognized3 earlier work by Richard Semon, a German scientist working at the turn of the twentieth century. Semon4 first emphasized the role of retrieval cues in remembering and introduced specific terminology to capture this process. Ecphory describes the memory retrieval process, and Semon argued that ecphory reflects the unique interplay between cues and stored memory traces at retrieval. He also coined the term engram’ to refer to such memory traces as biological entities; this may be considered his better-known contribution to the field5. Although engrams had not yet been identified empirically, the concept of ecphory became central to the cognitive psychology of memory retrieval6.
In the last decade, enormous progress has been made in identifying and manipulating engrams in rodents7–10. In large part, this progress may be attributed to the development of tools that allow researchers to map engrams to specific neuronal ensembles and manipulate these ensembles using genetically encoded actuators10–15 (Box 1). To date, these approaches have provided evidence for the existence of engrams at the cellular level7–9, but they may also shed light on the biological basis of memory retrieval16,17 (or, more precisely, ecphory). To the extent that ecphory involves reawakening specific engrams, the ability to identify and manipulate engrams is a prerequisite for gaining mechanistic insights into the retrieval process at the level of neuronal ensembles. Therefore, the recent progress in understanding engrams puts us in position to ask meaningful questions about the neurobiological basis of retrieval. Here we evaluate contemporary neuroscientific research on retrieval at the level of neuronal ensembles using the conceptual framework introduced by Semon and later elaborated by Tulving in his empirical and theoretical work. Although this research also has potentially interesting translational implications, they will not be covered here (but see ref.18).
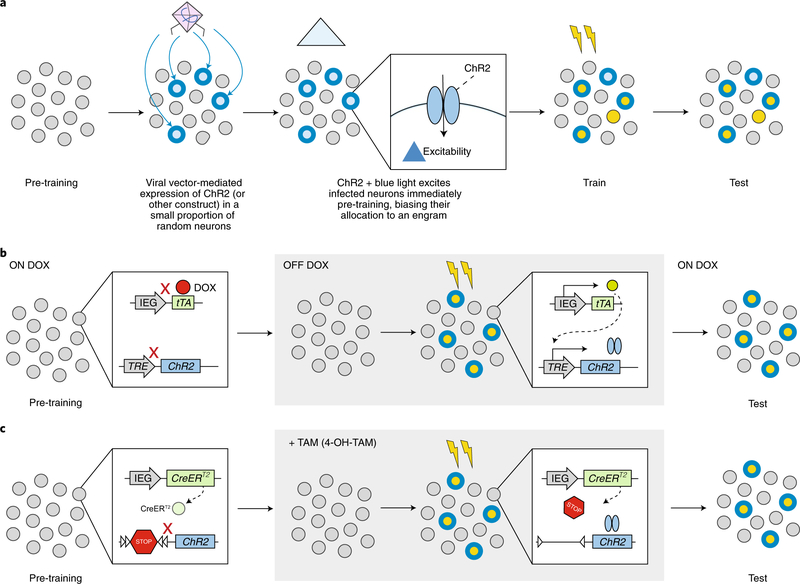
Box 1 |. Approaches for tagging and manipulating engrams in rodents.
Allocation
The allocation strategy takes advantage of the finding that, within a given brain region, eligible excitatory neurons compete for allocation to an engram. This strategy biases which neurons are allocated to an engram by artificially manipulating excitability before a training event. For example, before a training event, a small, random subpopulation of excitatory neurons (purple) is infected with a viral vector expressing a transgene that increases neuronal excitability, such as ChR2 (Box Fig. a)21,27 or CREB20,22,23,108,109. Infected neurons with relatively greater excitability at the time of training are biased for allocation to a resulting engram (red outline). Once allocated, these neurons become both necessary (indispensable) and sufficient (inducing) components of the engram supporting a memory.
Tagging
In the tagging strategy, neurons that happen to be sufficiently active (that would normally express an activity-dependent immediate early gene) at the time of training are tagged with an actuator (such as an excitatory or inhibitory opsin or chemogenetic construct). To tag active neurons, activity-dependent immediate early gene (IEG) promoters (c-Fos, Arc or others, including synthetic promoters such as E-SARE (enhanced synaptic activity-responsive element)110) are paired with an inducer that ‘opens the tagging window’. Two general types of inducers are used:
Tetracycline transactivator (tTA)-inducible tagging system. The initial studies13,26 using this approach took advantage of two transgenic mouse lines (but viral vectors can also be used14). In the first transgenic line, tTA (tetracycline-controlled transactivator) is expressed downstream of an IEG promoter. In active cells, neural activity results in tTA expression. However, this process is blocked in the presence of doxycycline (DOX). In second transgenic mouse line, the transgene of interest (depicted as ChR2 in Box Fig. b) is expressed downstream of a tetracycline response element (TRE). TRE is activated by tTA. Therefore, the absence of DOX opens the tagging window, allowing the transgene of interest to be expressed in active cells.
Cre recombinase-inducible tagging system. In this system, two transgene cassettes are generally used. In the first, a tamoxifen (TAM)-dependent Cre recombinase (CreERT2) is expressed under control of an IEG promoter while in the second, a loxP-flanked STOP signal is placed between a constitutive promoter and the transgene of interest (Box Fig. c). In the absence of TAM, the transgene is not expressed. However, in the presence of TAM, Cre recombinase translocates to the nucleus, cleaves the loxP sites, and removes the STOP signal, allowing expression of the transgene. TAM administration opens the tagging window allowing the transgene of interest to be expressed in active cells12,111.
Manipulating retrieval
Ecphory emphasizes that retrieval reflects interactions between cues, either external sensory or internally generated, and the engram. In other words, memory retrieval can be understood as cue-induced behavioral expression of the engram. It may occur in situations where we intentionally strive to recover a memory in relation to a specific cue (for example, trying to remember where we initially encountered a person we just again met). In other situations, cues may spontaneously trigger memory retrieval (for example, seeing a picture of Paris and remembering a recent visit there).
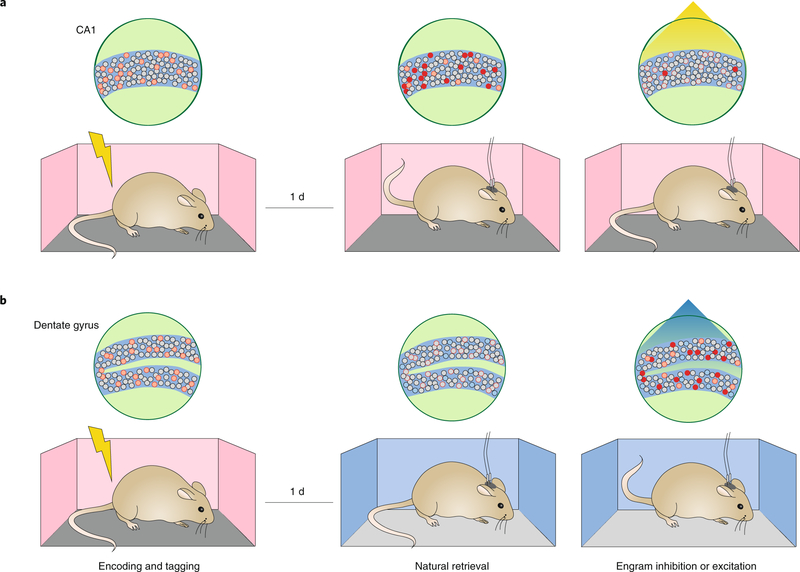
Contemporary engram studies have examined ecphory in three ways. The first type of experiment asked whether it is possible to prevent ecphory in the presence of external sensory retrieval cues (Fig. 2a). For instance, Tanaka and colleagues19 used a tetracycline-based system (TetTag) to label a contextual fear memory engram in mice, such that CA1 neuronal ensembles that were active during conditioning expressed an inhibitory opsin (ArchT). When subsequently placed back in the training context, mice typically freeze, indicating they recognized that this was the place where the footshock was previously administered. Critically, optogenetic inhibition of the ArchT-tagged neuronal ensemble during this test session reduced conditioned freezing levels (indicating impairment in memory retrieval). Of particular relevance, from the perspective of ecphory, is that the freezing behavior was context-specific (i.e., cue-specific). When a non-overlapping neuronal ensemble tagged in a different context (context B) was silenced during contextual fear testing in context A, mice froze, indicating that this intervention did not interfere with retrieval of the context A fear memory. Similar disruption of cue-induced retrieval by silencing corresponding engrams was observed across a variety of experimental conditions. These include silencing other brain regions (for example, the amygdala20–22 and insular cortex23), in tasks other than fear conditioning (for example, cocaine-cue memory24), as well as using alternate genetic ensemble tagging systems (for example, cre-inducible systems11,12,25).
Fig. 2 |. Preventing and inducing ecphory by direct manipulation of fear memory engrams.
a, In this experiment19, neuronal ensembles in the CA1 region of the hippocampus were tagged with the inhibitory opsin, ArchT, during contextual fear conditioning (left). When placed back into the training context (i.e., the retrieval cue), mice froze (middle). However, optogenetic inhibition of the tagged ensemble during this test reduced freezing levels (right), indicating that engram silencing can prevent ecphory even in the presence of natural retrieval cues. b, In this experiment26, neuronal ensembles in the DG region of the hippocampus were tagged with the excitatory opsin, ChR2, during contextual fear conditioning (left). When placed into a distinct context, mice did not freeze (middle). However, optogenetic activation of the tagged ensemble during this test induced freezing (right), indicating that engram activation, in the absence of natural retrieval cues, can induce ecphory.
The second type of experiment asked the converse question: is it possible to induce memory expression in the absence of sensory retrieval cues via direct stimulation of a tagged engram (Fig. 2b)? For instance, Liu and colleagues26 used a similar TetTag approach to express an excitatory opsin (ChR2) in neuronal ensembles that were active during contextual fear conditioning. Following conditioning, placing mice in a context distinct from the training context resulted in little freezing behavior. However, direct photostimulation of the ChR2-tagged neuronal ensemble in the dentate gyrus (DG) induced freezing. Subsequent studies generalized these findings across experimental conditions11,25 and in other brain regions (including the lateral amygdala (LA)27–30, basolateral amygdala (BLA)31 and retrosplenial cortex32). Together, these types of experiments indicate it is possible to bypass the requirement for natural retrieval cues in ecphory and to induce memory expression via direct stimulation of the putative engram. One interpretation is that stimulation reflects a reinstatement of an otherwise natural cue.
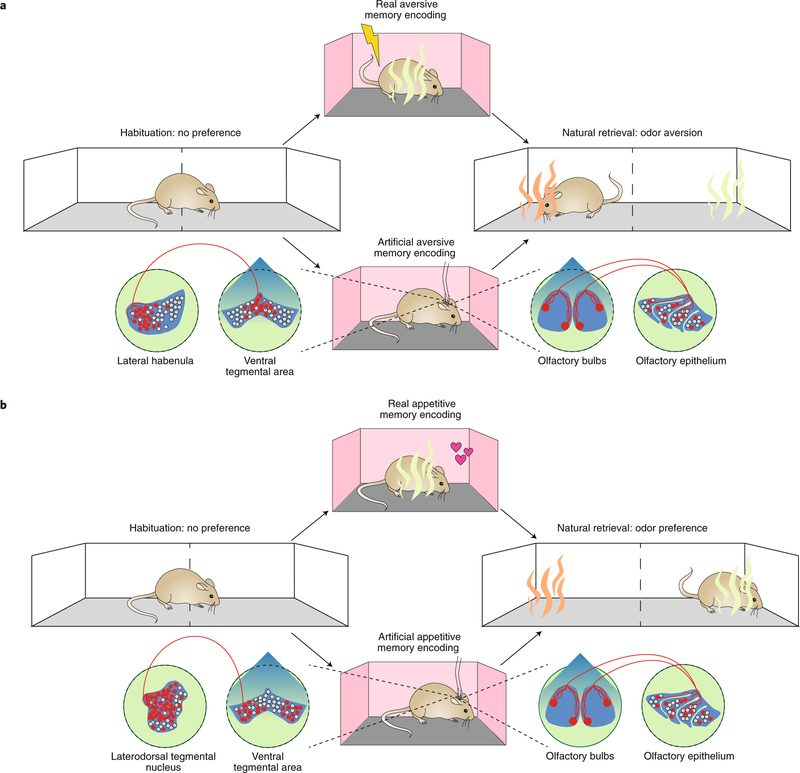
The first two types of studies used experience-dependent tagging approaches to label neurons that were endogenously active at the time of an event, and then used artificial means (for example, photostimulation) to either block or elicit ecphory. This begs the question of whether the opposite is possible: to create an engram by artificial means and then probe ecphory using natural cues. This question has been addressed in the third type of study considered here (Fig. 3). In this study33, photostimulation of a specific olfactory glomerulus (M72) was paired with photostimulation of specific projections that mediate aversion (from the lateral habenula to the ventral tegmental area (VTA)) to create an artificial engram. When mice were subsequently presented with a real M72-activating odorant (acetophenone), they exhibited conditioned avoidance, even though they had not encountered this odor previously. If, instead, M72 activation was paired with photostimulation of reward-mediating projections (laterodorsal tegmental nucleus → VTA), mice subsequently approached, rather than avoided, the M72 odorant, acetophenone. Retrieval of these artificially generated memories and real odor memories (in which acetophenone was actually paired with shock) engaged similar neural circuits, and suppressing neuronal activity in the BLA prevented expression of both artificial and real memories. Three aspects of this work illustrate nicely the tight interplay between engrams and retrieval cues, as initially suggested by Semon. First, artificial engram expression was demonstrated via presentation of a natural external sensory retrieval cue. Second, memory expression reflected the predicted content of the stored information (i.e., mice either approached or avoided acetophenone, depending on which VTA inputs, rewarding or aversive, were stimulated during the training phase). Third, behavioral responding was restricted to the trained cue and did not occur in the presence of unrelated cues.
Fig. 3 |. Ecphory for an artificially generated engram.
a, In these experiments33, mice formed either a real (top) or an artificial (bottom) odor aversion memory. For the real odor memory, an odor (acetophenone; green) was paired with shock during training. When mice were subsequently presented with the conditioned odor (acetophenone) or a distinct odor (carvone; orange), mice exhibited conditioned aversion to acetophenone. For the artificial odor memory, photostimulation of a specific olfactory glomerulus (M72) was paired with photostimulation of lateral habenula inputs into the VTA. When mice were subsequently tested, they avoided the M72 odorant acetophenone (green), preferring to spend time on the carvone (non-M72 odorant; orange) side of the apparatus. b, In these experiments33, mice formed either a real (top) or an artificial (bottom) odor attraction memory. For the real odor memory, an odor (acetophenone; green) was paired with food during training. When mice were subsequently presented with the conditioned odor (acetophenone) or a distinct odor (carvone; orange), mice exhibited conditioned attraction to acetophenone. For the artificial odor memory, photostimulation of the M72 olfactory glomerulus was paired with photostimulation of laterodorsal tegmental nucleus inputs into the VTA. When mice were subsequently tested, they approached (rather than avoided) the M72 odorant acetophenone (green), even though they had never had never encountered this odor previously.
Accessibility of engrams
The studies reviewed so far indicate that it is possible to both disrupt and to mimic ecphory by directly manipulating the activity of neuronal ensembles that were active during encoding. However, they do not address Tulving’s distinction between engram accessibility versus availability. Another category of studies speaks to this distinction, aiming to recover apparently ‘lost’ memories via direct optogenetic stimulation of the tagged engram. By doing so, these studies shed light on the biological mechanisms that distinguish whether a memory can be accessed in principle or not (i.e., when it is unavailable).
In one experiment, Ryan and colleagues30 tagged neuronal ensembles in either the DG or CA1 region of the hippocampus that were activated during contextual fear conditioning. Immediately following training, mice were treated with the protein synthesis inhibitor anisomycin and were tested 1 day later by returning mice to the training context. As expected, protein synthesis inhibition impaired consolidation and prevented subsequent memory expression. Despite this apparent amnesia in the presence of natural retrieval cues, however, optogenetic reactivation of the tagged neuronal ensemble enabled memory recovery30.
Similar recovery from amnesia has been observed across a range of conditions. For instance, following post-training protein synthesis inhibition, artificial engram reactivation in the DG or LA allows for recovery of place aversion or tone fear memories, respectively30,34. Moreover, memory recovery is not limited to amnestic states produced by protein synthesis inhibition during the consolidation period. Protein synthesis inhibition following natural memory retrieval blocks reconsolidation35,36, and this lost memory can be recovered via artificial engram reactivation30. Memory recovery has also been observed from other amnestic states, including in mouse models for studying Alzheimer’s disease37,38, infantile amnesia that naturally occurs in early development39, and following natural forgetting of social memories40.
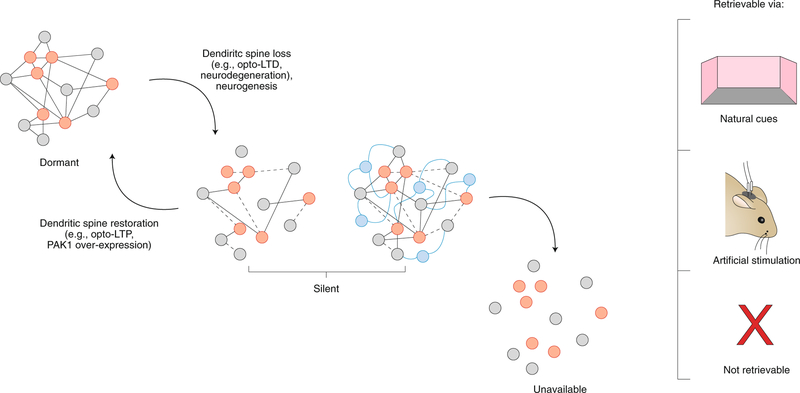
These results suggest that the underlying engram corresponding to the presumably forgotten event is not completely erased or, using Tulving’s terminology, unavailable. Rather, these engrams exist in otherwise inaccessible states, in which natural retrieval cues (such as exposure to the training context) typically are not sufficient to induce successful ecphory and resulting memory expression. Engrams in this state have been termed ‘silent’37. This is distinct from the notion of latent engrams introduced by Semon, which are both available and accessible through natural cues in principle, only not being accessed in the moment. By contrast, the silent engram is an in-between state: it is available, but nonetheless inaccessible by any natural means. Recent work shows that during engram formation, there is a specific increase in synapses between ‘engram cells’30,41,42. Maintaining these enhanced synaptic connections may be key to their later accessibility, as evidence suggests that weakening synaptic connections among the neurons of the critical ensembles and, additionally, between these ensembles and downstream regions, is associated with engram silencing30,34,37. Direct photostimulation of the silent engram may temporarily reinstate these weakened connections, leading to memory recovery.
While photostimulation of silent engrams induces memory expression, memory recovery is only transient: freezing behavior is typically only observed during photostimulation30,34,37–40. The absence of memory expression in the light-off epochs suggests that the engram remained inaccessible by natural cues. Might interventions that permanently reinstate connectivity shift an engram from a silent state back into a latent state, where it is available and accessible through natural cues? A number of strategies have been used to address this question. For instance, spine density is reduced on DG and CA1 neurons in mouse models for Alzheimer’s disease. High-frequency photostimulation of perforant path afferents (i.e., ‘opto-LTP’) restores spine density on these engram cells, as well as their connectivity to downstream targets (for example, in CA3 and BLA). Critically, in these experiments, presentation of natural cues (i.e., the training context) was now sufficient to induce memory expression in tests performed several days later, suggesting that the opto-LTP intervention had successfully transformed the engram from a silent to latent state37. Similarly, overexpression of a dominant active form of PAK1 in experience-tagged CA1 neurons restores spine density and allows memories lost through protein synthesis inhibition to be recovered by natural cues34. In related work, Nabavi and colleagues43 demonstrated that it was possible to modulate engram accessibility by manipulating the strength of synaptic inputs to the LA using opto-LTP (long-term potentiation) and opto-LTD (long-term depression) protocols.
The general picture emerging from this work is that engrams can differ in their degree of accessibility (Fig. 4) and that changes in accessibility reflect underlying changes in synaptic organization. Silent engrams are unique in that they can only be accessed by artificial means. The silent state may be transitional and mark the boundary between lack of engram accessibility and availability.
Fig. 4 |. Silencing of the engram. Engrams exist in different states of accessibility.
Engrams exist in a dormant state (where natural retrieval cues induce engram activation and successful retrieval), a silent state (where only direct optogenetic engram activation induces successful retrieval) and an unavailable state (where all information has been lost, and the memory is inaccessible regardless of the nature of access attempts). Transitions from dormant → silent→ unavailable likely reflect forgetting mechanisms (for example, weakening and loss of synaptic connectivity among engram cells or the addition of new connectivity as a consequence of neurogenesis). LTD, long-term depression.
Above we discussed the fact that some seemingly lost memories may simply be inaccessible by natural cues. Are some memories entirely unavailable? This is a difficult, if not impossible, question to answer. To the extent that any testing involves exploration with a finite number of cues, it is always a possibility that successful memory recovery could be achieved with cues that were not tested44. Similarly, failure to recover memories with optogenetic stimulation of tagged ensembles might simply reflect failure to test all stimulation protocols. Methods allowing for unambiguous labeling of specific engrams might one day offer researchers the unique opportunity to determine whether an engram has completely disappeared and is truly unavailable. While there are indeed techniques that allow permanent labeling of different components of engrams (for example, at the neuronal ensemble level45 or synapse level41), it is not clear at what point one could conclude that the absence of a marker indicates that the engram is completely gone. There might always be other markers that could point to remnants of the engram.
That being said, a large body of research shows that forgetting curves have canonical forms that ultimately approach zero (performance level) across whichever behavioral assessments are employed. Recent studies have identified a variety of active forgetting mechanisms at the neurobiological level, including dopamine-initiated signaling cascades, receptor trafficking and hippocampal neurogenesis, all of which could lead to erosion of the engram46–48. While this line of research is still in its infancy, this class of mechanisms may be of the kind that leads to silencing, ultimately rendering the engram unavailable over the course of forgetting, regardless of the nature of access attempts.
Retrieval as neuronal reinstatement
Recognition of the important distinction between accessibility and availability in cognitive psychology, which began with Tulving and Pearlstone’s findings1, led to critical insights on the relationship between encoding and retrieval. To understand what constitutes an effective retrieval cue, it is necessary to consider how the engram was initially formed. Specifically, Tulving and Thomson49 hypothesized that an engram is shaped by environmental features and internal cognitive or affective states during encoding. In turn, they argued, retrieval cues can only be successful to the extent that they overlap with these environmental features and internal states: that is, the greater the match between encoding and retrieval states, the higher the probability of retrieval success, a principle they termed ‘encoding specificity’. At the behavioral level, evidence in human and nonhuman species suggests that reinstatement of encoding context at the time of retrieval boosts recovery of information acquired in this context50–52. In fear conditioning, such context specificity provides the organism with adaptive flexibility, ensuring that expression of conditioned fear is usually limited to the training context (or very similar contexts)53,54.
In contemporary functional neuroimaging and recording studies in humans, the encoding specificity principle has been linked to neuronal reinstatement. Research asking to what extent neural activity patterns at encoding and retrieval overlap provides evidence for spatial and temporal forms of reinstatement that supports this principle55–62. Moreover, such studies reveal that the extent of this overlap impacts success and phenomenological attributes of retrieval. For instance, in visual cortex, increasing activation overlap predicts memory vividness during retrieval63. Interestingly, retrieval success may depend on concurrent hippocampal engagement, not only during encoding64 but also during retrieval65,66, with the latter perhaps reflecting a pivotal role of the hippocampus in pattern completion. The importance of neuronal reinstatement for context-specific retrieval has been demonstrated in work showing that its behavioral benefits are most pronounced when encoding and retrieval context match67.
The encoding specificity principle can also be evaluated in rodent studies in which cue-induced reactivation of neuronal ensembles active at encoding is examined at the cellular level. Initial work took advantage of a method that images the subcellular location of mRNA for the immediate early gene Arc, catFISH (cellular compartment analysis of temporal activity by fluorescent in situ hybridization), as a way to identify active neurons at two distinct time points. In this experiment68, rats were exposed consecutively to either identical (‘AA’ condition) or different (‘AB’ condition) environments, and neuronal ensembles activated by each exposure were assessed. In hippocampal CA1, higher levels of overlap in the AA, compared to the AB, condition suggested that retrieval re-engaged the neuronal ensemble active during initial encoding. While this study did not examine a behavioral readout of memory, subsequent studies linked behavioral expression of memory at retrieval to reactivation of the ensemble active at encoding using ensemble tagging approaches12–14,25,69–72. For instance, Reijmers and colleagues13 trained mice in a tone fear conditioning paradigm. Subsequent replacement in the training context reactivated neurons in the basal amygdala at above chance rates. Crucially, the rate of reactivation predicted memory strength, supporting the idea that greater similarity between encoding and retrieval states is associated with greater probability of retrieval success73.
In agreement with results examining context specificity in human neuroimaging67, studies in rodents reveal that neuronal ensembles activated at retrieval show context specificity related to behavior25,74. In one study74, a tone was paired with footshock in context A during training. Rats were subsequently given extinction training in context B, and then the tone conditioned stimulus (CS) was presented both in the extinction context (context B) and a third, distinct context (context C). Consistent with the idea that extinction is context-specific, rats froze in context C but not in context B (the extinction context) in these tests. At the neuronal level, presentation of the same tone CSs activated distinct populations of neurons in the B and C contexts. Moreover, activation of these different neuronal populations was critical for context-specific expression of extinction25.
Given that natural retrieval cues reactivate neural ensembles active at encoding and that the rate of reactivation relates to the strength of memory expression, we can ask whether the same holds for artificially induced memory retrieval. Recent studies30,34 have addressed this question. In these studies, during contextual fear conditioning, cells active in the CA3 and BLA were tagged. Posttraining, mice were administered a protein synthesis inhibitor to silence these engrams. As expected of a silent engram, no freezing was observed when the mice were placed back in the training context. However, optogenetic reactivation of the tagged DG cells produced freezing, and reactivation efficiency (i.e., the extent to which photostimulation induced reactivation of tagged encoding cells) predicted the strength of the artificially retrieved memory (i.e., freezing levels).
While many studies show that artificial reactivation of engrams induces memory expression, typically this expression is weaker than that evoked by natural cues. This finding is in agreement with the encoding specificity principle because it is unlikely that optogenetic stimulation fully recapitulates the state of the organism and the corresponding patterns of neural activity that occurred during encoding. While the local spatial features of activity patterns are preserved by optogenetic stimulation, temporal features are not faithfully reproduced. The development of holographic photostimulation approaches (that preserve both spatial and temporal patterning) may overcome this limitation of current optogenetic techniques75–77. In the future, closed-loop optogenetic systems could allow the recording and subsequent holographic reproduction of an endogenous ecphoric event78,79.
Although artificial engram manipulations are typically focal in nature, their effects may be more widespread. Experiences are encoded in hippocampal-cortical networks, and according to many contemporary accounts, the hippocampus plays a pivotal role both in the formation of memory as well as its recovery. At retrieval, the hippocampus is thought to reinstate patterns of activity in the cortex that were present at encoding80–83. Tanaka and colleagues19 tested this idea by tagging CA1 neuronal ensembles that were active during contextual fear conditioning. Silencing these tagged hippocampal cells during retrieval impaired memory expression and, critically, reduced reactivation of tagged cortical ensembles.
Conversely, activation, rather than inhibition, of tagged hippocampal neurons reinstates patterns of cortical activity present at encoding. For instance, Guskjolen and colleagues39 trained infant mice in contextual fear conditioning, tagging active ‘encoding’ ensembles with ChR2. When these mice were tested at later time points, they exhibited pronounced forgetting, a phenomenon resembling infantile amnesia in humans84. However, photostimulation of ChR2-tagged neurons in the DG induced memory recovery and reactivation of CA3, CA1 and cortical neurons that were tagged during training.
These types of findings support the idea that some engrams are distributed, spanning neuronal ensembles across subcortical and cortical brain regions85. Within this distributed network, each region may carry unique information about the encoded episode (for example, sensory, affective, spatial information), and the route by which network activation is triggered likely impacts phenomenological aspects of memory retrieval. The finding that activation of the hippocampus is essential for reinstating patterns of activity in the cortex that occurred during encoding (as also suggested by human neuroimaging studies65,66) additionally supports the view that the hippocampus is a critical hub within these distributed networks. However, it is unlikely that this region is the only hub with a critical role in reinstatement of neuronal states during retrieval32,86. Moreover, which regions serve as hubs likely changes over time, reflecting ongoing processes that modify the engram after initial memory formation, including consolidation and transformation87,88.
Equivalency
Artificially reactivating a naturally formed engram induces memory expression. But is ecphory induced by artificial means equivalent to natural ecphory? Next, we highlight four aspects of equivalency between artificially and naturally induced memories.
First, a naturally retrieved memory can serve as a CS for new learning89. A study by Ramirez and colleagues71 tested whether an artificially retrieved memory can similarly support new learning. In this experiment, neuronal ensembles activated by placing a mouse in a neutral context (context A) were tagged with ChR2. One day later, mice were foot-shocked in a second context (context B) while the tagged neuronal ensemble in the DG (corresponding to context A) was simultaneously reactivated. In subsequent testing, mice froze in context A (but not in a dissimilar context, C), even though context A had never been paired with footshock. A study by Ohkawa and colleagues90 went further. They used similar approaches to separately tag hippocampal and amygdala ensembles corresponding to context exposure (CS) and shock exposure (unconditioned stimulus, US), respectively. To create an artificial association between these ensembles corresponding to otherwise discontiguous events, the tagged CS and US ensembles were synchronously reactivated in the mouse’s home cage. Remarkably, when later placed in the original context, mice now froze even though they had never received a shock in this context.
Second, naturally retrieved memories extinguish. Repeated CS presentations in the absence of US lead to reduced conditioned responding. Khalaf and colleagues70 asked whether artificially retrieved fear memories similarly extinguish. To do this, they tagged hippocampal ensembles that were activated when mice were placed in a training context that had previously been paired with footshock. Repeated exposure to this training context led to a reduction in freezing behavior (i.e., extinction). However, reactivating the tagged hippocampal ensembles during extinction training accelerated extinction. Conversely, silencing this same population during extinction training slowed extinction. Recently, a related study tagged dorsal hippocampal ensembles during contextual fear conditioning. They then found that repeated, artificially induced retrieval, even in the absence of exposure to the training context, induced extinction of the contextual fear memory91.
Third, naturally retrieved memories reconsolidate. Retrieval destabilizes engrams, and protein synthesis is necessary for their restabilization (a process termed reconsolidation). Kim and colleagues28 asked whether a reconsolidation-like process occurs following artificially induced memory retrieval. In their experiment, CREB-overexpressing neurons in the LA were allocated to a tone fear memory during training. Artificially reactivating this allocated ensemble induced memory expression. However, pharmacological blockade of protein synthesis following artificial induction of ecphory impaired reconsolidation: when subsequently presented with the tone (i.e., the natural cue), mice treated with the protein synthesis inhibitor showed memory disruption. These results indicate that either artificial or natural retrieval destabilizes engrams, leading to the requirement for protein synthesis for their subsequent restabilization.
Fourth, naturally retrieved memories are subject to interference. If similar events are encountered either before or following the event in question, recovery of this target event can be compromised. That is, the ‘wrong’ (i.e., non-target) event or a merged event that combines the target and a similar lures could be recovered92. A similar phenomenon was observed following artificially induced retrieval in mice. Garner and colleagues93 tagged neuronal ensembles activated by exposure to a neutral context (context A) with the excitatory designer receptor exclusively activated by designer drug (DREADD) hM3Di. Mice were subsequently trained in a second context (context B) and tested 24 h later in the same context (context B). Chemogenetic activation of the context A ensemble while testing in context B reduced freezing levels, suggesting that reactivating the ‘wrong’ event interfered with natural cue-induced retrieval of the context A memory.
Retrieval over time: future challenges
This Review highlights the considerable progress made in gaining mechanistic insight into the process of memory retrieval at the biological level. This progress has been enabled by the development of new technologies that allow engrams to be visualized and manipulated in rodents at the level of neuronal ensembles. Combining this increased understanding of engrams with the cognitive theory developed by Endel Tulving2 permitted us to interpret contemporary research findings with respect to two major themes. First, when viewed in total, neurobiological findings support the cognitive theory that engram accessibility and memory retrieval success critically depend on interactions between engrams and retrieval cues (environmental or artificial). Second, the data also support the close ties between forming of engrams and their recovery, as captured by the notion of encoding specificity. However, the neurobiological study of retrieval is still in its infancy, and many important questions remain unanswered. We emphasize some of the most pressing issues in this remaining section.
Broadly speaking there is a dearth of knowledge as to how processes operating on engrams after their formation influence mechanisms of retrieval. Post-formation changes to the engram can be considered at two levels87. First, an engram for an individual episode or event changes over time. Second, multiple engrams (of distinct events or for the same re-encoded event) may interact. We assume that both types of change, which are likely not independent and are often considered together under the broad umbrella of systems consolidation, affect mechanisms of retrieval.
Psychological research suggests that forgetting is not indiscriminate and typically preserves gist over detail in the retention of events (for example, ref.94). It has been argued that this property is adaptive, with gist being particularly important when using memory to guide future behavior and make related predictions95. Currently, it is unclear how these dynamics and resulting changes in engram organization affect the neural mechanisms of retrieval. A shift toward more gist-like representation likely occurs hand-in-hand with large-scale shifts in network engagement during retrieval. For example, it has been proposed that retrieval of a gist-based representation (lacking episodic detail) may increasingly engage cortical regions over time, and, furthermore, hippocampal integrity may not be required for its retrieval96. At the level of neuronal ensembles, this shift toward more gist-like representation may involve partial silencing of hippocampal engrams. One recent study in mice97 labeled cells active during contextual fear conditioning in DG and medial prefrontal cortex (mPFC). When placed back in the context 1 day after training, only the DG engram was reactivated (whereas the mPFC engram was not). However, when tested 12 days after training, the mPFC engram, but not the DG engram, was engaged. Nonetheless, optogenetic stimulation of the DG engram (at the remote time point) or the mPFC engram (at the recent time point), respectively, induced artificial memory expression in an alternate context97. These changes can be understood as region-specific shifts in engram accessibility (rather than availability)98, which may go hand-in-hand with changes in the specificity of the memory expressed in behavior.
Beyond the fate of individual engrams, interactions between engrams may also influence subsequent memory retrieval. Indeed, there is a rich cognitive neuroscience literature focusing on the extraction of regularities across multiple experiences99 and the resulting changes in network engagement during retrieval. Data addressing this question at the level of neuronal ensembles, however, are only beginning to emerge. An initial study by Rashid and colleagues21 revealed that the engrams underlying two events experienced within a short period of time (<6 h) engage overlapping engrams and serve to link the two events, such that recall of one event produces recall of the other. In contrast, engrams supporting the same two events experienced with a longer intervening time (24 h) engage non-overlapping neural ensembles, and these events are remembered separately. Moreover, recalling an older event in the hours before experiencing a new event also links the two memories. Although these findings were initially reported for auditory fear memories and neural ensembles in the LA, other groups reported similar findings in the hippocampus supporting two context memories100 and a conditioned fear and conditioned taste aversion memory in the LA101. These findings provide evidence supporting the notion that once formed, engrams do not persist in isolation. However, as of yet the findings do not offer any insight that directly speaks to consequences for mechanisms engaged during retrieval.
One outcome of the extraction of regularities across multiple experiences is the development of schemas102. Schemas have received much attention in psychological research on retrieval, but have only recently been studied using neurobiological methods, albeit with promising initial results103,104. How schemas are organized at the level of neuronal ensembles, however, remains uncharted territory. It has been argued that the availability of a schema qualitatively changes the retrieval process; rather than directly accessing an engram, retrieval involves the reconstruction of a specific episode based on schema knowledge derived from multiple experiences105. It is difficult to determine, in particular for remote memories, the extent to which neuronal activity during retrieval reflects such reconstruction vs true engram reactivation106,107.
Here we have reviewed the current state of knowledge on the mechanisms of memory retrieval at the level of neuronal ensembles. Although recent progress in developing techniques for identifying and manipulating engrams at the level of neuronal ensembles has increased our understanding of engrams in the rodent brain, our understanding of the neurobiological underpinnings of retrieval remains rudimentary Guided by cognitive theories of ecphory, here we integrated and interpreted the findings of several studies taking advantage of the ability to tag and manipulate engrams. We hope this will spur further neuroscientific research into mechanisms underlying retrieval.
Acknowledgements
We thank A.Ramsaran and A.Park for drawing the figures, and we thank T. Ryan for comments on an earlier draft of this manuscript. This work was supported by Canadian Institutes of Health Research grants to P.W.F. (FDN-143227) and S.A.J. (FDN-388455) and a Natural Sciences and Engineering Research Council Discovery grant to S.K. (RGPIN-5770).
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information Nature Neuroscience thanks Stephen Maren and Steve Ramirez for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tulving E & Pearlstone Z Availability versus accessibility of information in memory for words. J. Verbal Learn. Verbal Behav. 5, 381–391 (1966). [Google Scholar]
- 2.Tulving E Ecphoric processes in episodic memory. Philos. Trans. R. Soc. Lond. B 302, 361–370 (1983). [Google Scholar]
- 3.Tulving E Elements of Episodic Memory. (Oxford University Press, 1983). [Google Scholar]
- 4.Semon R Die Mneme. (W. Engelmann, 1904). [Google Scholar]
- 5.Josselyn SA, Kohler S & Frankland PW Heroes of the engram. J. Neurosci 37, 4647–4657 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tulving E Episodic memory: from mind to brain. Annu. Rev. Psychol 53, 1–25 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Eichenbaum H Still searching for the engram. Learn. Behav 44,209–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Josselyn SA, Kohler S & Frankland PW Finding the engram. Nat. Rev. Neurosci 16, 521–534 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Tonegawa S, Liu X, Ramirez S & Redondo R Memory engram cells have come of age. Neuron 87, 918–931 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Tonegawa S, Pignatelli M, Roy DS & Ryan TJ Memory engram storage and retrieval. Curr. Opin. Neurobiol 35, 101–109 (2015). [DOI] [PubMed] [Google Scholar]
- 11.DeNardo LA et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci 22, 460–469 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny CA et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reijmers LG, Perkins BL, Matsuo N & Mayford M Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Sørensen AT et al. A robust activity marking system for exploring active neuronal ensembles. eLife 5, e13918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josselyn SA & Frankland PW Memory allocation: mechanisms and function. Annu. Rev. Neurosci 41, 389–413 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Yakov A, Dudai Y & Mayford MR Memory retrieval in mice and men. Cold Spring Harb. Perspect. Biol 7, a021790 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robins SK Memory and optogenetic intervention: separating the engram from the ecphory. Philos. Sci 85, 1078–1089 (2018). [Google Scholar]
- 18.Denny CA, Lebois E & Ramirez S From engrams to pathologies of the brain. Front. Neural Circuits 11, 23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka KZ et al. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84, 347–354 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Han JH et al. Selective erasure of a fear memory. Science 323, 1492–1496 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Rashid AJ et al. Competition between engrams influences fear memory formation and recall. Science 353, 383–387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci 12, 1438–1443 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sano Y et al. CREB regulates memory allocation in the insular cortex. Curr. Biol 24, 2833–2837 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiang HL et al. Manipulating a “cocaine engram” in mice. J. Neurosci 34, 14115–14127 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacagnina AF et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat. Neurosci 22, 753–761 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yiu AP et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron 83, 722–735 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Kwon JT, Kim HS, Josselyn SA & Han JH Memory recall and modifications by activating neurons with elevated CREB. Nat. Neurosci 17, 65–72 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Abdou K et al. Synapse-specific representation of the identity of overlapping memory engrams. Science 360, 1227–1231 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Ryan TJ, Roy DS, Pignatelli M, Arons A & Tonegawa S Memory. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redondo RL et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowansage KK et al. Direct reactivation of a coherent neocortical memory of context. Neuron 84, 432–441 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vetere G et al. Memory formation in the absence of experience. Nat. Neurosci 22, 933–940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy DS, Muralidhar S, Smith LM & Tonegawa S Silent memory engrams as the basis for retrograde amnesia. Proc. Natl. Acad. Sci. USA 114, E9972–E9979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nader K, Schafe GE & Le Doux JE Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Suzuki A et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci 24, 4787–4795 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy DS et al. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531, 508–512 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perusini JN et al. Optogenetic stimulation of dentate gyrus engrams restores memory in Alzheimer’s disease mice. Hippocampus 27, 1110–1122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guskjolen A et al. Recovery of “lost” infant memories in mice. Curr. Biol 28, 2283–2290.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Okuyama T, Kitamura T, Roy DS, Itohara S & Tonegawa S Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi JH et al. Interregional synaptic maps among engram cells underlie memory formation. Science 360, 430–435 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Gouty-Colomer LA et al. Arc expression identifies the lateral amygdala fear memory trace. Mol. Psychiatry 21, 364–375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nabavi S et al. Engineering a memory with LTD and LTP. Nature 511, 348–352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schacter DL Searching for Memory: The Brain, the Mind, and the Past. (Basic Books, 1996). [Google Scholar]
- 45.Roy DS, Okuyama T & Tonegawa S Tagging activated neurons with light. Nat. Biotechnol 35, 827–828 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Davis RL & Zhong Y The biology of forgetting—a perspective. Neuron 95, 490–503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frankland PW, Kohler S & Josselyn SA Hippocampal neurogenesis and forgetting. Trends Neurosci. 36, 497–503 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Hardt O, Nader K & Nadel L Decay happens: the role of active forgetting in memory. Trends Cogn. Sci 17, 111–120 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Tulving E & Thomson DM Encoding specificity and retrieval processes in episodic memory. Psychol. Rev 80, 352–373 (1973). [Google Scholar]
- 50.Eich E Mood as a mediator of place dependent memory. J. Exp. Psychol. Gen 124, 293–308 (1995). [DOI] [PubMed] [Google Scholar]
- 51.Godden DR & Baddeley AD Context-dependent memory in two natural environments: On land and underwater. Br. J. Psychol 66, 325–331 (1975). [Google Scholar]
- 52.Smith SM & Vela E Environmental context-dependent memory: a review and meta-analysis. Psychon. Bull. Rev 8, 203–220 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Bouton ME Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull 114, 80–99 (1993). [DOI] [PubMed] [Google Scholar]
- 54.Maren S, Phan KL & Liberzon I The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci 14, 417–428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jafarpour A, Fuentemilla L, Horner AJ, Penny W & Duzel E Replay of very early encoding representations during recollection. J. Neurosci 34, 242–248 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson JD, McDuff SG, Rugg MD & Norman KA Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis. Neuron 63, 697–708 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manning JR, Polyn SM, Baltuch GH, Litt B & Kahana MJ Oscillatory patterns in temporal lobe reveal context reinstatement during memory search. Proc. Natl. Acad. Sci. USA 108, 12893–12897 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polyn SM, Natu VS, Cohen JD & Norman KA Category-specific cortical activity precedes retrieval during memory search. Science 310, 1963–1966 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Ritchey M, Wing EA, LaBar KS & Cabeza R Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb. Cortex 23, 2818–2828 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staresina BP, Henson RN, Kriegeskorte N & Alink A Episodic reinstatement in the medial temporal lobe. J. Neurosci 32, 18150–18156 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Staresina BP et al. Hippocampal pattern completion is linked to gamma power increases and alpha power decreases during recollection. eLife 5, e17397 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yaffe RB et al. Reinstatement of distributed cortical oscillations occurs with precise spatiotemporal dynamics during successful memory retrieval. Proc. Natl. Acad. Sci. USA 111, 18727–18732 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.St-Laurent M, Abdi H & Buchsbaum BR Distributed patterns of reactivation predict vividness of recollection. J. Cogn. Neurosci 27, 2000–2018 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Danker JF, Tompary A & Davachi L Trial-by-trial hippocampal encoding activation predicts the fidelity of cortical reinstatement during subsequent retrieval. Cereb. Cortex 27, 3515–3524 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horner AJ, Bisby JA, Bush D, Lin WJ & Burgess N Evidence for holistic episodic recollection via hippocampal pattern completion. Nat. Commun 6, 7462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Staresina BP, Cooper E & Henson RN Reversible information flow across the medial temporal lobe: the hippocampus links cortical modules during memory retrieval. J. Neurosci 33, 14184–14192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Staudigl T, Vollmar C, Noachtar S & Hanslmayr S Temporal-pattern similarity analysis reveals the beneficial and detrimental effects of context reinstatement on human memory. J. Neurosci 35, 5373–5384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guzowski JF, McNaughton BL, Barnes CA & Worley PF Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci 2, 1120–1124 (1999). [DOI] [PubMed] [Google Scholar]
- 69.Deng W, Mayford M & Gage FH Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife 2, e00312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khalaf O et al. Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 360, 1239–1242 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Ramirez S et al. Creating a false memory in the hippocampus. Science 341, 387–391 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Tayler KK, Tanaka KZ, Reijmers LG & Wiltgen BJ Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol 23, 99–106 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Richards BA & Frankland PW The conjunctive trace. Hippocampus 23, 207–212 (2013). [DOI] [PubMed] [Google Scholar]
- 74.Orsini CA, Yan C & Maren S Ensemble coding of context-dependent fear memory in the amygdala. Front. Behav. Neurosci 7, 199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emiliani V, Cohen AE, Deisseroth K & Hausser M All-optical interrogation of neural circuits. J. Neurosci 35, 13917–13926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang SJ et al. Extended field-of-view and increased-signal 3D holographic illumination with time-division multiplexing. Opt. Express 23, 32573–32581 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang W & Yuste R Holographic imaging and photostimulation of neural activity. Curr. Opin. Neurobiol 50, 211–221 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Carrillo-Reid L, Han S, Yang W, Akrouh A & Yuste R Controlling visually guided behavior by holographic recalling of cortical ensembles. Cell 178, 447–457.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marshel JH et al. Cortical layer-specific critical dynamics triggering perception. Science 365, eaaw5202 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Squire LR & Alvarez P Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr. Opin. Neurobiol 5, 169–177 (1995). [DOI] [PubMed] [Google Scholar]
- 81.Teyler TJ & DiScenna P The hippocampal memory indexing theory. Behav. Neurosci 100, 147–154 (1986). [DOI] [PubMed] [Google Scholar]
- 82.McClelland JL, McNaughton BL & O’Reilly RC Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev 102, 419–457 (1995). [DOI] [PubMed] [Google Scholar]
- 83.Norman KA & O’Reilly RC Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol. Rev 110, 611–646 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Josselyn SA & Frankland PW Infantile amnesia: a neurogenic hypothesis. Learn. Mem 19, 423–433 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Wheeler AL et al. Identification of a functional connectome for long-term fear memory in mice. PLOS Comput. Biol 9, e1002853 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vetere G et al. Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron 94, 363–374.e4 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Moscovitch M, Cabeza R, Winocur G & Nadel L Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu. Rev. Psychol 67, 105–134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frankland PW & Bontempi B The organization of recent and remote memories. Nat. Rev. Neurosci 6, 119–130 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Rescorla RA Pavlovian Second-order Conditioning (Psychology Revivals): Studies in Associative Learning. (Psychology Press, 2014). [Google Scholar]
- 90.Ohkawa N et al. Artificial association of pre-stored information to generate a qualitatively new memory. Cell Rep. 11, 261–269 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Chen BK et al. Artificially enhancing and suppressing hippocampus-mediated memories. Curr. Biol 29, 1885–1894.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anderson MC Rethinking interference theory: Executive control and the mechanisms of forgetting. J. Mem. Lang 49, 415–445 (2003). [Google Scholar]
- 93.Garner AR et al. Generation of a synthetic memory trace. Science 335, 1513–1516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sekeres MJ et al. Recovering and preventing loss of detailed memory: differential rates of forgetting for detail types in episodic memory. Learn. Mem 23, 72–82 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Richards BA & Frankland PW The persistence and transience of memory. Neuron 94, 1071–1084 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Nadel L & Moscovitch M Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol 7, 217–227 (1997). [DOI] [PubMed] [Google Scholar]
- 97.Kitamura T et al. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tonegawa S, Morrissey MD & Kitamura T The role of engram cells in the systems consolidation of memory. Nat. Rev. Neurosci 19, 485–498 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Schapiro AC, Turk-Browne NB, Botvinick MM & Norman KA Complementary learning systems within the hippocampus: a neural network modelling approach to reconciling episodic memory with statistical learning. Philos. Trans. R. Soc. Lond. B 372, 20160049 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cai DJ et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yokose J et al. Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science 355, 398–403 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Gilboa A & Marlatte H Neurobiology of schemas and schema-mediated memory. Trends Cogn. Sci 21, 618–631 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Richards BA et al. Patterns across multiple memories are identified over time. Nat. Neurosci 17, 981–986 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Tse D et al. Schema-dependent gene activation and memory encoding in neocortex. Science 333, 891–895 (2011). [DOI] [PubMed] [Google Scholar]
- 105.Barry DN & Maguire EA Remote memory and the hippocampus: a constructive critique. Trends Cogn. Sci 23, 128–142 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Barry DN & Maguire EA Consolidating the case for transient hippocampal memory traces. Trends Cogn. Sci 23, 635–636 (2019). [DOI] [PubMed] [Google Scholar]
- 107.Moscovitch M & Nadel L Sculpting remote memory: enduring hippocampal traces and vmPFC reconstructive processes. Trends Cogn. Sci 23, 634–635 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Han JH et al. Neuronal competition and selection during memory formation. Science 316, 457–460 (2007). [DOI] [PubMed] [Google Scholar]
- 109.Rogerson T et al. Molecular and cellular mechanisms for trapping and activating emotional memories. PLoS One 11, e0161655 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kawashima T et al. Functional labeling of neurons and their projections using the synthetic activity-dependent promoter E-SARE. Nat. Methods 10, 889–895 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Guenthner CJ, Miyamichi K, Yang HH, Heller HC & Luo L Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]