Abstract
Purpose
Chronic lung disease is the most common cause of morbidity and mortality in patients with common variable immunodeficiency (CVID). While biomarkers exist to predict non-infectious complications, the unique features that define CVID patients with chronic lung disease are not well understood.
Methods
We analyzed data from CVID patients from the retrospective USIDNET (United States Immunodeficiency Network) patient database. Patients were categorized into 3 phenotypes for comparison: (1) CVID without chronic lung disease, (2) CVID with bronchiectasis only, and (3) CVID with interstitial lung disease (ILD) with or without bronchiectasis. Among these groups, differences were assessed in demographics, comorbidities, infections, treatments, and peripheral blood immune measures. We analyzed 1518 CVID patients which included 1233 (81.2%) without chronic lung disease, 147 (9.7%) with bronchiectasis only, and 138 (9.1%) with interstitial lung disease.
Results
Patients with ILD had lower CD3+ cell counts (P = .001), CD4+ cell counts (P < .05), and CD8+ cell counts (P < .001) compared with patients without lung disease. Additionally, there was significantly more CVID patients with ILD with pneumonia (P < .001), herpes viruses (P = .01) and fungal infections (P < .001) compared with patients with CVID without chronic lung disease.
Conclusion
This analysis suggests that patients with chronic lung disease may be more likely to have lower peripheral T cell counts and complications of those defects compared with CVID patients without chronic lung disease.
Keywords: Common variable immunodeficiency, CVID, granulomatous lymphocytic interstitial lung disease, GLILD, autoimmunity, USIDNET
Introduction
Common variable immunodeficiency (CVID) is a heterogeneous group of disorders, defined by low IgG at least 2 standard deviations below the mean for the age, and measured at least twice, 3 weeks apart [1]. Additionally, patients must have either low IgA or IgM and reduced or absent specific antibody production. The EUROClass trial in 2008 aimed to better characterize CVID phenotypes with clinical and immunologic features [2]. This study identified increased lymphoproliferation including splenomegaly and lymphadenopathy, granulomatous disease, and autoimmune defects introducing the subtype of immune dysregulation in CVID patients with poorer outcomes. In 2012, a large retrospective CVID cohort spanning 4 decades found an association between specific non-infectious complications, including chronic lung disease, and higher mortality rate despite immunoglobulin therapy [3]. More recently, Park et al. evaluated CVID patients with and without inflammatory complications and found an upregulation of interferon-associated genes in patients with inflammatory complications, suggesting a loss of adaptive immunity, with an expansion of the innate immune response [4]. Key questions remain regarding the clinical phenotype and outcomes of patients with an immune dysregulatory phenotype.
Resnick et al. reported chronic lung disease as a comorbidity in about a third of the patients, yet it was the top cause of death [3]. Chronic lung disease has long been appreciated as a comorbidity of CVID. Initial case reports described a “sarcoid like disease” in CVID patients [5]. Lung biopsies can be heterogeneous but typically show a peribronchiolar and interstitial infiltrate with areas of discrete T and B cell zones with almost near absence of T regulatory cells [6]. Bates et al. retrospectively evaluated a cohort of patients with granulomatous and lymphocytic interstitial lung disease (GLILD) and found they were more likely to have complications of splenomegaly, T cell lymphopenia, and reduced survival compared with other CVID groups [7]. In addition to some of the B cell defects seen in CVID patients, there are reports of T cell defects [8, 9]. There have even been reports which identified a decrease in the naïve T cell population in CVID patients with lymphoproliferation, autoimmune cytopenias, and enteropathy [10]. Together, this suggests patients with CVID complicated by interstitial lung disease have a broader immune dysregulation; however, the clinical and immunological phenotype of these patients in a large cohort has never been evaluated.
We thus aimed to determine the clinical phenotype and laboratory markers associated with CVID patients and ILD. We utilized data retrospectively collected from the United States Immunodeficiency Network (USIDNET) with patients enrolling from physicians over the USA to determine the clinical and immune phenotype of CVID patients with interstitial lung disease or bronchiectasis. Interestingly, we found that CVID patients with ILD more often have autoimmune cytopenia, T cell defects, and fungal infections.
Methods
USIDNET
The USIDNET is a national patient registry created in 2003 of patients with primary immunodeficiency diseases. Patients were registered by their physician at an institutional review board (IRB)–approved institution. Information in the registry includes comorbidities, infections, treatments, surgeries, laboratory values, among other information. Each individual site was responsible for consenting individual patients. At the time of consent, a healthcare member from the site manually entered data obtained from a patient’s chart. With the registry, the site in which each specific patient was recruited is blinded. Longitudinal data on patients is entered periodically by individual site investigators and registry staff. There is no system to verify diagnoses.
Query of USIDNET
For this study, a query was submitted to the USIDNET to examine patients with common variable immunodeficiency with and without chronic lung disease. Data that were provided by the USIDNET consisted of an excel file with 9 tabs of information including cohort demographics, infections, conditions, immunomodulators, surgery, other treatments, lab tests, and lab results. These files contained a common study ID and were imported to FileMaker™ Pro v15 (Santa Clara, CA, USA) application. This application links multiple data tables associated with a unique study ID. Filtering variables were used to divide the patients into 3 groups for comparison: no lung disease, bronchiectasis only, and interstitial lung disease with or without bronchiectasis.
Data Quantification
Within a single spreadsheet, all text-based and categorical variables were filtered as dichotomous. For continuous variables, such as labs which might have had more than one value present from different visits, the earliest lab value was utilized.
Exclusion Criteria
At the time of our query submission, there were 1526 patients with CVID. Eight patients were excluded from the primary analysis on the basis of having other primary pulmonary conditions, 1 with bronchiolitis obliterans, 5 with pulmonary alveolar proteinosis, and 2 with Wegener’s granulomatosis (see Fig. 1).
Fig. 1.
Consort diagram of CVID patients identified from USIDNET query with excluded patients along with breakdown into groups (1) CVID without lung disease, (2) CVID with bronchiectasis only, (3) CVID with ILD with or without bronchiectasis
Bronchiectasis and Interstitial Lung Disease Definitions
We defined the bronchiectasis group by inclusion of the filtering term bronchiectasis and exclusion of any phrases that would place them in the ILD group. The ILD group included filtering terms ‘bronchiolitis obliterans organizing pneumonia,’ ‘interstitial lung disease,’ ‘pulmonary fibrosis,’ ‘lung fibrosis,’ ‘interstitial pneumonia,’ ‘pneumonitis hypersensitivity,’ ‘interstitial pneumonitis,’ ‘Interstitial lung disease,’ ‘cryptogenic organizing pneumonia,’ ‘lymphocytic interstitial pneumonia (LIP),’ ‘GLILD,’ ‘Pulmonary Granuloma,’ or ‘multiple lung nodules (granulomas).’ Patients with both bronchiectasis and ILD were placed in the ILD group. We classified 285 from the 1518 analyzed as either bronchiectasis or ILD, while the remaining patients were considered to have no chronic lung disease (Fig. 1).
Statistical Analysis
Comparisons of dichotomous and categorical variables were done by a chi-square test of homogeneity. Comparison of continuous variables was done by a 1-way ANOVA with Tukey’s post hoc analysis. Analysis was between bronchiectasis to no lung disease and ILD and no lung disease. Statistical significance was set at P < .05. Statistical analysis was done using SPSS Statistics 24 (Armonk, NY, USA). Graphs were created using GraphPad Prism v8.0 (La Jolla, CA, USA).
Results
Patient Characteristics
We evaluated 1518 patients with CVID registered in the USIDNET at the time of our initial query. Entries came from 165 different clinical immunology specialists. Over 50% of the patients used came from 7 physicians. We found that 1233 of them had no chronic lung disease (81.2%), 147 (9.7%) had bronchiectasis, and 138 (9.1%) had interstitial lung disease with or without lung bronchiectasis (Fig. 1).
Demographics of CVID With and Without Chronic Lung Disease
We next investigated the demographics such as gender, ethnicity, and race between the cohort (Table 1). Importantly, the percentages were similar between these groups suggesting these clinical characteristics do not identify phenotypic differences. This suggests that chronic lung disease is an entity that is seen independent of these demographics.
Table 1.
No significant demographic differences in CVID patients analyzed in the USIDNET registry
| No lung disease | Bronchiectasis | P value | ILD | P value | |
|---|---|---|---|---|---|
| Age at CVID diagnosisa (mean ± SD) | 30.6 ± 20.8 | 30.8 ± 19.6 | 30.3 ± 15.8 | ||
| Age of Symptom onsetb (mean ± SD) | 20.9 ± 19.9 | 17.7 ± 16.1 | 20 ± 15.8 | ||
| Male, n (%) | 490/1233 (39.7%) | 61 /147 (41.5%) | .69 | 55/138 (39.9%) | .98 |
| Hispanic ethnicityc, n (%) | 35/721 (4.9%) | 3/108 (2.8%) | .34 | 1/87 (1.1%) | .11 |
| Raced | |||||
| African American, n (%) | 25/1080 (2.3%) | 5/127 (3.9%) | .27 | 2/122 (1.6%) | .63 |
| Asian or Pacific Islander, n (%) | 8/1080 (0.7%) | 1/127 (0.8%) | .95 | 0/122 (0%) | .34 |
| Caucasian/White, n (%) | 1039/1080 (96.2%) | 117/127 (92.1%) | .03 | 119/122 (97.5%) | .46 |
| American Indian/Native American, n (%) | 2/1080 (0.2%) | 1/127 (0.8%) | .20 | 0/122 (0%) | .63 |
| Other or more than one race, n (%) | 6/1080 (0.6%) | 3/127 (2.4%) | .03 | 1/122 (0.8%) | .72 |
Age at CVID diagnosis was available for 81.1%, 80.3%, and 87.7% respectively of the no lung disease, bronchiectasis, and ILD group
Age of Symptom onset was available for 64.1%, 53.7%, and 73.2% respectively of the no lung disease, bronchiectasis, and ILD group
Ethnicity was known for 916 (60.3%) of the patients
Race was known for 1329 (87.5%) of the patients
Medical Comorbidity Differences in Bronchiectasis and Interstitial Lung Disease
We next examined differences in the frequency of reported medical comorbidities between these groups. Interestingly, significant differences were seen in the autoimmune hematologic category defined by the following filtering terms: ‘Evan’s syndrome,’ ‘Autoimmune Hemolytic Anemia/AIHA,’ ‘Idiopathic/Immune Thrombotic Purpura,’ ‘Autoimmune Neutropenia.’ Autoimmune hematologic conditions were found in 5.7% of the no chronic lung disease cohort compared with 8.2% in the bronchiectasis cohort, and 17.4% of the ILD cohort (P < .001; Table 2). The higher rate of autoimmune hematologic conditions seen in the ILD cohort is consistent with previous reported data [3]. Significantly, more hypertension, chronic diarrhea, hepatomegaly, splenomegaly, and reports of underweight or weight loss were found in both the bronchiectasis group and the ILD group when compared with the group without chronic lung disease. Lymphadenopathy was significantly increased in the ILD group (34.8%) compared with the group without chronic lung disease (8.2%) or those with bronchiectasis (8.2%). These findings suggest there may be shared pathogenic mechanisms between the bronchiectasis and ILD groups while unique mechanisms exist in the ILD group compared with the CVID patients without lung disease.
Table 2.
Increased comorbidities in CVID patients with interstitial lung disease and bronchiectasis
| No lung disease (n = 1233) |
Bronchiectasis (n = 147) |
P value |
ILD (n = 138) |
P value |
|
|---|---|---|---|---|---|
| Autoimmune hematologica | 5.7% | 8.2% | .23 | 17.4% | < .001 |
| Hypertension | 13.4% | 23.8% | .001 | 21% | .02 |
| Chronic diarrhea | 11.4% | 19.7% | .004 | 18.1% | .02 |
| Hepatomegaly | 1.0% | 4.8% | < .001 | 10.9% | < .001 |
| Lymphadenopathy | 8.2% | 8.2% | .99 | 34.8% | < .001 |
| Splenomegaly | 5.6% | 12.9% | .001 | 36.2% | < .001 |
| Underweight or weight loss | 5.8% | 10.2% | .04 | 11.6% | .01 |
Autoimmune hematologic conditions included Evan’s syndrome, autoimmune hemolytic anemia/AIHA, idiopathic/immune thrombotic purpura, and autoimmune neutropenia
Distinguishing Infectious Comorbidities with Lung Disease
We next investigated the differences between groups in infection, a chronic comorbidity of CVID. There were no significant differences for bronchiectasis or ILD compared with the group without lung disease for rates of gastrointestinal infections, hepatitis, meningitis, encephalitis, and abscesses in deep and unspecified locations. Significant differences were found for abscesses in superficial locations in the bronchiectasis group (P < .05), lymphadenitis in the ILD group (P = .03), and pneumonia with a significantly higher percent in both the ILD and bronchiectasis groups compared with CVID without lung disease (Table 3). We next examined the specific type of microorganisms causing infection in our cohorts. No significant differences were found among the majority of microorganisms. However, the frequency of patients with ILD and bronchiectasis who had reports of Aspergillus, Candida infection, or other fungal infections was increased (P < .05). Fungal infections included the filtering terms ‘Aspergillus,’ ‘Candida,’ ‘Cryptococcus,’ ‘fungal,’ and excluded ‘tinea.’ Herpes virus infection was significantly increased in the ILD group compared with the group without lung disease (P = .01; Table 4). This included the filtering terms for ‘Human Herpes Virus (HHV),’ ‘Herpes Simplex Virus 1 or 2 (HSV),’ ‘Varicella Zoster Virus (VZV) (primary or reactivation),’ ‘Chickenpox,’ ‘Shingles,’ ‘Epstein-Barr virus,’ ‘Cytomegalovirus,’ ‘Roseola,’ and ‘Sixth disease.’ A significantly higher rate of Pseudomonas infections and Haemophilus influenza was seen in the bronchiectasis group when compared with the cohort without lung disease (P = .05; P < .001; Table 4). Taken together, patients with chronic lung disease are more likely to have a history of pneumonia infections and fungal infections compared with CVID patients without chronic lung disease. Although Haemophilus influenza was seen more commonly in the bronchiectasis group compared with those without lung disease, no difference was seen in the rate of Staphylococcus or Streptococcus in patients with chronic lung disease as might be expected due to the increased frequency of pneumonia in these patients.
Table 3.
Increase rates of infectious comorbidities in CVID patients with interstitial lung disease or bronchiectasis
| No lung disease (n = 1233) |
Bronchiectasis (n = 147) |
P value |
ILD (n = 138) |
P value |
|
|---|---|---|---|---|---|
| Abscess | |||||
| Deepa | 2.60% | 5.40% | .052 | 4.30% | .23 |
| Superficialb | 4.30% | 9.50% | .005 | 6.50% | .23 |
| Unspecified | 1.40% | 3.40% | .06 | 0.70% | .52 |
| GI infectionc | 14.50% | 20.40% | .06 | 12.30% | .48 |
| Hepatitis | 1.80% | 2% | .83 | 1.40% | .78 |
| Lymphadenitis | 2% | 2% | .99 | 5.10% | .03 |
| Meningitis/encephalitis | 4.90% | 4.80% | .96 | 5.80% | .63 |
| Pneumonia | 53% | 68% | .001 | 71% | < .001 |
Deep abscesses: Brain, dental, head and neck, inner ear, kidney, liver, lung, rectal, spleen, tonsillar, intradermal abscess, L. forearm, breast abscess, anal abscess with fistula;
Superficial abscesses: Abdominal wall, back and thigh, behind ear, groin, leg, lip, nostril, oral, perirectal, perianal, skin, Bartholin gland
GI infections: Gastrointestinal infections including infectious diarrhea, infectious enterocolitis, intestinal parasites, gastroenteritis, and small intestinal bacterial overgrowth
Table 4.
Significant differences in fungal and herpes virus infections between the interstitial lung disease and no lung disease cohorts
| No lung disease (n = 1233) |
Bronchiectasis (n = 147) |
P value |
ILD (n = 138) |
P value |
|
|---|---|---|---|---|---|
| Aspergillus | 0.6% | 2.7% | .006 | 2.2% | .04 |
| Candida | 4.9% | 9.5% | .02 | 11.6% | .001 |
| Fungal infectiona | 5.6% | 11.6% | .005 | 13.8% | < .001 |
| Giardia | 2.2% | 3.4% | .36 | 2.2% | .99 |
| Herpes virusb | 12% | 11.6% | .88 | 20.3% | .01 |
| Haemophilus influenza | 1.9% | 8.8% | < .001 | 2.9% | .45 |
| Pseudomonas | 1.7% | 4.1% | .05 | 1.4% | .83 |
| Staphylococcus | 5.8% | 9.5% | .08 | 7.2% | .51 |
| Streptococcus | 5.8% | 6.8% | .64 | 3.6% | .28 |
Fungal infection included Aspergillus, Candida, and Cryptococcus.
Herpes virus included Human herpes virus, herpes simplex virus 1 or 2, varicella zoster virus (primary or reactivation), chicken pox, shingles, Epstein-Barr Virus, cytomegalovirus, roseola, and sixth disease
Laboratory Measures of Immunity
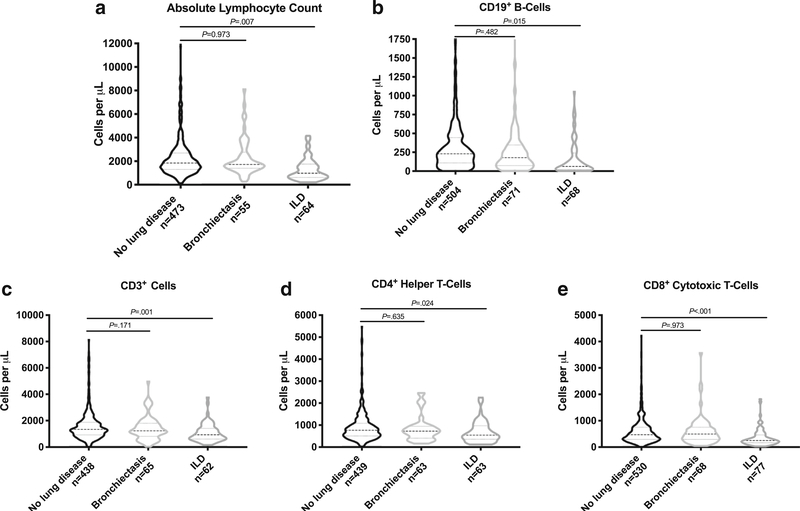
Finally, we evaluated whether laboratory markers of cellular and humoral immunity, commonly measured in clinical practice, differ between patients with bronchiectasis and ILD compared with no lung disease. Interestingly, we saw a significant difference between the no lung disease and ILD groups for absolute lymphocyte counts, CD19+ B cells, CD3+ cells, CD4+ helper T cells, and CD8+ cytotoxic T cells (Fig. 2). This supports a possible lack of cellular T cell–driven adaptive immunity that appears exacerbated in patients with interstitial lung disease. Given these interesting differences in cellular immunity between the groups, we evaluated differences in the downstream humoral function of immunoglobulin production. No significant difference was seen in IgA, IgM, and IgE levels (data not shown). Significance was seen between the bronchiectasis and no lung disease group with bronchiectasis patients having a higher serum IgG level; however, we provide caution in interpreting this as it is unclear whether these were baseline IgG levels or were after initiation of immunoglobulin replacement. Thus, we identified a reduction in T cell–associated cellular immunity in CVID patients with interstitial lung disease.
Fig. 2.
CVID patients with ILD have significantly lower numbers of lymphocyte subsets. There was significantly lower absolute lymphocyte counts (a), CD19+ B cells (b), CD3+ cells (c), CD4+ helper T cells (d), and CD8+ cytotoxic T cells (e) in CVID patients with ILD compared with CVID patients with no lung disease
Discussion
In this study, we found chronic lung disease in 19% of USIDNET patients with CVID, with half of these patients having interstitial lung disease with or without bronchiectasis and the remainder with bronchiectasis only. Furthermore, we found a significantly higher rate of comorbidities in CVID patients with chronic lung disease including autoimmune hematologic, hypertension, chronic diarrhea, hepatosplenomegaly, lymphadenopathy, and underweight. In addition, we identified a novel increased frequency of fungal infections in patients with chronic lung disease in addition to an increased frequency of infection with Haemophilus influenza or Pseudomonas in the bronchiectasis cohort, and increased Herpes virus infection in the ILD group. Finally, we found a significant decrease in B and T cells in the interstitial lung disease group compared with patients without chronic lung disease. Together, these findings suggest that patients with CVID and chronic lung disease have many other comorbidities that complicate their disease process with ILD patients being the most severe on a spectrum.
Our study identifies a potential cellular immune defect in patients with ILD compared with patients without any lung disease. These included reduced peripheral CD19+ B cells, CD3+ cells, and CD4+ helper T cells, with the most dramatic difference seen in the depressed numbers of CD8+ cytotoxic T cells in the ILD group compared with the no lung disease group. Similar cellular abnormalities of CVID patients were noted in the study by Bateman et al. who found low CD4+ helper T cells and CD8+ cytotoxic T cells in patients with GLILD compared with patients without lung disease [8]. Sarcoidosis and hypersensitivity pneumonitis patients were found to have elevated T cells in bronchoalveolar fluid with a peripheral blood lymphopenia, thought to be from a mechanism of dysfunctional leukocyte trafficking or cellular redistribution, but is poorly understood [11]. Interestingly, treatments that improved the disease state also improved the peripheral lymphopenia [12]. It is plausible that a similar immune-mediated mechanism is responsible for the lymphopenia seen in our CVID patients with ILD, and improves with treatment of the lung disease. Our study phenotyped CVID patients with chronic lung disease and examined T cell abnormalities, although CVID patients as a whole often have T cell lymphopenia [13–15]. Additionally, we identified an increase in fungal and viral infections for patients with lung disease, a potential complication of reduced cellular immunity. This was intriguing as some studies have shown a disruption in the Th17 subset in CVID patients [16, 17]. Additionally, we saw that patients with ILD were significantly more likely to have autoimmune cytopenias and hepatosplenomegaly which was similar to the overlap that was seen in the EUROClasstrial [2]. While the EUROClass study classified patients by B cell populations and determined phenotypes among different groups, our study phenotyped patients into different groups based on lung disease status. Recently, Unger et al. found that complicated CVID patients who have a more immune-dysregulated phenotype, including interstitial lung disease, have a predominant Th1 phenotype with interferon gamma expression [18]. This was directly related to CD21low B cells that were expanded in this group. Together, this supports an environment of more altered cellular immunity in patients with CVID and chronic lung disease.
The study contained several biases and limitations. The major limitation of this study was the self-reported nature of chronic lung disease in the USDINET registry and variability in the diagnosis of chronic lung disease. We performed quantification of free texted entries to identify lung comorbidities but the method for diagnosis may vary by site. While it is possible that pulmonary function testing, imaging including high-resolution computed tomography (HRCT), or lung biopsy confirmed the diagnosis, this information was not included in the registry. In contrast to prior studies where chronic lung disease was found in 25–51% of CVID patients, we found a significantly lower rate of chronic lung disease (19%), of which half had ILD with or without bronchiectasis while the remaining had isolated bronchiectasis [3, 7, 19]. We think the lower rate of chronic lung disease in the USIDNET likely reflects a failure of diagnosis by HRCT. In a previous study, HRCT scan was used to determine prevalence of chronic lung disease in CVID patients regardless of pulmonary symptoms where pulmonary abnormalities were seen in 51% of their cohort despite sometimes normal lung pulmonary function testing and chest x-rays [19]. Based on their findings and the lower rate of chronic lung disease found in the USIDNET, we strongly advocate for a baseline HRCT scan in all patients with CVID regardless of symptoms. A further limitation was that due to the nature of data collection in the registry, we were unable to determine how immunoglobulin replacement and other immunomodulating medications may have affected blood draws or infections. In addition, we did not have T and B cell phenotyping labs for a majority of the patients, leading to potential reporting bias of sicker patients. Lastly, the inherent bias comes from our use of the USIDNET study population who typically are seen at tertiary care centers and may not be representative of the general CVID population.
In conclusion, our analysis of the largest CVID patient registry in the USA identified patients with interstitial lung disease as more likely to have T and B cell lymphopenia, autoimmune hematologic conditions, and different types of infections. Importantly, we found a lower rate of chronic lung disease in the USIDNET than seen in other previously reported large cohorts of CVID, suggesting under diagnosis may occur due to lack of recognition or screening. Future studies should be done to longitudinally evaluate for changes to immune phenotype and function over time among CVID patients and the effect of these phenotypes on treatment response and natural history. The unique phenotype of interstitial lung disease warrants further endotyping to better understand unique pathways for which personalized medicine may be beneficial.
Acknowledgements
We gratefully acknowledge Julie Magnusson, Marla Goldsmith, and Tara Caulder for their continued support with the USIDNET. We also acknowledge and thank all contributing physicians and patients. This work was partially supported by K08DK09772 (to JBW).
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38–59. 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111(1):77–85. 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 3.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119(7):1650–7. 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park J, Munagala I, Xu H, Blankenship D, Maffucci P, Chaussabel D, et al. Interferon signature in the blood in inflammatory common variable immune deficiency. PLoS One. 2013;8(9):e74893 10.1371/journal.pone.0074893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fasano MB, Sullivan KE, Sarpong SB, Wood RA, Jones SM, Johns CJ, et al. Sarcoidosis and common variable immunodeficiency. Report of 8 cases and review of the literature. Medicine (Baltimore). 1996;75(5):251–61. [DOI] [PubMed] [Google Scholar]
- 6.Rao N, Mackinnon AC, Routes JM. Granulomatous and lymphocytic interstitial lung disease: a spectrum of pulmonary histopathologic lesions in common variable immunodeficiency–histologic and immunohistochemical analyses of 16 cases. Hum Pathol. 2015;46(9):1306–14. 10.1016/j.humpath.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol. 2004;114(2):415–21. 10.1016/j.jaci.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 8.Bateman EA, Ayers L, Sadler R, Lucas M, Roberts C, Woods A, et al. T cell phenotypes in patients with common variable immunodeficiency disorders: associations with clinical phenotypes in comparison with other groups with recurrent infections. Clin Exp Immunol. 2012;170(2):202–11. 10.1111/j.13652249.2012.04643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fevang B, Yndestad A, Sandberg WJ, Holm AM, Muller F, Aukrust P, et al. Low numbers of regulatory T cells in common variable immunodeficiency: association with chronic inflammation in vivo. Clin Exp Immunol. 2007;147(3):521–5. 10.1111/j.1365-2249.2006.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouillot G, Carmagnat M, Gerard L, Garnier JL, Fieschi C, Vince N, et al. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J Clin Immunol. 2010;30(5):746–55. 10.1007/s10875-010-9424-3. [DOI] [PubMed] [Google Scholar]
- 11.Semenzato G Immunology of interstitial lung diseases: cellular events taking place in the lung of sarcoidosis, hypersensitivity pneumonitis and HIV infection. Eur Respir J. 1991;4(1):94–102. [PubMed] [Google Scholar]
- 12.Crouser ED, Lozanski G, Fox CC, Hauswirth DW, Raveendran R, Julian MW. The CD4+ lymphopenic sarcoidosis phenotype is highly responsive to anti-tumor necrosis factor-{alpha} therapy. Chest. 2010;137(6):1432–5. 10.1378/chest.09-2576. [DOI] [PubMed] [Google Scholar]
- 13.Ochtrop ML, Goldacker S, May AM, Rizzi M, Draeger R, Hauschke D, et al. T and B lymphocyte abnormalities in bone marrow biopsies of common variable immunodeficiency. Blood. 2011;118(2):309–18. 10.1182/blood-2010-11321695. [DOI] [PubMed] [Google Scholar]
- 14.Farrant J, Spickett G, Matamoros N, Copas D, Hernandez M, North M, et al. Study of B and T cell phenotypes in blood from patients with common variable immunodeficiency (CVID).Immunodeficiency. 1994;5(2):159–69. [PubMed] [Google Scholar]
- 15.Wong GK, Huissoon AP. T-cell abnormalities in common variable immunodeficiency: the hidden defect. J Clin Pathol. 2016;69(8): 672–6. 10.1136/jclinpath-2015-203351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbosa RR, Silva SP, Silva SL, Melo AC, Pedro E, Barbosa MP, et al. Primary B-cell deficiencies reveal a link between human IL17-producing CD4 T-cell homeostasis and B-cell differentiation. PLoS One. 2011;6(8):e22848 10.1371/journal.pone.0022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganjalikhani-Hakemi M, Yazdani R, Sherkat R, Homayouni V, Masjedi M, Hosseini M. Evaluation of the T helper 17 cell specific genes and the innate lymphoid cells counts in the peripheral blood of patients with the common variable immunodeficiency. J Res Med Sci. 2014;19(Suppl 1):S30–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Unger S, Seidl M, van Schouwenburg P, Rakhmanov M, Bulashevska A, Frede N, et al. The TH1 phenotype of follicular helper T cells indicates an IFN-gamma-associated immune dysregulation in patients with CD21low common variable immunodeficiency. J Allergy Clin Immunol. 2018;141(2):730–40. 10.1016/j.jaci.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 19.Maarschalk-Ellerbroek LJ, de Jong PA, van Montfrans JM, Lammers JW, Bloem AC, Hoepelman AI, et al. CT screening for pulmonary pathology in common variable immunodeficiency disorders and the correlation with clinical and immunological parameters. J Clin Immunol. 2014;34(6):642–54. 10.1007/s10875-014-0068-6. [DOI] [PubMed] [Google Scholar]