Abstract
We report a new water-soluble fluorescent probe sensitive to aqueous fluoride. The probe shows spectral shifts and intensity changes in the presence of fluoride, in a wavelength ratiometric and colorimetric manner, enabling the detection of fluoride concentrations at visible wavelengths, in the concentration range ≈1–300 mM.
The probes response is based on the ability of the boronic acid group to interact with fluoride, changing from the neutral form of the boronic acid group [R–B(OH)2] to the anionic trifluoro form [R–B–F3], which is an electron donating group. The presence of an electron-deficient quaternary heterocyclic nitrogen center and a strong electron donating amino group in the 6-position, on the quinolinium backbone, provides for the spectral responses observed upon fluoride binding to the boronic acid group. Interestingly, the presence of the amino group on the 6-position of the quinolinium backbone suppresses the response of the boronic acid containing fluorophore towards monosaccharides, such as glucose and fructose, which are present in biological fluids and food stuffs, potentially allowing for the predominant fluoride sensitivity.
In addition to physiological sugars, we discuss the response of aqueous halides as potential interferents, or indeed analytes to be sensed, and show that the new probe responds well to aqueous fluoride in the presence of a high background of other species, such as in a biological cocktail of 50 mM glucose, 50 mM aqueous Cl− and 5 mM fructose.
Keywords: Boronic acid containing fluorophores, Fluoride, Monosaccharides
1. Introduction
The importance of fluoride detection and quantification can quite simply be judged by the vast amount of literature published over the last 20 or so years ([1–5] and refs. therein). Fluoride is present in biological fluids and tissues, and especially in bone and tooth. Fluoride is easily absorbed but is slowly excreted from the body, which can result in chronic poisoning, acute gastric and kidney disorders, dental and skeletal fluorosis, and even death. Fluoride can be accurately determined using fluoride-ion selective electrodes [6], spectrophotometry [7], gas chromatography [8], and even colorimetrically using boronic acid chemistry [9–10], although such systems are poisoned by the presence of sugars, such as by glucose or fructose [9–10]. It is due to the well-known high affinity between diol containing compounds and the boronic acid moiety that has lead to the development of carbohydrate sensors [11–17], chromatographic materials [18], and many glucose sensors [19–25].
One particular halide sensing mechanism that has attracted significant attention has been the quenching of fluorescence, which was first described by George Stokes as early as 1869 [1], when he observed the fluorescence of quinine in dilute sulphuric acid was reduced after the addition of hydrochloric acid, which is now commonly attributed to the dynamic quenching by aqueous chloride ions. Subsequently, there have been many halide-sensitive probes derived from quinine derivatives, most notably using the quinolinium nucleus, which display a modest sensitivity for chloride due to its relatively long lifetime when quaternised, which affords for chloride collisional quenching [1]. While the quinolinium nucleus is only sparingly water-soluble, its quaternised products are readily water-soluble and have subsequently been the transduction elements in many chloride/halide sensors [1,26–28].
It is not just the physiological significance of chloride that drives workers to mostly report the chloride sensitivity of some fluorescent probes, but because the quenching of fluorescence is not a selective process, and any fluorophore quenched by chloride is also quenched by bromide to a greater extent and also by iodide to an even greater extent. Therefore, for dynamic quenching, the sensitivity of fluorophores to halide is well known to be I− > Br− > Cl−. The explanation of this effect lies in the fact that the efficiency of intersystem crossing to the excited triplet state, promoted by spin-orbit coupling of the excited singlet fluorophore and halide upon contact, depends on the mass of the quencher atom, hence the expression “heavy-atom effect” is sometimes used [1,29]. It is for this reason that fluoride does not typically quench fluorescence. As such, traditional halide-sensitive probes are not very sensitive to fluoride and are therefore not suited for detecting fluoride <50 mM [1]. Only a few fluorescent probes can be found in the literature which are sensitive to fluoride [29], all based on either the benzene or naphthalene backbone, and therefore showing absorption in the deep UV (≈270 nm), which is not practical for many sensing applications [1,29,30].
Subsequently, we have quaternized the halide-sensitive 6-aminoquinoline heterocyclic nucleus with a o-boronic acid group (Fig. 1) to produce a novel fluoride-sensitive probe BAQBA. The resultant boronic acid-containing fluorophore (BAFs) shows both an absorption and emission wavelength ratiometric response to fluoride at mM concentrations, providing a much better alternative to the low extent of fluoride collisional quenching, which is inherent to most fluorophores. The origin of the fluoride response is due to the boronic acid group’s ability to interact with hard bases, such as F− [31], as shown in Fig. 2, to form the triboronate anion [R–BF3−], which is an electron donating group, the extent of which is dependent on the amount of fluoride present (Fig. 2). This in turn interacts with the electron-deficient quaternary heterocyclic nitrogen center on the quinolinium backbone, where the interaction is thought to provide for the wavelength shifts and intensity changes observed.
Fig. 1.
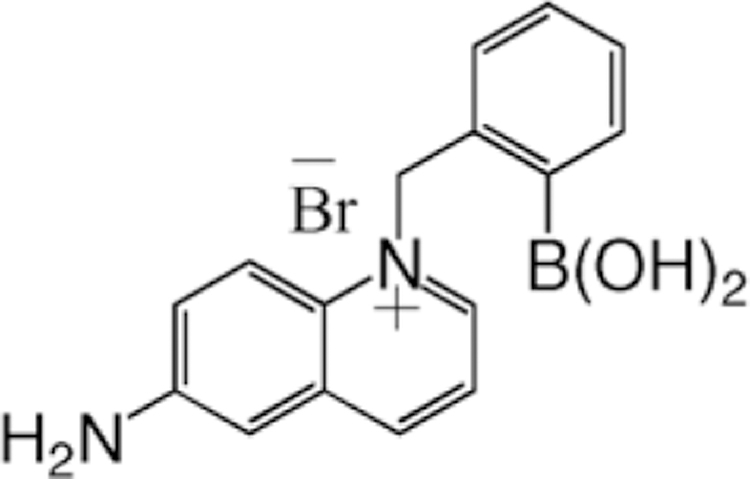
Molecular structure of BAQBA – N-(2-boronobenzyl)-6-aminoquinolinium bromide.
Fig. 2.
Equilibrium involved in the interaction between the boronic acid group and fluoride.
Ongoing studies in our laboratory have shown that by replacing the 6-amino group on the quinolinium backbone with less efficient electron donating groups, e.g. –OCH3, CH3, etc., in essence making the nitrogen center relatively more electron-deficient, then the dual emission bands at 450 and ≈546 nm are lost for a single emission band at ≈450 nm, eliminating the possibility of a ratiometric response. In contrast, a greater electron-deficient nitrogen center provides for a higher affinity for monosaccharides [32] (data not shown). Hence, our unique choice of backbone substituents affords for a high fluoride affinity with relatively little sugar response, even in the case of fructose, which is well known to have a high boronic acid affinity [19–25]. Thus, by additionally combining the well-known halide sensing capability of the quinolinium nucleus with the fluoride-binding boronic acid moiety, we have produced a probe sensitive to fluoride, chloride, bromide, and iodide, with the added feature of suppressed sugar response.
This new highly fluorescent water-soluble probe offers the attractive capability of sensing all halides at mM concentrations in un-degassed solutions, which to the best of our knowledge is not displayed by any other fluorescent probe/s.
In this paper, we characterize the fluoride response of BAQBA in the presence of interferences.
2. Experimental
2.1. Materials
All chemicals were purchased from Sigma. The preparation of BAQBA (Fig. 1) has recently been reported [32].
2.2. Methods
All solution absorption measurements were performed in a 4 cm × 1 cm × 1 cm quartz cuvette (Starna), using a Cary 50 spectrophotometer from Varian. Fluorescence spectra were similarly collected on a Varian Eclipse spectrofluorometer with solution optical densities less than 0.2 and λex = 358 nm.
Stability (KS ) and/or dissociation constants (KD) were obtained by fitting the titration curves with sugar or fluoride to the relation
| (1) |
where Imin and Imax are the initial (no sugar or fluoride) and final (plateau) fluorescence intensities of the titration curves, where KD (1/KS ).
Stern–Volmer constants, KSV (M−1) for aqueous halide, (Cl−, Br−, and I−) were obtained by plotting I0/IQ and τ0/τQ as a function of halide concentration [Q], where I0, τ0, and IQ, τQ are the intensities, mean lifetimes in the absence and presence of quencher Q, respectively
| (2) |
| (3) |
Time-resolved intensity decays were measured using reverse start–stop time-correlated single-photon counting (TC-SPC) with a Becker and Hickl GmBH 630 SPC PC card and a un-amplified MCP-PMT. Vertically polarized excitation at ≈372 nm was obtained using a pulsed LED source (1 MHz repetition rate) and a dichroic sheet polarizer. The instrumental response function was ≈1.1 ns fwhm. The emission was collected at the magic angle (54.7°), using a long pass filter (Edmund Scientific), which cut-off wavelengths below 380 nm. The use of a pulsed 372 nm LED provided for excitation near to the isosbestic point at 358 nm (Fig. 3).
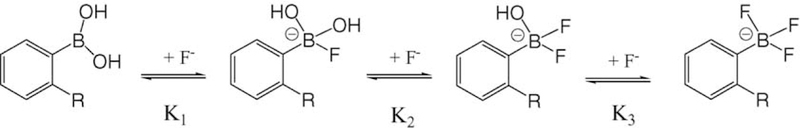
Fig. 3.
Absorption spectra of BAQBA with increasing fluoride concentration (top) and the respective wavelength ratiometric plot based on the A342/A388 absorption bands (bottom). Insert – enlarged 0–50 mM fluoride region.
The intensity decays were analyzed in terms of the multi-exponential model
| (4) |
where αi are the amplitudes and τi the decay times, Σ αi = 1.0. The fractional contribution of each component to the steady-state intensity is given by
| (5) |
The mean lifetime of the excited state is given by
| (6) |
and the amplitude-weighted lifetime is given by
| (7) |
The values of αi and τi were determined by non-linear least squares impulse reconvolution with a goodness-of-fit criterion.
3. Results
Fig. 3 shows the response of BAQBA to fluoride in Millipore water. As the concentration of fluoride increases, the absorption band at ≈388 nm decreases, while the band at ≈342 nm increases. We can also see a significant change in the 388 nm band with each 5 mM fluoride increment. Subsequently, Fig. 3 (bottom) shows the absorption wave-length ratiometric plot of the 342 and 388 nm bands. A linear response to fluoride was typically observed up to about 100 mM fluoride (Fig. 3, bottom insert). The non-linear response thereafter is thought to be due to both the third-order binding as depicted in Fig. 2 and saturation of the probe. As can be seen, the dynamic range for fluoride sensing is attractive where a ≈6-fold change in A342/A388 occurs up to ≈200 mM fluoride.
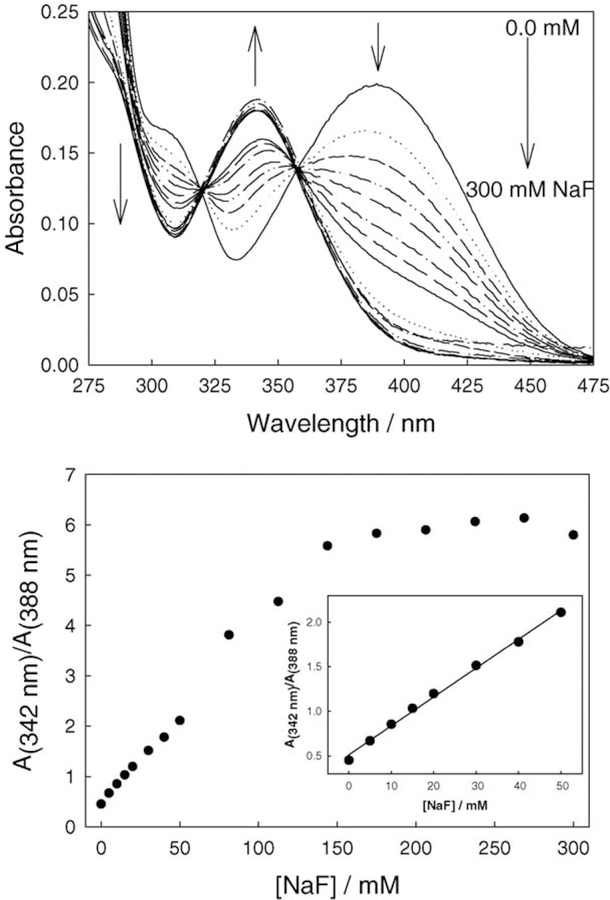
The fluorescence emission of BAQBA shows similar behavior (λex = 358 nm; Fig. 4), where the intensity of the band at 546 nm decreases while the emission band at 450 nm increases, noting the visible emission of the fluoride complexed form at 450 nm as compared to the red-shifted emission of the un-complexed form at 546 nm. This colorimetric response can also be seen visually in Fig. 5, where the vial on left contains no fluoride and the vial on the right is clear in the presence of 300 mM fluoride. For the data shown in Fig. 4 (top), we constructed the fluorescence emission ratiometric response (Fig. 4, bottom). Using Eq. (1), we were able to determine a dissociation constant for fluoride of 42 mM3 (KS 24 µ M−3). Interestingly, the ratiometric response plots (Figs. 3 and 4) both show different dynamic sensing ranges, reflecting the differences in extinction coefficients and quantum yields of the F− unbound and bound forms, respectively. Interestingly, the KD obtained from Fig. 3 (bottom) was ≈140 mM3, although the fit quality was poor due to the non-systematic increase shown in Fig. 3 (bottom), i.e. a deviation after 50 mM F−.
Fig. 4.
Fluorescence emission spectra of BAQBA with increasing fluoride concentrations, λex = 358 nm (top), and the respective wavelength ratiometric plot based on the I450/I546 emission bands (bottom).
Fig. 5.

Photograph of two vials containing equal concentrations of BAQBA and both 0 and 300 mM fluoride, left and right, respectively.
The affinity of boronic acid for diols is well known [11–25], hence for any fluoride sensor based on the boronic acid moiety, it is important to characterize the monosaccharide response. Subsequently, we tested the response of BAQBA towards both glucose (Fig. 6) and fructose (Fig. 7) at pH 7. A comparison of Fig. 6 (top) and Fig. 7 (top) shows the similar affinity for fructose as compared to glucose, where similar to fluoride, emission bands were observed at 450 and 546 nm, respectively. However, the 450 and 546 nm emission bands do not show increasing and decreasing intensities in the presence of sugar, but moreover show an overall spectral decrease as sugar concentration increases. Subsequently, Figs. 6 and 7 (bottom) show the response curves to sugars normalized by the response of BAQBA in the absence of sugar. Similarly to fluoride, we were able to determine the stability constants to be ≈1.04 and ≈0.06 mM−1 (KD 0.96, 16.67 mM) for glucose and fructose, respectively.
Fig. 6.
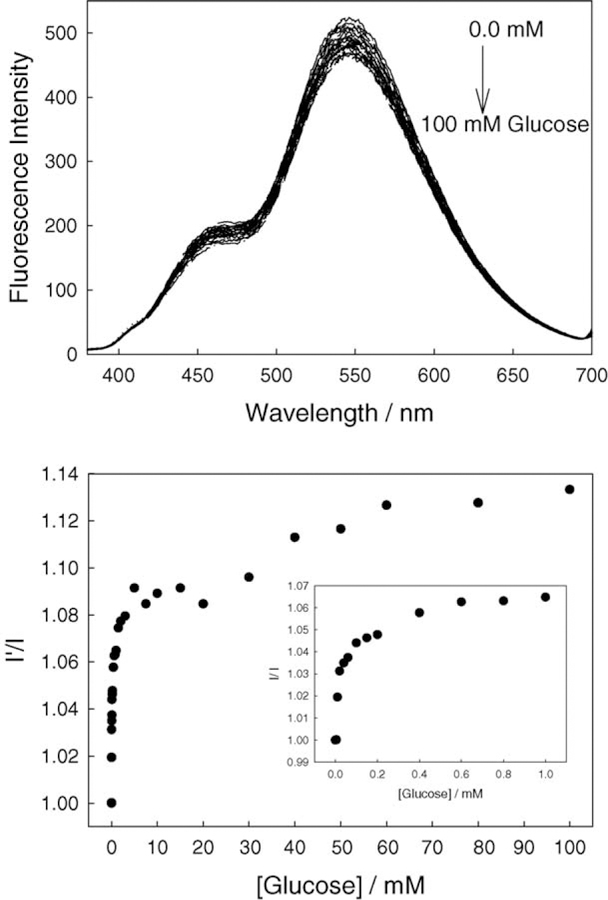
Emission spectra of BAQBA with increasing glucose concentrations (top) and the ratio plot in the absence of glucose I’, and in the presence of glucose I (bottom). Insert – enlarged region <1 mM glucose.
Fig. 7.
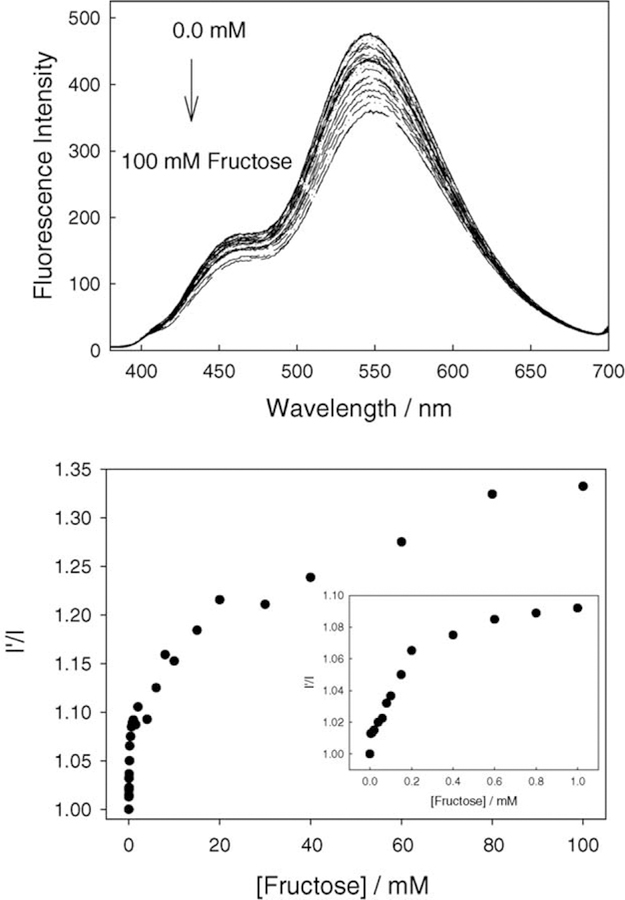
Emission spectra of BAQBA with increasing fructose concentrations (top) and the ratio plot in the absence of fructose I’, and in the presence of fructose I (bottom). Insert – enlarged region <1 mM fructose.
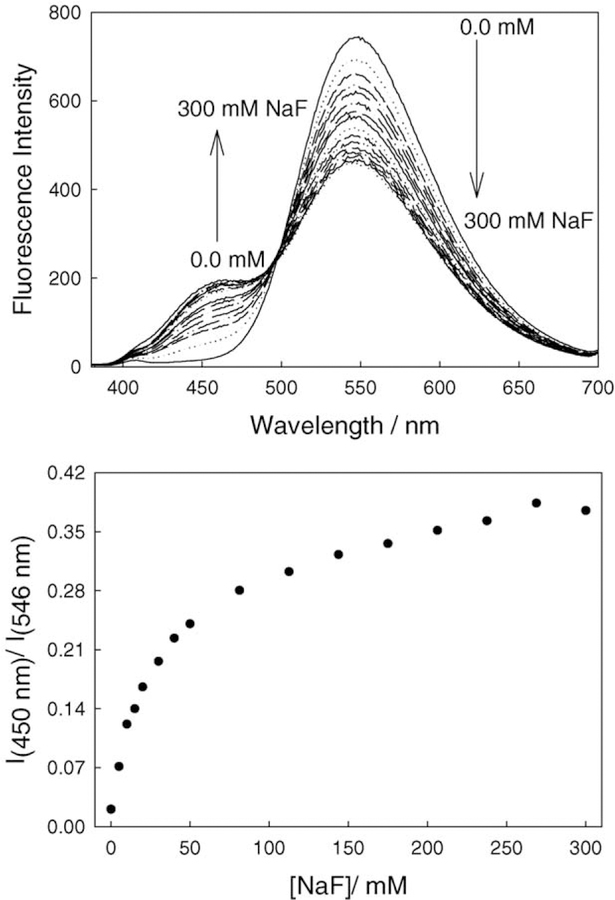
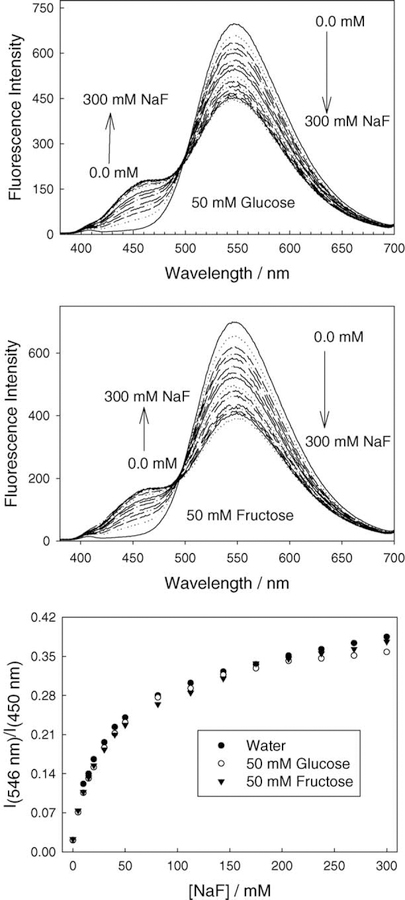
With a very slight sugar response evident, we tested the ability of BAQBA to sense aqueous fluoride in the presence of both 50 mM glucose and 50 mM fructose. Fig. 8 shows the absorption spectra of BAQBA in the presence of sugars with increasing fluoride concentration. Fig. 8 (bottom) shows the ratiometric plot, where we can see that the presence of sugar does not interfere with the fluoride response up to ≈50 mM F−. Even at higher fluoride concentrations, BAQBA responds well in the presence of sugar. It should be noted that the concentrations of glucose and fructose in blood for a normal healthy person are 2–8 mM and ≈1 mM, respectively, and in food products can be much higher [1]. The apparent deviations from the trend shown at ≈60 mM F− in Fig. 8 (bottom )is not a measurement artifact, but is indeed attributed to the complex binding of sugars and anions with boronic acid, and to some degree is also shown in Figs. 6 and 7 above 20 mM sugar.
Fig. 8.
Absorption spectra of BAQBA as a function of an increasing fluoride concentration with a background 50 mM glucose (top), a background 50 mM fructose (middle), and the respective ratiometric plots constructed from the A342/A388 nm bands as compared to that in water (bottom).
While the physiological fluoride levels for healthy people have been reported to be as low as 20–60 µg/L [2], it is the ability to track elevated fluoride levels that has been the focus of much research, as high fluoride levels can lead to poisoning by the blockage of enzymatic functions [1]. We do not expect this new fluorescent probe will sense aqueous fluoride, 2 orders of magnitude below its dynamic sensing range, nor are fluoride levels during poisoning likely to increase 1000-fold. However, these probes do offer the unique opportunity to ratiometrically or colorimetrically determine fluoride levels in the 1–300 mM range in the presence of sugars, and as we will later show, in the presence of other halides. To the best of our knowledge, this probe is amongst the most sensitive reported for fluoride to date [1], and may subsequently therefore find applications for food products or waste waters, where substantially elevated F− levels may be present and adverse to human health.
Fig. 9 shows fluorescence emission spectra for BAQBA for increasing fluoride concentrations in the presence of a high constant sugar background. By comparing with the response in water (no sugar), we can clearly see that the presence of sugar has little effect on overall fluoride response (Fig. 9, bottom), again reflecting the boronic acid moiety’s higher binding affinity for F− as compared to monosaccharides.
Fig. 9.
Emission spectra of BAQBA as a function of an increasing fluoride concentration with a background 50 mM glucose (top), a background 50 mM fructose (middle), and the respective ratiometric plots constructed from the I450/I546 nm bands as compared to that in water (bottom).
In addition to fluoride, we tested the response of BAQBA towards aqueous chloride, bromide, and iodide (Fig. 10) as the quinolinium nucleus is well known to be susceptible to heavy atom type collisional quenching [1]. As expected, the steady-state Stern–Volmer constant for iodide was the largest, KSV = 34 M−1, with bromide and chloride very similar and substantially smaller than for iodide, 1.4 and 1.0 M−1, respectively. We additionally measured the lifetime/s of BAQBA in the presence of halide to determine the dynamic quenching components (Fig. 10, bottom). Interestingly, the dynamic KSV values were slightly smaller, ≈27, 0.4, and 0.3 M−1 for I−, Br−, and Cl−, respectively, suggesting a small, but measurable, static quenching component. A very similar finding has recently been reported by Geddes et al. [28] for other quinolinium fluorophores, and was attributed to the presence of the halide counter ion.
Fig. 10.
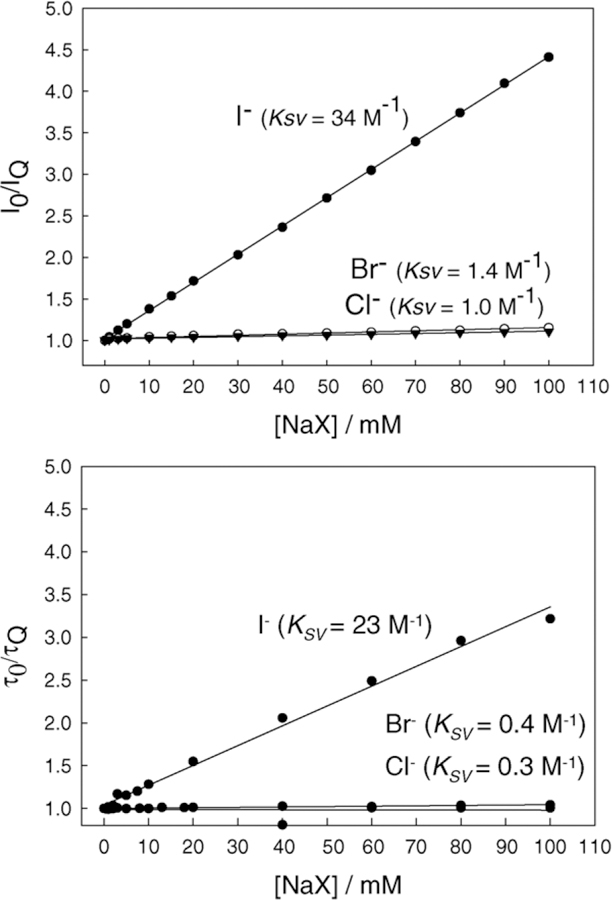
Steady-state Stern–Volmer plots using Eq. (2) for BAQBA with increasing concentrations of sodium halide (top) and the dynamic quenching Stern–Volmer plots constructed from the mean lifetime data in the presence of sodium halide (bottom), where and I0 and τ0 are the intensities and mean lifetime respectively in the absence of quencher Q.
Our time-resolved studies have revealed that BAQBA is bi-exponential in Millipore water with lifetimes of 1.87 and 2.97 ns, with amplitudes of 0.52 and 0.48, respectively (cf. Eq. (4)). Both the mean and amplitude weighted lifetimes were found to be 2.52 and 2.39 ns, respectively. Interestingly, the lower response of BAQBA towards aqueous halide can be attributed to its reduced mean lifetime as compared to other quinolinium type fluorophores [1,26–28], noting the weak response towards aqueous Cl−, which could be particularly advantages when using BAQBA in physiological or environmental fluids.
Finally, we tested the response of BAQBA towards aqueous fluoride in the presence of constant 50 mM glucose, 5 mM fructose, and a 50 mM chloride concentration (Fig. 11). The ratiometric response for the A342/A388 bands is almost linear showing an approximately five-fold change for 300 mM fluoride (Fig. 11, top), the trend deviations a result of the complex binding with species of different affinity. Similarly, the ratiometric plot of the I450/I546 fluorescence emission bands shows the utility of this probe (Fig. 11, bottom) in that it can readily determine fluoride concentrations in a high quenching background of potential interferents.
Fig. 11.
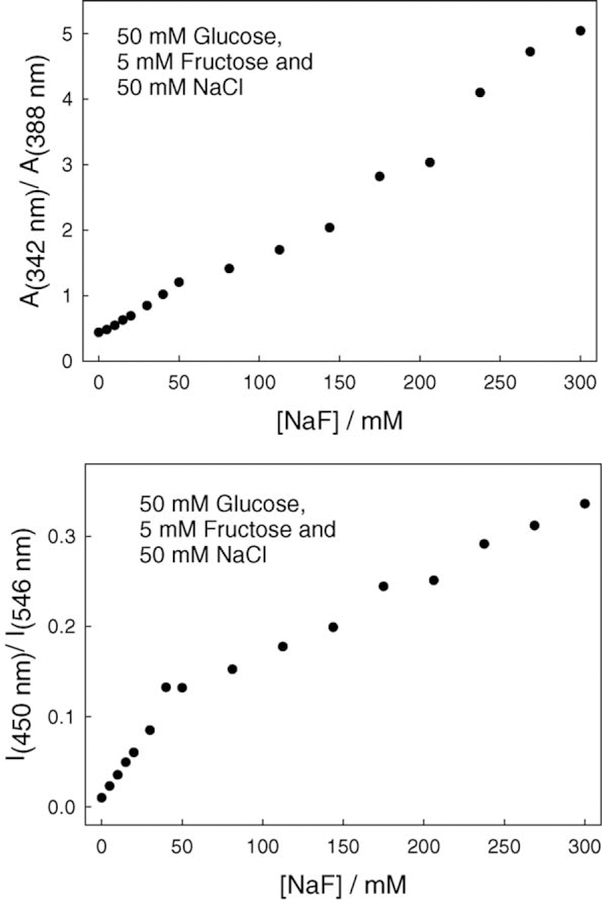
Absorption ratiometric plot for BAQBA with increasing fluoride concentrations, with a constant 50 mM glucose, 5 mM fructose, and 50 mM chloride (top), and the fluorescence emission ratiometric plot for the same with λex = 358 nm (bottom).
4. Discussion
It is widely recognized that ratiometric or lifetime-based methods offer intrinsic advantages for both chemical and biomedical sensing [29,33]. Fluorescence intensity measurements are typically unreliable away from the laboratory and can require frequent calibration/s due to a variety of chemical, optical, or other instrumental related factors [29,33]. Unfortunately, while fluorescent probes are known to be very useful for many applications [29,33], such as in fluorescence microscopy, DNA technology, and fluorescence sensing, most sensing fluorophores only display changes in intensity in response to analytes, and relatively few wavelength ratiometric and lifetime based probes are available [29,33]. Some useful wavelength ratiometric probes are available for pH, Ca2+, and Mg2+ [34,35], but the probes for Na+ and K+ generally display small spectral shifts and negligible lifetime changes, and are subsequently inadequate for quantitative sensing measurements. Dynamic quenchers such as O2 and the halides usually occur with a change in intensity and lifetime, but without an emission spectral shift. In addition, due to its low mass, the fluoride ion is not an effective collisional quencher and only a few probes are known to be quenched by fluoride in the low mM concentration range [1].
In this paper, we have shown that the boronic acid group can be relatively selective towards fluoride. Since the boronic acid group also interacts with other strong bases, such as OH− [31], BAQBA is likely to also have a pH-dependent response, although at pH 7, such as in physiological fluids or waste waters, the interaction is low (the concentration of OH− = 10−7 M), and environmental and physiological safeguard sensing applications do not experience notable pH changes.
While the many boronic acid containing fluorophores that have been synthesized to date [19–25] are likely to show a fluoride response, only a few will demonstrate spectral shifts and intensity changes, affording for ratiometric sensing, while even fewer will demonstrate an additional poor/non-sugar response. Further, BAQBA offers the additional possibility of lifetime-based halide sensing (collisional quenching). Hence, BAQBA offers the attractive opportunity for both ratiometric- and lifetime-based sensing of aqueous halide at neutral pH, in the mM halide concentration range. Moreover, because halide collisional quenching is an excited-state-based process and fluoride complexation with boronic acid is a ground state binding process, one has the future opportunity to potentially now simultaneously measure both fluoride (ratiometrically) and one other halide (lifetime based sensing), using one excitation wavelength, e.g. a 372 nm LED, and by monitoring two emission wavelengths, given the 94 nm emission band separation. It should be noted that fluoride will indeed collisionally quench quinolinium fluorescence, but not notably in the 10’s of mM fluoride concentration range. Such an approach may remove the requirement of needing two fluorescent probes, each with different KSV’s, for the simultaneous measurement of two halides, as first described by Wolfbeis and Urbano [36].
5. Conclusions
We have reported the ratiometric and colorimetric response of a highly fluorescent and water-soluble boronic acid containing fluorophore towards aqueous fluoride, in the presence of other potential sensing interferents known to bind boronic acid. This new probe displays a suppressed sugar response brought about by the amino substituent in the 6-position of the quinolinium backbone. While not shown here, by replacing the 6-amino group with other relatively less electron donating groups, then one does not observe the long wavelength 546 nm fluorescence emission band, eliminating the potential for ratiometric type sensing. In addition, a greater sugar response (affinity) is observed by introducing either CH3, –OCH3, and H groups into the 6-position. Hence, the unique combination of the amino group in the 6-position, coupled with the boronic acid moiety and a quinolinium nucleus, affords for a unique halide sensitive probe, sensitive to fluoride, chloride, bromide, and iodide in the mM concentration range, which is only slightly perturbed by sugars at a constant pH. In this paper, we have described the utility of this probe by its response to fluoride in the presence of other potential analytes/interferents, and the future opportunity for both simultaneous ratiometric and lifetime based analyte sensing.
Acknowledgements
This work was supported by the National Center for Research Resources, RR-08119.
Abbreviations:
- BA
boronic acid
- BAF and BAFs
boronic acid containing fluorophore/s
- BAQBA
N-(2-boronobenzyl)-6-aminoquinolinium bromide
- Imin
initial fluorescence intensity of BAQBA in the absence of sugar or fluoride
- Imax
final plateau fluorescence intensity of BAQBA in the presence of sugar or fluoride
- I0
fluorescence intensity in the absence of quencher
- IQ
fluorescence intensity in the presence of a quencher Q
- KSV
Stern–Volmer quenching constants
- LED
light emitting diode
- TC-SPC
time-correlated single photon counting
Biographies
Ramachandram Badugu is a postdoctoral fellow at the Center for Fluorescence Spectroscopy, University of Maryland School of Medicine in Baltimore. He has a B.Sc. in biological and chemical sciences and an M.Sc. in chemistry from Osmania University, Hyderabad, India, and Ph.D. in chemistry from University of Hyderabad, Hyderabad, India. Also he is a former JSPS fellow at Tohoku University, Sendai, Japan, and frontier research scientist at The Institute of Physical and Chemical Research (RIKEN), Sendai, Japan.
Joseph R. Lakowicz, Ph.D., is Director of the Center for Fluorescence Spectroscopy at the University of Maryland School of Medicine in Baltimore. He has a B.Sc. in chemistry from LaSalle University, and an M.Sc. and Ph.D. in Biochemistry from the University of Illinois at Urbana. He is also the founding editor of the Journal of Biomedical Optics and the Journal of Fluorescence. He is a co-founder and co-President of the Society of Fluorescence. He is also co-editor of the Who’s Who in Fluorescence and Annual Reviews in Fluorescence.
Chris D. Geddes, Ph.D., is an Associate Professor and Director of the Institute of Fluorescence, at the University of Maryland Biotechnology Institute, in Baltimore, USA. He has a B.Sc. from Lancaster University in England and a Ph.D. in physical chemistry (fluorescence spectroscopy) from the University of Wales Swansea. He is the Editor-in-Chief of the Journal of Fluorescence, and both editor and founding editor of the Who’s Who in Fluorescence and Annual Reviews in Fluorescence volumes. He is also executive director of the Society of Fluorescence.
References
- [1].Geddes CD, Meas. Sci. Technol 12 (2001) R53. [Google Scholar]
- [2].Kissa E, Clin. Chem 33 (1987) 253. [PubMed] [Google Scholar]
- [3].Michigami Y, Kurode Y, Ueda K, Yamamoto Y, Anal. Chim. Acta 274 (1993) 299. [Google Scholar]
- [4].Seifert K, Dominok B, Dominok GW, Fluoride 19 (1986) 22. [Google Scholar]
- [5].Tyler JE, Poole DFG, Arch. Oral. Biol 34 (1989) 995. [DOI] [PubMed] [Google Scholar]
- [6].Frant MS, Ross JW, Science 154 (1966) 1553. [DOI] [PubMed] [Google Scholar]
- [7].Culik B, Anal. Chim. Acta 189 (1986) 329. [Google Scholar]
- [8].Ikenishi R, Kitagawa T, Chem. Pharm. Bull 36 (1988) 810. [DOI] [PubMed] [Google Scholar]
- [9].Yamamoto H, Ori A, Ueda K, Dusemund C, Shinkai S, Chem. Commun 3 (1996) 407. [Google Scholar]
- [10].Ward CJ, Patel P, James TD, Chem. Lett 5 (2001) 406. [Google Scholar]
- [11].James TD, Sandanayake KRAS, Shinkai S, Nature 374 (1995) 345. [Google Scholar]
- [12].Norrild JC, Eggert H, J. Am. Chem. Soc 117 (1995) 1479. [Google Scholar]
- [13].Eggert H, Frederiksen J, Morin C, Norrild JC, J. Org. Chem 64 (1999) 3846. [Google Scholar]
- [14].Yang W, He H, Drueckhammer DG, Angew. Chem. Int. Ed 40 (2001) 1714. [PubMed] [Google Scholar]
- [15].Wang W, Gao S, Wang B, Org. Lett 1 (1999) 1209. [DOI] [PubMed] [Google Scholar]
- [16].Gao S, Wang W, Wang B, Bioorg. Chem 29 (5) (2001) 308. [DOI] [PubMed] [Google Scholar]
- [17].Lavigne JJ, Anslyn EV, Angew. Chem. Int. Ed 38 (1999) 3666. [PubMed] [Google Scholar]
- [18].Soundararajan S, Badawi M, Kohlrust CM, Hagerman JH, Anal. Biochem 178 (1989) 125. [DOI] [PubMed] [Google Scholar]
- [19].James TDK, Sandanayake KRAS, Shinkai S, Angew, Chem. Int. Ed. Engl 33 (1994) 2207. [Google Scholar]
- [20].James TD, Sandanayake KRAS, Iguchi R, Shinkai S, J. Am. Chem. Soc 117 (1995) 8982. [Google Scholar]
- [21].Bielecki M, Eggert H, Norrild JC, J. Chem. Soc. Perkin Trans 2 (1999) 449. [Google Scholar]
- [22].Dicesare N, Lakowicz JR, Anal. Biochem 294 (2001) 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dicesare N, Lakowicz JR, J. Photochem. Photobiol. A: Chem 143 (2001) 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dicesare N, Lakowicz JR, Org. Lett 3 (24) (2001) 3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dicesare N, Lakowicz JR, Tet. Lett 43 (2002) 2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Geddes CD, Sens. Actuators B: Chem 72 (2) (2001) 188. [Google Scholar]
- [27].Geddes CD, Douglas P, App. Poly. Sci 76 (5) (2000) 603. [Google Scholar]
- [28].Geddes CD, Karolin J, Apperson K, Birch DJS, Anal. Biochem 293 (1) (2001) 60. [DOI] [PubMed] [Google Scholar]
- [29].Lakowicz JR, Principles of Fluorescence Spectroscopy, 2nd ed., Kluwer/Academic Plenum Publishers, New York, 1997. [Google Scholar]
- [30].Cooper CR, Spencer N, James TD, Chem. Commun 13 (1998) 1365. [Google Scholar]
- [31].Dicesare N, Lakowicz JR, Anal. Biochem 301 (2002) 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Badugu R, Lakowicz JR, Geddes CD, Org. Lett 2004, submitted.
- [33].Gryczynski Z, Gryczynski I, Lakowicz JR, Meth. Enzymol 360 (2002) 44. [DOI] [PubMed] [Google Scholar]
- [34].Tsien RY, Rink TJ, Poenie M, Cell Calcium 6 (1985) 145. [DOI] [PubMed] [Google Scholar]
- [35].Kao JPY, Meth. Cell Biol 40 (1994) 155. [DOI] [PubMed] [Google Scholar]
- [36].Wolfbeis OS, Urbano E, Anal. Chem 55 (1983) 1904. [Google Scholar]