Abstract
Peripheral inflammation produces a long-lasting latent sensitization of spinal nociceptive neurons that is masked by tonic inhibitory controls. We explored mechanisms of latent sensitization with an established four-step approach: 1) induction of inflammation; 2) allow pain hypersensitivity to resolve; 3) interrogate latent sensitization with a channel blocker, mutant mouse, or receptor antagonist; 4) disrupt compensatory inhibition with a receptor antagonist so as to reinstate pain hypersensitivity. We found that the neuropeptide Y Y1 receptor antagonist BIBO3304 reinstated pain hypersensitivity, indicative of an unmasking of latent sensitization. BIBO3304-evoked reinstatement was not observed in AC1 knockout mice and was prevented with intrathecal co-administration of a pharmacological blocker to either: the N-methyl-D-aspartate receptor (NMDAR); adenylyl cyclase type 1 (AC1); protein kinase A (PKA); transient receptor potential cation channel A1 (TRPA1); channel V1 (TRPV1); or exchange protein activated by cAMP (Epac1 or Epac2). A PKA activator evoked both pain reinstatement and touch-evoked pERK expression in dorsal horn; the former was prevented with intrathecal co-administration of a TRPA1 or TRPV1 blocker. An Epac activator also evoked pain reinstatement and pERK expression. We conclude that PKA and Epac are sufficient to maintain long-lasting latent sensitization of dorsal horn neurons that is kept in remission by the NPY-Y1 receptor system. Furthermore, we have identified and characterized two novel molecular signaling pathways in the dorsal horn that drive latent sensitization in the setting of chronic inflammatory pain: NMDAR→AC1→PKA→TRPA1/V1 and NMDAR→AC1→Epac1/2. New treatments for chronic inflammatory pain might either increase endogenous NPY analgesia or inhibit AC1, PKA or Epac.
Introduction
Peripheral inflammation produces a central sensitization in the spinal cord that amplifies the intensity and extends the duration of nociception [36; 42]. After the initial hyperalgesia resolves, a silent long-lasting form of central sensitization, termed latent sensitization, continues to operate [17; 43; 68]. Latent sensitization contributes to the transition from acute to chronic pain, manifesting as vulnerability to recurrent allodynia or hyperalgesia upon subsequent injury or stressors [63]. However, the cellular and molecular mechanisms of latent sensitization remain largely unexplored.
By definition, latent sensitization is opposed by endogenous pain inhibitory mechanisms. A prime example is observed after disruption of the neuropeptide tyrosine (NPY) and NPY Y1 receptor (Y1R) signaling cascade (which we refer to as the NPY-Y1R axis). Solway et al. 2011 [58] reported that either genetic (NPY depletion) or pharmacological (intrathecal administration of an Y1R antagonist) disruption of the spinal NPY-Y1R reinstated hyperalgesia during the remission phase of latent sensitization, leading to the conclusion that the NPY-Y1R axis serves as an endogenous inhibitory brake on latent sensitization, thus keeping pain in remission. This project sought to unravel the underlying mechanisms that are kept in check by the NPY-Y1R axis.
Y1R is a member of the G-protein coupled receptor superfamily and couples to inhibitory G-proteins αi/o, leading to the inactivation of adenylyl cyclase and subsequent down regulation of cAMP [30; 56]. Ca2+/calmodulin-dependent adenylyl cyclase type 1 (AC1) is found in superficial laminae of spinal cord. Its activation by Ca2+ flux through N-Methyl-D-aspartate receptors (NMDAR) contributes to inflammatory pain [71; 73]. We reported that NMDAR-dependent AC1 superactivation contributes to the latent sensitization that is inhibited by μ-opioid receptor constitutive activity (MORCA) [17]. We propose an analogous inhibitory mechanism involving prolonged Y1R signaling after inflammation. Our first hypothesis is that endogenous NPY-Y1R axis maintains latent sensitization in the remission state by inhibiting NMDAR-dependent AC1.
cAMP is established to activate protein kinase A (PKA) and thus contribute to central sensitization [1; 42]. In addition, recent studies suggest a new exciting receptor target: the exchange protein directly activated by cAMP (Epac) [11; 20]. An emerging literature suggests that Epac critically contributes to pain [22; 23; 29; 31; 34; 57; 70]. These publications led to our second hypothesis that both PKA and Epac are essential to latent sensitization, which is silenced by the pain inhibitory NPY-Y1R axis.
Inflammation sensitizes the TRPA1 and TRPV1 channels [49; 50; 59] that are expressed on the central terminals of C and Aδ nociceptors [9; 32; 60; 61; 65], thus facilitating nociceptive transmission [3; 6; 8; 16; 18; 48; 49]. An estimated 35% of Y1R-expressing DRG neurons express TRPV1 [25], and Y1R activation down-regulates TPRV1/A1-induced Ca2+ mobilization [76; 77] or calcitonin-gene related peptide release [25–27]. PKA phosphorylation of either channel sensitizes them to inflammation [41; 52; 78]. Thus, our third hypothesis is that TRPA1 and V1 channels contribute to the latent sensitization that is silenced by the NPY-Y1R axis.
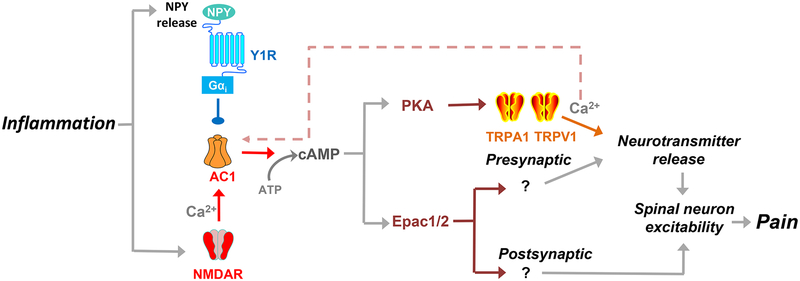
A schematic representation of these three hypotheses are illustrated in Figure 1.
Figure 1. Working hypotheses of the intracellular signaling pathways and mechanisms of latent sensitization that determine chronic inflammatory pain.
The endogenous NPY-Y1 receptor axis maintains latent sensitization in a state of remission (blue line and circle) through tonic inhibition of NMDAR-dependent AC1 (red arrows; Hypothesis 1), PKA and Epac (vermilion arrows; Hypothesis 2), and TRPA1 and TRPV1 channels (orange arrows; Hypothesis 3). In this model, a Y1 receptor antagonist will disinhibit NPY-Y1R, leading to pronociceptive neurotransmitter release, spinal neuron excitability and, ultimately, pain. The data of Figure 7 argues against a proposed pronociceptive feedback loop represented by the pink dashed arrow. Questions mark refer to unidentified postsynaptic mechanisms downstream of Epac 1/2. The current data suggest that inflammation sensitizes two pronociceptive signaling pathways that are silenced by the pain inhibitory NPY-Y1R axis: NMDAR → AC1 → PKA→ TRPA1/V1 at the presynaptic terminals of primary afferent neurons; and NMDAR → AC1 → Epac1/2 that operates within these presynaptic terminals and/or in post-synaptic sites on excitatory Y1R-expressing dorsal horn interneurons.
Materials and Methods
Animals
Male mice were housed 2–4 per cage, with littermates, in a light- (14-h light/dark cycle), temperature- (68–72° F) and humidity-controlled room with food and water provided ad libitum. Animals were allowed a minimum of one week to habituate to the facility prior to their entrance into the study. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky and the University of Pittsburgh, followed the guidelines for the treatment of animals of the International Association for the Study of Pain, and conducted in full compliance with the Association for Assessment and Accreditation of Laboratory Animal Care. Commercial mice. Male C57BL/6 mice weighing 18–20g were purchased from Charles Rivers (Indianapolis). AC1 knockout (KO) mice. As previously described [17], breeding pairs of AC1−/- mice were kindly provided by Dr. Daniel Storm (Washington University, Seattle, WA, USA), and bred at our facility to congenicity onto a C57BL/6 background. Male homozygous and their wildtype littermates were used in this study at the age of 8–12 weeks when the studies started. Genotype was confirmed before and after each study by tail-snip PCR.
Complete Freund’s Adjuvant (CFA) Model of Inflammatory Pain
Mice were injected subcutaneously with 5 μl CFA (1mg/ml, Sigma-Aldrich, St. Louis, MO) into the midplantar region of the left hindpaw with a 30G needle. Sham treatment involved restraint, with the left hindpaw extended for 1 min.
Mechanical Threshold Testing.
Animals were acclimated to a stainless-steel grid within individual Plexiglas tubes for at least 60 min prior to behavioral testing. To evaluate sensitivity to a non-noxious mechanical stimulus, we used an incremental series of 8 von Frey filaments (Stoelting, Inc, Wood Dale, IL) of logarithmic stiffness (0.008–6 grams). The 50% withdrawal threshold was determined using the up-down method [10]. Each filament was applied perpendicular to the central plantar surface of the hindpaw skin with sufficient force to cause a slight bending of the filament. A positive response was defined as a rapid withdrawal of the paw within a count of 5 seconds, as silently counted by the experimenter.
Intrathecal (i.t.) Drug Administration
Intrathecal injection was performed in lightly restrained unanesthetized mice as previously described [24]. Briefly, a 30 G needle attached to a Hamilton microsyringe was inserted between L5/L6 vertebrae, puncturing the dura (confirmed by presence of reflexive tail flick). We then injected a 5 μl volume of vehicle or drug. The data of Figure 2 were obtained 21 days after CFA induction, after two consecutive i.t. injections, each separated by 15 min. The data of Figure 3A include animals that were injected twice using a cross-over design with a 7-day separation between two injections. Animals receiving vehicle for the first injections received drug for the second, and animals receiving drug for the first injections received vehicle for the second. In all cases, group means of vehicle and drug did not differ on either injection day, and so were combined for final analysis.
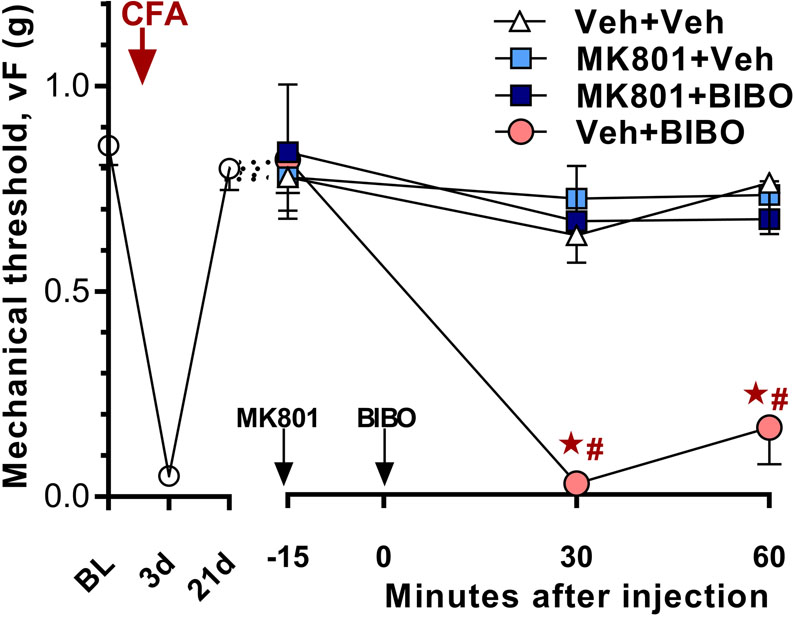
Figure 2. Inflammation-induced pain sensitization is tonically opposed by spinal Y1 signaling and requires NMDAR activation.
Time course of mechanical thresholds at pre-injection baseline, d3, d21 after CFA induction, and 30 and 60 min after the 2nd i.t. injection. Left) Progression and resolution of CFA-induced mechanical hyperalgesia (n=29). Right) 21 days after CFA intrathecal injection of MK801 (MK, 1 μg/5 μl) or vehicle (Veh) was followed 15 min later with injection of BIBO3304 (BIBO, 5 μg/5 μl) or vehicle. MK-801 prevented BIBO3304-evoked reinstatement of mechanical hypersensitivity. Post-injection n=6–8,★P < 0.05 (MK801 + BIBO vs Veh + BIBO), #P < 0.05 (Veh + BIBO vs Veh + Veh). Values represent mean ± SEM.
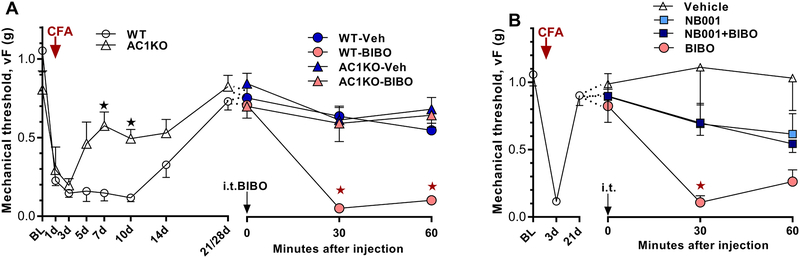
Figure 3. AC1 is necessary for the latent sensitization that is silenced by endogenous Y1R signaling.
Time course of mechanical thresholds at pre-injection baseline, after CFA induction, and 30 and 60 min after i.t. injection. A Left) Progression and resolution of CFA-induced mechanical hyperalgesia in AC1KO mice and their WT littermates. n=8, ★P < 0.05. A Right) Effect of BIBO3304 (BIBO, 5 μg/5 μl) when administered during pain remission. All animals were injected twice using a cross-over design with 7-day separation between the two i.t. injections. Post-injection n=13–16,★P < 0.05 (AC1KO-BIBO vs WT-BIBO). B) After the induction (3 days) and resolution (21 days) of CFA-induced hyperalgesia (n=22), co-administration of NB001 (1.5 μg) prevented BIBO3304-evoked reinstatement of mechanical hypersensitivity. Post-injection n=5–6, ★P < 0.05 (NB001+BIBO vs BIBO). Values represent mean ± SEM.
Drug Dosing
The following drugs and doses were used for intrathecal injections: BIBO 3304 trifluoroacetate (BIBO, Tocris Biosciences, United Kingdom), 5 μg/5 μl; (+)-MK-801 hydrogen maleate (MK-801, Sigma-Aldrich, St Louis, MO), 1 μg/5 μl (this dose of MK801 was devoid of overt motor effects); NB001 (Sigma-Aldrich, St Louis, MO), 1.5 μg/5 μl; N6- Benzoyladenosine- 3’, 5’- cyclic monophosphate (6-Bnz-cAMP), sodium salt membrane-permeant (6Bnz, BIOLOG Life Science Institute, Bremen, Germany), 10 nmol/5 μl; H-89 dihydrochloride hydrate (H89, Sigma-Aldrich, St Louis, MO), 10, 30 nmol/5 μl; 8-(4-Chlorophenylthio)-2’-O-methyladenosine 3’,5’-cyclic Monophosphate sodium salt (8cpt, Enzo Life Sciences, United Kingdom), 3 nmol/ 5 μl; ESI-09 and HJC0197 (synthesized by Dr. Jia Zhou, UTMB), 10 μg/5 μl; HJC0350 (synthesized by Dr. Jia Zhou, UTMB), 1 μg/5 μl; HC 030031 (Tocris Biosciences, United Kingdom) 10 μg/5 μl; AMG 9810 (Tocris Biosciences, United Kingdom) 1, 10 nmol/5 μl. Vehicle used for Figure 4A and 8A was saline. Vehicle used for Figure 5A, 6A, 8B–D, was ethanol: alkamuls EL-620 (Rhodia, Cranbury, NJ): saline in a volume ratio of 2:2:6. Vehicle used for other experiments was ethanol: alkamuls EL-620: saline in a volume ratio of 1:1:8.
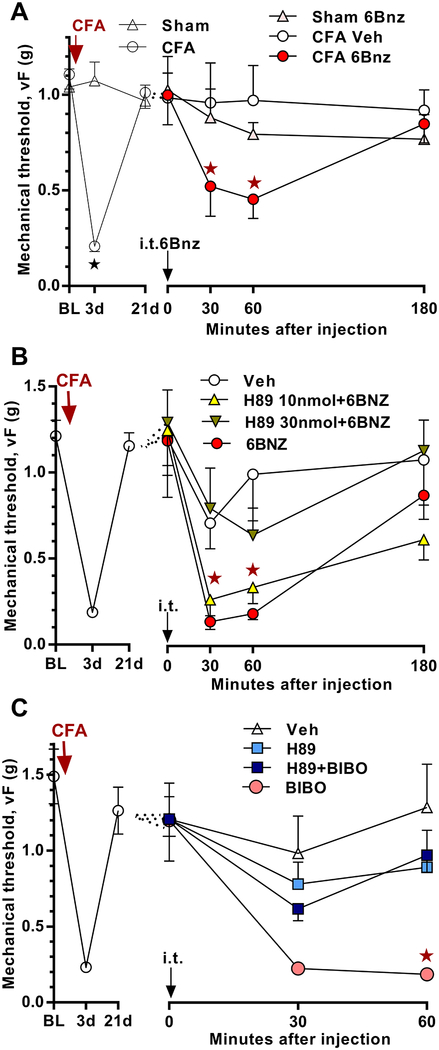
Figure 4. PKA activation is sufficient to reinstate mechanical hyperalgesia and contributes to the latent sensitization that is masked by NPY/Y1.
Time course of mechanical thresholds at pre-injection baseline, d3, d21 after CFA induction, and minutes after i.t. injection. A) CFA (n=28) but not sham injection (n=8) produced a mechanical hyperalgesia that peaked at 3 days and resolved within 21 days.★P < 0.05 (CFA vs Sham). 6Bnz (10 nmol) reinstated mechanical hyperalgesia in mice treated 21 days earlier with CFA but not sham controls. Post-injection n=6–8, ★P < 0.05 (CFA 6Bnz vs sham 6Bnz). B) After the induction (3 days) and resolution (21 days) of CFA-induced hyperalgesia (n=54), co-administration of H89 (30 nmol) blocked 6Bnz-evoked reinstatement. Post-injection n=4–12. ★P < 0.05 (30 nmol H89 + 6Bnz vs 6Bnz). C) H89 (10 nmol) attenuated BIBO3304 (BIBO, 5 μg)-evoked reinstatement of mechanical hyperalgesia when given 21 days after CFA induction. Post-injection n=8–12. ★P < 0.05 (H89 + BIBO vs BIBO). Values represent mean ± SEM.
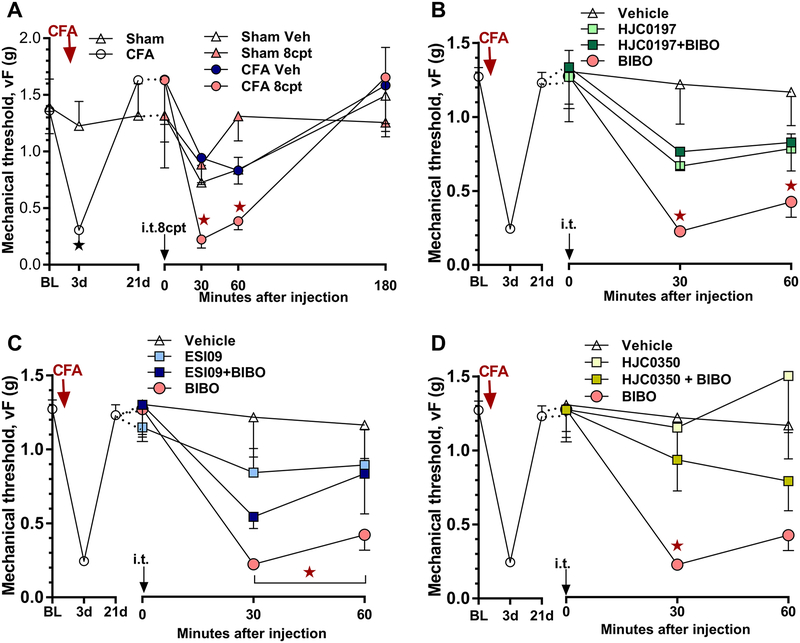
Figure 8. Epac activation is sufficient to reinstate mechanical hyperalgesia and contributes to the latent sensitization that is masked by NPY/Y1R.
Time course of mechanical thresholds at pre-injection baseline, d3, d21 after CFA induction, and minutes after i.t. injection. A) CFA (n=12) but not sham injection (n=11) produced a mechanical hyperalgesia that peaked at 3 days and resolved within 21 days, 8cpt (3 nmol) reinstated mechanical hyperalgesia when given 21 days after CFA induction but not in sham control mice. ★P < 0.05 (CFA vs sham). Post-injection n=5–6, ★P < 0.05 (CFA 8cpt vs sham 8cpt). B) After the induction (3 days) and resolution (21 days) of CFA-induced hyperalgesia (n=84), co-administration of HJC0197 (10 μg) attenuated BIBO3304 (BIBO, 5 μg)-induced reinstatement of mechanical hyperalgesia. Post-injection n=6–14. ★P < 0.05 (HJC0197 + BIBO vs BIBO). C) ESI-09 (10 μg) attenuated BIBO3304-evoked reinstatement. Post-injection n=10–14. ★P < 0.05 (ESI-09 + BIBO vs BIBO). D) HJC0350 (1 μg) attenuated BIBO3304-evoked reinstatement. Post-injection n=5–14. ★P < 0.05 (HJC0350 + BIBO vs BIBO). Values represent mean ± SEM.
Figure 5. TRPA1 and TRPV1 contributes to latent sensitization.
Time course of mechanical thresholds at pre-injection baseline, d3, d21 after CFA induction, and minutes after the i.t. injection. A) After the induction (3 days) and resolution (21 days) of CFA-induced hyperalgesia (n=34), Co-administration of HC030031 (HC, 10 μg) attenuated BIBO3304-evoked reinstatement of mechanical hypersensitivity. Post-injection n=6–10. ★P < 0.05 (HC+BIBO vs BIBO). B) AMG9810 (AMG, 10 nmol) attenuated BIBO3304-evoked reinstatement of mechanical hypersensitivity. Post-injection n=4–8. ★P < 0.05 (AMG+BIBO vs BIBO). Values represent mean ± SEM.
Figure 6. PKA-driven latent sensitization is mediated by TRPA1 and TPRPV1.
Time course of mechanical thresholds at pre-injection baseline, d3, d21 after CFA induction, and minutes after i.t. injection. A) After the induction (3 days) and resolution (21 days) of CFA-induced hyperalgesia (n=54), co-administration of HC030031 (10 μg) abolished 6Bnz (10 nmol)-induced reinstatement of mechanical hypersensitivity. Post-injection n=9–12. ★P < 0.05 (HC030031+6Bnz vs 6Bnz). B) Co-administration of AMG9810 (30 nmol) attenuated 6Bnz-induced reinstatement of mechanical hypersensitivity. Post-injection n=5–12. ★P < 0.05 (AMG9810+6Bnz vs 6Bnz). Values represent mean ± SEM.
Blinding procedure
The experimenter was blinded to drug treatments in all behavioral pharmacology experiments by a laboratory colleague. Briefly, all drugs and vehicle were made in identical tubes. A lab mate color-coded the tubes for the experimenter. The key for coding was kept hidden in a notebook until the completion of the experiment. The experimenter then obtained the key for data analysis.
Immunohistochemistry and quantification of pERK
Separate cohorts of mice were tested for baseline mechanical sensitivity and exposed to CFA or sham treatment. Mechanical thresholds were reassessed 3 days and/or 21 days later, followed by intrathecal injection of 6Bnz (10 nmol) 8cpt (3 nmol), or vehicle (5 ul). To determine the effect of vehicle or drug on pERK activation in the ipsilateral dorsal horn, a light touch stimulation protocol was initiated 30 min after the end of behavioral testing. As previously described [31; 33], mice were anesthetized with isoflurane (5% induction, 2% maintenance). The plantar surface of the left hindpaw was gently stroked in the longitudinal plane with a cotton-tip for 3 seconds out of every 5 seconds, for 5 min. After an additional 5 min wait time, mice received an intraperitoneal injection of sodium pentobarbital (>100 mg/kg, 0.2 ml, Fatal Plus ®) and were transcardially perfused with ice cold 1x phosphate buffered saline (PBS) with heparin (10,000 USP units/L) followed by ice-cold fixative (10% phosphate buffered formalin). The lumbar spinal cord was removed and post-fixed overnight in 10% phosphate buffered formalin and then cryoprotected in 30% sucrose in 0.1 M PBS for several days. Transverse sections (30 μm) centered at L4 were cut on a freezing microtome or cryostat and collected in antifreeze. The sections were washed three times in PBS and then pretreated with blocking solution (3% normal goat serum and 0.3% Triton X-100 in PBS) for 1 h. Sections were then incubated in blocking solution containing the primary antibody rabbit anti-pERK (1:1000, Phospho-p44/42 MAPK, Cell Signaling Technology #4370, RRID:AB_2315112) overnight at room temperature on a slow rocker. The sections were washed three times in PBS, and incubated in goat anti-rabbit secondary antibody (1:1000, Alexa Fluor 488, Invitrogen A11008, RRID:AB_143165) for 60 min, washed in PBS then 0.01 M phosphate buffer without saline, mounted onto Superfrost Plus slides, air dried, and cover-slipped with Hard Set Antifade Mounting Medium with DAPI (VECTASHIELD®)
All images were captured on a Nikon Eclipse Ti2 microscope using a 10x objective (numerical aperture 0.45) and analyzed using NIS-Elements Advanced Research software v5.02. We focused our quantification of the number of pERK immunopositive cell profiles within lamina I-II, where the majority of nociceptive peripheral afferents terminate within the dorsal horn (Corder et al., 2010), in left (ipsilateral to light touch stimulation) L4 spinal cord segments. Two observers who did not know the identity of the slides / sections (e.g. blinded to treatment) manually counted punctate immunoreactive profiles in 3–5 high quality randomly selected sections of L4 spinal cord from each animal. The manual counts for each L4 section were averaged between the two blinded quantifiers. These averaged section counts for each individual animal were then averaged for an overall animal mean of punctate immunoreactive profiles.
Statistics
Differences between means were analyzed by two-way analysis of variance (ANOVA) (Figure 2–6, 7A and 8), as indicated in the figure legends. Drug/Dose/Gene was a grouping factor and time was the repeated measure. If a significant interaction or group was found (p < 0.05), the ANOVA was followed by Bonferroni’s (for 2-group comparison) or Tukey’s post-hoc (for multi-group comparison) tests. Figure 7B was analyzed by Student’s t test (Graphpad Prism v7). Differences between means after intrathecal drug treatment (Figure 9J) were analyzed with a one-way analysis of variance (ANOVA) followed by Holm-Sidak post-hoc tests.
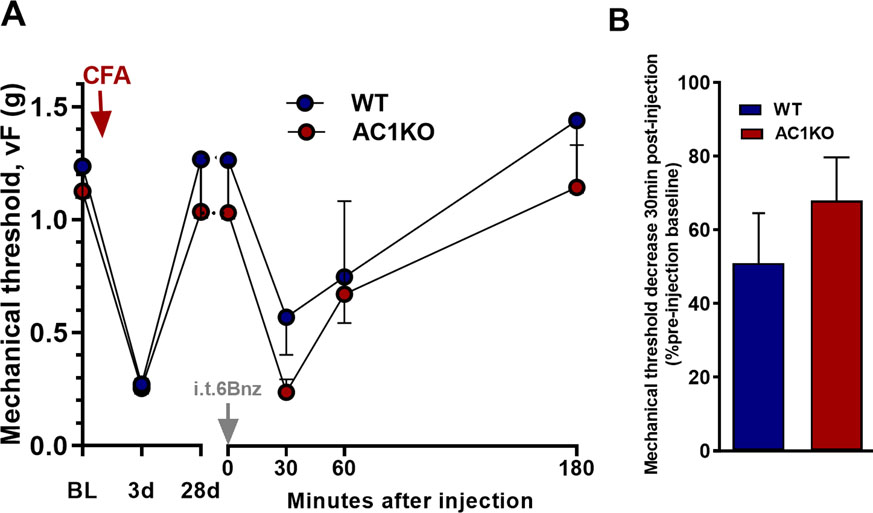
Figure 7. PKA-driven latent sensitization is not AC1-dependent.
A) Time course of mechanical thresholds at pre-injection baseline, d3, d28 after CFA induction, and 30, 60, and 180 min after i.t. injection. B) Decrease in mechanical threshold, 30 min after i.t. injection, plotted as percentage of pre-injection baseline. When injected after the resolution (28 days) of CFA-induced hyperalgesia, 6Bnz (10 nmol)-evoked a mechanical hyperalgesia that was similar between AC1KO and WT mice (p>0.05). n=7–9. Values represent mean ± SEM.
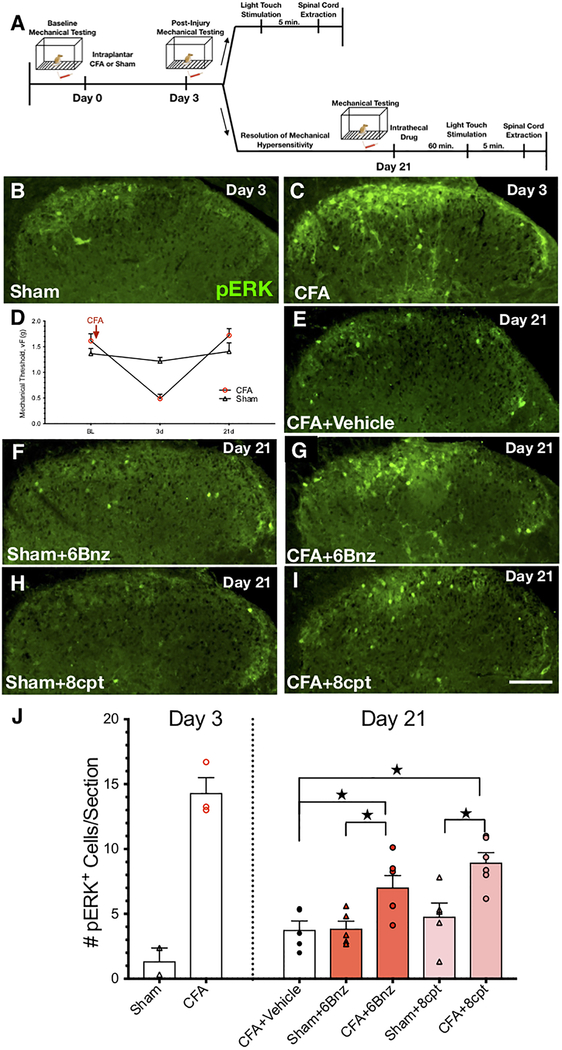
Figure 9. PKA or Epac activation is sufficient to reveal latent sensitization of dorsal horn neurons.
A) Timeline of experimental design for light touch mechanical stimulation-induced pERK immunoreactivity in mice 21 days after CFA. B-C) pERK immunoreactivity following light mechanical stimulation in representative transverse sections of L4 dorsal horn, 3 days after sham treatment (B) or intraplantar CFA injection (C). D) CFA (n=20) but not sham (n=10) produced mechanical hyperalgesia that peaked at 3 days and resolved by 21 days. E-I) pERK immunoreactivity following light mechanical stimulation in representative transverse sections of L4 dorsal horn 21 days after Sham or CFA, following intrathecal administration of vehicle in CFA mice (E), 10 nmol 6Bnz in Sham (F) or CFA mice (G), or 3nmol 8cpt in Sham (H) or CFA mice (I). J) Quantification of pERK. After the induction (Day 3) and resolution (Day 21) of CFA-induced hyperalgesia, intrathecal administration of 6Bnz or 8cpt increased the number of pERK-immunoreactive profiles in lamina I-II as compared to intrathecal administration of vehicle [★P < 0.05 (CFA-vehicle vs CFA-6Bnz, CFA-vehicle vs CFA-8cpt)], or as compared to sham controls [★P < 0.05, (Sham-6Bnz vs CFA-6Bnz, Sham-8cpt vs. CFA-8cpt)). n=5–6 mice per group. Values represent mean ± SEM. Scale bar 100 μm.
Results
Inflammation-induced pain sensitization is tonically opposed by spinal Y1 signaling and requires NMDAR activation.
As illustrated in Figure 2, mechanical hypersensitivity was confirmed three days after CFA injection, and then resolved by day 21. Consistent with previous reports [40; 58], the Y1R antagonist BIBO3304 did not change mechanical threshold in sham mice not given CFA, and, reinstated mechanical hyperalgesia at both 30 (p = 0.0004) and 60 (p = 0.0003) min after i.t. injection (Drug × Time; F3, 25 = 8.643; p = 0.0004).
NMDAR-Ca2+-AC1-cAMP signaling is required to maintain the inflammation-induced latent sensitization that is inhibited by μ-opioid receptor constitutive activity (MORCA) [17]. To test the hypothesis that prolonged endogenous NPY-Y1R signaling similarly suppresses latent sensitization, we first asked whether NMDAR activity is required for the induction of pain reinstatement produced by inhibition of Y1R signaling. Figure 2 illustrates that the activity-dependent NMDAR blocker MK801, but not vehicle, prevented the reinstatement evoked by BIBO3304 at both time points (p < 0.0001, p = 0.002, respectively). When given by itself, MK801 did not change mechanical threshold (P>0.05) and did not produce motor function side effects [17].
Latent sensitization requires AC1 activation.
Next, we used deletion mutant mice to ask whether AC1 is required for the latent sensitization that is masked by Y1R. Consistent with previous reports [17; 73], baseline mechanical thresholds were similar between AC1KO and WT mice (Figure 3A, P > 0.05). However, CFA produced a time-dependent difference in mechanical hyperalgesia (Gene × Time, F1, 14 = 4.932; p = 0.0434). While induction of hyperalgesia was similar across strains (d1–3, P > 0.05), mechanical thresholds returned to baseline sooner in AC1KO mice. These results indicate that AC1 is not required for the expression of acute nociception, but contributes to the later phase of CFA hyperalgesia. We next injected BIBO3304 after mechanical hypersensitivity had returned to baseline in both strains (21/28 days). BIBO3304 reinstated mechanical hyperalgesia in WT but not AC1KO (Gene × Time, F1, 26 = 20.5; p = 0.0001).
To determine whether the site of action of AC1 is at the spinal cord, we intrathecally co-injected BIBO3304 with NB001, a selective AC1 inhibitor [71]. As illustrated in Figure 3B, NB001 prevented the ability of BIBO3304 to reinstate mechanical hyperalgesia (Drug × Time, F1, 9 = 10.24; p = 0.0108).
PKA contributes to latent sensitization that is inhibited by the endogenous NPY-Y1R axis.
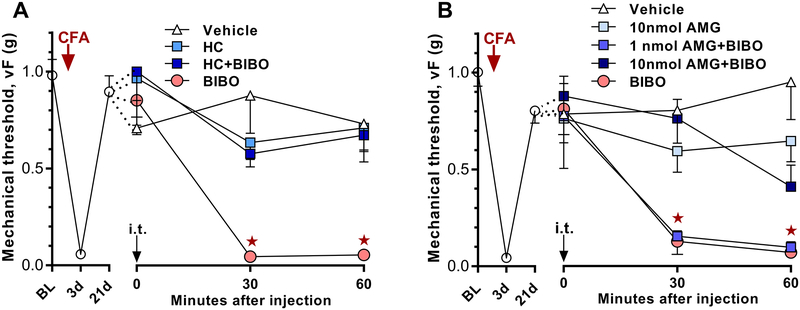
Corder et al. 2013 [17] reported that an NMDAR-Ca2+-AC1-cAMP signaling pathway drives the latent sensitization that is masked by MORCA but did not investigate signaling mechanisms downstream of cAMP. To determine whether PKA activation is sufficient to reinstate hyperalgesia, we administered the selective PKA activator, 6-Bnz-cAMP (6Bnz) to mice given CFA 21 days earlier and their sham controls. As illustrated in Figure 4A, we used a dose (10 nmol, i.t.) that did not produce mechanical hyperalgesia in sham mice. We found that 6Bnz reinstated mechanical hyperalgesia when given 21 days after CFA induction (Group × Time; F2, 20 = 5.571; p = 0.0119). To confirm that the reinstatement produced by 6Bnz is through the activation of PKA, we injected the PKA inhibitor H89. As illustrated in Figure 4B, H89 dose-dependently inhibited the reinstatement produced by 6Bnz (Group × Time; F3, 29 = 6.364; p = 0.0019).
To determine whether PKA contributes to the latent sensitization that is masked by NPY/Y1, we intrathecally administered H89 together with the Y1 antagonist BIBO3304 in mice given CFA 21 days earlier. As illustrated in Figure 4C, H89 attenuated BIBO3304-evoked reinstatement of mechanical hyperalgesia (Group × Time; F3, 38 = 5.965; p = 0.002).
TRPA1 and TRPV1 contribute to latent sensitization.
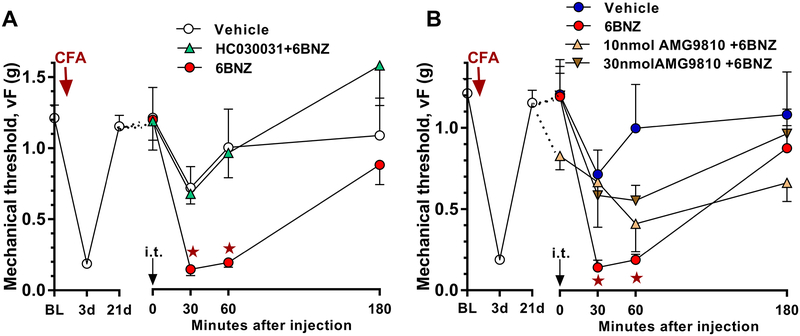
The above results suggest that an NMDAR-AC1-PKA signaling pathway drives latent sensitization. Phosphorylation by PKA is required for TRPA1 activation or sensitization as described in several rodent pain models [14; 54; 72]. To test the hypothesis that TRPA1 activity contributes to latent sensitization, we intrathecally injected HC030031, a TRPA1 antagonist, together with BIBO3304 in mice given CFA 21 days earlier. As illustrated in Figure 5A, HC030031 attenuated BIBO3304-evoked reinstatement of mechanical hyperalgesia (Group × Time, F3, 30 = 7.398; p = 0.0008).
Similar to TRPA1, PKA-dependent sensitization or reduction of desensitization of TRPV1 can increase the responsiveness of pain-processing neurons during pain perception. [5; 44; 46; 47; 51; 78]. To test the hypothesis that latent sensitization contributes to TRPV1 activation, we intrathecally injected AMG9810, a TRPV1 antagonist, together with BIBO3304 in mice given CFA 21 days earlier. As illustrated in Figure 5B, AMG9810 dose-dependently attenuated the BIBO3304-evoked reinstatement of mechanical hyperalgesia in the CFA model (Group × Time, F4, 29 = 19.38; p < 0.0001).
PKA-driven latent sensitization is TRPA1- and TRPV1-dependent.
It is unknown whether PKA-driven TRPA1 and TRPV1 activation contributes to latent sensitization. To test that PKA-driven latent sensitization is TRPA1- or TRPV1-dependent, we intrathecally delivered TRPA1 or TRPV1 antagonists, HC030031 or AMG9810 respectively, together with the PKA activator 6Bnz in mice given CFA 21 days earlier. As illustrated in Figure 6, HC030031 (10 μg) prevented the ability of 6Bnz to reinstate mechanical hypersensitivity (Figure 6A, Group × Time, F2, 30 = 10.25; p = 0.0004), while AMG9810 (30 nmol) attenuated 6Bnz-evoked reinstatement of mechanical hypersensitivity (Figure 6B, Group × Time, F3, 35 = 5.799; p = 0.0025).
PKA-driven latent sensitization is not AC1-dependent.
Both TRPA1 and TRPV1 are Ca2+ permeable cation channels, allowing Ca2+ influx into the cell upon activation. We posited that the resulting influx of Ca2+ could either activate a downstream calcium-dependent enzyme AC1, or cause further activation of the upstream AC1 thus forming a positive feedback loop that would then potentiate latent sensitization and pain reinstatement. To test this hypothesis, we delivered the PKA selective activator 6Bnz not only to wild type mice, but also to AC1 knockout mice at 21 days after CFA induction. We purposefully chose a dose of 6Bnz that did not produce mechanical hypersensitivity in uninflamed mice (Figure 4A). When conducted after the resolution (28 days) of CFA-induced hyperalgesia, reinstatement of mechanical hyperalgesia by the PKA activator 6Bnz was not reduced in AC1 knockout mice (Figure 7, P > 0.05).
Epac1 and Epac2 contribute to latent sensitization that is inhibited by the NPY-Y1R axis.
cAMP signaling is mediated not only by PKA, but also by exchange protein directly activated by cAMP (Epac). The two isoforms are isoforms 1 (Epac1, RapGef3, cAMP-GEF I) and isoform 2 (Epac2, RapGef4, cAMP-GEF II) [11; 20]. They catalyze GDP/GTP exchange in small G-proteins [19; 39], activating downstream signaling. An emerging literature indicates that both contribute to pain sensitization after inflammation or injury [22; 23; 29; 31; 33; 34; 45; 57; 70]. To determine whether Epac activation is sufficient to reinstate hyperalgesia, we administered the selective Epac activator, 8cpt, at a dose (3 nmol) that does not produce mechanical hyperalgesia in sham mice. As illustrated in Figure 8A, 8cpt reinstated mechanical hyperalgesia when tested after the resolution (21 days) of CFA-induced hyperalgesia (Figure 8A, Group × Time; F3, 19 = 5.555; p = 0.0066).
We next tested the hypothesis that endogenous latent sensitization is mediated by Epac1 and/or Epac2. After the resolution (21 days) of CFA-induced hyperalgesia, we injected BIBO3304 with either Epac1/2 dual antagonists HJC0197 and ESI-09, or with a highly isoform-selective Epac2 antagonist HJC0350 [2; 12; 13]. Each of these attenuated BIBO3304-evoked reinstatements of mechanical hyperalgesia: HJC0197 (Figure 8B, Group × Time; F3, 39 = 5.749; p = 0.0023); ESI-09 (Figure 8C, Group × Time; F3, 45 = 5.033; p = 0.0043); and HJC0350 (Figure 8D, Group × Time; F3, 36 = 6.071; p = 0.0019).
PKA and Epac contribute to latent sensitization of dorsal horn neurons.
Peripheral inflammation produces a cAMP-dependent latent central sensitization in the spinal cord that amplifies the intensity and extends the duration of nociception [36; 42][17; 43; 68][. To test the hypothesis that the cAMP receptors, PKA and Epac, contribute to latent sensitization of dorsal horn neurons, we evaluated stimulus-evoked expression of pERK. Dorsal horn pERK expression is widely used as a marker of central sensitization in models of persistent pain [13; 29; 31]. As illustrated by the timeline of Figure 9A, we injected 6Bnz (10 nmol), 8cpt (3 nmol), or vehicle (5 ul, i.t.) into mice 21 days after sham or CFA induction, applied light mechanical stimulation, and then extracted spinal cords for pERK immunohistochemistry in lamina I-II of the L4 dorsal horn. As illustrated in Fig 9B, the number of pERK+ cells was small when tested 3 days after sham treatment. By contrast, Fig 9C illustrates that light touch stimulation induced pERK expression is in a relatively large number of cells when tested 3 days after CFA. We next evaluated pERK expression after confirming that both hyperalgesia (Fig 9D) and stimulus-evoked increases in pERK expression (Fig 9E) had resolved by Day 21. As illustrated in Figs 9F–I, both 6Bnz (F4,22 =7.741, p=0.0200) and 8cpt (F4,22 =7.741, p=0.0008) increased stimulus-evoked pERK expression as compared to CFA 21d mice that received vehicle. Similarly, both 6Bnz (F4,22 =7.741, p= 0.0200) and 8cpt (F4,22 =7.741, p= 0.0048) increased pERK expression in CFA 21d mice as compared to Sham 21d mice.
Discussion
NPY is abundantly expressed in laminae I-III of the dorsal horn [28; 53], and is up-regulated on the side ipsilateral to paw inflammation [37](Ji et al. 1994), where it can inhibit inflammation-enhanced Y1R signaling and substance P release [64]. We targeted spinal NPY pain inhibition with intrathecal injection of the Y1R antagonist, BIBO3304. We cannot rule out diffusion along the dorsal root to the DRG; however, NPY is not expressed in the DRG after paw inflammation [66; 67]. Therefore, the present studies indicate that intrathecal BIBO reinstated hyperalgesia upon blockade of the actions of NPY that was released from spinal interneurons.
Endogenous NPY-Y1R axis maintains latent sensitization in remission by inhibiting NMDAR-dependent AC1.
The NMDAR is a non-selective cation channel (permeable to both Na+ and Ca2+) which can be found in almost every synapse in the superficial dorsal horn. Ca2+ stimulated isoforms of adenylyl cyclase are activated by NMDARs in brain [15], and NMDAR-derived Ca2+ increases are linked to cAMP signaling pathways [15; 75] necessary for LTP initiation [69], opiate physical dependence [79] and spinal synaptic facilitation [71; 74]. Continued activation of NMDAR leads to the induction and maintenance of central sensitization [42]. Furthermore, AC1 contributes to high frequency stimulation-induced LTP [71]. We report that during the pain remission stage after CFA, spinal delivery of the NMDAR activity blocker MK-801 silenced Y1R antagonist-induced reinstatement of mechanical hypersensitivity. Likewise, genetic (AC1 knockout) or pharmacological (NB001) interruption of AC1 prevented reinstatement. The interpretation of these results is two-fold: 1) Inflammation induces an NMDAR- and AC1-dependent latent sensitization, likely in dorsal horn; and 2) NMDAR- and AC1-dependent latent sensitization is inhibited by the NPY-Y1R axis. These conclusions are consistent with our previous report that an NMDAR-AC1-dependent latent sensitization is inhibited by MORCA [17]. As in the previous study, we reported that intrathecal MK-801 abolished an NTX-precipitated increase in cAMP when given 21 days after CFA induction [17]. Moreover, direct activation of spinal NMDARs with intrathecal NMDA increased spinal cAMP levels [17], indicating that NMDAR signaling is directly linked to downstream cAMP signaling during opioid receptor inverse agonism. In summary, we conclude that an NMDAR-cAMP linkage drives the pronociceptive latent sensitization that is kept in remission both by endogenous MORCA and NPY-Y1R signaling. Current studies, presented in abstract form, are finding that the intrathecal combination of a mu opioid receptor inverse agonist and a Y1R antagonist act synergistically to reinstate mechanical hypersensitivity [21]. This suggests that MORCA and NPY-Y1 signaling work together to restrain latent sensitization within a state of remission.
Inflammation produces a long-lasting latent sensitization of two cAMP effectors: PKA and Epacs
The current behavioral and pERK immunohistochemical results show that intrathecal injection of either a selective PKA (6Bnz) or Epac activator (8cpt) reinstated hyperalgesia and touch-evoked neuronal activation when given 21 days after CFA induction. These data indicate that inflammation induces a latent sensitization of dorsal horn neurons such that less PKA or Epac agonist is required to induce allodynia, and, ultimately, a state of vulnerability to the transition from acute to chronic pain. Possible underlying mechanisms could be: a) Sensitization of PKA and/or Epac, such that less robust activation of PKA or Epac is sufficient to produce hyperalgesia; or b) Increased affinity of PKA or Epac for agonist. Although G-protein coupled receptor pain inhibitory systems (MORCA, NPY/Y1R) are active 21 days after CFA induction, PKA and Epac activators are of sufficient power to override them. Our results are reminiscent of previous studies showing that direct activation of spinal NMDARs (with intrathecal NMDA) or AC (with intrathecal forskolin) produced enhanced spontaneous nocifensive behaviors when given 21 days after CFA induction [17].
Latent sensitization of PKA is maintained by TRPA1 and TRPV1 but not by AC1
Inflammation or injury-induced hyperalgesia is associated with PKA-induced phosphorylation and modulation of TRPA1 [14; 54; 72] and TRPV1 [5; 44; 46; 47; 51; 78]. We found that intrathecal injection of a TRPA1 antagonist (HC030031) or a TPRV1 antagonist (AMG9810) prevented the reinstatement of mechanical hyperalgesia produced by 6Bnz. We conclude that inflammation triggers a long-lasting sensitization of PKA-TRPA1 and PKA-TRPV1 signaling cascade that is masked by the NPY-Y1R axis.
Reinstatement of mechanical hyperalgesia by the PKA activator 6Bnz was not reduced in AC1 knockout mice. We conclude that Ca2+ influx through TRPA1 or TRPV1 channels, and any ensuing upstream activation of AC1, does not form a positive feedback loop that potentiates latent sensitization (Figure 1). Any Ca2+ signaling downstream of PKA is also AC1-independent.
NPY-Y1R axis maintains latent sensitization in remission by inhibiting PKA and Epac1/2.
When tested after the resolution (21 days) of CFA-induced hyperalgesia, we found that intrathecal injection of a PKA inhibitor (H89) or Epac inhibitors (ESI-09, HJC0350, or HJC0197) attenuated the reinstatement of mechanical hyperalgesia by a Y1R antagonist (BIBO3304). Because ESI-09 and HJC-0197 (nonselective Epac1/2 dual inhibitors) and HJC0350 (Epac2-specific inhibitor) attenuated BIBO3304 reinstatement, we conclude that Epac2 and possibly Epac1 are critical isoforms that contributes to latent sensitization. These data extend previous studies in rodent models of sub-chronic pain (e.g. behavioral testing conducted less than two weeks after inflammation or injury) that supported a pro-hyperalgesia actions of PKA [1] and Epacs [22; 23; 29; 31; 33; 34; 45; 57; 70]. For example, previous studies in pain models of inflammation, nerve-injury, or plantar incision indicated that pharmacological inhibition, genetic knockout, or antisense oligodeoxynucleotide knockdown of Epac1 (presumably in DRG neurons) reduced pain-like behavior [22; 31; 45; 70]. We conclude that PKA and Epacs are necessary for the cellular mechanisms that drive latent sensitization. This is consistent with previous studies suggesting that PKA mediates the hyperalgesic priming induced by repeated μ-opioid exposure [4], and that Epac mediates hyperalgesic priming induced by periphery inflammation (hindpaw carrageenan or ΨεRACK) [33; 70]. Our data describing inhibition of latent sensitization by intrathecal administration of HJC0350, ESI-09, and HJC0197 extend the therapeutic targeting of Epac1/2 inhibition to the dorsal horn of the spinal cord. In summary, our data have identified the downstream components of inflammation-induced latent sensitization and pain, beyond the spinal NMDAR-Ca2+-AC1-cAMP signaling pathway that was earlier described by Corder et al 2013 [17], and provide support for further investigation of the contribution of spinal Epac1 and Epac2 to chronic inflammatory pain.
NPY-Y1R axis maintains latent sensitization in remission by inhibiting TRPA1 and TRPV1.
The non-selective cation channels TRPA1 and TRPV1 are polymodal sensors located on nociceptor cell bodies and nerve terminals of primary afferent neurons. Channels on peripheral terminals detect somatosensory stimuli and transmit noxious information to the spinal cord [38], but the contribution of TRPA1 and TRPV1 on central terminals is poorly understood. We report that spinal delivery of a TRPA1 or TRPV1 antagonist attenuated Y1R antagonist-induced mechanical hyperalgesia reinstatement during latent sensitization and conclude that both TRPA1 and TRPV1 contribute to the inflammation-induced latent sensitization that is masked by the endogenous NPY-Y1R axis.
Following exposure of inflammatory mediators, TRPA1 [7] and TRPV1 [35; 55; 80] are phosphorylated by PKA through scaffolding protein A-kinase anchoring protein 79/150 (AKAP150). Furthermore, both AKAP150 and TRPA1 [7] or TRPV1 [62] are required in a carrageenan-induced hyperalgesic priming model. This suggests a possible role of AKAP for PKA-TRPA1/TRPV1 sensitization during inflammatory latent sensitization. AKAP may also mediate AC-PKA and cAMP-Epac signaling by providing an anchoring platform [33]. These studies suggest an interesting future direction to study the contribution of AKAP to latent sensitization.
Conclusions
Inflammation produces latent sensitization, which includes not only sensitization of neurons in the dorsal horn, but also a concomitant strengthening of endogenous inhibition. Together, these opposing systems underlie a vulnerability to episodic pain that is manifested when inhibitory controls fail [17; 43; 58]. In this paper, we identified and characterized two novel molecular signaling pathways (Figure 1) that drive latent sensitization at the spinal level, after inflammation: NMDAR→AC1→PKA→TRPA1/V1 and NMDAR→AC1→Epac1/2. We argue that both are silently supersensitive during latent sensitization: silent because of tonic inhibitory control by the spinal NPY-Y1R axis. To further test this working model, further studies are warranted to determine receptor levels (e.g. immunohistochemistry and western blot) and enzyme catalytic activity. In conclusion, the data described in this paper advance our understanding of the contribution of NMDAR, AC1, PKA, TRPA1, TRPV1, and Epacs to chronic pain. Spinal AC1 and Epacs are particularly attractive targets for the development of a new pharmacotherapy for chronic pain.
Conflict of interest statement
The authors have no conflict of interest to declare. This work was supported by R01DA37621 (BKT), R01NS45954 (BKT), K01DA031961 (SD), and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number KL2TR000116.
References
- [1].Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci 1999;19(6):2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F, Cheng X. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol 2013;83(1):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. The Journal of physiology 2006;575(Pt 2):555–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Araldi D, Ferrari LF, Levine JD. Repeated Mu-Opioid Exposure Induces a Novel Form of the Hyperalgesic Priming Model for Transition to Chronic Pain. J Neurosci 2015;35(36):12502–12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 2002;35(4):721–731. [DOI] [PubMed] [Google Scholar]
- [6].Bolcskei K, Helyes Z, Szabo A, Sandor K, Elekes K, Nemeth J, Almasi R, Pinter E, Petho G, Szolcsanyi J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain 2005;117(3):368–376. [DOI] [PubMed] [Google Scholar]
- [7].Brackley AD, Gomez R, Guerrero KA, Akopian AN, Glucksman MJ, Du J, Carlton SM, Jeske NA. A-Kinase Anchoring Protein 79/150 Scaffolds Transient Receptor Potential A 1 Phosphorylation and Sensitization by Metabotropic Glutamate Receptor Activation. Sci Rep 2017;7(1):1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000;288(5464):306–313. [DOI] [PubMed] [Google Scholar]
- [9].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389(6653):816–824. [DOI] [PubMed] [Google Scholar]
- [10].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [11].Chen H, Tsalkova T, Chepurny OG, Mei FC, Holz GG, Cheng X, Zhou J. Identification and characterization of small molecules as potent and specific EPAC2 antagonists. J Med Chem 2013;56(3):952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen H, Tsalkova T, Mei FC, Hu Y, Cheng X, Zhou J. 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg Med Chem Lett 2012;22(12):4038–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen H, Wild C, Zhou X, Ye N, Cheng X, Zhou J. Recent advances in the discovery of small molecules targeting exchange proteins directly activated by cAMP (EPAC). J Med Chem 2014;57(9):3651–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 2011;193:440–451. [DOI] [PubMed] [Google Scholar]
- [15].Chetkovich DM, Sweatt JD. nMDA receptor activation increases cyclic AMP in area CA1 of the hippocampus via calcium/calmodulin stimulation of adenylyl cyclase. J Neurochem 1993;61(5):1933–1942. [DOI] [PubMed] [Google Scholar]
- [16].Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 2001;411(6840):957–962. [DOI] [PubMed] [Google Scholar]
- [17].Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 2013;341(6152):1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000;405(6783):183–187. [DOI] [PubMed] [Google Scholar]
- [19].de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998;396(6710):474–477. [DOI] [PubMed] [Google Scholar]
- [20].Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 2005;437(7058):574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dos Santos D C-P L, Donahue R, Oliveira-Fusaro M,Taylor B. Does a long-lasting, analgesic synergy develop between spinal mu opioid receptors and neuropeptide Y1Rs after surgical incision? American Pain Society Annual Scientific Meeting, Vol. 18 Pittsburgh, PA: The Journal of Pain, 2017. p. S26. [Google Scholar]
- [22].Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, Ishikawa Y, Zwartkuis FJ, Cox JJ, Wood JN. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nature communications 2013;4:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Eijkelkamp N, Wang H, Garza-Carbajal A, Willemen HL, Zwartkruis FJ, Wood JN, Dantzer R, Kelley KW, Heijnen CJ, Kavelaars A. Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, protein kinase Cepsilon, and MEK/ERK. J Neurosci 2010;30(38):12806–12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Advanced drug delivery reviews 2003;55(8):1007–1041. [DOI] [PubMed] [Google Scholar]
- [25].Gibbs J, Flores CM, Hargreaves KM. Neuropeptide Y inhibits capsaicin-sensitive nociceptors via a Y1-receptor-mediated mechanism. Neuroscience 2004;125(3):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gibbs JL, Flores CM, Hargreaves KM. Attenuation of capsaicin-evoked mechanical allodynia by peripheral neuropeptide Y Y1 receptors. Pain 2006;124(1–2):167–174. [DOI] [PubMed] [Google Scholar]
- [27].Gibbs JL, Hargreaves KM. Neuropeptide Y Y1 receptor effects on pulpal nociceptors. Journal of dental research 2008;87(10):948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gibson SJ, Polak JM, Allen JM, Adrian TE, Kelly JS, Bloom SR. The distribution and origin of a novel brain peptide, neuropeptide Y, in the spinal cord of several mammals. The Journal of comparative neurology 1984;227(1):78–91. [DOI] [PubMed] [Google Scholar]
- [29].Griffin RS. An Epac-dependent pain pathway. J Neurosci 2005;25(36):8113–8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Grouzmann E, Meyer C, Burki E, Brunner H. Neuropeptide Y Y2 receptor signalling mechanisms in the human glioblastoma cell line LN319. Peptides 2001;22(3):379–386. [DOI] [PubMed] [Google Scholar]
- [31].Gu Y, Li G, Chen Y, Huang LY. Epac-protein kinase C alpha signaling in purinergic P2X3R-mediated hyperalgesia after inflammation. Pain 2016;157(7):1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 1999;11(3):946–958. [DOI] [PubMed] [Google Scholar]
- [33].Huang LY, Gu Y. Epac and Nociceptor Sensitization. Mol Pain 2017;13:1744806917716234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci 2005;25(26):6119–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 2008;138(3):604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003;26(12):696–705. [DOI] [PubMed] [Google Scholar]
- [37].Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hokfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. The Journal of neuroscience : the official journal of the Society for Neuroscience 1994;14(11 Pt 1):6423–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Julius D TRP channels and pain. Annu Rev Cell Dev Biol 2013;29:355–384. [DOI] [PubMed] [Google Scholar]
- [39].Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 1998;282(5397):2275–2279. [DOI] [PubMed] [Google Scholar]
- [40].Kuphal KE, Solway B, Pedrazzini T, Taylor BK. Y1 receptor knockout increases nociception and prevents the anti-allodynic actions of NPY. Nutrition 2008;24(9):885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lapointe TK, Altier C. The role of TRPA1 in visceral inflammation and pain. Channels (Austin) 2011;5(6):525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The journal of pain : official journal of the American Pain Society 2009;10(9):895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Le Roy C, Laboureyras E, Gavello-Baudy S, Chateauraynaud J, Laulin JP, Simonnet G. Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization Via a NMDA-dependent process. The journal of pain : official journal of the American Pain Society 2011;12(10):1069–1079. [DOI] [PubMed] [Google Scholar]
- [44].Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci 1998;18(16):6081–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Matsuda M, Oh-Hashi K, Yokota I, Sawa T, Amaya F. Acquired Exchange Protein Directly Activated by Cyclic Adenosine Monophosphate Activity Induced by p38 Mitogen-activated Protein Kinase in Primary Afferent Neurons Contributes to Sustaining Postincisional Nociception. Anesthesiology 2017;126(1):150–162. [DOI] [PubMed] [Google Scholar]
- [46].Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem 2003;278(50):50080–50090. [DOI] [PubMed] [Google Scholar]
- [47].Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem 2005;280(14):13424–13432. [DOI] [PubMed] [Google Scholar]
- [48].Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci 2003;23(14):6058–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Palazzo E, Luongo L, de Novellis V, Rossi F, Marabese I, Maione S. Transient receptor potential vanilloid type 1 and pain development. Current opinion in pharmacology 2012;12(1):9–17. [DOI] [PubMed] [Google Scholar]
- [50].Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, Ji RR. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014;82(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci 2002;22(11):4740–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rosenbaum T, Simon SA. TRPV1 Receptors and Signal Transduction In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL), 2007. [PubMed] [Google Scholar]
- [53].Rowan S, Todd AJ, Spike RC. Evidence that neuropeptide Y is present in GABAergic neurons in the superficial dorsal horn of the rat spinal cord. Neuroscience 1993;53(2):537–545. [DOI] [PubMed] [Google Scholar]
- [54].Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 2009;64(4):498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 2008;28(19):4904–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Silva AP, Cavadas C, Grouzmann E. Neuropeptide Y and its receptors as potential therapeutic drug targets. Clinica chimica acta; international journal of clinical chemistry 2002;326(1–2):3–25. [DOI] [PubMed] [Google Scholar]
- [57].Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, Mei FC, Zhu Y, Cheng X, Hawke D, Mayor F Jr., Murga C, Heijnen CJ, Kavelaars A. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proceedings of the National Academy of Sciences of the United States of America 2016;113(11):3036–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. Proceedings of the National Academy of Sciences of the United States of America 2011;108(17):7224–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Spahn V, Stein C, Zollner C. Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Mol Pharmacol 2014;85(2):335–344. [DOI] [PubMed] [Google Scholar]
- [60].Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacological reviews 1999;51(2):159–212. [PubMed] [Google Scholar]
- [61].Szallasi A, Nilsson S, Farkas-Szallasi T, Blumberg PM, Hokfelt T, Lundberg JM. Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain research 1995;703(1–2):175–183. [DOI] [PubMed] [Google Scholar]
- [62].Szteyn K, Rowan MP, Gomez R, Du J, Carlton SM, Jeske NA. A-kinase anchoring protein 79/150 coordinates metabotropic glutamate receptor sensitization of peripheral sensory neurons. Pain 2015;156(11):2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Taylor BK, Corder G. Endogenous analgesia, dependence, and latent pain sensitization. Curr Top Behav Neurosci 2014;20:283–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Taylor BK, Fu W, Kuphal KE, Stiller CO, Winter MK, Chen W, Corder GF, Urban JH, McCarson KE, Marvizon JC. Inflammation enhances Y1 receptor signaling, neuropeptide Y-mediated inhibition of hyperalgesia, and substance P release from primary afferent neurons. Neuroscience 2014;256:178–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Valtschanoff JG, Rustioni A, Guo A, Hwang SJ. Vanilloid receptor VR1 is both presynaptic and postsynaptic in the superficial laminae of the rat dorsal horn. The Journal of comparative neurology 2001;436(2):225–235. [PubMed] [Google Scholar]
- [66].Wakisaka S, Kajander KC, Bennett GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett 1991;124(2):200–203. [DOI] [PubMed] [Google Scholar]
- [67].Wakisaka S, Kajander KC, Bennett GJ. Effects of peripheral nerve injuries and tissue inflammation on the levels of neuropeptide Y-like immunoreactivity in rat primary afferent neurons. Brain research 1992;598(1–2):349–352. [DOI] [PubMed] [Google Scholar]
- [68].Walwyn WM, Chen W, Kim H, Minasyan A, Ennes HS, McRoberts JA, Marvizon JC. Sustained Suppression of Hyperalgesia during Latent Sensitization by mu-, delta-, and kappa-opioid receptors and alpha2A Adrenergic Receptors: Role of Constitutive Activity. J Neurosci 2016;36(1):204–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wang GD, Zhuo M. Synergistic enhancement of glutamate-mediated responses by serotonin and forskolin in adult mouse spinal dorsal horn neurons. J Neurophysiol 2002;87(2):732–739. [DOI] [PubMed] [Google Scholar]
- [70].Wang H, Heijnen CJ, van Velthoven CT, Willemen HL, Ishikawa Y, Zhang X, Sood AK, Vroon A, Eijkelkamp N, Kavelaars A. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. The Journal of clinical investigation 2013;123(12):5023–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang H, Xu H, Wu LJ, Kim SS, Chen T, Koga K, Descalzi G, Gong B, Vadakkan KI, Zhang X, Kaang BK, Zhuo M. Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Sci Transl Med 2011;3(65):65ra63. [DOI] [PubMed] [Google Scholar]
- [72].Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 2008;131(Pt 5):1241–1251. [DOI] [PubMed] [Google Scholar]
- [73].Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW, Pineda VV, Xu HM, Chen ZF, Storm DR, Muglia LJ, Zhuo M. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 2002;36(4):713–726. [DOI] [PubMed] [Google Scholar]
- [74].Wei F, Vadakkan KI, Toyoda H, Wu LJ, Zhao MG, Xu H, Shum FW, Jia YH, Zhuo M. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci 2006;26(3):851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron 1999;23(4):787–798. [DOI] [PubMed] [Google Scholar]
- [76].Xu J, Li M, Shen P. A G-protein-coupled neuropeptide Y-like receptor suppresses behavioral and sensory response to multiple stressful stimuli in Drosophila. J Neurosci 2010;30(7):2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Xu J, Sornborger AT, Lee JK, Shen P. Drosophila TRPA channel modulates sugar-stimulated neural excitation, avoidance and social response. Nature neuroscience 2008;11(6):676–682. [DOI] [PubMed] [Google Scholar]
- [78].Yao X, Kwan HY, Huang Y. Regulation of TRP channels by phosphorylation. Neurosignals 2005;14(6):273–280. [DOI] [PubMed] [Google Scholar]
- [79].Zachariou V, Liu R, LaPlant Q, Xiao G, Renthal W, Chan GC, Storm DR, Aghajanian G, Nestler EJ. Distinct roles of adenylyl cyclases 1 and 8 in opiate dependence: behavioral, electrophysiological, and molecular studies. Biol Psychiatry 2008;63(11):1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008;59(3):450–461. [DOI] [PubMed] [Google Scholar]