Abstract
Background
In neurocysticercosis, the larval form of the pork tapeworm Taenia solium appears to evolve through three phases—active, degenerative and sometimes calcification—before disappearance. The antihelmintic drug, albendazole, has been shown to hasten the resolution of active cysts in neurocysticercosis. Little is known about the time cysts take to progress through each phase, with or without treatment.
Methods
We reconfigured brain imaging data from patient level to cyst level for 117 patients in a randomized clinical trial of albendazole in which images were taken at baseline, 1, 6, 12 and 24 mo. Applying a multistate model, we modelled the hazard of a cyst evolving to subsequent cyst phases before the next imaging (vs no change). We examined the impact of albendazole treatment overall and by patient and cyst characteristics on the hazard.
Results
Albendazole accelerated the evolution from the active to degenerative phase (HR=2.7, 95% CI 1.3 to 6.5) and from the degenerative phase to disappearance (HR=1.9, 95% CI 1.1 to 3.9). Albendazole’s impact was stronger for patients who were male, did not have calcified cysts at baseline and who had multiple cysts in different locations.
Conclusions
This research provides a better understanding of where in the cyst trajectory albendazole has the greatest impact.
Keywords: albendazole, brain, central nervous system, multistate model, neurocysticercosis, Taenia solium
Introduction
Neurocysticercosis (NC) is an infection of the central nervous system with the larval form of the pork tapeworm Taenia solium.1 NC is the most frequent preventable cause of epilepsy worldwide and in endemic countries it is estimated to cause 30% of all epilepsy cases.2
When located in the human brain, the larval stage of T. solium appears to pass through three distinct stages of evolution before total disappearance.3,4 In the first stage, the parasite is viable or alive. These cysts are classified as being in the ‘active’ phase. In the second phase, the parasite is degenerating (colloidal and granular nodular forms) and is targeted by the host’s immune system. This stage is called the ‘transitional’ or ‘degenerative’ phase and is most frequently associated with symptomatic disease. After the parasite dies, a calcified nodule sometimes remains in its place rather than the cyst evolving directly from the degenerating phase to total disappearance; this is termed the ‘calcified’ phase. Although a pattern of cyst evolution through these three phases before total disappearance has been identified, the natural history of cysts in the brain is not well understood and little is known about the amount of time cysts take to progress through each phase, with or without treatment.5
There have been randomized clinical trials (RCTs) suggesting that antihelmintic drugs, such as albendazole (ALB), decrease the burden of active cysts,6–8 but the role of degenerative cysts remains controversial.9,10 Recent guidelines state that ALB is probably effective in decreasing the number of cysts in patients with parenchymal NC. 11,12 However, the impact of ALB on cysts in other brain locations has not been definitively determined,9 although a 2019 study found an association between complete cyst disappearance and higher concentrations of ALB in patients with extraparenchymal NC.13
Most studies of NC have looked at cysts in an aggregated form, examining outcomes such as the presence, number or percentage of cysts of a specified type or in a specific location in the patient’s brain identified through imaging (CT or MRI). To our knowledge, the only studies that have looked at changes over time in individual cysts within the brain (as opposed to a summary of all cysts in the brain) have been restricted to patients with solitary cysts.14–16 However, it is possible that cyst evolution is impacted, in part, by the overall cyst burden of the patient such that solitary cysts evolve differently from cysts in a brain that hosts other NC cysts in various locations and phases. A patient may present with multiple cysts at different phases and with varying immunological responses around those cysts, suggesting either multiple infections over time or that different cysts may evolve differently within the same individual.17
In this paper, we evaluate the impact of treatment with ALB on the evolution of individual NC cysts from 117 patients over 24 mo using multistate models. The data come from an RCT comparing the impact of ALB plus prednisone to placebo plus prednisone conducted by the Ecuadorian Neurocysticercosis Group between 2001 and 2005.6 In contrast to previous studies, our sample included patients with multiple cysts in multiple locations, as well as parenchymal and extraparenchymal cysts. Also, we explore if ALB has a different impact on cyst evolution depending on the patient’s gender, age and cyst burden, cyst location and presence of calcified cysts at baseline. To our knowledge, this is the first study examining individual NC cyst evolution among patients with more than one cyst over 24 mo and using multistate models.
Materials and methods
Participants
The trial is registered with Clinicaltrials.gov (NCT00283699, available at https://clinicaltrials.gov/ct2/show/NCT00283699?cond=Neurocysticercosis&cntry=EC&rank=1) and its design has been described elsewhere.6 Briefly, this randomized, double-blind, placebo-controlled clinical trial recruited symptomatic patients with newly diagnosed NC from six hospitals in Ecuador. Patients with at least one cyst in the active or degenerative phase in any location in the brain were included (n=172) and randomized to receive albendazole 400 mg or placebo given orally every 12 h for 8 d. All patients also received prednisone. Patients had CT or MRI scans at baseline and the same type of scan at 1, 6 and 12 mo of follow-up for comparison; 59.8% of patients had CT scans and 40.2% had MRIs. Patients were followed clinically for 24 mo. Although the protocol for the original RCT only called for imaging through 12 mo, the usual protocol for treating NC patients in Ecuador calls for imaging at 24 mo. The thickness of the CT scan cuts was approximately 3–5 mm for infratentorial cuts and 5x8 mm for supratentorial cuts and was consistent over time. We acquired the 24-mo image data, digitized and integrated it with the trial data. For each scan, a radiologist, who was blind to the patient’s treatment status and the results of previous scans, documented the phase and location of each cyst and summarized the total number of cysts overall and by phase and location in the brain.
Measures
We disaggregated data from the patient to the cyst level. Our sample of cysts included those from patients with a single cyst and those from patients with multiple cysts at baseline. For the multiple cyst cases, we restricted our selection of individual cysts to those in locations that had only one cyst to be able to easily follow the evolution of the cyst over time. In each treatment arm, we traced the cyst phase over the five possible scan times (baseline, 1, 6, 12 and 24 mo) and kept only the cysts with non-reverse transitions, in which the cyst either stayed in the same phase or evolved progressively to another phase forward on the evolutionary track at next scan time. We call this procedure ‘serialization’.
The treatment group was coded as an indicator for ALB vs placebo. Patient gender was categorized as male or female and age at baseline was summarized as a continuous variable and dichotomized at the median as <40 vs 40+ y for regression models. Cyst location was classified as parenchymal or extraparenchymal, where the extraparenchymal cysts included those that were in intraventricular, cisternal and subarachnoid locations. To evaluate the impact of cyst burden, we created a patient level binary indicator for having multiple cysts in multiple locations (MCMLs) vs a single cyst and we looked at an indicator for whether the patient had any calcified cysts at baseline (yes/no).
Statistical analysis
Analyzing NC cyst evolution presents several challenges. First, the four possible NC cyst phases (active, degenerative, calcification and disappearance) construct a multivariate outcome structure; therefore, using standard statistical methods, such as the Cox Proportional Hazards, leads to inaccurate results due to competing risks. Second, study designs using fixed schedules for imaging are common and consequently the exact cyst evolving times that are in between the fixed schedules cannot be determined. In addition, the variation in rates of cyst progression and long intervals between subsequent imaging can generate a latent path problem, alternative unobservable evolution pathways that a cyst might take to reach the next observed stage.
Multistate models (MSMs) are a generalization of the survival process where survival is the ultimate outcome, but intermediate states are also of interest. A comprehensive overview of MSMs is available elsewhere.18,19 Briefly, the MSM’s flexible framework allows for defining several states and modelling the probability of evolving from one state to another over time. The unobserved evolving and latent path problem can be addressed with the interval-censoring schema in MSMs. Compared with separate Cox models, multistate models allow for dissection of prognostic factors and intermediate events in the analysis of cause-specific endpoints and can yield new insights into disease progression and associated pathways. Given the presence of four distinct NC cyst stages with disappearance as the absorbing state under a fixed schedule for imaging, MSM is a natural choice to model NC cyst evolution.
Therefore, we used a four-state multistate Markov model with three transient states (active, degenerative and calcification) and one absorbing state (disappearance) to model the risk of remaining in the current cyst phase or changing to the subsequent phases over the study period. Figure 1 shows the expected NC cyst life course we used for the treatment evaluation. Due to the fixed imaging times, we observed certain ‘direct’ evolutions. For example, cysts that were observed to ‘progress’ from the active phase to disappearance directly, while in fact the intermittent states likely occurred, but were not observed. For those cases, the interval-censoring schema was applied in MSM with prespecified ‘allowable’ transitions. Evolution intensities, representing the instantaneous risk of moving from one state to another (vs remaining in the same state), were estimated from the identified evolutions. The estimates were then used to calculate HRs for the impact of ALB vs placebo to evolve to a subsequent phase. Subgroup analyses of patient characteristics (age and gender) and cyst characteristics (location, MCMLs and presence of calcified cysts at baseline) were also conducted. Specifically, we tested for interactions between ALB and each patient and cyst characteristic described above by adding the interaction term to the MSM model and ran stratified models if the interaction term was significant.
Figure 1.
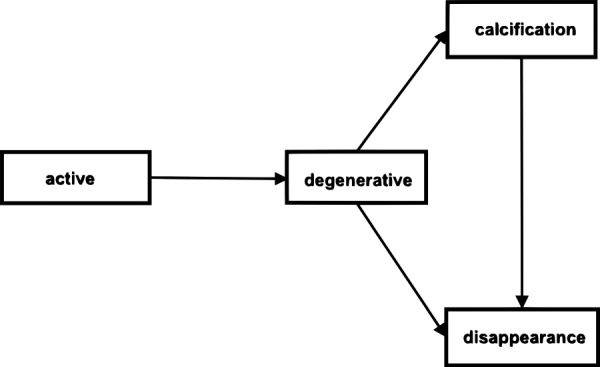
NC cyst progressive evolution with four states.
When multiple NC cysts evolutions were abstracted from the same patient, those evolutions were correlated. Although not affecting the point estimates for our analyses, biased results with respect to the CIs and p-values can be induced when the correlation is ignored. In this analysis, we accounted for the within-patient correlation with the bootstrap method.20
Analyses were conducted using R software version 3.3.3 (R Foundation of Statistical Computing, Vienna, Austria) and the multistate models were fitted with the msm package.21 The likelihood ratio test was used to assess the treatment effect on the entire trajectory where α=0.05 was used, except for tests of interaction, which were tested at the α=0.1 level because of the decreased statistical power associated with testing for interaction.22
Results
The serialization gave us data on 221 individual cysts (and their evolution paths) from 117 patients; 62 were under ALB treatment and 55 were treated with placebo. We excluded 14 cysts due to the appearance of reverse evolution, in which cysts moved to a previous phase of cyst evolution, and 13 racemous cysts, because it is difficult to tell if a racemous cyst is one or multiple cysts and thus difficult to follow their pathways. Among the sample, there were 27 people with single cysts and 90 people with MCMLs. There were 132 parenchymal cysts and 89 extraparenchymal cysts. In the analytic data, 53% of the 117 patients were male and the mean age was 40 y (SD 17.2, range 3.1 to 82.1 y). Sixty-nine patients had imaging data available at 24 mo and thus a fair proportion of participants were censored at some point in the 24-mo period (Table 1). Of the 221 cysts, 124 were in patients from the ALB arm and 97 from patients in the placebo arm at baseline.
Table 1.
Percentage of cysts in each phase at each image time point during follow-up as well as the corresponding total number of cysts and the number of patients across treatment groups
| Albendazole | Placebo | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage of cysts in each phase | Total | Number | Percentage of cysts in each phase | Total | Number | |||||||
| Active | Degenerativec | Calcification | Disappearance | number | of | Active | Degenerativec | Calcification | Disappearance | number | of | |
| of cysts | patients | of cysts | patients | |||||||||
| 0 mo (baseline) | 54.8 | 22.6 | 22.6 | 0.0 | 124 | 62 | 42.3 | 38.1 | 19.6 | 0.0 | 97 | 55 |
| 1 moa | 16.3 | 17.9 | 19.5 | 46.3 | 123 | 62 | 27.8 | 32.9 | 14.6 | 24.7 | 97 | 55 |
| 6 mo | 9.3 | 9.3 | 13.8 | 67.6 | 108 | 56 | 24.7 | 21.2 | 11.8 | 42.3 | 85 | 52 |
| 12 mo | 7.7 | 9.6 | 8.7 | 74.0 | 104 | 56 | 9.9 | 11.9 | 9.7 | 68.5 | 92d | 52 |
| 24 mob | 0.0 | 3.1 | 1.4 | 95.6 | 68 | 37 | 3.7 | 1.9 | 3.7 | 90.7 | 54 | 32 |
Loss-to-follow-up occurred to both groups. For example:
aat 6 mo: six patients were lost to follow-up from the treatment group and three from the placebo group;
bat 24 mo: an additional 19 patients were lost to follow-up in the treatment group and 20 from the placebo group.
cdegenerative includes the colloidal and granular nodular phases of the cyst evolution.
dthe increase of cysts from 6 mo to 12 mo is due to certain patients who missed 6-mo scans, but returned for 12-mo scans.
In Table 1, we describe the percentage of cysts in each phase at each time point and the total number of cysts and of patients between treatment arms, a cross-sectional summary of the data.
Table 2 presents the transition frequency over the study period across the imaging visits. From the 221 cysts, there were 259 ‘evolutions’ in which the cyst was observed to have stayed within its initial phase and 203 evolutions in which the cyst moved to a subsequent phase. Fifty active to disappearance evolutions were observed under ALB treatment. Under placebo, there were only 24 such evolutions.
Table 2.
Evolution frequency, overall and by treatment group observed over imaging visitsa
| Evolution | Overall | Albendazole | Placebo |
|---|---|---|---|
| Active → Active | 97 | 38 | 59 |
| Active → Degenerativeb | 19 | 11 | 8 |
| Active → Calcification | 2 | 2 | 0 |
| Active → Disappearance | 74 | 50 | 24 |
| Degenerative → Degenerative | 87 | 33 | 54 |
| Degenerative → Calcification | 7 | 2 | 5 |
| Degenerative → Disappearance | 66 | 32 | 34 |
| Calcification → Calcification | 75 | 45 | 30 |
| Calcification → Disappearance | 35 | 20 | 15 |
| Total | 462 | 233 | 229 |
anote that a single cyst may contribute multiple transitions. For example, an active cyst at baseline that progresses to degenerative between 6 mo and 12 mo and stays in the stage to the end of the study contributes four transitions: 1: baseline to 1 mo (as active to active), 2: 1 mo to 6 mo (as active to active), 3: 6 mo to 12 mo (as active to degenerative) and 4: 12 mo to 24 mo (as degenerative to degenerative).
bdegenerative includes the colloidal and granular nodular phases of the cyst evolution.
The results of the MSM for the treatment comparison overall and stratified by patient and cyst characteristics that modify the treatment effect are presented in Table 3. Overall, ALB had a significant impact on the entire trajectory (likelihood ratio test p<0.01). In particular, cysts in the ALB arm were more likely to evolve from the active phase to the degenerative phase than those in the placebo arm (HR=2.7, 95% CI 1.3 to 6.5, p<0.01). Cysts in the ALB arm were also more likely to evolve from the degenerative phase to the disappearance phase compared with those in the placebo arm (HR=1.9, 95% CI 1.1 to 3.9, p<0.05). There were no significant differences between the ALB and placebo arms in the evolution of cysts from the degenerative phase to the calcification phase or from the calcification phase to disappearance.
Table 3.
Impact of albendazole (ALB) on the cyst trajectory and evolution for the entire sample and stratified by patient and cyst characteristics
| Active→Degenerative | Degenerative → Calcification | Degenerative → Disappearance | Calcification → Disappearance | |||||
|---|---|---|---|---|---|---|---|---|
| Models | HRa | (95% CI) | HRa | (95% CI) | HRa | (95% CI) | HRa | (95% CI) |
| Entire sample (p<0.01) | ||||||||
| ALB | 2.7 | (1.3 to 6.5)** | 1.1 | (0.0 to 5.8) | 1.9 | (1.1 to 3.9)* | 1.1 | (0.5 to 2.2) |
| Impact of ALB stratified by patient gender | ||||||||
| female | 1.5 | (0.2 to 5.5) | 0.3 | (0.0 to Inf) | 1.2 | (0.4 to 4.1) | 1.3 | (0.1 to Inf) |
| male | 4.4 | (1.8 to 14.6)** | 3.8 | (0.0 to Inf) | 3.8 | (1.6 to 15.9)** | 1.3 | (0.5 to 3.3) |
| Impact of ALB stratified by patient age at baseline (y) | ||||||||
| < 40 | 3.6 | (1.2 to 16.2)* | 0.3 | (0.0 to Inf) | 0.8 | (0.2 to 2.3) | 0.7 | (0.1 to 2.2) |
| ≥40 | 1.6 | (0.7 to 4.8) | 1.9 | (0.0 to Inf) | 4.4 | (1.5 to 19.9)** | 1.6 | (0.5 to 6.7) |
| Impact of ALB stratified by the cyst burden in the brain at baseline | ||||||||
| single cyst | 0.6 | (0.2 to 1.5) | 1.1 | (0.0 to Inf) | 0.9 | (0.1 to 10.1) | 2.8 | (0.0 to Inf) |
| multiple cysts | 4.1 | (1.8 to 13.1)** | 1.1 | (0.0 to 10.8) | 2.4 | (1.1 to 5.9)* | 1.0 | (0.4 to 2.3) |
| Impact of ALB stratified by the presence of calcified cysts at baseline | ||||||||
| No | 2.5 | (1.1 to 7.1))** | 1.3 | (0.0 to Inf) | 1.3 | (0.7 to 3.7) | 2.5 | (0.0 to Inf) |
| Yes | 2.8 | (0.8 to 14.7) | 1.4 | (0.0 to 32.8) | 3.6 | (1.1 to 24.3) | 1.0 | (0.4 to 2.3) |
| Impact of ALB stratified by the location of cyst | ||||||||
| parenchymal | 3.1 | (1.4 to 9.3)* | 0.9 | (0.0 to 8.7) | 1.4 | (0.7 to 3.4) | 1.1 | (0.4 to 3.4) |
| extraparenchymal | 1.6 | (0.6 to 6.7) | 1.4 | (0.0 to Inf) | 5.4 | (1.4 to 99.5)* | 0.7 | (0.0 to 3.3) |
aHR refers to the impact of ALB on the specific evolution; Inf: a value larger than 1000.00
*0.01<p-value<0.05; **p-value<0.01
We found significant interaction between albendazole treatment and patient gender (interaction p<0.01), age (interaction p<0.01), MCMLs (interaction p<0.01) and location of cyst (interaction p=0.03). Overall, the impact of ALB on cyst evolution was much stronger among males than females. Among younger patients (aged <40 y), ALB was associated with a significantly faster rate of evolution from active to degenerative (HR=3.6, 95% CI 1.2 to 16.2) and a slower rate of other evolutions, which were not statistically significant. Among older patients, ALB was associated with a faster rate of evolution for all cyst evolutions, but only the evolution from degenerative to disappearance was significant (HR=4.4, 95% CI 1.5 to 19.9). Among those with MCMLs at baseline, ALB had a stronger and significant impact on the rates of evolution from active to degenerative (HR=4.1, 95% CI 1.8 to 13.1) and degenerative to disappearance (HR=2.4, 95% CI 1.1 to 5.9).
The impact of ALB on cyst evolution also differed significantly between cysts in parenchymal vs extraparenchymal regions with the impact of ALB stronger for cysts in parenchymal regions for the evolution from active to degenerative (HR=3.1, 95% CI 1.4 to 9.3).
Discussion
We present a four-state MSM for the serialized evolutions of individual NC cysts abstracted from the patient level data of an RCT on the impact of ALB treatment. By considering several states and related evolutions simultaneously, we may better understand the points on the evolutionary pathway of NC cysts where ALB works and whether the impact of ALB on that evolution varies by patient and cyst characteristics. The data for these analyses comes from a trial that has been deemed one of only two high quality trials on the impact of ALB for treatment of NC.23
The prolonged imaging data in this analysis depicted a more complete NC cyst trajectory. Empirically, we observed that by 24 mo, nearly all cysts were resolved, regardless of the treatment (95.6% in the ALB arm and 90.7% in the placebo arm), although ALB seems to resolve the cysts faster. This is consistent with our previous findings in which we found that more active cysts had resolved in the ALB group compared with placebo at 1, 6 and 12 mo follow-up, but the difference between the two groups decreased over time.6 With the addition of the 24-mo scans, we see that that difference continues to decrease over time as the cysts in the placebo group resolve naturally.
Using MSMs, we were able to provide a better depiction of the life course of NC cysts. For example, the treatment significantly accelerated the evolution from the active phase to the degenerative phase and the evolution from the degenerative phase to disappearance. While it is well known that ALB kills viable cysts, our finding suggests that ALB also may have effects on degenerative cysts and that warrants further research. With regards to the impact of ALB on calcification, we found that ALB hastened the move of active cysts to the degenerative stage and from there the majority of cysts transited into disappearance, not calcification. Therefore, we infer that ALB does not lead to calcification. This finding is generally consistent with the existing literature, where one previous study found an increase in cyst calcification over follow-up in patients receiving ALB treatment, but the statistical significance of that difference was not reported,7 and two other studies found no association between cyst calcification and ALB treatment.24,25
Although NC cyst calcifications may remain for years, there is evidence that they occasionally resolve,26 and that is consistent with our observation. ALB, however, did not have a significant impact on the evolution of calcified cysts. We found that ALB was more effective among men than women. Such results could be related to previous findings that women are more likely to initiate an immune response to NC cysts without treatment, as evidenced by a higher probability of presenting with edema around the NC cysts.27,28 We also found differential effects by patient age, which is consistent with previous studies of solitary cysts that found that ALB may accelerate NC cyst degeneration in children and young adults.8,14 In our study, however, the association with age appears to be complicated. We found that ALB treatment worked well for younger patients in facilitating the active to degenerative evolution but not afterward; while among older patients the drug tended to speed up the later phase evolutions.
The impact of ALB was significantly stronger for cysts in parenchymal regions of the brain on evolutions from the active to the degenerative phase. This finding supports the results from the patient-level analysis.6 However, with MSM, we found that for cysts in extraparenchymal regions, ALB had significant effects on evolutions from the degenerative phase to cyst disappearance. The number of NC cysts and, at borderline significance, the presence of calcified cysts at baseline also had an impact on the effect of ALB on cyst trajectory, findings that have not been previously reported and that warrant further exploration. Because the evolution of NC cysts is closely tied to the host’s ability to recognize the parasite, the absence of calcifications at baseline may suggest a strong host immune response.
Limitations
This study had several limitations. We only included cysts that were located in unique identifiable brain locations so that we could easily follow each cyst. If the evolutionary trajectory of NC cysts is influenced by having other NC cysts in close proximity, we may be less able to detect this in our sample of cysts. Thus, our findings may not apply to cysts that share a location with other NC cysts, which may resolve at a different rate. It could be that having other NC cysts located nearby prolongs the time it takes for those cysts to resolve, perhaps because having other cysts that are able to hide from the host’s immune system in proximity makes it less likely that the immune system will identify any of the cysts in the locale. Future studies should include all NC cysts and look at whether the cysts evolve differently depending on cyst burden within specific brain regions rather than in the brain overall. To include cysts that share a location, we would need the radiologist to reread the images and collect data on the trajectory of each cyst in the shared location. Fourteen cysts were excluded during serialization due to ‘reverse evolutions’. This reverse evolving could also be an error in reading the scan, which can be challenging due to the variations in edema around the cysts over time. Future studies should have the brain images read at the cyst level to make it possible to follow each cyst over time.
Our dichotomization of cyst location into parenchymal vs extraparenchymal may have led to some heterogeneity within the category. For example, cysts located over the convexity of the hemispheres were classified as extraparenchymal based on anatomy, but may respond to ALB more like parenchymal cysts.9 In our data, cysts over the convexity were classified as being in cisternal locations and could not be separated out from other cisternal cysts for analysis.
It is important to note that our scan data included both CT and MRI scans. The original RCT study was conducted in Ecuador, where the availability of MRI and CT scans varied by hospital. CT scans are more sensitive for detecting calcifications while MRIs are better for identifying cysts in other phases.29 Misclassification of calcification-related evolutions may be more likely in MRI while misclassification of cysts in other phases may be more likely in CT scans. Of the 35 calcification to disappearance transitions, 29 were documented on CT scans and 6 on MRIs. Of the 110 calcification-related transitions, 77% were detected by CT scans (Table 2). However, any measurement error is unlikely to be related to treatment group due to randomization and thus this misclassification should bias the results towards the null.
We were likely underpowered for a few analyses, as indicated by very wide CIs for some estimates, especially in the stratified models, which had smaller sample sizes. Thus, we may not have been able to detect weaker associations between ALB and specific cyst evolutions. Also, we had a fair amount of missing data, i.e. loss to follow-up. In total, 48 patients were lost to follow-up from the study in the late months, leading to a high degree of uncertainty for the long-term results from MSM modelling as well as the cross-sectional summary. However, despite the low statistical power, we did find a number of significant associations. Accurate estimation requires appropriate handling of loss to follow-up with MSM, which is challenging since it involves advanced statistical modelling and numerical algorithms such as expectation and maximization. Multiple imputations and inverse probability weighting usually do not work well when the missing data is not random, as it likely is in our data.30 Therefore, methods to handle missing data with MSM are needed.
Conclusions
Few studies have assessed changes to individual NC cysts over time and none have included cysts from patients with multiple cysts. Using the MSM model, we were able to identify where in the trajectory ALB has the greatest impact and which patient and cyst characteristics modify that impact.
Authors’ contributions
WAH, AC and EAK conducted the original clinical trial. AJ read the 24-mo CT and MRI scans. HZ determined the analytic plan and MR conducted the data formatting and statistical analyses. MAM, EAK and HZ wrote the manuscript and all coauthors reviewed drafts and approved the final manuscript.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (R01-NS39403 to WAH) and Ruth L. Kirschstein National Research Service Award (5F31NS051946 to EAK). Glaxo Wellcome/SmithKline Beecham and Acromax supplied active drugs and placebos. The analyses presented here were supported by the City University of New York (PSC-CUNY ENHC 47 to HZ and EAK) and a grant from the University of Cuenca, Ecuador, to AC.
Competing interests
None declared.
Ethical approval
The Institutional Review Board (IRB) of Columbia University, the Office for Human Research Protection of the National Institutes of Health and the ethics committees at the recruiting hospitals in Ecuador approved the original RCT. The IRB of the City University of New York deemed these analyses as IRB exempt because the data provided to the researchers were deidentified.
References
- 1. Gripper LB, Welburn SC. The causal relationship between neurocysticercosis infection and the development of epilepsy - a systematic review. Infect Dis Poverty. 2017;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Taeniasis/cysticercosis: key facts. Available from: https://www.who.int/en/news-room/fact-sheets/detail/taeniasis-cysticercosis (accessed 30 May 2019).
- 3. Carpio A, Placencia M, Santillan F, Escobar A. A proposal for classification of neurocysticercosis. Can J Neurol Sci. 1994;21:43–7. [DOI] [PubMed] [Google Scholar]
- 4. Escobar A. The pathology of neurocysticercosis In: Palacios E, Rodriquez-Carbajal J, Taveras JM, editors. Cysticercosis of the Central Nervous System. Springfield, IL: Thomas, 1983; p. 27–54. [Google Scholar]
- 5. Carpio A. Neurocysticercosis: an update. Lancet Infect Dis. 2002;2:751–62. [DOI] [PubMed] [Google Scholar]
- 6. Carpio A, Kelvin EA, Bagiella E et al. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2008;79:1050–5. [DOI] [PubMed] [Google Scholar]
- 7. Garcia HH, Pretell EJ, Gilman RH et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350:249–58. [DOI] [PubMed] [Google Scholar]
- 8. Sharma SR, Agarwal A, Kar AM et al. Evaluation of role of steroid alone and with albendazole in patients with epilepsy with single-small enhancing computerized tomography lesions. Ann Indian Acad Neurol. 2007;10:39–43. [Google Scholar]
- 9. Carpio A, Fleury A, Romo ML, Abraham R. Neurocysticercosis: the good, the bad, and the missing. Expert Rev Neurothera. 2018;18:289–301. [DOI] [PubMed] [Google Scholar]
- 10. Singh G, Rajshekhar V, Murthy J et al. A diagnostic and therapeutic scheme for a solitary cysticercus granuloma. Neurology. 2010;75:2236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abba K, Ramaratnam S, Ranganathan LN. Anthelmintics for people with neurocysticercosis (review). Cochrane Database Syst Rev. 2010;3:CD000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baird RA, Wiebe S, Zunt JR, et al. Evidence-based guideline: treatment of parenchymal neurocysticercosis: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:1424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osorio R, Carrillo-Mezo R, Romo ML et al. Factors associated with cysticidal treatment response in extraparenchymal neurocysticercosis. J Clin Pharmacol. 2019;59:548–56. [DOI] [PubMed] [Google Scholar]
- 14. Baranwal AK, Singhi PD, Khandelwal N, Singhi SC. Morphometry of single small enhancing computed tomographic lesions: outcome and effect of albendazole therapy. J Trop Pediatr. 2002;48:219–24. [DOI] [PubMed] [Google Scholar]
- 15. Souza A, Nalini A, Kovoor JME et al. Natural history of solitary cerebral cysticercosis on serial magnetic resonance imaging and the effect of albendazole therapy on its evolution. J Neurol Sci. 2010;288:135–41. [DOI] [PubMed] [Google Scholar]
- 16. Goel D, Mittal M, Bansal KK, Singhal A. Natural history of solitary cerebral cysticercosis cases after albendazole therapy: a longitudinal follow-up study from India. Acta Neurol Scand. 2010;121:204–8. [DOI] [PubMed] [Google Scholar]
- 17. Carpio A, Escobar A, Hauser WA. Cysticercosis and epilepsy: a critical review. Epilepsia 1998;39:1025–40. [DOI] [PubMed] [Google Scholar]
- 18. Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods in Med Res. 2002;11:91–115. [DOI] [PubMed] [Google Scholar]
- 19. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430. [DOI] [PubMed] [Google Scholar]
- 20. Efron B. Bootstrap methods: another look at jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 21. Jackson C. The msm package for R. J Stat Softw. 2011;38:1–28. [Google Scholar]
- 22. Marshall SW. Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. White AC Jr, Coyle CM, Rajshekhar V et al. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Disease Society of America (IDSA) and American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2018;66:1159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gulati S, Jain P, Sachan D et al. Seizure and radiological outcomes in children with solitary cysticercosis granulomas with and without albendazole therapy: a retrospective case record analysis. Epilepsy Res. 2014;108:1212–20. [DOI] [PubMed] [Google Scholar]
- 25. Otte W, Singla M, Sander J, Singh G. Drug therapy for solitary cysticercosis granuloma: a systematic review and meta-analysis. Neurology. 2013;80:152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meneses Quiroz LJP, Gonzales I, Pretell EJ et al. Occasional resolution of multiple parenchymal brain calcifications in patients with neurocysticercosis. Neurol Clin Pract. 2015;5:531–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fleury A, Dessein A, Preux PM et al. Symptomatic human neurocysticercosis–age, sex and exposure factors relating with disease heterogeneity. J Neurol. 2004;251:830–7. [DOI] [PubMed] [Google Scholar]
- 28. Kelvin EA, Carpio A, Bagiella E et al. The association of host age and gender with inflammation around neurocysticercosis cysts. Ann Trop Med Parasitol. 2009;103:487–99. [DOI] [PubMed] [Google Scholar]
- 29. Lerner A, Shiroishi MS, Zee CS et al. Imaging of neurocysticercosis. Neuroimaging Clin N Am. 2012;22:659–76. [DOI] [PubMed] [Google Scholar]
- 30. Hout A. Multistate Survival Models for Interval-Censored Data. New York, NY: Chapman and Hall; 2016. [Google Scholar]


