Abstract
STUDY QUESTION
Did weight reduction in obese women scheduled for IVF increase cumulative live birth rate (CLBR) after 2 years?
SUMMARY ANSWER
Weight loss prior to IVF did not increase CLBR.
WHAT IS KNOWN ALREADY
Few studies have investigated the effect of weight reduction in obese infertile women scheduled for IVF. In a recent randomized controlled trial (RCT), including one IVF cycle, we found no increase in live birth rate after weight reduction. Weight regain after obesity reduction treatment often occurs, and children born to obese women have a higher risk of childhood obesity.
STUDY DESIGN, SIZE, DURATION
A 2-year follow-up of a multicenter, RCT running between 2012 and 2018 was performed. Out of 317 women randomized to weight reduction followed by IVF treatment or IVF treatment-only, 305 remained in the full analysis set. Of these women, 90.5% (276/305) participated in this study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Nine infertility clinics in Sweden, Denmark and Iceland participated in the RCT. Obese women under 38 years of age having a BMI ≥30 and < 35 kg/m2 were randomized to weight reduction and IVF or IVF-only. In all, 160 patients were randomized to a low calorie diet for 12 weeks and 3–5 weeks of weight stabilization, before IVF and 157 patients to IVF-only. Two years after randomization, the patients filled in a questionnaire regarding current weight, live births and ongoing pregnancies.
MAIN RESULTS AND THE ROLE OF CHANCE
42 additional live births were achieved during the follow-up in the weight reduction and IVF group, and 40 additional live births in the IVF-only group, giving a CLBR, the main outcome of this study, of 57.2% (87/152) and 53.6% (82/153), respectively (P = 0.56; odds ratio (OR) 1.16, 95% CI: 0.74–1.52). Most of the women in the weight reduction and IVF group had regained their pre-study weight after 2 years. The mean weight gain over the 2 years was 8.6 kg, while women in the IVF-only group had a mean weight loss of 1.2 kg. At the 2-year follow-up, the weight standard deviation scores of the children born in the original RCT (index cycle) were 0.218 (1.329) (mean, SD) in the weight reduction and IVF group and − 0.055 (1.271) (mean, SD) in the IVF-only group (P = 0.25; mean difference between groups, 0.327; 95% CI: −0.272 to 0.932).
LIMITATIONS, REASON FOR CAUTION
All data presented in this follow-up study were self-reported by the participants, which could affect the results. A further limitation is in power for the main outcome. The study is a secondary analysis of a large RCT, where the original power calculation was based on live-birth rate after one cycle and not on CLBR.
WIDER IMPLICATIONS OF THE FINDINGS
The follow-up indicates that for women with a BMI ≥30 and < 35 kg/m2 and scheduled for IVF, the weight reduction did not increase their chance of a live birth either in the index cycle or after 2 years. It also shows that even in this highly motivated group, a regain of pre-study weight occurred.
STUDY FUNDING/COMPETING INTEREST(S)
The 2-year follow-up was financed by grants from the Swedish state under the agreement between the Swedish Government and the county councils, the ALF-agreement (ALFGBG-70940 and ALFGBG-77690), Merck AB, Solna, Sweden (an affiliate of Merck KGaA, Darmstadt, Germany), Hjalmar Svensson Foundation. Ms Kluge has nothing to disclose. Dr Bergh has been reimbursed for lectures and other informational activities (Ferring, MSD, Merck, Gedeon Richter). Dr Einarsson has been reimbursed for lectures for Merck and Ferring. Dr Thurin-Kjellberg reports grants from Merck, and reimbursement for lectures from Merck outside the submitted work. Dr Pinborg has been reimbursed for lectures and other informational activities (Ferring, MSD, Merck, Gedeon Richter). Dr Englund has nothing to disclose.
TRIAL REGISTRATION NUMBER
ClinicalTrials.gov number, NCT01566929.
Keywords: IVF, obesity, weight loss, infertility, follow-up, cumulative live birth rate
WHAT DOES THIS MEAN FOR PATIENTS?
This study looks at whether losing weight before In Vitro Fertilization (IVF) makes it more likely that very overweight (obese) women will have a child within two years. Many earlier studies have shown that obese women have a reduced chance of having a child after IVF.
In our previous trial, infertile obese (women with a BMI—a measure of appropriate weight for their height of 30 or over and under 35 kg/m2) women were divided into two groups: the first to a weight reduction program before starting IVF treatment and the second group started IVF treatment immediately. Even though the women in the weight reduction group lost more than nine kilograms, they did not have more children compared with the women who did not lose weight. In this study, we looked at these women 2 years after the first trial.
The women were given a questionnaire and almost all of the patients from the main study answered it. We found that over half of the women in the weight reduction group had a child (57.2%), and the result was about the same for the other group (53.6%). At this point, the two groups had a similar BMI.
This research shows that obese women who lose weight before IVF are not more likely to have a child in the following 2 years compared with women who do not lose weight before IVF, and that most of the women who had lost weight had put it back on.
Introduction
Obesity has been shown to have a compromising effect on both pregnancy and live birth rate for women undergoing ART, such as IVF (Maheshwari et al., 2007; Luke et al., 2011; Bellver et al., 2013; Petersen et al., 2013; Provost et al., 2016). When compared with women with a normal BMI, obese women have an increased miscarriage rate and they require higher doses of gonadotrophins, illustrating an impaired response to ovarian stimulation (Fedorcsak et al., 2004; Metwally et al., 2008). A weight loss of 5–10% in obese women has been demonstrated to be effective in normalizing menstruation, ovulation and spontaneous pregnancy rates (Norman et al., 2004).
Earlier studies have shown that children born to obese women have a higher risk for childhood obesity (Olson et al., 2010; Ruager-Martin et al., 2010; Woo Baidal et al., 2016), and obese children have a higher risk of adult adiposity, adult morbidity and premature mortality (Reilly et al., 2011).
Until recently, only few studies (Moran et al., 2011; Sim et al., 2014; Becker et al., 2015) have investigated the effect of weight reduction in obese infertile women preceding infertility treatments. However, these trials were not powered for pregnancy or live birth. A large Dutch randomized controlled trial (RCT) found that life style interventions, preceding fertility treatment, had no effect on cumulative live birth rate (CLBR) after 24 months in infertile obese women, when including ongoing pregnancies that ended after the follow-up period. The mean weight loss in that study was modest, at 4.4 kg in the intervention group and 1.1 kg in the control group, a difference between the groups of 3.3 kg (Mutsaerts et al., 2016). In our RCT where 317 obese women (BMI ≥30 and <35 kg/m2) were randomized to weight reduction with a low calorie diet (880 kcal/day) for 12 weeks before IVF or to IVF-only, we found no significant difference in live birth rate between groups (Einarsson et al., 2017) although the mean weight change between the groups was substantially higher at 9.44 kg.
Weight regain after obesity reduction treatment is common and well known. It is usually a challenge for the patient to maintain the new lower weight (Franz et al., 2007; Kraschnewski et al., 2010; Johansson et al., 2014), even though some studies show that weight maintenance is possible (Vogels et al., 2007; Montesi et al., 2016). Little is known about weight maintenance after weight reduction using a low calorie diet specifically preceding an IVF treatment.
The aim of this follow-up study was to evaluate if weight reduction in obese women scheduled for IVF increased the CLBR rates assessed as having at least one live birth during a period of 2 years after randomization and if the achieved weight reduction remained.
We also wanted to evaluate if a large weight loss in the women would affect the weight development of the children born in the index cycle, i.e. the cycle included in the RCT.
Materials and Methods
The present study was a 2-year follow-up of a prospective, multicentre, RCT, performed between 2010 and 2016 in the Nordic countries (Einarsson et al., 2017). A total of 317 obese infertile women under 38 years of age were randomized to two groups: weight reduction and IVF or IVF-only. The weight reduction and IVF group started with 12 weeks of low calorie diet (880 kcal/day) and thereafter 2–5 weeks of re-introduction to solid foods before IVF. The IVF-only group started the IVF as soon as possible after randomization and was not given any dietary counselling. In the full analysis set (FAS) population (n = 305), there was no significant difference regarding live birth between the two groups: 29.6% (45/152) in the weight reduction and IVF group and 27.5% (42/153) in the IVF-only group, but the women in the intervention group had significantly more spontaneously conceived pregnancies.
Knowing the risk of weight regain, the women in the weight reduction and IVF group were offered complementary dietary counselling by the dietician for 1 year from randomization, to help them to maintain their achieved lower weight.
In the follow-up study, a questionnaire was sent, 2 years after randomization, to all patients in the FAS population. The follow-up was performed from 2012 to 2018. The questionnaire covered the current weight of the woman at 2 years after randomization and if she had any pregnancies or live births after the index cycle in the randomized trial. The questionnaire also covered general health, last measured weight and height of the children born in the RCT and data of the children born after the RCT (birth date, mode of conception). The follow-up questionnaire did not contain any questions concerning current or past diet.
The primary outcome was CLBR in the FAS population, defined as at least one child born alive, after infertility treatment or a spontaneous pregnancy, during the 2-year follow-up from randomization, and to assess if the weight reduction that was achieved remained at the 2-year follow-up. The time of 2 years made it possible to include frozen embryo transfers (FETs) from the index cycle and additional IVF or other fertility treatments performed during the follow-up period. Furthermore, the risk of patients not answering a questionnaire was anticipated to be low after a period of 2 years.
Secondary outcomes were ongoing pregnancies and a total number of fertility treatments. Data concerning the number of treatments were retrieved from patient records. Dietary-related measurements included weight change between last weight measured in the RCT and weight reported at the 2-year follow-up. A further secondary outcome was follow-up of the children born in the index cycle concerning general health, and weight and height measured at last visit at the Child Health Care Centre.
Statistics
The main analysis in the present study was performed on the FAS population. Twenty-nine women in the FAS population did not participate in the follow-up. Of these, six women in the weight reduction and IVF group and three women in the IVF-only group had achieved a live birth in the index cycle. Except for these births, we assumed that the women who did not participate in the follow-up had not succeeded in having a live birth.
The weights of the women with ongoing pregnancies at the time of the follow-up were excluded from the weight calculations. One woman (in the IVF-only group) answered the questionnaire during the postpartum period, while all other women had passed this period at time of follow-up. There are missing data concerning any additional IVF/FET performed at one Danish clinic, and we have assumed that these patients did not undergo any further treatments. There are also missing data concerning follow-up weight for children born in the index cycle and missing data concerning weight of some women at the follow-up. These patients were not included in the weight calculations. No imputations were performed.
Descriptive statistics are given by mean, SD, median, maximum and minimum for continuous variables and number and percentage for categorical variables. For comparison between the two randomized groups, Fisher’s exact test was used (lowest 1-sided P-value multiplied by 2) for all dichotomous variables, the Mantel-Haenszel Chi Square test was used for ordered categorical variables, Chi Square test was used for non-ordered categorical variables and the Mann-Whitney U-test was used for continuous variables. The CI for dichotomous variables was the unconditional exact confidence limits. If no exact limits could be computed, the asymptotic Wald confidence limits with continuity correction was calculated instead. The CI for the mean difference between groups was based on Fisher's non-parametric permutation test. Weight standard deviation scores (SDS) were calculated according to Marsal et al. (1996).
A P-value less than 0.05 or a 95% CI not including 1.0 was considered significant.
According to a post hoc power calculation, it was possible to detect a difference in cumulative live birth of 15% between groups, when 152 and 153 women were included in the two groups (α = 0.05, β = 0.20). Data were analyzed using SPSS version 22.0 (IBM Corp, Armonk, NY, USA).
Ethical approval
Research ethics committees in Sweden, Denmark and Iceland approved the trial. All participants provided written informed consent for the 2-year follow-up.
Results
From the FAS population, 90.5% (276/305) of the patients participated in the follow-up: 137/152 in the weight reduction and IVF group and 139/153 in the IVF-only group. The patient’s characteristics were comparable in the two groups except for the termination of pregnancy, which was significantly higher in the IVF-only group (Table I). Forty-two additional live births were achieved during the follow-up in the weight reduction and IVF group and 40 additional live births in the IVF-only group, giving a CLBR of 57.2% (87/152) and 53.6% (82/153), respectively (P = 0.56; odds ratio (OR) 1.16, 95% CI: 0.74–1.52).
Table I.
Characteristics of the patients in the 2-year follow-up.
| Variable | Weight reduction intervention and IVF (n = 137) | IVF only (n = 139) | p-value |
|---|---|---|---|
| Age of the woman at randomization (years) | 31.6 (4.3) 32.0 (22.3; 38.0) | 31.6 (4.2) 31.8 (22.7; 38.0) | 0.97 |
| Weight at randomization (kg) | 92.8 (8.1) 92.1 (74.3; 111) | 91.6 (8.0) 91.6 (73.1; 114.7) | 0.35 |
| BMI (kg/m2) at randomization | 33.1 (1.4) 33.4 (29.9; 35.1) | 33.0 (1.4) 33.3 (30; 35.1) | 0.95 |
| Duration of infertility (months) | 38.2 (24.5) 30.0 (6.0; 168.0) | 38.9 (21.7) 36.0 (1.0; 180.0) | 0.22 |
| Cause for infertility | |||
| Tubal factor | 12 (8.8) | 11 (7.9) | |
| Endometriosis | 6 (4.4) | 2 (1.4) | |
| Hormonal factor | 2 (1.5) | 9 (6.5) | |
| Polycystic ovary syndrome | 33 (24.1) | 28 (20.1) | |
| Male factor | 43 (31.4) | 41 (29.5) | |
| Unexplained infertility | 39 (28.5) | 44 (31.7) | |
| Other | 2 (1.5) | 4 (2.9) | 0.24 |
| Smoking | 16 (11.7) | 12 (8.6) | 0.52 |
| Ethnicity | |||
| Caucasian | 127 (92.7) | 128 (92.1) | |
| African | 1 (0.7) | 2 (1.4) | |
| Asian | 2 (1.5) | 2 (1.4) | |
| Other | 7 (5.1) | 7 (5.0) | 0.96 |
| History of previous pregnancies | |||
| Live birth | 9 (6.6) | 10 (7.2) | 1.00 |
| Miscarriage | 7 (5.1) | 7 (5.0) | 1.00 |
| Termination of pregnancy | 11 (8.0) | 28 (20.1) | 0.006 |
| Ectopic pregnancy | 0 (0.0) | 3 (2.2) | 0.25 |
| History of previous treatment with IVF | |||
| 0 | 113 (82.5) | 117 (84.2) | |
| 1 | 13 (9.5) | 14 (10.1) | |
| 2 | 11 (8.0) | 8 (5.8) | 0.56 |
For categorical variables, n (%) is presented. For continuous variables, Mean (SD)/Median (Min; Max) is presented. For comparison between groups, Fisher’s Exact test (lowest 1-sided p-value multiplied by 2) was used for dichotomous variables, the Mantel-Haenszel Chi Square test was used for ordered categorical variables, Chi Square test was used for non-ordered categorical variables and the Mann-Whitney U-test was used for continuous variables.
In addition, one women in the intervention group had a second child, and a further 19 ongoing pregnancies were reported in the weight reduction and IVF group and 16 in the IVF-only group, including both first pregnancies and pregnancies after a previous live birth (Table II). In total, in the weight reduction and IVF group and the IVF-only group, 63.8% (97/152) and 58.2% (89/153), respectively, achieved a live birth or first ongoing pregnancy (P = 0.34; OR 1.28, 95% CI: 0.8–2.01). The pregnancies achieved after the index cycle were the result of either new fresh IVF treatments, FET, spontaneous conceptions, egg donation or low-dose gonadotrophin treatment (Table II).
Table II.
Outcome of the FAS population at the 2-year follow-up.
| Weight reduction and IVF group | IVF only group | p-value odds ratio (95%CI) | |
|---|---|---|---|
| Cumulative live birth rate * | 87/152 (57.2) | 82/153 (53.6) | 0.56 1.16 (0.74 to 1.52) |
| No. of participants in the follow-up | 137 | 139 | |
| No. of additional live births after index cycle | 42 | 40 | |
| Additional live birth rate | 42/137 (30.7) | 40/139 (28.8) | |
| Way of conception | |||
| IVF/ICSI | 20/42 (47.6) | 20/40 (50) | |
| FET | 11/42 (26.2) | 9/40 (22.5) | |
| Spontaneous pregnancies | 9/42 (21.4) | 9/40 (22.5) | |
| Oocyte donation/ | 1/42 (2.4) | 0 (0.0) | |
| Gonadotrophin stimulation | 1/42 (2.4) | 0 (0.0) | |
| Unknown | 0 (0.0) | 2/40 (5.0) | |
| Ongoing pregnancies | 19 | 16 | |
| ‐ Expecting first child in the study | 10/137 (7.3) | 7/139 (5.0) | |
| ‐ Expecting second child in the study | 9/137 (6.6) | 9/139 (6.5) | |
| Way of conception | |||
| IVF/ICSI | 6/19 (31.6) | 4/16 (25.0) | |
| FET | 4/19 (21.1) | 1/16 (6.3) | |
| Spontaneous pregnancy | 9/19 (47.4) | 9/16 (56.3) | |
| Oocyte donation/ | 0 (0.0) | 1/16 (6.3) | |
| Unknown | 0 (0.0) | 1/16 (6.3) | |
| Cumulative live birth rate/ongoing pregnancy rate | 97/152 (63.8) | 89/153 (58.2) | 0.34 1.28 (0.8 to 2.01) |
| Total no. of started IVF/ICSI cycles ** | 214 | 244 | |
| Total no. of FET ** | 70 | 74 |
FAS: full analysis set, FET: frozen embryo transfer.
For categorical variables, n (%) is presented.
For comparison between groups, Fisher’s Exact test (lowest 1-sided p-value multiplied by 2) was used for dichotomous variables.
*Cumulative live birth defined as at least one child born alive. Calculated on all FAS patients 152/153.
**Missing data from one Danish clinic concerning any follow-up treatments performed by the nine patients in the weight reduction and IVF group and the seven patients from the IVF-only group.
In total, 214 IVF/ICSI fresh treatments were performed in the weight reduction and IVF group and 244 in the IVF-only group, and 70 and 74 FET cycles, respectively.
The majority of women in the weight reduction and IVF group had regained their pre-study weight after 2 years with a mean weight gain of 8.6 kg, while women in the IVF-only group had a mean weight loss of 1.2 kg, P < 0.0001 (Fig. 1; Table III). The opportunity for complementary dietary counselling by a dietician in the year after randomization was utilized by 48% of the patients (21%; 4–7 visits, 27%; 1–3 visits, 52%; no visits). In the weight reduction and IVF group, 23.3% (27/116) had a BMI <30 kg/m2 at the 2-year follow-up, compared with 10.9% (13/119) in the IVF-only group (P = 0.019). However, the mean BMI did not differ between the groups at the 2-year follow-up (Table III).
Figure 1.
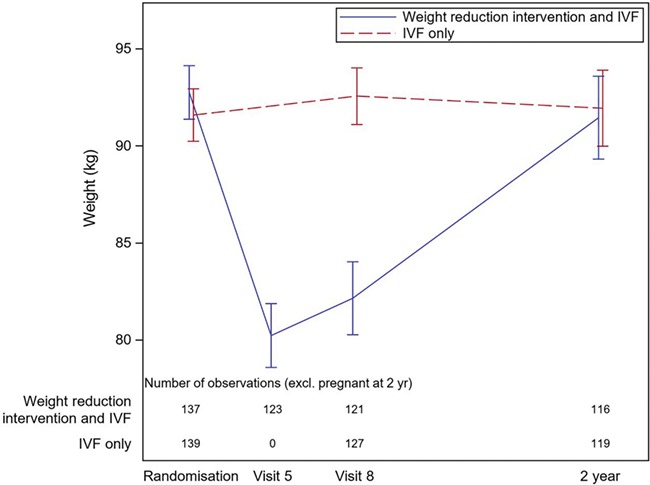
Weight of women from randomization until 2-year follow-up. Visit 5 = week 15 of the diet. Visit 8 = oocyte retrieval. Missing data visit 5, on 14 patients in the weight reduction and IVF group. Missing data visit 8, on 16 patients in the weight reduction and IVF group and 12 patients in the IVF only group. Excluding ongoing pregnant women at the 2-year follow-up. Missing data, at the 2-year follow-up on two patients from the weight reduction and IVF group and four patients from the IVF-only group.
Table III.
Weight changes at 2-year follow-up, excluding ongoing pregnant women at the time for the follow up.
| Weight reduction and IVF group n = 116 | IVF only group n = 119 | p-value | |
|---|---|---|---|
| Weight change from last assessment in the RCT until 2 year follow up * | 8.57 (9.55) 8.40 (−33.50; 30.60) | −1.18 (7.05) -0.60 (−29.10; 16.60) | <0.0001 |
| BMI (kg/m2) at 2 year follow up * | 32.5 (3.5) 33.2 (22.7; 39.1) | 33.1 (3.0) 33.3 (23.7; 42.2) | 0.44 |
| ‐ No. of women with BMI <30 at the 2 year follow up | 27 (23.1) | 13 (10.9) | 0.019 |
| ‐ No. of women with BMI ≥30—<34.9 at the 2 year follow up | 52 (44.4) | 70 (58.8) | 0.044 |
| ‐ No. of women with BMI ≥35 at the 2 year follow up | 37 (32.5) | 36 (30.3) | 0.895 |
For categorical variables, n (%) is presented. For continuous variables, Mean (SD)/Median (Min; Max) is presented. For comparison between groups, the Fisher’s Exact test (lowest 1-sided p-value multiplied by 2) was used for dichotomous variables and the Mann-Whitney U-test was used for continuous variables.
*Missing weight data of two patients in the weight reduction and IVF group and four patients in the IVF-only group.
At the 2-year follow-up, the weight SDS for the children born in the index cycle was 0.218 (1.329) (mean, SD) in the weight reduction and IVF group and −0.055 (1.271) (mean, SD) in the IVF-only group (P = 0.25; mean difference between groups, 0.327; 95% CI −0.272 to 0.932) (Fig. 2; Table IV). The children born in the index cycle were generally healthy at the 2-year follow-up. Adverse medical conditions were reported for four children in the weight reduction and IVF group, while in the IVF-only group, two children were affected.
Figure 2.
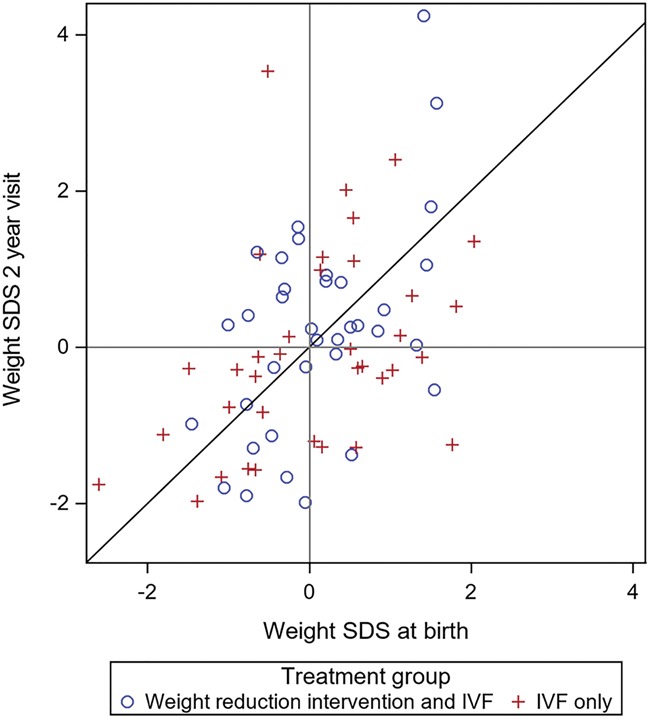
Weight SDS of children born in the index cycle. SDS: standard deviation scores. Each child is represented by one dot.
Table IV.
Follow-up of children born in the index cycle (FAS live births excluding one set of twins).
| Variable | Weight reduction and IVF group n = 45 | IVF only group n = 41 | p-value | Mean difference between groups (95% CI) |
|---|---|---|---|---|
| Female | 25 (55.6) | 22 (53.7) | 1.00 | |
| Birth weight (g) | 3486 (523) 3560 (1820; 4384) | 3584 (509) 3638 (2140; 4820) | 0.46 | |
| Weight standard deviation score at birth | −0.021 (0.868) -0.050 (−1.615; 1.571) | 0.073 (1.073) 0.061 (−2.605; 2.282) | 0.61 | 0.104 (−0.332; 0.526) |
| Weight standard deviation score 2 year visit* | 0.218 (1.329) 0.245 (−1.986; 4.241) | −0.055 (1.271) -0.258 (−1.973; 3.530) | 0.25 | 0.327 (−0.272; 0.932) |
For categorical variables, n (%) is presented. For continuous variables, Mean (SD)/Median (Min; Max)/n = is presented. For comparison between groups, Fisher’s Exact test (lowest 1-sided p-value multiplied by 2) was used for dichotomous variables and the Mann-Whitney U-test was used for continuous variables. The CI for dichotomous variables is the unconditional exact confidence limits. If no exact limits can be computed, the asymptotic Wald confidence limits with continuity correction are calculated instead. The CI for the mean difference between groups is based on Fishers non-parametric permutation test.
*Six women in the weight reduction and IVF group and three in the IVF-only group, who had a live birth in the index cycle, did not participate in the follow-up. Missing weight data at the 2-year follow-up: three in the weight reduction and IVF group and two in the IVF-only group.
Discussion
In this 2-year follow-up of a RCT, no significant difference in CLBR was observed between the weight reduction and IVF group compared with the IVF-only group. It also showed that most of the women in the weight reduction and IVF group had regained their pre-study weight. We found no difference in weight SDS development between children born in the RCT despite a considerable difference in maternal weight between groups.
Our results are in line with the results of Mutsaerts et al., (2016), where no difference in live birth rate was shown after a lifestyle intervention preceding infertility treatment, when including ongoing pregnancies that ended after the follow-up period. Mutsaerts discussed that a larger weight loss might have led to a higher live birth rate, and in our study, the difference in weight loss was almost three times as large, at 9.44 kg compared with 3.3 kg in the Dutch study. Despite this difference in weight loss, the CLBR in the present study did not differ significantly between groups, although the 95% CI for OR of CLBR indicated that clinical valuable differences between groups may exist.
Several studies have shown that it is common to regain lost weight after a low calorie diet (Franz et al., 2007; Kraschnewski et al., 2010; Johansson et al., 2014). This was confirmed in our 2-year follow-up, where most women in the weight reduction and IVF group had regained the weight lost during the intervention. Our hypothesis before the study was that the women in this highly motivated group of patients, performing IVF-treatments, might be able to keep the lower post-study weight in order to improve outcome.
However, significantly more women in the weight reduction and IVF group had a BMI < 30 kg/m2 at the 2-year follow-up: 23.3% (27/116) compared with 10.9% (13/119) the IVF-only group (P = 0.019).
It is well known that children born to obese women have a higher risk of childhood obesity (Olson et al., 2010; Woo Baidal et al., 2016) and that obese children have a higher risk of adult adiposity, adult morbidity and premature mortality (Reilly et al., 2011). At the present 2-year follow-up, there was no significant difference between the groups in weight SDS of the children born in the index cycle, despite a considerable difference in maternal weight (Fig. 2; Table IV).
The strength of this follow-up study is the high participation rate of above 90%. Another strength is that the study is a follow-up of a RCT in a field that is quite difficult to explore.
A limitation is that data are self-reported by questionnaires, yet a recently published study showed that bias in self-reported weight is negligible (Seijo et al., 2018). It is a challenge to assess weight in this group of women. They might recently have given birth or are still pregnant at the time of assessment. We chose to exclude women with ongoing pregnancies when presenting data on the weights at the 2-year follow-up. No weight assessment was made during the follow-up time, and therefore, we have no data concerning when the women regained the lost weight, which is a limitation. Although 90.5% of the women in the RCT participated in the follow-up, it is obviously a limitation that not all women participated.
There is also some limitation in power to detect clinical differences in CLBR. One could argue that even a smaller difference in CLBR would be valuable for the patient. However, we believe that a rather large difference in CLBR is required to motivate young women to participate in a rather demanding trial, which this 2-year follow-up is based on. This statement is well supported by the randomization process in the main RCT, indicating a high decline rate. Thus, we consider the power in the present study as reasonable.
In conclusion, no significant difference in CLBR between the groups at the 2-year follow-up was observed. Most patients in the weight reduction and IVF group had regained the weight they lost during the weight reduction. There was no difference at the follow-up between the groups in weight SDS for the children born in the index cycle. The follow-up indicated that for women scheduled for IVF showing World Health Organization class I obesity (BMI ≥30 and < 35 kg/m2), the weight reduction did not increase their chance of a live birth either in the index cycle or after 2 years. It also shows that even in this highly motivated group, a regain of pre-study weight occurred.
Acknowledgements
We thank statisticians Mattias Molin and Henrik Albrektsson for valuable statistical support and Niklas Svensson, who provided IT-assistance. We also thank all co-workers at the following participating clinics, both IVF and obesity units:
Sweden: Sahlgrenska University Hospital, Gothenburg; Karolinska University Hospital, Stockholm; Skåne University Hospital, Malmö; Örebro University Hospital, Örebro.
Denmark: Rigshospitalet, Copenhagen University Hospital, Copenhagen; Hvidovre Hospital, Copenhagen University Hospital, Copenhagen; Herlev Hospital, Copenhagen University Hospital, Copenhagen; Holbaek Hospital, Copenhagen University Hospital, Copenhagen and.
Department of Nutrition, Exercise and Sports of Copenhagen University, Copenhagen. Iceland: Livio Reykjavik, Reykjavik.
Authors’ roles
LK, ATK, CB and SE designed the study, participated in enrolment of patients, LK, ATK and CB participated in analyzing and interpretation of data, writing of the manuscript and approval of the final version, SE participated in interpretation of data, revising the manuscript and approval of the final version and AP ALME participated in enrolment of patients, revising the manuscript and approval of the final version.
Funding
Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-70 940 and ALFGBG-77690); Merck AB, Solna, Sweden (an affiliate of Merck KGaA, Darmstadt, Germany); Hjalmar Svensson Foundation.
Conflict of interest
Ms Kluge has nothing to disclose. Dr Bergh has been reimbursed for lectures and other informational activities (Ferring, MSD, Merck, Gedeon Richter). Dr Einarsson has been reimbursed for lectures for Merck and Ferring. Dr Thurin-Kjellberg reports grants from Merck, and reimbursement for lectures from Merck outside the submitted work. Dr Pinborg has been reimbursed for lectures and other informational activities (Ferring, MSD, Merck, Gedeon Richter). Dr Englund has nothing to disclose.
References
- Becker GF, Passos EP, Moulin CC. Short-term effects of a hypocaloric diet with low glycemic index and low glycemic load on body adiposity, metabolic variables, ghrelin, leptin, and pregnancy rate in overweight and obese infertile women: a randomized controlled trial. Am J Clin Nutr 2015;102:1365–1372. [DOI] [PubMed] [Google Scholar]
- Bellver J, Pellicer A, Garcia-Velasco JA, Ballesteros A, Remohi J, Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril 2013;100:1050–1058. [DOI] [PubMed] [Google Scholar]
- Einarsson S, Bergh C, Friberg B, Pinborg A, Klajnbard A, Karlström PO, Kluge L, Larsson I, Loft A, Mikkelsen-Englund AL et al. Weight reduction intervention for obese infertile women prior to IVF: a randomized controlled trial. Hum Reprod 2017;32:1621–1630. [DOI] [PubMed] [Google Scholar]
- Fedorcsak P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, Omland AK, Abyholm T, Tanbo T. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod 2004;19:2523–2528. [DOI] [PubMed] [Google Scholar]
- Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, Bowman JD, Pronk NP. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 2007;107:1755–1767. [DOI] [PubMed] [Google Scholar]
- Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very-low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2014;99:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, Sciamanna CN. Long-term weight loss maintenance in the United States. Int J Obes 2010;34:1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod 2011;26:245–252. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology--a systematic review. Hum Reprod Update 2007;13:433–444. [DOI] [PubMed] [Google Scholar]
- Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843–848. [DOI] [PubMed] [Google Scholar]
- Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril 2008;90:714–726. [DOI] [PubMed] [Google Scholar]
- Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle Grave R. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes 2016;9:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran L, Tsagareli V, Norman R, Noakes M. Diet and IVF pilot study: short-term weight loss improves pregnancy rates in overweight/obese women undertaking IVF. Aust N Z J Obstet Gynaecol 2011;51:455–459. [DOI] [PubMed] [Google Scholar]
- Mutsaerts MA, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WK, Perquin DA, Koks CA, van Golde R, Kaaijk EM, Schierbeek JM et al. Randomized trial of a lifestyle program in obese infertile women. N Engl J Med 2016;374:1942–1953. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update 2004;10:267–280. [DOI] [PubMed] [Google Scholar]
- Olson CM, Demment MM, Carling SJ, Strawderman MS. Associations between mothers' and their children's weights at 4 years of age. Child Obes 2010;6:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jorgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril 2013;99:1654–1662. [DOI] [PubMed] [Google Scholar]
- Provost MP, Acharya KS, Acharya CR, Yeh JS, Steward RG, Eaton JL, Goldfarb JM, Muasher SJ. Pregnancy outcomes decline with increasing body mass index: analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008-2010 Society for Assisted Reproductive Technology registry. Fertil Steril 2016;105:663–669. [DOI] [PubMed] [Google Scholar]
- Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes 2011;35:891–898. [DOI] [PubMed] [Google Scholar]
- Ruager-Martin R, Hyde MJ, Modi N. Maternal obesity and infant outcomes. Early Hum Dev 2010;86:715–722. [DOI] [PubMed] [Google Scholar]
- Seijo M, Minckas N, Cormick G, Comande D, Ciapponi A, BelizAn JM. Comparison of self-reported and directly measured weight and height among women of reproductive age: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2018;97:429–439. [DOI] [PubMed] [Google Scholar]
- Sim KA, Dezarnaulds GM, Denyer GS, Skilton MR, Caterson ID. Weight loss improves reproductive outcomes in obese women undergoing fertility treatment: a randomized controlled trial. Clin Obes 2014;4:61–68. [DOI] [PubMed] [Google Scholar]
- Vogels N, Westerterp-Plantenga MS. Successful long-term weight maintenance: a 2-year follow-up. Obesity 2007;15:1258–1266. [DOI] [PubMed] [Google Scholar]
- Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med 2016;50:761–779. [DOI] [PubMed] [Google Scholar]


