Abstract
Background and Aims
The long-term risk of high-grade dysplasia [HGD] and colorectal cancer [CRC] following low-grade dysplasia [LGD] in inflammatory bowel disease [IBD] patients is relatively unknown. We aimed to determine the long-term cumulative incidence of advanced neoplasia [HGD and/or CRC], and to identify risk factors for advanced neoplasia in a nationwide IBD cohort with a history of LGD.
Methods
This is a nationwide cohort study using data from the Dutch National Pathology Registry [PALGA] to identify all IBD patients with LGD between 1991 and 2010 in the Netherlands. Follow-up data were collected until January 2016. We determined the cumulative incidence of advanced neoplasia and identified risk factors via multivariable Cox regression analysis.
Results
We identified 4284 patients with colonic LGD with a median follow-up of 6.4 years after initial LGD diagnosis. The cumulative incidence of subsequent advanced neoplasia was 3.6, 8.5, 14.4 and 21.7%, after 1, 5, 10 and 15 years, respectively. The median time to develop advanced neoplasia after LGD was 3.6 years. Older age [≥ 55 years] at moment of LGD (hazard ratio [HR] 1.73, 95% confidence interval [CI] 1.44–2.06), male sex [HR 1.33, 95% CI 1.10–1.60], and follow-up at an academic [vs non-academic] medical centre [HR 1.37, 95% CI 1.07–1.76] were independent risk factors for advanced neoplasia following LGD.
Conclusions
In a large nationwide cohort with long-term follow-up of IBD patients with LGD, the cumulative incidence of advanced neoplasia was 21.7% after 15 years. Older age at LGD [≥55 years], male sex and follow-up by a tertiary IBD referral centre were independent risk factors for advanced neoplasia development after initial LGD.
Keywords: High-grade dysplasia, colorectal cancer, ulcerative colitis, Crohn’s disease
1. Introduction
Colorectal cancer [CRC] is one of the most detrimental complications in patients with inflammatory bowel disease [IBD], and CRC risk is increased compared to the general population.1 It develops through an inflammation—low-grade dysplasia [LGD]—high-grade dysplasia [HGD] pathway to carcinoma.2 Endoscopic surveillance is advocated to detect and remove precancerous lesions before CRC develops. After removal of LGD lesions, patients have an increased risk for development of subsequent advanced neoplasia [HGD and/or CRC].3,4 Current guidelines recommend colectomy or intensified surveillance following LGD detection, but the optimal surveillance strategy remains under debate.5,6
Data regarding the risk of advanced neoplasia after LGD are scarce. One recent study reported that 33 of 172 [19.1%] IBD patients with LGD developed advanced neoplasia.7 However, lower but also higher rates up to 54% have been reported as well.8–12 A recent meta-analysis calculated a pooled CRC rate after LGD of 0.8 per 100 patient-years follow-up.4 Most of the included studies had limited numbers of LGD patients [range 2–172] and no studies including >60 patients exceeded a median follow-up duration of 5 years. Moreover, most data were collected in tertiary referral centres and these cohorts are at risk of selection bias. As a consequence, point estimates of the long-term risk of advanced neoplasia following LGD remain unclear.
To determine the long-term advanced neoplasia risk in IBD patients with a history of LGD, we established a nationwide cohort of IBD patients with a history of LGD. In this cohort we aimed to [1] determine the cumulative incidence of advanced neoplasia in IBD patients following LGD, and [2] identify the risk factors for developing advanced neoplasia.
2. Methods
2.1 Study design
We studied the cumulative incidence and risk factors for advanced neoplasia following LGD in a nationwide multicentre cohort study.
2.2 Study population
A nationwide search was conducted to identify all patients with IBD and neoplasia using the nationwide network and registry of histopathology and cytopathology in the Netherlands [PALGA].13 PALGA has collected all pathology reports in the Netherlands since 1971 and has complete national coverage since 1991 of both academic and non-academic hospitals. All reports can be tracked to an individual patient using a unique identifier, allowing follow-up on an individual basis even when biopsies are performed at different institutes. We have previously shown that a search strategy in the PALGA database was able to identify 95% of all IBD patients correctly, as confirmed by individual patient files.3 A PALGA search was performed including terms for IBD [‘ulcerative colitis’, ‘Crohn’s disease’, ‘indeterminate colitis’ and ‘chronic idiopathic inflammatory bowel disease’], combined with terms for colorectal neoplasia [‘indefinite for dysplasia’, ‘low-grade dysplasia’, ‘high-grade dysplasia’, ‘carcinoma in situ’, ‘colorectal cancer’ and ‘dysplasia’] located in the colon, rectum or appendix. The PALGA search was restricted to patients with neoplastic lesions between 1991 and 2010, allowing long-term follow-up. Follow-up pathology reports were collected until January 1, 2016. All individual pathology reports were carefully evaluated to confirm inclusion or exclusion. The study was approved by the PALGA ethical committee [lzv-1215].
2.3 Inclusion and exclusion criteria
All patients with a diagnosis of IBD (ulcerative colitis [UC], Crohn’s disease [CD] and IBD-unclassified [IBD-U]) with colonic LGD [after IBD diagnosis] from January 1991 to December 2010 were eligible for inclusion. IBD diagnosis was based on histology from both biopsies and resection specimens. Exclusion criteria were defined as follows: advanced neoplasia diagnosis before IBD development, advanced neoplasia diagnosis before LGD development, no histological follow-up, and patients with hereditary CRC syndromes such as Lynch syndrome and familial adenomatous polyposis. Furthermore, patients who underwent [sub]total colectomy before LGD diagnosis [in the residual colon] were excluded because this significantly impacts CRC risk.14 Subtotal colectomy was defined as a colon resection, only leaving the rectum in place.
2.4 Data collection
The variables extracted from the PALGA database included sex, date and type of neoplasia, date and type of colon resection, number of colonoscopies, origin of histology [academic vs non-academic centre] and type of IBD diagnosis. The diagnosis IBD-U was used when no clear distinction between a diagnosis of UC or CD could be made. Date of IBD diagnosis could not be accurately extracted from PALGA and was therefore not included in our analysis. The highest grade of dysplasia at baseline was considered the initial neoplastic lesion. Patients with a LGD diagnosis in an academic centre, and/or with subsequent follow-up in an academic centre were considered as ‘academic’. To confirm whether patients who were identified in our PALGA search indeed had a diagnosis of IBD, a verification cohort was compiled. The verification cohort consisted of all IBD patient with LGD [identified with our PALGA search] from one academic [Radboud University Medical Centre Nijmegen] and two non-academic centres [Jeroen Bosch Hospital s’Hertogenbosch and Rijnstate Hospital Arnhem]. All patient charts were reviewed to confirm IBD diagnosis.
2.5 Statistics
Cumulative incidences of advanced neoplasia were counted with 1 minus Kaplan–Meier curves, censoring patients at the end of follow-up. End of follow-up was defined as the date of the last pathology report or, if performed, the date of [sub]total colectomy given the impact on CRC risk.14 A sensitivity analysis was performed excluding patients who developed advanced neoplasia within a year of LGD diagnosis, as these lesions might represent missed advanced lesions instead of new advanced neoplasia. To calculate incidence rates of advanced neoplasia, the number of patients with advanced neoplasia was divided by the total number of follow-up years from index LGD until the first event or censoring [i.e. last follow-up colonoscopy or colectomy]. Thus, patients who developed both HGD and CRC were counted once [only the first event]. Continuous outcomes are presented as means including standard deviation [SD] if normally distributed and as medians with interquartile range [IQR] if non-normally distributed. To identify risk factors for developing advanced neoplasia, potential risk factors were univariably compared with Kaplan–Meier curves and log-rank tests. We used the mean age at LGD in the total cohort as the cut-off to determine if older age was associated with a greater risk of advanced neoplasia. Risk factors with a p-value <0.2 in univariable analysis were included in a multivariable Cox regression model with backward elimination. The proportional hazards assumption was tested for all variables in our model by testing time–covariate interactions and visual inspection of log-minus log plots. A p-value of <0.05 was considered to be statistically significant.
Data analysis was performed using the SPSS statistical software [version 22, IBM]. Incidence rates of advanced neoplasia with 95% confidence interval [CI] were determined using OpenEpi software.15
3. Results
3.1 Patient selection
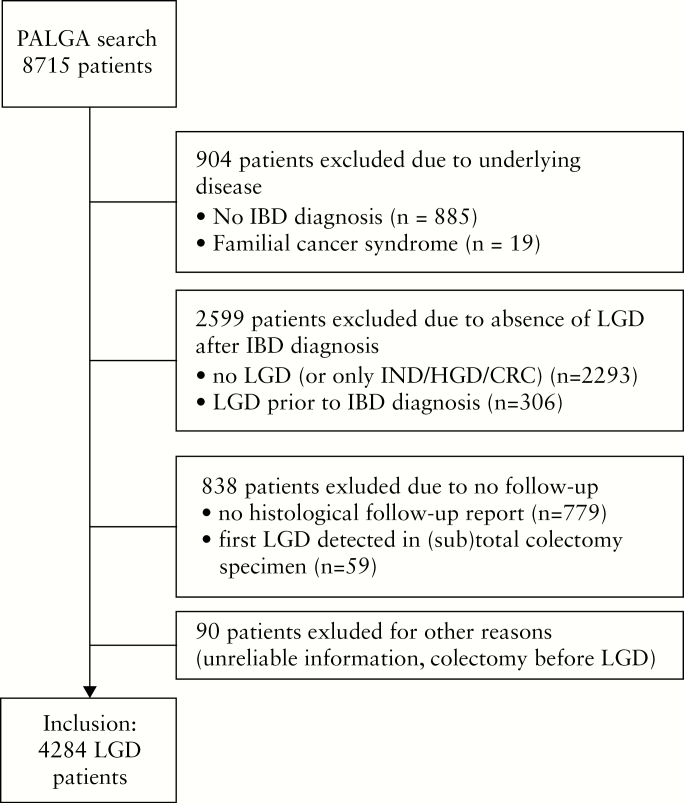
Our initial PALGA search yielded 8715 patients. A total of 4284 IBD patients with LGD were available for inclusion [Figure 1]. The cumulative follow-up for the entire cohort from date of LGD diagnosis to date of last available report was 33 401 patient-years, with a median follow-up of 6.4 years [3.2–11.3], including 2655 patients with a follow-up duration of >5 years and 1301 patients with >10 years of follow-up. Total follow-up until censoring or event was 30 154 patient-years. A total of 24 796 colonoscopies were performed in our cohort [median five per patient]. Overall, the median time between consecutive colonoscopies after LGD was 1.4 [0.8–2.3] years, and the median time to first follow-up after LGD was 1.5 [0.7–3.2] years. The majority of patients were male [61.4%] and had a diagnosis of UC [71.5%] [Table 1]. Mean age at LGD diagnosis was 55.3 [±14.8] years.
Figure 1.
Patient inclusion flowchart.
IBD = inflammatory bowel disease, LGD = low-grade dysplasia, IND = indefinite for dysplasia, HGD = high-grade dysplasia, CRC = colorectal cancer.
Table 1.
Baseline characteristics of included patients with low-grade dysplasia
| Characteristic | Patients with LGD [n = 4284] |
|---|---|
| Male sex, n [%] | 2630 [61.4] |
| Disease | |
| Ulcerative colitis, n [%] | 3065 [71.5] |
| Crohn’s disease, n [%] | 970 [22.6] |
| IBD-unclassified, n [%] | 249 [5.8] |
| First year of dysplasia, n [%] | |
| 1990–2000 | 1655 [38.6] |
| 2001–2010 | 2629 [61.4] |
| Age at LGD, mean [±SD] | 55.3 [14.8] |
| Follow-up after dysplasia in years, median [IQR] | 6.4 [3.2–11.3] |
| Follow-up after IBD diagnosis in years, mean [±SD] | 13.8 [8.2] |
| Centre | |
| Academic | 504 [11.8] |
| Non-academic | 3780 [88.2] |
IBD = inflammatory bowel disease, LGD = low-grade dysplasia, SD = standard deviation, IQR = interquartile range.
3.2 Verification cohort
Our verification cohort consisted of 235 patients. A diagnosis of IBD could be confirmed in 216/235 [91.9%] patients. Patients without a confirmed IBD diagnosis had unspecified colonic inflammation, diverticulitis and/or abdominal tuberculosis. The mean first date of histologically confirmed IBD as found in PALGA was 2.4 [±6.8] years later than reported in medical charts and thus IBD duration was excluded from analysis.
3.3 Cumulative incidence of advanced neoplasia
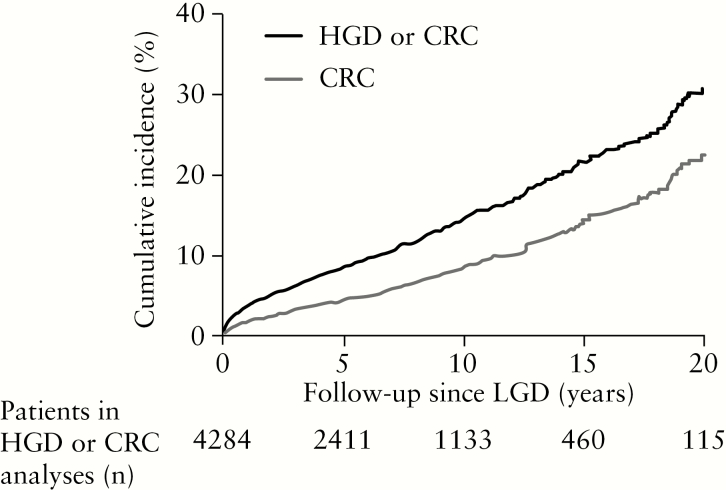
In our cohort, 526 of 4284 LGD patients developed advanced neoplasia [378 UC, 126 CD, 22 IBD-U]. Within this subgroup, 211/526 patients developed HGD as the highest grade of dysplasia and 315/526 patients developed CRC at a median age of 62 years [51–74]. Seventy-one of 315 patients were diagnosed with HGD preceding CRC. The median time to develop advanced neoplasia after LGD was 3.6 [0.8–8.7] years. The median time between colonoscopies until advanced neoplasia was 0.9 [0.3–1.7] years. A total of 38.6% of patients who developed advanced neoplasia had more than one LGD finding before advanced neoplasia. Figure 2 shows the cumulative incidence of both advanced neoplasia and CRC after LGD. The 1-, 5-, 10- and 15-year cumulative incidence of advanced neoplasia was 3.6, 8.5, 14.4 and 21.7%, respectively. The cumulative incidence of CRC was 2.1, 4.9, 8.6 and 14.0%, respectively. Sensitivity analysis, excluding patients who developed advanced neoplasia within 1 year following LGD [n = 146], revealed a cumulative incidence of advanced neoplasia of 18.8% after 15 years [Supplementary Figure 1]. The incidence rate of advanced neoplasia was 17.4 [95% CI 16.0–19.0] per 1000 patient-years (incidence rate of HGD 7.0 [95% CI 6.1–8.0] per 1000 patient-years; incidence rate of CRC 10.4 [95% CI 9.3–11.7] per 1000 patient-years).
Figure 2.
Kaplan–Meier plot showing the cumulative incidence of high-grade dysplasia or colorectal cancer combined, and colorectal cancer only, both after initial low-grade dysplasia.
LGD = low-grade dysplasia, HGD = high-grade dysplasia, CRC = colorectal cancer.
A total of 551/4284 [12.9%] of LGD patients underwent [sub]total colectomy during follow-up and 175 of 551 underwent [sub]total colectomy within a year after LGD. Histological evaluation of the 175 resection specimens revealed CRC in 28 patients [16%] and HGD in 13 patients [7%]. Twelve patients developed advanced neoplasia after [sub]total colectomy in the residual rectum [five HGD, seven CRC]. The median time to develop advanced neoplasia after [sub]total colectomy was 6.0 years [4.8–8.8]. Patients with LGD before the year 2000 underwent colectomy more frequently than after the year 2000 [17.8% vs 10.2%, p < 0.001].
3.4 Risk factors for advanced neoplasia development
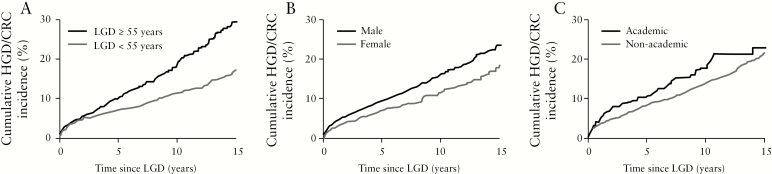
Table 2 shows the results of the univariable analyses. Male sex (hazard ratio [HR] 1.38, 95% CI 1.15–1.67; p = 0.001) and LGD at age 55 years or older [HR 1.72, 95% CI 1.44–2.05; p < 0.001] were significant risk factors [Figure 3]. A histopathology report from an academic centre [HR 1.26, 95% CI 0.99–1.62, p = 0.06] and a history of indefinite for dysplasia before LGD [HR 1.69, 95%CI 0.84–3.40, p = 0.14] were not significant risk factors in univariable analysis, but were included in multivariable analysis as the p-value was below the prespecified threshold of 0.2. A diagnosis of LGD before the year 2000 was not identified as a risk factor in univariable analysis. Likewise, the type of IBD diagnosis [UC/CD/IBD-U] was not significantly different between patients with and without advanced neoplasia.
Table 2.
Univariable and multivariable analysis of potential demographic risk factors for high-grade dysplasia or colorectal cancer
| Baseline | HR univariable | 95% CI | p-value | HR multivariable [final model] | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Diagnosis | ||||||
| UC | 1 | |||||
| CD | 1.13 | 0.92–1.39 | 0.258 | - | - | - |
| IBD-U | 0.84 | 0.57–1.23 | 0.365 | - | - | - |
| Sex | ||||||
| Female | 1 | 1 | ||||
| Male | 1.38 | 1.15–1.67 | 0.001 | 1.33 | 1.10–1.60 | 0.003 |
| LGD diagnosis | ||||||
| Before 2001 | 1 | – | – | – | ||
| After 2000 | 1.12 | 0.93–1.36 | 0.237 | – | – | – |
| Academic centre | 1.26 | 0.99–1.62 | 0.063 | 1.37 | 1.07–1.76 | 0.012 |
| Age at first LGD | ||||||
| <55 years old | 1 | 1 | 1 | |||
| >55 years old | 1.72 | 1.44–2.05 | <0.001 | 1.73 | 1.44–2.06 | <0.001 |
| IND before LGD | 1.69 | 0.84–3.40 | 0.14 | – | – | NS |
UC = ulcerative colitis, CD = Crohn’s disease, IBD-U = IBD-unclassified, LGD = low-grade dysplasia, IBD = inflammatory bowel disease, IND = indefinite for dysplasia, NS = not significant.
Figure 3.
Kaplan–Meier plot showing the cumulative incidence of advanced neoplasia after low-grade dysplasia for [A] age ≥55 years at moment of low-grade dysplasia, [B] male sex and [C] academic centre.
LGD = low-grade dysplasia, HGD = high-grade dysplasia, CRC = colorectal cancer, IBD = inflammatory bowel disease.
Multivariable analysis identified LGD at age 55 years or older [HR 1.73, 95% CI 1.44–2.06], male sex [HR 1.33, 95% CI 1.10–1.60] and academic centre [HR 1.37, 95% CI 1.07–1.76] as independent risk factors for advanced neoplasia development. Multivariable analyses for development of CRC identified only age >55 years at LGD diagnosis as a single independent risk factor [Supplementary Table 1].
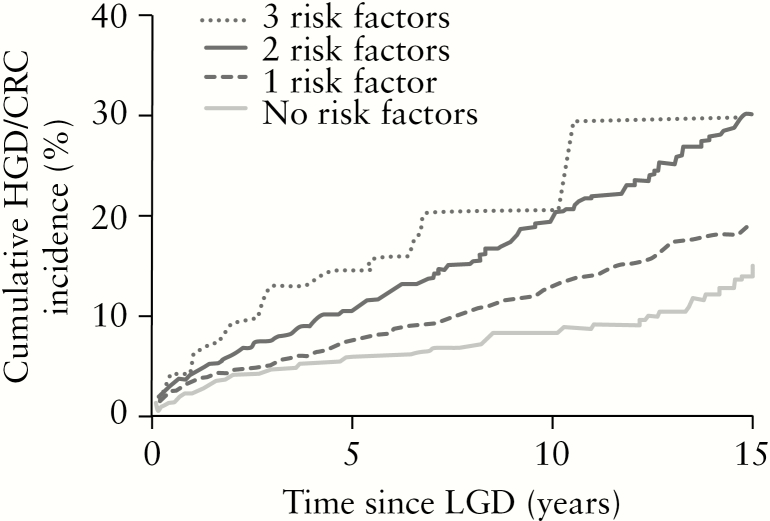
The number of risk factors [including LGD age >55 years, male sex and academic centre] corresponded with a higher cumulative risk of advanced neoplasia [1 vs 0 risk factors: p = 0.007; 2 vs 1: p < 0.001; 3 vs 2: p = 0.21]. The 10-year cumulative advanced incidences of neoplasia [Figure 4] were 8.3% [no risk factors], 12.9% [one risk factor], 19.8% [two risk factors] and 20.4% [all three risk factors].
Figure 4.
Kaplan–Meier plot showing the cumulative incidence of advanced neoplasia in patients with different numbers of risk factors.
Number of patients: 0 risk factors [n = 724], 1 risk factor [n = 1883], 2 risk factors [n = 1557], 3 risk factors [n = 120]. LGD = low-grade dysplasia, HGD = high-grade dysplasia, CRC = colorectal cancer, IBD = inflammatory bowel disease.
4. Discussion
In this nationwide cohort study including IBD patients with long-term follow-up, the cumulative incidence of developing advanced neoplasia after colonic LGD was 21.7% after 15 years. Risk factors for developing advanced neoplasia included older age at LGD [≥55 years], male sex and a histological follow-up at an academic centre.
LGD is a frequent finding in IBD patients, with reported rates of 11–21%.16–18 Given the high prevalence of LGD, more insight into cancer risk after LGD is needed to determine an optimal surveillance strategy for these patients. Previously reported incidence rates of advanced neoplasia after LGD showed a wide range between 0% and 54%.7–12 One study including 172 UC patients with LGD showed a cumulative risk of advanced neoplasia of 27.1% after 10 years.7 This is considerably higher than the reported 14.4% in our cohort, which may be explained by our nationwide study approach, better representing the general IBD population and avoiding selection bias. Indeed, we found a higher risk of advanced neoplasia in LGD patients from academic centres. Similar to previous study findings, this might be the result of a more complex IBD population in academic centres resulting in a higher CRC risk. In addition, a recent meta-analysis reported that the risk of CRC was higher when LGD was diagnosed by expert gastrointestinal pathologists than by community pathologists.4
One meta-analysis including 671 UC patients reported a pooled incidence rate of advanced neoplasia of 18 per 1000 patient-years, which is in line with the incidence rate of 17.4 per 1000 patient-years in our cohort. The annual incidence of CRC in our cohort was 1.0%, compared to a previously reported incidence of 0.3% in patients with ‘non-dysplastic’ UC19 and 0.017–0.041% in a general non-IBD screening population.20,21
We found that older age at LGD diagnosis was associated with a higher risk of advanced neoplasia. This possibly reflects the overall higher CRC risk at older age. Previous studies reported an increased risk when dysplasia developed at older age,22 although this was not always statistically significant.7,23 In addition, we identified male sex as an independent risk factor for advanced neoplasia development. Male sex is an established risk factor for development of metachronous high-risk adenoma in non-IBD CRC screening.24 Moreover, a large Danish population-based study also reported a higher CRC risk in male IBD patients. It was suggested that hormonal effects such as oestrogens may protect females from developing CRC.25,26
The results of our nationwide cohort may impact IBD surveillance recommendations after LGD detection. The American Gastroenterological Association guidelines6 recommend an intensified surveillance in IBD patients with LGD, but do not specify an interval. The British Society of Gastroenterology [BSG] guidelines5 define intensified surveillance as performing yearly colonoscopy during the subsequent 5 years. This recommendation is supported by our finding that the majority of cases with advanced neoplasia developed within 5 years of LGD diagnosis [median time between LGD and advanced neoplasia: 3.6 years]. Given our finding that a large proportion of advanced neoplasia cases were detected within 1 year after LGD diagnosis [146/526, 28%], we would recommend performing a first surveillance colonoscopy within 1 year after LGD detection. Moreover, 23% of IBD patients who underwent a colectomy within 1 year after LGD detection had synchronous or metachronous advanced neoplasia in the colectomy specimen. This illustrates the risk of missed neoplasia when LGD is detected. Similarly, another study reported 38.9% advanced neoplasia in colectomy specimens of patients who underwent colectomy for LGD.7
Furthermore, risk stratification allows the tailoring of endoscopic surveillance following LGD in IBD patients towards a more individualized approach. For example, high-risk patients [male, LGD >55 years, follow-up in an academic centre] may be recommended to undergo yearly surveillance in the 5 years following LGD diagnosis, similar to the BSG guidelines. In addition, one can speculate based on the current literature that patients with other high-risk features [not assessed in our study], such as multifocal or metachronous LGD, non-polypoid LGD, lesions >1 cm, or LGD that occurs in IBD patients with strictures or concomitant primary sclerosing cholangitis (PSC),4,7 may also benefit from such an intensive surveillance strategy. Indeed, one study already reported an increased advanced neoplasia risk based on the number of risk factors.7 Similarly, we observed that patients with multiple risk factors were at the greatest risk of developing advanced neoplasia. By contrast, low-risk patients who have no new abnormalities at first follow-up colonoscopy may well need a less intensified surveillance strategy. The optimal surveillance interval and whether the latter period of 5 years may be shortened for a subgroup of relatively low-risk patients following LGD requires additional studies and is beyond the scope of our work.
Our study has several strengths, including the relatively long-term follow-up [median 6.4 years, with 2655 patients with >5 years of follow-up of whom 1301 had >10 years of follow-up], the nationwide population-based study approach reducing selection bias, and the large cohort of 4284 LGD patients. In addition, we established a verification cohort to confirm the reliability of IBD diagnosis in our nationwide IBD cohort. This study also comes with some limitations. First, our results reflect the overall long-term outcomes of LGD over more than two decades, representing a mix of colonoscopy practices and quality of endoscopes. In addition, due to the retrospective nature of our study no standardized surveillance strategy was followed, which may result in selection bias. However, overall patients received frequent follow-up colonoscopies [median interval 1.4 years]. Second, the use of a histopathology database without clinical data from patient files did not allow us to evaluate known potential risk factors, such as LGD location and size/morphology [flat or raised LGD], concomitant diseases such as PSC, disease duration, extent and activity, as well as type of IBD treatment. Moreover, it is unknown how LGD was approached [wait and see, polypectomy, colectomy], which subsequently may impact the risk of advanced neoplasia. Third, our results are based on data from IBD patients with LGD detected between 1991 and 2010. As such, we could establish long-term risk with a median follow-up of 6.4 years, much longer than in previous studies. However, the incidence of advanced neoplasia might be overestimated because currently more advanced endoscopic techniques [such as high-definition endoscopes and the use of chromo-endoscopy] with updated surveillance guidelines are available, as well as a host of new therapeutic options. Indeed, a meta-analysis has reported a declining CRC risk in IBD over recent decades.27 We did not find a significantly different advanced neoplasia risk between patients who developed LGD before 2000 and those with LGD between 2000 and 2010. One can hypothesize that higher colectomy rates before 2000 may contribute to these findings. Finally, in the past histopathology specimens were not always re-assessed by a second pathologist. It is well established that there is substantial interobserver variation in the grading of dysplasia between pathologists24,28 and that misclassification of LGD might lead to lower incidence rates of advanced neoplasia.29 Therefore, re-evaluation by a second pathologist for dysplasia detection is recommended.30
In conclusion, we found in a nationwide IBD cohort that the cumulative incidence of advanced neoplasia after LGD is 21.7% after 15 years [incidence rate 17 per 1000 patient-years]. Potential risk factors include older age [≥55 years] at the moment of LGD, male sex and follow-up at an academic centre. These findings support current guidelines that recommend initial yearly surveillance following detection of LGD in IBD patients.
Funding
This work was not supported by any company or grants.
Conflict of Interest
All authors declare no conflicts of interest.
Author Contributions
No additional writing assistance was used for this manuscript. F.H., L.D., L.N., I.N., W.K. and M.J. all contributed to the design of the study. S.T. and M.J. collected data, and M.J. analysed the data. C.H. and T.R. assisted in data collection. L.D. and M.J. drafted the manuscript. F.H. and J.D. critically revised the manuscript for important intellectual content. All authors have approved the final version of the manuscript.
Supplementary Material
References
- 1. Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med 2015;373:195. [DOI] [PubMed] [Google Scholar]
- 2. Riddell RH, Goldman H, Ransohoff DF, et al.. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol 1983;14:931–68. [DOI] [PubMed] [Google Scholar]
- 3. Derikx LA, Kievit W, Drenth JP, et al.; Dutch Initiative on Crohn and Colitis Prior colorectal neoplasia is associated with increased risk of ileoanal pouch neoplasia in patients with inflammatory bowel disease. Gastroenterology 2014;146:119–28.e1. [DOI] [PubMed] [Google Scholar]
- 4. Fumery M, Dulai PS, Gupta S, et al.. Incidence, risk factors, and outcomes of colorectal cancer in patients with ulcerative colitis with low-grade dysplasia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2017;15:665–674.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cairns SR, Scholefield JH, Steele RJ, et al.; British Society of Gastroenterology; Association of Coloproctology for Great Britain and Ireland Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666–89. [DOI] [PubMed] [Google Scholar]
- 6. Farraye FA, Odze RD, Eaden J, et al.; AGA Institute Medical Position Panel on Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010;138:738–45. [DOI] [PubMed] [Google Scholar]
- 7. Choi CH, Ignjatovic-Wilson A, Askari A, et al.. Low-grade dysplasia in ulcerative colitis: risk factors for developing high-grade dysplasia or colorectal cancer. Am J Gastroenterol 2015;110:1461–71; quiz 1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim CH, Dixon MF, Vail A, Forman D, Lynch DA, Axon AT. Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut 2003;52:1127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Befrits R, Ljung T, Jaramillo E, Rubio C. Low-grade dysplasia in extensive, long-standing inflammatory bowel disease: a follow-up study. Dis Colon Rectum 2002;45:615–20. [DOI] [PubMed] [Google Scholar]
- 10. Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology 1994;107:934–44. [DOI] [PubMed] [Google Scholar]
- 11. Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet 1994;343:71–4. [DOI] [PubMed] [Google Scholar]
- 12. Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology 2003;125:1311–9. [DOI] [PubMed] [Google Scholar]
- 13. Casparie M, Tiebosch AT, Burger G, et al.. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007;29:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Derikx LAAP, Nissen LHC, Smits LJT, Shen B, Hoentjen F. Risk of neoplasia after colectomy in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:798–806.e20. [DOI] [PubMed] [Google Scholar]
- 15. Dean AG, Sullivan K, Soe MM.. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. www.OpenEpi.com [Google Scholar]
- 16. Choi CH, Rutter MD, Askari A, et al.. Forty-year analysis of colonoscopic surveillance program for neoplasia in ulcerative colitis: an updated overview. Am J Gastroenterol 2015;110:1022–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mooiweer E, van der Meulen-de Jong AE, Ponsioen CY, et al.; Dutch Initiative on Crohn’s and Colitis Incidence of interval colorectal cancer among inflammatory bowel disease patients undergoing regular colonoscopic surveillance. Clin Gastroenterol Hepatol 2015;13:1656–61. [DOI] [PubMed] [Google Scholar]
- 18. Shah SC, Ten Hove JR, Castaneda D, et al.. High risk of advanced colorectal neoplasia in patients with primary sclerosing cholangitis associated with inflammatory bowel disease. Clin Gastroenterol Hepatol 2018;16:1106–1113.e3. [DOI] [PubMed] [Google Scholar]
- 19. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Cancer Institute. Surveillance, Epidemiology, and Ends Results Program. http://seer.cancer.gov/statfacts/html/colorect.html [Google Scholar]
- 21. International Agency for Research in Cancer. Geneva: World Health Organization; Published February 2019. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx [Google Scholar]
- 22. Lai KK, Horvath B, Xie H, et al.. Risk for colorectal neoplasia in patients with inflammatory bowel disease and mucosa indefinite for dysplasia. Inflamm Bowel Dis 2015;21:378–84. [DOI] [PubMed] [Google Scholar]
- 23. Pekow JR, Hetzel JT, Rothe JA, et al.. Outcome after surveillance of low-grade and indefinite dysplasia in patients with ulcerative colitis. Inflamm Bowel Dis 2010;16:1352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melville DM, Jass JR, Morson BC, et al.. Observer study of the grading of dysplasia in ulcerative colitis: comparison with clinical outcome. Hum Pathol 1989;20:1008–14. [DOI] [PubMed] [Google Scholar]
- 25. Brenner H, Hoffmeister M, Arndt V, Haug U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer 2007;96:828–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Söderlund S, Granath F, Broström O, et al.. Inflammatory bowel disease confers a lower risk of colorectal cancer to females than to males. Gastroenterology 2010;138:1697–703. [DOI] [PubMed] [Google Scholar]
- 27. Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 2013;19:789–99. [DOI] [PubMed] [Google Scholar]
- 28. Dixon MF, Brown LJ, Gilmour HM, et al.. Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology 1988;13:385–97. [DOI] [PubMed] [Google Scholar]
- 29. van Schaik FD, ten Kate FJ, Offerhaus GJ, et al.; Dutch Initiative on Crohn and Colitis Misclassification of dysplasia in patients with inflammatory bowel disease: consequences for progression rates to advanced neoplasia. Inflamm Bowel Dis 2011;17:1108–16. [DOI] [PubMed] [Google Scholar]
- 30. Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R; SCENIC Guideline Development Panel SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148:639–51.e28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.