Abstract
Background
After proximal humerus resection for bone tumors, restoring anatomy and shoulder function remains demanding because muscles and bone are removed to obtain tumor-free surgical margins. Current modes of reconstruction such as anatomic modular prostheses, osteoarticular allografts, or allograft-prosthetic composites and arthrodeses are associated with relatively poor shoulder function related to loss of the deltoid and rotator cuff muscles. Newer prosthetic designs like the reverse total shoulder arthroplasty (RTSA) are felt to be useful in other reconstructions where rotator cuff function is compromised, so it seemed logical that it might help in tumor reconstructions as well in patients where the deltoid muscle and its innervation can be preserved.
Questions/purposes
In patients with a tumor of the proximal humerus that can be resected with preservation of the deltoid muscle, (1) What complications are associated with tumor resection and reconstruction with a modular RTSA? (2) What are the functional results of modular RTSA in these patients?
Methods
From January 2011 to January 2018, we treated 52 patients for bone tumors of the proximal humerus. Of these, three patients were treated with forequarter amputation, 14 were treated with standard modular proximal humerus implants, seven were treated with allograft-prosthetic composites (RTSA-APC), and 28 were treated with a modular RTSA. Generally, we used anatomic modular prosthetic reconstruction if during the tumor resection none of the abductor mechanism could be spared. Conversely, we preferred reconstruction with RTSA if an innervated deltoid muscle could be spared, but the rotator cuff and capsule could not, using RTSA-APC or modular RTSA if humeral osteotomy was distal or proximal to deltoid insertion, respectively. In this study, we retrospectively analyzed only patients treated with modular RTSA after proximal humerus resection. We excluded three patients treated with modular RTSA as revision procedures after mechanical failure of previous biological reconstructions and three patients treated after December 2016 to obtain an expected minimum follow-up of 2 years. There were nine men and 13 women, with a mean (range) age of 55 years (18 to 71). Reconstruction was performed in all patients using silver-coated modular RTSA protheses. Patients were clinically checked according to oncologic protocol. Complications and function were evaluated at final follow-up by the treating surgeon (PR) and shoulder surgeon (AC). Complications were evaluated according to Henderson classification. Functional results were assessed with the Musculoskeletal Tumor Society score (range 0 points to 30 points), Constant-Murley score (range 0 to 100), and American Shoulder and Elbow Surgeons score (range 0 to 100). The statistical analysis was performed using Kaplan-Meier curves.
Results
Complications occurred in five of 22 patients; there was a shoulder dislocation (Type I) in four patients and aseptic loosening (Type II) in one. Function in these patients on the outcomes scales we used was generally satisfactory; the mean Musculoskeletal Tumor Society score was 29, the mean Constant score was 61, and the mean American Shoulder and Elbow Surgeons score was 81.
Conclusions
Although this was a small series of patients with heterogeneous diagnoses and resection types, and we were not able to directly compare the results of this procedure with those of other available reconstructions, we found patients treated with RTSA achieved reasonable shoulder function after resection and reconstruction of a proximal humerus tumor. It may not be valuable in all tumor resections, but in patients in whom the deltoid can be partly spared, this procedure appears to reasonably restore short-term shoulder function. However, future larger studies with longer follow-up are needed to confirm these findings.
Level of Evidence
Level IV, therapeutic study.
Introduction
Restoring anatomy and shoulder function after proximal humerus resection for a bone tumor is demanding because wide soft-tissue margins and bone are needed to minimize the potential for local recurrence. Historically, different reconstructive techniques such as arthrodesis [38, 41, 54], massive osteoarticular allografts [2, 3, 9, 19, 21-23, 31, 35, 42, 51, 54], and anatomic modular prostheses [10, 12, 16, 27, 35, 39, 44, 45, 49, 58, 59] have been proposed. These techniques may preserve the function of the elbow, wrist, and hand, but shoulder motion and strength are often lost because the rotator cuff muscles or tendon insertions are resected to achieve a satisfactory tumor margin.
Reverse total shoulder arthroplasty (RTSA) was developed to treat patients with severe cuff tear arthropathy [26]. Indeed, this type of arthroplasty, which inverts the joint geometry, leads to better recruitment of deltoid fibers and allows for an increase of active motion, compensating for the lack of a functional rotator cuff [4-6, 57]. Because excellent results were obtained in patients with nononcologic conditions and because there is similarity in residual shoulder biomechanics between cuff arthropathy and some tumor resections, allograft-prosthetic composite RTSA was introduced as a reconstruction option after proximal humerus resection [1, 7, 11,15, 16, 20, 25, 29, 33, 34, 48]. Currently, if the axillary nerve and deltoid muscle can be spared, modular RTSA may be used, which offers both the advantages of modularity and a prosthesis with inverse geometry.
However, to our knowledge, only few studies have used this approach [28, 29, 36, 53], and the data reported did not completely evaluate functional results. Because of this, we considered whether this reconstructive approach might be useful for patients who underwent tumor resections that involved the rotator cuff without involvement of deltoids muscles and its innervation.
We therefore asked: (1) What complications are associated with resection and reconstruction in patients with modular RTSA? (2) What are the functional results of using modular RTSA in these patients?
Patients and Methods
From January 2011 to January 2018, we treated 52 patients for bone tumors of the proximal humerus in our institution. Of these, three patients were treated with forequarter amputation, 14 were treated with standard modular proximal humerus implants, seven were treated with allograft-prosthetic composites (RTSA-APC), and 28 were treated with a modular RTSA. Generally, we used modular prosthetic reconstruction (hemiarthroplasty) as a “spacer” if during the tumor resection none of the abductor mechanism can be spared. Conversely, we preferred reconstruction with RTSA if an innervated deltoid muscle could be spared, but the rotator cuff and capsule could, using RTSA-APC or modular RTSA if humeral osteotomy was distal or proximal to deltoid insertion, respectively (Fig. 1). We included in the study patients treated for bone tumors of the proximal humerus, excluding (1) three patients treated with amputation, (2) 21 patients reconstructed with other techniques (standard proximal humerus prosthesis or allograft prosthesis composite), (3) three patients treated with modular RTSA as a revision procedure after mechanical failure of previous biological reconstructions, (4) three patients treated after December 2016 who were not expected to obtain a minimum follow-up of 2 years expected (Fig. 2). In this study, we retrospectively analyzed only patients treated with modular RTSA after proximal humerus resection. All patients were surgically treated by the senior author (PR). Twenty-two patients were included in this study; 39% (nine) were men and 61% (13) were women with a mean (range) age of 55 years (18 to 71).
Fig. 1.
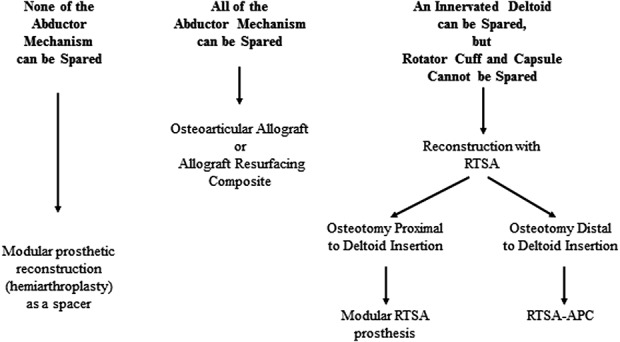
Our proposed decision-making algorithm for the reconstructive technique after proximal humerus resection for bone tumors is shown. RTSA = reverse total shoulder arthroplasty; RTSA-APC = reverse shoulder arthroplasty with allograft-prosthesis composites.
Fig. 2.
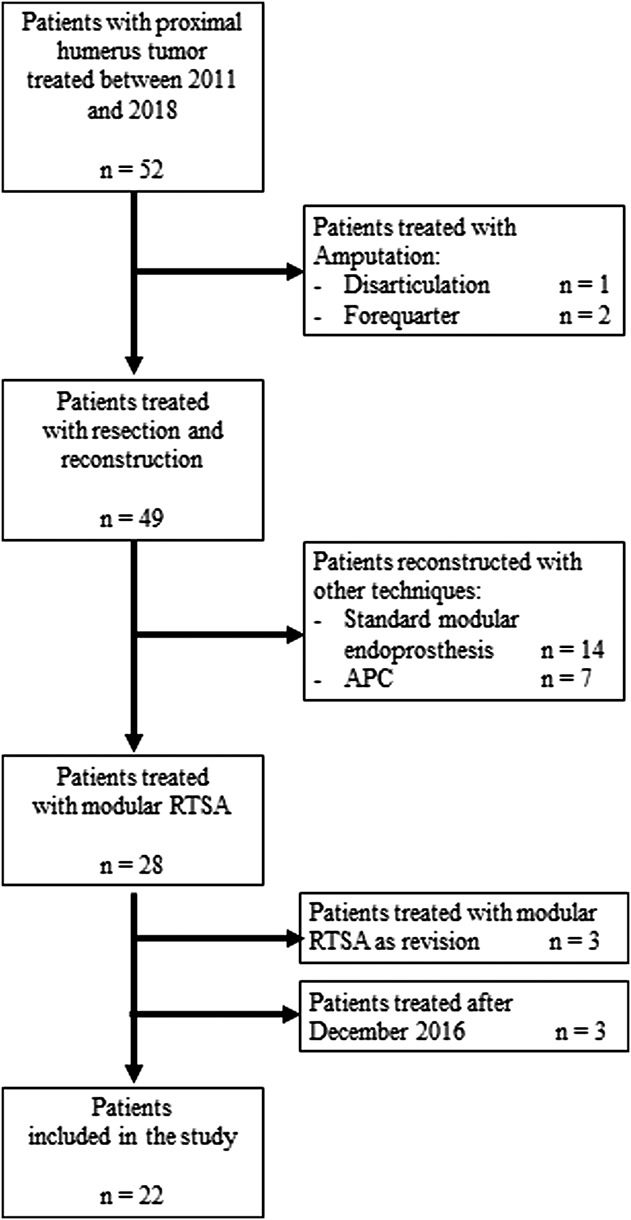
This flow diagram describes our inclusion and exclusion criteria for patients enrolled in the study.
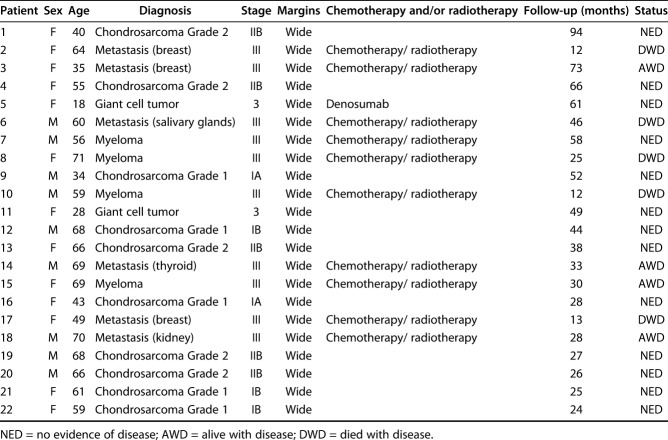
The diagnoses were chondrosarcoma in 10 patients, metastasis in six (breast in three patients, and kidney, salivary glands and thyroid in one each), multiple myeloma in four, and giant cell tumors in two. According to the Surgical Staging System for musculoskeletal tumors [17], two patients had Stage 3 (benign) tumors, two had Stage IA, three had Stage IA, five had Stage IIB, and 10 had Stage III (Table 1).
Table 1.
Demographic and oncologic data of patients undergoing reconstruction with modular reverse total shoulder arthroplasty (n = 22)
The choice of operative strategy was based on a multidisciplinary evaluation by our oncologic team and adjuvant treatments were administered as appropriate for the diagnosis. Chemotherapy was used in patients who had metastatic lesions and myeloma according to the primary cancer type, and these patients additionally underwent radiotherapy. Denosumab was administered preoperatively in one patient with a giant cell tumor. In all patients, intra-articular Type 1 resection [37] was performed using an extended deltopectoral approach, protecting the pectoralis major insertion, biceps tendon, axillary nerve and deltoid muscle. The latissimus insertion and rotator cuff (supraspinatus and infraspinatus muscles) were sacrificed in all patients; whereas the subscapularis insertion was preserved in eight patients. The mean (range) resection length, planned preoperatively on MRI according to tumor extension, was 10 cm (6 to 14), and oncologic surgical margins were wide in all patients. The attempt to achieve wide surgical margins was made also in patients with metastatic carcinoma and myeloma because of the staging revealed a solitary/oligometastatic setting. Two patients with a benign lesion (giant cell tumor) were treated with wide resection due to the high risk of local recurrence despite treatment with denosumab.
Proximal humeral reconstruction was performed in all patients using a silver-coated modular RTSA prosthesis (MUTARS® Reverse Shoulder Modular Prosthesis, Implantcast®, Buxtude, Germany) because we believed this implant might reduce the risk of postoperative infection. This implant is a metal-backed glenosphere stabilized with one to four compression screws and is available in lengths from 26 to 34 mm. Only one size of glenosphere (40 mm) with a single radius of curvature is available. We prefer to implant the metal back caudal to the glenoid equator trying to obtain a 1- to 2-mm overhang under the inferior edge of the glenoid, to avoid scapular notching. No inferior tilt was performed. The humeral component was always implanted with a retroversion range from 15° to 30°, according to the version of the glenosphere to provide stability. A press-fit stem was used in young patients with primary bone tumors (10 of 22), whereas cemented stem implants were used in the other 12 patients with metastatic tumors and hematologic malignancies (considering the adjuvant effect of cement and the need of postoperative radiation therapy) and in those with poor bone quality. Soft tissue reconstruction consisted of subscapularis tendon reattachment with nonabsorbable suture on the prosthesis (eight patients) and pectoralis major tendon reattachment to the deeper layer of deltoid. Reconstruction was carefully performed to avoid excessive muscular tension and carefully testing implant stability.
Postoperative immobilization was used in all patients, with a 30° abduction brace for 4 weeks; early free mobilization of the elbow, wrist, and hand was encouraged. After 1 month, the brace was removed and patients started active mobilization with pendulum movements limited to 30° of abduction, forward flexion and extension, avoiding forced passive-assisted motion by a therapist for the first weeks, then full ROM exercises. Once patients achieved active ROM, they were allowed to perform exercises against a weight or force. Six to 7 weeks postoperatively, patients began exercises to strengthen the scapulothoracic muscles, using isometric exercise to emphasize lower trapezius and serratus anterior activation and reduce upper trapezius activation.
Routine follow-up evaluations, including a physical examination, shoulder radiography, and chest CT, were performed every 3 months in the first 3 years, every 4 months in the fourth year, every 6 months in the fifth year, and then annually. Outcome, complications and functional results were collected at the time of clinical checks and all alive patients were independently re-evaluated at final follow-up by tumor surgeons (PR, GT) and a shoulder surgeon (AC).
Oncologic results were assessed regarding local recurrence, metastases, or death (Table 1). At a mean (range) follow-up duration of 3 years (1 to 8), all patients treated for a primary bone tumor were continuously without evidence of disease (NED), while five patients treated for metastasis or myeloma were alive with disease (AWD) at a mean (range) of 38 months (25 to 73 months), and five died with disease (DWD) at a mean (range) of 22 months (12 to 46 months).
We evaluated and classified complications and prosthetic failures according to the method described by Henderson et al. [30]. Type I was classified as soft tissue failure, Type II as aseptic loosening, Type III as structural failure, Type IV as infection, and Type V as local recurrence. We analyzed implant survival with Kaplan-Meier curves, which we appraised as the time from surgery to the last clinic visit or failure.
We performed functional evaluation using three different scoring systems: the Musculoskeletal Tumor Society (MSTS) functional rating system [18], the American Shoulder and Elbow Surgeons (ASES) shoulder score [40, 46, 49], and the Constant score (CS) [13-14]. The MSTS score system is usually used to evaluate functional outcome in oncologic patients and it assigns a score from 0 to 5 to each of five categories (pain, function, emotional acceptance, hand positioning, dexterity and lifting ability); 30 is the maximum obtainable score. Then, we assessed functional results as a percentage of the maximum score; these were defined as excellent (76% to 100%), good (51% to 75%), fair (26% to 50%), poor (0 to 25%) [18]. The ASES score ranges from 0 (pseudo-paralytic shoulder) to 100 (essentially normal shoulder function) regarding pain (seven items) and activities of daily living (10 items) [40, 46, 49]. The Constant score ranges from 0 points (most disability) to 100 points (least disability) with four domains, including pain (15 points), activities of daily living (20 points), mobility (40 points), and strength (25 points) [13-14]. Postoperative results were considered as “poor” if the Constant score was less than 30 points, “fair” if the Constant score was 30 to 39, “good” if the Constant score was 40 to 59, “very good” if the Constant score was 60 to 69, and “excellent” if the Constant score was at least 70 [8]. Then we normalized the Constant score, measured according to sex and age, using a procedure described by Katolik et al. [32], and compared the normalized Constant score of the healthy side with that of the affected side (defined as the individual normalized Constant score).
The data were recorded in a Microsoft 1 Excel 2003 (Microsoft Corp, Redmond, WA, USA) spreadsheet and analyzed using Med-Calc Software Version 11.1 (MedCalc Software, Mariakerke, Belgium).
Results
Complications occurred in five of 22 patients at a mean (range) time of 4.3 months postoperatively (0.1 to 25). Implant survival to all complications was 76% at 3 and 5 years (Fig. 3). Type I complications (all dislocations) occurred in four of the 22 patients (18%) at a mean (range) time of 1 month postoperatively (0.36 to 2.27). In all four patients, dislocation was successfully treated with surgical revision: change of prosthetic spacers, liner augmentation, retroversion of a humeral component, or a combination of all three. Implant survival after Type I complications was 81% at 3 and 5 years postoperatively, Type I complications occurred in three of eight patients with humeral resection resections longer than 10 cm and one of 14 of those with resections shorter than 10 cm (Fig. 4). Type II complications occurred in one of 22 patients at 25 months after surgery. This patient had pain during active and passive movements; radiographs showed a radiolucent line around the humeral stem, and the result of a three-phase technetium-99 m bone scan showed an increased uptake around the stem. We successfully treated this patient with aseptic loosening with surgical revision by removing 2 cm of the damaged humeral diaphysis, elongating the prosthesis, and inserting a cemented stem. We did not observe any Types III, IV, or V complications. Also, we did not detect local recurrences in these patients. Implant survival in patients with major complications (aseptic loosening, breakage, and infection) was 94% at 3 and 5 years (Fig. 5). None of our patients had signs of scapular notching; however, we observed radiolucent lines, which remained stable over time, on the radiographs of three patients (one with radiolucent lines in Zone 4, one with lines in Zone 3, and one with lines in Zone 2) around the humeral stem. We did not observe radiolucent lines around the metal back of the glenoid in any patient, according to the method of Gilot et al. [24].
Fig. 3.
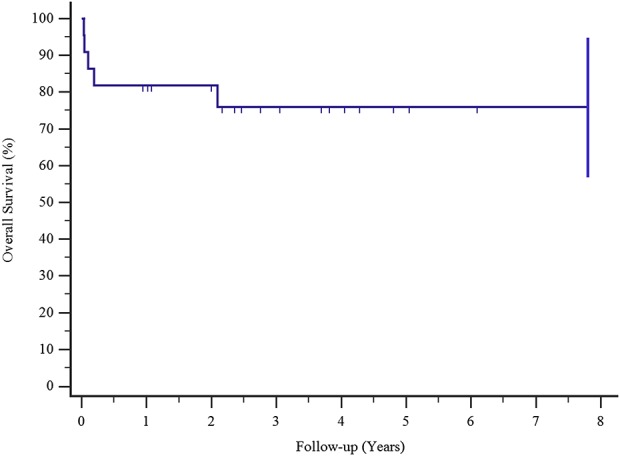
Kaplan-Meier survival curves show a 76% proportion of implant survival after all complications at 3 and 5 years.
Fig. 4.
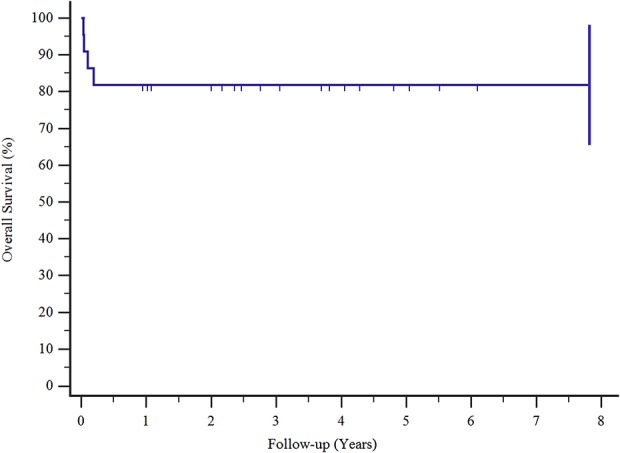
Kaplan-Meier survival curves show that the survival rates of patients with Type I complications were 81% at 3 and 5 years.
Fig. 5.
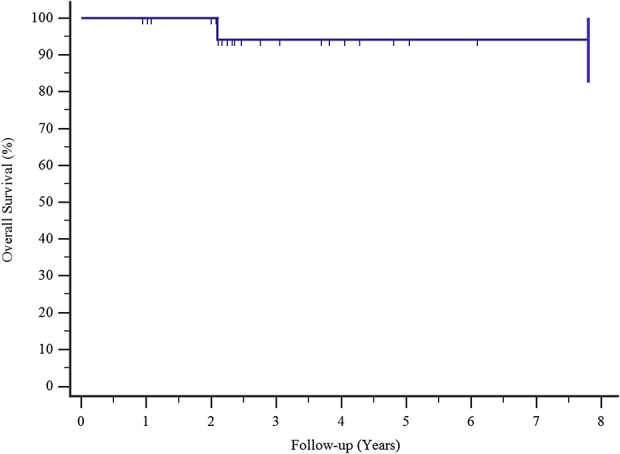
Kaplan-Meier survival curves show that the survival rate of patients with major complications (loosening, breakage, and infection) was 94% at 3 and 5 years.
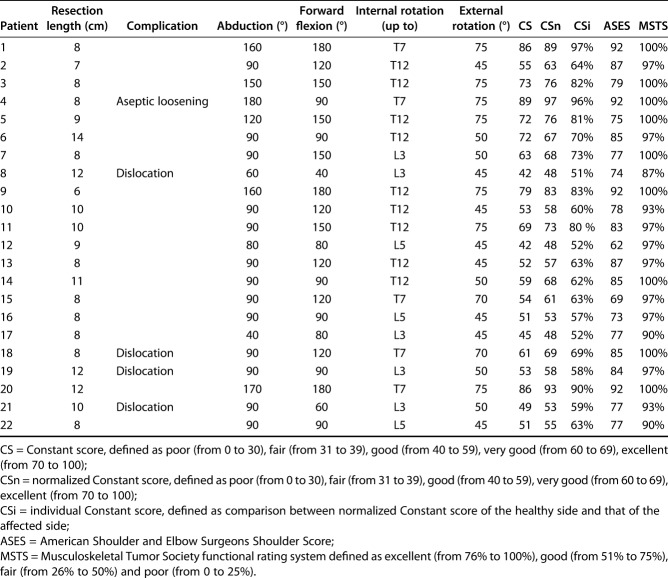
Functional outcomes were generally good with all scales, though not excellent. The mean (range) Constant score was 61 points (42 to 89), the mean (range) normalized Constant score was 66 points (48 to 97), and the mean (range) individual Constant score was 69 points (51 to 97). The mean ASES score was 81 points (62 to 92) and the mean MSTS score was 29% (26 to 30). Moreover, the active ROM was satisfactory (Table 2): the mean abduction angle was 103° (40 to 180), the mean forward flexion angle was 117° (40 to 180), the mean internal-rotation angle reached T12 (L5 to T7), and the mean external rotation angle was 58° (45 to 75). No differences related to diagnosis, gender or age were found, while scores remained satisfactory in those patients where complication occurred (Fig. 6 A-D).
Table 2.
Complications and functional results of patients who underwent reconstruction with modular reverse total shoulder arthroplasty (n = 28)
Fig. 6 A-D.
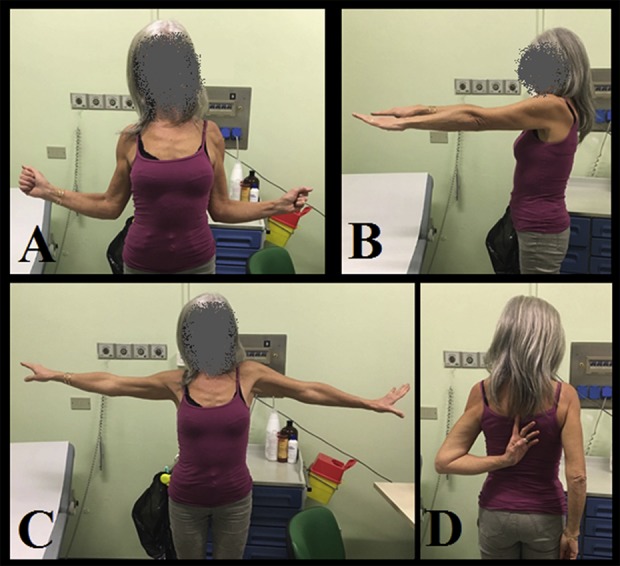
These images show a 55-year-old woman who was treated with modular RTSA after proximal humerus resection for Grade 2 chondrosarcoma. She had revision for aseptic loosening after 2 years. Her functional results at 5.5 years of follow-up were excellent with MSTS score of 30, CSn score of 97, ASES score of 92 and (A) external rotation of 75°, (B) forward flexion of 90°, (C) abduction of 90°, and (D) internal rotation that reached T7.
Discussion
Reconstruction after proximal humerus resection for tumor is still challenging, despite the different techniques proposed [1-3, 7, 9, 10, 12, 15-16, 19-23, 25, 27, 29, 31, 33-37, 39, 41-45, 47-48, 50-51, 53-56, 58-59]; all of these have comparable oncologic results in terms of survival and local recurrence rates, although most patients have poor functional results [1-3, 7, 9, 10, 12, 15-16, 19-23, 25, 27, 29, 31, 33-37, 39, 41-45, 47-48, 50-51, 53-56, 58-59]. Many of these studies included resections that were more aggressive and did not spare the deltoid muscle. After satisfactory results were obtained in patients with nononcologic conditions, RTSA was introduced as a reconstruction option after proximal humerus resection, since there are similar residual shoulder biomechanics between cuff arthropathy and some tumor resections [1, 7, 11,15,16, 20, 25, 29, 33-34, 48]. Some authors [28, 29, 36, 53] reported their experience using modular RTSA prostheses, which combine the advantages of modular prosthesis (immediate stability, early mobilization, and ready-to-use components that are adaptable to the length of resected bone) with those of inverse geometry that compensates for the absence of the rotator cuff, with satisfactory shoulder ROM; however, the data reported on these small series with a limited follow-up did not completely evaluate complications and functional outcome using dedicate shoulder score [28, 29, 36, 53]. As a result, we reviewed our experience with modular RTSA after proximal humerus resection that involved the rotator cuff but not the deltoid muscle and its innervation. In a group of 22 patients, despite a relative high incidence of dislocation, we found generally good results on the Constant and MSTS scores.
Limitations
This study had several limitations. First, this is a retrospective, nonrandomized patient series with potential selection biases. At our institution, we use the modular RTSA after proximal humerus resection only when it is possible to preserve the deltoid muscle and the axillary nerve. Avoiding this reconstruction in larger resections when the surgeon cannot spare an innervated deltoid muscle to obtain oncologically correct margins may result in functional outcomes that look better than if RTSA had been used unselectively. However, we think that if none of the abductor mechanism can be spared, the reconstruction would act only as a “spacer” to preserve function of the elbow, wrist, and hand and it would not require a more expensive option such as RTSA.
Second, we performed this reconstruction both in patients with metastatic disease who have potentially poor prognosis and in patients with primary bone tumors. This resulted in a very heterogeneous population, but this is not unexpected considering the rarity of the pathology treated. Consequently, since the patients’ diagnoses were heterogeneous, a survival analysis was precluded. However, analysis of patient survival was not an objective of our study, since difference in survival between primary and secondary tumors is not related to the type of reconstructions but with the different biological aggressiveness of the tumors. Considering good functional results and quality of life, we think that patients with metastatic disease and poor prognosis, especially those expected to survive longer than 1 year, should be also be offered the opportunity for a functional reconstructive option.
Third, we presented results only at short term; however, insofar as our key goal here was to describe early complications, we believe our follow-up duration was sufficient. Future studies will need to assess reconstructive durability. Dislocation was the most frequent complication and occurred primarily in the immediate postoperative period, but complications at longer term could not be assessed here and may yet prove to be important. Further studies are needed to assess long-term complications and function.
Complications
In our group of 22 patients, five had complications resulting in revision, and Type I failure was the most frequent. Dislocation occurred postoperatively in four of the 22 patients, and three of them were in patients treated with resections longer than 10 cm. Modular RTSA is primarily used in patients in whom it is deemed possible to preserve the axillary nerve and deltoid muscle and still achieve a wide tumor margin. We believe that surgical planning should be based on a careful assessment of the tumor extension to aim for oncologic margins and not jeopardize the patient’s survival. We sacrificed the rotator cuff and capsule, preserving an innervated deltoid in all patients and obtaining wide surgical margins; no local recurrences were observed. When a functional deltoid muscle can be spared, modular RTSA appears to be a reasonable reconstruction option. We believe the proportion of patients with complications is comparable to or possibly fewer than those previously reported using other techniques—biological reconstructions [2, 3, 9, 19, 21, 22, 23, 31, 41-43, 51, 52, 54, 56], prostheses [10, 12, 26, 27, 35, 39, 44, 50, 58, 59], allograft-prosthetic composites with standard or RTSA prosthesis [7, 11, 15, 35, 47, 48], and modular RTSA reconstructions [28, 29, 36, 53]—but we also realize that we cannot directly compare our results with those of other studies because the types of resection, diagnoses, and indications for the procedure differ. Biological reconstructions such as arthrodesis [41, 43, 56], vascularized fibular grafts [3, 21, 31, 51, 52], clavicle pro humerus [2, 9], and osteoarticular allografts [19, 22, 23, 42, 54] were reported to have a high incidence of complications ranging from 30% to more than 50% [2, 3, 9, 16, 19, 21-23, 31, 35, 39, 41-44, 51, 54, 56, 58], mainly represented by infection, fracture, nonunion or incomplete integration of the graft. Standard mega-prosthetic reconstructions may have a lower incidence of complications (ranging from 17.5% to 30%); dislocation was the most frequent, occurring in nearly 26% of patients, followed by infection [10, 12, 27, 35, 39, 44, 50, 58, 59]. Allograft-prosthesis composites with standard [1, 44, 55] or RTSA prostheses [7, 11, 15, 35, 47, 48] are reported to have an incidence of complications ranging from 12% to 44%; the most frequent are graft-related complications (nonunion and fracture), aseptic loosening, infection, and dislocation [1, 7, 11, 15, 35, 44, 48, 55]. This proportion of complications may not be different from those after a modular RTSA prosthesis, but the types of complications may be different. Dislocation is the most frequent complication in our patients (Table 3), occurring in the early postoperative period and with a reported incidence of up to 22% [25, 32, 46], probably due to differences in restoration of soft tissue tension or a surgical technique. The main reason for dislocation in our series was insufficient humeral component retroversion or inadequate deltoid tension, associated with postoperative hematomas, especially in resections longer than 10 cm. When the deltoid is detached near the native humerus, as occurs in longer resections, it may be difficult to reattach the insertion and achieve the same tension as would occur in shorted resections where the deltoid attachment is preserved. More patients will be needed to confirm this supposition. In patients with dislocations, surgical revision was usually easy and successful, performed with modified prosthetic spacers, liner augmentation, or retroversion of a humeral component. Infection did not occur in our series; we believe that this is related to the use of silver-coating, but more patients will need to be treated to confirm this observation.
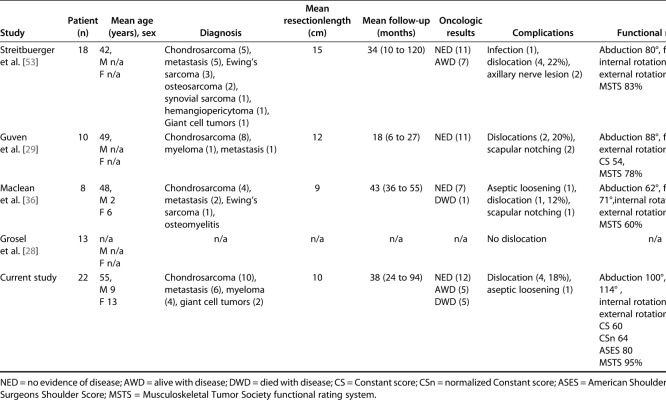
Table 3.
Summary of the published studies reporting on modular RTSA reconstructions after proximal humerus resection: systematic review
Outcomes Scores and Range of Motion
Patients in this small series achieved outcomes scores (Constant, ASES, and MSTS scores) that generally were good to very good, but usually were not excellent, which is consistent with what one might expect in patients undergoing proximal humerus resections that also sacrificed the rotator cuff. These results may be defined as good to very good for a complex reconstruction procedure such as modular RTSA, according to Booker et al. [8] classification; however, we were able to preserve an innervated deltoid in all our patients, making our population a selected group. Although these appear to be reasonable functional results, the shoulder function of our patients was by no means normal. These results may be superior to the results of other reports in terms of ROM [1-3, 7, 9, 10, 12, 15-16, 19-23, 25, 27, 29, 31, 33-37, 39, 41-45, 47-48, 50-51, 53-56, 58-59], but in the absence of head-to-head comparisons, it is impossible to be certain; moreover, resection in our patients retained the deltoid, which may not have been the case in other reports. No reports have directly compared this prosthesis to other options when patients have this type of deltoid-sparing resection. The reported functional outcome of the restored shoulder joint remains poor [10, 12, 26, 35, 39, 44, 50, 58, 59], and in most reports, the prosthesis works only as a “spacer,” preserving the function of the elbow, wrist, and hand, but not active shoulder motion. In the rare instances in which it is possible to preserve an innervated deltoid and the rotator cuff, the best functional results (MSTS score ranging from 70% to 82%) were obtained with allograft-prosthesis composites [44, 47, 48]. This reconstructive procedure combines the advantages of biological reconstruction (better anchorage of the soft tissues) with those of prosthetic reconstruction [1, 20, 25, 34, 47]. Owing to the reported excellent functional results and low incidence of complications in patients with RTSA in a nononcologic setting, inverse geometry has also been introduced after proximal humerus resection when an innervated deltoid is spared [28, 29, 36, 53]. Initially, non-modular RTSA was combined with allografts (RTSA-allograft-prosthesis composites). Functional results were satisfactory, with a reported mean Constant score of nearly 76, mean MSTS of nearly 67%, mean abduction angle of nearly 157°, mean flexion angle of nearly 122°, and external rotation ranging from 8° to 31° [7, 11, 15, 35, 48]. Successively, to obtain satisfactory functional results and avoid graft-related complications, modular RTSA was introduced. All previous studies on modular RTSA [28, 29, 36, 53] (Table 3) reported satisfactory functional results according to MSTS score (mean 74%; range 43% to 97%), despite a wide variation of ROM value and depending on whether or not the deltoid muscle and axillary nerve were totally or partially preserved. In our study, the functional results appear comparable or possibly better, and this is likely due to the fact that we were able to preserve an innervated deltoid muscle in all patients. Moreover, since the MSTS score usually overestimates functional results because the system is not selective enough and it does not measure meaningful differences in patients, we decided to add evaluations of functional outcomes with specific shoulder scores in patients undergoing standard prosthetic reconstruction (the Constant score and the ASES score), giving a more objective assessment.
Conclusions
Modular RTSA appears to be a reasonable reconstruction option after proximal humerus resections if an innervated deltoid muscle is spared. Inverse geometry allows for good function and modularity provides the possibility to restore bone loss after resection. We cannot conclude that an RTSA prosthesis is superior to other reconstruction options, but our promising preliminary results indicate the need for a study that will directly compare this procedure with other techniques for the same type of resection. We believe that RTSA may offer some advantages, and because of these, it deserves further investigation.
Acknowledgments
None.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abdeen A, Hoang BH, Athanasian EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91:2406-2015. [DOI] [PubMed] [Google Scholar]
- 2.Barbier D, De Billy B, Gicquel P, Bourelle S, Journeau P. Is the clavicula pro humero technique of value for reconstruction after resection of the proximal humerus in children? Clin Orthop Relat Res. 2017;475:2550-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilgin SS. Reconstruction of proximal humeral defects with shoulder arthrodesis using free vascularized fibular graft. J Bone Joint Surg Am. 2012;94:e94. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P, Trojani C, Walch G, Krishnan SG, Romeo A, Sinnerton R. Shoulder arthroplasty for the treatment of the sequelae of fractures of the proximal humerus. J Shoulder Elbow Surg. 2001;10:299-308. [DOI] [PubMed] [Google Scholar]
- 5.Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14:147S-161S. [DOI] [PubMed] [Google Scholar]
- 6.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527-540. [DOI] [PubMed] [Google Scholar]
- 7.Bonnevialle N, Mansat P, Lebon J, Laffosse JM, Bonnevialle P. Reverse shoulder arthroplasty for malignant tumors of proximal humerus. J Shoulder Elbow Surg. 2015;24:36-44. [DOI] [PubMed] [Google Scholar]
- 8.Booker S, Alfahad N, Scott M, Gooding B, Wallace WA. Use of scoring systems for assessing and reporting the outcome results from shoulder surgery and arthroplasty. World J Orthop. 2015;6:244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvert GT, Wright J, Agarwal J, Jones KB, Randall RL. Is claviculo pro humeri of value for limb salvage of pediatric proximal humerus sarcomas? Clin Orthop Relat Res. 2015;473:877-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon CP, GU Paraliticci, Lin PP, Lewis VO, Yasko AW. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg. 2009;18:705-710. [DOI] [PubMed] [Google Scholar]
- 11.Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91:119-127. [DOI] [PubMed] [Google Scholar]
- 12.Chen CM, Wu PK, Tsai SW, Chen CF, Chen WM. Prognosis-based shoulder hemiarthroplasty after resection of proximal humeral malignancy. Artif Organs. 2017;41:1162-1172. [DOI] [PubMed] [Google Scholar]
- 13.Constant CR, Gerber C, Emery RJ, Søjbjerg JO, Gohike F, Boileau P. A review of the Constant score: modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17:355-361. [DOI] [PubMed] [Google Scholar]
- 14.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160-164. [PubMed] [Google Scholar]
- 15.De Wilde L, Boileau P, Van der Bracht H. Does reverse shoulder arthroplasty for tumors of the proximal humerus reduce impairment? Clin Orthop Relat Res. 2011;469:2489-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubina A, Shiu B, Gilotra M, Hasan SA, Lerman D, Ng VY. What is the optimal reconstruction option after the resection of proximal humeral tumors? A systematic review. Open Orthop J . 2017;11:203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enneking WF. A system of staging musculoskeletal neoplasm. Clin Orthop Relat Res. 1986;204:9-24. [PubMed] [Google Scholar]
- 18.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;241-246. [PubMed] [Google Scholar]
- 19.Farfalli GL, Ayerza MA, Muscolo L D., Aponte-Tinao LA. Proximal humeral osteoarticular allografts: technique, pearls and pitfalls, outcomes. Curr Rev Musculoskelet Med. 2015;8:334-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautam D, Malhotra R. Megaprosthesis versus allograft prosthesis composite for massive skeletal defects. J Clin Orthop Trauma. 2018;9:63-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebert C, Hillmann A, Schwappach A, Hoffmann Ch, Hardes J, Kleinheinz J, Gosheger G. Free vascularized fibular grafting for reconstruction after tumor resection in the upper extremity. J Surg Oncol. 2006;94:114-127. [DOI] [PubMed] [Google Scholar]
- 22.Gebhardt MC, Roth YF, Mankin HJ. Osteoarticular allografts for reconstruction in the proximal part of the humerus after excision of a musculoskeletal tumor. J Bone Joint Surg Am. 1990;72:334-345. [PubMed] [Google Scholar]
- 23.Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81:1138-1146. [DOI] [PubMed] [Google Scholar]
- 24.Gilot G, Alvarez-Pinzon AM, Wright TW, Flurin PH, Krill M, Routman HD, Zuckerman JD. The incidence of radiographic aseptic loosening of the humeral component in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:1555-1559. [DOI] [PubMed] [Google Scholar]
- 25.Gitelis S, Piasecki P. Allograft prosthetic composite arthroplasty for osteosarcoma and other aggressive bone tumors. Clin Orthop Relat Res. 1991;270:197-201. [PubMed] [Google Scholar]
- 26.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65-68. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths D, Gikas PD, Jowett C, Bayliss L, Aston W, Skinner J, Cannon S, Blunn G, Briggs TW, Pollock R. Proximal humeral replacement using a fixed-fulcrum endoprosthesis. J Bone Joint Surg Br. 2011;93:399-403. [DOI] [PubMed] [Google Scholar]
- 28.Grosel TW, Plummer DR, Mayerson JL, Scharschmidt TJ, Barlow JD. Oncologic reconstruction of the proximal humerus with a reverse total shoulder arthroplasty megaprosthesis. J Surg Oncol. 2018;118:867-872. [DOI] [PubMed] [Google Scholar]
- 29.Guven MF, Aslan L, Botanlioglu H, Kaynak G, Kesmezacar H, Babacan M. Functional outcome of reverse shoulder tumor prosthesis in the treatment of proximal humerus tumors. J Shoulder Elbow Surg. 2016;25:e1-6. [DOI] [PubMed] [Google Scholar]
- 30.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418-429. [DOI] [PubMed] [Google Scholar]
- 31.Kassab M, Dumaine V, Babinet A, Ouaknine M, Tomeno B, Anract P. Twenty nine shoulder reconstructions after resection of the proximal humerus for neoplasm with mean 7-year follow-up. [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:15-23. [DOI] [PubMed] [Google Scholar]
- 32.Katolik LI, Romeo AA, Cole BJ, Verma NN, Hayden JK, Bach BR. Normalization of the Constant score. J Shoulder Elbow Surg. 2005;14:279-285. [DOI] [PubMed] [Google Scholar]
- 33.King JJ, Nystrom LM, Reimer NB, Gibbs CP, Jr, Scarborough MT, Wright TW. Allograft-prosthetic composite reverse total shoulder arthroplasty for reconstruction of proximal humerus tumor resections. J Shoulder Elbow Surg. 2016;25:45-54. [DOI] [PubMed] [Google Scholar]
- 34.Lazerges C, Dagneaux L, Degeorge B, Tardy N, Coulet B, Chammas M. Composite reverse shoulder arthroplasty can provide good function and quality of life in cases of malignant tumour of the proximal humerus. Int Orthop. 2017;41:2619-2625. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Zhang Q, Guo X, Zhang X, Li Z, Li X. Treatment and outcome of malignant bone tumors of the proximal humerus: biological versus endoprosthetic reconstruction. BMC Musculoskelet Disord. 2014;7:15:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maclean S, Malik SS, Evans S, Gregory J, Jeys L. Reverse shoulder endoprosthesis for pathologic lesions of the proximal humerus: a minimum 3-year follow-up. J Shoulder Elbow Surg. 2017;26:1990-1994. [DOI] [PubMed] [Google Scholar]
- 37.Malawer MM, Meller I, Dunham WK. A new surgical classification system for shoulder-girdle resections. Analysis of 38 patients. Clin Orthop Relat Res. 1991;267:33-44. [PubMed] [Google Scholar]
- 38.Marulanda GA, Henderson E, Cheong D, Letson GD. Proximal and total humerus reconstruction with the use of an aortograft mesh. Clin Orthop Relat Res. 2010;468:2896-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meijer ST, Paulino Pereira NR, Nota SPFT, Ferrone ML, Schwab JH, Lozano Calderón SA. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J Shoulder Elbow Surg. 2017;26:931-938. [DOI] [PubMed] [Google Scholar]
- 40.Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg . 2002;11:587-594. [DOI] [PubMed] [Google Scholar]
- 41.Mimata Y, Nishida J, Sato K, Suzuki Y, Doita M. Glenohumeral arthrodesis for malignant tumor of the shoulder girdle. J Shoulder Elbow Surg. 2015;24:174-178. [DOI] [PubMed] [Google Scholar]
- 42.Muscolo DL, Ayerza M, Aponte-Tinao LA. Massive allograft use in orthopedic oncology. Orthop Clin North Am. 2006;37:65-74. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor MI, Sim FH, Chao EY. Limb salvage for neoplasms of the shoulder girdle: intermediate reconstructive and function results. J Bone Joint Surg Am. 1996;78:1872-1888. [DOI] [PubMed] [Google Scholar]
- 44.Potter BK, Adams SC, Pitcher JD, Jr, Malinin TI, Temple HT. Proximal humerus reconstructions for tumors. Clin Orthop Relat Res. 2009;467:1035-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raiss P, Kinkel S, Sauter U, Bruckner T, Lehner B. Replacement of the proximal humerus with MUTARS tumor endoprostheses. Eur J Surg Oncol. 2010;36:371-377. [DOI] [PubMed] [Google Scholar]
- 46.Richards RR, An KN, LU Bigliani, Friedman RJ, Gartsman GM, Gristina AG, Iannotti JP, Mow VC, Sidles JA, Zuckerman JD. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg . 1994;3:347-352. [DOI] [PubMed] [Google Scholar]
- 47.Ruggieri P, Mavrogenis AF, Guerra G, Mercuri M. Preliminary results after reconstruction of bony defects of the proximal humerus with an allograft-resurfacing composite. J Bone Joint Surg Br. 2011;93:1098-1103. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Sotelo J, Wagner ER, Sim FH, Houdek MT. Allograft-prosthetic composite reconstruction for massive proximal humeral bone loss in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2017;99:2069-2076. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt S, Ferrer M, González M, González N, Valderas JM, Alonso J, Escobar A, Vrotsou K. Evaluation of shoulder-specific patient-reported outcome measures: a systematic and standardized comparison of available evidence. J Shoulder Elbow Surg . 2014;23:434-444. [DOI] [PubMed] [Google Scholar]
- 50.Schmolders J, Koob S, Schepers P, Kehrer M, Frey SP, Wirtz DC, Pennekamp PH, Strauss AC. Silver-coated endoprosthetic replacement of the proximal humerus in case of tumour-Is there an increased risk of periprosthetic infection by using a trevira tube? Int Orthop. 2017;41:423-428. [DOI] [PubMed] [Google Scholar]
- 51.Shammas RL, Avashia YJ, Farjat AE, Catanzano AA, Levin LS, Eward WC, Brigman BE, Erdmann D. Vascularized fibula-based physis transfer: a follow-up study of longitudinal bone growth and complications. Plast Reconstr Surg Glob Open. 2017;5:e1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevenson JD, Doxey R, Abudu A, Parry M, Evans S, Peart F, Jeys L. Vascularized fibular epiphyseal transfer for proximal humeral reconstruction in children with a primary sarcoma of bone. Bone Joint J. 2018;100:535-541. [DOI] [PubMed] [Google Scholar]
- 53.Streitbuerger A, Henrichs M, Gosheger G, Ahrens H, Nottrott M, Guder W, Dieckmann R, Hardes J. Improvement of the shoulder function after large segment resection of the proximal humerus with the use of an inverse tumour prosthesis. Int Orthop. 2015;39:355-61. [DOI] [PubMed] [Google Scholar]
- 54.Teunis T, Nota SP, Hornicek FJ, Schwab JH, Lozano-Calderón SA. Outcome after reconstruction of the proximal humerus for tumor resection: a systematic review. Clin Orthop Relat Res. 2014;472:2245-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van de Sande MA, Dijkstra PD, Taminiau AH. Proximal humerus reconstruction after tumour resection: biological versus endoprosthetic reconstruction. Int Orthop. 2011;35:1375-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viehweger E, Gonzalez JF, Launay F, Legre R, Jouve JL, Bollini G. Shoulder arthrodesis with vascularized fibular graft after tumor resection of the proximal humerus. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:523-529. [DOI] [PubMed] [Google Scholar]
- 57.Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clin Orthop Relat Res. 2011;469:2440-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang B, Wu Q, Liu J, Yang S, Shao Z. Endoprosthetic reconstruction of the proximal humerus after tumour resection with polypropylene mesh. Int Orthop. 2015;39:501-506. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Guo Z, Li J, Li XD, Sang HX. Functional outcomes and complications of reconstruction of the proximal humerus after intra-articular tumor resection. Orthop Surg. 2010;2:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]