Abstract
Background
We attempted to resect peripheral chondrosarcoma of the pelvis with clear margins. Because of the proximity of vessels or organs, there is still concern that narrow surgical margins may have an adverse effect on disease outcomes. Although current guidelines recommend resection of histologic Grade II or Grade III chondrosarcomas with a “wide” margin, there are no specific recommendations for the adequate width of a surgical margin.
Questions/purposes
(1) What is the disease-specific and local recurrence-free survival of patients with peripheral chondrosarcoma of the pelvis treated with resection or amputation? (2) Is the width of a surgical margin associated with the outcome of disease in patients with peripheral chondrosarcoma of the pelvis? (3) Does the histologic grade as determined with a preoperative biopsy correlate with the final grade after resection? (4) What are surgical complications in these patients?
Methods
We retrospectively reviewed records from three international collaborating hospitals. Between 1983 and 2017, we resected 262 pelvic chondrosarcomas of all types. After reviewing the pathologic reports of these patients, we included 52 patients with peripheral chondrosarcomas of the pelvis who had an osteochondroma-like lesion at the base of the tumor and a cartilage cap with malignant cells in resected specimens. To be eligible for this study, a patient had to have a minimum of 1 year of follow-up. Two patients were excluded because they had less than 1 year of follow-up, leaving 50 patients for inclusion in this study. The median follow-up duration was 7.0 years (interquartile range 2.1-10 years). The median age was 37 years (IQR 29-54 years). The ilium was the most frequently affected bone (in 36 of 50 patients; 72%). The histologic status of the surgical margin was defined as microscopically positive (0 mm), negative < 1 mm, or negative ≥ 1 mm. Thirteen of the 50 patients (26%) had local recurrence. Seven of 34 patients had Grade I tumors, five of 13 had Grade II tumors, and one of three had a Grade III tumor. Nine of 16 patients had multiple local recurrences. Two patients with Grade I tumors and two with Grade II tumors died because of pressure effects caused by local recurrence.
Results
The 10-year disease-specific and local recurrence-free survival rates were 90% (95% confidence interval, 70-97) and 69% (95% CI, 52-81), respectively. A surgical margin ≥ 1 mm (n = 16) was associated with a better local recurrence-free survival rate than a surgical margin < 1 mm (n = 17) or 0 mm (n = 11) (10-year local recurrence-free survival: resection margin ≥ 1 mm = 100% versus < 1 mm = 52% [95% CI, 31 to 70]; p = 0.008). No patients with a surgical margin ≥ 1 mm had local recurrence, metastasis, or disease-related death, irrespective of tumor grade. Patients with local recurrence (n = 13) showed worse disease-specific survival than those without local recurrence (n = 37) (10-year disease-specific survival: local recurrence [+] = 59% [95% CI, 16 to 86] versus local recurrence [-] = 100%; p=0.001]). The preoperative biopsy results correctly determined the tumor grade in 15 of 41 patients (37%). The most frequent complication after surgery was local recurrence (13 of 50 patients, 26%). Deep infection was the most frequent nononcologic complication (four patients).
Conclusions
We found a high local recurrence rate after surgical treatment of a peripheral pelvic chondrosarcoma, which was related to the width of the surgical margin. These local recurrences led to inoperable recurrent tumors and death. The tumor grade as determined by preoperative biopsy was inaccurate in 2/3 of patients compared with the final histologic assessment. Therefore, we believe every attempt should be made to achieve a negative margin during the initial resection to lessen the likelihood of local recurrence of peripheral chondrosarcoma of the pelvis of all grades. A margin of 1 mm or more appeared to be sufficient in these patients.
Level of Evidence
Level III, therapeutic study.
Introduction
The most frequently affected site of peripheral chondrosarcoma is the pelvis [1, 9]. Surgery of peripheral chondrosarcoma of the pelvis is challenging because of the exophytic nature of tumor growth, poor compartmentalization, and the proximity of major vessels and organs. Current guidelines recommend resection of Grade II or III chondrosarcomas with “wide” surgical margins [3]. However, there is no specific recommendation for the width of the margin, nor is there an exact definition of a negative margin. Moreover, a previous report showed that patients with peripheral pelvic chondrosarcomas had good overall survival and suggested that intralesional margins in histologic Grade I tumors may be acceptable [5], which is in contrast to the recommended wide margins for primary central pelvic chondrosarcoma of any grade [2]. Histologic data on the surgical margin should be evaluated to determine what margin thickness is necessary to reduce local recurrence after resection, and these data might be useful for preoperative counseling of patients, planning surgical resections, and determining which patients are at the most risk of recurrence postoperatively.
There is still a debate regarding the survival outcomes and treatment of peripheral chondrosarcoma of the pelvis; no previous studies that we know of have focused on this subgroup of pelvic chondrosarcomas. Previous studies have combined the results of different subtypes of pelvic chondrosarcoma, such as the primary central type [4, 5, 10, 13, 15]. Furthermore, there is no consensus about the role of preoperative biopsy in pelvic peripheral chondrosarcoma because determining the tumor grade with small specimens is difficult.
We therefore asked the following questions: (1) What is the disease-specific survival (DSS) and local recurrence-free survival (LRFS) of patients with peripheral chondrosarcoma of the pelvis treated with resection or amputation? (2) Is the width of a surgical margin associated with the outcome of disease in patients with peripheral chondrosarcoma of the pelvis? (3) Does the histologic grade as determined with a preoperative biopsy correlate with the final grade after resection? (4) What are surgical complications in these patients?
Patients and Methods
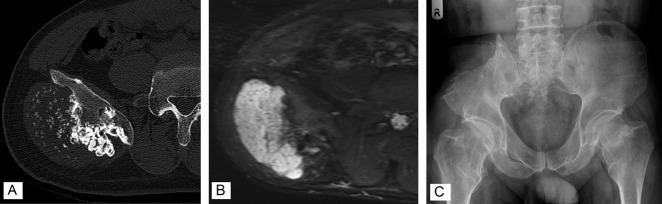
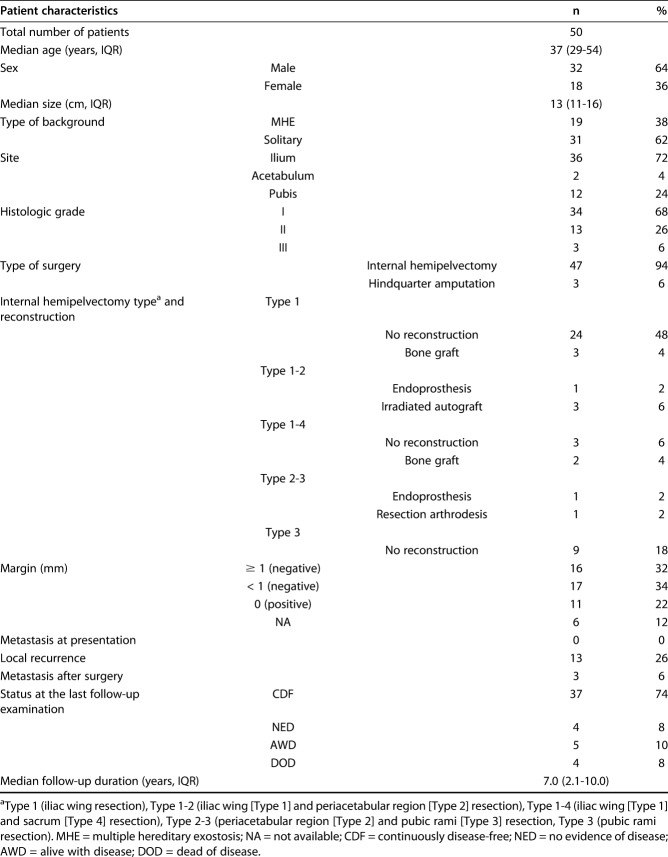
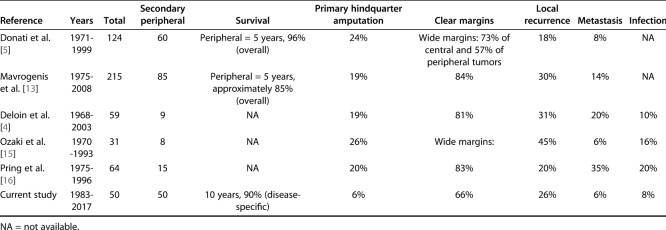
We retrospectively reviewed records and collected data from three international collaborative hospitals. Between 1983 and 2017, 262 patients with chondrosarcoma of the pelvis of any type underwent tumor resection. After we reviewed the pathologic reports of these patients, we included 52 patients with peripheral chondrosarcoma of the pelvis who had an osteochondroma-like lesion at the base of the tumor and a cartilage cap with malignant cells in resected specimens. Eligibility criteria included a minimum of 1 year of follow-up; consequently, two patients with follow-up of less than 1 year were excluded. The histologic diagnosis and treatment plan were made in a multidisciplinary discussion in each hospital after a review of histopathology and radiology reports (Fig. 1A-C). Histologic grades were determined based on cellularity, nuclear size, and the presence of an abundant hyaline cartilage matrix or mucomyxoidmatrix and mitoses [8, 12]. We collected the data of grades or surgical margins from pathologic reports. If there were no data in pathologic reports, we reevaluated histology slides. All tumors were resected to achieve wide surgical margins.
Fig. 1.
A 36-year-old man had a histologic Grade II peripheral chondrosarcoma. (A) A preoperative CT image shows a bone tumor arising from the right ilium. (B) MRI shows a 5.0-cm-thick cartilage cap. (C) This radiograph was taken postoperatively. The tumor and attached iliac wing were resected.
Patients’ Demographics
The median follow-up duration was 7.0 years (interquartile range 2.1 to 10 years). A clear description of symptoms at the initial presentation was available for 42 patients; these symptoms were pain in 12 patients (29%) and a lump or swelling in 30 (71%). Of these 30 patients, 21 described a lump that was increasing in size. The ilium was the most commonly involved bone (36 of 50 patients; 72%) (Table 1). The histologic grades were Grade I in 34 of 50 patients (68%), Grade II in 13 (26%), and Grade III in three (6%). Nineteen of 50 patients (38%) had an underlying diagnosis of multiple hereditary exostosis; tumors were Grade I in 12 of these 19 patients, Grade II in six, and Grade III in one. The largest median tumor dimension was 13 cm (IQR 11-16 cm).
Table 1.
Patients’ demographics
The primary treatment was internal hemipelvectomy in 47 of the 50 total patients (94%) and hindquarter amputation in three. The types of internal hemipelvectomy were Type 1 (iliac wing resection) in 27 of 50 patients (52%), Types 1 and 2 (iliac wing [Type 1] and periacetabular region [Type 2] resection) in four, Types 1 and 4 (iliac wing [Type 1] and sacrum [Type 4] resection) in five, Types 2 and 3 (periacetabular region [Type 2] and pubic rami [Type 3] resection) in two, and Type 3 (pubic rami resection) in nine. Ten of 47 patients who underwent hemipelvectomy (21%) underwent reconstruction, including five with a fibula autograft, two with metallic implants (a coned hemipelvis prosthesis in one and a custom-made endoprosthesis in the other [both from Stryker Stanmore, Elstree, UK]), and three with excision and extracorporeal irradiation as well as reimplantation of the resected bone segment. No patients received chemotherapy. Of 13 patients with local recurrence, one patient underwent palliative radiotherapy to control local recurrence of a Grade II tumor after resection of five previous local recurrences.
MRI findings before surgery were available for 32 patients; some images had been destroyed because of the length of the study period. The median cartilage cap for all tumors was 6 cm (IQR 4 to 10 cm; range 2 to 27 cm), as seen on MRI.
All patients were observed according to a standard protocol, with radiographs of the chest and pelvis at 3-month intervals for 2 years postoperatively, 6-month intervals from 2 to 5 years postoperatively, and annually thereafter to 10 years postoperatively [3]. After 10 years, we followed patients at 2-year intervals to monitor their recovery. Chest CT images were obtained at that point if the chest radiograph showed a nodule.
Primary and Secondary Study Outcomes
Our primary study outcome of interest was DSS and LRFS after surgical resection. Any suspected local recurrence was assessed by MRI and histologically confirmed with a biopsy.
Our secondary study outcomes were the rates of disease-specific death, metastasis, and local recurrence, stratified by histologically defined surgical margins. Surgical margin was measured on histologic slides in millimeters from the resection surface to the nearest viable tumor [11, 19]. The histologic status of the surgical margin was defined as microscopically positive (0 mm), negative < 1 mm, and negative ≥ 1 mm. Because Enneking et al.’s [7] definition of marginal and wide excision is inherently subjective and may vary depending on who is assessing the margin [20], we assessed the width of the surgical margin in millimeters for this study.
Statistical Analysis
Kaplan-Meier curves were used to estimate DSS, metastasis-free survival (MFS), and LRFS. DSS was defined as the time from diagnosis to disease-related death and was censored at the date of the latest follow-up examination or death of other causes. LRFS and MFS were defined as the time from the surgical procedure to local recurrence or metastasis, respectively, and were censored at the date of the latest follow-up visit or death. A log-rank test was used to compare the survival distributions. A two-tailed p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Oncologic Outcomes
The 10-year DSS rate was 90% (95% CI, 70 to 97) (Fig. 2A). The 10-year LRFS rate was 69% (95% CI, 52 to 81) (Fig. 2B). At the time of the latest follow-up examination, 46 of 50 patients (92%) were alive; 37 of 50 (74%) continuously had no evidence of disease, four had no evidence of disease after treatment of local recurrence, and five were alive with disease. Four patients died of local pressure effects from a local, recurrent tumor during the follow-up period. Three of these four patients had lung metastases with fewer than five nodules. Two of 34 patients with Grade I tumors and two of 13 patients with Grade II tumors had risks of disease-related death. Thirteen of 50 patients (26%) had local recurrence; seven of 34 patients had histologic Grade I tumors, five of 13 had Grade II tumors, and one of three had a Grade III tumor. Four recurrent tumors in these 13 patients were of a higher grade than the original tumor. Recurrent lesions were diagnosed after a median of 1.8 years (IQR 0.8-2.7 years) after surgical resection of the index tumor; eight were diagnosed within 2 years, 12 were diagnosed within 5 years, and a single patient had local recurrence after 7 years. Nine of these 13 patients had multiple local recurrences (mean of three recurrences; range one to five). Disease was controlled in only four of the 13 patients at the final follow-up examination. Patients with local recurrence (n = 13) showed worse DSS than those without local recurrence (n = 37) (10-year DSS, local recurrence [+] = 59% [95% CI, 16-86] versus local recurrence [-] = 100%; p = 0.001) (Fig. 3). The 10-year MFS rate was 91% (95% CI, 74 to 97). Lung metastases were diagnosed in three patients after a median of 5.0 years (range 0.5 to 7.5 years) after definitive surgery. One of the 34 patients with Grade I tumors and two of the 13 patients with Grade II tumors had metastases. All metastases developed after resection of local recurrences.
Fig. 2.
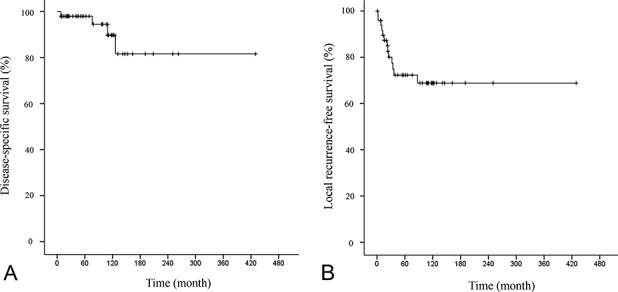
This figure shows Kaplan-Meier curves of (A) disease-specific survival and (B) local recurrence-free survival in all patients.
Fig. 3.
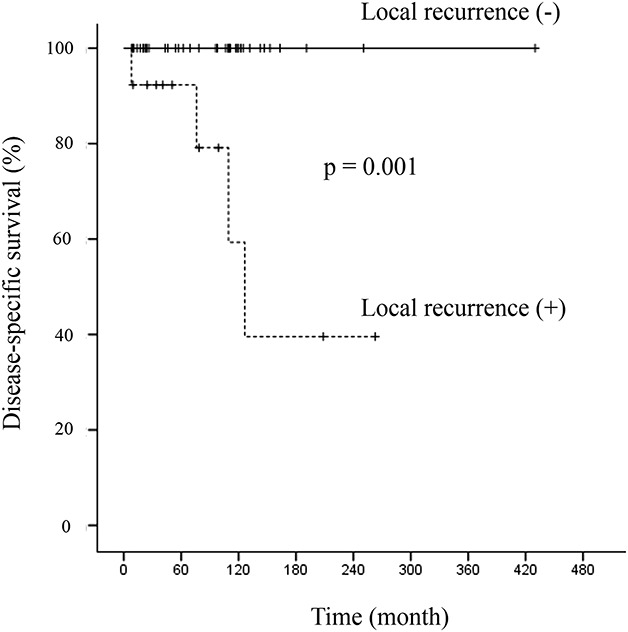
This figure shows a Kaplan-Meier curve of disease-specific survival, stratified by the occurrence of local recurrence.
The Association Between the Width of Surgical Margins and Disease Outcomes
No patients, including those with histologic Grade II or Grade III tumors who underwent resection with a ≥ 1 mm margin, had local recurrence, metastasis, or disease-related death (Table 2). A surgical margin ≥ 1 mm (n = 16) was associated with a better LRFS rate than a surgical margin < 1 mm (n = 28) (10-year LRFS, resection margin ≥ 1 mm = 100% versus ≤ 1 mm = 52% [95% CI, 31-70]; p = 0.008) (Fig. 4). Information on surgical margins was not obtained for six patients because we could not evaluate the surgical margin due to reimplantation of an irradiated autograft (n = 3) or because pathologic slides were lost due to a long study period (n = 3).
Table 2.
The relationship between surgical margin and oncological outcomes
Fig. 4.
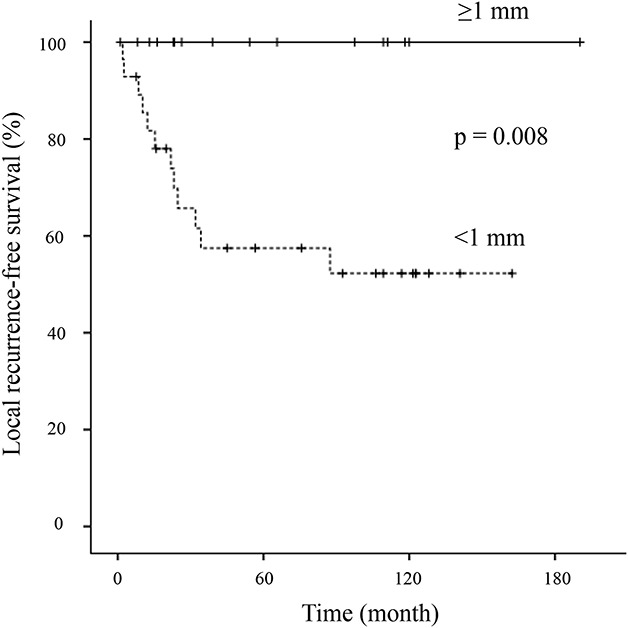
This image shows Kaplan-Meier curves of local recurrence-free survival, stratified by tumor margins.
Preoperative Biopsies
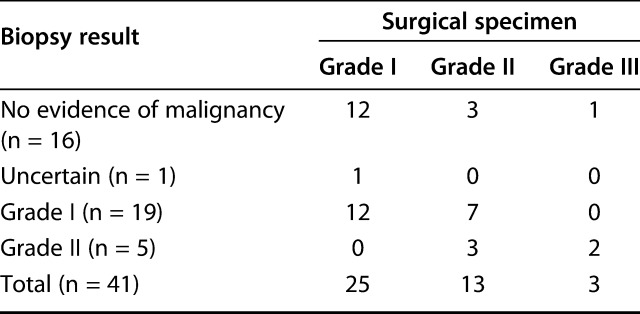
Pathologic reports of biopsy samples and surgical excision specimens were available for 41 patients; the data for the other nine patients were lost because of the long study period. In 37 patients (90%), a needle biopsy with or without imaging guidance (radiography or CT) was conducted. The biopsy specimen and resected tumor were evaluated by the same pathologists. The preoperative biopsy results confirmed the presence of a cartilage tumor in all patients. However, a malignancy was present in 24 patients (59%). The biopsy results correctly predicted the final histologic grade as noted with a resection specimen in only 15 of 41 patients (37%) (Table 3). Among these 41 patients, 26 biopsies underestimated the final histologic grade. Furthermore, only five of 16 patients with high-grade tumors determined on full surgical resection specimens (Grade II or III) had a high-grade tumor at the initial biopsy. Of 19 patients with a Grade I tumor determined with biopsy, grades from resected specimens were Grade I in 12 and Grade II in seven. Of five patients with Grade II tumors determined with biopsy, grades from resected specimens were Grade II in three and Grade III in two.
Table 3.
Concordance of histologic grade between biopsy and resection specimens
Surgical Complications
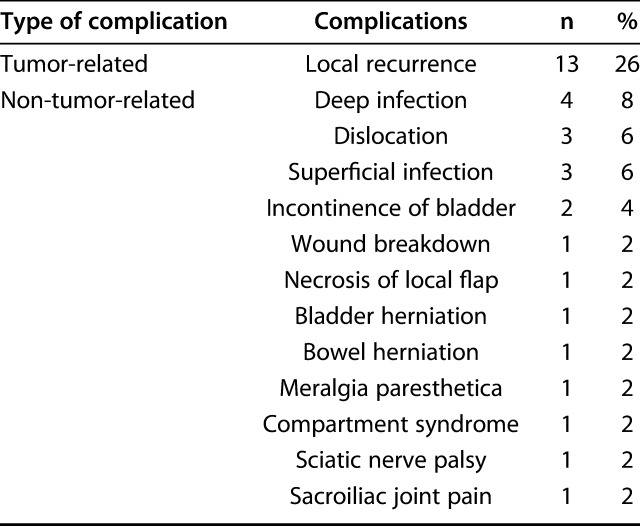
The most common complication after surgery was local recurrence (13 of 50 patients, 26%) (Table 4). Deep infection occurred in four of the 50 patients (8%). One of these four patients underwent extracorporeal irradiation and reimplantation, while the other patients underwent excision without reconstruction. Other complications were hip dislocation in three, bladder incontinence in two, wound breakdown in one, necrosis of a local flap in one, bladder herniation in one, bowel herniation in one, meralgia paresthesia in one, compartment syndrome in one, sciatic nerve palsy in one, and severe sacroiliac pain in one. Eighteen of 50 patients (36%) underwent a further operation. The indications for reoperation were local recurrence (12 of 18 patients), deep infection (four patients), and reconstruction-related complications (one patient). Two patients underwent a secondary hindquarter amputation because of local recurrence in one patient and infection in the other. However, the latter patient had local recurrence after undergoing hindquarter amputation and died of sarcoma. Three patients underwent hindquarter amputation as their initial surgery. Limb salvage was achieved in 45 of 50 patients (90%) after all procedures.
Table 4.
Evaluation of complications (n = 50)
Discussion
Surgical treatment of a peripheral chondrosarcoma of the pelvis is challenging because of the exophytic nature of growth and the tumor’s proximity to major vessels and organs. Although current guidelines recommend wide resection of Grade II or III chondrosarcomas [3], there is no specific recommendation for the width of the surgical margin, nor is there an exact definition of a negative margin. Moreover, it is unclear how wide the minimum resection margin should be to lessen the likelihood of local recurrence after resection, especially in patients with low-grade tumors [5]. We determined the adequate surgical margin in patients with peripheral chondrosarcomas of the pelvis. We also assessed the role of preoperative biopsy. In our study, no patients who underwent resection with a ≥ 1 mm margin had local recurrence, metastasis, or disease-related death. Local recurrence was associated with worse DSS. However, because of sampling errors, the biopsy results correctly predicted the final histologic grade noted with a resection specimen in only 37% patients.
We acknowledge the limitations of the study. First, it is difficult to determine a surgical margin to the nearest millimeter during surgery. Therefore, our results of a margin ≥ 1 mm indicate that an adequate “wide” surgical margin is anything but intralesional during surgery. However, clarifying the association between the surgical margin and oncologic outcomes is crucial to counsel patients, predict prognosis, and plan surgical resection. Second, our results should be interpreted cautiously because of the small number of patients who were included, particularly in each surgical margin category (11 in the 0 mm group, 17 in the < 1 mm group, and 16 in the ≥ 1 mm group). Missing data for surgical margin (six patients) or biopsy results (nine patients) could have influenced our results. Moreover, the small number of local recurrences (n = 13) may have led to the probability of a Type II error in the log-rank test. Larger studies are therefore needed to confirm these results. Third, the distribution of patients during the 34-year observation period was uneven, and only six of the 50 patients had more than 15 years of follow-up, which may have affected our analysis of cumulative survival. Fourth, evolution of surgical reconstruction and imaging techniques during the 34-year study period likely influenced our results. Surgical advances such as computer navigation [14] and improvements in cross-sectional imaging, particularly MRI, have enabled more accurate preoperative planning and can determine the likelihood of malignant change in pelvic osteochondromas more precisely. Finally, tumor grades were subjectively determined for the purposes of the study, and the interobserver reliability of classifying chondrosarcoma has been reported to be poor [6, 18]. However, we believe that specialized pathologists at our referral centers reduced this bias.
We found that the DSS and LRFS rates of peripheral chondrosarcomas of the pelvis were 90% (95% CI, 70 to 97) and 69% (95% CI, 52-81). We focused on peripheral chondrosarcomas of the pelvis, which show distinct features from the primary central type [2], in contrast to previous studies combining the results of all subtypes of pelvic chondrosarcoma [4, 5, 10, 13, 16, 17, 21]. Donati et al. [5] reported the 5-year overall survival rate was 96% in patients with peripheral pelvic chondrosarcoma. In 2013, the same group analyzed 215 patients with chondrosarcomas of the pelvis, including 85 patients with peripheral chondrosarcomas [13]. In that report, the mean follow-up duration was 8.6 years (range 2 to 31 years) and the 10-year overall survival rate in patients with peripheral chondrosarcoma was approximately 85%, which is comparable to our results (Table 5).
Table 5.
Summary of relevant research
Achieving a surgical resection margin of greater than 1 mm was associated with no local recurrence in our patients. A previous report [5] concluded that contaminated margins in histologic Grade I peripheral chondrosarcomas may be acceptable. However, we recommend that all peripheral chondrosarcomas of the pelvis, irrespective of grade, should be resected with adequate margins because Grade I tumors recurred locally in seven of 34 patients in the present study. Local disease was not controlled in five of these seven patients after a mean of four surgical excisions at the last follow-up. Two of the 34 patients who had Grade I tumors at the time of the initial resection subsequently died of local recurrence. Based on our results, we recommend using a negative margin at the initial resection to lessen the likelihood of local recurrence of peripheral chondrosarcomas of the pelvis. A margin of 1 mm or more appeared to be sufficient.
Preoperative biopsy results underreported the histologic tumor grade and were accurate in only 37% of the studied patients, presumably because of the potential for a biopsy sampling error. Based on our results, we conclude that a preoperative biopsy should not be relied on to provide an accurate diagnosis if a peripheral chondrosarcoma of the pelvis is suspected based on MRI findings. This is similar to previous reports highlighting the unreliability of biopsy results of chondrosarcomas, which should be interpreted with caution when assessing the malignant potential of the tumor [12]. Emphasis should be placed on clinical (pain or a growing lump) and radiologic features (progressive tumor growth on serial imaging studies or a cartilage cap thickness greater than 2 cm on MRI) when making a multidisciplinary diagnosis. However, we believe biopsy has still a role because it can provide evidence of a cartilage tumor before challenging pelvic resection. Additionally, not all biopsy results are incorrect. Thus we still perform biopsies in these patients.
Patients who undergo pelvic resection and reconstruction have a high risk of postoperative complications. In our analysis, deep infection was the most frequent non-tumor-related complication (four of 50 patients, 8%), the incidence of which was slightly lower than in other reports (10% to 20%) (Table 5) [4, 5, 13, 15, 16]. This is presumably because most of our patients had a tumor of the ilium, where reconstruction is not often used.
In conclusion, we found the most frequent postoperative complication was local recurrence of pelvic peripheral chondrosarcomas, which in some patients was related to death because of an inoperable locally recurrent tumor. Because of the high rates of local recurrence, which can lead to death even in patients with Grade I tumors, and the difficulty of distinguishing grades preoperatively on biopsy, every attempt should be made to achieve a negative margin at the initial resection to lessen the likelihood of local recurrence. A margin of 1 mm or more appeared to be sufficient, irrespective of histologic tumor grade. Although we collected data from three referral centers, the number of patients was still small. A larger study is needed to confirm our findings.
Acknowledgments
We thank Yoichi Kaneuchi for his role in gathering clinical information and assisting with the revising the manuscript.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Royal Orthopaedic Hospital, Birmingham, UK.
References
- 1.Ahmed AR, Tan TS, Unni KK, Collins MS, Wenger DE, Sim FH. Secondary chondrosarcoma in osteochondroma: report of 107 patients. Clin Orthop Relat Res . 2003;411:193-206. [DOI] [PubMed] [Google Scholar]
- 2.Bus MP, Campanacci DA, Albergo JI, Leithner A, van de Sande MA, Gaston CL, Caff G, Mettelsiefen J, Capanna R, Tunn PU, Jeys LM, Dijkstra PD. Conventional primary central chondrosarcoma of the pelvis: prognostic factors and outcome of surgical treatment in 162 patients. J Bone Joint Surg Am . 2018;21:316-325. [DOI] [PubMed] [Google Scholar]
- 3.Casali PG, Bielack S, Abecassis N, Aro HT, Bauer S, Biagini R, Bonvalot S, Boukovinas I, Bovee JV, Brennan B, Brodowicz T, Broto JM, Brugières L, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Dhooge C, Eriksson M, Fagioli F, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gaspar N, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hecker-Nolting S, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kager L, Kasper B, Kopeckova K, Krákorová DA, Ladenstein R, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Morland B, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Strauss SJ, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WT, Whelan J, Wardelmann E, Zaikova O, Blay JY; ESMO Guidelines Committee, PaedCan and ERN EURACAN. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;1:29:iv79-iv95. [DOI] [PubMed] [Google Scholar]
- 4.Deloin X, Dumaine V, Biau D, Karoubi M, Babinet A, Tomeno B, Anract P. Pelvic chondrosarcomas: surgical treatment options. Orthop Traumatol Surg Res . 2009;95:393-401. [DOI] [PubMed] [Google Scholar]
- 5.Donati D, El Ghoneimy A, Bertoni F, Di Bella C, Mercuri M. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J Bone Joint Surg Br. 2005;87:1527-1530. [DOI] [PubMed] [Google Scholar]
- 6.Eefting D, Schrage YM, Geirnaerdt MJ, Le Cessie S, Taminiau AH, Bovee JV, Hogendoorn PC; EuroBoNeT Consortium. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am J Surg Pathol. 2009;33:50-57. [DOI] [PubMed] [Google Scholar]
- 7.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res . 1980;153:106–120. [PubMed] [Google Scholar]
- 8.Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818-831. [DOI] [PubMed] [Google Scholar]
- 9.Garrison RC, Unni KK, McLeod RA, Pritchard DJ, Dahlin DC. Chondrosarcoma arising in osteochondroma. Cancer . 1982;49:1890-1897. [DOI] [PubMed] [Google Scholar]
- 10.Guo W, Li D, Tang X, Ji T. Surgical treatment of pelvic chondrosarcoma involving periacetabulum. J Surg Oncol. 2010;101:160-165. [DOI] [PubMed] [Google Scholar]
- 11.Jeys LM, Thorne CJ, Parry M, Gaston CL, Sumathi VP, Grimer JR. A novel system for the surgical staging of primary high-grade osteosarcoma: the Birmingham classification. Clin Orthop Relat Res. 2017;475:842-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laitinen MK, Stevenson JD, Parry MC, Sumathi V, Grimer RJ, Jeys LM. The role of grade in local recurrence and the disease-specific survival in chondrosarcomas. Bone Joint J. 2018;100:662-666. [DOI] [PubMed] [Google Scholar]
- 13.Mavrogenis AF, Angelini A, Drago G, Merlino B, Ruggieri P. Survival analysis of patients with chondrosarcomas of the pelvis. J Surg Oncol. 2013;108:19-27. [DOI] [PubMed] [Google Scholar]
- 14.Nandra R, Matharu G, Stevenson J, Parry M, Grimer R, Jeys L. Long-term outcomes after an initial experience of computer-navigated resection of primary pelvic and sacral bone tumours: soft-tissue margins must be adequate to reduce local recurrences. Bone Joint J . 2019;101:484-490. [DOI] [PubMed] [Google Scholar]
- 15.Ozaki T, Hillmann A, Lindner N, Blasius S, Winkelmann W. Chondrosarcoma of the pelvis. Clin Orthop Relat Res . 1997;337:226-239. [DOI] [PubMed] [Google Scholar]
- 16.Pring ME, Weber KL, Unni KK, Sim FH. Chondrosarcoma of the pelvis. A review of sixty-four cases. J Bone Joint Surg Am. 2001;83:1630-1642. [PubMed] [Google Scholar]
- 17.Sheth DS, Yasko AW, Johnson ME, Ayala AG, Murray JA, Romsdahl MM. Chondrosarcoma of the pelvis. Prognostic factors for 67 patients treated with definitive surgery. Cancer. 1996;78:745-750. [DOI] [PubMed] [Google Scholar]
- 18.Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg Am . 2007;89:2113-2123. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson JD, Laitinen MK, Parry MC, Sumathi V, Grimer RJ, Jeys LM. The role of surgical margins in chondrosarcoma. Eur J Surg Oncol. 2018;44:1412-1418. [DOI] [PubMed] [Google Scholar]
- 20.Trovik CS, Skjeldal S, Bauer H, Rydholm A, Jebsen N. Reliability of margin assessment after surgery for extremity soft tissue sarcoma: the SSG experience. Sarcoma . 2012;2012:290698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wuisman PI, Jutte PC, Ozaki T. Secondary chondrosarcoma in osteochondromas. Medullary extension in 15 of 45 cases. Acta Orthop Scand . 1997;68:396-400. [DOI] [PubMed] [Google Scholar]