Abstract
Background
Periprosthetic joint infection (PJI) is one of the most devastating complications of total joint arthroplasty. Given the mortality and morbidity associated with PJI and the challenges in treating it, there has been increased interest in risk factors that can be modified before surgery. In this study, we used a novel mouse model to consider the role of the gut microbiome as a risk factor for PJI.
Questions/purposes
(1) Does the state of the gut microbiota before surgery influence the likelihood of developing an established infection in a mouse model of PJI? (2) How does the state of the gut microbiota before surgery influence the local and systemic response to the presence of an established infection in a mouse model of PJI?
Methods
Male C57Bl/6 mice were divided into two groups: those with modified microbiome ∆microbiome (n = 40) and untreated mice (n = 42). In ∆microbiome mice, the gut flora were modified using oral neomycin and ampicillin from 4 weeks to 16 weeks of age. Mice received a titanium tibial implant to mimic a joint implant and a local inoculation of Staphylococcus aureus in the synovial space (102 colony forming units [CFUs]). The proportion of animals developing an established infection in each group was determined by CFU count. The local and systemic response to established infection was determined using CFU counts in surrounding joint tissues, analysis of gait, radiographs, body weight, serum markers of inflammation, and immune cell profiles and was compared with animals that received the inoculation but resisted infection.
Results
A greater proportion of animals with disrupted gut microbiota had infection (29 of 40 [73%]) than did untreated animals (21 of 42 [50%]; odds ratio, 2.63, 95% CI, 1.04–6.61; p = 0.035). The immune response to established infection in mice with altered microbiota was muted; serum amyloid A, a marker of systemic infection in mice, was greater than in mice with disrupted gut microbiota with infection (689 µg/dL; range, 68–2437 µg/dL, p < 0.05); infection associated increases in monocytes and neutrophils in the spleen and local lymph node in untreated mice but not were not observed in mice with disrupted gut microbiota.
Conclusions
The findings from this in vivo mouse model suggest that the gut microbiota may influence susceptibility to PJI.
Clinical Relevance
These preclinical findings support the idea that the state of the gut microbiome before surgery may influence the development of PJI and justify further preclinical and clinical studies to develop appropriate microbiome-based interventions.
Introduction
Periprosthetic joint infection (PJI) is one of the most devastating complications after THA or TKA. Although PJI is relatively rare, occurring in as few as 1% of all total joint arthroplasties [20, 35], infection is the primary reason for revision TKA and the third-leading reason for revision THA. The incidence of PJI is expected to grow as the number of total joint arthroplasties increases in the coming years [5, 28], and PJI is associated with an increased risk of death [2, 44]. Treatment of PJI is challenging and success rates range from 0% to 89% [23]. Given the mortality and morbidity associated with PJI and the challenges in treating it, there has been increased interest in risk factors that can be modified before surgery. In this study, we considered the role of the gut microbiome as a risk factor for PJI.
The microbiome consists of the genetic components of microbial organisms (bacteria, archea, and eukaryotes) that inhabit the body. The gut contains most commensal microorganisms in mammals. An individual’s gut microbiome typically consists of more than 1000 distinct microbial species that interact with one another and the host [26]. Disruption of a healthy gut microbiome is associated with an imbalance in the constituents and/or functional interactions of commensal microbes, a state referred to as dysbiosis. Dysbiosis leads to changes in host-microbe interactions, especially the microbial stimuli provided to immune cell populations in the gut lining [30], and is associated with chronic medical conditions that are also risk factors for PJI including obesity, diabetes, and inflammatory bowel disease [24-26].
Commensal microbes in the skin and nasal microbiome have long been known to be a source of pathogens involved in PJI and are targeted by some interventions [4, 36]. However, the majority of the mammalian microbiome is in the gut and the gut microbiota have may also influence PJI by stimulating the immune system. By altering host-microbe interactions at the gut lining, modifications to the constituents of the gut microbiome may lead to reductions in the number and/or effectiveness of host macrophages and reduced ability to respond to systemic bacterial challenge [25]. The composition of the gut microbial community can differ among individuals due to host genotype, diet, exposure early in life, and history of antibiotic use [16]. Although oral antibiotics are key to treating and preventing infection (particularly prophylactic antibiotics associated with orthopaedic surgery), a course of antibiotics can cause dramatic changes in the constituents of the microbiome that remain long after the antibiotics have cleared the system [26]. The cumulative effect of multiple courses of oral antibiotics throughout life may cause the loss of key species from the gut flora and overall reductions in gut flora diversity [3] that can then influence the outcomes of procedures. In humans, reduced diversity of the gut microbial community has been associated with an increased risk of infection after hematopoietic stem cell transplantation [41, 42].
Although the constituents of the gut microbiome are known to influence the risk of enteric and systemic infections, the relationship between the gut microbiome and the severity of PJI remains unknown. Determining how the state of the gut microbiome before surgery may influence PJI in patients is challenging given the small rates of infection. Preclinical models provide a means of testing the potential effect of the microbiome on PJI. Recently, a mouse model of total joint arthroplasty using an intraarticular titanium implant has been used to study PJI [12]. Here we use the mouse model to test the concept that the state of the microbiome before surgery can influence PJI development.
Specifically, we asked: (1) Does the state of the gut microbiota before surgery influence the likelihood of developing an established infection in a mouse model of PJI? (2) How does the state of the gut microbiota before surgery influence the local and systemic response to the presence of an established infection in a mouse model of PJI?
Materials and Methods
We performed the animal experiments after our local institutional animal care and use committee approved the study; institutional veterinarians monitored the animals. We performed the animal work in an animal facility that has been accredited by the Association for Assessment and Accreditation of Laboratory Animals International. This study involved 86 adult (16 weeks old) male C57Bl/6 mice. Male animals were studied to allow comparison with prior work with this animal model [12]. We purchased breeders from a commercial provider (Jackson Laboratory, Bar Harbor, ME, USA). The experimental animals were bred in a conventional facility using trio breeding and raised in plastic cages with sterilized bedding (SANI-Chips, PJ Murphy Forest Products, Montville, NJ, USA) and chow (Picolab Rodent Diet 20, LabDiet, St. Louis, MO, USA) in a 12-hour light/dark cycle. At weaning (4 weeks old), animals were divided evenly into two groups selected to uniformly distribute animals among litters/dams: modified gut microbiome (∆microbiome) and untreated animals. Animals were housed in cages with animals from the same group.
Experimental Overview
In this experiment, mice with modified microbiome (∆microbiome) and mice with unmodified microbiomes (untreated) underwent surgery to receive a tibial component in one limb. Immediately after surgery an inoculation of Staphylococcus aureus was injected into the joint to provide bacterial challenge. Five days after surgery/inoculation the animals were examined for signs of infection noninvasively and tissue was collected. A proportion of the mice from each group displayed PJI (as assessed by CFU count at the implant surface). We examined serum markers of inflammation and immune cell profiles in animals that developed PJI and those that were uninfected in each of the two groups (∆microbiome and untreated).
Disruption of the Gut Microbiota
The gut microbiota of the animals in the ∆microbiome group were disrupted using antibiotics in drinking water (ampicillin, 1.0 g/L and neomycin, 0.5 g/L). We selected ampicillin and neomycin because these two antibiotics have poor oral bioavailability, thereby limiting the effects of the antibiotics to the gut microbiome and avoiding systemic effects of the antibiotics [15, 31]. Neomycin directly targets Gram-negative organisms and ampicillin is broad spectrum. The use of chronic oral antibiotics is a common experimental approach to generate large but consistent long-term modifications to the constituents of the gut microbiome that is analogous to differences in the microbiota among individuals. The approach is particularly useful for initial studies testing the effect of the microbiome on a phenotype [19, 29]. A histological examination of the gut after euthanasia indicated that chronic oral exposure to these antibiotics does not lead to signs of gastrointestinal inflammation [17]. We provided antibiotics continuously for 3 months (solutions were refreshed every 3 days) and stopped dosing 4 days before surgery to ensure the antibiotics cleared the system (the half-life of these drugs is ∼ 1 hour [31]) .
Establishment of PJI and Sample Size Determination
At skeletal maturity (16 weeks old) all animals underwent surgical placement of a titanium tibial component (Fig. 1A) [12]. After arthrotomy closure, we injected an inoculation of methicillin-sensitive S. aureus (Xen36 strain) in 2 µL of phosphate-buffered saline into the knee (Fig. 1B) [12]. We used Xen36 because of its known resistance to kanamycin, permitting it to be identified later through culturing samples on kanamycin-infused plates [12]. We verified bacterial dosing at inoculation by plating three identical inoculation doses for each animal. After surgery, we provided mice with subcutaneous buprenorphine (0.05 mg/kg) for 48 hours and did not restrict their activity.
Fig. 1 A-C.
(A) A titanium tibial implant for the mice is shown. (B) After the wound was surgically closed, an inoculation of Staphylococcus aureus was applied to the joint space. (C) An example radiograph of an animal with resistance to infection and intact bone-implant interface is shown on the left and a radiograph of a mouse with infection is shown on the right illustrating bone loss around the implant (arrow).
We chose an inoculation of 102 colony forming units (CFUs) after a pilot experiment in which the relationship between the bacterial inoculation and resulting bacterial load on the implant was determined (see Figure; Supplemental Digital Content 1, http://links.lww.com/CORR/A196). The results indicated that, in an infection, the bacterial load on the implant surface was not correlated with the magnitude of the inoculation (inoculations across a range of 102 to 105 CFUs). However, the size of the inoculation influenced the proportion of animals developing an infection and that an inoculation of 102 CFUs resulted in infection in only a subset of the animals. Therefore, we designed the study to detect differences in the proportion of animals with an infection. A power analysis indicated that a sample size of 43 animals per group would be able to detect an effect of treatment in which the proportion of animals developing an infection increased from 50% to 81% or more (chi-square test, alpha = 0.05, power = 0.80).
Experimental Outcomes
Our primary study endpoints were the proportion of animals in each group (∆microbiome and untreated) to develop PJI. To assess this, we measured the bacterial load (CFUs) at the implant surface.
Our secondary study endpoints included a serum marker of systemic inflammation (serum amyloid A, the mouse analog to C-reactive protein), profiles of immune cell populations in the spleen and local (popliteal) lymph nodes, gait score, radiograph score and reductions in body weight after surgery/infection. Systemic markers were compared between both groups (∆microbiome versus untreated) as well as within groups (established infection versus uninfected/resisting infection).
PJI Analysis
Five days after surgery or inoculation, the mice were evaluated noninvasively for signs of infection. We took lateral radiographs (Faxitron, Tucson, AZ, USA) that were graded by blinded observers (ASS, JK, MS) using an established quantitative radiographic score for PJI in mice [12] (scored from 0 (no boney erosion) to 4 (severe boney erosion), Fig. 1C). We collected videos of mice ambulating in their cages for 10 to 30 seconds at 240 frames per second using a handheld device (iPhone 7, Apple, Cupertino, CA, USA). Blinded observers (ASS, JK, MS) evaluated gait using the following scoring system [12]: (0) full weightbearing, indicated as full knee motion and full foot contact with the cage floor, with no clear preference for ambulating on the contralateral limb; (1) partial weightbearing, indicated as full knee motion and at least midfoot contact with the cage floor with a clear limp; (2) toe-touch weightbearing, indicated as partial knee motion and forefoot contact with the cage floor; or (3) non-weightbearing, indicated as no knee motion and disuse or retraction of the limb.
We euthanized the animals 5 days after surgery. Immediately before administering euthanasia, we measured body weight and collected fecal pellets in sterile tubes. We collected blood through a terminal cardiac puncture with the mice under anesthesia. We stored fecal specimens and serum at -80° C until further analysis. Immediately after administering euthanasia, we cleaned the operative limb with 10% povidone-iodine and amputated it. We opened the joint, removed the implant, and placed it in a sterile tube containing 200 µL of phosphate-buffered saline at 4° C. The tube was sealed, vortexed for 30 seconds, and subjected to ultrasonic stimulation for 15 minutes to remove bacteria from the implant surface. We resuspended the resulting solution in tryptic soy broth and plated it in triplicate for overnight culture to determine implant CFU counts. The proximal tibia, knee extensor tissue, and distal femur were collected, placed in 2 mL of sterile phosphate-buffered saline, and homogenized for 5 minutes (Bullet Blender; Next Advance, Troy, NY, USA). The resulting homogenized solution was diluted with 4° C tryptic soy broth and plated in triplicate for overnight culture to achieve tissue CFU counts. We measured serum amyloid A, a marker of systemic inflammation analogous to C reactive protein in humans, using blood collected by cardiac puncture in a subset of animals (23 in the ∆microbiome group and 28 untreated animals, selected at random) using an ELISA kit (#KMA0021; Invitrogen, Waltham, MA, USA).
We sequenced fecal specimens to determine differences in microbiota between the groups. We selected a subset of the fecal pellets after infection was detected, enabling us to examine the gut microbiota in animals with and without an established infection (six untreated animals that were uninfected or resistant to infection, eight untreated animals with an established infection, four in the ∆microbiome group that were uninfected or resistant to infection, and 12 in the ∆microbiome group with an established infection). We isolated DNA from fecal pellets (DNeasy PowerSoil DNA Isolation Kit, MO BIO Laboratories Inc, Carlsbad, CA, USA) with the recommended proteinase K step to assist in cell lysis. We prepared 16S rRNA libraries using the Earth Microbiome Project protocol [11] with primers as described by Walters et al. [47]. We imported paired-end 150 x 150 reads into QIIME2 (https://qiime2.org)[10] and demultiplexed them. We used DADA2 as a quality control method [9] to remove chimeric sequences and retain unique de novo sequence variants. We trimmed poor-quality bases separately for the forward and reverse reads. We used the core-metrics-phylogenetic method to compute alpha diversity (Shannon Diversity Index) using unweighted UniFrac distances and principal coordinates analysis plots using Emperor. We assigned taxonomies using QIIME’s machine learning classifier trained on Greengenes sequences.
We harvested the spleen and popliteal lymph node adjacent to the infected joint from a subset of animals to investigate local and systemic immune responses using flow cytometry. We determined neutrophil and monocyte cell numbers from recovered spleen (12 ∆microbiome and 14 untreated mice) and lymph node samples (six ∆microbiome and 24 untreated mice). After the spleen and lymph nodes were harvested, the hematopoietic cells were extruded with a 3-mL syringe plunger, the total cell count was determined using a particle counter (Coulter Counter Z1, Beckman Coulter, Brea, CA, USA) and stained with antibodies for Ly6C and CD11b to identify Ly6Cmed CD11b+ SSchi neutrophils and Ly6Chi CD11b+ FSchi monocytes on flow cytometric analysis [37, 43], similar to the protocol of a previous study [1]. We also stained cells for CD3, CD4 and CD8 to identify CD3+CD4+ T cells and CD3+CD8+ T cells [43] (see Figure, Supplemental Digital Content 2, http://links.lww.com/CORR/A197). We ran samples on a FACSCanto (BD Biosciences, San Jose, CA, USA) and analyzed them using FlowJo software (Tree Star, Ashland, OR, USA). The number of each type of immune cell per organ was calculated by multiplying the percentage of the total gated population by the total cell count.
Six animals (all from the ∆microbiome group) died because of complications associated with surgery (four animals before recovery from anesthesia and two animals within 24 hours of surgery) and were removed from the study analysis, leaving a sample size of 82 animals (40 ∆microbiome and 42 untreated mice). Of the remaining animals, eight died 24 to 96 hours after surgery (seven ∆microbiome and one untreated) and had implant and tissue CFU counts but none of the other markers for us to assess infection.
Statistical Analysis
We determined that infection had developed using CFU counts at the implant surface (as determined in preliminary studies (see Figure; Supplemental Digital Content 1, http://links.lww.com/CORR/A196). We determined differences in the proportion of animals with established infection in each group using the chi-square test (JMP Pro 10.0.2, SAS Institute Inc, Cary, NC, USA). We determined differences in continuous parameters that were normally distributed using ANOVA and the Holm post-hoc test for multiple comparisons. We used nonparametric tests (Wilcoxon and Steel-Dwass all pairs) for measures that were not normally distributed. We performed a Spearman’s rank correlation analysis to detect associations among parameters with nonnormal distributions. Receiver operating characteristic curves were generated and area under the curve (AUC) was calculated for diagnosis of PJI based on logistic regression models using noninvasive assays as predictors (reduction in body weight, gait score, radiograph score). Unless otherwise stated, results are presented as the mean ± SD or median (range) and p < 0.05 was considered significant.
Results
Bacterial Load and Proportion of Animals Developing Infection
The proportion of animals in the ∆microbiome group that developed established infection (29 of 40, 72.5%) was greater than that in untreated mice (21 of 42, 50%; odds ratio, 2.63; 95% CI, 1.04–6.61; p = 0.03, difference 22.5%, Fig. 2A).
Fig. 2 A-C.

Indicators of infection surrounding the prosthesis are shown. (A) Bacterial load on the implant surface at 5 days after surgery/inoculation showed a binary response indicating infection or uninfected/resistance to infection. A greater proportion of animals in the ∆microbiome group developed established infection than did those in the untreated group. (B) Bacterial load in the tissue surrounding the implant was greater in ∆microbiome mice than in untreated mice, and (C) there was a strong correlation between bacterial load at the implant surface and in the surrounding joint tissue in animals with established infection (p < 0.001).
Several animals in each group developed PJI as determined by CFU counts. Bacterial load on the implant clustered into two distinct ranges: 0 – 20 CFUs and 1.3*103 – 2.0*107 CFUs, which were classified as uninfected/resisting infection and established infection, respectively (Fig. 2A). Bacterial load in the surrounding joint tissues was greater in animals with established infection (median 1.1*107 range: 5.5*105,1.4*108 CFUs) than animals that were uninfected (median 267 range: 0, 5.1*104 CFUs, p < 0.001, Fig. 2B). In animals with PJI, the bacterial load in the surrounding joint tissues was correlated with implant CFUs in animals with PJI (R2 = 0.42, p < 0.001, Fig. 2C ).
Local and Systemic Response to Infection
Gait score, radiograph score, body weight, serum markers of infection and systemic and local T cell populations were associated with the presence of infection across both ∆microbiome and untreated mice. Animals with PJI had poorer gait score (median, 2.5; range, 1–3) than animals that were uninfected/resisting infection (median 0.5; range, 0–2; p < 0.001, difference 2). Radiographs demonstrated greater bony destruction in animals with infection; radiograph score in mice with PJI (median, 2; range, 0–4) was greater than that in uninfected animals (median, 0; range, 0–3; p < 0.001, difference 2). The reductions in body weight after surgery was greater in animals with PJI (median, 3 g; range, -1 to 8 g) than in uninfected animals (median, 2 g; range, -4 to 5 g; p = 0.013, difference 1 g). Serum amyloid A in mice with PJI (median, 885 µg/dL; range, 68–2437 µg/dL) was greater than that in uninfected mice (median, 68 µg/dL; range, 12–352 µg/dL; p < 0.001, difference 817 µg/dL, Fig. 3A). Serum amyloid A was correlated with tissue CFU counts (Fig. 3B) (ρ = 0.75; p < 0.001) and CFU counts in implants (ρ = 0.72; p < 0.001) (Table 1). Gait score and reduction in body weight were weakly correlated with CFU counts and serum amyloid A (see Figure; Supplemental Digital Content 3, http://links.lww.com/CORR/A198). Gait score, recorded 5 days after surgery, was the single best noninvasive indicator of infection (AUC 0.935, p < 0.001), although radiograph score was also predictive (AUC 0.913, p < 0.001) (see Figure; Supplemental Digital Content 4, http://links.lww.com/CORR/A199).
Fig. 3 A-D.
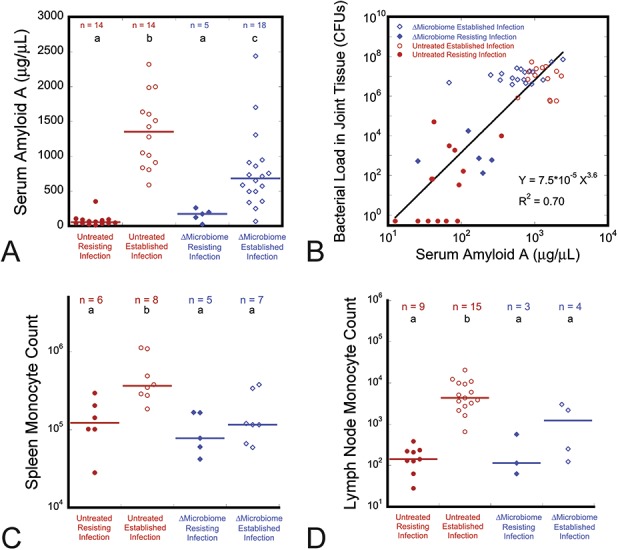
Animals with disrupted gut microbiome had a reduced systemic response to infection of the prosthesis compared with untreated animals. (A) The increase in serum amyloid A levels seen with established infection (as compared with uninfected/resisting infection) was not as great in the ∆microbiome group as in the uninfected group. (B) Serum amyloid A was related to the bacterial load in the surrounding joint tissue (p < 0.001). Although established infection led to increases in monocyte counts in (C) the spleen and (D) local lymph node in both untreated and ∆microbiome animals, the increase in untreated animals was greater indicating reduced immune cell response to infection. Groups with different lowercase letters above the data are different than one another (p < 0.05, Steel-Dwass all pairs), horizontal lines indicate median.
The immune response to PJI differed between ∆microbiome and untreated animals (Table 2). In untreated animals, PJI was also associated with increases in the numbers of inflammatory monocytes in the spleen (Fig. 3C) and lymph nodes (Fig. 3D) as well as increases in other immune cell populations (see Figure; Supplemental Digital Content 5, http://links.lww.com/CORR/A200), likely reflecting the activation of immune cell populations to help fight infection. In mice with PJI, serum amyloid A was greater in untreated mice (median, 1352 µg/dL; range, 589–2320 µg/dL) than in ∆microbiome mice (median, 683 µg/dL; range, 68–2437 µg/dL; p = 0.013, difference 669 µg/dL) (Fig. 3A). Furthermore, in contrast to observations in untreated animals, in ∆microbiome animals, infection was not associated with increased monocyte counts in the spleen (Fig. 3C) and popliteal lymph node (Fig. 3D) nor with increased neutrophils or lymph node T cells (see Figure; Supplemental Digital Content 5, http://links.lww.com/CORR/A200).
The composition of the gut microbiota in ∆microbiome and untreated animals differed considerably (Fig. 4). At the phyla level, the gut flora was dominated by Bacteroidetes and Firmicutes (Fig. 4A). Principal coordinate analysis indicated that ∆microbiome and untreated animals clustered separately, demonstrating that the composition of the microbial community of the ∆microbiome and untreated groups was distinct (Fig. 4B). Within the ∆microbiome and untreated groups, the principal coordinate analysis did not demonstrate a clear distinction between the microbial community structure when comparing established infection with resistance to infection. The diversity of the gut microbiota, as measured by the Shannon Diversity index, in the Δmicrobiome mice (2.36 ± 0.57) was less than that in untreated animals (3.87 ± 0.28; p < 0.001, difference 1.51) (Fig. 4C).
Fig. 4 A-C.
We characterized the gut microbiota using fecal samples collected at the time we administered euthanasia to the mice. (A) The relative abundance differs considerably between ∆microbiome and untreated animals. (B) The principal coordinate analysis (weighted) of the microbial constituents by phyla shows large differences in the gut microbiota between ∆microbiome and untreated animals, without noticeable differences associated with infection. (C) Microbial diversity was greater in the untreated animals than in the ∆microbiome animals. Solid colored lines on dot plots represent the mean.
Discussion
Periprosthetic joint infection (PJI) is one of the most devastating complications of total joint arthroplasty. Alterations to the gut microbiome are associated with many of the patient risk factors for PJI. Here we use a preclinical model to test the idea that modifications to the gut microbiome can influence the development of PJI. Animals in which the microbiome was disrupted (∆microbiome) were more likely to develop an established infection than animals with unmodified gut microbiota. Animals with disrupted gut flora showed a reduced systemic response to infection.
The limitations of the current study must be considered when interpreting its findings. First, we modified the gut microbiota in growing mice, so we cannot ignore the possibility that our findings were influenced by changes in the development of the host immune system early in life. Alterations in the gut microbiome at later ages may have different effects on the response to bacterial challenge. Second, as with all murine studies, there are limitations in extrapolating findings to humans, including differences between the two species in the constituents of the microbiome [26] and immune system [33]. However, our primary conclusion that the microbiome can influence PJI remains, even if the specific microbes that link the microbiome to implant infection in mice differ from those in humans. A better understanding of the mechanisms involved in mice is needed to identify analogous mechanisms in humans. Third, the current study only examined male mice and it is possible that the response differs in females. Fourth, we modified the constituents of the microbiome using chronic oral antibiotics. Chronic antibiotics are useful for modifying the gut microbiome experimentally. However, in general, few patients undergo antibiotic treatment for such a long period (3 months). Clinically, differences in the gut flora among individuals may be milder than those observed here. Nevertheless, our findings with chronic antibiotics indicate that the gut microbiome may influence the risk of implant infection and supports further work examining the underlying mechanisms. Similarly, while the study design was sufficient to address the primary research question regarding infection and the microbiome, the study design was not sufficient to isolate the specific mechanisms such as the changes in the microbiota and immune cell populations in uninfected animals. Additional baseline and control groups would be required to isolate these mechanisms. Lastly, while neomycin has zero oral bioavailability, ampicillin has negligible oral bioavailability in rodents [45], suggesting that trace amounts may be distributed in the animals. However, ampicillin dosing was stopped 4 days before surgery, providing sufficient time to clear the system (the half-life in serum is ∼ 1 hour [31]).
There are several notable methodological aspects of the study. First, the mouse model provides a meaningful translational representation to clinical PJI in that it uses a titanium load-bearing implant that separates the intramedullary and articular spaces. In contrast, other available PJI models use nonloadbearing implants, place implants far from the joint space, or use implants made of nonmetallic components [13]. Second, the animals were bred and raised in our facility with consistent housing, bedding, and diet, thereby allowing us to reasonably control these three factors, which are known to influence the constituents of the microbiome [26]. Finally, we modified the microbiome using chronic oral antibiotics over a 3-month period, thereby avoiding transient changes in the microbiome and bone physiology that can occur during shorter durations of oral antibiotic exposure [49].
Our study demonstrates that the constituents of the gut microbiome before surgery have the potential to influence the development of PJI after bacterial challenge. We attribute the increased infection rate in the ∆microbiome group to disruption of the gut microbiome and not the direct effects of oral antibiotics because the antibiotic dosing was terminated 4 days before inoculation, and the antibiotics used in the experiment (ampicillin and neomycin) are poorly absorbed in the gut lining and therefore are not sufficiently distributed through the systemic circulation. Changes in the gut microbiome can alter the way in which the microbiome stimulates the host’s systemic immune system, which, over time, can lead to changes in immune cell populations and function, thus modulating the response to bacterial challenge [24]. Our findings are consistent with prior preclinical work demonstrating the effects of alterations of the gut microbiome on infection. A history of oral antibiotic treatment has long been associated with an increased risk of enteric infections [24, 34]. More recently, the microbiome and history of antibiotic exposure has been associated with the risk of systemic infection [24]. Germ-free mice (animals raised in the absence of live microbes) and mice receiving chronic oral antibiotics have reduced immune cell populations and are more susceptible to systemic infection by Listeria monocytogenes or S. aureus [25]. Additionally, our findings in mice are consistent with observations that reduced diversity of human gut flora was associated with an increased risk of infection after allogenic stem cell transplantation [18, 40, 48]. How microbial diversity influences the response to infection is not well understood. More detailed studies are required to identify the mechanism through which disruption of the gut flora alters the risk of PJI and to identify microbial taxa that greatly influence the process.
Our secondary findings associated disruption of the gut microbiome with reduced systemic response to PJI. In our study, infection led to increases in monocyte, neutrophil, and T cell counts in untreated mice but not in ∆microbiome mice. Increases in serum amyloid A (the mouse analog to C-reactive protein) associated with PJI were more not as large in ∆microbiome mice as in untreated mice. These findings provide preliminary support to the idea that modifications to the gut microbiota can influence the development of PJI by modulating the ability of the host to respond to bacterial challenge. Further experiments are required to confirm these preliminary findings and to isolate the specific mechanisms involved.
Our results do not suggest that prophylactic antibiotics place patients at an elevated risk for acquiring PJI. Prophylactic antibiotics are well known to reduce surgery-associated infections, including infection after total joint arthroplasty [8, 32]. However, antibiotic prophylaxis is usually administered intravenously instead of orally and is therefore expected to have less drastic effects on the gut microbiome. Furthermore, clinically, prophylactic antibiotics are administered for a short time perioperatively (16 to 24 hours) [32] and it is unlikely that such a short period of exposure would cause large changes in the capacity of immune cells to respond to bacterial challenge. The chronic oral dosing applied to the ∆microbiome mice resulted in long-term modification to the gut microbiota, thereby mimicking differences in the gut microbiome seen in humans that can be caused by differences in early life exposure, habitual diet, or the cumulative effect of multiple courses of antibiotics over a lifetime [3].
The current study in mice suggests that the constituents of the microbiome may be associated with the risk of PJI. Clinically, patient-related factors associated with PJI include obesity, diabetes, rheumatologic disease, and anemia associated with malnutrition [4, 6, 7, 21, 27], a set of conditions associated with and contributed to by the gut microbiome [14, 22, 38, 46]. The association between the gut microbiome and PJI in the current study is intriguing because the microbiome is modifiable and may therefore be a target for preventive interventions. For example, disruption of the gut microbiome may be reversed by replenishing the microbiome (in the extreme case by a fecal transplant) and thereby help to promote healthy microbial diversity that is associated with resistance to bacterial challenge [39, 42]. Replenishment of the microbiome is increasingly considered useful for mediating the adverse effects of oral antibiotics on beneficial microbes [3, 39] and may become standard postsurgical practice to encourage a healthy microbiome. Additional preclinical studies are required to understand the mechanisms relating the microbiome to PJI and to determine the magnitude of the effect under more modest changes in the constituents of the microbiome than are shown here. Additionally, the variability in the gut microbiota of patients presenting for total joint arthroplasty is not known and would inform the appropriateness of microbiome-based interventions before surgery.
Acknowledgments
We thank Marjolein van der Meulen PhD, for her helpful comments and constructive criticism.
Footnotes
This publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (US) under Award Numbers AR0671534 and AR068061 and National Institute of Allergy and Infectious Diseases under Award Number R01AI079178. The content of the work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The institution of one or more of the authors (CJH, XY, GJ, YN, ASS, JK, MS, MWF, SC, TML, FPR, TTL, AVC, and MPGB) has received, during the study period, funding from the National Institutes of Health (USA) and the US Department of Defense. One author (CJH) has received personal funds from the Northern California Institute for Bone Health, Inc. One author (MPGB) has received personal fees in an amount of USD 10,000 to 100,000 from Smith & Nephew (Watford, Hertfordshire, UK) and fees associated with the editorial board in an amount of USD 10,000 to 100,000 from the HSS Journal (New York, NY, USA) and fees for service on the board of the American Austrian Foundation (New York, NY, USA) in an amount of USD 10,000 to 100,000. All other authors certify that neither he or she, nor any member of his or her immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Hospital for Special Surgery, New York, NY, USA.
References
- 1.Benahmed F, Chyou S, Dasoveanu D, Chen J, Kumar V, Iwakura Y, Lu TT. Multiple CD11c+ cells collaboratively express IL-1beta to modulate stromal vascular endothelial growth factor and lymph node vascular-stromal growth. J Immunol. 2014;192:4153-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berend KR, Lombardi AV, Jr., Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471:510-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke-Grauls CM, Roosendaal R, Troelstra A, Box AT, Voss A, van der Tweel I, van Belkum A, Verbrugh HA, Vos MC. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9-17. [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128-133. [DOI] [PubMed] [Google Scholar]
- 6.Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin Orthop Relat Res. 2012;470:130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozic KJ, Lau E, Kurtz S, Ong K, Rubash H, Vail TP, Berry DJ. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am. 2012;94:794-800. [DOI] [PubMed] [Google Scholar]
- 8.Bryson DJ, Morris DL, Shivji FS, Rollins KR, Snape S, Ollivere BJ. Antibiotic prophylaxis in orthopaedic surgery: difficult decisions in an era of evolving antibiotic resistance. Bone Joint J. 2016;98-B:1014-1019. [DOI] [PubMed] [Google Scholar]
- 9.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carli AV, Bhimani S, Yang X, Shirley MB, de Mesy Bentley KL, Ross FP, Bostrom MP. Quantification of peri-implant bacterial load and in vivo biofilm formation in an innovative, clinically representative mouse model of periprosthetic joint infection. J Bone Joint Surg Am. 2017;99:e25. [DOI] [PubMed] [Google Scholar]
- 13.Carli AV, Ross FP, Bhimani SJ, Nodzo SR, Bostrom MP. Developing a clinically representative model of periprosthetic joint infection. J Bone Joint Surg Am. 2016;98:1666-1676. [DOI] [PubMed] [Google Scholar]
- 14.Chassaing B, Aitken JD, Gewirtz AT, Vijay-Kumar M. Gut microbiota drives metabolic disease in immunologically altered mice. Adv Immunol. 2012;116:93-112. [DOI] [PubMed] [Google Scholar]
- 15.Ferrier L, Berard F, Debrauwer L, Chabo C, Langella P, Bueno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24:392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guss JD, Horsfield MW, Fontenele FF, Sandoval TN, Luna M, Apoorva F, Lima SF, Bicalho RC, Singh A, Ley RE, van der Meulen MC, Goldring SR, Hernandez CJ. Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner Res. 2017;32:1343-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haak BW, Littmann ER, Chaubard JL, Pickard AJ, Fontana E, Adhi F, Gyaltshen Y, Ling L, Morjaria SM, JU Peled, van den Brink MR, Geyer AI, Cross JR, Pamer EG, Taur Y. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood. 2018;131:2978-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez CJ, Guss JD, Luna M, Goldring SR. Links between the microbiome and bone. J Bone Miner Res. 2016;31:1638-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91:38-47. [DOI] [PubMed] [Google Scholar]
- 21.Jamsen E, Nevalainen P, Kalliovalkama J, Moilanen T. Preoperative hyperglycemia predicts infected total knee replacement. Eur J Intern Med. 2010;21:196-201. [DOI] [PubMed] [Google Scholar]
- 22.Kane AV, Dinh DM, Ward HD. Childhood malnutrition and the intestinal microbiome. Pediatr Res. 2015;77:256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387:386-394. [DOI] [PubMed] [Google Scholar]
- 24.Khosravi A, Mazmanian SK. Disruption of the gut microbiome as a risk factor for microbial infections. Curr Opin Microbiol. 2013;16:221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight R, Callewaert C, Marotz C, Hyde ER, Debelius JW, McDonald D, Sogin ML. The Microbiome and Human Biology. Annu Rev Genomics Hum Genet. 2017;18:65-86. [DOI] [PubMed] [Google Scholar]
- 27.Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD, Team I. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. PLoS One. 2016;11:e0150866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27:61-65 e61. [DOI] [PubMed] [Google Scholar]
- 29.Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev. 2016;40:117-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis. 1997;24:457-467. [DOI] [PubMed] [Google Scholar]
- 32.Meehan J, Jamali AA, Nguyen H. Prophylactic antibiotics in hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91:2480-2490. [DOI] [PubMed] [Google Scholar]
- 33.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731-2738. [DOI] [PubMed] [Google Scholar]
- 34.Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science. 2016;352:535-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943-948. [DOI] [PubMed] [Google Scholar]
- 36.Rao N, Cannella BA, Crossett LS, Yates AJ, Jr., McGough RL, 3rd, Hamilton CW. Preoperative screening/decolonization for Staphylococcus aureus to prevent orthopedic surgical site infection: prospective cohort study with 2-year follow-up. J Arthroplasty. 2011;26:1501-1507. [DOI] [PubMed] [Google Scholar]
- 37.Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A. 2012;81:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scher JU, Littman DR, Abramson SB. Review: Microbiome in inflammatory arthritis and human rheumatic diseases. Arthritis Rheumatol. 2016;68:35-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taur Y, Coyte K, Schluter J, Robilotti E, Figueroa C, Gjonbalaj M, Littmann ER, Ling L, Miller L, Gyaltshen Y, Fontana E, Morjaria S, Gyurkocza B, Perales MA, Castro-Malaspina H, Tamari R, Ponce D, Koehne G, Barker J, Jakubowski A, Papadopoulos E, Dahi P, Sauter C, Shaffer B, Young JW, Peled J, Meagher RC, Jenq RR, van den Brink MRM, Giralt SA, Pamer EG, Xavier JB. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB, Ponce DM, Barker JN, Giralt S, van den Brink M, Pamer EG. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taur Y, Jenq RR, Ubeda C, van den Brink M, Pamer EG. Role of intestinal microbiota in transplantation outcomes. Best Pract Res Clin Haematol. 2015;28:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taur Y, Pamer EG. Microbiome mediation of infections in the cancer setting. Genome Med. 2016;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terstappen LW, Mickaels RA, Dost R, Loken MR. Increased light scattering resolution facilitates multidimensional flow cytometric analysis. Cytometry. 1990;11:506-512. [DOI] [PubMed] [Google Scholar]
- 44.Toulson C, Walcott-Sapp S, Hur J, Salvati E, Bostrom M, Brause B, Westrich GH. Treatment of infected total hip arthroplasty with a 2-stage reimplantation protocol: update on "our institution's" experience from 1989 to 2003. J Arthroplasty. 2009;24:1051-1060. [DOI] [PubMed] [Google Scholar]
- 45.Tsuji A, Nakashima E, Kagami I, Yamana T. Intestinal absorption mechanism of amphoteric beta-lactam antibiotics I: Comparative absorption and evidence for saturable transport of amino-beta-lactam antibiotics by in situ rat small intestine. J Pharm Sci. 1981;70:768-772. [DOI] [PubMed] [Google Scholar]
- 46.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [DOI] [PubMed] [Google Scholar]
- 47.Walters W, Hyde ER, Berg-Lyons D, Ackermann G, Humphrey G, Parada A, Gilbert JA, Jansson JK, Caporaso JG, Fuhrman JA, Apprill A, Knight R. Improved bacterial 16S rRNA Gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber D, Jenq RR, Peled JU, Taur Y, Hiergeist A, Koestler J, Dettmer K, Weber M, Wolff D, Hahn J, Pamer EG, Herr W, Gessner A, Oefner PJ, van den Brink MRM, Holler E. Microbiota disruption induced by early use of broad-spectrum antibiotics is an independent risk factor of outcome after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23:845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016;113:E7554-E7563. [DOI] [PMC free article] [PubMed] [Google Scholar]




