Abstract
Genetic alterations in lung cancer are distinctly represented in non-small cell lung carcinoma (NSCLC) and small cell lung carcinoma (SCLC). Mutation of the RB1 and CDKN2A genes, which are tightly associated with cell cycle regulation, is exclusive to SCLC and NSCLC cells, respectively. Through the systematic analysis of transcriptome and proteome datasets for 318 cancer cell lines, we characterized differential gene expression and protein regulation in RB1-mutant SCLC and CDKN2A-mutant NSCLC. Many of the genes and proteins associated with RB1-mutant SCLC cell lines belong to functional categories of gene expression and transcription, whereas those associated with CDKN2A-mutant NSCLC cell lines were enriched in gene sets of the extracellular matrix and focal adhesion. These results indicate that the loss of RB1 and CDKN2A function induces distinctively different signaling cascades in SCLC and NSCLC cells. In addition, knockdown of the RB1 gene in CKDN2A-mutant cell lines (and vice versa) synergistically inhibits cancer cell proliferation. The present study on the exclusive role of RB1 and CDKN2A mutations in lung cancer subtypes demonstrates a synthetic lethal strategy for cancer regulation.
Keywords: cancer cell line panel, RB1, CDKN2A, gene expression, reverse-phase protein array, lung cancer
Introduction
Understanding heterogeneous genetic alterations in tumors is recognized as a key factor in advancing cancer therapy (1–3). Lung cancers are classified into two subtypes, non-small cell lung carcinoma (NSCLC) and small cell lung carcinoma (SCLC), which harbor exclusive specific mutations: RB1 in SCLC and CDKN2A (p16INK4A) in NSCLC (4). Both the RB1 and CDKN2A genes are tightly associated with cell cycle regulation, and CDKN2A regulates RB1 phosphorylation through cyclin E and D1 (5,6). The crucial role of RB1 as a regulator in cell cycle progression has been intensively investigated (7–9). Accumulated data have demonstrated mutually exclusive mutation patterns for genes encoding proteins that function in the same biological pathway. For instance, mutations of the KRAS or BRAF gene, which are downstream of the EGFR signaling pathway, have not been found in EGFR-mutated NSCLC (10,11), and co-mutations of the TP53 and PIK3CA pair (12) or the RB1 and CDKN2A pair (13) rarely occur in the same tumors. However, the biological meaning of such mutually exclusive mutation patterns is not fully understood, even though this exclusiveness does serve as an attractive target for the development of novel therapeutics (14).
An understanding of differential regulation along with distinct mutations in RB1 and CDKN2A is required to identify molecular characteristics of the progression of SCLC and NSCLC subtypes. Large-scale cell line-based high-throughput transcriptome and proteome datasets facilitate the understanding of molecular characterization of cancers through genome-wide functional analyses. The National Cancer Institute (NCI) released well-annotated sets of both DNA microarray data to detect the gene expression and reverse-phase protein array (RPPA) data to detect the total protein and phosphorylation on 60 well-characterized cancer cell lines (15). Diverse omics datasets on an expanded panel of >300 cancer cell lines were also generated by GlaxoSmithKline (GSK) (16). Together with these datasets, the extensive mutation profile on individual cell lines is available from the COSMIC (Catalogue of Somatic Mutations in Cancer) database (17). Through mutation-oriented association studies on cell line-based omics data, we have reported new targets and mechanisms for cancer regulation (3,18,19).
In the present study, the regulation of gene and protein levels driven by RB1 or CDKN2A mutations in lung cancer was analyzed using transcriptome and proteome datasets obtained from 318 diverse cancer cell lines. We attempted to identify the differentially regulated gene/protein signatures and functional pathways specific to RB1 and CDKN2A mutations. Furthermore, we experimentally investigated whether double or complementary knockdown of RB1 or CDKN2A gene expression has a specific effect on the reciprocal mutant subtype in lung cancer cell lines. We expect that this study will provide a useful resource for the regulation of lung cancer progression using synergistic mechanisms of exclusive RB1 or CDKN2A mutations.
Materials and methods
Data acquisition
The large-scale transcriptome dataset on 318 cancer cell lines was obtained from the Cancer Biomedical Informatics Grid (caBIG) website (https://cabig.nci.nih.gov/caArray_GSKdata) (16). This dataset, also known as the GlaxoSmithKline (GSK) dataset, has 950 arrays performed in triplicate for each cell line with the Affymetrix U133 Plus 2.0 Array chip. It was normalized to MAS5 and then transformed to a log2 scale.
The reverse-phase protein array (RPPA) dataset to detect protein expression and phosphorylation was generated in the Functional Proteomics Core of the M.D. Anderson Cancer Center using a total of 179 cancer cell lines, which were included in the transcriptome dataset. These cell lines were purchased from several vendors (American Type Culture Collection; Developmental Therapeutics Program, National Cancer Institute; German Resource Centre for Biological Material and European Collection of Animal Cell Cultures) and grown in standard culture media as recommended by the vendor. The genetic identity of cell lines was determined by cross comparing all cell lines in this set (16,20). The cells were maintained in RPMI-1640 supplemented with 5% fetal bovine serum at 37°C in a humidified 5% CO2 atmosphere. Proteins were harvested when the cells reached ~70% confluence. The cells were lysed in buffer containing 1% Triton X-100, 50 mM HEPES pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM ethylene glycol tetraacetic acid, 100 mM NaF, 10 mM NaPPi, 10% glycerol, 1 mM Na3VO4 and complete protease inhibitor cocktail (Roche Diagnostics). Protein supernatants were isolated using standard methods (21), and the protein concentration was determined using the bicinchoninic acid assay (22). Samples were diluted to a uniform protein concentration and denatured in 1% sodium dodecyl sulfate for 10 min at 95°C. Samples were stored at −80°C until use. RPPA analysis was performed as described previously (21,23,24). A logarithmic value reflecting the relative amount of each protein in each sample was generated for subsequent analyses. The RPPA analysis was performed using a total of 115 antibodies.
The annotation of somatic mutation on all cell lines was organized by the COSMIIC (Catalogue of Somatic Mutations in Cancer) database (http://cancer.sanger.ac.uk/cosmic) (17).
Enrichment analysis of somatic mutations
To describe the selectivity of mutation occurrence, we calculated enrichment scores using an odds ratio between the observed odds and expected odds. The observed odds score is the ratio for the number of mutated cell lines in a specific cancer type via the number of cell lines in a specific cancer type. The expected odds score is the ratio for the number of mutated cell lines vs. the total number of cell lines. In addition, the probability of an odds ratio was calculated by the Fisher exact test using the R open-source computing language, version 2.15. The Fisher exact test uses a hypergeometric distribution to determine the significance of the agreement between individual question pairs (25).
Mutation-specific gene and protein expression analysis
For the selection of RB1 and CDKN2A mutation-specific gene and protein expression markers together with excluding the subtype-dependent expressions, lung cancer cell lines were classified into two groups: NSCLC and SCLC. Then, we divided the cell lines of each subtype into two groups based on the mutational status of RB1 and CDKN2A. CDKN2A-mutant and wild-type cell lines were mainly considered in the NSCLC type. RB1-mutant and wild-type cell lines were considered in the SCLC type. As a result, in the transcriptome dataset, we classified 9, 16, 22 and 24 cell line samples into the following four groups, respectively: RB1wt SCLC; RB1mt SCLC; CDKN2Awt NSCLC; and CDKN2Amt NSCLC. In the RPPA dataset, we classified 4, 7, 4 and 16 cell line samples into four groups, respectively: RB1wt SCLC; RB1mt SCLC; CDKN2Awt NSCLC; and CDKN2Amt NSCLC. The gene expression was detected using a log2 fold change value for the average difference of mutant and wild-type cell lines. The significance was confirmed by a t-test.
The patterns of gene expression were analyzed through a hierarchical clustering method. The clustering and its visualization on a heatmap were performed using the software QCanvas (26). QCanvas can be downloaded freely from the website http://compbio.sookmyung.ac.kr/~qcanvas.
Gene set enrichment analysis
Pathway analysis was performed using the GSEA (Gene Set Enrichment Analysis) method (27). Gene sets, integrated from Reactome, PID, KEGG, and Biocarta database, were obtained from the online pathway database, MSigDB v3.1 (http://www.broadinstitute.org/gsea/msigdb). The significantly (p<0.01) enriched gene sets among the results of the GSEA were reorganized based on major functional categories in each database.
Cell culture
NCI-60 lung cancer cell lines (NCI-H460, A549, NCI-H322M, NCI-H226, EKVX, and NCI-H23) were obtained from National Cancer Institute (NCI DTP), USA. NCI-H1993, NCI-H1935, NCI-H82 and NCI-H524 were obtained from American Type Culture Collection (ATCC). All cells were grown in RPMI-1640 medium (HyClone, USA) with 10% FBS (HyClone) and 1% penicillin/streptomycin (Gibco, USA), and maintained at 37°C in a humidified atmosphere at 5% CO2
siRNA transfection and cell viability assay
To detect cell viability after siRNA transfection, the cells were seeded in a 96-well plate at a density of 5,000 cells per well. After adhering for 24 h, target siRNAs were added in transfection medium (Gibco) for 6 h at 37°C in a CO2 incubator. siRB1 (L-003296-02), siCDKN2A (L-011007-00) and non-targeting siRNA (D-001810-10) were purchased from Dharmacon Inc. (Lafayette, CO, USA). After being cultured for 72 h at 37°C, 5% CO2, cell viability was detected using a CellTiter-Blue Cell Viability Assay (Promega, Madison, WI, USA).
Results and Discussion
RB1 and CDKN2A mutations in SCLC and NSCLC cell lines
Genetic alterations affecting the same biological pathway are generally not found in the same cancer cell. Accordingly, exclusive mutation patterns of RB1 and CDKN2A genes have been observed in the lung cancer subtypes SCLC and NSCLC (4,13). Based on the analysis of mutation frequencies across 318 cell lines, we found the general exclusiveness of RB1 and CDKN2A mutations in diverse cancer lineages (Fig. 1A). RB1 mutations were significantly enriched in urinary tract and lung cancer cell lines yet rarely found in liver, renal, pancreatic and skin cancers, in which CDKN2A mutations were frequent. Furthermore, among 71 lung cancer cell lines, 25 SCLC-derived cells were significantly enriched with RB1 mutations, whereas 46 NSCLCs predominantly contained STK11, KRAS and CDKN2A mutations (Fig. 1B). Taken together, the mutations of RB1 and CDKN2A genes, which belong to a common functional pathway, were clearly exclusive from each other among frequently mutated genes in diverse cancer cell lines (Fig. 1C).
Figure 1.
RB1 and CDKN2A mutation frequency in the cancer cell line panel. (A) Enrichment score of RB1 and CDKN2A mutation frequency among diverse cancer lineages. The enrichment score was calculated by the odds ratio between the observed and expected odds. The observed odds ratio is the ratio for RB1 or CDKN2A mutation among each cancer type. The expected odds ratio is the ratio for RB1 or CDKN2A mutation among all 318 cell lines. (B) The enrichment score of major mutation frequency in non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). The observed odds ratio is the ratio for each mutation frequency in 46 NSCLC or 25 SCLC cell lines. The expected odds ratio is the ratio for each mutation frequency in all 318 cell lines. (C) The distribution of major gene mutations in lung cancer cell lines. Statistical significance of *p<0.05 and **p<0.01, respectively.
Differential gene expression profiles between RB1mt SCLCs and CDKN2Amt NSCLCs
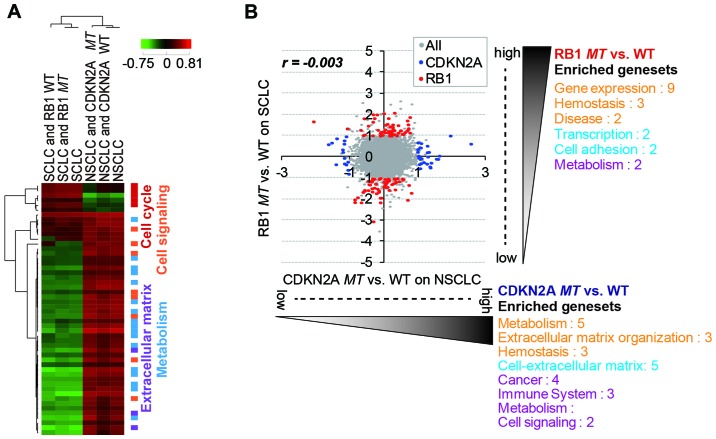
To find lineage-independent, mutation-specific gene expression patterns, we classified 9, 16, 22 and 24 cell line samples into four groups, RB1wt SCLC, RB1mt SCLC, CDKN2Awt NSCLC and CDKN2Amt NSCLC, and analyzed the group-specific gene expression patterns using DNA microarray data. There was no general correlation of gene expression between the SCLC and NSCLC cell lines (Fig. 2A), and significantly enriched gene sets were also different between the lung subtypes. However, RB1mt SCLC and CDKN2Amt NSCLC cells showed a negative correlation in gene expression (Fig. 2B), whereas RB1wt SCLC and CDKN2Awt NSCLC exhibited a positive correlation (Fig. 2C). This observation indicated that RB1 and CDKN2A mutations caused lineage-specific distinctive changes in gene expression.
Figure 2.
Comparison of gene expression in SCLC and NSCLC with the mutational status of RB1 and CDKN2A. (A) The expression change of a total of 22,357 gene probes in SCLC and NSCLC cell lines. Major categories of the gene sets significantly (p<0.01) over-enriched for each gene expression were analyzed using gene set enrichment analysis (GSEA). Gene sets from different pathway DBs (orange, Reactome; sky-blue, PID; purple, KEGG; and green, Biocarta) were collected. The number indicates the number of gene sets per category. (B) Comparison of gene expressional change in RB1-mutated SCLC and CDKN2A-mutated NSCLC. Red represents 1,208 RB1-mutated SCLC-specific gene signatures and blue represents 159 RB1-mutated SCLC-specific gene signatures (>2-fold change and p<0.01). A total of 42 gene probes were commonly selected between them. (C) Comparison of gene expression change in SCLC and NSCLC cell lines with RB1 and CDKN2A wild-type, respectively. The colored gene signatures were consistent with (B). The expressional change for each gene probe was calculated by the log2 fold change via the median across 318 cell lines. The r value represents the Pearson correlation coefficient.
Our analysis showed that the lineage difference was generally more important than RB1 and CDKN2A mutational status in the differential gene expression pattern (Fig. 3A). Thus, we attempted to identify RB1mt- and CDKN2Amt-specific gene signatures by separately analyzing SCLC and NSCLC cells (Fig. 3B). As a result, we were able to identify distinct mutation-specific gene signatures for which expression was significantly regulated (>2-fold change and p<0.05) in each subtype (Tables I and II). Of note, the significantly over-enriched (p<0.01) gene sets (functional categories of selected gene signatures) generally did not overlap between the two mutation groups (Fig. 3B). The upregulated gene sets with RB1 mutation in SCLC cell lines mainly belonged to functional categories of transcription. The hit list included known target genes of E2F, which are released and activated upon RB1 inactivation (28). The upregulated genes upon CDKN2A mutation in NSCLC cell lines were largely enriched in the gene sets of extracellular matrix and metabolism. Genes related to the extracellular matrix are known to be important factors for enhancing tumorigenicity and promoting metastasis (29). Although CDKN2A and RB1 are known to function in the same pathway of cell cycle regulation, inactivation of the mutations might have a different functional role in cancer development or progression in SCLC and NSCLC subtypes.
Figure 3.
RB1mt- and CDKN2Amt-specific gene signatures in SCLC and NSCLC cells. (A) Enrichment score (ES) map of significantly (p<0.01) over-enriched gene sets in SCLC and NSCLC cell lines. Each subtype was further divided by RB1 or CDKN2A mutational status. The color index on the right side represents the functional category of clustered gene sets. The individual gene expression level in Fig. 2 was used for gene set enrichment analysis (GSEA). (B) Comparison of gene expression change along RB1 and CDKN2A mutations. The red color represents 159 transcription markers specific to the RB1 mutation, and blue represents 122 transcription markers specific to the CDKN2A mutation (>2-fold change and p<0.05). The complete list of the selected markers is available in Table I and II. The scale of the plot is log2 fold change of differential gene expression. It was calculated by the differences of average log2 gene expression between mutation and wild-type cell lines in a given subtype. Major categories of the gene sets significantly (p<0.01) over-enriched for each gene expression were analyzed by GSEA. Gene sets from different pathway DBs (orange, Reactome; sky-blue, PID; purple, KEGG; and green, Biocarta) were collected. The value next to each category indicates the number of sub-gene sets. The r value represents the Pearson correlation coefficient.
Table I.
The RB1mt-specific gene signatures in SCLC.
| Upregulated genes specific to RB1mt | Downregulated genes specific to RB1mt | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| ProbeID | Symbol | log2 fold change | p-value | ProbeID | Symbol | log2 fold change | p-value |
| 231736_x_at | MGST1 | 2.083 | 0.001 | 202834_at | AGT | −3.059 | 0 |
| 218847_at | IGF2BP2 | 2.058 | 0.011 | 1566764_at | MACC1 | −2.16 | 0.035 |
| 202620_s_at | PLOD2 | 2.01 | 0.002 | 205501_at | PDE10A | −2.159 | 0.004 |
| 213139_at | SNAI2 | 2.002 | 0.016 | 204044_at | QPRT | −2.149 | 0.004 |
| 206332_s_at | IFI16 | 1.879 | 0.03 | 239503_at | Unknown | −2.041 | 0 |
| 235763_at | SLC44A5 | 1.829 | 0.009 | 208891_at | DUSP6 | −2.019 | 0.006 |
| 204646_at | DPYD | 1.828 | 0.005 | 1560652_at | Unknown | −1.943 | 0.019 |
| 202016_at | MEST | 1.817 | 0.003 | 203881_s_at | DMD | −1.937 | 0.006 |
| 226225_at | MCC | 1.717 | 0.045 | 208892_s_at | DUSP6 | −1.921 | 0.005 |
| 217028_at | CXCR4 | 1.675 | 0.023 | 206218_at | MAGEB2 | −1.732 | 0.013 |
| 214597_at | SSTR2 | 1.655 | 0.038 | 203132_at | RB1 | −1.709 | 0.006 |
| 210839_s_at | ENPP2 | 1.557 | 0.04 | 205305_at | FGL1 | −1.67 | 0.006 |
| 203038_at | PTPRK | 1.531 | 0.001 | 201328_at | ETS2 | −1.663 | 0.005 |
| 222553_x_at | OXR1 | 1.528 | 0.003 | 205110_s_at | FGF13 | −1.651 | 0.036 |
| 1558217_at | SLFN13 | 1.515 | 0.045 | 209365_s_at | ECM1 | −1.61 | 0.014 |
| 1565162_s_at | MGST1 | 1.493 | 0.016 | 210102_at | VWA5A | −1.597 | 0.005 |
| 204620_s_at | VCAN | 1.47 | 0.011 | 209468_at | LRP5 | −1.583 | 0.001 |
| 221731_x_at | VCAN | 1.44 | 0.018 | 1558882_at | LOC401233 | −1.574 | 0.032 |
| 218197_s_at | OXR1 | 1.409 | 0.006 | 219750_at | TMEM144 | −1.572 | 0.034 |
| 205229_s_at | COCH | 1.338 | 0.006 | 223748_at | SLC4A11 | −1.552 | 0.002 |
| 203184_at | FBN2 | 1.338 | 0.026 | 205601_s_at | HOXB5 | −1.511 | 0.023 |
| 205027_s_at | MAP3K8 | 1.311 | 0.004 | 209803_s_at | PHLDA2 | −1.495 | 0.038 |
| 204030_s_at | SCHIP1 | 1.308 | 0.038 | 212268_at | SERPINB1 | −1.467 | 0.001 |
| 241400_at | Unknown | 1.296 | 0.006 | 1569191_at | ZNF826 | −1.448 | 0.022 |
| 1555788_a_at | TRIB3 | 1.274 | 0.034 | 212188_at | KCTD12 | −1.43 | 0.002 |
| 211675_s_at | MDFIC | 1.272 | 0.012 | 241672_at | C13orf36 | −1.414 | 0.033 |
| 229465_s_at | PTPRS | 1.255 | 0.008 | 219305_x_at | FBXO2 | −1.337 | 0.015 |
| 225093_at | UTRN | 1.255 | 0.042 | 1554472_a_at | PHF20L1 | −1.317 | 0 |
| 205122_at | TMEFF1 | 1.251 | 0.01 | 203028_s_at | CYBA | −1.308 | 0.047 |
| 219489_s_at | NXN | 1.238 | 0.035 | 228726_at | Unknown | −1.303 | 0.01 |
| 225056_at | SIPA1L2 | 1.237 | 0.011 | 204158_s_at | TCIRG1 | −1.302 | 0.006 |
| 208949_s_at | LGALS3 | 1.235 | 0.021 | 211538_s_at | HSPA2 | −1.279 | 0.035 |
| 201063_at | RCN1 | 1.229 | 0.033 | 220082_at | PPP1R14D | −1.259 | 0.008 |
| 235244_at | CCDC58 | 1.184 | 0.032 | 203005_at | LTBR | −1.257 | 0.011 |
| 210978_s_at | TAGLN2 | 1.184 | 0.005 | 229964_at | C9orf152 | −1.23 | 0.036 |
| 233903_s_at | SGEF | 1.182 | 0.003 | 203961_at | NEBL | −1.212 | 0.032 |
| 205123_s_at | TMEFF1 | 1.177 | 0.019 | 224577_at | ERGIC1 | −1.206 | 0.002 |
| 200897_s_at | PALLD | 1.164 | 0.018 | 238021_s_at | CRNDE | −1.189 | 0.022 |
| 200916_at | TAGLN2 | 1.161 | 0.015 | 223041_at | CD99L2 | −1.182 | 0.001 |
| 215127_s_at | RBMS1 | 1.143 | 0.03 | 205586_x_at | VGF | −1.182 | 0.008 |
| 202887_s_at | DDIT4 | 1.141 | 0.005 | 239278_at | Unknown | −1.163 | 0.013 |
| 212636_at | QKI | 1.137 | 0.014 | 213689_x_at | FAM69A | −1.157 | 0.005 |
| 214877_at | CDKAL1 | 1.134 | 0.03 | 232099_at | PCDHB16 | −1.153 | 0.028 |
| 227197_at | SGEF | 1.129 | 0.005 | 219256_s_at | SH3TC1 | −1.153 | 0.005 |
| 224918_x_at | MGST1 | 1.12 | 0.02 | 227943_at | Unknown | −1.141 | 0.004 |
| 227522_at | CMBL | 1.08 | 0.007 | 210538_s_at | BIRC3 | −1.138 | 0.024 |
| 206385_s_at | ANK3 | 1.073 | 0.042 | 1568838_at | LOC100132169 | −1.117 | 0.032 |
| 226464_at | C3orf58 | 1.072 | 0.01 | 229872_s_at | LOC100132999 | −1.099 | 0.021 |
| 1568720_at | ZNF506 | 1.054 | 0.04 | 1555579_s_at | PTPRM | −1.09 | 0.043 |
| 201656_at | ITGA6 | 1.04 | 0.028 | 224997_x_at | H19 | −1.082 | 0.032 |
| 212190_at | SERPINE2 | 1.034 | 0.037 | 213005_s_at | KANK1 | −1.081 | 0.01 |
| 204995_at | CDK5R1 | 1.022 | 0.017 | 219371_s_at | KLF2 | −1.076 | 0.013 |
| 210512_s_at | VEGFA | 1.02 | 0.037 | 37408_at | MRC2 | −1.074 | 0.01 |
| 226419_s_at | FLJ44342 | 1.015 | 0.001 | 224391_s_at | SIAE | −1.059 | 0.01 |
| 210735_s_at | CA12 | 1.011 | 0.032 | 201329_s_at | ETS2 | −1.053 | 0.022 |
| 65588_at | LOC388796 | 1.002 | 0.009 | 205016_at | TGFA | −1.049 | 0.007 |
| 213857_s_at | CD47 | 1.001 | 0.002 | 227384_s_at | LOC727820 | −1.043 | 0.002 |
| 208622_s_at | EZR | 1.001 | 0.001 | 228010_at | PPP2R2C | −1.033 | 0.031 |
| 209500_x_at | TNFSF12/TNFSF13 | −1.031 | 0.019 | ||||
| 224576_at | ERGIC1 | −1.031 | 0.007 | ||||
| 236719_at | Unknown | −1.025 | 0.004 | ||||
| 227001_at | NIPAL2 | −1.021 | 0.006 | ||||
| 230722_at | BNC2 | −1.019 | 0.047 | ||||
| 204682_at | LTBP2 | −1.007 | 0.024 | ||||
Table II.
The CDKN2Amt-specific gene signatures in NSCLC.
| Upregulated genes specific to CDKN2Amt | Downregulated genes specific to CDKN2Amt | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| ProbeID | Symbol | log2 fold change | p-value | ProbeID | Symbol | log2 fold change | p-value |
| 236694_at | CYorf15A | 2.585 | 0.001 | 228956_at | UGT8 | −1.618 | 0.001 |
| 211980_at | COL4A1 | 1.978 | 0.006 | 209644_x_at | CDKN2A | −1.52 | 0.004 |
| 213725_x_at | XYLT1 | 1.654 | 0.023 | 225681_at | CTHRC1 | −1.396 | 0.014 |
| 204971_at | CSTA | 1.615 | 0.022 | 1554242_a_at | COCH | −1.373 | 0.008 |
| 209970_x_at | CASP1 | 1.566 | 0.001 | 218820_at | C14orf132 | −1.199 | 0.012 |
| 225688_s_at | PHLDB2 | 1.412 | 0.017 | 200884_at | CKB | −1.191 | 0.001 |
| 202638_s_at | ICAM1 | 1.388 | 0.011 | 227623_at | Unknown | −1.156 | 0.018 |
| 222453_at | CYBRD1 | 1.377 | 0.016 | 207558_s_at | PITX2 | −1.151 | 0.014 |
| 1562102_at | AKR1C1 | 1.344 | 0.048 | 236302_at | PPM1E | −1.151 | 0.009 |
| 208782_at | FSTL1 | 1.312 | 0.014 | 209198_s_at | SYT11 | −1.145 | 0.007 |
| 211340_s_at | MCAM | 1.299 | 0.002 | 1560023_x_at | Unknown | −1.097 | 0.01 |
| 210004_at | OLR1 | 1.299 | 0.01 | 214321_at | NOV | −1.094 | 0.035 |
| 202008_s_at | NID1 | 1.286 | 0.004 | 205229_s_at | COCH | −1.061 | 0.031 |
| 202350_s_at | MATN2 | 1.197 | 0.012 | 223551_at | PKIB | −1.045 | 0.046 |
| 239999_at | Unknown | 1.126 | 0.033 | 230130_at | Unknown | −1.04 | 0.049 |
| 205407_at | RECK | 1.117 | 0.014 | 212706_at | LOC100286937/LOC100287164/RASA4 | −1.019 | 0.003 |
| 203304_at | BAMBI | 1.113 | 0.012 | ||||
| 228698_at | SOX7 | 1.104 | 0.014 | ||||
| 227051_at | Unknown | 1.088 | 0.036 | ||||
| 201939_at | PLK2 | 1.082 | 0.017 | ||||
| 209087_x_at | MCAM | 1.081 | 0.007 | ||||
| 206165_s_at | CLCA2 | 1.067 | 0.025 | ||||
| 227178_at | CUGBP2 | 1.067 | 0.012 | ||||
| 227253_at | CP | 1.044 | 0.015 | ||||
| 212262_at | QKI | 1.043 | 0.002 | ||||
| 202998_s_at | LOXL2 | 1.039 | 0.006 | ||||
| 214022_s_at | IFITM1 | 1.021 | 0.034 | ||||
| 211366_x_at | CASP1 | 1.019 | 0.001 | ||||
| 222446_s_at | BACE2 | 1.014 | 0.009 | ||||
Specific change in total proteins and phosphoproteins in RB1 and CDKN2A mutations
We characterized the differential regulation of RB1 and CDKN2A mutations at the protein level using RPPA data of 77 pan- and 38 phospho-antibodies for 89 proteins across 179 cancer cell lines. Consistent with the patterns of gene expression data, the overall protein expression and phosphorylation status were inversely correlated between RB1mt SCLC and CDKN2Amt NSCLC cell lines (Fig. 4). Thus, the mutational effect of RB1 and CDKN2A genes were separately analyzed in SCLC and NSCLC cell lines (Fig. 5). The results showed that β-catenin was commonly over expressed in both RB1 and CDKN2A mutants. Wnt/β-catenin overexpression has been extensively reported in lung cancer (30), and the overexpression of β-catenin might be maintained by the mutational effect of both RB1 and CDKN2A genes. The RB1 mutation specifically regulated PTEN, STAT, mTOR, p53 expression and MAPK phosphorylation in SCLC cells. However, the CDKN2A mutation altered the expression of JNK2 and cKIT and the phosphorylation status of AKT, STAT3 and AMPKa.
Figure 4.
Comparison of protein expression and phosphorylation in SCLC and NSCLC with the mutational status of RB1 and CDKN2A. Protein expression change of a total of 77 pan-antibodies was compared between (A) SCLC and NSCLC, (B) RB1-mutated SCLC and CDKN2A-mutated NSCLC, and (C) SCLC and NSCLC with RB1 and CDKN2A wild-type cell lines, respectively. Protein phosphorylation change of a total of 38 phospho-antibodies was compared between (D) SCLC and NSCLC, (E) RB1-mutated SCLC and CDKN2A-mutated NSCLC, and (F) SCLC and NSCLC with RB1 and CDKN2A wild-type cell lines, respectively. The phosphorylation change for each protein phospho-antibody was calculated by the log2 fold change via the median across 179 cell lines. The r value represents the Pearson correlation coefficient.
Figure 5.
Protein and phosphoprotein signatures specific to RB1 or CDKN2A mutations. (A) Comparison of differential protein expression (77 pan-antibody data) between RB1 and CDKN2A mutations. (B) Comparison of differential protein phosphorylation (38 phospho-antibody data) between RB1 and CDKN2A mutations. The red color represents protein markers specific to the RB1 mutation, and blue represents protein markers specific to the CDKN2A mutation (>1-fold change and p<0.05). The scale of the plots is the log2 fold change of protein expression and phosphorylation, respectively. The value was calculated by the differences of average log2 expression or phosphorylation level between mutation and wild-type cell lines in the given subtype. The r value represents the Pearson correlation coefficient.
MAPK (T202), which is significantly (p<0.05) phosphorylated in RB1-mutated SCLC cancer cell lines, has an important role in transcriptional regulation of targeting transcription factors such as c-Jun, c-Fos, and c-Myc (31). This observation is consistent with the DNA microarray data (Fig. 3B) for RB1mt SCLC cells, which are enriched in the functional categories of transcription. AKT is specifically phosphorylated (S473, T308) in CDKN2Amt NSCLC and related to focal adhesion (32), which is the enriched gene set of CDKN2Amt NSCLC from DNA microarray analysis. Furthermore, PTEN, which was overexpressed in RB1mt SCLC cells (Fig. 5A), is a well-known negative regulator of AKT activation (33), suggesting that AKT-mediated signaling might be exclusively activated by CDKN2Amt in NSCLC, not by RB1mt in SCLC. Both proteome and transcriptome data analyses demonstrated that exclusive RB1 and CDKN2A mutations in different subtypes of lung cancer included a differential change of gene expression and protein regulation, even though RB1 and CDKN2A are in the same cell cycle-related pathway.
Synthetic lethality of reciprocal regulation of RB1 and CDKN2A expression
Through the systematic analysis of transcriptome and proteome data, we found unique mRNA and protein regulation patterns induced by the mutation of either the RB1 gene or the CDKN2A gene (Fig. 6A). Furthermore, we investigated the synergistic negative effect on cancer growth by simultaneous functional loss (or knockdown) of these two genes. We performed a viability assay with diverse lung cancer cell lines with the combined knockdown of RB1 and CDKN2A genes using siRNA-mediated gene depletion. As a result, the knockdown of one of these genes decreased the viability of cells harboring a mutation of the other gene (Fig. 6B). The viability of CDKN2A-mutant cell lines was significantly decreased by knockdown of RB1; similarly, RB1-mutant cell lines were inhibited by CDKN2A depletion. Consistently, the simultaneous depletion of RB1 and CDKN2A genes significantly decreased the viability of lung cell lines harboring wild-types of these genes (Fig. 6C). However, the single knockdown of either the RB1 gene or the CDKN2A gene did not effectively reduce viability in these wild-type cell lines. In conclusion, the functional inhibition of the RB1 or CDKN2A gene in CDKN2Amt or RB1mt cancer cells, respectively, might be a promising therapeutic approach in SCLC or NSCLC lung cancers. The present study on differential proteome and transcriptome profiles between two mutant groups provides mechanistic insights into the synthetic lethality of RB1 and CDKN2A mutations.
Figure 6.
Differential viability change of lung cancer subtypes induced by reciprocal knockdown of the RB1 and/or CDKN2A genes. (A) A schematic summary of biological functions dysregulated by the mutation status of RB1 and CDKN2A. (B) The effects of siRB1 and siCDKN2A in lung cell lines with CDKN2A mutations (NCI-H82 and NCI-H524) and RB1 mutations (NCI-H460, A549, NCI-H322M, and NCI-H226), respectively. (C) The effect of single- or double-knockdown of RB1 and CDKN2A gene expression on the viability of RB1- and CDKN2A-positive cell lines (EKVX, NCI-H23, NCI-H1395, and NCI-H1993). The cells were incubated in siRB1 and siCDKN2A and cultured for 72 h. Cell viability was determined using a CellTiter-Blue assay. The siRNA-mediated gene depletion efficacy of RB1 or CDKN2A in each tested cell line was evaluated (data not shown). The % cell viability was calculated using siNC as the negative control for each siRNA treatment. *p<0.05 and **p<0.01 between the compared groups.
Acknowledgements
This study was supported by Sookmyung Women's University Research Grant 1-1303-0160.
References
- 1.Kim N, He N, Kim C, Zhang F, Lu Y, Yu Q, Stemke-Hale K, Greshock J, Wooster R, Yoon S, et al. Systematic analysis of genotype-specific drug responses in cancer. Int J Cancer. 2012;131:2456–2464. doi: 10.1002/ijc.27529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Settleman J. Cell culture modeling of genotype-directed sensitivity to selective kinase inhibitors: Targeting the anaplastic lymphoma kinase (ALK) Semin Oncol. 2009;36(Suppl 1):S36–S41. doi: 10.1053/j.seminoncol.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 3.He N, Kim N, Song M, Park C, Kim S, Park EY, Yim HY, Kim K, Park JH, Kim KI, et al. Integrated analysis of transcriptomes of cancer cell lines and patient samples reveals STK11/LKB1-driven regulation of cAMP phosphodiesterase-4D. Mol Cancer Ther. 2014;13:2463–2473. doi: 10.1158/1535-7163.MCT-14-0297. [DOI] [PubMed] [Google Scholar]
- 4.Knudsen ES, Knudsen KE. Tailoring to RB: Tumour suppressor status and therapeutic response. Nat Rev Cancer. 2008;8:714–724. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie Z, Liu D. Pathogenesis of molecular signaling pathways changes in smoking-induced lung cancer. Zhongguo Fei Ai Za Zhi. 2009;12:1202–1205. doi: 10.3779/j.issn.1009-3419.2009.11.14. in Chinese. [DOI] [PubMed] [Google Scholar]
- 6.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 7.Hernando E, Nahlé Z, Juan G, Diaz-Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald W, Benezra R, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 8.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 9.Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2:910–917. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- 10.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: Biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 11.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 12.Singh B, Reddy PG, Goberdhan A, Walsh C, Dao S, Ngai I, Chou TC, O-Charoenrat P, Levine AJ, Rao PH, et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002;16:984–993. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco R, Iwakawa R, Tang M, Kohno T, Angulo B, Pio R, Montuenga LM, Minna JD, Yokota J, Sanchez-Cespedes M. A gene-alteration profile of human lung cancer cell lines. Hum Mutat. 2009;30:1199–1206. doi: 10.1002/humu.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etemadmoghadam D, Weir BA, Au-Yeung G, Alsop K, Mitchell G, George J, Davis S, D'Andrea AD, Simpson K, Hahn WC, et al. Australian Ovarian Cancer Study Group. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc Natl Acad Sci USA. 2013;110:19489–19494. doi: 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoemaker RH, Monks A, Alley MC, Scudiero DA, Fine DL, McLemore TL, Abbott BJ, Paull KD, Mayo JG, Boyd MR. Development of human tumor cell line panels for use in disease-oriented drug screening. Prog Clin Biol Res. 1988;276:265–286. [PubMed] [Google Scholar]
- 16.Greshock J, Bachman KE, Degenhardt YY, Jing J, Wen YH, Eastman S, McNeil E, Moy C, Wegrzyn R, Auger K, et al. Molecular target class is predictive of in vitro response profile. Cancer Res. 2010;70:3677–3686. doi: 10.1158/0008-5472.CAN-09-3788. [DOI] [PubMed] [Google Scholar]
- 17.Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39(Database):D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim N, He N, Yoon S. Cell line modeling for systems medicine in cancers (Review) Int J Oncol. 2014;44:371–376. doi: 10.3892/ijo.2013.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong E, He N, Park H, Song M, Kim N, Lee S, Yoon S. MACE: mutation-oriented profiling of chemical response and gene expression in cancers. Bioinformatics. 2015;31:1508–1514. doi: 10.1093/bioinformatics/btu835. [DOI] [PubMed] [Google Scholar]
- 20.McDermott U, Sharma SV, Dowell L, Greninger P, Montagut C, Lamb J, Archibald H, Raudales R, Tam A, Lee D, et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci USA. 2007;104:19936–19941. doi: 10.1073/pnas.0707498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craft CS, Zou W, Watkins M, Grimston S, Brodt MD, Broekelmann TJ, Weinbaum JS, Teitelbaum SL, Pierce RA, Civitelli R, et al. Microfibril-associated glycoprotein-1, an extra-cellular matrix regulator of bone remodeling. J Biol Chem. 2010;285:23858–23867. doi: 10.1074/jbc.M110.113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, Kornblau SM. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 25.Agresti A John Wiley & Sons. Wiley Series in Probability and Statistics. Wiley-Interscience; New York: 2002. Categorical Data Analysis. [Google Scholar]
- 26.Kim N, Park H, He N, Lee HY, Yoon S. QCanvas: An Advanced Tool for Data Clustering and Visualization of Genomics Data. Genomics Inform. 2012;10:263–265. doi: 10.5808/GI.2012.10.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 2001;264:42–55. doi: 10.1006/excr.2000.5149. [DOI] [PubMed] [Google Scholar]
- 29.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extra-cellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazieres J, He B, You L, Xu Z, Jablons DM. Wnt signaling in lung cancer. Cancer Lett. 2005;222:1–10. doi: 10.1016/j.canlet.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Basson MD. Akt directly regulates focal adhesion kinase through association and serine phosphorylation: Implication for pressure-induced colon cancer metastasis. Am J Physiol Cell Physiol. 2011;300:C657–C670. doi: 10.1152/ajpcell.00377.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]