Abstract
Background:
Pediatric seizures have been linked to psychiatric disorders in childhood, but there is a lack of large-scale population-based studies of psychiatric comorbidity in later life. This study examines the relation between childhood seizures and the risk of psychiatric disorders in adolescence and early adulthood.
Methods:
We carried out a register-based cohort study of all individuals born in Denmark in 1978–2002. Using diagnostic information from the Danish National Patient Register, all cohort members were categorized according to occurrence of febrile seizures and epilepsy, prior to entering follow-up on the child’s 10th birthday. The children were followed until onset of mental illness, death, emigration, or the end of the study period on December 31, 2012. Cox regression analyses were used to estimate the risk of five pre-defined groups of psychiatric disorders (substance abuse disorders, schizophrenia, mood disorder, anxiety, and personality disorder), separately and combined. The models were adjusted for relevant confounders.
Findings:
We followed 1,291,679 individuals, including 43,148 with febrile seizures, 10,355 with epilepsy, and 1,696 with both, for a total of ~15 million person years and identified 83,735 cohort members with at least one of the psychiatric disorders of interest. The risk of any psychiatric disorder was elevated in individuals with a history of febrile seizures (hazards ratio (HR): 1.12, 95% confidence interval (CI): 1.08–1.17), with epilepsy (HR: 1.34, 95% CI: 1.25–1.44), and with both (HR: 1.50, 95% CI: 1.28–1.75). Excess risk of psychiatric illness associated with childhood seizures was present across a range of different disorders, most notably schizophrenia, but also anxiety and mood disorders. The associations did not differ between males and females (p-value: p=0.30), but increased with increasing numbers of hospitalizations for febrile seizures (p-value < 0.001) and with later onset of childhood epilepsy (p-value <0.001).
Interpretation:
Children with epilepsy as well as febrile seizures - with and without concomitant epilepsy - are at elevated risk of developing a broad range of psychiatric disorders in later life. Clarification of the underlying mechanisms responsible for these associations are needed to identify potentials for prevention.
Funding:
This study was supported by the Novo Nordisk Foundation (grant number: NNF16OC0019126), the Danish Epilepsy Association, the Central Denmark Region, the Lundbeck Foundation (grant no R102-A9118 and R155-2014-1724), and the Stanley Medical Research Institute.
Introduction
Pediatric seizures including febrile seizures and epilepsy affect 4–5% of all children, and is thereby some of the most common neurological conditions in childhood.1 Febrile seizures have generally been considered to have a favorable prognosis, with little or no long-lasting implications for child development.2 However, epilepsy has been associated with an increased risk of adverse neuro-psychiatric development. In the youngest children in particular, associations between epilepsy and psychiatric disorders have been demonstrated, including associations with Attention Deficit/Hyperactive Disorder (ADHD)3,4 and autism spectrum disorders.5–7 In older children and younger adults, psychiatric comorbidity encompasses a broad spectrum of conditions, including behavioral disorders, mood disorders, personality disorders, anxiety disorders, and psychotic disorders.8–10
The nature of the relationship between seizures in early childhood and psychiatric disorders is poorly understood. Some hypotheses have evolved around direct effects of the seizures themselves, while others suggest that psychiatric disorders may arise due to the psychosocial disadvantage of having to live with the condition.11 Many studies have documented a bidirectional relation between seizures and psychiatric illness, which may suggest that the associations arise from a shared underlying genetic susceptibility and pathophysiology.12 Psychiatric comorbidity has been shown to be a driving factor of premature mortality among individuals with epilepsy,13 and it is consequently important to clarify whether a similar psychiatric comorbidity, which might also be associated with premature mortality, is seen in children with febrile seizures.
So far, studies have generally been based on clinical or small population samples,14–16 and there is a lack of large-scale studies examining the full range of psychiatric comorbidities following childhood seizures. We conducted a large population-based study to examine the relation between febrile seizures and childhood epilepsy and subsequent risk of a broad spectrum of psychiatric comorbidities in adolescence and early adulthood.
Methods
Study design and study population
This study was conducted as a population-based follow-up study. Using data from the Danish Civil Registration System, we established a cohort of all children born in Denmark between January 1st 1978 and December 31st 2002, and followed them from their 10th birthday. The Danish Civil Registration System includes virtually all individuals living in Denmark, and it contains continuously updated information on vital status and place of residence (including emigrations and immigrations). Each person in the register is assigned a unique personal identification number, which enables accurate linkage between a range of nationwide health and social registries. The study population was restricted to persons who were alive and residing in Denmark on their 10th birthday and where both parents were also born in Denmark, resulting in a sample of n=1,291,679 children.
Assessment of seizures in childhood
Information on diagnoses of febrile seizures and epilepsy in childhood was obtained by linkage to the Danish National Patient Register. This register covers all admissions to hospitals in Denmark from 1977, and all outpatient and emergency room contacts are included from 1995 and onwards. Diagnoses are based on the World Health Organization’s International Classification of Diseases, 8th revision (ICD-8) (1977–1993) and the 10th revision (ICD-10) from 1994 until the end of the study period in 2012. A child was considered as having epilepsy if he or she received any epilepsy diagnosis before the age of 10 years (ICD-8: 345 excluding 345.29, ICD-10 G40) and as having had a febrile seizure if the diagnosis (ICD-8: 780.21, ICD-10: R56.0) was given between the age of 3 months and 5 years, and if there was no recorded diagnosis of epilepsy, cerebral palsy (ICD-8: 343.99, 344.99, ICD-10: G80), intracranial tumors (ICD-8: 191, 225, ICD-10: C70-C71, D32-D33), severe head traumas (ICD-8: 851, 854, ICD-10: S06.1-S06.9), or intracranial infections (ICD-8: 320, 323, ICD-10: G00-G09) prior to the febrile seizure.17 Children were categorized into four groups depending on the occurrence of seizures before the child’s 10th birthday: 1) a history of neither febrile seizures nor epilepsy, 2) a history of febrile seizures only, 3) a history of epilepsy only, and 4) a history of febrile seizures and subsequent epilepsy. The number of hospital contacts with febrile seizures was defined as none, one, two, or ≥ three. We distinguished between febrile seizures occurring before or after the median age at onset (16 months), and additionally defined a group of late onset febrile seizures (i.e. ≥3 years of age).18 For epilepsy, we defined four distinct categories of age at onset, of approximately equal size (<1 years, 1–3 years, 4–6 years, and 7–9 years).
Assessment of psychiatric disorders
Information on incident psychiatric diagnoses in all cohort members was obtained by linkage to the Danish Psychiatric Central Research Register. This register is considered to have complete coverage of all admissions to mental hospitals and psychiatric departments in Denmark from 1970; and from 1995, all outpatient and emergency room contacts are included as well. Individuals found in this register thus represent patients with psychiatric disorders treated in secondary care. To avoid issues related to bidirectionality we did not consider disorders such as ADHD or autism spectrum disorders that are frequently diagnosed in the early years of childhood (i.e. in the same period where we assessed the febrile seizures and epilepsy). Instead we focused on disorders that typically do not develop until adolescence or early adulthood.19 For all cohort members, we considered the following disorders: mental and behavioral disorders due to psychoactive substance abuse (ICD-10: F10-F19), schizophrenia and related disorders (ICD-10: F20-F29), mood disorders (ICD-10: F30-F39), anxiety, stress-related and somatoform disorders (ICD-10: F40-F48), and specific personality disorders (ICD-10: F60-F60.9). For equivalent ICD-8 codes, we refer to Pedersen et al.19 Individuals with multiple disorders were included in the analyses of each specific disorder. The date of onset for each of the disorders was defined as the first date of the first contact.
Statistical analyses
Cox regression models were used to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for psychiatric disorders according to history, number, and age at onset of seizures. Analyses were performed separately for each group of psychiatric disorders, as well as combined (any psychiatric disorder). In all models, the child’s age was used as the underlying time scale. Follow-up of children at risk, (i.e. children with no prior diagnosis of the psychiatric disorder of interest), began on their 10th birthday and continued until diagnosis, emigration from Denmark, death, or end of follow-up on December 31st 2012, whichever came first. All models were stratified by sex, allowing for different baseline hazards in boys and girls. The proportionality assumption was tested using Schoenfeld-scaled residuals and was satisfied for all main exposures. To account for possible confounding, we furthermore adjusted all HRs for a range of perinatal, demographic, and socio-economic factors. Covariates included in the analyses were birth weight, gestational age, Apgar score at 5 minutes, and parental age at birth. Further adjustment was made for maternal education (highest completed) and paternal income in the year the child turned 10. We considered these two different measures of maternal and paternal socio-economic status, as we believed these would each be more stable across the childhood years. Continuous variables were categorized, as none of the associations could be more adequately described by a linear model. Parental psychiatric disorders were treated as time-varying covariates, estimated as time since first diagnosis (unexposed, and subsequently time since diagnosis in intervals increasing from 1 month to 5 years). Similarly, calendar time during follow-up was split into 3–4-year intervals to account for calendar period effects. All other covariates were considered time-independent. The analyses were carried out as complete case analyses and robust standard errors were used to correct for dependency between (maternal) siblings in the population. To estimate the cumulative incidences of psychiatric disorders, we used competing risk regression.
Sensitivity analyses
We performed analyses excluding children with a) status epilepticus (ICD-8: 345.29 and ICD-10: G41), b) seizure onset in the neonatal period (first 28 days after birth), and c) potentially structural causes of epilepsy (head trauma, intracranial infections, intracranial bleedings, cerebral palsy, and intracranial tumors). Children below 10 years of age with epilepsy without these structural lesions will include a relatively high proportion of children with unknown cause of epilepsy i.e. “idiopathic epilepsy”.20–22 We also examined the association separately for epilepsies with focal and generalized onset. To account for epilepsy with onset after the beginning of follow-up (i.e. onset in adolescence and adulthood) we furthermore carried out a sensitivity analysis, where epilepsy was treated as a time-varying exposure. Furthermore, because anxiety, stress-related and somatoform disorders may be diagnosed prior to age 10,19 some children with very early onset of these disorders were excluded in the main models because they were not at risk of incident disease at the beginning of follow-up. In a sensitivity analysis we ignored all diagnoses of anxiety and stress-related disorder given between the age of 5 and 10 years, and followed them from age 10, until the first diagnosis given after this point. In this way, we allowed children that were diagnosed in early childhood (i.e. before age 10) and who were later admitted to a psychiatric department or outpatient clinic (i.e. after age 10) to also be included in the risk estimates. Finally, as an alternative to the complete case analysis, which may induce some selection in the fully adjusted models, we included a missing category in the variables without complete coverage (birth weight, gestational age, Apgar score, maternal education, paternal income), in order to include the entire study population in the analyses.
All analyses were performed using Stata, version 13 (StataCorp, College Station, Texas, USA).
Results
Among the 1,291,679 children born in Denmark between 1978 and 2002 who were alive at their 10th birthday, 43,148 (3.3%) had been diagnosed with febrile seizures, 10,355 (0.8%) had been diagnosed with epilepsy, and 1,696 (0.1%) had been diagnosed with both. The proportion of boys was higher among the children with febrile seizures and epilepsy than among those without, and children with seizures generally had worse perinatal and sociodemographic characteristics. Similarly, at the beginning of follow-up the children with seizures were also more likely to have a diagnosis of ADHD and autism spectrum disorders (e–Table 1). The cohort was followed for more than 15 million person years, and the median age at the end of follow-up was 21.2 years (10th percentile: 12.5 years, 90th percentile: 32.3 years).
During the follow-up, a total of 83,735 cohort members were registered with at least one of the psychiatric disorders of interest (Table 1). Compared to the group of children without seizures, the risk of developing a psychiatric disorder was marginally elevated for children with a history of febrile seizures (HR: 1.12, 95% CI: 1.08–1.17) and moderately elevated for those with childhood epilepsy, with (HR: 1.50, 95% CI: 1.28–1.75) or without febrile seizures (HR: 1.34, 95% CI: 1.25–1.44). The association with febrile seizures remained unchanged when we accounted for epilepsies with onset after the beginning of follow-up (HR: 1.12, 95% CI: 1.08–1.17, e–Table 2). In the group of children with no seizures, the risk of having at least one psychiatric diagnosis by the age of 30 was 11.5% (95% CI:11.4–11.6). By comparison, the corresponding risks among children with febrile seizures, epilepsy, or both febrile seizures and epilepsy were 13.4% (95% CI: 12.9–13.9), 17.0% (95% CI: 15.8–18.2), and 18.8% (95% CI: 15.9–21.8), respectively. The cumulative incidence of psychiatric disorders was higher among females than among males, but the impact of childhood seizures was independent of sex (p-value for interaction, p=0.30).
Table 1:
Relative and absolute risks of any psychiatric disorder associated with a history of seizures among 1,291,769a children born in Denmark (1978–2002).
| Exposure | Any psychiatric disorder (n) | Hazard ratioc (95% confidence interval) |
Cumulative incidence at age 30, % (95% CI) | ||
|---|---|---|---|---|---|
| basic adjustment | full adjustmentd | ||||
| Total | 83,735 | - | - | 11.6 (11.6–11.7) | |
| All | None | 79,676 | 1.00 (ref). | 1.00 (ref.) | 11.5 (11.4–11.6) |
| Febrile seizures | 2,954 | 1.17 (1.12–1.21) | 1.12 (1.08–1.17) | 13.4 (12.9–13.9) | |
| Epilepsy | 934 | 1.55 (1.45–1.65) | 1.34 (1.25–1.44) | 17.0 (15.8–18.2) | |
| Febrile seizures and epilepsy | 171 | 1.74 (1.50–2.02) | 1.50 (1.28–1.75) | 18.8 (15.9–21.8) | |
| Femalesb | None | 47,896 | 1.00 (ref.) | 1.00 (ref.) | 13.9 (13.8–14.1) |
| Febrile seizures | 1,664 | 1.18 (1.12–1.24) | 1.14 (1.08–1.20) | 16.8 (16.0–17.7) | |
| Epilepsy | 510 | 1.48 (1.36–1.62) | 1.30 (1.18–1.42) | 20.9 (19.0–22.8) | |
| Febrile seizures and epilepsy | 89 | 1.64 (1.33–2.02) | 1.37 (1.10–1.70) | 22.4 (17.9–27.3) | |
| Malesb | None | 31,780 | 1.00 (ref.) | 1.00 (ref.) | 9.2 (9.1–9.3) |
| Febrile seizures | 1,290 | 1.15 (1.09–1.21) | 1.10 (1.04–1.17) | 10.7 (10.1–11.4) | |
| Epilepsy | 424 | 1.64 (1.49–1.81) | 1.41 (1.27–1.56) | 13.7 (12.4–15.2) | |
| Febrile seizures and epilepsy | 82 | 1.86 (1.50–2.31) | 1.66 (1.32–2.09) | 15.6 (12.4–19.7) | |
The number of individuals included in the fully adjusted analyses was 1,198,831, due to missing information on covariates in 92,938 individuals.
P-value for interaction between sex and childhood seizures, p=030
All analyses are stratified by sex and adjusted for calendar year
Further adjusted for: birth weight, gestational age at delivery, Apgar score (5 minutes), maternal education, paternal income, parental age at birth, and parental psychiatric disease
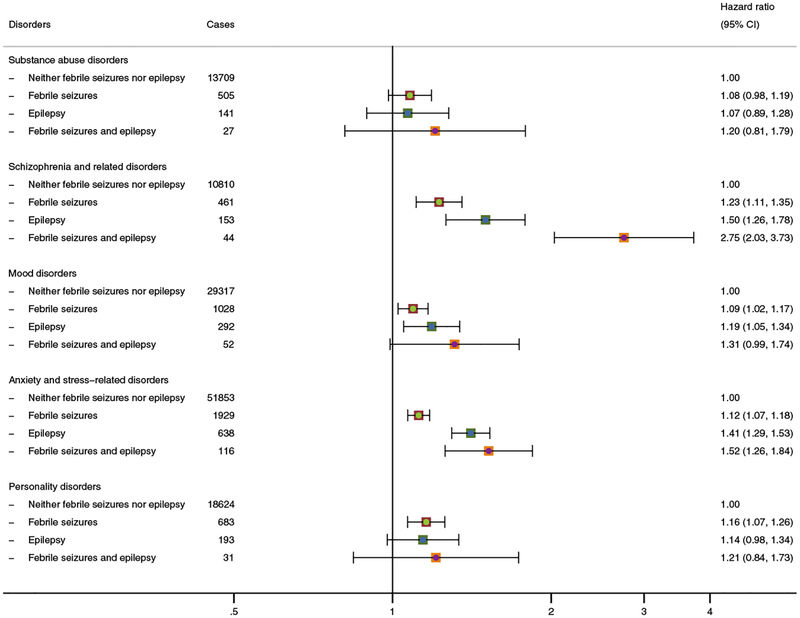
An excess risk of psychiatric illness associated with childhood seizures was present across a range of different disorders (Figure 1). The strongest associations were observed for schizophrenia and related disorders (for febrile seizures, HR: 1.23 (95% CI: 1.11–1.35), for epilepsy, HR: 1.50 (95% CI; 1.26–1.78), and for febrile seizures and epilepsy, HR: 2.75 (95% CI: 2.03–3.73)); similar, although less pronounced, associations were present for mood-, anxiety-, and stress-related disorders. The estimates for anxiety and stress-related disorders were largely unaffected, when children with a prior diagnosis of these disorders (n=2175) were included in the analyses (e–Table 3). The risk of substance abuse disorders was not significantly increased for any of the groups of children with childhood seizures, and the association with febrile seizures was statistically significant for personality disorders only. With the exception of schizophrenia, the risks of specific psychiatric disorders were generally elevated with a factor 1.1–1.15 among children with a history of febrile seizures, and with a factor 1.1–1.4 among those with epilepsy. The group of children with a history of both febrile seizures and epilepsy generally had the highest risk of all of the disorders considered in this study. However, given the limited size of this group, these estimates are associated with a high degree of uncertainty.
Figure 1: Adjusted relative risks of psychiatric disorders associated with a history of febrile seizures and epilepsy in childhood among 1,291,769 children born in Denmark (1978–2002).
Footnote: All analyses are adjusted for sex, calendar year, birth weight, gestational age at delivery, Apgar score (5 minutes), maternal education, paternal income, parental age at birth, and parental psychiatric disease.
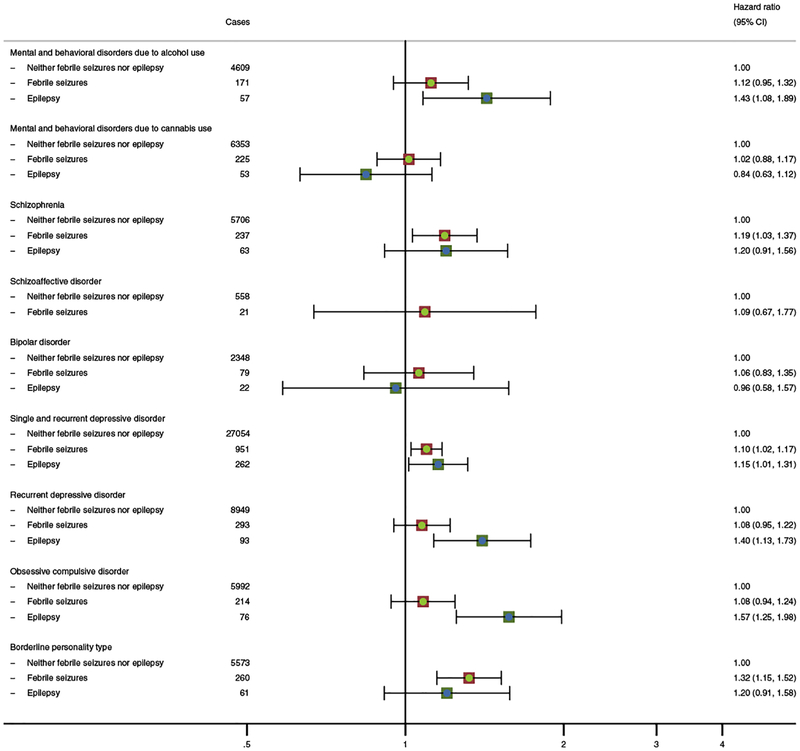
Figure 2 shows the associations for the subgroupings of persons diagnosed with substance abuse disorders, schizophrenia and related disorders, mood disorders, anxiety and stress-related disorders, and specific personality disorders. These findings are largely consistent with those presented in Figure 1, but reveal that there are slight differences in how childhood seizures are associated with the risks of specific diagnoses. For instance, the risk of substance abuse disorders due to alcohol use is elevated for those with childhood epilepsy (HR: 1.43, 95% CI: 1.08–1.89). However, this is not the case for substance abuse disorders due to cannabis use (HR: 0.84, 95% CI: 0.63–1.12). In addition, for mood disorders, it seems that the overall association is driven more by depressive disorders than by bipolar disorders.
Figure 2: Adjusted relative risks of subgroupings of psychiatric disorders associated with a history of febrile seizures and epilepsy in childhood, among 1,291,769 children born in Denmark (1978–2002).
Footnotes:
The following classification of disorders were used: Mental and behavioral disorders due to alcohol use (ICD-10: F10, ICD-8: 291.x9, 303.x9, 303.20, 303.28, 303.90), Mental and behavioral disorders due to cannabis use (ICD-10: F12, ICD-8: 304.59), Schizophrenia (ICD-10: F20, ICD-8: 295.x9 (excluding 295.79)), Schizoaffective disorder (ICD-10: F25, ICD-8: 295.79, 296.89), Bipolar disorders (ICD-10: F30-F31, ICD-8: 296.19, 296.39, 298.19), Single and recurrent depressive disorder (ICD-10: F32-F33, ICD-8: 296.09, 296.29, 298.09, 300.49), Recurrent depressive disorder (ICD-10: F33, ICD-8: 296.09, 296.29, 298.09, 300.49b), Obsessive compulsive disorder (ICD-10: F42, ICD-8: 300.39), Borderline personality type (ICD-10: F60.31, ICD-8: 301.84)
For recurrent depression, onset was defined as the second admission that occurred at least 8 weeks after the last discharge with these ICD-8 codes.
All analyses are adjusted for sex, calendar year, birth weight, gestational age at delivery, Apgar score (5 minutes), maternal education, paternal income, parental age at birth, and parental psychiatric disease.
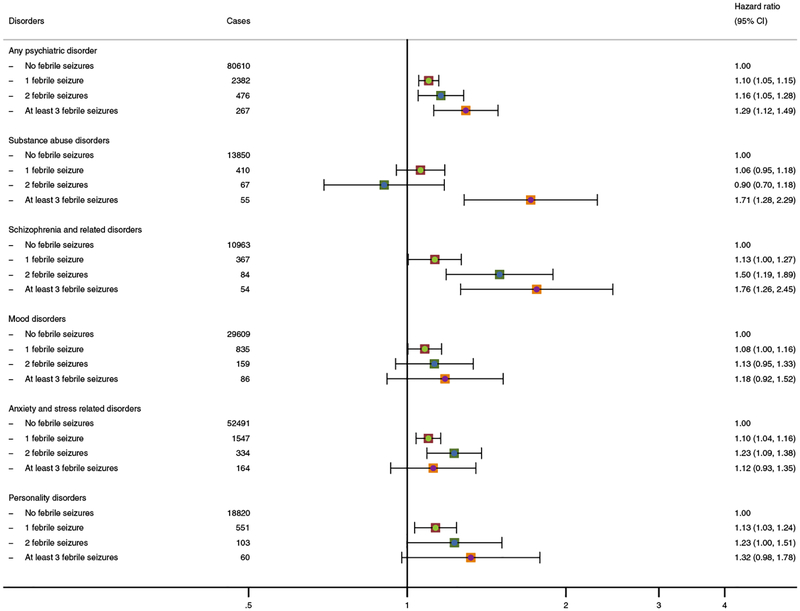
For epilepsy, we found no association between number of admissions and later development of psychiatric disorders (data not shown). However, the risk of psychiatric disorders generally increased progressively with increasing numbers of hospital contacts for febrile seizures (Figure 3). The HR of psychiatric disorders was 1.10 (95% CI: 1.05–1.15) after a single admission with febrile seizure, 1.16 after two febrile seizures (95% CI: 1.05–1.28), and 1.29 for three or more febrile seizures (95% CI: 1.12–1.49).
Figure 3: Adjusted relative risks of psychiatric disorders according to number of admissions with febrile seizures, among 1,291,769 children born in Denmark (1978–2002).
Footnote: All analyses are adjusted for sex, calendar year, birth weight, gestational age at delivery, Apgar score (5 minutes), maternal education, paternal income, parental age at birth, and parental psychiatric disease.
The association between the child’s age at seizure onset and the risk of psychiatric disorders was examined for febrile seizures and epilepsy. The median age at onset for febrile seizures in this sample was ~16 months (10th percentile: ~9 months, 90th percentile: ~3 years); and for epilepsy ~4.5 years (10th percentile: ~6 months, 90th percentile: ~9 years). For children with febrile seizures, there was no difference between onset of seizures <16 months of age (HR: 1.10, 95% CI: 1.04–1.17) and ≥16 months to 3 years of age (HR: 1.11, 95% CI 1.04–1.17) with regard to the risk of developing psychiatric disease. However, a somewhat higher risk was observed in children where the first febrile seizure did not occur until after the age of 3 years (HR: 1.25, 95% CI: 1.12–1.41). Similarly, for epilepsy, higher age at epilepsy onset was also associated with higher risks of psychiatric disorders (Table 2).
Table 2:
Risk of psychiatric disorder after the age of 10 in 1,291,769 children born in Denmark (1978–2002), according to age at onset of childhood seizures.
| Hazard ratioa (95% confidence interval) |
|||
|---|---|---|---|
| Age at onset | n | Basic adjustment | Full adjustmentb |
| Febrile seizures | |||
| None | 1,246,925 | 1.00 (ref.) | 1.00 (ref.) |
| <16 months | 20,706 | 1.13 (1.07–1.19) | 1.10 (1.04–1.17) |
| 16 months to 3 years | 19,975 | 1.16 (1.10–1.23) | 1.11 (1.04–1.17) |
| ≥3 years | 4,163 | 1.33 (1.19–1.49) | 1.25 (1.12–1.41) |
| Epilepsy | |||
| None | 1,279,718 | 1.00 (ref.) | 1.00 (ref.) |
| 0–1 years | 2,247 | 1.37 (1.17–1.60) | 1.20 (1.01–1.42) |
| 1–4 years | 3,279 | 1.43 (1.25–1.63) | 1.17 (1.01–1.35) |
| 4–7 years | 3,132 | 1.61 (1.42–1.83) | 1.40 (1.22–1.59) |
| 7–10 years | 3,393 | 1.69 (1.51–1.89) | 1.52 (1.35–1.71) |
All analyses are stratified by sex and adjusted for calendar year
Further adjusted for: birth weight, gestational age at delivery, Apgar score (5 minutes), maternal education, paternal income, parental age at birth, and parental history of psychiatric disease
The risk of psychiatric disorders after epilepsy remained essentially unchanged when we excluded individuals with status epilepticus (HR: 1.35, 95% CI: 1.25–1.44), seizure onset in the neonatal period (HR: 1.35, 95% CI: 1.26–1.44), and potentially structural causes (HR: 1.40, 95% CI: 1.30–1.49). Furthermore, the association did not seem to differ in epilepsies with focal (HR: 1.42, 95% CI: 1.25–1.62) and generalized (HR: 1.36, 95% CI: 1.19–1.56) onset. Finally, when children with missing values in covariates were included in the fully adjusted models, the association with psychiatric disorders did not change (febrile seizures: HR: 1.13, 95% CI: 1.08–1.17; epilepsy: HR: 1.35, 95% CI: 1.26–1.44; and both: HR: 1.46, 95% CI: 1.25–1.70).
Discussion
In this large nation-wide follow-up study, individuals who experienced seizures during childhood were at increased risk of developing psychiatric disease in adolescence and early adulthood. Febrile seizures were generally associated with a low to moderate excess risk of psychiatric morbidity, whereas epilepsy seemed to confer a somewhat greater risk. These findings thereby suggest that children experiencing seizures not only are susceptible to mental illnesses in childhood,3–7 but that this susceptibility carries on into later stages of life.
These findings contribute to the growing body of evidence on comorbidity between epilepsy and psychiatric disorders.8,9,23 In the present study, people with childhood onset epilepsy were at a higher risk of a range of different disorders in later life, including schizophrenia, mood disorders, and anxiety and stress-related disorders. However, while we generally found no more than a 1.5-fold excess risk of psychiatric disease in people with childhood epilepsy, meta-analyses have frequently reported much higher risk ratios,23 suggesting that people with epilepsy have a 2–3-fold excess risk of depression24, and even a nearly 8-fold increased risk of psychotic illness.25 In contrast to our sample, which was population-based, much of the current evidence on psychiatric comorbidity in individuals with epilepsy stems from studies using clinical samples. It is possible that the association with psychiatric comorbidity is more pronounced in such samples, since they tend to include the more severe cases of epilepsy. Our findings, on the other hand, may more accurately reflect the risk among the general population of people with epilepsy. Furthermore, the association between epilepsy and mental illness was also somewhat stronger in the analyses, where epilepsy with onset after the age of 10 years was considered, suggesting that later onset epilepsy may be associated with a greater risk of psychiatric comorbidity.
We furthermore found that children with childhood epilepsy were at higher risk of mental disorders due to alcohol use, but not due to cannabis use. Substance abuse disorders have previously been linked to epilepsy,26 but the reason for the difference between alcohol- and cannabis related substance abuse disorders found in the present study is not entirely clear. These findings needs further replication.
Much evidence suggests that the relationship between epilepsy and psychiatric disorders is bidirectional.26 In this study, we aimed to estimate the psychiatric risk following seizures (and not vice versa). We therefore exclusively studied seizures occurring during early childhood and psychiatric disorders occurring in later life in order to clearly separate in time the onset seizures from the onset of psychiatric disorders. We used the date of the first diagnosis to define onset of the disorders. While this date is likely to be accurate in measuring onset of febrile seizures where the admissions often occur directly in relation to the seizure, this is seldom the case for epilepsy and psychiatric disorders. For these disorders, there will often be a delay between the onset of symptoms and the time of diagnosis. In the analyses of age at onset, we observed that the association between epilepsy and psychiatric disorders seemed to become stronger with later onset of epilepsy (i.e. the highest risk was found in epilepsies with onset at ages 7–9 years). We cannot exclude that this is a reflection of bidirectionality, given that some individuals with epilepsy may have had psychiatric symptoms (that were not yet diagnosed) preceding epilepsy onset in this age group. However, this is unlikely to be the case for febrile seizures and for the epilepsies with onset at early age.
The psychiatric comorbidity of febrile seizures has been studied less, possibly because febrile seizures have been considered largely benign with a favorable prognosis in terms of further neurocognitive development and mortality.2,27 In the present study, we have shown that children with febrile seizures do seem to be at slightly higher risk of developing psychiatric disorders as teenagers and young adults even in the absence of subsequent epilepsy. This excess risk was present across a range of different disorders, such as schizophrenia, mood-, anxiety-, personality-, and stress-related disorders. These findings are in line with other register-based studies, suggesting that children with febrile seizures had a 1.3- (95% CI: 1.2–1.4) and 1.5-fold (95% CI: 0.9–2.4) increased risk of ADHD,3,5 a 2.5-fold (95% CI: 1.5–4.1) increased risk of autism spectrum disorders,5 and a 1.4-fold (95% CI: 1.1–2.0) increased risk of schizophrenia,28 which could not be explained by concomitant epilepsy. On the contrary, multiple studies on neurocognitive and behavioral outcomes suggest that individuals with a history of febrile seizures perform similarly to those without in several cognitive and behavioral assessments.11,14,15,29 We observed that the association with mental illness was stronger in individuals with recurrent febrile seizures and with onset of febrile seizures after the age of 3 years. It is possible that the excess risk of psychiatric disease we observed is attributable, mainly to a subset of individuals with febrile seizures (e.g. individuals with prolonged or otherwise complex febrile seizures and individuals with genetic predisposition), or that we were simply able to detect more subtle differences than most other studies as our study was very well-powered, particularly in the analyses of febrile seizures.
Febrile seizures have been associated with premature mortality in children with complex febrile seizures in the first few years after the onset of febrile seizures. This excess mortality was partly explained by underlying neurological disorders, including epilepsy.27,30 We followed children from age 10 years and thus only included children who survived until that age. However, only very few children died and this is consequently unlikely to influence the association between febrile seizures and psychiatric disorders in adults
The mechanisms responsible for the observed associations are not clear. It is possible that the seizures (febrile and afebrile) themselves or their associated treatment harm the developing brain and thereby predispose individuals to mental illness in later life.10,31,32 However, the relation described between seizures and many psychiatric disorders seems to be bidirectional, which suggests that common underlying etiological factors may exist. While we adjusted all analyses for a range of perinatal and sociodemographic characteristics, it is possible that residual or unmeasured confounding may explain our findings, in part or in whole. In particular, genetic factors may cause functional or structural neuronal changes, leading to both seizures and subsequent mental illness. Elucidating to what extent a shared genetic basis may explain our findings, and whether there might be mediating or modifying conditions (e.g. parental mental illness, cognitive function, educational attainment etc.) are therefore important priorities in future research.33
Strengths and limitations
Several important strengths and limitations are worth noting. This study was designed as a population-based follow-up study, and included all children born in Denmark during a 25-year period. Given this large population size, we were able to address a broad spectrum of psychiatric comorbidities in adolescence and early adulthood associated with childhood seizures. The study was entirely based on information from nation-wide registries, and owing to the virtually complete coverage of the population registers, bias arising from sampling and attrition was therefore minimal. For the covariates included in the analyses of the present study, the proportion of children with missing values was generally very low (<2%), with the exception of gestational age (4–5%). However, more than 85% of the missing values for gestational age were in children born in the beginning of the study period (1978–1981), where changes in the reporting was implemented. The distribution of gestational age in that period has been shown to be similar to that in other periods, and the children without information on gestational age were similar to children with that information, in terms of birth weight.34 Thus, there does not seem to be any systematic pattern in the missingness of gestational age in that period, which reduces the risk of bias. Furthermore, the results of the present study did not change in the sensitivity analysis where children with missing values were included, suggesting that the complete case analyses were not biased by this issue in any substantial manner.
In the present study, psychiatric diagnoses were retrieved from the Danish Psychiatric Central Research Register. This register contains information on individuals treated in secondary mental health care. Some individuals suffering from mental illness may not need treatment in a secondary healthcare facility but may be seen in primary care only, particularly if the prognosis is mild. These individuals will not appear in the register. However, as the threshold for admission to secondary mental health care services is not likely to depend on seizure history, misclassification would most likely be non-differential and bias our estimates towards the null. Nevertheless, the cumulative incidences are likely conservative estimates of the actual risk of developing mental health problems.
The National Patient Register was used to identify children with febrile seizures and epilepsy. Validation studies have shown fairly high positive predictive values of diagnoses of 93% (95% CI: 89–96%) for febrile seizures35 and 81% (95% CI: 75–87%) for epilepsy, but much lower for epilepsy subtypes.36 We were unable to identify a risk difference between focal epilepsy and generalized epilepsy. This may possibly be due to the uncertainty of the epilepsy subtype classification and differences in the terminology used, since the distinction between generalized epilepsies and focal epilepsies are made on clinical grounds, often supported by findings on neuroimaging and interictal EEG discharges.22 However, this is often insufficient to classify the type of epilepsy. In addition, children with generalized epilepsy may have EEGs with focal features and incidental focal findings on MRI. Accordingly, the differentiation between the two main types of epilepsy is associated with some degree of uncertainty. Further, the classification and terminology used in the ICD-8 and ICD-10 do not match the classification and terminology used by the International League Against Epilepsy20,22,36 and the ICD 8/10 do not list etiology in any detail.21
Registration and diagnostic practices of psychiatric disorders, febrile seizures and epilepsy have changed during the study period (e.g. introduction of the ICD-10 classification system in 1994 and inclusion of outpatient and emergency room contacts from 1995 and onwards). Issues arising from such changes were addressed by adjusting all analyses for calendar period effects. In the analyses, we also treated all continuous variables, including age at onset of febrile seizures and epilepsy, as categorical variables. However, categorizing continuous variables, e.g. age at onset, inevitably leads to loss of information. There is rarely consensus about the choice of the exact cut-off points, and this may limit the generalizability of the findings.
Finally, the present study was limited by a lack of important clinical information. For instance, it would have been of interest to examine in more detail how the associations varied with duration or frequency of the seizures, whether the children later became seizure-free, and whether they experienced side effects of medication.
Conclusion
In this large prospective population-based study, we found that individuals with febrile seizures and epilepsy in childhood are at excess risk of developing a broad range of psychiatric disorders in later life, such as anxiety, mood disorders, and psychotic disorders. While these findings quantify the psychiatric comorbidity in the general population of individuals with childhood seizures, further research is needed to clarify the underlying mechanisms.
Research in context.
Evidence before this study
Febrile seizures and epilepsy are the two most common seizure disorders in children, but are thought to have very different impact on subsequent neuro-psychiatric development. Epidemiological and clinical studies have shown that children with epilepsy frequently will develop comorbid psychiatric disease over the life-course, but febrile seizures have usually been thought to have little or no long-lasting implications for the development of the child. However, large-scale population-based studies addressing the long-term risk of psychiatric comorbidities are lacking. We searched Medline and Web of Science for articles published before October 1st 2018, using the MeSH terms and key words “Seizures, Febrile”, “Epilepsy”, “Mental disorders”, “Comorbidity”, with no restrictions on date or language. Furthermore, we used a snowballing technique in which we pursued references of references to detect reports of studies not identified in the database search. Details of the literature search is provided in online appendix 1.
Added value of this study
In this study, we follow a population-based cohort of children for more than 15 million person-years, including children with a history of febrile seizures, epilepsy and both, and report the relative and cumulative risk of developing psychiatric disorders in adolescence and early adulthood. We show that children experiencing seizures during childhood are at excess risk of developing a broad range of psychiatric disorders in later life, such as anxiety, mood disorders, psychotic disorders, and personality disorders. These findings confirms and extends the range of psychiatric disorders previously associated with childhood epilepsy, and document a novel association with febrile seizures without epilepsy, that increase in strength with recurrent febrile seizures. We also provide new evidence that the risk of psychiatric comorbidity increase in children with febrile seizures who later develop epilepsy. Compared to previous reports, the extent of the psychiatric comorbidity in children with seizures found in this study was more modest. Much of the existing evidence from previous reports is based on studies using clinical samples that may tend to comprise more severe cases, while our findings likely reflects the risk of psychiatric disorders in the general population of people with epilepsy and febrile seizures.
Implication of all the available evidence
Febrile seizures account for approximately half of all pediatric seizures, and is by far the most common type of seizures in childhood. Previously, there has been little evidence to suggest that febrile seizures, without any underlying neurological morbidity (including epilepsy), have lasting implications for intellectual or behavioral development. Our study shows that children with febrile seizures, and in particular those with recurrent febrile seizures, do in fact seem to have a higher risk of a wide range of psychiatric comorbidities, in a similar, although less pronounced, manner as children with epilepsy. These findings suggest that children experiencing seizures in the first 10 years of their life, seems to be somewhat more susceptible to mental disorders throughout adolescence and early adulthood.
Role of the funding source
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Potential conflicts of interest: Dr. Dreier and Dr. Christensen reports grants from the Novo Nordisk Foundation, the Danish Epilepsy Association, and the Central Denmark Region, during the conduct of the study; Dr. Christensen reports personal fees for giving lectures for UCB Nordic, and for Eisai, outside the submitted work. Dr. Bøcker Pedersen reports grants from The Lundbeck Foundation, and Stanley Medical Research Institute, during the conduct of the study. Dr. Cotsapas has nothing to disclose.
Appendix 1
Literature search:
Study: Childhood seizures and risk of psychiatric disorders in adolescence and early adulthood. A Danish nation-wide cohort study.
Medline search strategy
Date of last search, October 5, 2018
624 results
MeSH terms used
“Seizures, Febrile”, “Epilepsy”, “Mental disorders”, “Comorbidity”
Restrictions
Years: None
Language: None
Final search strategy:
((“epilepsy”[MeSH Major Topic] OR “seizures, febrile”[MeSH Major Topic]) AND “mental disorders”[MeSH Major Topic]) AND “comorbidity”[MeSH Terms]
Web of Science search strategy
Date of last search, October 5, 2018
715 results
Topic words
“Epilepsy”, “febrile seizures”, “psychiatric disorder”, “mental illness”, “comorbid*”
Restrictions
Document type: Article
Years: None
Language: None
Final search strategy:
(TS=((epilepsy OR febrile seizures) AND (psychiatric disorder OR mental illness) AND(comorbid*))) AND DOCUMENT TYPES: (Article)
Furthermore, we used a snowballing technique in which we pursued references of references to detect reports of studies not identified in the database search.
e-Table 1:
Characteristics of study population (n = 1,291,769) according to history of febrile seizures and epilepsy. Born in Denmark, 1978–2002 and alive and residing in Denmark on 10th birthday.
| No epilepsy or febrile seizure n =1,236,570 n (%) |
Febrile seizure n = 43,148 n (%) |
Epilepsy n = 10,355 n (%) |
Both epilepsy and febrile seizure n =1,696 n (%) |
|
|---|---|---|---|---|
| Female subjects | 604,514 (48.9) | 19,101 (44.3) | 4,736 (45.7) | 739 (43.6) |
| Gestational age, weeks | ||||
| ≤33 | 16,341 (1.3) | 840 (2.0) | 407 (3.9) | 60 (3.5) |
| 34–36 | 45,412 (3.7) | 2,060 (4.8) | 591 (5.7) | 102 (6.0) |
| 37–41 | 1,015,870 (82.2) | 35,331 (81.9) | 8,082 (78.1) | 1,345 (79.3) |
| ≥42 | 105,728 (8.6) | 3,476 (8.1) | 926 (8.9) | 132 (7.8) |
| Missing | 53,219 (4.3) | 1,441 (3.3) | 349 (3.4) | 57 (3.4) |
| Birth weight, g | ||||
| <1500 | 6,981 (0.6) | 365 (0.9) | 225 (2.2) | 34 (2.0) |
| 1500–2499 | 52,306 (4.2) | 2,404 (5.6) | 807 (7.8) | 112 (6.6) |
| 2500–2999 | 152,357 (12.3) | 5,959 (13.8) | 1,482 (14.3) | 282 (16.6) |
| 3000–3999 | 809,068 (65.4) | 27,585 (63.9) | 6,132 (59.2) | 1,024 (60.4) |
| ≥4000 | 209,716 (17.0) | 6,612 (15.3) | 1,629 (15.7) | 236 (13.9) |
| Missing | 6,142 (0.5) | 223 (0.5) | 80 (0.8) | 8 (0.5) |
| Apgar score (at 5 minutes) | ||||
| ≤7 | 16,640 (1.4) | 710 (1.7) | 457 (4.4) | 43 (2.5) |
| 8–9 | 65,800 (5.3) | 2,541 (5.9) | 812 (7.8) | 134 (7.9) |
| 10 | 1,141,048 (92.3) | 39,471 (91.5) | 8,921 (86.2) | 1,504 (88.7) |
| Missing | 13,082 (1.1) | 426 (1.0) | 165 (1.6) | 15 (0.9) |
| Maternal age at birth, years | ||||
| <20 | 31,758 (2.6) | 1,233 (2.9) | 373 (3.6) | 59 (3.5) |
| 20–24 | 260,543 (21.1) | 9,180 (21.3) | 2,430 (23.5) | 419 (24.7) |
| 25–29 | 492,120 (39.8) | 17,298 (40.1) | 3,929 (37.9) | 652 (38.4) |
| 30–34 | 331,203 (26.8) | 11,223 (26.0) | 2,670 (25.8) | 412 (24.3) |
| 35–39 | 106,333 (8.6) | 3,724 (8.6) | 830 (8.0) | 131 (7.7) |
| ≥40 | 14,613 (1.2) | 490 (1.1) | 123 (1.2) | 23 (1.4) |
| Paternal age at birth, years | ||||
| <20 | 8,668 (0.7) | 360 (0.8) | 89 (0.9) | 14 (0.8) |
| 20–24 | 134,968 (10.9) | 4,881 (11.3) | 1,333 (12.9) | 224 (13.2) |
| 25–29 | 410,920 (33.2) | 14,486 (33.6) | 3,386 (32.7) | 577 (34.0) |
| 30–34 | 408,627 (33.1) | 14,041 (32.5) | 3,249 (31.4) | 529 (31.2) |
| 35–39 | 191,797 (15.5) | 6,559 (15.2) | 1,535 (14.8) | 242 (14.3) |
| ≥40 | 81,590 (6.6) | 2,821 (6.5) | 763 (7.4) | 110 (6.5) |
| Maternal education, start of follow-up | ||||
| Primary education | 331,420 (26.8) | 11,420 (26.5) | 3,375 (32.6) | 590 (34.8) |
| Secondary education | 512,570 (41.5) | 18,155 (42.1) | 4,162 (40.2) | 661 (39.0) |
| Undergraduate education | 303,863 (24.6) | 10,395 (24.1) | 2,190 (21.2) | 348 (20.5) |
| Graduate education | 75,565 (6.1) | 2,711 (6.3) | 504 (4.9) | 82 (4.8) |
| Missing | 13,152 (1.1) | 467 (1.1) | 124 (1.2) | 15 (0.9) |
| Paternal income, start of follow-up | ||||
| 1st quintile | 243,647 (19.7) | 8,800 (20.4) | 2,597 (25.1) | 444 (26.2) |
| 2nd quintile | 244,352 (19.8) | 8,560 (19.8) | 2,184 (21.1) | 380 (22.4) |
| 3rd quintile | 244,601 (19.8) | 8,544 (19.8) | 2,011 (19.4) | 321 (18.9) |
| 4th quintile | 244,870 (19.8) | 8,537 (19.8) | 1,780 (17.2) | 289 (17.0) |
| 5th quintile | 245,327 (19.8) | 8,251 (19.1) | 1,644 (15.9) | 239 (14.1) |
| Missing | 13,773 (1.1) | 456 (1.1) | 139 (1.3) | 23 (1.4) |
| Maternal psychiatric diagnosis | ||||
| Yes | 128,422 (10.4) | 5,183 (12.0) | 1,657 (16.0) | 275 (16.2) |
| No | 1,108.148 (89.6) | 37,965 (88.0) | 8,698 (84.0) | 1,421 (83.8) |
| Paternal psychiatric diagnosis | ||||
| Yes | 105,076 (8.5) | 4,155 (9.6) | 1,227 (11.9) | 208 (12.3) |
| No | 1,131,494 (91.5) | 38,993 (90.4) | 9,128 (88.2) | 1,488 (87.7) |
| Diagnosis of ADHD, start of follow-up | ||||
| Yes | 5,990 (0.5) | 343 (0.8) | 215 (2.1) | 57 (3.4) |
| No | 1,230,580 (99.5) | 42,805 (99.2) | 10,140 (97.9) | 1,639 (96.6) |
| Diagnosis of ASD, start of follow-up | ||||
| Yes | 4,814 (0.4) | 251 (0.6) | 309 (3.0) | 58 (3.4) |
| No | 1,231,756 (99.6) | 42,897 (99.4) | 10,046 (97.0) | 1,638 (96.6) |
e-Table 2:
Relative risks of any psychiatric disorder associated with seizures among 1,291,769 children born in Denmark (1978–2002). Allowing for time-varying changes in exposure status.
| Exposurea | Any psychiatric disorder (n) | Hazard ratiob (95% confidence interval) |
|
|---|---|---|---|
| basic adjustment | full adjustmentc | ||
| Total | 83,735 | - | - |
| None | 78,526 | 1.00 (ref). | 1.00 (ref.) |
| Febrile seizures | 2,879 | 1.17 (1.12–1.21) | 1.12 (1.08–1.17) |
| Epilepsy | 2084 | 1.83 (1.75–1.92) | 1.64 (1.56–1.72) |
| Febrile seizures and epilepsy | 246 | 1.74 (1.53–1.97) | 1.53 (1.34–1.75) |
In this model epilepsy is treated as a time-varying co-variate.
All analyses are stratified by sex and adjusted for calendar year
Further adjusted for: birth weight, gestational age at delivery, Apgar score (5 minutes), maternal education, paternal income, parental age at birth, and parental history of psychiatric disease.
e-Table 3:
Relative risk of anxiety, stress-related and somatoform disorders associated with a history of seizures. Main and sensitivity analysis.
| Hazard ratioa (95% confidence interval) |
||
|---|---|---|
| Exposure | Main analysis (cases excluded with onset of anxiety and stress-related disorders before age 10) | Sensitivity analysis (cases included with onset of anxiety and stress-related disorders before age 10) |
| None | 1.00 (ref) | 1.00 (ref) |
| Febrile seizures | 1.12 (1.07–1.17) | 1.12 (1.07–1.18) |
| Epilepsy | 1.41 (1.29–1.53) | 1.42 (1.31–1.54) |
| Febrile seizures and epilepsy | 1.52 (1.26–1.84) | 1.56 (1.29–1.88) |
Analyses are stratified by sex and adjusted for calendar year, birth weight, gestational age at delivery, Apgar score (5 minutes), maternal education, paternal income, parental age at birth, and parental history of psychiatric disease.
Footnotes
Ethical approval
The study was approved by the Danish Data Protection Agency. According to Danish legislation, no further ethical approval or collection of informed consent is required for research projects entirely based on public administrative registries.
References
- 1.Friedman MJ, Sharieff GQ. Seizures in children. Pediatr Clin North Am 2006; 53(2): 257–77. [DOI] [PubMed] [Google Scholar]
- 2.Nelson KB, Ellenberg JH. Prognosis in children with febrile seizures. Pediatrics 1978; 61(5): 720–7. [PubMed] [Google Scholar]
- 3.Bertelsen EN, Larsen JT, Petersen L, Christensen J, Dalsgaard S. Childhood Epilepsy, Febrile Seizures, and Subsequent Risk of ADHD. Pediatrics 2016; 138(2). [DOI] [PubMed] [Google Scholar]
- 4.Brikell I, Ghirardi L, D’Onofrio BM, et al. Familial Liability to Epilepsy and Attention-Deficit/Hyperactivity Disorder: A Nationwide Cohort Study. Biol Psychiatry 2018; 83(2): 173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillberg C, Lundström S, Fernell E, Nilsson G, Neville B. Febrile Seizures and Epilepsy: Association With Autism and Other Neurodevelopmental Disorders in the Child and Adolescent Twin Study in Sweden. Pediatric Neurology 2017; 74(Supplement C): 80–6.e2. [DOI] [PubMed] [Google Scholar]
- 6.Sundelin HEK, Larsson H, Lichtenstein P, et al. Autism and epilepsy. Neurology 2016; 87(2): 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen J, Overgaard M, Parner ET, Vestergaard M, Schendel D. Risk of epilepsy and autism in full and half siblings-A population-based cohort study. Epilepsia 2016; 57(12): 2011–8. [DOI] [PubMed] [Google Scholar]
- 8.Rodenburg R, Stams GJ, Meijer AM, Aldenkamp AP, Dekovic M. Psychopathology in children with epilepsy: a meta-analysis. J Pediatr Psychol 2005; 30(6): 453–68. [DOI] [PubMed] [Google Scholar]
- 9.Verrotti A, Carrozzino D, Milioni M, Minna M, Fulcheri M. Epilepsy and its main psychiatric comorbidities in adults and children. J Neurol Sci 2014; 343(1–2): 23–9. [DOI] [PubMed] [Google Scholar]
- 10.Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta neurologica Scandinavica 2004; 110(4): 207–20. [DOI] [PubMed] [Google Scholar]
- 11.Kariuki SM, Newton CR, Prince MJ, Das-Munshi J. The Association Between Childhood Seizures and Later Childhood Emotional and Behavioral Problems: Findings From a Nationally Representative Birth Cohort. Psychosom Med 2016; 78(5): 620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg AT, Altalib HH, Devinsky O. Psychiatric and behavioral comorbidities in epilepsy: A critical reappraisal. Epilepsia 2017; 58(7): 1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazel S, Wolf A, Langstrom N, Newton CR, Lichtenstein P. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet 2013; 382(9905): 1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visser AM, Jaddoe VW, Ghassabian A, et al. Febrile seizures and behavioural and cognitive outcomes in preschool children: the Generation R study. Developmental medicine and child neurology 2012; 54(11): 1006–11. [DOI] [PubMed] [Google Scholar]
- 15.Chang YC, Guo NW, Huang CC, Wang ST, Tsai JJ. Neurocognitive attention and behavior outcome of school-age children with a history of febrile convulsions: a population study. Epilepsia 2000; 41(4): 412–20. [DOI] [PubMed] [Google Scholar]
- 16.Berg AT, Caplan R, Hesdorffer DC. Psychiatric and neurodevelopmental disorders in childhood-onset epilepsy. Epilepsy Behav 2011; 20(3): 550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Febrile Seizures: Long-Term Management of Children with Fever-Associated Seizures. Pediatrics 1980; 66(6): 1009–12. [PubMed] [Google Scholar]
- 18.Annegers JF, Hauser WA, Elveback LR, Kurland LT. The risk of epilepsy following febrile convulsions. Neurology 1979; 29(3): 297-. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen CB, Mors O, Bertelsen A, et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry 2014; 71(5): 573–81. [DOI] [PubMed] [Google Scholar]
- 20.Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1989; 30(4): 389–99. [DOI] [PubMed] [Google Scholar]
- 21.Shorvon SD. The etiologic classification of epilepsy. Epilepsia 2011; 52(6): 1052–7. [DOI] [PubMed] [Google Scholar]
- 22.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017; 58(4): 512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josephson CB, Jette N. Psychiatric comorbidities in epilepsy. Int Rev Psychiatry 2017; 29(5): 409–24. [DOI] [PubMed] [Google Scholar]
- 24.Fiest KM, Dykeman J, Patten SB, et al. Depression in epilepsy: a systematic review and meta-analysis. Neurology 2013; 80(6): 590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clancy MJ, Clarke MC, Connor DJ, Cannon M, Cotter DR. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry 2014; 14: 75-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Annals of neurology 2012; 72(2): 184–91. [DOI] [PubMed] [Google Scholar]
- 27.Vestergaard M, Pedersen MG, Ostergaard JR, Pedersen CB, Olsen J, Christensen J. Death in children with febrile seizures: a population-based cohort study. Lancet 2008; 372(9637): 457–63. [DOI] [PubMed] [Google Scholar]
- 28.Vestergaard M, Pedersen CB, Christensen J, Madsen KM, Olsen J, Mortensen PB. Febrile seizures and risk of schizophrenia. Schizophr Res 2005; 73(2–3): 343–9. [DOI] [PubMed] [Google Scholar]
- 29.Norgaard M, Ehrenstein V, Mahon BE, Nielsen GL, Rothman KJ, Sorensen HT. Febrile seizures and cognitive function in young adult life: a prevalence study in Danish conscripts. J Pediatr 2009; 155(3): 404–9. [DOI] [PubMed] [Google Scholar]
- 30.Hesdorffer DC, Crandall LA, Friedman D, Devinsky O. Sudden unexplained death in childhood: A comparison of cases with and without a febrile seizure history. Epilepsia 2015; 56(8): 1294–300. [DOI] [PubMed] [Google Scholar]
- 31.Bergen DC. Do seizures harm the brain? Epilepsy Curr 2006; 6(4): 117–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N Engl J Med 1990; 322(6): 364–9. [DOI] [PubMed] [Google Scholar]
- 33.Brainstorm C, Anttila V, Bulik-Sullivan B, et al. Analysis of shared heritability in common disorders of the brain. Science 2018; 360(6395). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.a Rogvi R, Mathiasen R, Greisen G. Defining smallness for gestational age in the early years of the Danish Medical Birth Registry. PLoS One 2011; 6(1): e16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vestergaard M, Obel C, Henriksen TB, et al. The Danish National Hospital Register is a valuable study base for epidemiologic research in febrile seizures. J Clin Epidemiol 2006; 59(1): 61–6. [DOI] [PubMed] [Google Scholar]
- 36.Christensen J, Vestergaard M, Olsen J, Sidenius P. Validation of epilepsy diagnoses in the Danish National Hospital Register. Epilepsy Res 2007; 75(2–3): 162–70. [DOI] [PubMed] [Google Scholar]