Abstract
Neuropeptide Y (NPY) modulates nociception in the spinal cord, but little is known about its mechanisms of release. We measured NPY release in situ using the internalization of its Y1 receptor in dorsal horn neurons. Y1 receptor immunoreactivity was normally localized to the cell surface, but addition of NPY to spinal cord slices increased the number of neurons with Y1 internalization in a biphasic fashion (EC50s of 1 nM and 1 μM). Depolarization with KCl, capsaicin, or the protein kinase A activator 6-benzoyl-cAMP also induced Y1 receptor internalization, presumably by releasing NPY. NMDA receptor activation in the presence of BVT948, an inhibitor of protein tyrosine phosphatases, also released NPY. Electrical stimulation of the dorsal horn frequency-dependently induced NPY release; and this was decreased by the Y1 antagonist BIBO3304, the Nav channel blocker lidocaine, or the Cav2 channel blocker ω-conotoxin MVIIC. Dorsal root immersion in capsaicin, but not its electrical stimulation, also induced NPY release. This was blocked by CNQX, suggesting that part of the NPY released by capsaicin was from dorsal horn neurons receiving synapses from primary afferents and not from the afferent themselves. Mechanical stimulation in vivo, with rub or clamp of the hindpaw, elicited robust Y1 receptor internalization in rats with spared nerve injury but not sham surgery. In summary, NPY is released from dorsal horn interneurons or primary afferent terminals by electrical stimulation and by activation of TRPV1, PKA or NMDA receptors in. Furthermore, NPY release evoked by noxious and tactile stimuli increases after peripheral nerve injury.
Keywords: C-fiber, neuropathic pain, nociception, neurotransmitter release
1. Introduction
Neuropeptide Y (NPY) is an amidated 36 amino acid peptide that modulates a variety of physiological processes through G protein-coupled Y receptors (Hokfelt et al., 1998; Wan and Lau, 1995). Two NPY receptors, Y1 and Y2, are present in regions involved in pain transmission (Brumovsky et al., 2007; Gibson et al., 1984; Ji et al., 1994). In the spinal cord, Y1 receptors are expressed in dorsal horn neurons and in primary afferents terminals (Brumovsky et al., 2006; Brumovsky et al., 2002), whereas Y2 receptors are localized in primary afferents terminals (Brumovsky et al., 2005) and possibly on a few interneurons (Haring et al., 2018). Nerve injury, but not inflammation, increases NPY immunoreactivity in medium to large primary afferent neurons (Smith et al., 2007; Solway et al., 2011; Wakisaka et al., 1992). These studies place NPY and its Y1 receptor at key spinal cord sites involved in nociception.
Pharmacological studies have revealed that NPY given intrathecally acts at Y1 and Y2 receptors to reduce not only mechanical and cold hypersensitivity in a neuropathic pain model (Intondi et al., 2008), but also c-Fos expression and hyperalgesia after inflammation or nerve injury (Intondi et al., 2008; Kuphal et al., 2008; Mahinda and Taylor, 2004; Taiwo and Taylor, 2002). Ablation of Y1-expressing dorsal horn neurons with NPY-saporin produced antinociception (Lemons and Wiley, 2012) and reduced mechanical and cold hypersensitivity in the spared nerve injury model (Nelson et al., 2019), suggesting that they are pro-nociceptive neurons. NPY also participates in the transition from acute to chronic pain, because conditional depletion of NPY during the remission period of latent sensitization reinstated hyperalgesia (Solway et al., 2011). We recently reported that Y1 receptors inhibit hyperalgesia and substance P release in the spinal cord, and that these effects increase during inflammation (Taylor et al., 2014). These functional studies clearly assign NPY and its receptors as key sites of pain modulation in the spinal cord.
Despite this, very little is known about NPY release in the dorsal horn under normal and chronic pain conditions. One study used antibody-coated microprobes (Colvin and Duggan, 2001) to demonstrate that nerve injury increased the spontaneous release of NPY-immunoreactivity in the deep dorsal horn of anesthetized rats; local anesthetic block of the injured nerve did not reduce the spontaneous release of NPY, suggesting that it was independent of primary afferent firing. Most other studies on NPY release has been conducted in the hippocampus (Gemignani et al., 1997; King et al., 1999) and hypothalamus (Martin, 1996; Stricker-Krongrad et al., 1997).
Receptor internalization has been extensively used as a measure in situ of neuropeptide release. This requires that the receptor is present at the cell surface in the absence of neuropeptide and that selective antibodies to label the receptor are available. Accordingly, internalization of the neurokinin 1 receptor (NK1R) has been widely used to measure substance P release (Allen et al., 1997; Chen et al., 2014b; Honore et al., 1999; Kondo et al., 2005; Liu et al., 1997; Mantyh et al., 1995; Marvizon et al., 1997; Marvizon et al., 2003a). Likewise, internalization of μ-opioid receptors has been used to measure opioid release (Chen and Marvizon, 2009; Chen et al., 2014a; Chen et al., 2007; Marvizon et al., 1999; Song and Marvizon, 2003a; Song and Marvizon, 2003b; Trafton et al., 2000). Here, we used Y1 receptor internalization to study the properties of NPY release in rat spinal cord slices and in vivo.
2. Material and methods
2.1. Animals
Rats were male adult (2-4 months old) Sprague-Dawley (Harlan, Indianapolis, IND). All animal procedures were approved by the Institutional Animal Care and Use Committee of the Veteran Affairs Greater Los Angeles Healthcare System and the University of Kentucky, and conform to NIH guidelines. Efforts were made to minimize the number of animals used and their distress.
2.2. Chemicals
Alanine-pyrrolidine-nitrile (Ala-Pyrr-2CN) was a gift from Dr. Sherwin Wilk (Mount Sinai School of Medicine, New York, NY). NPY was from AnaSpec (Fremont, CA). Amastatin, captopril, capsaicin, D-serine (D-Ser), Ile-Pro-Ile (diprotin A), lidocaine, N-methyl-D-aspartate (NMDA), thiorphan and common reagents were from Sigma-Aldrich (St. Louis, MO). BIBO3304 trifluoroacetate, BVT948 and ω-conotoxin (CTX) MVIIC were from Tocris (Ellisville, MO). Drugs were prepared as stock solutions of 1-10 mM in the appropriate solvent (DMSO or water) and then diluted in aCSF.
2.3. Spared nerve injury (SNI) model of peripheral nerve trauma
Rats were anaesthetized with isoflurane. At the dorsal upper thigh level, the skin was cut and the muscle separated to expose the trifurcation of the sciatic nerve where the sciatic nerve splits into the sural, common peroneal and tibial nerves. The tibial and common peroneal nerves were ligated using 6-0 silk suture and then cut on either side of the suture to remove 2-3 mm of each nerve. Sham surgery consisted of exposing the trifurcation of the sciatic nerve without cutting any of the nerves. The muscle fascia was closed in a separate layer from the skin in a simple continuous pattern. The opening in the skin was closed in a subcuticular intradermal pattern. Rats were given daily injections of the analgesic carprofen for 3 days.
2.4. Measures of mechanical hyperalgesia
Mechanical allodynia in rats was measured with a two-out-of-three method (Jarahi et al., 2014; Kingery et al., 2000; Michot et al., 2012). Rats were habituated for periods of 30 min for 3 days to acrylic enclosures on an elevated metal grid (IITC Life Science Inc., CA). A series of von Frey filaments (‘Touch-Test’, 0.8, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10, 15 g) were applied in ascending order to the plantar surface of the hind paw for a maximum period of 3 s. A withdrawal response was counted only if the hind paw was completely removed from the customized platform. Each filament was applied three times, and the minimal value that caused at least two responses was recorded as the PWT. The 15 g filament was taken as cut-off threshold.
2.5. Noxious and non-noxious stimulation
All procedures were performed under isoflurane anesthesia (2-3%) and consisted of either: no stimulation (n=5); non-noxious rubbing of the sural receptor field of the hindpaw ipsilateral to SNI with the thump of the investigator, 2 s on, 2 s off, for 2 min; or noxious clamp of the ipsilateral hindpaw with a binder clip (2 cm-wide) for 2 min (n=7). Five minutes after paw stimulation, rats were euthanized with an overdose of isoflurane or pentobarbital (120 mg/kg, Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI) followed by bilateral thoracotomy, and then perfused for immunohistochemistry.
2.6. Spinal cord slices
Spinal cord slices were prepared as described (Adelson et al., 2009; Lao et al., 2003; Marvizon et al., 2003a; Song and Marvizon, 2003b). Briefly, the spinal cord was extracted from adult rats under pentobarbital anesthesia (0.15-0.2 ml of Fatal Plus, Vortech Pharmaceuticals, Dearborn, Michigan, corresponding to 58-78 mg of pentobarbital per rat). Transverse slices (400 μm, 3-8 per rat) were cut from the lumbar spinal cord (L2-L4) with a vibratome (Integraslice 7550PSDS, Lafayette Instruments, Lafayette, IN) using low advance speed and fast vibration. For stimulation of the dorsal root, slices were prepared with one contiguous dorsal root of >8 mm. Slices were kept in artificial cerebrospinal fluid (aCSF), which contained (in mM) 124 NaCl, 1.9 KCl, 26 NaHCO3, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2 and 10 glucose, and was bubbled with 95% O2 / 5% CO2. Vibratome sectioning was done in sucrose-aCSF (NaCl was substituted with 5 mM KCl and 215 mM sucrose). Slices were left to recover at 35°C for 1 h in oxygenated aCSF with 5 mM KCl, and then used within 3 h of preparation.
Slices were incubated with drugs as described (Chen et al., 2018; Chen et al., 2014b): they were placed on a nylon net glued to a ring inserted halfway down a plastic tube, which contained 5 ml aCSF superficially gassed with 95% O2/5% CO2 and was kept at 35 °C using a metal block incubator (IncuBlock, Denville Scientific Inc., Metuchen, NJ).
We used methods previously described to electrically stimulate the dorsal horn (Song and Marvizon, 2003b) or the dorsal root (Adelson et al., 2009) of the slices. Slices were superfused at 3–6 ml/min with aCSF at 35 °C in a custom-made chamber. Electrical stimulation was generated with a Master-8 stimulator and an Iso-Flex stimulus isolating unit (AMP Instruments, Jerusalem, Israel) and consisted of square pulses of 20 V and 0.4 msec delivered in a single train of 1000 pulses. For dorsal horn stimulation, a slice without roots was held vertically with pins, dorsal side up. One dorsal horn was inserted between the poles of a bipolar hook electrode (1 mm pole separation, 1 mm hook diameter) made of 0.25 mm thick platinum/iridium wires (Frederick Haer, Bowdoinham, ME) and mounted on a manipulator. For dorsal root stimulation, the root was drawn into a side compartment of the chamber through a hole in a movable partition sealed with vacuum grease and placed on top of a bipolar platinum electrode (0.5 mm wire diameter, 1 mm separation). The electrode compartment was then emptied of aCSF and filled with mineral oil. To stimulate the dorsal root with capsaicin, the electrode compartment was filled with aCSF containing 1 μM capsaicin. The stimulated side of the slice was marked with a round hole in the ventral horn. At the end of the experiment (5 min after electrical stimulation), slices were fixed by immersion in ice-cold fixative (4% paraformaldehyde, 0.18% picric acid) overnight.
2.7. Characterization of the Y1 receptor antiserum
The rabbit antiserum against the Y1 receptor was initially a gift from J. H. Urban, Rosalind Franklin University of Medicine and Science, North Chicago, IL (Wolak et al., 2003) and was later purchased from ImmunoStar (#24506). It was raised against a synthetic peptide corresponding to amino acids 356–382 of the rat Y1 receptor (LKQASPVAFKKISMNDNEKI), which was coupled to keyhole limpet hemocyanin and bovine thyroglobulin. The antibody was affinity purified. The antibody was characterized as follows (Wolak et al., 2003). When used in Western blots of SK-N-MC cells it showed an immunoreactive band of 42 kD, which corresponds to the predicted molecular weight of the Y1 receptor. In Western blots of rat brain homogenates two bands were observed at 42 kDa and 85 kDa. When used for immunohistochemistry in rat brain, the staining correlated well with in situ hybridization and receptor autoradiography. Preincubation of the antibody with the immunizing peptide blocked the staining.
2.8. Immunohistochemistry
Spinal cord slices were fixed and processed as described (Adelson et al., 2009; Chen et al., 2018; Chen et al., 2014b; Marvizon et al., 2003a). Rats were euthanized with pentobarbital (100 mg/kg) and fixed by aortic perfusion of 100 ml phosphate buffer (0.1 M sodium phosphate, pH 7.4) containing 0.01% heparin followed by 400 ml of ice-cold fixative (4% paraformaldehyde, 0.18% picric acid in phosphate buffer). The slices or a L4-L6 spinal cord segment were post-fixed, cryoprotected, frozen and sectioned at 25 μm in the coronal plane using a cryostat. Sections were washed four times and then incubated overnight at room temperature with the Y1 receptor antiserum diluted 1:500 in phosphate-buffered saline containing 0.3% Triton X-100, 0.001% thimerosal and 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA). After three washes, the secondary antibody (1:2000, goat anti-rabbit, Alexa Fluor 488, Alexa Fluor 546 or Alexa Fluor 568, Molecular Probes-Invitrogen, Eugene, OR) was applied at for 2 hours at room temperature. Sections were washed four more times, mounted on glass slides, and coverslipped with Prolong Gold (Molecular Probes-Invitrogen).
2.9. Quantification of Y1 receptor internalization
We used Y1R internalization as a measure in situ of NPY release following an approach used to measure the release of substance P with NK1R internalization (Abbadie et al., 1997; Adelson et al., 2009; Allen et al., 1997; Honore et al., 1999; Hughes et al., 2007; Mantyh et al., 1995; Marvizon et al., 2003a; Trafton et al., 1999) and the release of opioid peptides with μ-opioid receptor (MOR) internalization (Chen et al., 2007; Marvizon et al., 1999; Song and Marvizon, 2003a; Song and Marvizon, 2003b; Trafton et al., 2000). The amount of Y1R internalization was quantified by visually counting Y1 receptor neurons in laminae I-II and classifying them as either with or without internalization, using a Zeiss Axio-Imager A1 (Carl Zeiss, Inc., Thornwood, NY) fluorescence microscope with a 63x (1.40 numerical aperture) objective. The criterion for internalization was the presence in the neuronal soma of five or more Y1 receptor-positive endosomes, defined as a small region of bright staining separated from the cell surface. The person counting the neurons was blinded to the treatment. The number of Y1 receptor neurons in the dorsal horn of each section were counted. The average of four histological sections per spinal segment or slice was taken as one data point for data analysis and data presentation. . Results were expressed as the percentage of Y1 receptor neurons with Y1 receptor internalization.
2.10. Confocal microscopy
Confocal images were acquired using a Zeiss LSM 710 confocal microscope (Carl Zeiss, Inc., Thornwood, NY) with objectives of 20x (numerical aperture 0.8) and 63x oil (numerical aperture 1.4). The pinhole was always set at 1.0 Airy unit, as determined by the confocal microscope software. For the Alexa Fluor 488 fluorophore, the excitation was 488 nm (Ar laser), the emission window was 500-560 nm, and the pinhole was 31.5 μm for 20x and 50.7 μm for 63x. For the Alexa Fluor 546 fluorophore, the excitation was 561 nm (diode laser), the emission window was 568-612 nm, and the pinhole was 35.6 μm for 20x and 56.3 μm for 63x. Images were acquired in grayscale as confocal stacks of sections of 1024x1024 pixels. Each section was averaged 2 or 4 times to reduce noise. Photomultiplier gain was adjusted to avoid pixel saturation. The separation between confocal sections was determined by the confocal microscope software using the Nyquist formula: for the 20x objective it was 0.85 μm for the Ar laser and 0.96 nm for the diode laser; for the 63x objective it was 0.38 μm for the Ar laser and 0.42 nm for the diode laser.
2.11. Image processing
An image of the entire dorsal horn was first acquired with a 20x objective; this provided the location of the single neurons imaged with the 63x objective. Imaris 6.1.5 (x64, Bitplane AG, Zurich, Switzerland) was used to crop the images in three dimensions and generate projection pictures which were then imported into Adobe Photoshop 5.5 (Adobe Systems Inc., Mountain View, CA), for the composition of multi-panel figures and to add text, frames and arrows.
2.12. Statistical Analysis
Data were analyzed using Prism 8 (GraphPad Software, San Diego, CA). Statistical significance was set at 0.05. Error bars indicate standard error of the mean.
Experiments in spinal cord slices:
Statistical analyses consisted of one-way or two-way ANOVA and Holm-Sidak’s post-hoc tests. Non-linear regression analyses were used to analyze concentration-response data, which were simultaneously fitted to monophasic and biphasic dose-response functions. Akaike’s Information Criterion to determine which of those two functions were better fitted by the data. Baseline measures (zero concentration of drug) were included in the non-linear regression by assigning them a concentration value three log units lower than the lowest concentration of drug. Parameter constraints were: 0% < top < 100%, 0% < bottom.
Experiments in vivo:
Rats were randomly assigned to treatment. The investigator quantifying the Y1 receptor internalization was blinded to treatment. Statistical analyses consisted of repeated measures two-way or three-way ANOVA followed by Holm-Sidak’s post-hoc tests. Behavioral data were analyzed by 3-way ANOVA with SNI as the between-subject factor, Side as a within-subjects factor, and Time as the repeated measure.
3. Results and Discussion
3.1. Y1 receptor internalization induced by NPY
The anti-Y1 receptor antibody produced bright staining within lamina II of the rat lumbar spinal cord, mainly in the medial and central regions (Figure 1). In this area we found numerous Y1 receptor-positive cell profiles. In an untreated spinal cord slice (Figure 1A), Y1 receptor immunoreactivity was found at the cell surface and was absent from the cytoplasm and nucleus. Figure 2A shows a series of confocal sections taken with a 63x objective, 0.38 μm apart, through one of these neurons. The Y1 receptor staining is present at the cell surface with a few discontinuities and hot spots. In contrast, in a spinal cord slice incubated at 35 °C for 10 min with 1 μM NPY, Y1 receptor immunoreactivity was scarce at the cell surface and was present in bright dots (endosomes) in the cytoplasm but not in the nucleus (Figure 2B). This endosomal pattern is quite similar to that observed following substance P-induced internalization of NK1Rs (Abbadie et al., 1997; Adelson et al., 2009; Allen et al., 1997; Honore et al., 1999; Hughes et al., 2007; Mantyh et al., 1995; Marvizon et al., 2003a; Trafton et al., 1999) and opioid agonist-induced internalization of MORs (Chen et al., 2007; Marvizon et al., 1999; Song and Marvizon, 2003a; Song and Marvizon, 2003b; Trafton et al., 2000).
Figure 1 -. Y1 receptor immunoreactivity in the rat spinal cord and its internalization by endogenously released NPY.
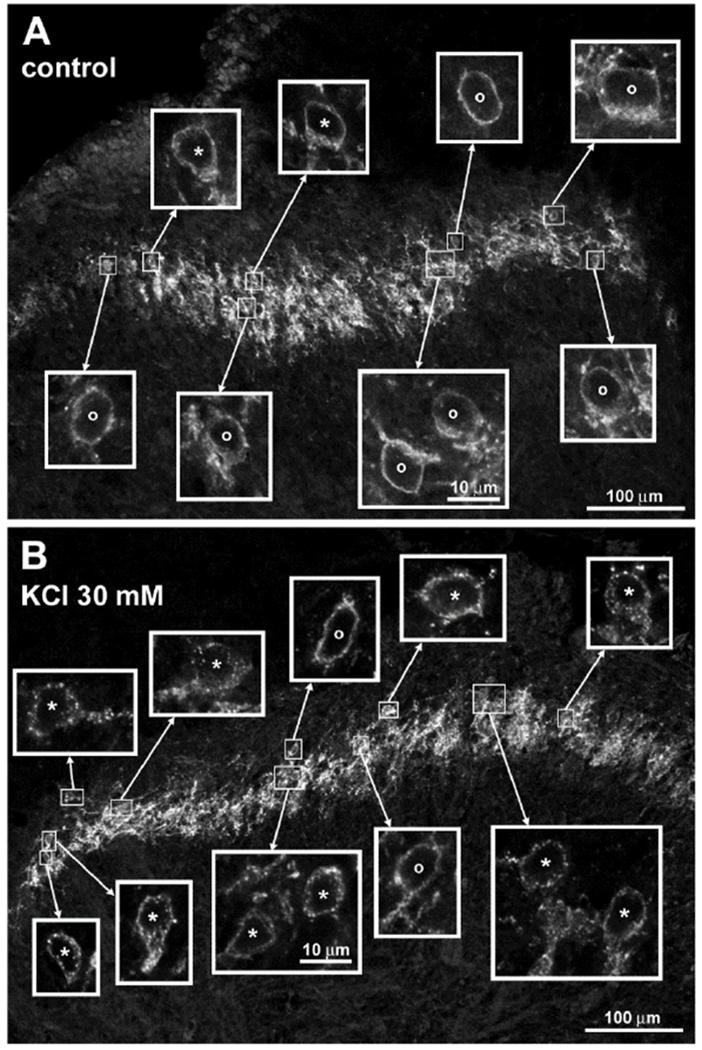
A. Untreated rat spinal cord slice. B. Spinal cord slice incubated for 2 min with 30 mM KCl and then for 8 min in aCSF. In histological sections from the slices the Y1 receptor antibody labeled cells and fibers in lamina II. The fluorophore was Alexa Fluor 546. Main panels are images taken with a 20x objective and a zoom of 0.6, 10 optical sections (A) and 9 optical sections (B) separated 0.96 μm. Insets are images taken with a 63x objective and a zoom of 2.0, 4 optical sections separated 0.42 μm. Cells labeled ‘o’ and ‘*’ were considered without and with Y1 receptor internalization, respectively. All insets are at the same scale.
Figure 2 -. Lamina II neurons without and with Y1 receptor internalization.
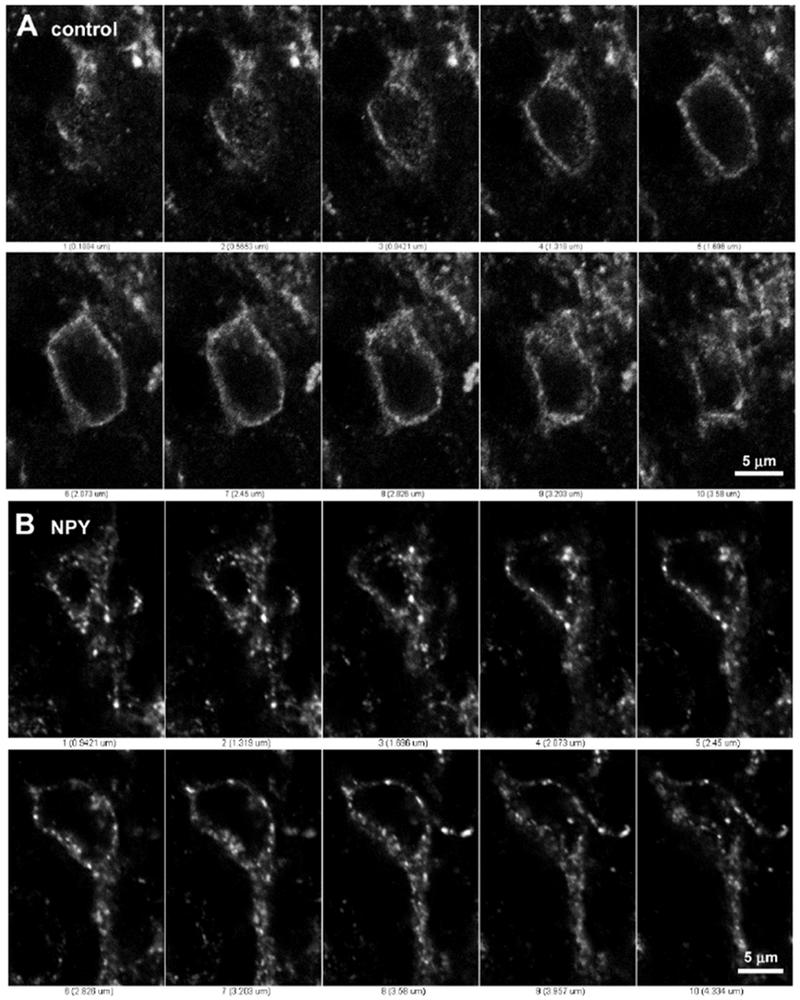
Series of confocal optical sections separated 0.38 μm taken with a 63x objective through two lamina II neurons labeled with the Y1 receptor antibody and Alexa Fluor 488 secondary antibody. A: Neuron from a control spinal cord slice; the Y1 receptor is located at the cell surface and no endosomes are observed. B: Neuron from a spinal cord slice incubated with 1 μM NPY for 10 min; the Y1 receptor is located in endosomes inside the cytoplasm and one dendrite, but not in the nucleus.
A recent study used massive transcriptional profiling (Sathyamurthy et al., 2018) to identify 43 classes of neurons in the mouse spinal cord. In it, the lamina II neurons expressing the Y1 receptor were denominated as DE-2 excitatory interneurons (“D” stands for “dorsal”, “E” for “excitatory”). DE-2 neurons also express gastrin-releasing peptide (GRP) and are probably central transient cells (Dickie et al., 2019; Peirs and Seal, 2016). Another class of central transient excitatory neurons with GRP express MORs (DE-1) (Dickie et al., 2019; Peirs and Seal, 2016; Sathyamurthy et al., 2018). DE-1 neurons express low levels of Y1 receptors, while the DE-2 neurons express low levels of MORs (Dickie et al., 2019; Sathyamurthy et al., 2018), underscoring the similarity of these two types of neurons. In fact, in another transcriptional study (Haring et al., 2018), the DE-1 and DE-2 neurons expressing GRP were included together in the Glut8 type of excitatory neurons.
3.2. NPY induces Y1 receptor internalization in a concentration-dependent manner
To determine the potency of NPY to induce Y1 receptor internalization, we incubated spinal cord slices with varying concentrations of NPY (Figure 3). In control slices, about 20% of the Y1 receptor neurons had enough endosomes to meet our criterion for internalization. This is slightly higher than the percentage of NK1R neurons with internalization (~10 %) (Chen et al., 2018; Chen et al., 2014b; Marvizon et al., 2003a) and MOR neurons with internalization (~5 %) (Marvizon et al., 1999; Song and Marvizon, 2003a) in control rat spinal cord slices. At concentrations higher than 0.1 nM, NPY increased the number of neurons with internalization, reaching 100% of the neurons at 10 μM. The concentration-response data were fitted simultaneously with a monophasic function and a biphasic function. Akaike’s Information Criterion was used to compare the fittings to the two functions, yielding a 98% probability that the biphasic function was the correct model. Therefore, NPY interacts with Y1Rs to induce their internalization with two potencies. The first potency corresponds to an EC50 of 1.25 nM and accounts for 31 ± 6% of the Y1R internalization (‘Frac’, Figure 3). The second potency corresponds to an EC50 of 0.98 μM and accounts for 69% of the Y1R internalization. These results suggest that there are two states of the Y1R with a 1000-fold difference in their affinity for NPY. These two states may correspond to different phosphorylation states, different association with G proteins, or homodimers or heterodimers of the Y1 receptor.
Figure 3 -. Concentration-response curve for neuropeptide Y (NPY) to induce Y1 receptor internalization.
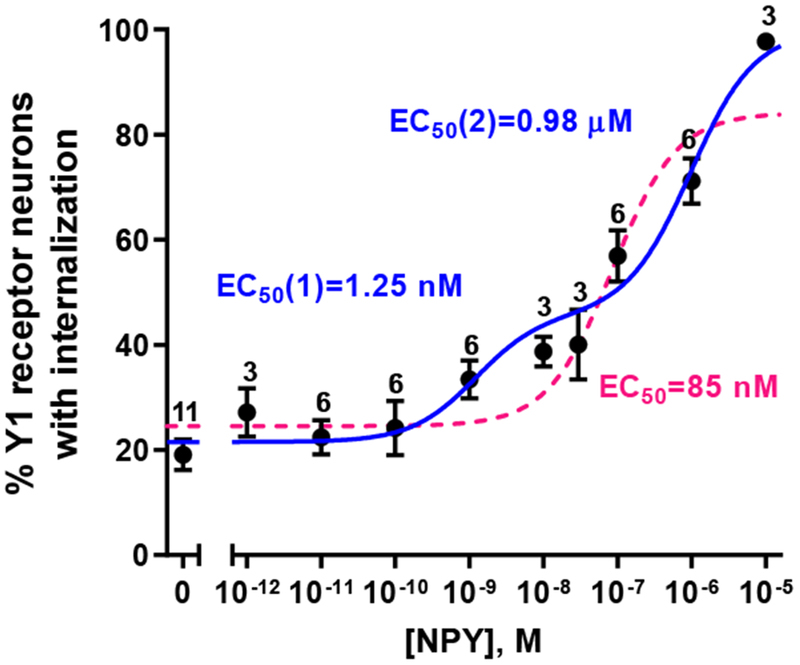
Rat spinal cord slices were incubated at 35 °C for 10 min in aCSF alone (0 M) or with NPY at the concentrations indicated. Slices were fixed and immunofluorescence with the Y1 receptor antibody was used to measure Y1 receptor internalization. Y1 receptor neurons were counted in laminae I and II. Numbers are the number of slices at each concentration of NPY. Non-linear regression fitting of a biphasic dose-response function to the data (98 % probability, Akaike’s Information Criterion): Log EC50(1) = −8.90 ± 0.35 (EC50 = 1.25 nM), Log EC50(2) = −6.01 ± 0.23 (EC50 = 0.98 μM), Frac = 0.31 ± 0.06, top = 100 % (hit the constraint top ≤ 100 %), bottom = 21.5 ± 2.1 %, R2 = 0.844. The dotted curve represents fitting to a monophasic concentration-response curve.
3.3. Peptidase inhibitors only marginally increase the ability of NPY to induce Y1 receptor internalization
The rapid degradation of most endogenous opioids by peptidases (Kishioka et al., 1994) is a major factor when studying their ability to induce internalization of the MOR. For example, peptidase degradation reduced the potency of Leu-enkephalin to induce MOR internalization by two orders of magnitude (Song and Marvizon, 2003a). This makes the use of peptidase inhibitors necessary to detect opioid release through MOR internalization (Chen and Marvizon, 2009; Song and Marvizon, 2003b). In contrast, peptidases cleave substance P and neurokinin A much less readily, so they do not affect the ability of NK1R internalization to measure their release (Marvizon et al., 2003b). Therefore, we asked whether endogenous peptidases significantly reduce the apparent potency of NPY to induce Y1 receptor internalization.
The main enzyme that degrades NPY is dipeptidyl-peptidase IV (Baticic et al., 2011; Buljevic et al., 2013; Mentlein et al., 1993; Rosmaninho-Salgado et al., 2012), so we incubated spinal cord slices with a submaximal concentration of NPY (0.1 μM) in the absence and presence of the dipeptidyl peptidase IV inhibitors alanine-pyrrolidine-nitrile (Ala-Pyrr-CN, 10 μM) (Li et al., 1995) and Ile-Pro-Ile (10 μM) (Mentlein et al., 1993). These inhibitors did not significantly increase NPY-induced Y1 receptor internalization (Figure 4). To explore whether other peptidases can significantly degrade NPY in the rat spinal cord, we added to 0.1 μM NPY a mixture of inhibitors (10 μM each) of dipeptidyl-peptidase IV (Ala-Pyrr-CN), aminopeptidase (amastatin), dipeptidyl carboxypeptidase I (captopril) and neutral endopeptidase (thiorphan). This mixture produced only a small increase in the Y1 internalization induced by NPY. Therefore, peptidase degradation has a negligible effect on the ability of NPY to induce Y1 receptor internalization in the dorsal horn.
Figure 4 -. Effect of peptidase inhibitors on Y1 receptor internalization induced by NPY.
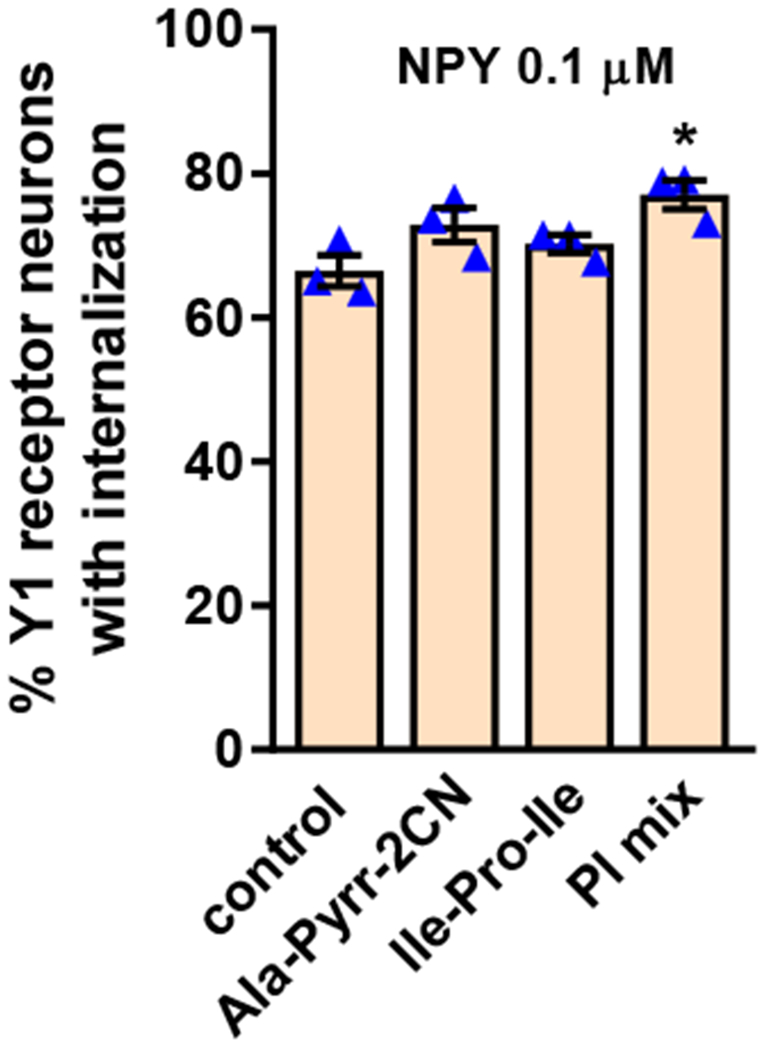
Rat spinal cord slices were incubated at 35 °C for 10 min with 100 nM NPY, alone (control), with the dipeptidyl-peptidase IV inhibitors Ala-pyrrolidine-2-CN (Ala-Pyrr-2CN, 10 μM) or Ile-Pro-Ile (10 μM), or with a mixture of peptidase inhibitors (PI mix) consisting of Ala-pyrrolidine-2-CN, amastatin, captopril and thiorphan (all 10 μM). Slices were fixed and a Y1 receptor antibody was used for immunofluorescence. Y1 receptor neurons were counted in laminae I and II. ANOVA, p=0.0306. Holm-Sidak’s post-hoc test: *p=0.0166 compared to control.
3.4. Chemical stimulation induces Y1 receptor internalization.
Next, we used Y1 receptor internalization in spinal cord slices to determine which chemical stimuli can induce NPY release. First, to induce widespread depolarization, we incubated spinal cord slices for 2 min with 30 mM KCl, a stimulus that induced abundant opioid release in spinal cord slices (Chen et al., 2008). KCl depolarization induced internalization in >80% of Y1 receptor neurons (Figure 5), an amount comparable to that induced by exogenous NPY at 1 μM (Figure 2). Examples of Y1 receptor neurons in a control slice and in a slice incubated with 30 mM KCl are shown in Figure 1A and B, respectively.
Figure 5 -. Y1 receptor internalization induced by capsaicin, NMDA and high K+.
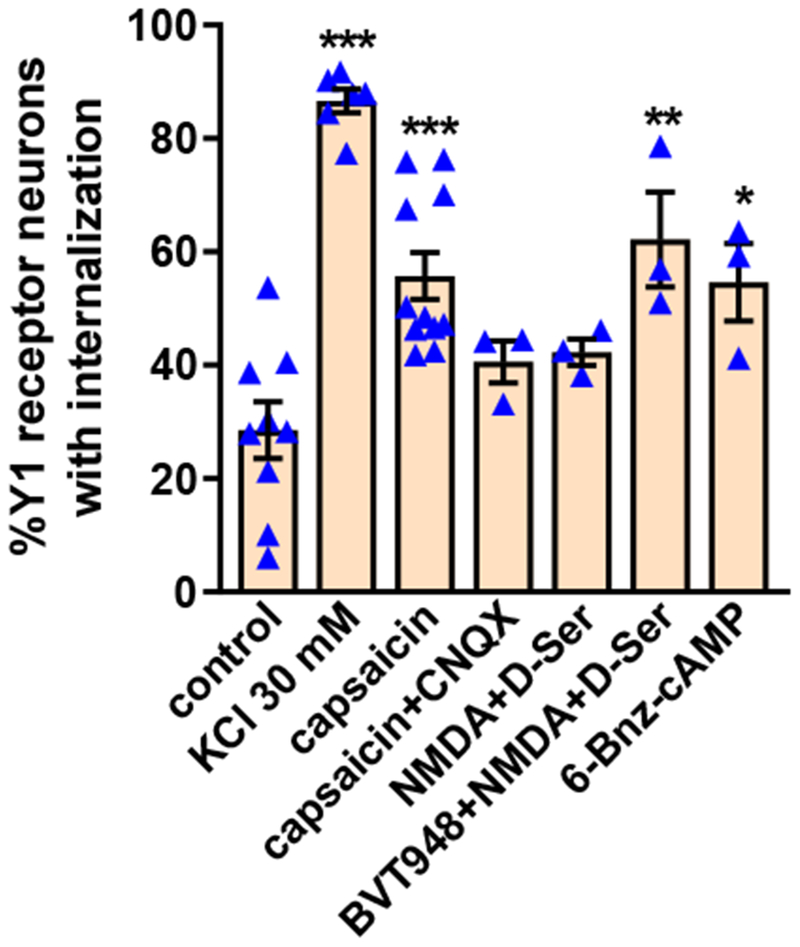
Rat spinal cord slices were untreated (control) or incubated at 35 °C as follows: 2 min in aCSF containing 30 mM KCl and 8 min in aCSF (KCl 30 mM); 10 min with 1 μM capsaicin (capsaicin); 10 min with 1 μM capsaicin and 10 μM CNQX (capsaicin + CNQX); 2 min with 10 μM NMDA and 10 μM D-serine and 8 min in aCSF (NMDA+D-Ser); 60 min with 10 μM BVT948 (protein tyrosine phosphatase inhibitor), 2 min with 10 μM NMDA + D-serine and 8 min in aCSF (BVT948+NMDA+D-Ser), or 60 min with 6-Bnz-cAMP 10 nM. Slices were fixed and the Y1 receptor antibody was used for immunofluorescence. Y1 receptor neurons were counted in laminae I and II. ANOVA, p<0.0001; Holm-Sidak’s post-hoc test: *** p<0.001, ** p<0.01 compared to control.
Capsaicin is one of the most efficacious stimuli to induce substance P release (Lao et al., 2003; Marvizon et al., 2003a; Yaksh et al., 1979), but not opioid release (Song and Marvizon, 2003b), in the dorsal horn. This reflects the fact that substance P is released by primary afferents that express TRPV1 (Usoskin et al., 2015) and by excitatory dorsal horn neurons receiving synaptic input from them (Dickie et al., 2019; Gutierrez-Mecinas et al., 2017), whereas enkephalins are released from dorsal horn interneurons (Chen and Marvizon, 2009; Haring et al., 2018; Marvizon et al., 2009; Marvizon et al., 2007; Sathyamurthy et al., 2018; Song and Marvizon, 2003b). Figure 5 illustrates that incubation of spinal cord slices with 1 μM capsaicin induced Y1 receptor internalization in approximately 50% of Y1 receptor-immunoreactive neurons. This indicates that capsaicin induces NPY release from TRPV1-expressing primary afferents (Wakisaka et al., 1992) or from neurons that receive synaptic inputs from them. To distinguish between these two possibilities, we incubated slices with capsaicin together with CNQX (10 μM), an antagonist of AMPA and kainate receptors, in order to block synaptic transmission from primary afferents. CNQX decreased the Y1R internalization induced by capsaicin to near control levels (Figure 5), suggesting that part of the NPY released by capsaicin was from interneurons receiving synaptic input from TRPV1-expressing primary afferents.
Activation of NMDA receptors induces substance P release in the spinal cord (Chen et al., 2014b; Chen et al., 2010; Liu et al., 1997; Malcangio et al., 1998; Marvizon et al., 1997) and also inhibit the release of opioids that produce MOR internalization (Song and Marvizon, 2005). Figure 5 illustrates that NMDA and D-serine (D-Ser, both 10 μM), applied to the slices for 2 min to activate NMDA receptors, produced a trend to increase Y1 receptor internalization. We next tried BVT948, an inhibitor of protein tyrosine phosphatases that increases NMDA receptor activation by augmenting the phosphorylation of Tyr1472 of the NR2B subunit, and also increases substance P release (Chen et al., 2014b; Chen et al., 2010). Preincubation of slices for 60 min with BVT948 (10 μM) followed by NMDA+D-Ser increased Y1 receptor internalization to the same extent as capsaicin (Figure 5). These data indicate that NPY release is likely driven by the same NR2B-containing NMDA receptors that induce substance P release (Marvizon et al., 2002).
Another class of stimuli that induce substance P release in the dorsal horn are cell-permeable cAMP analogs like 8-Br-cAMP (Chen et al., 2018). Here we found that a selective activator of protein kinase A, the cAMP analog, 6-benzoyl-cAMP (6-Bnz-cAMP, 10 nM, 60 min) (Hewer et al., 2011), induced a substantial amount of Y1 receptor internalization (Figure 5). These data indicate that activation of protein kinase A releases NPY in the dorsal horn.
3.5. Y1 receptor internalization induced by electrical stimulation of the dorsal horn
Electrical stimulation of the dorsal horn of spinal cord slices is an effective stimulus to induce both substance P and opioid release (Song and Marvizon, 2003b), so we used the same approach to study NPY release. One dorsal horn was inserted between the poles of a bipolar hook electrode. Electrical stimulation consisted of 1000 pulses of 20 V and 0.4 ms delivered at the frequencies of 1, 5, 30, 100 and 500 Hz. Control slices were superfused in the slice chamber without electrical stimulation. Substantial Y1 receptor internalization on the side ipsilateral to stimulation was detected at all frequencies except 1 Hz (Figure 6A). The amount of Y1 receptor internalization increased with frequency up to 30 Hz - 100 Hz, then it decreased at 500 Hz but was still higher than control. Electrical stimulation did not change Y1 receptor internalization above control levels at the non-stimulated (contralateral) dorsal horn; this indicates that any current leakage beyond the electrodes was unable to trigger NPY release at distant sites. This inverted U-shape profile of frequency dependence with a peak at 100 Hz (and less release at 500 Hz) was also observed in substance P release studies (Adelson et al., 2009). By contrast, opioid release in the dorsal horn continued to increase at frequencies higher than 100 Hz (Song and Marvizon, 2003b).
Figure 6 -. NPY release induced by electrical stimulation to the dorsal horn.
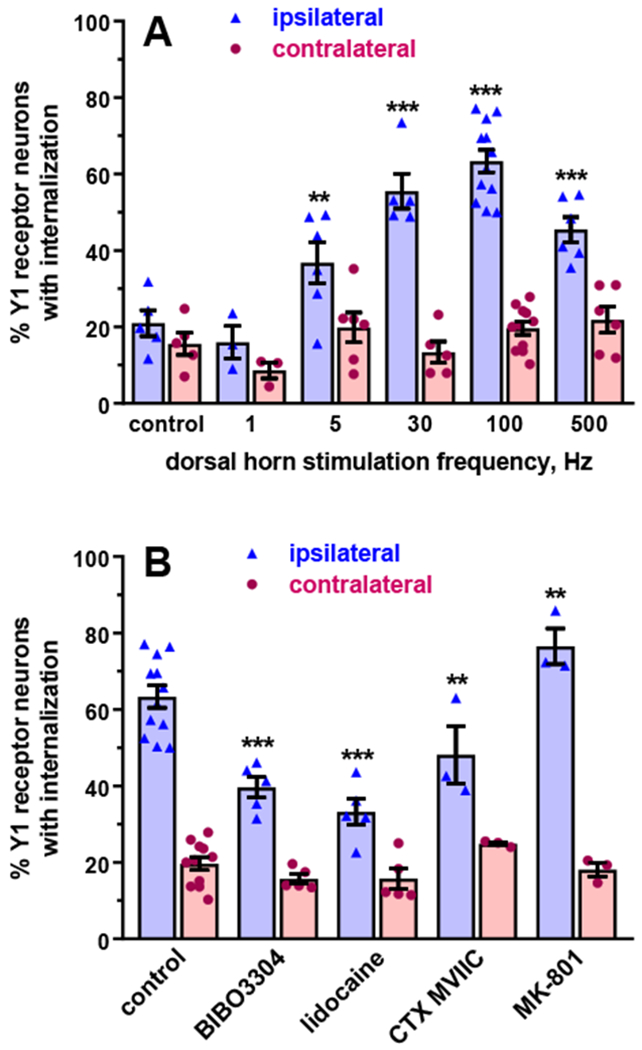
A. One dorsal horn (‘ipsilateral’) of spinal cord slices was inserted between the poles of a hook bipolar electrode. Electrical stimulation consisted of 1000 pulses of 20 V and 0.4 ms delivered at the frequencies indicated. After the stimulation, slices were superfused in the chamber for 10 min. Control: slices superfused in the chamber for 10 min. Data analysis by a mixed-effects model (maximum likelihood method) yielded significant effects of frequency p<0.0001. side (ipsilateral vs. contralateral) p<0.0001 and their interaction p<0.0001. B. Spinal cord slices were stimulated at 100 Hz (1000 pulses of 20 V, 0.4 ms) while being superfused with aCSF (control), 100 nM BIBO3304 (Y1 receptor antagonist), 1 μM ω-conotoxin (CTX) MVIIC (Cav2.2 channel blocker), 1 mM lidocaine or 10 μM MK-801 (NMDA receptor blocker). Compounds were superfused starting 2 min (BIBO3304 and CTX MVIIC) or 10 min (lidocaine and MK-801) before the stimulus. Analysis by a mixed-effects model yielded significant effects of compounds p<0.0001, side p<0.0001 and interaction p<0.0001. Holm-Sidak’s post-hoc test: *** p<0.001, ** p<0.01, compared to control.
To determine whether Y1 internalization induced by electrical stimulation was due to agonist activation of the Y1 receptor, we stimulated spinal cord slices at 100 Hz in the presence of the selective Y1 receptor antagonist BIBO3304 (100 nM for 2 min). As illustrated in Figure 6B, BIBO3304 decreased evoked Y1 receptor internalization. This indicates that electrical stimulation induces Y1 receptor internalization by releasing NPY, which then binds to and activates the Y1 receptor.
We next characterized the contribution of sodium, calcium and NMDA channels to evoked NPY release. First, we superfused the dorsal horn for 10 min with the sodium channel blocker lidocaine (1 mM), and then stimulated the slices at 100 Hz. Lidocaine robustly decreased Y1 receptor internalization (Figure 6B), indicating that NPY release is dependent upon the firing of action potentials. Second, we stimulated the dorsal horn (100 Hz) in the presence of the Cav2 channel blocker ω-conotoxin (CTX) MVIIC (1 μM). CTX partially decreased evoked Y1 receptor internalization (Figure 6B), indicating that Ca2+ entry through Cav2 channels is necessary for NPY release, though other Cav channels may contribute as well. Third, we stimulated the dorsal horn (100 Hz) in the presence of the NMDA receptor blocker MK-801 (10 μM). In contrast to previous studies indicating that presynaptic NMDA receptors contribute to substance P release evoked by electrical stimulation (Chen et al., 2018; Chen et al., 2014b; Chen et al., 2010; Liu et al., 1997; Malcangio et al., 1998; Marvizon et al., 1997), we found that MK-801 did not decrease stimulus-evoked Y1 receptor internalization. Instead, MK-801 produced a small increase in internalization at the ipsilateral dorsal horn (but not the contralateral dorsal horn, indicating that it does not induce NPY release by itself). These data suggest that NMDA receptor activity inhibits, rather than facilitates, NPY release from dorsal horn neurons. We speculate that presynaptic NR2B-containing NMDA receptors increase NPY release from primary afferents, whereas extrasynaptic NMDA receptors without the NR2B subunit inhibit NPY release from dorsal horn interneurons. This explanation is consistent with previous findings showing that extrasynaptic NMDA receptors without the NR2B subunit inhibit opioid release from dorsal horn neurons by opening large conductance Ca2+-sensitive K+ channels and thus hyperpolarizing them (Song and Marvizon, 2005), and with results of Figure 5 showing that NMDA receptor activation with NMDA+D-Ser (together with preincubation with BVT948 to promote phosphorylation of the NR2B subunit) induced NPY release.
3.6. Y1 receptor internalization induced by electrical stimulation of the dorsal root
Electrical stimulation of the dorsal horn recruits axons of both intrinsic dorsal horn neurons and primary afferents. To selectively study NPY release driven by primary afferent firing, we stimulated the dorsal root electrically or with capsaicin. It should be noted that either approach can drive NPY release not only from the primary afferents themselves, but also from dorsal horn neurons receiving synaptic input from those afferents. To enable consistent stimulation of the dorsal root and not the dorsal horn, we used a two-chamber arrangement that prevents shunting of current outside of the root, thus effectively isolating the stimulated root from the slice (Adelson et al., 2009). Electrical stimulation consisted of 1000 pulses of 0.4 ms duration. Controls slices without roots were superfused with aCSF in the slice chamber. To exclusively recruit A-fibers we used pulses of 1 V delivered at 100 Hz (Adelson et al., 2009). As illustrated in Figure 7, this produced negligible Y1 receptor internalization. To recruit both A-fibers and C-fibers, we used pulses of 20 V (Adelson et al., 2009) delivered at low (5 Hz) or high (100 Hz) frequency. Y1 receptor internalization was not significantly different from the control slices, or between the ipsilateral and the contralateral sides (Figure 7A).
Figure 7 -. Y1 receptor internalization induced by dorsal root stimulation.
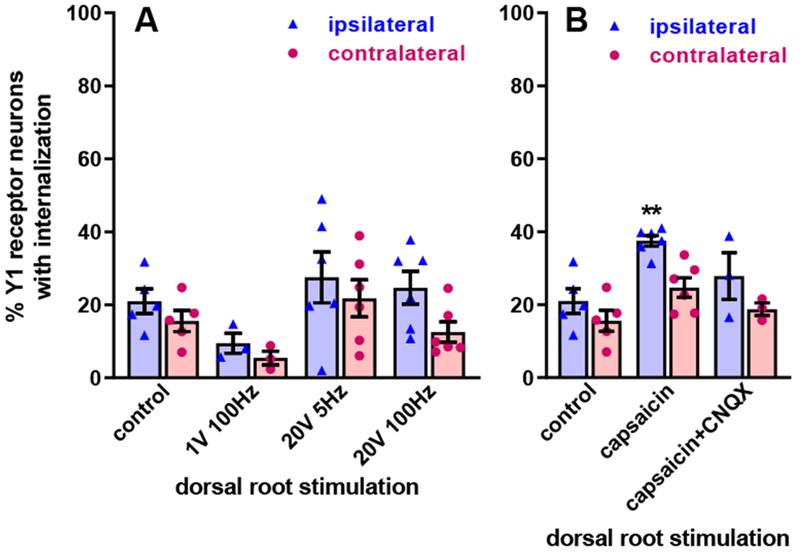
A. Electrical stimulation: one dorsal root contiguous with the spinal cord slice was put on a side chamber through a grease bridge, placed on a bipolar electrode and covered with mineral oil. Then the root was stimulated with 1000 pulses of 0.4 ms duration, 1 V or 20 V, delivered at 5 Hz or 100 Hz, as indicated. After the stimulation, slices were superfused in the chamber for 10 min. “Control” slices are the same in both panels and were superfused in the chamber for 10 min. Repeated measures 2-way ANOVA: stimulation p=0.14, side (ipsilateral vs. contralateral) p=0.0015, interaction p=0.36. B. “Capsaicin”: the dorsal root was put into the side chamber and covered with 1 μM capsaicin for 10 min. “Capsaicin + CNQX”: the dorsal root was covered with 1 μM capsaicin while the slice was superfused with 10 μM CNQX for 10 min. Repeated measures 2-way ANOVA: stimulation p=0.010, side p=0.0005, interaction p=0.23. Holm-Sidak’s post-hoc test: ** p<0.01, compared to control.
3.7. Y1 receptor internalization induced by capsaicin stimulation of the dorsal root
To selectively stimulate TRPV1-expressing nociceptors (which are mostly C-fibers), we incubated the dorsal root in capsaicin (1 μM) for 10 min in a side chamber that was separated from the slice superfusion chamber by a grease bridge, thus preventing capsaicin from reaching the dorsal horn. There is an important difference between incubating the whole slice with capsaicin as opposed to the dorsal root: when added to the whole slice, capsaicin can induce neuropeptide release by letting Ca2+ into the presynaptic terminal through TPRV1 channels; when added to the dorsal root, capsaicin has no access to the presynaptic terminals and thus induces neuropeptide release by TRPV1-mediated axon depolarization and firing of action potentials (Lao et al., 2003). Stimulation of the dorsal root with capsaicin evoked substantial Y1 receptor internalization, which was significantly higher than in control slices (Figure 7B). Superfusion of the slices with 10 μM CNQX to suppress synaptic transmission eliminated the effect of capsaicin, suggesting that part of the NPY released by capsaicin was from dorsal horn neurons receiving synapses from TRPV1-expressing primary afferents and not from the afferent themselves. These results are consistent with those obtained by incubating slices with capsaicin (Figure 5), but harder to reconcile with the fact that electrical stimulation of the dorsal root produced negligible NPY release. The larger scattering of the individual data points in Figure 7 suggests that electrical stimulation of the root provides a stimulus that is weaker and less consistent than capsaicin stimulation.
Taken together, our data indicate that NPY is released from dorsal horn interneurons receiving synapses from TRPV1-expressing peptidergic C-fibers (Figure 10). Indeed, Polgar et al. (2013) reported that NPY-expressing interneurons are one of the four classes of inhibitory interneurons in the dorsal horn, the other three being the ones expressing galanin, parvalbumin and neuronal nitric oxide synthase (nNOS). Noxious stimuli like pinch, heat, capsaicin and formalin induced pERK only in the galanin and NPY neurons, supporting the idea that they receive synaptic input from nociceptors. In the RNA-seq transcriptional profiling study by Sathyamurthy et al. (2018), the NPY inhibitory interneurons were denominated as DI-4 (“D” stands for “dorsal”, “I” for “inhibitory”). They seem to correspond to lamina II islet cells that make synapses with the NK1R projection neurons (Peirs and Seal, 2016; Polgar et al., 1999). However, the other RNA-seq transcriptional study (Haring et al., 2018) found that NPY is expressed in 14 out of their 15 classes of inhibitory GABAergic neurons in the dorsal horn, but in none of their 15 classes of excitatory neurons.
Figure 10 -. Diagram with the proposed circuit formed by the neuropeptide Y (NPY) and the Y1 receptor (Y1R) dorsal horn neurons.
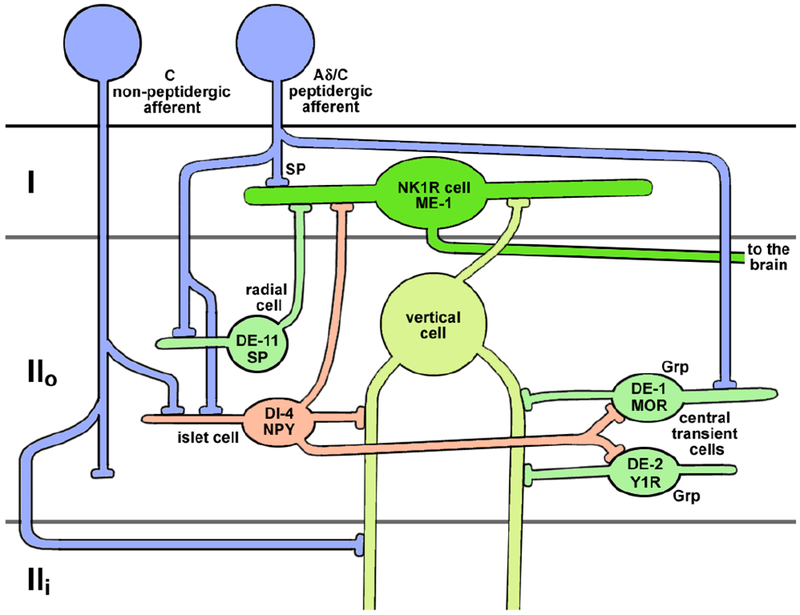
DE-1, DE-2, DE-1, ME-1 and DI-4 are the denominations for dorsal horn (D) excitatory (E, green) and inhibitory (I, red) neurons proposed by Sathyamurthy et al. Grp: gastrin-releasing peptide; MOR: μ-opioid receptor; NK1R: neurokinin-1 receptor; SP: substance P. Laminae I, II outer (IIo) and II inner (IIi) are indicated.
3.8. Nerve injury increases Y1 receptor internalization induced by dorsal horn stimulation in spinal cord slices
Nerve injury dramatically increases the content of NPY in myelinated primary afferents and the dorsal horn (Ma and Bisby, 1998; Wakisaka et al., 1992). To test the hypothesis that this would translate into an increase in evoked NPY release, we used the spared nerve injury (SNI) model of peripheral neuropathic pain. As illustrated in Figure 8A, rats with SNI (n=6), but not sham-operated rats (n=7), developed mechanical hyperalgesia at the ipsilateral hind paw by day 3 that was still present at day 14. Repeated measures 3-way ANOVA revealed significant effects of time (p=0.0008, F3, 33=7.19) and side (p=0.0029, F1, 11=14.53), but not SNI (p=0.49, F1, 11=0.508), and significant interactions of time x side (p=0.0044, F3, 33=5.27), time x SNI (p=0.0173, F3, 33=3.90), side x SNI (p=0.0002, F1, 11=29.6) and time x side x SNI (p=0.0006, F3, 33=7.45). Therefore, SNI increased hyperalgesia over time at the ipsilateral paw.
Figure 8 -. Y1 receptor internalization in spinal cord slices after spared nerve injury.
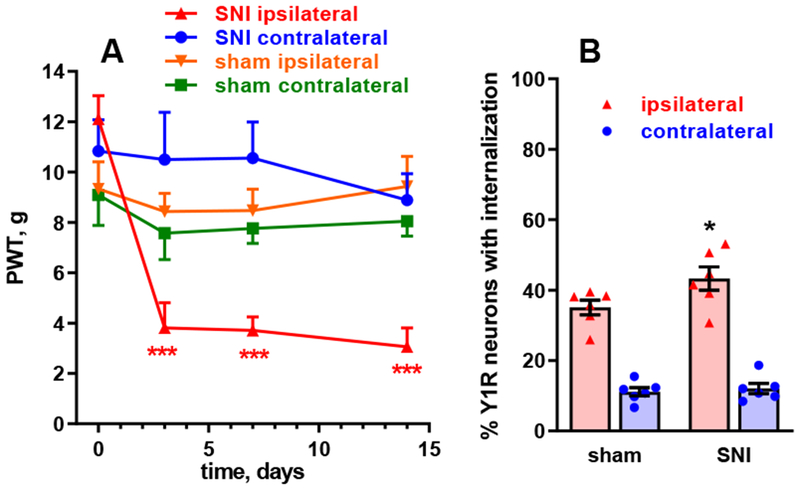
A. Rats received spared nerve injury (SNI) consisting in cutting the common peroneal and tibial nerves (n=6) or sham surgery (n=7). The development of mechanical hyperalgesia was followed for 14 days as responses to von Frey filament in the hind paws ipsilateral and contralateral to SNI. Repeated measures (by time and side) 3-way ANOVA yielded significant effects of time (p=0.0008) and side (p=0.0029), but not SNI (p=0.49), and significant interactions time x side (p=0.0044), time x SNI (p=0.0173), side x SNI (p=0.0002) and time x side x SNI (p=0.0006). Holm-Sidak’s post-hoc test: ***, p<0.0001 compared to baseline. B. On day 14, 3 spinal cord slices were obtained from each rat, which were stimulated at the ipsilateral dorsal horn with 1000 pulses of 20 V, 0.4 ms, delivered at 5 Hz. Y1R internalization was measured in sections from the slices. Repeated measures 2-way ANOVA yielded significant effects of side (p<0.0001) and interaction (p=0.0386), but not SNI (p=0.116). Holm-Sidak’s post-hoc test: *, p=0.0289 compared with sham.
At day 14, we prepared spinal cord slices (3 slices per rat). To induce NPY release, one dorsal horn was electrically stimulated with 1000 pulses of 20 V, 0.4 ms. The stimulation frequency was set at a sub-maximal stimulus, 5 Hz (Figure 5), to avoid a ceiling effect and thus allow detection of increases in NPY release. Y1 receptor internalization was measured in the ipsilateral and contralateral dorsal horn of each slice, and the mean from the three slices of each rat was taken as the value for that rat. We detected a small increase in stimulus-evoked Y1 receptor internalization in the ipsilateral dorsal horn of the rats with SNI as compared with sham-operated rats (Figure 8B). Repeated measures 2-way ANOVA yielded significant effects of side (p<0.0001, F1, 10=324.3) and interaction (p=0.0386, F1, 10=5.66), but not of SNI (p=0.116, F1, 10=2.95).
3.9. Nerve injury increases Y1 receptor internalization induced by noxious and non-noxious stimulation in vivo
In a parallel experiment, rats also received SNI, but they were subjected to natural mechanical stimuli in vivo to induce NPY release. SNI produced mechanical hyperalgesia at the hindpaw ipsilateral (Figure 9A) but not contralateral to SNI (Figure 9B). For release studies, the stimulus consisted of no stimulation (n=5 rats), rubbing the paw ipsilateral to SNI with a brush (n=6 rats), or clamping the ipsilateral paw with a binder clip for 2 min (n=7 rats). No stimulation resulted in negligible (baseline) Y1 receptor internalization in both the sham and the SNI rats (Figure 9). In sham rats, paw rub or paw clamp did not significantly increase Y1 receptor internalization above control levels. In contrast, in the SNI rats both rubbing and clamping the paw increased Y1 receptor internalization. Interestingly, both non-noxious (rub) and noxious (clamp) stimulation were equally effective in releasing NPY. Furthermore, paw rub increased Y1 receptor internalization at the contralateral dorsal horn of SNI rats as well. Repeated measures two-way ANOVA yielded significant effects of stimulus (p=0.0002, F2, 16=15.16), side (p<0.0001, F1, 16=44.32) and stimulus x side interaction (p=0.0048, F2, 16=7.60). Taken together with the above in situ studies, these results indicate that nerve injury increases stimulus-evoked release of NPY, a result that is consistent with a concomitant and robust increase in NPY expression in large myelinated afferents and dorsal horn (Ma and Bisby, 1998; Wakisaka et al., 1992).
Figure 9 -. Y1 receptor internalization in vivo after spared nerve injury.
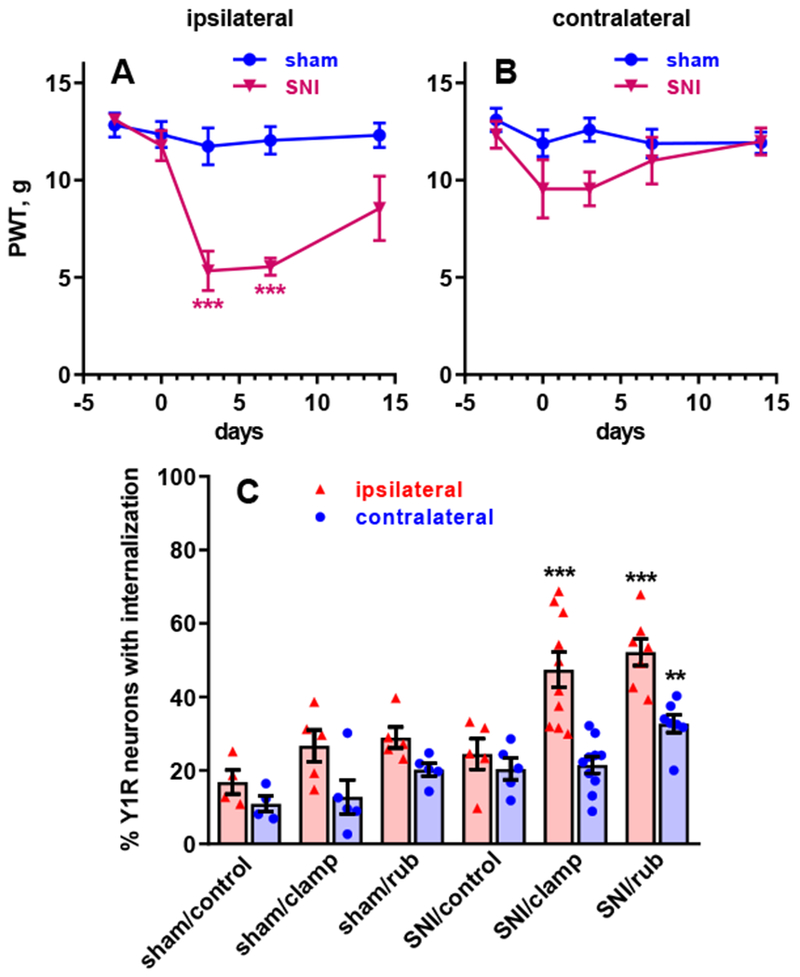
Rats received sham surgery or spared nerve injury (SNI), consisting in cutting the common peroneal and tibial nerves unilaterally. The development of mechanical hyperalgesia was followed for 14 days as responses to von Frey filaments in the hind paws ipsilateral (A) and contralateral (B) to SNI. Repeated measures 2-way ANOVA, A: time p<0.0001, SNI p = 0.0246, interaction p <0.0001; B: time p=0.12, SNI p=0.22, interaction p =0.29. C. On day 14, rats with sham surgery or SNI received no stimulation (“control”), clamping of the ipsilateral hind paw with a binder clip for 2 min (“clamp”), or rubbing of the ipsilateral hind paw (“rub”). Repeated measures 2-way ANOVA: stimulus/SNI p<0.0001, side p<0.0001, interaction p=0.0053. Holm-Sidak’s post-hoc tests: ** p<0.01, *** p<0.0001 compared with sham (A) or sham/control (C).
4. Conclusions
Y1 receptor internalization is a fast, precise and sensitive technique to measure NPY release in slices and in vivo. Exogenous NPY induces Y1 receptor internalization in dorsal horn neurons with a biphasic concentration-response curve with EC50s of 1 nM and 1 μM. Peptidases in the dorsal horn do not substantially limit Y1R activation by NPY. Overall, our data paint a complex picture of NPY release in the dorsal horn, with multiple sources that include capsaicin-sensitive C-fibers and dorsal horn interneurons. NPY release in the dorsal horn increases dramatically after nerve injury and can be triggered by both noxious and non-noxious stimuli. Figure 10 is a diagram of the proposed circuit formed by these neurons, based on Peirs and Seal (2016) and Sathyamurthy et al. (2018).
Acknowledgements
This study was completed under the umbrella of three UCLA institutes: Brain Research Institute, CURE: Digestive Diseases Research Center, and the Oppenheimer Family Center for Neurobiology of Stress and Resilience.
Funding: This work was supported by the National Institute of Health (grant numbers R01-NS045954 and R01-DA037621 to BKT and R01-DA033059 to JCM); and the Department of Veterans Affairs Rehabilitation Research & Development Service (grant number 1I01RX001646A to JCM).
Abbreviations
- 8-Br-cAMP
8-bromoadenosine-3′,5′-cyclic monophosphate
- 6-Bnz-cAMP
6-benzoyl-3′,5′-cyclic monophosphate
- ANOVA
analysis of variance
- BVT948
4-hydroxy-3,3-dimethyl-2H-benz[g]indole-2,5(3H)-dione
- Cav channel
voltage-gated calcium channel
- CTX
ω-conotoxin
- D-Ser
D-serine
- MK-801
dizocilpine, (5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate
- MOR
μ-opioid receptor
- NK1R
neurokinin 1 receptor
- NMDA
N-methyl-D-aspartate
- NPY
neuropeptide Y
- TRPV1
transient receptor potential vanilloid 1
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds
Amastatin (PubChem CID: 439518); BIBO3304 (PubChem CID: 5311022); BVT948 (PubChem CID: 6604934); capsaicin (PubChem CID: 1548943); captopril (PubChem CID: 44093); ω-conotoxin MVIIC (PubChem CID: 56841670); D-serine (PubChem CID: 71077); diprotin A (Ile-Pro-Ile, PubChem CID: 94701); lidocaine (PubChem CID: 6314); MK-801 (PubChem CID: 180081); neuropeptide Y (PubChem CID: 16132352); NMDA (PubChem CID: 22880); thiorphan (PubChem CID: 3132).
References
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI, 1997. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. Journal of Neuroscience 17, 8049–8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson DW, Lao L, Zhang G, Kim W, Marvizón JC, 2009. Substance P release and neurokinin 1 receptor activation in the rat spinal cord increases with the firing frequency of C-fibers. Neuroscience 161, 538–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW, 1997. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J. Neurosci 17, 5921–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baticic L, Detel D, Kucic N, Buljevic S, Pugel EP, Varljen J, 2011. Neuroimmunomodulative properties of dipeptidyl peptidase IV/CD26 in a TNBS-induced model of colitis in mice. J Cell Biochem 112, 3322–3333. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Hofstetter C, Olson L, Ohning G, Villar M, Hokfelt T, 2006. The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord. Neuroscience 138, 1361–1376. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Shi TS, Landry M, Villar MJ, Hokfelt T, 2007. Neuropeptide tyrosine and pain. Trends Pharmacol Sci 28, 93–102. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Stanic D, Shuster S, Herzog H, Villar M, Hokfelt T, 2005. Neuropeptide Y2 receptor protein is present in peptidergic and nonpeptidergic primary sensory neurons of the mouse. J Comp Neurol 489, 328–348. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Shi TJ, Matsuda H, Kopp J, Villar MJ, Hokfelt T, 2002. NPY Y1 receptors are present in axonal processes of DRG neurons. Exp Neurol 174, 1–10. [DOI] [PubMed] [Google Scholar]
- Buljevic S, Detel D, Pucar LB, Mihelic R, Madarevic T, Sestan B, Varljen J, 2013. Levels of dipeptidyl peptidase IV/CD26 substrates neuropeptide Y and vasoactive intestinal peptide in rheumatoid arthritis patients. Rheumatol Int 33, 2867–2874. [DOI] [PubMed] [Google Scholar]
- Chen W, Ennes HS, McRoberts JA, Marvizon JC, 2018. Mechanisms of mu-opioid receptor inhibition of NMDA receptor-induced substance P release in the rat spinal cord. Neuropharmacology 128, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Marvizón JC, 2009. Acute inflammation induces segmental, bilateral, supraspinally mediated opioid release in the rat spinal cord, as measured by μ-opioid receptor internalization. Neuroscience 161, 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, McRoberts JA, Marvizón JCG, 2014a. μ-Opioid receptor inhibition of substance P release from primary afferents disappears in neuropathic pain but not inflammatory pain. Neuroscience 267, 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Song B, Lao L, Perez OA, Kim W, Marvizon JCG, 2007. Comparing analgesia and μ-opioid receptor internalization produced by intrathecal enkephalin: Requirement for peptidase inhibition. Neuropharmacology 53, 664–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Song B, Zhang G, Marvizon JC, 2008. Effects of veratridine and high potassium on μ-opioid receptor internalization in the rat spinal cord: stimulation of opioid release versus inhibition of internalization. J Neurosci Methods 170, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Walwyn W, Ennes H, Kim H, McRoberts JA, Marvizon JC, 2014b. BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur. J. Neurosci 39, 1439–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang G, Marvizon JC, 2010. NMDA receptors in primary afferents require phosphorylation by Src family kinases to induce substance P release in the rat spinal cord. Neuroscience 166, 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin LA, Duggan AW, 2001. The effect of conduction block on the spinal release of immunoreactive-neuropeptide Y (ir-NPY) in the neuropathic rat. Pain 91, 235–240. [DOI] [PubMed] [Google Scholar]
- Dickie AC, Bell AM, Iwagaki N, Polgar E, Gutierrez-Mecinas M, Kelly R, Lyon H, Turnbull K, West SJ, Etlin A, Braz J, Watanabe M, Bennett DLH, Basbaum AI, Riddell JS, Todd AJ, 2019. Morphological and functional properties distinguish the substance P and gastrin-releasing peptide subsets of excitatory interneuron in the spinal cord dorsal horn. Pain 160, 442–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemignani A, Marchese S, Fontana G, Raiteri M, 1997. Neuropeptide Y release from cultured hippocampal neurons: stimulation by glutamate acting at N-methyl-D-aspartate and AMPA receptors. Neuroscience 81, 23–31. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Allen JM, Adrian TE, Kelly JS, Bloom SR, 1984. The distribution and origin of a novel brain peptide, neuropeptide Y, in the spinal cord of several mammals. J. Comp. Neurol 227, 78–91. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Bell AM, Marin A, Taylor R, Boyle KA, Furuta T, Watanabe M, Polgar E, Todd AJ, 2017. Preprotachykinin A is expressed by a distinct population of excitatory neurons in the mouse superficial spinal dorsal horn including cells that respond to noxious and pruritic stimuli. Pain 158, 440–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lonnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O, Lagerstrom MC, Linnarsson S, Ernfors P, 2018. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci 21, 869–880. [DOI] [PubMed] [Google Scholar]
- Hewer RC, Sala-Newby GB, Wu YJ, Newby AC, Bond M, 2011. PKA and Epac synergistically inhibit smooth muscle cell proliferation. Journal of molecular and cellular cardiology 50, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Broberger C, Zhang X, Diez M, Kopp J, Xu Z, Landry M, Bao L, Schalling M, Koistinaho J, DeArmond SJ, Prusiner S, Gong J, Walsh JH, 1998. Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev 26, 154–166. [DOI] [PubMed] [Google Scholar]
- Honore P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW, 1999. Spinal cord substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J. Neurosci 19, 7670–7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DI, Scott DT, Riddell JS, Todd AJ, 2007. Upregulation of substance P in low-threshold myelinated afferents is not required for tactile allodynia in the chronic constriction injury and spinal nerve ligation models. J Neurosci 27, 2035–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intondi AB, Dahlgren MN, Eilers MA, Taylor BK, 2008. Intrathecal neuropeptide Y reduces behavioral and molecular markers of inflammatory or neuropathic pain. Pain 137, 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarahi M, Sheibani V, Safakhah HA, Torkmandi H, Rashidy-Pour A, 2014. Effects of progesterone on neuropathic pain responses in an experimental animal model for peripheral neuropathy in the rat: a behavioral and electrophysiological study. Neuroscience 256, 403–411. [DOI] [PubMed] [Google Scholar]
- Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hokfelt T, 1994. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. J Neurosci 14, 6423–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King PJ, Widdowson PS, Doods HN, Williams G, 1999. Regulation of neuropeptide Y release by neuropeptide Y receptor ligands and calcium channel antagonists in hypothalamic slices. J Neurochem 73, 641–646. [DOI] [PubMed] [Google Scholar]
- Kingery WS, Guo TZ, Davies MF, Limbird L, Maze M, 2000. The alpha(2A) adrenoceptor and the sympathetic postganglionic neuron contribute to the development of neuropathic heat hyperalgesia in mice. Pain 85, 345–358. [DOI] [PubMed] [Google Scholar]
- Kishioka S, Miyamoto Y, Fukunaga Y, Nishida S, Yamamoto H, 1994. Effects of a mixture of peptidase inhibitors (amastatin, captopril and phosphoramidon) on Met-enkephalin-, beta-endorphin-, dynorphin-(1-13)- and electroacupuncture-induced antinociception in rats. Jap. J. Pharmacol 66, 337–345. [DOI] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL, 2005. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J. Neurosci 25, 3651–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal KE, Solway B, Pedrazzini T, Taylor BK, 2008. Y1 receptor knockout increases nociception and prevents the anti-allodynic actions of NPY. Nutrition (Burbank, Los Angeles County, Calif 24, 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao L, Song B, Marvizon JCG, 2003. Neurokinin release produced by capsaicin acting on the central terminals and axons of primary afferents: relationship with NMDA and GABAB receptors. Neuroscience 121, 667–680. [DOI] [PubMed] [Google Scholar]
- Lemons LL, Wiley RG, 2012. Neuropeptide Y receptor-expressing dorsal horn neurons: role in nocifensive reflex and operant responses to aversive cold after CFA inflammation. Neuroscience 216, 158–166. [DOI] [PubMed] [Google Scholar]
- Li J, Wilk E, Wilk S, 1995. Aminoacylpyrrolidine-2-nitriles: potent and stable inhibitors of dipeptidyl-peptidase IV (CD 26). Archives of Biochemistry and Biophysics 323, 148–154. [DOI] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI, 1997. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature 386, 721–724. [DOI] [PubMed] [Google Scholar]
- Ma W, Bisby MA, 1998. Partial and complete sciatic nerve injuries induce similar increases of neuropeptide Y and vasoactive intestinal peptide immunoreactivities in primary sensory neurons and their central projections. Neuroscience 86, 1217–1234. [DOI] [PubMed] [Google Scholar]
- Mahinda TB, Taylor BK, 2004. Intrathecal neuropeptide Y inhibits behavioral and cardiovascular responses to noxious inflammatory stimuli in awake rats. Physiol Behav 80, 703–711. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Fernandes K, Tomlinson DR, 1998. NMDA receptor activation modulates evoked release of substance P from rat spinal cord. Br. J. Pharmacol 125, 1625–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, 1995. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science 268, 1629–1632. [DOI] [PubMed] [Google Scholar]
- Martin JR, 1996. Evidence of systemic neuropeptide Y release after carbachol administration into the posterior hypothalamic nucleus. J Cardiovasc Pharmacol 28, 447–457. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Chen W, Murphy N, 2009. Enkephalins, dynorphins and β-endorphin in the rat dorsal horn: an immunofluorescence colocalization study. J. Comp. Neurol 517, 51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Waszak-McGee J, Mayer EA, 1999. Internalization of μ-opioid receptors in rat spinal cord slices. Neuroreport 10, 2329–2334. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Martinez V, Grady EF, Bunnett NW, Mayer EA, 1997. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J. Neurosci 17, 8129–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA, 2002. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J. Comp. Neurol 446, 325–341. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Perez OA, Song B, Chen W, Bunnett NW, Grady EF, Todd AJ, 2007. Calcitonin Receptor-Like Receptor and Receptor Activity Modifying Protein 1 in the rat dorsal horn: localization in glutamatergic presynaptic terminals containing opioids and adrenergic α2C receptors. Neuroscience 148, 250–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Wang X, Matsuka Y, Neubert JK, Spigelman I, 2003a. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience 118, 535–545. [DOI] [PubMed] [Google Scholar]
- Marvizon JCG, Wang X, Lao L, Song B, 2003b. Effect of peptidases on the ability of exogenous and endogenous neurokinins to produce neurokinin 1 receptor internalization in the rat spinal cord. Br. J. Pharmacol 140, 1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentlein R, Dahms P, Grandt D, Kruger R, 1993. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul Pept 49, 133–144. [DOI] [PubMed] [Google Scholar]
- Michot B, Bourgoin S, Viguier F, Hamon M, Kayser V, 2012. Differential effects of calcitonin gene-related peptide receptor blockade by olcegepant on mechanical allodynia induced by ligation of the infraorbital nerve vs the sciatic nerve in the rat. Pain 153, 1939–1948. [DOI] [PubMed] [Google Scholar]
- Nelson TS, Fu W, Donahue R, Corder G, Hokfelt T, Wiley R, Taylor BK, 2019. Facilitation of neuropathic pain by the NPY Y1 receptor-expressing population of excitatory interneurons in the dorsal horn. Scientific reports (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Seal R, 2016. Neural circuits for pain: Recent advances and current views. Science 354, 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Sardella TC, Tiong SY, Locke S, Watanabe M, Todd AJ, 2013. Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn. Pain 154, 2606–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Shehab SA, Watt C, Todd AJ, 1999. GABAergic neurons that contain neuropeptide Y selectively target cells with the neurokinin 1 receptor in laminae III and IV of the rat spinal cord. J. Neurosci 19, 2637–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmaninho-Salgado J, Marques AP, Estrada M, Santana M, Cortez V, Grouzmann E, Cavadas C, 2012. Dipeptidyl-peptidase-IV by cleaving neuropeptide Y induces lipid accumulation and PPAR-gamma expression. Peptides 37, 49–54. [DOI] [PubMed] [Google Scholar]
- Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC, Kelley MW, Levine AJ, 2018. Massively Parallel Single Nucleus Transcriptional Profiling Defines Spinal Cord Neurons and Their Activity during Behavior. Cell Rep 22, 2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK, 2007. Spinal mechanisms of NPY analgesia. Peptides 28, 464–474. [DOI] [PubMed] [Google Scholar]
- Solway B, Bose SC, Corder G, Donahue RR, Taylor BK, 2011. Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci U S A 108, 7224–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JC, 2003a. Peptidases prevent μ-opioid receptor internalization in dorsal horn neurons by endogenously released opioids. J Neurosci 23, 1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JCG, 2003b. Dorsal horn neurons firing at high frequency, but not primary afferents, release opioid peptides that produce μ-opioid receptor internalization in the rat spinal cord. J Neurosci 23, 9171–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JCG, 2005. NMDA receptors and large conductance calcium-sensitive potassium channels inhibit the release of opioid peptides that induce μ-opioid receptor internalization in the rat spinal cord. Neuroscience 136, 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Kozak R, Burlet C, Nicolas JP, Beck B, 1997. Physiological regulation of hypothalamic neuropeptide Y release in lean and obese rats. Am J Physiol 273,R2112–2116. [DOI] [PubMed] [Google Scholar]
- Taiwo OB, Taylor BK, 2002. Antihyperalgesic effects of intrathecal neuropeptide Y during inflammation are mediated by Y1 receptors. Pain 96, 353–363. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Fu W, Kuphal KE, Stiller CO, Winter MK, Chen W, Corder GF, Urban JH, McCarson KE, Marvizon JC, 2014. Inflammation enhances Y1 receptor signaling, neuropeptide Y-mediated inhibition of hyperalgesia, and substance P release from primary afferent neurons. Neuroscience 256, 178–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marchand S, Mantyh PW, Basbaum AI, 1999. Spinal opioid analgesia: how critical is the regulation of substance P signaling? Journal of Neuroscience 19, 9642–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marek K, Basbaum AI, 2000. Postsynaptic signaling via the mu-opioid receptor: Responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J. Neurosci 20, 8578–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P, 2015. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennett GJ, 1992. Effects of peripheral nerve injuries and tissue inflammation on the levels of neuropeptide Y-like immunoreactivity in rat primary afferent neurons. Brain Res 598, 349–352. [DOI] [PubMed] [Google Scholar]
- Wan CP, Lau BH, 1995. Neuropeptide Y receptor subtypes. Life Sci 56, 1055–1064. [DOI] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH, 2003. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol 464, 285–311. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Farb DH, Leeman SE, Jessell TM, 1979. Intrathecal capsaicin depletes substance P in the rat spinal cord and produces prolonged thermal analgesia. Science 206, 481–483. [DOI] [PubMed] [Google Scholar]


