Abstract
Background:
The benefit of adjuvant treatment for esophageal cancer patients with positive lymph nodes following induction therapy and surgery is uncertain. This in-depth multicenter study assessed the benefit of adjuvant therapy in this population.
Methods:
A retrospective cohort study from 9 institutions included patients who: received neoadjuvant treatment, underwent esophagectomy from 2000–2014, and had positive lymph nodes on pathology. Factors associated with administration of adjuvant therapy were assessed using multilevel random-intercept modeling to account for institutional variation in practice. Kaplan-Meier analyses were performed based on adjuvant treatment status. Variables associated with survival were identified using Cox proportional hazards modeling.
Results:
1,082 patients were analyzed with node positive cancer following induction therapy and esophagectomy. 209 (19.3%) received adjuvant therapy and 873 (80.7%) did not. Administration of adjuvant treatment varied significantly from 3.2% to 50.0% between sites (p<0.001). Accounting for institution effect, factors associated with administration of adjuvant therapy included clinically positive and negative prognostic characteristics: younger age, higher pathologic stage, pathologic grade, no neoadjuvant radiation, non-smoking status, and absence of postoperative infection. On Kaplan-Meier analysis, patients receiving adjuvant therapy had a longer median survival: 2.6 years vs 2.3 years, p=0.02. Cox modeling identified adjuvant treatment as independently associated with improved survival, with a 24% reduction in mortality (HR=0.76, p=0.005).
Conclusions:
Adjuvant therapy was associated with improved overall survival. Therefore, consideration should be given to administration of adjuvant therapy to esophageal cancer patients who have persistent node positive disease after induction therapy and esophagectomy, and are able to tolerate additional treatment.
The incidence of esophageal cancer in the United States has increased in recent decades, and most patients present with regional or metastatic disease [1]. Neoadjuvant treatment prior to esophagectomy for locoregionally-advanced cancers has been established as standard of care for operable patients. Results from a prospective, randomized trial of induction chemotherapy compared to surgery alone for esophageal cancer (OEO2) showed improved overall and disease-free survival with the addition of neoadjuvant chemotherapy [2]. The Preoperative Chemoradiotherapy for Esophageal Cancer (CROSS) randomized controlled trial demonstrated significantly improved survival in patients receiving trimodality therapy compared to surgery alone, with no increase in postoperative complications or mortality and higher rates of complete resection [3]. Consequently, induction therapy followed by surgery is the standard of care for patients who present with clinical locoregionally advanced cancer of the middle or distal esophagus and gastroesophageal junction [4]. For patients who follow this treatment paradigm, the poor prognostic significance of residual positive lymph nodes has been established [5,6].
However, it remains unclear how to best treat patients with residual nodal disease after multimodality therapy. A pair of small institutional series have evaluated the effect of adjuvant chemotherapy in this population with mixed results [7,8], and National Cancer Database (NCDB) studies have suggested there may be a benefit [9,10]. While these studies have been helpful in generating some data to guide this clinical decision, they are limited, either by sample size or granularity of data, in their ability to adjust for factors that may affect receipt of adjuvant therapy or patient outcomes.
To answer this important clinical question and address the limitations of prior studies, we assembled a collaborative, multicenter, retrospective cohort study to collect detailed patient data on factors influencing the administration of adjuvant treatment and patient outcomes. The purpose of this study was to determine if there was a benefit to administering adjuvant treatment to esophageal cancer patients who have undergone induction chemotherapy or chemoradiation and still have positive lymph nodes on pathologic exam. We hypothesized that administration of adjuvant treatment would be independently associated with improved survival.
PATIENTS AND METHODS
Patient Population
This retrospective cohort study included patients from nine collaborating institutions: Washington University in St. Louis, MD Anderson Cancer Center, Mayo Clinic, Weill Cornell Medicine, Michigan Medicine, University of Toronto, Johns Hopkins University School of Medicine, Emory University School of Medicine, and Boston Medical Center. Institutional Review Board (IRB) approval was obtained from Washington University. Data Use Agreements were established with participating institutions. Data were captured and shared between sites using Research Electronic Data Capture (REDCap).
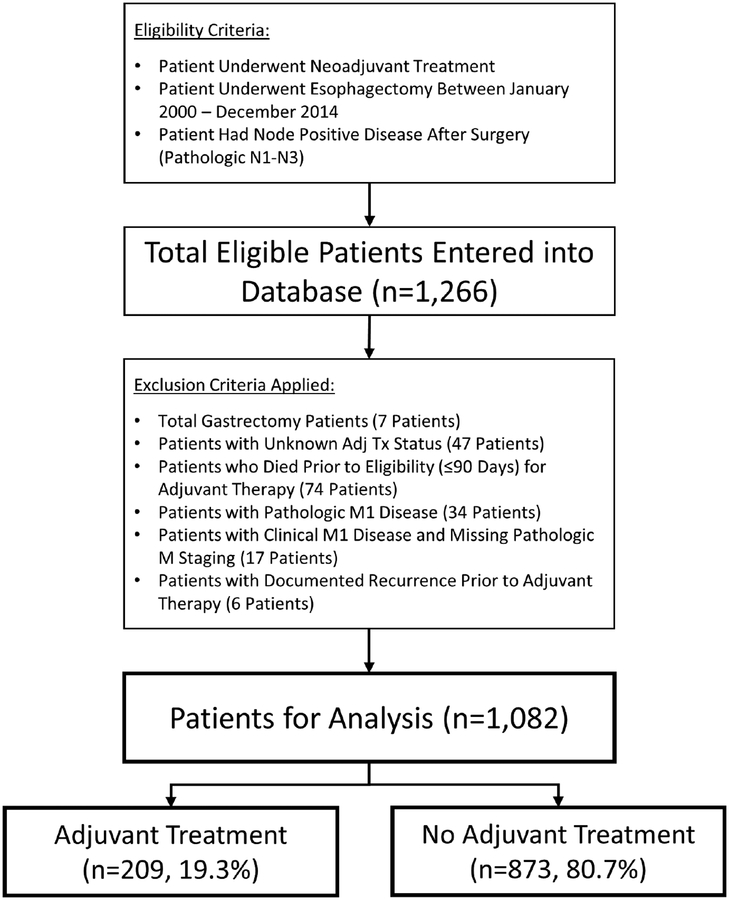
Patients were eligible for inclusion in the study if they received neoadjuvant treatment, underwent esophagectomy between January 2000 and December 2014 at any of the participating centers, and had node positive disease after surgery (pathologic stage N1-N3). Detailed data on the number of positive lymph nodes was collected and patients were staged according to the American Joint Committee on Cancer (AJCC) 7th edition. Patients were excluded if they: underwent total gastrectomy, had unknown adjuvant treatment status, died prior to eligibility (≤90 days) for adjuvant therapy, had pathologic M1 disease, had clinical M1 disease with missing pathologic M staging, or had a documented recurrence of cancer prior to administration of adjuvant therapy. All esophageal cancer histology types were included, and were classified adenocarcinoma, squamous cell, or other. Patients were then divided into ‘adjuvant’ or ‘no adjuvant’ treatment groups for analysis. Patients were classified as receiving adjuvant treatment if they received chemotherapy, radiation, or both within six months of their operation prior to any documented recurrence of cancer. A summary of the inclusion and exclusion criteria can be seen in our CONSORT diagram (Figure 1). Detailed patient demographic data, comorbidities, clinical staging information, operative characteristics, postoperative complications, and pathologic staging information was collected on all patients. Long-term survival and recurrence data were also tracked with time measured from the date of surgery.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) Diagram. Adjuvant treatment was defined as chemotherapy, radiation, or both given within 6 months of surgery.
Statistical Analyses
All statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC). Descriptive statistics were performed using t-tests, Wilcoxon rank sum tests, chi-square tests, or Fisher’s exact tests as appropriate. Univariable analysis was performed to identify factors associated with receipt of adjuvant therapy. Multilevel random-intercept modeling was also performed to look for institutional effects on the analyses. Additional variables were included in the hierarchical multivariable model via forward selection with significance set at p<0.05. Kaplan-Meier analysis was performed to compare overall survival. Since long-term follow up was available, both Wilcoxon p-values (which weight survival by the number of patients at risk at each time, and therefore are more sensitive for detecting differences earlier on the Kaplan Meier curve) and log-rank p-values (which weight all time points equally) are reported. Cox proportional hazard modeling was performed to assess for association with mortality hazard. A decision was made a priori to include the clinically relevant variables of: adjuvant treatment status, age, sex, histologic subtype, ECOG score, and pathologic stage. The remaining variables were determined via stepwise selection with a threshold of p<0.25 for entry into the model and p<0.15 to stay. A Cox model including frailty explored whether there was a correlation between survival time and institution. The variance was very small (<0.01) and did not reach statistical significance (p>0.05), indicating that, after accounting for the clinical variables in the model, there was not much variation between sites. Consequently, the results using standard Cox modeling are presented.
RESULTS
Neoadjuvant Treatment
After application of inclusion and exclusion criteria, 1,082 patients with node positive cancer (N1-N3) following induction therapy and esophagectomy were available for analysis. Neoadjuvant chemotherapy was documented for 1,080 (99.8%) patients and neoadjuvant radiation for 942 (87.1%): therefore 940 (87%) patients received induction chemoradiation, 140 (13%) received induction chemotherapy alone, and 2 (0.2%) may have received induction radiation alone. Neoadjuvant chemotherapeutic regimens were most commonly platinum based regimens (83.4%) combined with 5-FU (61.8%), taxanes (21.3%), or epirubicin (4.9%), (Supplementary Table 1). Radiation doses ranged from 12.6–70Gy, with the most common doses including: 50.4Gy (n=514, 54.6%), 50Gy (n=70, 7.4%), 45Gy (n=130, 13.8%), 41.4Gy (n=29, 3.1%), and unknown (n=136, 14.4%). Staging was documented with an endoscopic ultrasound (EUS) in 780 (72.1%), computed tomography (CT) scan in 967 (89.4%), and positron emission tomography (PET) in 969 (89.6%).
Adjuvant Treatment
Adjuvant therapy was administered in 209 (19.3%) patients. Of these, 167 (79.9%) received adjuvant chemotherapy alone, 30 (14.4%) received chemoradiation, and 12 (5.7%) received radiation alone. 873 (80.7%) patients received no adjuvant therapy. Administration of adjuvant treatment ranged from a low of 3.2% (11/339) to a high of 50.0% (8/16) among the nine participating sites (Table 1, p<0.001), demonstrating the wide variability in practice patterns and relevance of this study. Adjuvant chemotherapeutic regimens included classes of agents similar to neoadjuvant regimens (Supplementary Table 1). When compared to neoadjuvant chemotherapies, the same category of regimen was used in 39/209 (19%) patients, a different category of regimen was used in 99/209 (47%), and it could not be determined in 71/209 (34%). Adjuvant radiation doses ranged from 30–59.4Gy.
Table 1.
Site Contributions and Adjuvant Treatment Practice Variability
| Site |
Adjuvant n=209 |
No Adjuvant n=873 |
|---|---|---|
| A | 50.0% (8) | 50.0% (8) |
| B | 49.6% (66) | 50.4% (67) |
| C | 47.8% (11) | 52.2% (12) |
| D | 46.1% (48) | 53.9% (56) |
| E | 26.5% (22) | 73.5% (61) |
| F | 12.8% (34) | 87.2% (231) |
| G | 11.1% (2) | 88.9% (16) |
| H | 6.9% (7) | 93.1% (94) |
| I | 3.2% (11) | 96.8% (328) |
Patient, tumor, and operative characteristics by adjuvant treatment status are displayed in Table 2. Patients receiving adjuvant therapy were more likely to have: younger age, ECOG score of 0, clinical M1 status, transhiatal or three-hole esophagectomy, more positive lymph nodes, higher positive lymph node ratio, higher pathologic N status, and higher overall pathologic stage. Patients receiving adjuvant therapy were less likely to have: coronary artery disease, neoadjuvant radiation, Ivor Lewis esophagectomy, or infectious complications including empyema, pneumonia, and urinary tract infection. A random-intercept model was used to identify factors independently associated with receipt of adjuvant therapy while accounting for the intra-site correlation of using adjuvant therapy. That analysis revealed that 35.9% of the variation in adjuvant treatment administration is accounted for by institutional practice patterns. Other significant factors (all p<0.05) included both positive and negative prognostic characteristics: younger age, higher pathologic stage, pathologic grade, no neoadjuvant radiation, non-smoking status, and absence of postoperative pneumonia or urinary tract infection (multivariable analysis: Table 3, univariable analysis: Supplementary Table 2).
Table 2.
Patient, Tumor, and Operative Characteristics by Adjuvant Treatment Status
| Characteristic | Adjuvant | No Adjuvant | p-value |
|---|---|---|---|
| n=209 (19.3%) | n=873 (80.7%) | ||
| Age in years, Median (IQR) | 58 (51–64) | 62 (54–68) | <0.001 |
| Body Mass Index, Median (IQR) | 26.9 (23.8–29.8) | 26.9 (24.0–30.3) | 0.8 |
| Female | 16.3% (34) | 13.6% (119) | |
| ≥4 | 5.6% (9) | 2.8% (23) | |
| ≥2 | 1.6% (3) | 2.5% (13) | |
| Comorbidities | |||
| Coronary Artery Disease | 8.6% (18) | 14.2% (124) | 0.03 |
| Congestive Heart Failure | - | 1.3% (11) | 0.1 |
| COPD | 6.2% (13) | 6.5% (57) | 0.9 |
| Diabetes | 14.8% (31) | 16.7% (146) | 0.5 |
| ESRD | 0.5% (1) | 0.3% (3) | 0.6 |
| Hypertension | 44.5% (93) | 42.0% (367) | 0.5 |
| Neoadjuvant Chemotherapy | 100% (209) | 99.8% (871) | 1.0 |
| Neoadjuvant Radiation | 66.5% (139) | 91.9% (802) | <0.001 |
| Other Major Comorbidity | 30.6% (64) | 22.6% (197) | 0.01 |
| Peripheral Vascular Disease | 2.4% (5) | 2.0% (17) | 0.7 |
| Prior Cancer | 12.4% (26) | 13.9% (121) | 0.6 |
| Prior Cardiothoracic Surgery | 3.8% (8) | 3.6% (31) | 0.8 |
| Pulmonary Hypertension | 0.5% (1) | 0.1% (1) | 0.3 |
| Smoking | 67.0% (140) | 70.8% (618) | 0.3 |
| Steroid Use | - | 0.5% (4) | 1.0 |
| Stroke | 2.4% (5) | 2.2% (19) | 0.8 |
| Transient Ischemic Attack | 1.0% (2) | 0.2% (2) | 0.2 |
| Other | 1.4% (3) | 2.4% (21) | |
| cTX | 2.4% (5) | 3.0% (26) | |
| cNX | 4.8% (10) | 6.2% (54) | |
| cM1 | 9.1% (19) | 4.9% (43) | |
| Other/Unknown | 2.9% (6) | 3.1% (27) | |
| Median Nodes Resected (IQR) | 20 (14–28) | 20 (14–28) | 0.3 |
| Median Positive Nodes (IQR) | 3 (1–6) | 2(1–4) | <0.001 |
| Median LN Ratio (IQR) | 0.14 (0.06–0.30) | 0.11 (0.06–0.22) | 0.01 |
| R1/2 | 9.6% (20) | 10.1% (88) | |
| pTX | - | 0.3% (3) | |
| pN3 | 19.6% (41) | 10.8% (94) | |
| IIIC | 21.5% (45) | 12.4% (108) | |
| X | 10.5% (22) | 16.3% (142) | |
| Operative Complications | |||
| Anastomotic Leak | 9.6 (20) | 10.7% (93) | 0.6 |
| ARDS | 0.5% (1) | 2.1% (18) | 0.1 |
| Atrial Arrhythmia | 13.9% (29) | 16.4% (143) | 0.4 |
| Bleeding | 7.2% (15) | 6.9% (60) | 0.8 |
| Chylothorax | 3.8% (8) | 6.5% (57) | 0.1 |
| DVT | 1.0% (2) | 1.8% (16) | 0.6 |
| Empyema | - | 2.2% (19) | 0.03 |
| Gastric Outlet Obstruction | 1.4% (3) | 0.8% (7) | 0.4 |
| Myocardial Infarction | - | - | - |
| Other Complication* | 19.1% (40) | 14.6% (127) | 0.1 |
| Pulmonary Embolism | 1.0% (2) | 2.0% (17) | 0.6 |
| Pneumonia | 6.2% (13) | 11.1% (97) | 0.04 |
| Renal Failure | 1.0% (2) | 1.4% (12) | 1.0 |
| Reoperation | 9.5% (20) | 10.2% (89) | 0.8 |
| Respiratory Failure | 5.2% (11) | 5.4% (47) | 0.9 |
| Surgical Site Infection | 6.2% (13) | 8.1% (71) | 0.4 |
| Stroke | 1.4% (3) | 0.8% (7) | 0.4 |
| UTI | 0.5% (1) | 3.3% (29) | 0.02 |
| Ventricular Arrhythmia | 1.4% (3) | 1.1% (9) | 0.7 |
| Median LOS (IQR) | 10 (8–13) | 9 (8–14) | 0.2 |
| ICU Stay | 0 (0–1) | 0 (0–1) | 0.4 |
Other Complications included a wide variety of conditions. The most common were: vocal cord paralysis, ileus, c. difficile infection, urinary retention, delirium, pleural effusion, and pneumothorax but also included more serious rare events such as sepsis, conduit necrosis, aspiration, and intraoperative injuries.
Table 3.
Multivariable Analysis of Factors Associated with Administration of Adjuvant Therapy
| Variable | Multivariable OR (95% CI) | p-value |
|---|---|---|
| Age | 0.97 (0.95–0.98) | <0.001 |
| IIIC | 2.74 (1.53–4.90) | |
| X | 0.39 (0.12–1.27) | |
| Neoadjuvant Radiation | 0.40 (0.21–0.75) | 0.004 |
| Smoking | 0.63 (0.42–0.96) | 0.03 |
| Pneumonia | 0.45 (0.23–0.90) | 0.02 |
| UTI | 0.05 (0.01–0.40) | 0.005 |
Multilevel random-intercept (site) analysis was performed to account for practice patterns between institutions. The intraclass correlation coefficient (ICC) was 0.359, meaning 35.9% of the variation in adjuvant treatment administration is accounted for by institutional effect.
Survival Analyses
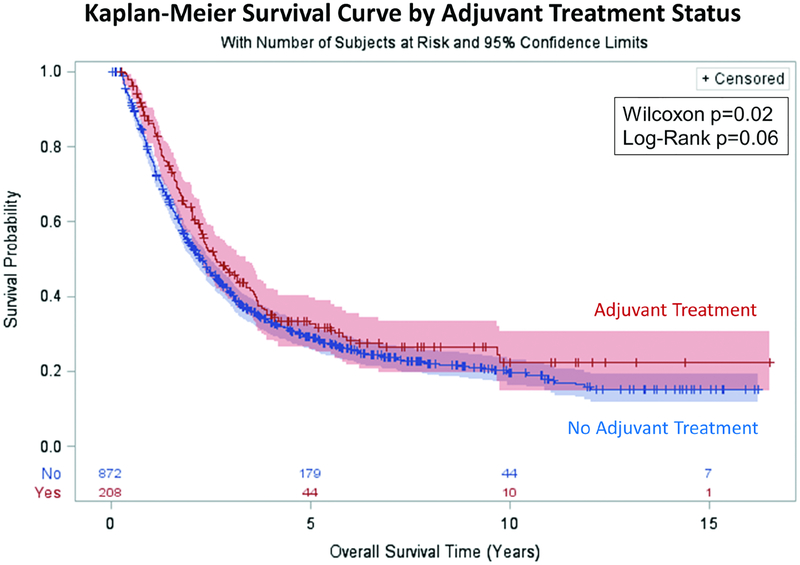
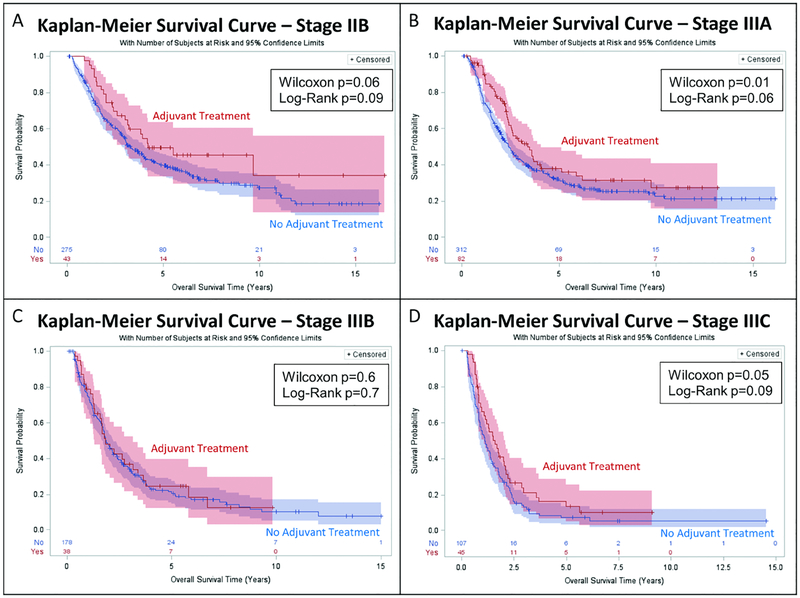
Patients receiving adjuvant therapy had a longer median post-resection Kaplan-Meier survival (Figure 2): 2.6 years (95%CI: 2.3–3.4) vs 2.3 years (95%CI: 2.0–2.5), with the significant survival benefit most evident in the early portion of the curves (Wilcoxon p=0.02, Log-Rank p=0.06). Kaplan-Meier analysis stratified by pathologic stage (Figure 3) revealed longer median survival that approached statistical significance for stages IIB, IIIA, and IIIC, while no significant difference was seen for stage IIIB (T3N2 patients). A greater absolute survival benefit was seen for patients with earlier stage disease. Unadjusted survival by stage for adjuvant vs no adjuvant treatment, respectively, was: Stage IIB: 4.2 years vs 3.3 years (Wilcoxon p=0.06), Stage IIIA: 3.4 years vs 2.4 years (Wilcoxon p=0.01), Stage IIIB: 1.8 years vs 1.9 years (Wilcoxon p=0.6), Stage IIIC: 1.6 years vs 1.1 years (Wilcoxon p=0.05).
Figure 2.
Kaplan-Meier Analysis of Overall Survival by Adjuvant Treatment Status. Median survival for the adjuvant treatment group was 2.6 years (95% CI: 2.3–3.4) vs 2.3 years (95% CI: 2.0–2.5) for the no adjuvant treatment group.
Figure 3.
Kaplan Meier Analysis of Overall Survival by Adjuvant Treatment Status, Stratified by Pathologic Stage. Median survival estimates:
A: Stage IIB: Adjuvant: 4.2 years (95% CI: 3.0-unknown) vs No Adjuvant: 3.3 years (95% CI: 2.9–4.0).
B: Stage IIIA: Adjuvant: 3.4 years (95% CI: 2.4–4.1) vs No Adjuvant: 2.4 years (95% CI: 2.1–2.8).
C: Stage IIIB: Adjuvant: 1.8 years (95% CI: 1.3–3.2) vs No Adjuvant: 1.9 years (95% CI: 1.7–2.2).
D: Stage IIIC: Adjuvant: 1.6 years (95% CI: 1.1–2.1) vs No Adjuvant: 1.1 years (95% CI: 0.9–1.4).
Cox proportional hazards modeling identified adjuvant treatment as independently associated with improved survival, with a 24% reduction in mortality (hazard ratio (HR)=0.76 (95%CI: 0.62–0.92, p=0.005) when adjusting for: age, sex, histologic subtype, ECOG score, total number of lymph nodes resected, number of positive lymph nodes, presence of a positive margin, pathologic stage, comorbid diabetes or peripheral vascular disease, and complications of anastomotic leak, atrial arrhythmia, bleeding, reoperation, or surgical site infection (Table 4).
Table 4.
Cox Proportional Hazards Model
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Adjuvant Treatment (Yes vs No) | 0.76 (0.62–0.92) | 0.005 |
| Age (per year) | 1.01 (1.00–1.02) | 0.004 |
| Sex (Female vs Male) | 0.93 (0.74–1.16) | 0.5 |
| Other | 0.92 (0.55–1.54) | |
| Missing | 0.93 (0.77–1.11) | |
| Nodes Resected (per node) | 0.99 (0.98–1.00) | 0.005 |
| Positive Nodes (per node) | 1.07 (1.04–1.10) | <0.001 |
| Margin (Positive vs Negative) | 1.63 (1.28–2.06) | <0.001 |
| IIIC | 1.73 (1.22–2.47) | |
| Diabetes | 1.18 (0.97–1.44) | 0.1 |
| Peripheral Vascular Disease | 1.41 (0.89–2.26) | 0.1 |
| Anastomotic Leak | 1.27 (1.00–1.60) | 0.05 |
| Atrial Arrhythmia | 1.19 (0.98–1.45) | 0.08 |
| Bleeding | 1.33 (1.01–1.75) | 0.04 |
| Reoperation | 1.33 (1.05–1.70) | 0.02 |
| Surgical Site Infection | 1.23 (0.95–1.61) | 0.1 |
A decision was made a priori to include the clinically relevant variables: Adjuvant Treatment Status, Age, Sex, Histologic Subtype, ECOG Score, and Pathologic Stage. Univariable analysis is included in Supplementary Table 3.
The observed benefit of adjuvant therapy was durable to multiple sensitivity analyses, with comparable benefits in both mortality hazard and unadjusted survival seen as in the overall cohort. In our Supplementary Material, we present the results of Kaplan Meier analyses and Cox Proportional Hazards modeling exploring the effect of adjuvant treatment in four additional contexts: 1. Adjuvant chemotherapy vs radiation, 2. Known vs unknown interval to adjuvant treatment, 3. Positive vs negative margins, and 4. Neoadjuvant chemoradiation vs neoadjuvant chemotherapy alone.
COMMENT
The relative lack of data and absence of a consensus on the most appropriate treatment for patients who are node positive after multimodality therapy is reflected in clinical practice guidelines. The National Comprehensive Cancer Network (NCCN) guidelines suggest both observation until disease progression and adjuvant treatment with chemotherapy ± radiation as potential treatment options [4]. The Society for Thoracic Surgeons (STS) guidelines on multimodality treatment for esophageal cancer recommend adjuvant therapy for patients with node positive disease if they received surgery alone, but do not address the optimal treatment for node positive patients who have already received multimodality therapy [11]. As a result, there is substantial variability in clinical practice, and the clinical decision about adjuvant treatment has not been strongly evidence-based.
Within this multicenter study, we observed that utilization of adjuvant therapy ranged widely from 3.2% to 50%. Clustered analysis demonstrated that institutional effect accounted for 35.9% of the variability in practice. The remainder of variables predicting administration of adjuvant therapy included both positive and negative prognostic factors, possibly indicating that the decision is being made on a case-by-case basis for many patients with substantial influence from institutional habits. These findings demonstrate the relevance of our study and the importance of stronger data to guide clinical decision making.
This study demonstrates that adjuvant treatment conferred a survival advantage and there was a 24% reduction in mortality hazard. These findings were consistent and durable when patient cohorts were examined stratified by pathologic stage and subject to sensitivity analyses. This is an important result that supports consideration of adjuvant treatment for all patients with node positive disease after trimodality therapy who are acceptable candidates for additional chemotherapy, providing more sound evidence upon which to base postoperative treatment recommendations than has previously been available.
Prior institutional studies have included small cohorts of patients and reached varied conclusions on the benefit of adjuvant treatment. In 101 patients, Brescia et al found that adjuvant therapy was associated with an improved overall median survival of 24 vs 18 months (p=0.03), with adjuvant therapy conferring a 42% reduction in mortality risk on Cox modeling (p=0.05) [7]. In contrast, in 96 patients, Stiles et al found that adjuvant therapy was not a significant prognostic factor for overall survival (p=0.9), and instead clinical and pathological tumor and nodal staging data was most important [8]. These studies, while providing important preliminary data, were limited by small sample size and therefore could not allow robust multivariable analyses. Retrospective studies of the NCDB have been performed with much larger patient populations, but the comorbidity and surgical complication data that may both contribute to patient selection for adjuvant treatment and affect outcomes is missing from this dataset. Still, these series have demonstrated an approximately 30% reduction in mortality hazard [9,10] with adjuvant therapy.
This study builds on previous work through creation and utilization of a larger and more detailed database to specifically answer this question. We evaluated data on specific patient comorbidities, functional status, operative characteristics, postoperative complications, and staging information. We adjusted for significant factors in our statistical models when evaluating the effect of adjuvant therapy, thereby minimizing the effect of selection bias. It is possible that unmeasured confounders, including postoperative patient condition and severity of adverse events, may still affect this retrospective analysis. By incorporating detailed data on postoperative complications and measures of morbidity, we hoped to control for this metric as much as possible. We also were able to test for institutional effect and incorporate this into our analyses. These attributes strengthen our findings that adjuvant therapy confers a survival benefit.
Certainly patient clinical factors and values still need to be considered when making this decision. Patient condition following induction therapy and esophagectomy needs to be assessed, and the risk of recurrence needs to be balanced with the cost and quality of life consequences of more chemotherapy. Additional research into the optimal adjuvant regimens for effectiveness and tolerability is warranted. However, to improve overall survival, consideration should be given to administration of adjuvant therapy to esophageal cancer patients who have persistent node positive disease after induction therapy and esophagectomy, and are able to tolerate additional treatment.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Bharat A, Crabtree T. Management of Advanced-Stage Operable Esophageal Cancer. Surg Clin North Am 2012;92:1179–97. doi: 10.1016/j.suc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- [2].Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062–7. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- [3].van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, Henegouwen MI van B, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- [4].Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, et al. NCCN Guidelines Version 2.2018 Esophageal and Esophagogastric Junction Cancers NCCN Evidence Blocks™. 2018. [Google Scholar]
- [5].Gu Y, Swisher SG, Ajani JA, Correa AM, Hofstetter WL, et al. The number of lymph nodes with metastasis predicts survival in patients with esophageal or esophagogastric junction adenocarcinoma who receive preoperative chemoradiation. Cancer 2006;106:1017–25. doi: 10.1002/cncr.21693. [DOI] [PubMed] [Google Scholar]
- [6].Depypere LP, Vervloet G, Lerut T, Moons J, De Hertogh G, et al. ypT0N+: the unusual patient with pathological complete tumor response but with residual lymph node disease after neoadjuvant chemoradiation for esophageal cancer, what’s up? J Thorac Dis 2018;10:2771–8. doi: 10.21037/jtd.2018.04.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brescia AA, Broderick SR, Crabtree TD, Puri V, Musick JF, et al. Adjuvant Therapy for Positive Nodes After Induction Therapy and Resection of Esophageal Cancer. Ann Thorac Surg 2016;101:200–8–10. doi: 10.1016/j.athoracsur.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stiles BM, Christos P, Port JL, Lee PC, Paul S, Saunders J, et al. Predictors of survival in patients with persistent nodal metastases after preoperative chemotherapy for esophageal cancer. J Thorac Cardiovasc Surg 2010;139:387–94. doi: 10.1016/j.jtcvs.2009.10.003. [DOI] [PubMed] [Google Scholar]
- [9].Samson P, Puri V, Lockhart AC, Robinson C, Broderick S, Patterson GA, et al. Adjuvant chemotherapy for patients with pathologic node-positive esophageal cancer after induction chemotherapy is associated with improved survival. J Thorac Cardiovasc Surg 2018. doi: 10.1016/j.jtcvs.2018.05.100. [DOI] [PubMed] [Google Scholar]
- [10].Burt BM, Groth SS, Sada YH, Farjah F, Cornwell L, Sugarbaker DJ, et al. Utility of Adjuvant Chemotherapy After Neoadjuvant Chemoradiation and Esophagectomy for Esophageal Cancer. Ann Surg 2017;266:297–304. doi: 10.1097/SLA.0000000000001954. [DOI] [PubMed] [Google Scholar]
- [11].Little AG, Lerut AE, Harpole DH, Hofstetter WL, Mitchell JD, Altorki NK, et al. The Society of Thoracic Surgeons Practice Guidelines on the Role of Multimodality Treatment for Cancer of the Esophagus and Gastroesophageal Junction. Ann Thorac Surg 2014;98:1880–5. doi: 10.1016/j.athoracsur.2014.07.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.