Abstract
A 50-year-old Indigenous woman, on home haemodialysis, was found to have a large mycotic aneurysm of the proximal left anterior descending coronary artery at the site of a previous drug-eluting stent. Blood cultures grew methicillin-sensitive Staphylococcus aureus bacteraemia. She underwent a complex operation involving resection of the mycotic aneurysm, removal of the stent and a coronary artery bypass graft to the distal left anterior descending (LAD) coronary artery using the left internal mammary artery. She had a complicated intensive care unit admission with pericardial tamponade on day 1 postoperatively requiring reopening and removal of clot and type 1 respiratory failure requiring reintubation on day 10 postoperatively. Once extubated, she developed prolonged hyperactive delirium and a significant decline in mobility. Over the course of a 6-week hospital admission, she received extensive multidisciplinary care and was discharged for rehabilitation to a peripheral hospital. She was discharged home after rehabilitation with ongoing follow-up with infectious diseases.
Keywords: ischaemic heart disease, interventional cardiology, infectious diseases, cardiothoracic surgery
Background
The incidence of aneurysms of the coronary arteries ranges between 1.4% and 4.9%.1 2 A mycotic aneurysm is a dilatation of an artery due to infection.3 Mycotic coronary artery aneurysms are even more rare, accounting for 3% of all coronary artery aneurysms.4 Patients with immunodeficiencies, such as diabetes, HIV and underlying cancer, or on immunosuppressive therapy have a higher incidence of mycotic aneurysms.5
Mycotic coronary artery aneurysms are generally thought to be associated with infective endocarditis or infection of a coronary stent.6 The most common organisms responsible for the infection are Staphylococcus aureus and Streptococcus viridans. Coronary stent infections are most commonly due to an underlying bacteraemia from a complication of venous access.7 8 A prospective study looked at 960 patients who underwent percutaneous coronary intervention (PCI). Of the 960 patients, 0.16% had a clinically important infection with none related to the PCI.8
Clinically, most patients present with non-specific symptoms, such as fever, chills or lethargy. Presentations suggestive of an acute coronary syndrome are usually due to complications of the aneurysm, such as rupture or thrombosis.9 10 Without aggressive management, the prognosis is poor with high morbidity and mortality.10 11 Management usually involves surgical resection with aortocoronary bypass grafting and intravenous antibiotics. The perioperative mortality is high at 38.9%, with approximately half being intraoperative deaths.12 Without intervention, the mortality rate is higher at 63.9%.12 Therefore, urgent surgical intervention is recommended.
Case presentation
A 50-year-old Indigenous woman, on home haemodialysis, presented to emergency department with central chest pain, fever and lethargy. This was on a background of a recent admission to hospital a month earlier where she had similar symptoms with an angiogram showing non-obstructive coronary disease and a patent LAD stent, which was inserted 5 years before presentation. Prior to this, she lived at home with her partner and was independent of her activities of daily living. She had new ST elevations in the lateral leads with an elevated troponin; however, she was initially diagnosed with myopericarditis. Due to fever, blood cultures were taken and grew methicillin-sensitive S. aureus and she was commenced on intravenous flucloxacillin on day 1 of admission. She experienced further chest pain with new T-wave inversion in her precordial leads and rising troponin. She then underwent a repeat angiogram which showed a large aneurysm of the mid-LAD at the distal end of the drug-eluting stent (figures 1 and 2).
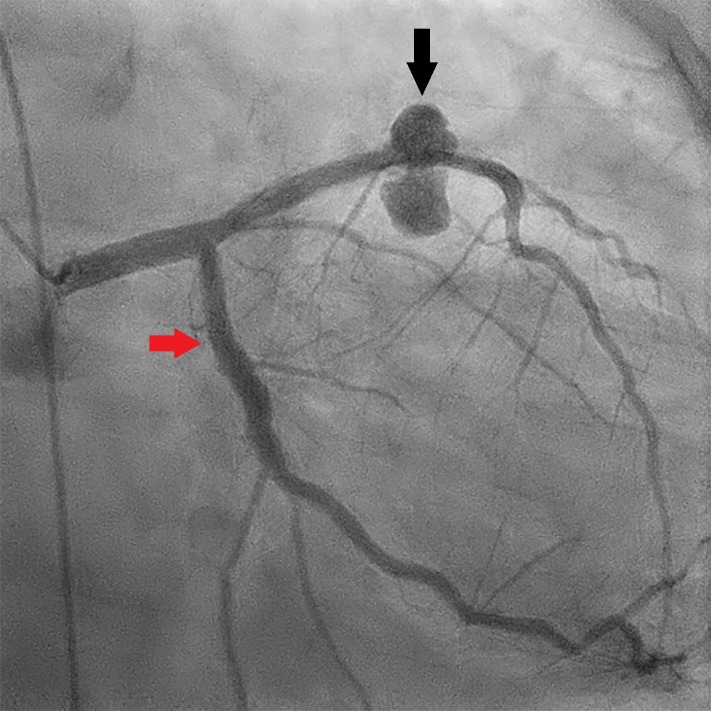
Figure 1.
Coronary angiogram (right anterior oblique 2° caudal 27°). The black arrow demonstrates the large mycotic aneurysm of the left anterior descending coronary artery. The red arrow demonstrates the circumflex coronary artery.
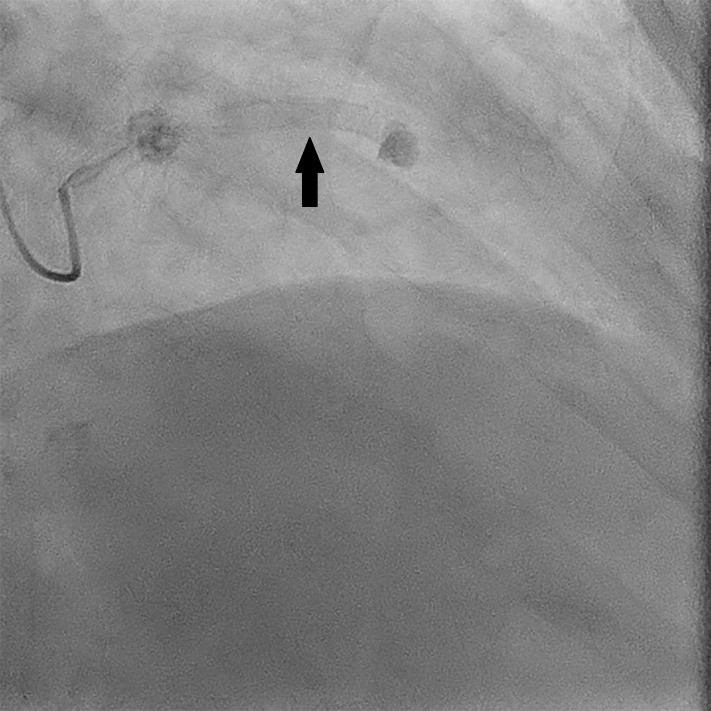
Figure 2.
Coronary angiogram (right anterior oblique 2° caudal 27°). The black arrow demonstrates the drug-eluting stent in the left anterior descending coronary artery.
She was referred to the cardiothoracic surgery department and after a lengthy consultation and consent, surgery was scheduled. A median sternotomy was performed and the left internal mammary artery (LIMA) was harvested. Well-formed extensive pericardial adhesions over the anterior left ventricle (figure 3) were encountered and divided to allow for routine cannulation for cardiopulmonary bypass (CPB). Heparin was administered routinely to prevent clotting during CPB. The ascending aorta was cannulated followed by the right atrial appendage with a two-stage venous cannula. An antegrade cannula was then placed for cardioplegia, a solution administered to stop the heart. The patient was then commenced on CPB. The aortic cross-clamp was placed, antegrade cardioplegia was administered and the patient cooled to 34°C.
Figure 3.
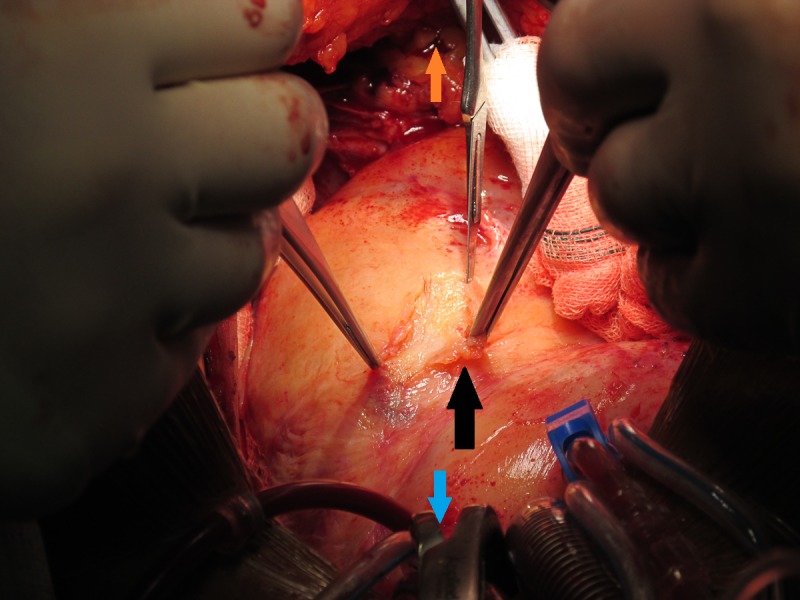
Intraoperative image demonstrating the well-formed pericardial adhesions over the left ventricle, shown by the black arrow. The blue arrow is pointing towards the head of the patient and the orange arrow is pointing towards the patient’s feet.
On exploration of the LAD territory, a large abscess cavity was found (figure 4) and drained. Swabs were sent for microbiology. The LAD was followed proximally, and further detachment of the abscess cavity was performed. The stented segment of the proximal LAD was identified and removed. The left main coronary artery was temporarily ligated to control bleeding and then the LAD was ligated proximally. The septal branches of the excised aneurysmal LAD were oversewn. A further abscess cavity was identified to the right of the LAD, which was opened and drained. The LIMA was then anastomosed to the mid-LAD.
Figure 4.
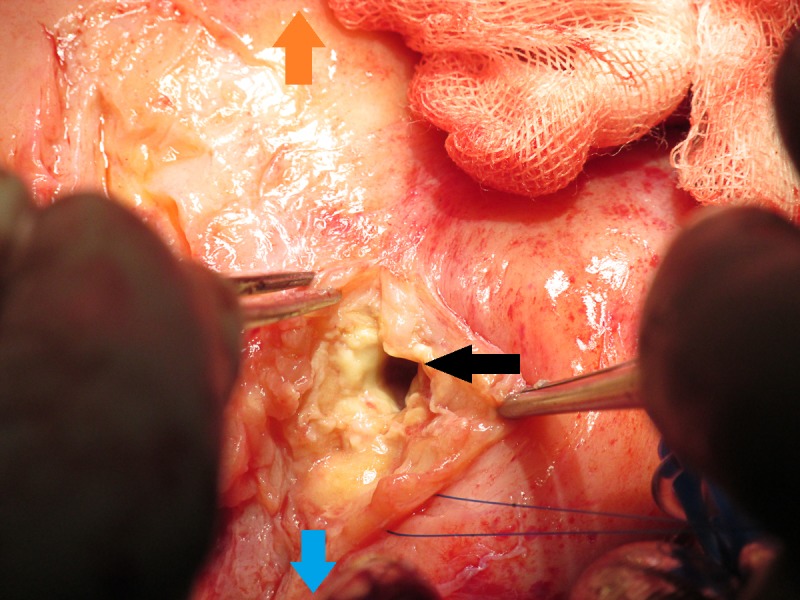
Intraoperative image demonstrating one of the large abscess cavities encountered at the LAD territory, shown by the black arrow. The blue arrow is pointing towards the head of the patient and the orange arrow is pointing towards the patient’s feet.
She was then weaned off bypass with the routine administration of protamine to reverse the effects of heparin and allow for adequate haemostasis. The patient was coagulopathic and required administration of platelets and cryoprecipitate. Four drains were inserted, and the sternum was closed in a routine fashion. She was transferred to the intensive care unit (ICU).
The following morning, she had escalating inotrope requirements in the setting of a pericardial collection. She was taken back to theatre urgently; her sternotomy was reopened, and a small amount of clot was removed with no bleeding identified. Her sternotomy was then closed in a regular fashion, and she returned to the ICU.
She was extubated on day 8 postoperatively. On day 10 postoperative, she developed type 1 respiratory failure due to hospital-acquired pneumonia with increasing norepinephrine requirement, necessitating reintubation. On day 14 postoperative, she was successfully re-extubated but was delirious. She otherwise demonstrated no focal neurology and a CT scan of the brain showed no acute intracranial abnormalities.
She was transferred to the ward on day 17 postoperative and had ongoing delirium. A peripherally inserted central catheter was placed for long-term antibiotics in her right arm and she was reviewed regularly by the infectious diseases team. Her white cell count normalised from a value of 42.33×109/L preoperatively and her C reactive protein level went from 600 mg/L preoperatively to 19 mg/L. She underwent haemodialysis regularly via a Hickman catheter.
Due to her long intubation time, she experienced dysphagia requiring a nasogastric tube. With regular speech pathology input, she was progressed to a normal diet. Over the course of her admission, her delirium resolved; however, she was still requiring assistance for mobility. She was discharged from our hospital 6 weeks postoperatively to a peripheral rehabilitation hospital where she stayed for 2 weeks. She was switched to oral amoxycillin on discharge to rehabilitation for a further 6 weeks with ongoing follow-up with the infectious disease team.
Investigations
Blood cultures: S. aureus sensitive to flucloxacillin.
LAD stent microscopy, culture and sensitive: S. aureus sensitive to flucloxacillin.
LAD mycotic aneurysm swab: S. aureus sensitive to flucloxacillin.
Outcome and follow-up
After a 6-week hospital admission, our patient required a further 2 weeks in rehabilitation. She was then discharged home and continued with haemodialysis at a dialysis centre via her central line. She made a complete recovery and returned to her baseline functioning.
Discussion
Our case highlights the diagnostic and management challenges associated with mycotic aneurysms of the coronary arteries. Our patient presented with a history of non-specific symptoms for greater than a month. It is likely that the source of her bacteraemia was from home dialysis. Our case demonstrates the aggressive nature of such aneurysms. Despite a normal coronary angiogram 1 month earlier for atypical symptoms, persistence of these symptoms was associated with the development of a large mycotic aneurysm highlighting the aggressive destruction this infection can cause.
Our patient made an exceptional recovery. She had a 6-week admission and was discharged for ongoing physiotherapy at a smaller peripheral hospital where she spent a further 2 weeks after which she was discharged home.
Case reports and series form most of the literature surrounding this topic. Although smaller aneurysms may be managed conservatively, larger aneurysms usually require surgical treatment.13 Unfortunately, there are no guidelines to define the exact measurements of a small or large aneurysm to guide management; therefore, we suggest multidisciplinary input. Surgical resection of mycotic coronary artery aneurysms is associated with lower mortality rates than conservative management. There is no clear answer regarding the timing of surgery; however, the literature suggests urgent surgery to avoid rupture of the aneurysm.14 This patient underwent surgery 3 days after the diagnosis.
Management involved a multidisciplinary approach with cardiothoracic surgery, cardiology, infectious diseases, ICU and the renal team.
Given the variable natural history of mycotic coronary artery aneurysms, there is no universal management plan. It has been suggested that smaller aneurysms may be managed conservatively with intravenous antibiotics; however, larger aneurysms require surgical intervention.13 A review of 26 cases found that 19 cases were managed surgically.15 This case illustrates the successful management of a coronary artery mycotic aneurysm. From our experience, mycotic aneurysms of the coronary artery should be managed with a multidisciplinary approach. Although antibiotic therapy may control small aneurysms, a demonstrated increase in size of the aneurysm will likely necessitate surgical intervention. The degree of cardiac destruction is one significant factor in the difficulty of the surgical repair and the severity of the septic illness also impacts on mortality.
Learning points.
Mycotic aneurysms of the coronary artery are a rare occurrence with high mortality and morbidity.
Patients with pre-existing coronary stents and underlying bacteraemia are at risk of developing mycotic aneurysms.
Early diagnosis is essential in the successful management of mycotic coronary artery aneurysms.
Intravenous antibiotics should be administered as soon as the diagnosis is suspected or confirmed.
Surgical intervention with resection of the aneurysm and coronary artery bypass grafting should be undertaken for large aneurysms.
Footnotes
Twitter: @drnikkistamp
Contributors: RL was the primary surgeon. NS was the first assistant. UA wrote the article and was involved in the patient’s care.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Daoud AS, Pankin D, Tulgan H, et al. Aneurysms of the coronary artery. Report of ten cases and review of literature. Am J Cardiol 1963;11:228–37. 10.1016/0002-9149(63)90064-x [DOI] [PubMed] [Google Scholar]
- 2. Robertson T, Fisher L. Prognostic significance of coronary artery aneurysm and ectasia in the coronary artery surgery study (CASS) registry. Prog Clin Biol Res 1987;250:325–39. [PubMed] [Google Scholar]
- 3. Bisdas T, Teebken OE. Mycotic or infected aneurysm? time to change the term. Eur J Vasc Endovasc Surg 2011;41:570–1. 10.1016/j.ejvs.2010.11.036 [DOI] [PubMed] [Google Scholar]
- 4. Garg N, Garg R, Gordon C, et al. Acute coronary syndrome caused by coronary artery mycotic aneurysm due to late stent infection localized with radiolabeled autologous leukocyte imaging. Clin Nucl Med 2009;34:753–5. 10.1097/RLU.0b013e3181b7dbc7 [DOI] [PubMed] [Google Scholar]
- 5. Reece IJ, al Tareif H, Tolia J, et al. Mycotic aneurysm of the left anterior descending coronary artery after aortic endocarditis. A case report and brief review of the literature. Tex Heart Inst J 1994;21:231–5. [PMC free article] [PubMed] [Google Scholar]
- 6. Buono A, Maloberti A, Bossi IM, et al. Mycotic coronary aneurysms. J Cardiovasc Med 2019;20:10–15. 10.2459/JCM.0000000000000734 [DOI] [PubMed] [Google Scholar]
- 7. Ahn C-M, Hong B-K, Kim J-Y, et al. Incidence and natural history of coronary artery aneurysm developing after drug-eluting stent implantation. Am Heart J 2010;160:987–94. 10.1016/j.ahj.2010.07.013 [DOI] [PubMed] [Google Scholar]
- 8. Banai S, Selitser V, Keren A, et al. Prospective study of bacteremia aftercardiac catheterization. Am J Cardiol 2003;92:1004–7. 10.1016/S0002-9149(03)00990-1 [DOI] [PubMed] [Google Scholar]
- 9. Morimoto N, Nishioka N, Yoshida M, et al. Rapid expansion of mycotic aneurysm of left coronary sinus of Valsalva causing myocardial ischemia: report of a case. Gen Thorac Cardiovasc Surg 2016;64:160–2. 10.1007/s11748-014-0410-1 [DOI] [PubMed] [Google Scholar]
- 10. Chhabra L, Kruger MA, Kuraganti G, et al. Rapidly progressing mycotic aortic aneurysm masquerading as acute coronary syndrome. Can J Cardiol 2013;29:1742.e17–20. 10.1016/j.cjca.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 11. Dieter RS. Coronary artery stent infection. Clin Cardiol 2000;23:808–10. 10.1002/clc.4960231129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bosman WMPF, Borger van der Burg BLS, Schuttevaer HM, et al. Infections of intravascular bare metal stents: a case report and review of literature. Eur J Vasc Endovasc Surg 2014;47:87–99. 10.1016/j.ejvs.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 13. Yamamoto Y, Ushijima T, Yamaguchi S, et al. Mycotic aneurysm of the left anterior descending coronary artery after coronary artery bypass graft surgery. Ann Thorac Surg 2011;91:1601–3. 10.1016/j.athoracsur.2010.11.018 [DOI] [PubMed] [Google Scholar]
- 14. Omoto T, Saito K, Kashima T, et al. Mycotic aneurysm of the right coronary artery. Asian Cardiovasc Thorac Ann 2006;14:331–2. 10.1177/021849230601400413 [DOI] [PubMed] [Google Scholar]
- 15. Goldblatt J, Doi A, Negri J, et al. Mycotic Pseudoaneurysms of the coronary arteries. J Card Surg 2015;30:555–9. 10.1111/jocs.12563 [DOI] [PubMed] [Google Scholar]