Abstract
Neurodevelopmental disorders such as fragile X syndrome (FXS) result in lifelong cognitive and behavioural deficits and represent a major public health burden. FXS is the most frequent monogenic form of intellectual disability and autism, and the underlying pathophysiology linked to its causal gene, FMR1, has been the focus of intense research. Key alterations in synaptic function thought to underlie this neurodevelopmental disorder have been characterized and rescued in animal models of FXS using genetic and pharmacological approaches. These robust preclinical findings have led to the implementation of the most comprehensive drug development programme undertaken thus far for a genetically defined neurodevelopmental disorder, including phase IIb trials of metabotropic glutamate receptor 5 (mGluR5) antagonists and a phase III trial of a GABAB receptor agonist. However, none of the trials has been able to unambiguously demonstrate efficacy, and they have also highlighted the extent of the knowledge gaps in drug development for FXS and other neurodevelopmental disorders. In this Review, we examine potential issues in the previous studies and future directions for preclinical and clinical trials. FXS is at the forefront of efforts to develop drugs for neurodevelopmental disorders, and lessons learned in the process will also be important for such disorders.
Fragile X syndrome (FXS) is an X-linked neurodevelopmental disorder caused by a CGG repeat expansion exceeding 200 repeats in the promoter region of FMR1. This mutation results in hyper methylation and silencing of FMR1 and absence or reduction of its gene product, fragile X mental retardation protein 1 (FMRP)1,2. In a small fraction (less than 1%) of patients with FXS, the inactivation of FMR1 is caused by other non-trinucleotide repeat mutations3. The developmental trajectory in patients with FXS is slower than in healthy neurotypical children and adolescents and typically results in a relative decline in IQ and adaptive behaviour scores throughout childhood without actual regression4–6. Beyond the intellectual disabilities, there is a fairly consistent pattern of cognitive weaknesses and strengths in individuals with FXS. Relative weaknesses include visuospatial skills, working memory, processing of sequential information and attention, whereas simultaneous processing and visual memory are relative strengths7,8. Females are on average less affected than males. The majority of males with FXS present with mild to severe intellectual disability9 with an average IQ of 35–40 (REF. 10) (although this may be higher for those with mosaicism) and a mental age of about 5–6 years for adult males. By contrast, females present with an average IQ of 75–80 and a much broader range of involvement, from severe impairment to normal cognitive skills. About a third of women with FXS present with intellectual disabilities, and at least an additional third are diagnosed with learning disabilities11. Over 50% of males and 20% of females with FXS meet the diagnostic criteria for autism spectrum disorder (ASD)12,13. Pragmatic language deficits, reduced eye contact, social and generalized anxiety, sensory oversensitivity, difficulty with regulation of attention and activity level, self-injurious behaviour and aggression are common symptoms, some of which may drive the ASD diagnosis in individuals with FXS. Patients with FXS typically have minimal medical problems other than their cognitive disabilities and behavioural issues. Interestingly, gene-disrupting mutations identified in individuals with ASD are enriched in genes that code for mRNAs binding to FMRP14. This finding suggests that many gene products controlled by FMRP (at the translational level) are also individually associated with ASD. A large percentage of the approximately 800 target mRNAs of FMRP encode synaptic proteins and are thought to have a major role in neuroplasticity15.
Current treatments for FXS focus on symptomatic management of the disease. Very few randomized clinical trials (RCTs) have been conducted for symptomatic management in FXS, but drugs used off-label include: psychostimulants for attention deficit16, hyperactivity, distractibility and impulsivity; α2 adrenergic receptor agonists for sensory over-stimulation, hyperarousal, hyperactivity and sleep disturbances17; anticonvulsants for seizures and mood instability18; selective serotonin reuptake inhibitors for anxiety; and antipsychotics and antidepressants for aggression, anxiety and sleep disturbance18,19.
There is no approved or effective treatment that targets the mechanisms underlying FXS. Trials in idiopathic ASD are carried out in genetically and mechanistically heterogeneous groups of patients defined by behavioural criteria, and usually without an animal model aligned with the patient population. By contrast, robust preclinical findings (TABLE 1) from two decades of basic research on the function of FMRP have led to the implementation of the most comprehensive drug development programme undertaken thus far for a genetically defined neurodevelopmental disorder. However, what may have seemed to be an optimal translational scenario in FXS has not led to the expected results. In this Review, we discuss the RCTs that have been conducted in children, adults and adolescents with FXS in parallel. Some of these trials were led and sponsored by the pharmaceutical industry and some were led by academic investigators, funded by public and philanthropic sources and sponsored by pharmaceutical companies. In an effort to understand which mechanisms hold therapeutic potential and to clarify the barriers to translation from preclinical to clinical findings, we also examine potential issues at different steps of the drug development process. Finally, we analyse the current status of the field and propose mid-term and long-term objectives to extrapolate the lessons learned from this important drug development effort to the entire neurodevelopmental field.
Table 1 |.
Preclinical studies in Fmr1-KO mice, outcome measures and reported effects
| Outcome measure | mGluR5* | mGluR5‡ | GABABR activation§ | Statins‖ | Lithium | STEP‡ | MMP9¶ | S6K# | S6K‡ | CB1** | PAK‡‡ | AMPAR modulation§§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular | ||||||||||||
| Increased protein synthesis | >3 | 1 | 1 | 1 | >3 | ND | ND | 1 | 1 | ND | ND | ND |
| Increased ERK-mTOR-PI3K activity | >3 | ND | ND | 1 | >3 | ND | 1 | 1 | 1 | 1 | ND | ND |
| Synapse | ||||||||||||
| Altered synapse architecture | >3 | 1 | 1 | ND | 2–3 | ND | >3 | 1 | 1 | 1 | 1 | ND |
| Altered synaptic plasticity | >3 | 1 | ND | 1 | >3 | ND | ND | ND | 1 | ND | ND | ND |
| Behaviour | ||||||||||||
| Increased seizure incidence | >3 | 1 | 1 | 1 | >3 | 1 | 1 | ND | ND | 1 | 1 | ND |
| Impaired sensorimotor gating | 1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Hyperactivity | 1 | ND | 1 | ND | >3 | 1 | 1 | ND | ND | ND | 1 | ND |
| Impaired memory and cognition | >3 | 1 | ND | ND | >3 | ND | ND | 1 | 1 | 1 | ND | ND |
| Impaired social interactions | >3 | ND | ND | ND | >3 | 1 | ND | 1 | 1 | ND | ND | ND |
| Physiology | ||||||||||||
| Macroorchidism | 1 | - | ND | ND | 1 | ND | ND | 1 | 1 | ND | ND | ND |
| Elevated body growth | >3 | 1 | ND | ND | ND | ND | ND | 1 | 1 | ND | ND | ND |
| Clinical research? | Yes | No | Yes | Yes | Yes | No | Yes | No | No | No | No | Yes |
Numbers indicate the number of laboratories independently reporting the phenotype correction. A dash (−) indicates a lack of phenotype correction reported for the intervention (that is, explicit negative results were reported). AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CB1, cannabinoid receptor 1; GABABR, GABAB receptor; KO, knockout; mGluR5, metabotropic glutamate receptor 5; MMP9, matrix metalloproteinase 9; mTOR, mechanistic target of rapamycin; ND, not determined; PAK, p21-activated kinase; PI3K, phosphoinositide 3-kinase; S6K, ribosomal protein 6 kinase; STEP, striatal-enriched protein-tyrosine phosphatase.
Pharmacological inhibition.
Genetic correction.
GABAB receptor activation studies were conducted with arbaclofen.
Studies conducted with lovastatin.
Pharmacological inhibition conducted with minocycline treatment (minocycline has been reported to have more pharmacological effects than lowering MMP9 activity).
Pharmacological S6K inhibition conducted with PF-4708671 and FS-115122.
CB1 inhibition was studied with rimonabant129.
PAK inhibition was studied with FRAX48653.
The AMPAR modulator assessed was the AMPAkine, CX586, an investigational drug that was also studied clinically in schizophrenia and Alzheimer disease.
Molecular pathophysiology of FXS
Two decades of basic research on the function of FMRP have led to the characterization of several mechanisms that may underlie FXS (BOX 1). FMRP is an RNA-binding protein that regulates the synthesis of many proteins involved in synaptic function15. One of the most extensively studied functions of FMRP is its role in translational control and in long-term synaptic and spine morphological plasticity, which require rapid protein synthesis. Considerable efforts have focused on rescuing the synaptic plasticity that is dependent on protein synthesis in mouse models of FXS by manipulating receptors that regulate local mRNA translation. The two primary targets in preclinical studies and clinical trials have been group 1 metabotropic glutamate receptors (mGluRs)1,5 and GABA receptors (FIG. 1).
Box 1 |. Secondary or novel therapeutic targets in fragile X syndrome with no or little human trial data.
Two decades of basic research on the function of FMRP have led to the characterization of several mechanisms that may underlie fragile X syndrome (FXS):
3-Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase is inhibited by lovastin, which is also used to treat hypercholesterolaemia. Statins attenuate the activity of RAS proteins (FIG. 1), which are upstream of overactive protein synthesis in FXS. Lovastatin acts as a mild inhibitor of RAS by interfering with its farnesylation and recruitment to the cell membrane and consequently dampens activation of the extracellular-signal-regulated kinase (ERK) signalling pathway that drives fragile X mental retardation protein 1 (FMRP)-regulated protein synthesis. Lovastatin has been tested in preclinical trials122, and clinical trials are ongoing123,124.
Metformin, a widely prescribed treatment for type 2 diabetes, can also reduce ERK pathway activation. Lovastatin122 and metformin125 treatment of Fmr1-knockout (KO) mice corrects several phenotypes, including excessive protein synthesis122.
Matrix metalloproteinase 9 (MMP9) (FIG. 1) is an extracellular MMP that is overabundant in the brain tissue of Fmr1-KO mice and believed to degrade proteins required for synapse maturation and stabilization. Genetic deletion of Mmp9 or treatment of Fmr1-KO mice with minocycline (which normalizes MMP9 levels in Fmr1-KO brain tissue50) corrects multiple phenotypes50,126. Minocycline is being evaluated in clinical trials123,124.
Lithium (FIG. 1) can inhibit glycogen synthase kinase 3 (GSK3)127, which is involved in protein synthesis regulation. Chronic treatment of Fmr1-KO mice with lithium has been reported to correct multiple phenotypes127–129, and it has also been evaluated in clinical trials130.
Striatal-enriched protein-tyrosine phosphatase (STEP) (FIG. 1) is expressed in neurons in several brain areas and acts on multiple targets, including the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor and NMDA (N-methyl-D-aspartate) receptor subunits, as well as on several kinases including ERK, tyrosine-protein kinase Fyn (FYN), protein-tyrosine kinase 2β (PYK2; also known as PTK2B) and p38 mitogen-activated protein kinase131. Step mRNA is under translational control of FMRP, and increased Step expression levels are reported in the brain tissue of Fmr1-KO mice132, which may alter synaptic function and several behavioural phenotypes. Genetic ablation of Step in Fmr1-KO mice was reported to correct several phenotypes133. STEP inhibitors suitable for human applications have not been reported.
Ribosomal protein S6 kinase (S6K) (FIG. 1) is essential for regulating cellular protein synthesis and metabolism, and it is crucial for the phosphorylation of FMRP134 and linked to the increased protein synthesis rate in the absence of FMRP135. Genetic reduction49 or pharmacological inhibition136 of S6K1 in Fmr1-KO mice corrects multiple phenotypes. There are currently no S6K inhibitors suitable for human applications reported.
Cannabinoid receptor 1 (CB1) mediates long-term depression (LTD) triggered by the production of endocannabinoids as a consequence of the activation of metabotropic glutamate receptor 5 (mGluR5)137. Administration of the CB1 inhibitors rimonabant and NESS0327 (FIG. 1) corrects elevated CB1-mediated signalling and several phenotypes in Fmr1-KO mice52,138. In patients, although rimonabant was originally developed for the treatment of obesity139, approval was withdrawn based on severe neuropsychiatric adverse events, which were deemed to be related to its mechanism of action140,141.
The family of p21-activated kinases (PAKs) (FIG. 1) are effector proteins for RAC1 and cell division control protein 42 homologue (CDC42), which are both small RHO GTPases involved in modulating cytoskeletal function, cell division, motility and survival. Altered PAK signalling was reported in Fmr1-KO mice142, and genetic ablation of PAK55 or administration of the PAK inhibitor FRAX486 (REF. 53) corrected several phenotypes of Fmr1-KO mice. There are currently no inhibitors suitable for human applications reported.
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors143 (FIG. 1) constitute a family of postsynaptic ionotropic glutamate receptors and are the backbone for mediating fast glutamatergic neurotransmission. AMPAkines, such as CX516, are positive allosteric modulators of AMPA receptors144,145. In FXS, defective LTD24 — a form of synaptic plasticity that relies on the modulation of AMPA receptor function — and reduced mGluR1 AMPA receptor subunit cell surface expression in neurons146 has been reported, indicating that enhancing AMPA receptor function could have therapeutic utility. No preclinical studies have been conducted in Fmr1-KO mice, and CX516 showed no efficacy in one clinical trial147.
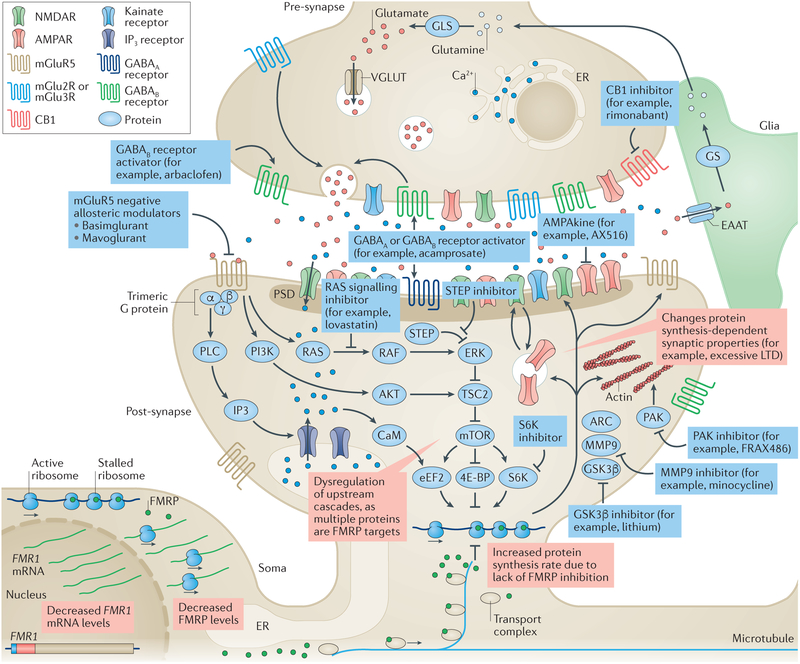
Figure 1 |. Drug targets in fragile X syndrome under investigation.
Glutamate activates a range of ionotropic and metabotropic receptors, including metabotropic glutamate receptor 5 (mGluR5). Activation of mGluR5 leads to activation of Gαq/o and formation of IP3 via phospholipase C (PLC) and intracellular Ca2+ mobilization. mGluR5 also acts (among other effects) on the phosphoinositide 3-kinase (PI3K)–AKT and RAS–ERK pathways, thereby increasing mTOR and ribosomal protein S6 kinase (S6K) activity, ultimately modulating protein synthesis, which is key for regulating synaptic strength. mGluR5 also interacts with NMDA receptors (NMDARs) by way of phosphorylation and receptor trafficking, and with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs) by modulating membrane insertion and receptor subunit composition. Fragile X mental retardation protein 1 (FMRP) is an RNA-binding protein that regulates protein biosynthesis by inhibiting mRNA translation through ribosomal stalling. In patients with fragile X syndrome (FXS) (red boxes), an increase in CGG repeat length and subsequent hypermethylation of FMR1 result in reduced FMR1 transcript levels and decrease FMRP levels. The lack of FMRP at the synapse leads to an increased rate of protein synthesis of FMRP targets. These changes alter downstream protein synthesis-dependent processes, causing long-term depression (LTD), probably due to increased AMPA receptor exocytosis. Blue boxes represent interventions under consideration. mGlu5 negative allosteric modulators can correct multiple aspects of the molecular pathophysiology, including increased phosphorylation of S6K and mTOR, and the rate of protein biosynthesis. AMPAkines can counterbalance the increased AMPAR internalization, enhancing AMPAR sensitivity to glutamate. GABAB receptor activators inhibit glutamate release into the synaptic cleft, which in turn reduces activation of mGluR5 and other glutamate receptors. RAS–ERK signalling inhibitors target the downstream cascades of mGluR5. Genetic reduction of striatal-enriched protein-tyrosine phosphatase (STEP) levels can correct multiple phenotypes in Fmr1-knockout (KO) mice133. Glycogen synthase kinase 3β (GSK3β) is a key target of lithium, which can ameliorate multiple phenotypes in Fmr1-KO mice (see REF. 129). Matrix metalloproteinase 9 (MMP9) is upregulated in FXS, and MMP9 inhibitors correct multiple Fmr1-KO phenotypes126. p21-activated kinase (PAK) is a small G protein that modulates actin dynamics, and PAK inhibitors can revert multiple phenotypes in Fmr1-KO mice53. S6K is essential for regulating cellular protein synthesis, and genetic reduction49 or pharmacological inhibition136 of S6K1 corrected multiple phenotypes in Fmr1-KO mice. Increased cannabinoid receptor 1 (CB1)-mediated signalling has been reported in Fmr1-KO mice, and administration of rimonabant corrected several phenotypes in these mice52,138. Acamprosate, which activates GABAB and GABAA receptors, also ameliorated several phenotypes in Fmr1-KO mice. A more detailed discussion of novel drug targets for FXS is covered in recent reviews61,148,149. 4E-BP, eukaryotic translation initiation factor 4E-binding protein; ARC, activity-regulated cytoskeleton-associated protein; CaM, calmodulin; EAAT, excitatory amino acid transporter; eEF2, elongation factor 2; ER, endoplasmic reticulum; GLS, glutaminase; GS, glutamine synthetase; PSD, postsynaptic density protein; TSC2, tuberin; VGLUT, vesicular glutamate transporter.
The mGluR theory of FXS20 posits that abnormal synaptic function and certain aspects of aberrant behaviour in FXS are a result of exacerbated group 1 mGluR-dependent protein synthesis. This hypothesis is based on multiple observations: first, mGluR stimulation triggers de novo protein synthesis21; second, FMRP functions as an RNA-binding protein and attenuator of protein biosynthesis22,23; and third, Fmr1-knockout (KO) mice show increased mGluR-dependent hippocampal long-term synaptic plasticity24,25. Genetic reduction of mGluR5 activity achieves correction of multiple phenotypes in Fmr1-KO mice and dfmr1−/− flies26. Multiple pharmacological preclinical studies using mGluR5 negative allosteric modulators (referred to as mGluR5 antagonists throughout this Review) such as 2-methyl-6-(phenylethynyl)pyridine (MPEP)27, fenobam28, 2-chloro-4-((2,5-dimethyl-1-(4-(trifluoromethoxy)phenyl)-1H-imidazol-4-yl)ethynyl)pyridine (CTEP)29 and mavoglurant30–35 have demonstrated that dampening mGluR5 signalling rescues protein synthesis and many of the classic outcome measures (TABLE 1) used in the mouse and fly models30–35.
GABA is the predominant inhibitory neurotransmitter in the brain. This neurotransmitter acts through GABAA receptors, which are ligand-regulated chloride channels that cause hyperpolarization in mature neurons upon activation, and GABAB receptors, which are heterodimeric G protein-coupled receptors (GPCRs) that are expressed mostly presynaptically throughout the brain. GABAB receptor activation dampens presynaptic glutamate release and causes hyperpolarization of postsynaptic neurons by activation of G protein-activated inward rectifying potassium channels (GIRKs), thereby collectively reducing glutamatergic signalling at excitatory synapses36. Administration of GABAB agonists, such as baclofen or arbaclofen, corrects exacerbated protein synthesis and multiple phenotypes in Fmr1-KO mice37–39. Acamprosate, which activates GABAB and GABAA receptors40, also ameliorates several phenotypes in Fmr1-KO mice41. GABAA family receptors and enzymes required for the production of GABA are expressed at reduced levels in Fmr1-KO mice compared with wild-type mice42, and this phenotype can be rescued by introducing a yeast artificial chromosome (YAC) containing the ‘healthy’ human FMR1 genomic region into Fmr1-KO mice43. Preclinical studies have investigated ganaxolone, a neurosteroid and positive GABAA modulator with sedative, anxiolytic and anticonvulsant properties44, which addresses several phenotypes of Fmr1-KO mice43. Ganaxolone and acamprosate are currently being clinically tested in patients with FXS45,46.
Conclusions from preclinical studies
Translational research in neurodevelopmental disorders (NDDs) is in its infancy relative to other biomedical fields and will likely struggle with similar issues, including challenges in translation from mice to humans. For example, in oncology, in which the knowledge of mechanisms is much more advanced than in NDDs, the rate of translation from preclinical models to clinical application is approximately 8%47, and even in the area of targeted molecular approaches, studies often have quite different outcomes in mice and humans48. On the bright side, our knowledge on genetic aetiologies, molecular mechanisms and contributing factors is rapidly increasing, allowing relevant animal models to be established to study underlying mechanisms. As a result, several conclusions can be drawn from the preclinical studies conducted so far in Fmr1-KO mice.
Among the different outcome measures used in FXS preclinical studies, protein synthesis, dendritic spine density and morphology, long-term depression (LTD) and audiogenic seizures are some of the most robust phenotypes observed in FXS mice. One striking conclusion of the broad array of preclinical studies is that these core deficits can be consistently rescued by more than ten genetic approaches and multiple classes of pharmacological compounds including mGluR antagonists (MPEP, fenobam, mavoglurant and CTEP), GABA agonists, inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA), mechanistic target of rapamycin (mTOR) inhibitors and specific targets of FMRP (such as Mmp9, which encodes matrix metalloproteinase 9; class 1A phosphatidylinositol 3-kinase isoform p110β; App, which encodes amyloid-β precursor protein; Agap2 (also known as Centg1), which encodes phosphoinositide 3-kinase enhancer (PIKE; also known as AGAP2); Step (also known as Ptpn5), which encodes striatal-enriched protein-tyrosine phosphatase; Bkca (also known as Kcnma1), which encodes calcium-activated potassium channel subunit α1; and Kcnd2, which encodes voltage-gated potassium channel subunit KV4.2)26,31,32,49–59. Together, these studies have delineated a signalling pathway that couples neural activity to FMRP-regulated protein synthesis and have additionally led to the discovery of novel roles for FMRP in the regulation of ion channels60 (FIG. 1).
These accomplishments notwithstanding, a central issue in the use of the Fmr1-KO mouse model of FXS is the variability and small effect size of the mouse phenotypes in the area of cognitive defects. Standard learning and memory tasks for mice — including the Morris water maze, fear conditioning, passive avoidance, novel object recognition, visual discrimination and delayed non-matching to position — detected deficits in Fmr1-KO mice in some reports, but normal performance in others, both within and across laboratories and unrelated to the genetic background (FVB/NJ, FVB/AntJ or C57BL/6J)61–63. As intellectual disability is a core feature of FXS, inconsistent and small cognitive deficits in the Fmr1-KO mouse model may limit its value for evaluating the efficacy of pharmacological interventions in the cognitive domain (of note, to date, behavioural traits, but not cognition, have been used as primary outcome measures in human trials). Further, many of the other classic behavioural phenotypes used as outcome measures in preclinical studies are inconsistently observed in Fmr1-KO mice (such as open field, rotarod, elevated plus-maze, marble burying, self-grooming and most social paradigms). As a result, the rescue of these behavioural phenotypes has been difficult to consolidate and to use as a guide for clinical studies30,49,56,64,65.
The duration and age at treatment is likely to influence the rescue of cognitive and behavioural deficits substantially. However, windows of plasticity (specific age ranges when neuronal connections are most modifiable and a specific form of learning can occur most easily) have not been clearly documented in preclinical studies of FXS (age-dependent plasticity is discussed below). Moreover, a comprehensive correction of Fmr1-KO phenotypes was achieved with mGluR5 antagonist treatment of young adult or fully adult mice30,32.
Conclusions from clinical studies
Large clinical trials were conducted between 2008 and 2014 (FIG. 2) for two mGluR5 antagonists (basimglurant66 and mavoglurant67) as well as a GABAB agonist (arbaclofen68) (BOX 2). Mavoglurant was tested in two phase IIb double-blind, placebo-controlled, parallel-group studies that included 175 adults aged 18–45 years and 139 adolescents with FXS. In both trials, participants were stratified by methylation status and randomly assigned to receive mavoglurant (25, 50 or 100 mg twice daily (b.i.d.)) or placebo over 12 weeks69. Arbaclofen was tested in two parallel randomized, double-blind, placebo-controlled studies in 125 adolescents and adults aged 12–50 years and in 172 children aged 5–11 years. In the combined adolescent and adult study, arbaclofen was flexibly titrated from 5 mg to the maximum tolerated dose (10 mg b.i.d., 10 mg three times daily (t.i.d.) or 15 mg t.i.d.), whereas participants in the child study were randomly assigned to three fixed doses (5 mg b.i.d., 10 mg b.i.d. or 10 mg t.i.d. or placebo over 8 weeks. Several conclusions can be drawn from the data collected through these unprecedented efforts. Many questions also arose and remain unanswered.
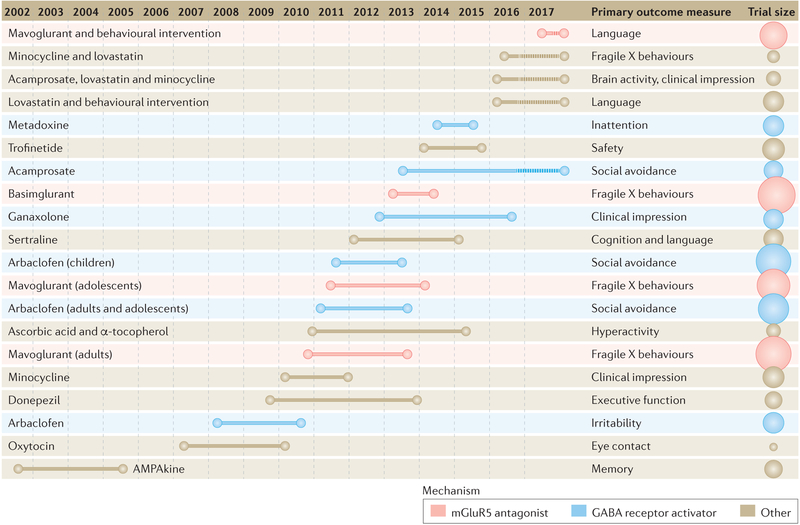
Figure 2 |. Clinical trials performed since 2002 in fragile X syndrome.
Trial duration and primary outcome measures are displayed for each trial. On the far right, the size of the circles is proportional to the number of participants enrolled in the study. mGluR5, metabotropic glutamate receptor 5. Adapted with permission from Nicolas Rapp, Spectrum (https://spectrumnews.org/news/despite-setbacks-fragile-x-drugs-file-clinical-trials/).
Box 2 |. Conclusions from mGluR5 antagonist and GABAB agonist trials.
Basimglurant and mavoglurant (metabotropic glutamate receptor 5 (mGluR5) inhibitors) do not modulate behaviour within 3 months of the treatment period; however, arbaclofen (a GABAB agonist), which seemed to address behaviour, showed trends of efficacy in children in an analysis of the primary and secondary trial outcomes, without invoking a post-hoc analysis.
The trials with mGluR5 inhibitors and GABAB agonists were sufficiently long to measure behavioural changes related to potential symptomatic effects of the drugs. Indeed, clinically active drugs in autism and other psychiatric conditions show efficacy for behavioural symptoms in adults, adolescents and children at treatment intervals shorter than 3 months (typically 4 weeks or less).
The broad age range (12–40 years) should have enabled the detection of age-related therapeutic benefits, and age did not co-vary with response to mavoglurant in patients with fragile X syndrome (FXS) aged 12 years and older in these studies69. Of note, many psychotropic medications effective in adults also show some efficacy in adolescent patients. However, these medications, unlike arbaclofen, are largely targeted at behavioural support and not the underlying disease. In the arbaclofen studies, a possible signal of efficacy was seen in children aged 5–11 years, but not in adults and adolescents, suggesting the possibility that treatment needs to commence at younger ages to demonstrate disease modification.
Enrolment of more than 100 participants was required to reach unequivocal negative findings, which calls into question the utility of smaller trials in neurodevelopmental disorders (NDDs), as these trials almost invariably identify significant improvement in one of the multiple post-hoc exploratory analyses. The additional burden of dose finding drastically decreases the power of these studies and should be taken into account.
The methodology and design of the trials highlighted key issues.
Windows of plasticity: very young patients were not included in the studies reviewed above. Plasticity is expected to be much higher in young children, and this may be the only group in which effects of a disease-modifying agent targeting cognition and development can be seen in the time period assessable by a placebo-controlled trial. Trials in adults and adolescents may be able to detect drug effects only if there is a direct effect on a specific area of behaviour.
Measuring change: primary outcome measures were mostly questionnaires performed by caregivers and showed large placebo response. Objective measures of core phenotypes rather than secondary behaviours, such as direct assessments of cognition and language that are less subject to placebo response and have less inherent variability than caregiver-rated scales, need to be implemented in future trials.
Measuring disease modification in NDDs: efforts may need to be redirected towards the implementation of longer trials in younger children accompanied by learning interventions measuring cognitive and developmental outcomes
Therefore, it cannot be excluded that mGluR5 antagonists might show improvement of the developmental trajectory and cognition when tested in very young subjects with longer treatment duration.
Tolerability and target
The study drugs (mavoglurant, basimglurant and arbaclofen) were overall well tolerated at the dose levels tested. Mild side effects (headaches, dizziness, insomnia and vomiting) were observed in about 20% of FXS patients with mavoglurant at higher dose levels69. A wealth of preclinical data and direct receptor occupancy measures for basimglurant in mice66 and healthy subjects70 demonstrated that the study drugs entered the brain, engaged their target receptors and showed pharmacodynamic effects.
Efficacy
In the two mavoglurant phase IIb studies, behaviour was not improved by the mGluR5 antagonist in a 3-month time period as tested by primary outcome measures (Aberrant Behaviour Checklist (ABC) total score (FXS algorithm)) compared with placebo. Secondary end points included Clinical Global Impression (CGI), Repetitive Behaviour Scale total score and Social Responsiveness Scale (total score) (TABLE 2). A pre-specified stratification of the DNA methylation status (complete versus partial) did not show improvement in either stratum. Results from post-hoc analyses of a mavoglurant phase IIa trial71 showed improvement in patients with FXS and full methylation but were not replicated in the two phase IIb trials. In the mavoglurant phase IIb trials, a broad array of post-hoc analyses were carried out, including the investigation of many secondary behavioural outcome measures, with or without stratification of the DNA methylation status. In these well-powered studies, the data did not support efficacy of different doses of mavoglurant versus placebo in any of these behavioural outcome measures for any of the subgroups. Exploratory biomarkers and endophenotypes tested in a substudy (n = 56) of the mavoglurant trials suggests an improvement relative to placebo in gaze towards the eye region on an eye-tracking task and in performance on a computerized executive function battery69. Cognition was not formally investigated in the overall trial. A computerized measure of cognition used at a few sites in the mavoglurant trials was too challenging for patients with FXS. Language was not directly tested in a study powered to evaluate change.
Table 2 |.
Clinical trials for fragile X syndrome
| Design | Drug | Phase | Treatment duration (months) | n (F/M) | Age (years) | Status | Efficacy | Primary outcomes and biomarkers | Refs |
|---|---|---|---|---|---|---|---|---|---|
| mCluR5 receptor | |||||||||
| OL | Fenobam | IIa | NA, single dose | 12 (6/6) | 18–31 | Completed | Changes reported on a biomarker | PPI (improved over test-retest controls) | 150 |
| Mavoglurant‡ | IIa | 1 | 30 (0/30) | 18–35 | Completed | Efficacy reported in a post-hoc analysis |
|
71 | |
| 175 (11/164) | 18–45 | Completed | Lack of efficacy reported |
|
69 | ||||
| 139 (15/124) | 12–17 | Completed | Lack of efficacy reported |
|
69 | ||||
| Mavoglurant | II/III | >12 | 148 (10/138) | 18+ | Terminated | Lack of efficacy reported | CGI-I | 151 | |
| Mavoglurant | II/III | >12 | 119 (13/106) | 12–18 | Terminated | Lack of efficacy reported | CGI-I | 152 | |
| Basimglurant | IIa | 1.5 | 40 | 18–50 | Completed | Results not yet published | ADAMS | 76 | |
| Basimglurant | IIb | 3 | 185 (34/151) | 14–50 | Completed | Lack of efficacy reported |
|
73 | |
| Basimglurant | IIa | 3 | 47 | 5–13 | Completed | Results not yet published | No outcomes at this point | 74 | |
| Intracellular signaling | |||||||||
| OL | Lithium | lla | 2 | 16 | 6–30 | Completed | Efficacy reported (ERK and other outcome measures such as ABC-T.CGI.VAS and RBANS) |
|
130 |
| NNZ-2256 | II | 1.5 | 72 (0/72) | 12–45 | Completed | Results not yet published |
|
153 | |
| Metadoxine§ | II | 1.5 | 62 (15/47) | 15–55 | Completed | Results not yet published |
|
154 | |
| Lovastatin and PILI | II | 5 | 60 | 10–17 | Ongoing | Ongoing study |
|
123 | |
| Proteins regulated by FMRP | |||||||||
| Minocycline and lovastatin | II | 3 | 26 | 13–45 | Ongoing | Ongoing study |
|
124 | |
| Minocycline | IIa | 2 | 20 (2/18) | 13–35 | Completed | Efficacy reported on ABC | ABC | 155 | |
| RCT | Minocycline | II | 3 | 55 (8/47) | 3.5–16 | Completed | Modest efficacy reported on CGI-I |
|
156 |
| AMPA receptor | |||||||||
| RCT | CX516¶ | II | 1 | 49 (11/38) | 18–50 | Completed | Lack of efficacy reported | Memory# | 147 |
| GABA modulators | |||||||||
| Arabaclofen** | II | 1 | 63 (8/55) | 6–40 | Completed | Efficacy reported in post-hoc analysis on ABC |
|
68 | |
| Arabaclofen | III | 2 | 125 (26/99) | 12–50 | Completed | Lack of efficacy reported | ABC | 72 | |
| Arabaclofen | III | 2 | 172 (25/144) | 5–11 | Completed | Lack of efficacy reported | ABC | 72 | |
| Arbaclofen | II | 12 | 45 | 6–40 | Terminated | Results not yet published | ABC | 157 | |
| Arbaclofen | III | >12 | 357 | 5–50 | Terminated | Results not yet published | Open-label study for safety | 158 | |
| Acamprosate | III | 2.5 | 12 (2/10) | 5–17 | Completed | Efficacy reported on the CGI and behavioural scales |
|
159 | |
| Donepezil | I | 1.5 | 8 (2/6) | 14–44 | Completed | Efficacy reported | CNT | 160 | |
| Acamprosate | II/III | 2.5 | 48 | 5–23 | Ongoing | Ongoing study |
|
46 | |
| Ganaxolone | II | 1.5 | 59 (9/50) | 6–17 | Completed | Lack of efficacy |
|
161 | |
| Donepezi | II | 3 | 42 (15/27) | 12–29 | Completed | Results not yet published | CNT | 162 | |
| Donepezil | II | 3 | 20 (0/20) | 6–15 | Completed | Lack of efficacy reported | IQ§§ | 163 | |
ABC, Aberrant Behaviour Checklist; ADAMS, Anxiety Depression and Mood Scale total score; ADHDRS, attention-deficit hyperactivity disorder Rating Scale IV; AE, adverse events; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AP, auditory processing; APP, amyloid-β precursor protein; BDNF, brain-derived neurotrophic factor; CGI-I, Clinical Global Impression-Improvement; CNT, Contingency Naming Test; ELS, Expressive Language Sampling; ERK, extracellular-signal-regulated kinase; ERP, event-related potential; ES, efficacy study; ET, eye tracking; F, female; FMRP, fragile X mental retardation protein 1; HR, heart rate; HRV, heart rate variability; M, male; mGluR5, metabotropic glutamate receptor 5; MMP9, matrix metalloproteinase 9; NA, not applicable; OL, open label; PK, pharmacokinetics; PPI, prepulse inhibition; RCT, randomized clinical trial; RSA, respiratory sinus arrhythmia; VAS, visual analogue scale.
Stratification strategy. In the AFQ056 studies, participants were divided into completely methylated and partially methylated groups. In the arbaclofen studies, social withdrawal was used for stratifying participants.
AFQ056 studies phase IIb: eye tracking was performed only at some sites.
Metadoxine also targets the GABA modulators group.
Neuroimaging includes functional magnetic resonance imaging (fMRI) and transcranial magnetic stimulation.
Z-scores reported for all outcome measures.
Memory includes test of visual perceptual skills (TVPS), Woodcock–Johnson memory for words test (W–JMem) and repeatable battery for the assessment of neuropsychological status (RBANS).
Improvement in the ABC-CFX-Social Avoidance subscale over placebo, but not in the primary end point ABC-I.
Lack of efficacy reported.
In this trial, the Stanford–Binet Intelligence Scale used is the Hindi adaptation by Kulshrestha.
In the arbaclofen phase III studies, no improvement over placebo was demonstrated for the primary outcome measures. In the phase III study carried out in children, the primary end point (ABC-CFX Social Avoidance subscale) narrowly missed significant improvement (P = 0.08)72. Of note, the primary outcome measure was chosen on the basis of previous post-hoc analyses that showed improvement in the phase II trial68. In the phase III study, children receiving the highest dose of arbaclofen also showed improvement over placebo on the secondary end points of the ABC-CFX Irritability subscale (P = 0.03) and the Parenting Stress Index (P = 0.03). This trial was limited by a lack of full enrolment due to financial issues for the sponsor, the use of fixed rather than flexible dosing and possible inflation of symptoms by families in order to meet inclusion criteria72. Multiple end points in this trial showed effect sizes of 0.3–0.5 in favour of arbaclofen, which highlights the importance of properly powered studies and new statistical designs encompassing clusters of several end points as a primary outcome.
Basimglurant was tested in two phase II clinical trials in adult and adolescent patients aged 14–50 years73 and in children aged 5–13 years74. Both studies were designed as randomized, double-blind, placebo-controlled, parallel-design trials testing two doses of basimglurant over a 12-week treatment period in male and female patients.
In the paediatric study, a total of 47 patients were randomly assigned to 2 body weight-adjusted doses of basimglurant. The primary objective of the study was to explore safety and tolerability of basimglurant in this age range. A suite of efficacy and biomarker assessments were included in the study which, given the sample size, was exploratory. This study is not published yet, and we therefore need to defer an in-depth discussion of this trial to a later time.
The adult and adolescent FragXis study73 included 185 outpatients for whom the FXS diagnosis was confirmed based on Southern blot at the start of the study. Study participants showed a level of behavioural symptoms of the ABC (total) ≥20 as reported by caregivers and were at least ‘mildly ill’ (CGI of Severity of Illness (CGI-S) scale >3) based on a clinician’s assessment. The ABC entry score was chosen to ensure a minimum level of symptoms and to enable an adequate representation of female patients, who often show less severe symptoms than male patients. Stable prescription medications were permitted with the exception of drugs with GABAergic or glutamatergic mechanisms (including other mGluR5 modulators administered within 18 months before screening) that might potentially interfere with the activity of the study drug. Patients were randomly assigned to receive basimglurant (0.5 or 1.5 mg once a day (q.d.)) or placebo, with stratification by gender and age group (14–17 and 18–50 years) to ensure that about one-third of participants were adolescents.
The primary efficacy outcome measure was the Anxiety Depression and Mood Scale (ADAMS) total score, which was recorded every 3 weeks by the same person throughout the study; the end point was ADAMS total change from baseline at 12 weeks compared with placebo. Secondary outcome measures included the ABC total, Social Responsiveness Scale (SRS), Visual Analogue Scale (VAS) measure of the patient’s most troubling symptoms assessed at baseline, CGI-S and CGI-Improvement, Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and Vineland Adaptive Behavior Scale (VABS-II) scores; an exploratory assessment of caregiver-related outcomes was recorded using the Caregiver Burden Inventory-Modified75.
The choice of the primary end point was partly motivated by the results from a smaller, exploratory trial76. This double-blind, parallel-design, placebo-controlled trial was conducted in 40 adult male and female patients with FXS aged 18–49 years of age, testing a dose range of 0.1–1.5 mg of basimglurant q.d. for a 6-week treatment period. The explored dose range of basimglurant was well tolerated, and even though this trial was not powered to detect differences in the outcome measures, it showed trends of efficacy, warranting further exploration in a larger sample76.
Several factors were incorporated into the study design with the aim of minimizing variability, subjectivity and placebo effects. To mention just two points, selected outcome measures were recorded by caregivers (ADAMS, ABC, SRS and VAS) and clinicians (CGI-S, CGI-I, RBANS and VABS-II). Furthermore, the primary outcome measure (ADAMS) and behavioural entry criterion (ABC) were designed to be different, with the objective of minimizing baseline score inflation.
In addition to the outcome measure, a suite of biomarker measures were carried out, including measurements of levels of FMRP protein and FMR1 mRNA in blood, as well as the genomic DNA methylation status in a stretch of the FMR1 untranslated region.
In the primary analysis of ADAMS total change from baseline to the 12-week time point, neither of the basimglurant treatment groups showed improvement over placebo. Similarly, in the secondary end point analysis, all treatment groups improved, but neither of the basimglurant groups showed improvement over placebo. Extensive post-hoc analysis using biomarker data did not show efficacy in the subgroups analysed. Basimglurant was overall well tolerated in the trial, with most adverse events classified as psychiatric disorders. No clinically relevant changes in mean laboratory parameters, vital signs and electrocardiography related to the treatment were recorded.
Guidelines for future research
The FXS field is at the forefront of biomedical research in NDDs. The lessons learned from this important drug development effort and the subsequent best practice guidelines are important for the entire neurodevelopmental field. In this section, we attempt to define our current position and propose mid-term and long-term objectives.
Preclinical studies
Many compounds, including those tested in the large clinical trials mentioned above, can rescue altered translational control and dendritic structure, as well as biochemical parameters and behaviour in animal models, and these synaptic markers are widely studied in ASD and NDDs. Additional levels of evidence are recommended for future preclinical studies, which should attempt to incorporate translatable measures, such as electroencephalogram (EEG) recordings and functional magnetic resonance imaging (fMRI)77, which can be applied in smaller clinical trials to demonstrate brain functional changes. Incorporating these measures will increase the cost of preclinical studies and may require consortia typically used in human trials (BOX 3).
Box 3 |. Suggested level of preclinical evidence to justify randomized clinical trials in humans.
Preclinical studies have indicated various phenotype corrections in Fmr1-knockout (KO) mice with well over ten diverse interventions; this observation might suggest that the Fmr1-KO mouse model is over-predictive. The aforementioned negative trial results, despite their limitations, suggest that the Fmr1-KO mouse model alone is of limited value for predicting the therapeutic potential of novel mechanisms of action or outcome of trials as they have been designed so far (short duration and behavioural outcomes). It is therefore strongly recommended that preclinical studies be carried out in different genetic backgrounds (such as C57BL/6 and FVB/NJ) and additional disease models (such as Fmr1-KO rats). Patient-derived induced pluripotent stem cells are promising, but they do not reflect the complexity of mammalian organisms, and the ability to translate findings to inform design of human trials has yet to be established.
Reproducibility: preclinical results suggesting therapeutic benefits should be reproduced by at least two independent laboratories.
Meaningful phenotypes and readouts: disease pathophysiology and correction will manifest differently in different species, but new end points such as electroencephalogram measures could align across preclinical and clinical studies.
Broad phenotyping: new interventions should be assessed broadly for their effects in disease models, because readouts often ‘cross-validate’; improvements in several cognitive paradigms interrogating the same or related cognitive domains may increase the confidence that the findings might translate.
Improved technical design standards: randomization and blinding should be used to exclude time of day and rater-bias effects.
Dose: appropriate potency on the target, pharmacokinetic properties and brain penetration must be ensured. Several doses should be studied to define the minimally active and the maximally efficacious dose and to unmask nonlinear effects on outcomes.
Combination therapy: if the patient population is treated with one or several psychotropic drugs, which can have an effect on intended outcome measures, such as vigilance and cognitive performance, these drug combinations also need to be assessed preclinically. Combination studies need to include careful monitoring of drug exposure, as simultaneous administration of two or more drugs can influence the clearance and ultimately exposure of the individual drugs.
Power: studies need to be sufficiently powered. This requires mathematical simulation incorporating the variance of the trait chosen as an outcome typically observed in the animal model and the expected effect size of the intervention. This is particularly relevant for Fmr1-KO mouse phenotypes, which are often subtle without a clearly reported effect size.
Reporting negative data: negative data are vital to judge the confidence in new mechanisms and to determine which assessments in preclinical studies and human trials are most meaningful. In this context, it is important that entirely negative studies are being reported (such as when a novel intervention failed to improve on any outcome, which in the future could be used as negative control), as well as studies in which a tested intervention failed on some of the assessment (when some intervention only acts on select phenotypes, whereas others act more broadly). Preclinical studies need to include all experimental details relevant for the experimental procedures.
Windows of plasticity.
Thorough investigation of the effect of gene reinstatement and pharmacological treatment carried out at multiple time points and using multiple outcome measures should be required in preclinical studies. Such approaches have been studied in detail for other models. For example, reinstatement of ubiquitin protein ligase E3A (Ube3a) in Ube3a-KO mice can restore synaptic plasticity at any age, but behaviour can only improve when reinstatement occurs during early development78. Gene reinstatement shows similar results in the Syngap1-KO model, which lacks the RAS/RAP GTPase-activating protein SYNGAP79. By contrast, as mentioned above, treatment with mGluR antagonists (mavoglurant) and GABAB agonists (arbaclofen), as well as gene reinstatement in Fmr1-KO mice starting in young adult or adult mice, can fully correct most behavioural, physiological, biochemical and neuroanatomical alterations30,32,37. Similarly, adult activation of the gene encoding methyl-CpG-binding protein 2 (Mecp2) in an inducible mouse model of Rett syndrome can rescue behavioural alterations and synaptic plasticity deficits, suggesting that there is a broader window of therapeutic opportunity in other genetic defects. Similar corrections with late-onset treatment in mice have been reported in tuberous sclerosis80 and spinocerebellar ataxia type 1 (SCA1)81. However, it is yet to be demonstrated that improvement of neurocognitive functioning in patients with tuberous sclerosis complex (TSC) can be achieved with the rapamycin derivative sirolimus, especially given its limited brain penetration. No human data are available for SCA1 in the absence of a drug suitable for clinical trials30,32,37.
Translatable outcome measures.
An important goal for future translational research is to better connect preclinical measures and human clinical outcomes. In particular, phenotypes addressed in Fmr1-KO mice, such as the rate of protein synthesis, spine morphology, LTD or audiogenic seizures have not been addressed in clinical studies because the readouts are either inaccessible or very difficult to obtain in patients. Other readouts, such as open field exploration or self-grooming, are quite distant from the human symptoms that they are trying to mimic. Measures such as EEG recordings82,83 and fMRI77 — which can be applied in preclinical models and clinically and thus could help to improve translation — should be applied more broadly.
Regarding the Fmr1-KO mouse as a translational disease model, the authors of this Review expressed divergent views. Some of us are of the opinion that the clear discrepancies between preclinical and clinical findings with mGluR5 inhibitors and arbaclofen to date suggest that the Fmr1-KO mouse line is not useful as a translational preclinical disease model. The observations that multiple therapeutic interventions correct the same phenotypes in the Fmr1-KO mouse model (TABLE 1) further support the notion that either the Fmr1-KO mouse model, the outcome measures currently used or both factors combined are over-predictive of clinical efficacy84. There is a need to develop novel disease models, preferably in a non-rodent species, which may be closer to the human pathophysiology (including features such as DNA methylation) and/or to develop assessments that are translatable to the clinical outcome measures such as EEG and event-related potentials (ERPs). Generally, the confidence in the therapeutic potential of a new mechanism of action is considerably strengthened when consistent findings are obtained in at least two distinct disease models, preferably in two different species. As far as the Fmr1-KO mouse is concerned, for the time being, this mouse model should no longer be viewed as sufficient to predict the therapeutic utility of novel or known interventions.
Other medical fields have faced similar issues. The reproducibility of preclinical results has been problematic in the field of amyotrophic lateral sclerosis (ALS). The ALS Therapy Development Institute rigorously retested more than 100 drug candidates in the superoxide dismutase 1 (SOD1) mouse model, and they were unable to replicate many of the previously reported preclinical efficacy findings85. Lack of reproducibility in preclinical models and lack of translation from preclinical efficacy for drugs tested in the SOD1 mouse model to patients with ALS explain why the profiling of drug candidates in the SOD1 mouse model poorly translated into clinical efficacy85.
By contrast, some of us believe that the Fmr1-KO mouse remains a valid model for both mechanistic and preclinical studies. The available data strongly suggest that the behavioural phenotypes commonly studied in the Fmr1-KO mouse are of limited value for predicting therapeutic utility in short-term clinical trials that focus on behavioural symptoms. However, many argue that the methodology to conduct robust ‘negative’ clinical trials also needs to be examined, and some results are ambiguous (such as the arbaclofen efficacy results). As discussed above, there are multiple issues that may account for the negative trials in FXS, and the negative trial results thus do not persuasively invalidate the preclinical models and drug treatments. In particular, conserved pathophysiology and treatment responses in flies and mice of different genetic backgrounds suggest the validity of these models. In FXS, clinical trials have not yet been designed to investigate the neurodevelopmental potential of these drugs.
Clinical trials
Assessing behaviour.
Behaviour is often the primary motive for referral and will remain a major objective for treatment. However, it is hypothesized that if a disease-modifying drug restores underlying neural mechanisms, the subsequent behavioural changes may be pleiotropic and may occur later in the course of the treatment. Of note, we cannot exclude the possibility that symptomatic effects can have substantial positive consequences on the disease course and result in a disease-modifying outcome. Behavioural measures used in FXS trials showed very large placebo effects. Improvement was also recorded using the ABC in previous ASD trials evaluating risperidone86,87. It is therefore unlikely that robust behavioural improvement escaped the very broad array of measures used in FXS trials. Nevertheless, further research is warranted to improve quantification of behaviour, with an emphasis on mitigating placebo effects and direct capture to avoid sole reliance on caregiver report.
Evaluating cognition.
There is consensus on cognitive domains that are crucial to FXS outcomes (such as attention and response inhibition and working memory) but not on which specific measures should be used to evaluate changes in cognition. Intensive work is underway to establish the validity, reliability and sensitivity of cognitive measures in FXS for clinical trials (such as the National Institutes of Health Toolbox Cognitive Battery and others). Related areas of research have struggled with the same issues. As an example, Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) was a multipronged effort led by the National Institute of Mental Health (NIMH) that included academia, the Food and Drug Administration (FDA) and industry to improve the assessment of cognitive impairment and its treatment in patients with schizophrenia.
Evidence of cognitive improvement has not yet been unequivocally demonstrated in RCTs that evaluate pharmacological treatments in NDDs. In ASD and attention deficit hyperactivity disorder, improvement in measures of cognitive processing (verbal learning and cancellation task) and IQ have been reported in pharmacological RCTs after 3–12 months of treatment and behavioural interventions88–91. However, these studies were conducted in individuals who were intellectually higher functioning than typical male patients with FXS. The latter often perform at or below the floor of many standardized cognitive tests, which are normalized primarily for subjects without moderate or severe intellectual disabilities. Whereas the average IQ range spans from approximately 85 to 115, the average IQ for males with FXS is estimated to be in the low 40s (4 s.d. below normal), which represents the floor of most standardized tests. New methods to properly measure cognition in populations with intellectual disability and methods for normalization of standardized tests in the intellectual disability range are required. Work is ongoing in these areas, and re-scoring of the Wechsler Intelligence Scale for Children (WISC)92 and Stanford–Binet10 for populations with intellectual disability has been completed, yielding much more sensitive estimates of true ability below the traditional test floor. Any studies using IQ measurements in FXS should use these methods to avoid data rendered uninterpretable by floor effects and improve sensitivity to the level of deficit, strengths and weaknesses, and changes in IQ over time. New measures such as Expressive Language Sampling (ELS)93 have improved the capture of conversational language improvements, which is one aspect of cognition that parents often cite as improving in pharmacological studies but has been difficult to capture in the past. ELS is a quantitative measure of the number of utterances, utterance planning, articulation, syntax and vocabulary obtained after taping and subsequently coding language in a standardized format. ELS has excellent test and retest validity after weeks, and it has been validated against the expressive language subtest of the VABS94. The ELS procedure has been used in a treatment study of intensive language intervention through distance videoconferencing by McDuffie et al.94. It is currently being used in several trials combining pharmacological treatment with behavioural intervention and training in FXS. Additional new measures — including the Kidde Test of Attentional Performance (KiTAP)95, SimpleMatrices (a visual analogical reasoning task)96 and the NIH Toolbox Cognitive Battery97 — are being adapted for use in patients with FXS and intellectual disability and are expected to enable more meaningful cognitive assessments in at least a subgroup of patients. Defining the subgroup for which these assessments are valid, and developing new measures for those too low-functioning or too young to complete these adapted measures, will be a crucial goal in order to be able to optimize outcome measures for interventional trials in very young children95.
Evaluating disease modification.
In NDDs, there is currently no consensus on what constitutes disease modification, and there is currently no definition recognized by regulatory agencies. A disease-modifying treatment implies direct targeting of causal pathophysiological processes in a manner that enduringly modifies its progression. It may be defined as an intervention, which improves the neurodevelopmental trajectory and translates into meaningful improvement of everyday functioning. To measure changes in core deficits (learning, adaptive behaviour, cognition) across developmental trajectories, one would likely need longer studies involving learning paradigms that are focused on younger patients. Cognitive remediation trials are under way and might be good platforms for measuring the effect of drugs on learning rate. Whether a drug needs to show cognitive benefit independently of a behavioural and/or educational intervention before testing its capacity to accelerate learning in the context of a specific behavioural and/or educational treatment, for example, will be an important question for investigators to consider. Several biomarkers (such as ERP and eye tracking) have been studied but have not yet been validated as core deficits and linked to quality of life or clinical measures.
It is debated, however, whether disease-modifying treatments need to directly target the underlying pathophysiological processes. It is conceivable that an effective improvement of symptoms over a sufficiently long period of time could result in long-term benefits and meaningful improvements of the developmental trajectory, irrespective of whether the treatment actually targets the core pathophysiology or not. For instance, a stimulant such as methylphenidate, which is commonly used to treat the symptoms of hyperactivity and attention deficits in FXS18, may target symptoms as well as core neurobiological deficits in FXS. Indeed, evidence at a cellular level suggests that dopamine release is dysregulated in neuronal culture in full-mutation neurons, and this is improved with the addition of either FMRP or methylphenidate to the cell culture98. Potential disease-modifying effects of sertraline, a symptomatic treatment for anxiety in children99, were recently tested in a double-blind, placebo-controlled trial in 57 children with FXS aged 2–6 years100. There were no improvements in primary outcome measures, the CGI-I or the Expressive Language subtest of the Mullen Scales of Early Learning (MSEL). However, the secondary exploratory analyses — specifically the Visual Reception and Fine Motor subtests of the MSEL — demonstrated improvements in the group receiving sertraline compared with patients receiving placebo. Post-hoc analysis demonstrated that patients with ASD and FXS receiving sertraline showed improvements in the Expressive Language subtest compared with patients receiving placebo100.
At the cellular level, it is possible, and perhaps likely, that the neurobiological rescue observed in Fmr1-KO animal models by the aforementioned targeted treatments will also occur in patients treated with the same compounds. However, outcome measures are not comparable across species. For example, the reported rescue of phenotypes such as audiogenic seizures, epileptiform bursts, open field hyperactivity and prepulse inhibition in mice does not map well onto the mechanisms underlying complex aberrant behaviours in patients with FXS as measured in the trials. Indeed, these behaviours arise in patients as a result of complex interactions between the resultant effects of FMRP deficiency on brain functioning and variability in environmental factors — such as family environment, parenting, the school or learning environment and other variables — whereas the genetic background and environmental factors in animal models are held constant. We argue that new outcome measures that tap similar neuroanatomical pathways and processes in both mice and humans are needed to potentially increase signal over noise in clinical trial analyses. These new measures — such as EEG and ERP and fMRI signatures at baseline and in response to particular stimuli, various biomarkers and cognitive tasks — would need to be similarly abnormal in patients with FXS and in the animal models, correlate with clinical aspects of the disease and be relatively stable over time. As an example, available mouse operant touchscreen paradigms seem to have face validity63,89,101–104 relative to human cognitive touchscreen tasks97, although there is concern that the cognitive profile of Fmr1-KO mice may not emulate the cognitive deficits in the human syndrome63. Similarly, the above mentioned EEG82,83 and fMRI77 recordings can be applied to both Fmr1-KO mice and patients with FXS. Clinical and preclinical scientists will need to work more collaboratively to ensure translation of animal results to human trials and backwards translation of key findings in human studies to inform development of new phenotypic measures in mice. Finally, biomarker development in patients is warranted, in particular, cellular phenotypes related to the major putative mechanisms in FXS such as protein synthesis regulation and intracellular signalling cascades (such as extracellular-signal-regulated kinase 1 (ERK1) signalling) that relate to neuro development and clinical manifestations in patients and the general population.
Designing clinical trials
Among the many challenges of clinical trials in NDDs, quality and power are particularly problematic (BOX 4). A review carried out in 2015 identified 169 trials assessing dietary interventions and drug treatments to address cognitive function in patients with 32 genetic disorders. In 44% of these studies, authors reported potential efficacy, but this led to only two approved treatments: dietary restriction for phenylketonuria and miglustat for Niemann–Pick disease type C105. The median sample size for RCTs was 25 patients (range: 2–537), and less than a third of RCTs had an acceptable Jadad score exceeding 3. These issues also apply to FXS trials, many of them being statistically underpowered and open label. Inconclusive studies may inhibit new efforts and investments in the development of novel medicines. The neurodevelopmental field will be faced with difficult choices in prioritizing the implementation of clinical trials, as many new targets and corresponding compounds will be identified by preclinical research. In the past, other medical fields tried to address similar problems by increasing the volume of trials. This approach was based on a simple hypothesis: if one drug was launched for every ten candidates entering clinical development, then doubling the number of candidates entering development should increase the number of drugs approved. In reality, research and development costs increased while the number of drugs approved remained static106,107.
Box 4 |. A framework for prioritizing clinical trials.
To increase the quality of trials in fragile X syndrome (FXS) and maintain patient safety and community engagement, we propose criteria to prioritize new clinical trials based in part on previous publications106.
Target mechanism: evidence supporting target selection is one of the most challenging aspects. Preclinical data should be reviewed using the guidelines detailed in BOX 3. Efforts to develop biomarkers should be prioritized.
Tissue and target exposure: an in-depth understanding of pharmacokinetics and pharmacodynamics is required.
Safety and risk–benefit consideration: the safety and toxicological data set needs to support the use of investigational drugs for the targeted age range and treatment duration. Juvenile toxicology studies are a mandatory requirement for paediatric clinical studies to assess the potential of unique toxic effects in younger age groups.
Trial design: given the high placebo response rate, objective performance-based outcome measures should be used, and open-label trials should be avoided except in particular instances (such as safety data or to establish the validity of an important biomarker).
Statistical power: a single well-powered study is more useful than several smaller inconclusive efforts. Exploratory outcome measures are often important aspects of phase II trials and require large sample sizes or replication. Power will represent a serious logistical and financial hurdle for future trials in FXS and other ‘genetically defined’ neurodevelopmental disorders. Adaptive multistage Bayesian Design trials are strategies that may be used in the context of dose findings, but clear end points or biomarkers are required to implement phase II and III trials. n-of-1 trials are a promising method that will also require objective and valid measures that can be extensively repeated.
A meta-analysis including all placebo-controlled, double-blinded RCTs conducted in patients with a genetic diagnosis of intellectual disability showed a placebo response with an effect size of 0.5 (moderate). This is similar to the placebo response in adult patients without intellectual disability108. Of note, placebo effects are higher in open-label studies than in placebo-controlled trials (matched on drug category) in patients with intellectual disability and a genetic diagnosis109. The certainty of receiving the real drug in open-label trials may therefore increase patients’ treatment expectations and placebo effect.
Small sample size will become a pervasive issue across NDDs with the development of precision medicine and the discovery of many contributing mechanisms involved in small groups of patients. Promising methods to deal with this problem include n-of-1 trials, which are multiple crossover (ABABABA) studies conducted in single individuals. Series of n-of-1 trials can be combined across participants, providing a substitute for traditional parallel-group RCTs. Randomization in n-of-1 trials is used to generate the order in which the study interventions are given over time. Statistical power is leveraged through repeated measures110 (between 20 and 512 in a recent review of 108 studies)111. Power remains a critical issue, and only 22% of n-of-1 studies have led to either negative or positive conclusive results. This highlights the need in the neurodevelopmental field for measures that can be repeated extensively.
In addition, phase II trials in FXS were developed without knowing which clinical or endophenotypic measures were most sensitive to the targeted mechanism. This situation can improve only with the development of translational animal models and with the use of preclinical assessments translating to clinical outcome measures. As a result, a broad range of secondary outcome measures were tested to search for a sensitive measure or subgroup of responders to inform the design of subsequent phase IIb and III trials68,71. This stepwise approach has been ineffective because most phase II studies were grossly underpowered (n ranging from 30 to 60) to adequately explore utility of the secondary measures. Conversely, the larger studies of mavoglurant, basimglurant and arbaclofen included over 100 participants and were able to provide conclusions across secondary outcome measures69. In order to achieve the level of quality and power required to draw unequivocal conclusions on the benefits of a given compound, trials will have to be conducted through large international consortia.
The risk–benefit consideration should be carefully evaluated on a case-by-case basis, taking into consideration any safety concerns, the burden for patients and caregivers, and the potential gain for the individual patient and the patient population as a whole. Testing drugs is associated with health risks even if the risks are considered acceptable in view of preclinical profiling and/or experience in healthy individuals or in patients diagnosed with a different disease. Potential benefit is different if a clinical trial can lead to the approval of a novel medicine as opposed to methodological exploration in a small open-label study.
Regulatory framework for RCTs in children
A challenge for drug development in NDDs is the very limited precedence of approved medicines compared with drugs for other indications such as schizophrenia or major depressive disorder. Thus, in the area of NDDs, the regulatory environment is less well established, leading to uncertainties in clinical trial design. Closer dialogue between the pharmaceutical industry, academic partners, patient organizations, payers and regulatory authorities (the FDA or the European Medicines Agency (EMA)) may help to attain more clarity on the regulatory requirements and pathways to evaluate the safety and efficacy of new investigational drugs for NDDs, as well as regulators’ views on the acceptability of new trial designs and outcome measures.
When to conduct trials in children?
For treatment of early-onset NDDs, it is possible that interventions starting at early developmental stages (in children under the age of 12, for instance) may achieve better overall long-term efficacy than treatments starting in adulthood. Yet safety requirements become even more crucial and complex in the long-term treatment of children, as the mechanism of action (such as inhibition of an enzyme or receptor) itself may have adverse and potentially irreversible effects.
Therefore, for new investigational drugs in non-life-threatening indications, both the FDA112 and the EMA113 usually require that safety and efficacy first be demonstrated in adults before moving to adolescents (from 12 to 16–18 years) and children (2–11 years). This procedure is built on the rationale that data obtained in adults related to safety and efficacy of drugs can be used to inform paediatric development112,113.
The extension down from adults and adolescents to an age of 5 years with basimglurant and arbaclofen and the extended open-label trial with mavoglurant were possible thanks to the safety and pharmacokinetic information available from clinical trials with the same drugs in other indications, the development of a paediatric formulation, and an extensive toxicology package including juvenile toxicological studies, as well as chronic carcinogenicity studies. The EMA recently set out a framework to evaluate when, to what extent and how data collected in adult and adolescent patients can be used to guide development in children114. In essence, the EMA will evaluate paediatric development on a case-by-case basis, taking into account the totality of available information, including scientific rationale, preclinical and clinical efficacy and safety data and the severity of the indication, as well as ethical risk–benefit considerations. This approach provides some flexibility and may facilitate an early progression into clinical paediatric studies. However, the absence of a clear default paediatric development path, and the hesitancy of regulators to commit to study plans tailored to each drug enabling paediatric clinical trials, causes some of the uncertainties that make the planning and execution of paediatric medicine development a challenge.
Longer trials in younger patients.
For the treatment of lifelong NDDs, we expect that short trials may not be sufficient to evaluate the full impact of study drugs on the developmental trajectory.
In this context, partially diverging views were expressed among our focus group. One view supports a stepwise approach starting in adults and/or adolescents with subsequently longer trials in gradually younger patients as discussed above. This rationale is based on the fact that psychotropic drugs currently used off-label in children show efficacy in adults and adolescents, and the bulk of preclinical data in FXS suggest that starting treatment in late adolescence is sufficient to achieve reversal of most phenotypes studied. Stepwise approaches present lower risks to the patients, and information about effective dose range and symptom domains sensitive to the treatment in adults also allows a refined study design for subsequent trials in children.
Others argue that the efficacy seen in adolescents and adults in the above noted studies of psychotropic medication is a supportive behavioural effect, which is not attributable to direct targeting of the disease mechanism or reversal of the developmental disorder itself. It cannot be known whether drugs that fail or show minimal short-term effects in adolescents and adults will be effective in children with a developmental disorder. If the decision to test the drug in children must always be based on a positive result in older patients, it may be impossible to ever develop successful, truly mechanistically targeted treatments in NDDs. The ultimate goal of changing the actual developmental trajectory and improving cognitive outcomes will require a paradigm shift in the strategy for drug development and registration in NDDs. An example of this process in FXS is the study in young children of mavoglurant, which already has juvenile toxicity data and PK data in children with FXS. This trial, to be conducted through the NIH-funded Neuronxt network115, will study children aged 3–6 years with a drug exposure time of over a year, while simultaneously using a uniform intensive language learning intervention and focusing on objective outcome measures for language, cognition and development, to assay potential learning enhancement by mavoglurant. The implementation of learning measures in clinical trials testing novel medicines will require validation through such exploratory trials.
The EMA has recently encouraged longer clinical trial durations to ensure that patients indeed benefit from treatment116. These long-term trials should ideally be preceded by shorter-duration exploratory studies, and double-blind, randomized, placebo-controlled, fixeddose trials are the preferred design. Trials of new investigational drugs in paediatric populations and longer trial durations require specific preclinical toxicological and safety examination as outlined below.
Preclinical safety requirements
Regulatory requirements for safety and toxicological data117,118 include information on maximum tolerated dose or exposure for any given treatment duration, the type of adverse drug effects, the target organs affected when the highest tolerated dose or exposure is exceeded, drug metabolism, pharmacokinetic properties and the potential of the drug to interact with co-medication.
The general toxicity programme for a drug is typically composed of sequential studies of increasing duration (typical increments are 4, 13, 26 and 52 weeks) in rats and a non-rodent species, ultimately leading to chronic toxicity studies of 6–24 months duration. Toxicity studies must identify both a dose or exposure level that produce no toxicological findings of concern and a dose or exposure level that causes relevant toxicological findings. The sequential approach of studies with increasing treatment duration is necessary because the maximal tolerated dose or exposure often decreases and the number and/or severity of safety-relevant findings often increases with treatment duration.
The permitted duration of clinical trials then usually correlates 1:1 with the length of the successfully completed general toxicity studies. For example, clinical trials with treatment durations of up to 6 months are possible only once general toxicity studies with a duration of 6 months have successfully demonstrated an acceptable safety margin for the anticipated therapeutic dose or drug exposure. In paediatric populations, clinical trials also require dedicated juvenile toxicological studies118,119. Unlike the tightly prescribed core of the general toxicity studies, the design of juvenile toxicological studies is typically developed in collaboration with the regulatory agencies. Because their design is informed by results from general toxicity and pharmacokinetic studies conducted in adult animals, juvenile toxicological studies are usually conducted towards the end of the comprehensive toxicological and safety programme for a given drug. Toxicological studies from start to finish take much longer than the actual treatment duration (generally 6–9 months for a study with a 4 week treatment), so it takes several years of sequential studies for the completion of the comprehensive toxicology or safety programme for a new drug.
Although options to reduce the time required for completion of the actual toxicological and safety studies for new investigational drugs are limited, procedures could be expedited by faster reviewing timelines, as well as earlier and more specific guidance from regulators. Furthermore, whereas the EMA requires sponsors to provide a detailed paediatric investigation plan (PIP; also known as paediatric study plan (PSP)) early in their development programme, streamlining the PIP process, as well as offering incentives to sponsors for the front-loading of paediatric drug development activities, could facilitate an earlier consideration of clinical trials in the paediatric population.
Selection of clinical end points
The FDA and EMA currently require phase II and phase III clinical trials to select only one assessment as the primary end point against which the success of the trial is measured. In recent FXS trials, the choice of end point was hampered by the fact that readouts from preclinical studies did not match established clinical outcome measures and by the lack of a truly mechanistic understanding of the link between the molecular and physiological disease mechanism and behavioural symptoms. Instead, the choice of clinical outcome measures was largely informed by feedback from families on disruptive behaviours and by the applicability of scales and measures to patient symptoms, as well as the acceptance of particular scales by regulators based mostly on their use in other NDD populations. In addition, summary total behaviour scores were chosen from instruments (ABC and ADAMS) with very diverse symptoms (such as depression, anxiety and mania) even though empirically derived subscales are well established. As such, treatments with prominent beneficial effects in a particular domain would likely be missed by such a heterogeneous combination of behaviours captured by a total score that has little psychometric foundation. However, for the mGluR5 antagonist trials, for example, even the focus on subscales would not have changed the outcome of the trials. In the future, confidence in the robustness of treatment effects tested on more than one end point could be increased by backing up clinical measures with validated objective surrogate outcome measures (such as eye tracking, EEG and fMRI) or target engagement biomarkers, which also increase the understanding of the processes by which a target impacts on behaviour. In addition, the robustness of detecting a treatment effect may also be increased by introducing aggregate measures composed of several individual outcome measures, thereby increasing the chance of capturing improvement of symptoms in a heterogeneous patient population with differential response to treatment.
It is expected that regulatory acceptance of novel outcome measures as primary end points will require a significant body of clinical validation in naturalistic and drug intervention studies.
Possible solutions and conclusions
It is clear that new trial designs and outcome measures need to be scientifically validated and receive regulatory acceptance to be effectively implemented in future proof-of-concept trials. This approval will require the dedicated effort of academic and industrial partners working together with regulators, as well as a combination of observational trials (that is, without drug intervention) and trials applying well-characterized interventions.
Factors that may have contributed to the negative findings reported above are not unique to research on FXS, and other medical fields are facing the same challenges. We prioritize four key points to improve the quality and validity of preclinical and clinical studies in FXS and NDD. First, drugs considered for clinical testing should be prioritized based on solid, reproducible preclinical data obtained in more than one species. Causes underlying differential response in different animal models should be valued as specific research aims. Second, clinical trials need to be double-blind, placebo-controlled and sufficiently powered. Extensive exploratory aims should be de-prioritized if the power of the trial is insufficient to make these exploratory readouts unambiguous and if the inclusion of exploratory readouts risks compromising the quality of those readouts that are the main focus of the trial. Third, demonstration of disease modification in FXS and developmental disorders may require trials in children and new trial designs; paths to registration trials in children that involve cognitive and learning outcomes and do not require prior demonstration of efficacy in older patients need to be considered and developed. Fourth, studies that investigate the issues of inter-patient variability and test–retest validation as well as regulatory acceptance of new outcome measures should be carried out in observational trials. This area of research is particularly well suited for large consortia of academic investigators.
Recently, funders and regulators have recognized that addressing these challenges requires strategic approaches and have set up initiatives to bring together academics, industry, patient organizations and other stakeholders. For example, the National Academies of Sciences, Engineering, and Medicine have organized a series of workshops to discuss opportunities for improving the integrity, efficiency and validity of clinical trials of CNS disorders, including the implementation of cutting-edge technologies in future trials120. Another example of a public–private partnership joining forces in the area of NDDs is the Innovative Medicines Initiative (IMI-2), which has set out to develop a strategic framework for the development of pharmacotherapies for ASD121. This work comprises three key stages that include: validation and qualification of biomarkers and development of objective outcome measures to test drug responses in relevant patient subgroups; development of a European-wide clinical trial network trained to good clinical practice standards to facilitate large-scale clinical trials — including trials with specific patient subgroups — and to minimize site and/or investigator effects; and finally, on the basis of clinical studies, the achievement of a better understanding of the translatability of molecular mechanisms and drug effects between different preclinical disease models.
In summary, translating the emerging knowledge on mechanisms underlying NDDs has been a challenging process. A series of large human trials in FXS were not able to demonstrate efficacy of several compounds despite a large body of data demonstrating efficacy in preclinical studies. These landmarks studies will have profound implications at every step of the drug development process for NDDs. Considerable efforts should be devoted to methods for the detection of treatment effects valid across species and neurodevelopment. It is expected that regulatory acceptance of novel study designs and primary end points will require a substantial body of clinical validation in naturalistic and drug intervention studies as well as close collaboration with the FDA and the EMA.
Mosaicism.
The presence of cell populations with a full mutation or premutation expansions. Methylation mosaicism is defined as some cells carrying fully methylated alleles and others carrying unmethylated alleles. Approximately 40% of male patients with fragile X syndrome present with size-mosaicism.
Rotarod.
A performance test in which the rodent is placed on a rotating rod to examine motor skills and coordination.
Aberrant Behaviour Checklist.
(ABC). A caregiver-rated symptom checklist that assesses problem behaviours via a 58-item and 5-subscale questionnaire. Each item is attributed a score from 0 (“not at all a problem”) to 3 (“problem is severe in degree”), resulting in total score ranks from 0 to 174.
Endophenotypes.
Phenotypes that bear a closer relationship to the biological processes underlying the clinical manifestation.
ABC-CFX Social Avoidance subscale.
The ABC-CFX is a modified version of the ABC-C, with 55 items and 6 subscales (irritability, lethargy/withdrawal, stereotypic behaviour, hyperactivity, inappropriate speech and social avoidance). The total score ranks from 0 to165, and a negative change from baseline indicates improvement.
Vineland Adaptive Behavior Scale.
(VABS). A test that measures adaptive behaviour across lifespan and contains five domains (communication, daily living skills, socialization, motor skills and maladaptive behaviour) each with 2–3 subdomains, such as expressive language.
Audiogenic seizures.
Convulsions caused by prolonged exposure to high frequency sound in, for example, rodents.
National Institutes of Health Toolbox.
A battery of extensively validated computer-administered cognitive, emotional, motor and sensory tests with utility across the lifespan.
Cancellation task.
A test of attention span in which the participants cancel the target figure and leave all other figures uncancelled (in other words, it is a test of the number of correct detections).
Wechsler Intelligence Scale for Children.
(WISC). An intelligence test for children between 6 and 16 years of age.
Stanford–Binet.
A cognitive ability and intelligence test used for individuals aged 2 to 85+ years.
Jadad score.
A score that ranks the quality of clinical trials with respect to randomization, blinding and placebo control on a score from 0–5, with 5 being the maximum score.
Open label.
A type of clinical trial in which the treatment being administered is known to both the researchers and participants.
Bayesian Design trials.
A theory of statistical inference in clinical trials.
Sequential studies.
Studies that combine longitudinal and cross-sectional designs by following several different age cohorts over time.
Test–retest validation.
A measure of reliability obtained by administering the same test twice over a period of time to a group of individuals.
Acknowledgements
The authors acknowledge the patients, caregivers and global fragile X syndrome community of scientists and investigators who contribute to the body of knowledge that continues to inform the treatment and care of this special patient population. The authors thank Spectrum and artist N. Rapp for FIG. 2. Selected research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement number 115300, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution. V.D.P. and A.C. thank the Clinical Center of Investigation (CIC), 1407 INSERM, Hospices Civils de Lyon, 69002, Lyon, France. Some work presented here has been facilitated by the Fragile X Clinical and Research Consortium (FXCRC) in the USA. E.B.K. is supported by U01NS096767. A.E.J. is the recipient of grants from Odense University Hospital Free Research Fund (grant number 15-A857) and of the Region of Zealand and Region of Southern of Denmark joint research fund (journal no. 14-001308) as well as a PhD scholarship from the Region of Southern of Denmark and the Faculty of Health Sciences, University of Southern of Denmark. M.F.B. acknowledges past and ongoing support and encouragement from FRAXA and NIH grant number R01-MH106469. J.N.C. is the recipient of NIH grant numbers R01NS085709 and U54HD079125-04 and Autism Speaks PACT targeted grant number 8603. E.L. is supported by EU-AIMS (European Autism Interventions), which receives support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115300, the resources of which are composed of financial contributions from the European Union’s Seventh Framework Programme (grant FP7/2007-2013), from the European Federation of Pharmaceutical Industries and Associations. R.H. is funded by The MIND Institute IDDRC U54 HD079125, HRSA grants R40MC22641 and R40MC27701 and DOD PR101054. S.J. is the recipient of a Bursary Professor fellowship of the Swiss National Science Foundation (SNSF), the Jeanne et Jean Louis Levesque Research Chair and the Canadian Research Chair.
Footnotes
Competing interests statement
The authors declare competing interests: see Web version for details.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oberle I et al. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252, 1097–1102 (1991). [DOI] [PubMed] [Google Scholar]
- 2.Pieretti M et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66, 817–822 (1991). [DOI] [PubMed] [Google Scholar]; References 1 and 2 are seminal papers of the simultaneous discovery of FMR1 by several research groups.
- 3.Handt M et al. Point mutation frequency in the FMR1 gene as revealed by fragile X syndrome screening. Mol. Cell Probes 28, 279–283 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Dykens EM, Hodapp RM & Leckman JF Strengths and weaknesses in the intellectual functioning of males with fragile X syndrome. Am. J. Ment. Defic 92, 234–236 (1987). [PubMed] [Google Scholar]
- 5.Fisch GS et al. Longitudinal changes in cognitive-behavioral levels in three children with FRAXE. Am. J. Med. Genet 84, 291–292 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Klaiman C et al. Longitudinal profiles of adaptive behavior in fragile X syndrome. Pediatrics 134, 315–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgardner TL, Reiss AL, Freund LS & Abrams MT Specification of the neurobehavioral phenotype in males with fragile X syndrome. Pediatrics 95, 744–752 (1995). [PubMed] [Google Scholar]
- 8.Mazzocco MM, Pennington BF & Hagerman RJ The neurocognitive phenotype of female carriers of fragile X: additional evidence for specificity. J. Dev. Behav. Pediatr 14, 328–335 (1993). [PubMed] [Google Scholar]
- 9.Kaufmann WE, Abrams MT, Chen W & Reiss AL Genotype, molecular phenotype, and cognitive phenotype: correlations in fragile X syndrome. Am. J. Med. Genet 83, 286–295 (1999). [PubMed] [Google Scholar]
- 10.Sansone SM et al. Improving IQ measurement in intellectual disabilities using true deviation from population norms. J. Neurodev. Disord 6, 16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vries BB et al. Mental status of females with an FMR1 gene full mutation. Am. J. Hum. Genet 58, 1025–1032 (1996). [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis P et al. Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. J. Intellect. Disabil. Res 50, 532–545 (2006). [DOI] [PubMed] [Google Scholar]
- 13.McDuffie A et al. Autism spectrum disorder in children and adolescents with fragile X syndrome: within-syndrome differences and age-related changes. Am. J. Intellect. Dev. Disabil 115, 307–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iossifov I et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darnell JC et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified a stringent set of 842 FMRP target transcripts, many of them associated with neuropsychiatric disorders. This highlights the fact that FMRP has broad regulatory roles at the synapse.
- 16.Hagerman RJ, Murphy MA & Wittenberger MD A controlled trial of stimulant medication in children with the fragile X syndrome. Am. J. Med. Genet 30, 377–392 (1988). [DOI] [PubMed] [Google Scholar]
- 17.Ingrassia A & Turk J The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders — a case series. Eur. Child Adolesc. Psychiatry 14, 34–40 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Hagerman RJ et al. Advances in the treatment of fragile X syndrome. Pediatrics 123, 378–390 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erickson CA, Stigler KA, Posey DJ & McDougle CJ Aripiprazole in autism spectrum disorders and fragile X syndrome. Neurotherapeutics 7, 258–263 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bear MF, Huber KM & Warren ST The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 (2004). [DOI] [PubMed] [Google Scholar]; This is one of the seminal papers to propose a role for FMRP in the regulation of synaptic function and plasticity through regulation of long-term depression of synaptic strength involving stimulation of mGluR5. Excessive mGluR5 signalling has been targeted in several studies in rodents and humans.
- 21.Weiler IJ et al. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc. Natl Acad. Sci. USA 94, 5395–5400 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashley CT Jr, Wilkinson KD, Reines D & Warren ST FMR1 protein: conserved RNP family domains and selective RNA binding. Science 262, 563–566 (1993). [DOI] [PubMed] [Google Scholar]
- 23.Li Z et al. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 29, 2276–2283 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber KM, Gallagher SM, Warren ST & Bear MF Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl Acad. Sci. USA 99, 7746–7750 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auerbach BD & Bear MF Loss of the fragile X mental retardation protein decouples metabotropic glutamate receptor dependent priming of long-term potentiation from protein synthesis. J. Neurophysiol 104, 1047–1051 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dölen G et al. Correction of fragile X syndrome in mice. Neuron 56, 955–962 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates in the fragile X mouse model the rescue of a range of phenotypes relevant to the human disorder by reducing mGluR5 expression.
- 27.Gasparini F et al. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 38, 1493–1503 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Porter RH et al. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J. Pharmacol. Exp. Ther 315, 711–721 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Lindemann L et al. CTEP: a novel, potent, long-acting, and orally bioavailable metabotropic glutamate receptor 5 inhibitor. J. Pharmacol. Exp. Ther 339, 474–486 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Gantois I et al. Chronic administration of AFQ056/Mavoglurant restores social behaviour in Fmr1 knockout mice. Behav. Brain Res 239, 72–79 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Gross C et al. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J. Neurosci 30, 10624–10638 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michalon A et al. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 74, 49–56 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported efficacy of a long-acting mGluR5 antagonist in adult Fmr1-KO mice. The comprehensive phenotype correction after development of the phenotype sparked great interest for testing mGluR5 antagonists in adult and adolescent patients with FXS.
- 33.Osterweil EK, Krueger DD, Reinhold K & Bear MF Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J. Neurosci 30, 15616–15627 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pop AS et al. Rescue of dendritic spine phenotype in Fmr1 KO mice with the mGluR5 antagonist AFQ056/Mavoglurant. Psychopharmacology 231, 1227–1235 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Yan QJ, Rammal M, Tranfaglia M & Bauchwitz RP Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49, 1053–1066 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Gassmann M & Bettler B Regulation of neuronal GABA(B) receptor functions by subunit composition. Nat. Rev. Neurosci 13, 380–394 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Henderson C et al. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofen. Sci. Transl Med 4, 152ra128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman JL et al. GABAB receptor agonist R-baclofen reverses social deficits and reduces repetitive behavior in two mouse models of autism. Neuropsychopharmacology 40, 2228–2239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin M et al. R-Baclofen reverses a social behavior deficit and elevated protein synthesis in a mouse model of fragile X syndrome. Int. J. Neuropsychopharmacol 18, pyv034 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mann K, Kiefer F, Spanagel R & Littleton J Acamprosate: recent findings and future research directions. Alcohol. Clin. Exp. Res 32, 1105–1110 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Schaefer TL et al. Acamprosate in a mouse model of fragile X syndrome: modulation of spontaneous cortical activity, ERK1/2 activation, locomotor behavior, and anxiety. J. Neurodev. Disord 9, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Hulst C et al. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS). Brain Res. 1253, 176–183 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Braat S et al. The GABAA receptor is an FMRP target with therapeutic potential in fragile X syndrome. Cell Cycle 14, 2985–2995 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter RB et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J. Pharmacol. Exp. Ther 280, 1284–1295 (1997). [PubMed] [Google Scholar]
- 45.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01725152 (2016).
- 46.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01911455 (2016).
- 47.Mak IW, Evaniew N & Ghert M Lost in translation: animal models and clinical trials in cancer treatment. Am. J. Transl Res 6, 114–118 (2014). [PMC free article] [PubMed] [Google Scholar]
- 48.Lee MJ et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc. Natl Acad. Sci. USA 109, 7859–7864 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhattacharya A et al. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron 76, 325–337 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bilousova TV et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J. Med. Genet 46, 94–102 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Boda B, Mendez P, Boury-Jamot B, Magara F & Muller D Reversal of activity-mediated spine dynamics and learning impairment in a mouse model of Fragile X syndrome. Eur. J. Neurosci 39, 1130–1137 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Busquets-Garcia A et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat. Med 19, 603–607 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Dolan BM et al. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc. Natl Acad. Sci. USA 110, 5671–5676 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gross C et al. Increased expression of the PI3K enhancer PIKE mediates deficits in synaptic plasticity and behavior in fragile X syndrome. Cell Rep. 11, 727–736 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayashi ML et al. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc. Natl Acad. Sci. USA 104, 11489–11494 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hebert B et al. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule. Orphanet J. Rare Dis 9, 124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas AM et al. Genetic reduction of group 1 metabotropic glutamate receptors alters select behaviors in a mouse model for fragile X syndrome. Behav. Brain Res 223, 310–321 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian M et al. 7, 8-Dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome. Neuropharmacology 89, 43–53 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Udagawa T et al. Genetic and acute CPEB1 depletion ameliorate fragile X pathophysiology. Nat. Med 19, 1473–1477 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laura J, Stoppel EKO & Bear MF in Fragile X Syndrome From Genetics to Targeted Treatment (eds Willemsen R& Kooy FR) 173–204 (Academic Press, 2017). [Google Scholar]
- 61.Gross C, Hoffmann A, Bassell GJ & Berry-Kravis EM Therapeutic strategies in fragile X syndrome: from bench to bedside and back. Neurotherapeutics 12, 584–608 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kazdoba TM, Leach PT, Silverman JL & Crawley JN Modeling fragile X syndrome in the Fmr1 knockout mouse. Intractable Rare Dis. Res 3, 118–133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leach PT, Hayes J, Pride M, Silverman JL & Crawley JN Normal performance of Fmr1 mice on a touchscreen delayed nonmatching to position working memory task. eNeuro 10.1523/eneuro.0143-15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Esch CE et al. Fragile X mice have robust mGluR5-dependent alterations of social behaviour in the automated tube test. Neurobiol. Dis 75, 31–39 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Westmark CJ et al. Reversal of fragile X phenotypes by manipulation of AβPP/Aβ levels in Fmr1-KO mice. PLoS ONE 6, e26549 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindemann L et al. Pharmacology of basimglurant (RO4917523, RG7090), a unique metabotropic glutamate receptor 5 negative allosteric modulator in clinical development for depression. J. Pharmacol. Exp. Ther 353, 213–233 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Vranesic I et al. AFQ056/mavoglurant, a novel clinically effective mGluR5 antagonist: identification, SAR and pharmacological characterization. Bioorg. Med. Chem 22, 5790–5803 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Berry-Kravis EM et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci. Transl Med 4, 152ra127 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Berry-Kravis E et al. Mavoglurant in fragile X syndrome: results of two randomized, double-blind, placebo-controlled trials. Sci. Transl Med 8, 321ra325 (2016). [DOI] [PubMed] [Google Scholar]; This article presents the results of two of the largest randomized, placebo-controlled studies undertaken in adolescents and adults with FXS. These studies did not show efficacy of mavoglurant, but they have stimulated further study of mavoglurant in younger children with FXS.
- 70.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01483469 (2016).
- 71.Jacquemont S et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci. Transl Med 3, 64ra61 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Berry-Kravis E et al. Arbaclofen in fragile X syndrome: results of phase 3 trials. J. Neurodev. Disord 9, 3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Youssef EA et al. Effect of the mGluR5-NAM basimglurant on behavior in adolescents and adults with fragile X syndrome in a randomized, double-blind, placebo-controlled trial: fragXis phase 2 results. Neuropsychopharmacology 10.1038/npp.2017.177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This reference reports the large randomized, double-blind, placebo-controlled phase II trial with the mGluR5 antagonist basimglurant in adult and adolescent patients with FXS.
- 74.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01750957 (2016).
- 75.Caserta MS, Lund DA & Wright SD Exploring the caregiver burden inventory (CBI): further evidence for a multidimensional view of burden. Int. J. Aging Hum. Dev 43, 21–34 (1996). [DOI] [PubMed] [Google Scholar]
- 76.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01015430 (2016).
- 77.Michalon A et al. Chronic metabotropic glutamate receptor 5 inhibition corrects local alterations of brain activity and improves cognitive performance in fragile X mice. Biol. Psychiatry 75, 189–197 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Silva-Santos S et al. Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model. J. Clin. Invest 125, 2069–2076 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barnes SA et al. Convergence of hippocampal pathophysiology in syngap+/− and Fmr1-/y mice. J. Neurosci 35, 15073–15081 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ehninger D et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat. Med 14, 843–848 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zu T et al. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J. Neurosci 24, 8853–8861 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Radwan B, Dvorak D & Fenton AA Impaired cognitive discrimination and discoordination of coupled theta-gamma oscillations in Fmr1 knockout mice. Neurobiol. Dis 88, 125–138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ethridge LE et al. Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in fragile X syndrome. Transl Psychiatry 6, e787 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scharf SH, Jaeschke G, Wettstein JG & Lindemann L Metabotropic glutamate receptor 5 as drug target for fragile X syndrome. Curr. Opin. Pharmacol 20, 124–134 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Scott S et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph. Lateral Scler 9, 4–15 (2008). [DOI] [PubMed] [Google Scholar]
- 86.Dove D et al. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 130, 717–726 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCracken JT et al. Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med 347, 314–321 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Aman MG et al. Cognitive effects of risperidone in children with autism and irritable behavior. J. Child Adolesc. Psychopharmacol 18, 227–236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dawson G et al. Randomized, controlled trial of an intervention for toddlers with autism: the early start denver model. Pediatrics 125, e17–e23 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Estes A et al. Long-term outcomes of early intervention in 6-year-old children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 54, 580–587 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gillberg C et al. Long-term stimulant treatment of children with attention-deficit hyperactivity disorder symptoms. A randomized, double-blind, placebo-controlled trial. Arch. Gen. Psychiatry 54, 857–864 (1997). [DOI] [PubMed] [Google Scholar]
- 92.Hessl D et al. A solution to limitations of cognitive testing in children with intellectual disabilities: the case of fragile X syndrome. J. Neurodev. Disord 1, 33–45 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berry-Kravis E et al. Development of an expressive language sampling procedure in fragile X syndrome: a pilot study. J. Dev. Behav. Pediatr 34, 245–251 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McDuffie A et al. A spoken-language intervention for school-aged boys with fragile X syndrome. Am. J. Intellect. Dev. Disabil 121, 236–265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knox A et al. Feasibility, reliability, and clinical validity of the test of attentional performance for children (KiTAP) in fragile X syndrome (FXS). J. Neurodev. Disord 4, 2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Curie A et al. A novel analog reasoning paradigm: new insights in intellectually disabled patients. PLoS ONE 11, e0149717 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hessl D et al. The NIH toolbox cognitive battery for intellectual disabilities: three preliminary studies and future directions. J. Neurodev. Disord 8, 35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang LW, Berry-Kravis E & Hagerman RJ Fragile X: leading the way for targeted treatments in autism. Neurotherapeutics 7, 264–274 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giles LL & Martini DR Challenges and promises of pediatric psychopharmacology. Acad. Pediatr 16, 508–518 (2016). [DOI] [PubMed] [Google Scholar]
- 100.Greiss Hess L et al. A randomized, double-blind, placebo-controlled trial of low-dose sertraline in young children with fragile X syndrome. J. Dev. Behav. Pediatr 37, 619–628 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brigman JL, Bussey TJ, Saksida LM & Rothblat LA Discrimination of multidimensional visual stimuli by mice: intra- and extradimensional shifts. Behav. Neurosci 119, 839–842 (2005). [DOI] [PubMed] [Google Scholar]
- 102.Brigman JL, Graybeal C & Holmes A Predictably irrational: assaying cognitive inflexibility in mouse models of schizophrenia. Front. Neurosci 10.3389/neuro.01.013.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bussey TJ et al. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology 62, 1191–1203 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bussey TJ et al. The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn. Mem 15, 516–523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Vaart T, Overwater IE, Oostenbrink R, Moll HA & Elgersma Y Treatment of cognitive deficits in genetic disorders: a systematic review of clinical trials of diet and drug treatments. JAMA Neurol 72, 1052–1060 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Cook D et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat. Rev. Drug Discov 13, 419–431 (2014). [DOI] [PubMed] [Google Scholar]
- 107.Pammolli F, Magazzini L & Riccaboni M The productivity crisis in pharmaceutical R&D. Nat. Rev. Drug Discov 10, 428–438 (2011). [DOI] [PubMed] [Google Scholar]
- 108.Curie A et al. Placebo responses in genetically determined intellectual disability: a meta-analysis. PLoS ONE 10, e0133316 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jensen KB et al. Certainty of genuine treatment increases drug responses among intellectually disabled patients. Neurology 88, 1912–1918 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Punja S et al. N-Of-1 trials are a tapestry of heterogeneity. J. Clin. Epidemiol 76, 47–56 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Gabler NB, Duan N, Vohra S & Kravitz RL N-Of-1 trials in the medical literature: a systematic review. Med. Care 49, 761–768 (2011). [DOI] [PubMed] [Google Scholar]
- 112.US Food and Drug Administration. Specific requirements on content and format of labelling for human prescription drugs: revision of ‘pediatric’ use’ subsection in the labelling: final rule. FDA https://www.fda.gov/ohrms/dockets/ac/01/briefing/3778b1_Tab6_7-21CFR%20Part%20201.pdf (1994).] [Google Scholar]
- 113.European Medicines Agency. ICH topic E 11 clinical investigation of medicinal products in the paediatric population. European Medicines Agency http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002926.pdf (2001). [Google Scholar]
- 114.European Medicines Agency. Extrapolation of efficacy and safety in paediatric medicine development. European Medicines Agency http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_001367.jsp&mid=WC0b01ac0580029572 (2016). [Google Scholar]
- 115.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02920892 (2017).
- 116.European Medicines Agency. Guideline on the clinical development of medicinal products for the treatment of autism spectrum disorder (ASD). European Medicines Agency http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/03/WC500202650.pdf (2013). [Google Scholar]
- 117.US Food and Drug Administration. Pharmacology/toxicology. FDA http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm065014.htm (2017). [Google Scholar]
- 118.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Safety guidelines. ICH http://www.ich.org/products/guidelines/safety/article/safety-guidelines.html (2017). [Google Scholar]
- 119.US Food and Drug Administration. Guidance for industry: nonclinical safety evaluation of pediatric drug products. FDA http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm079247.pdf (2006). [Google Scholar]
- 120.National Academies of Sciences, Engineering and Medicine. Neuroscience Trials of the Future: Proceedings of a Workshop (The National Academies Press, 2016). [PubMed] [Google Scholar]
- 121.Innovative Medicines Initiative. IMI2 10 th call for proprosals. Innovative Medicines Initiative http://www.imi.europa.eu/sites/default/files/uploads/documents/IMI2Call10/IMI2_Call10_TopicsText.pdf (2016). [Google Scholar]
- 122.Osterweil EK et al. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model. Neuron 77, 234–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02642653 (2016).
- 124.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02680379 (2016).
- 125.Gantois I et al. Metformin ameliorates core deficits in a mouse model of fragile X syndrome. Nat. Med 23, 674–677 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Sidhu H, Dansie LE, Hickmott PW, Ethell DW & Ethell IM Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J. Neurosci 34, 9867–9879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chiu CT & Chuang DM Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol. Ther 128, 281–304 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu ZH et al. Lithium reverses increased rates of cerebral protein synthesis in a mouse model of fragile x syndrome. Neurobiol. Dis 45, 1145–1152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuskaitis CJ et al. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem. Pharmacol 79, 632–646 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berry-Kravis E et al. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J. Dev. Behav. Pediatr 29, 293–302 (2008). [DOI] [PubMed] [Google Scholar]
- 131.Braithwaite SP et al. Synaptic plasticity: one STEP at a time. Trends Neurosci. 29, 452–458 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goebel-Goody SM et al. Taking STEPs forward to understand fragile x syndrome. Results Probl. Cell Differ 54, 223–241 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goebel-Goody SM et al. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 11, 586–600 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klann E & Dever TE Biochemical mechanisms for translation regulation in synaptic plasticity. Nat. Rev. Neurosci 5, 931–942 (2004). [DOI] [PubMed] [Google Scholar]
- 135.Narayanan U et al. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J. Biol. Chem 283, 18478–18482 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bhattacharya A et al. Targeting translation control with p70 S6 kinase 1 inhibitors to reverse phenotypes in fragile X syndrome mice. Neuropsychopharmacology 41, 1991–2000 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kano M et al. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev 89, 309–380 (2009). [DOI] [PubMed] [Google Scholar]
- 138.Gomis-Gonzalez M, Matute C, Maldonado R, Mato S & Ozaita A Possible therapeutic doses of cannabinoid type 1 receptor antagonist reverses key alterations in fragile X syndrome mouse model. Genes 7, E56 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xie S et al. The endocannabinoid system and rimonabant: a new drug with a novel mechanism of action involving cannabinoid CB1 receptor antagonism — or inverse agonism — as potential obesity treatment and other therapeutic use. J. Clin. Pharm. Ther 32, 209–231 (2007). [DOI] [PubMed] [Google Scholar]
- 140.Christensen R et al. Efficacy and safety of the weight-loss drug rimonabrant: a meta analysis of randomised trials. Lancet 370, 1706–1713 (2007). [DOI] [PubMed] [Google Scholar]
- 141.[No authors listed.] Rimonabant: suicide and depression. Depression and suicidal tendencies are about twice as frequent with rimonabant as with placebo. Prescrire Int 16, 250 (2007). [PubMed] [Google Scholar]
- 142.Chen LY et al. Physiological activation of synsptic Rac>PAK (p-21activated kinase) signalling is defective in a mouse model of fragile X syndrome. J. Neurosci 30, 10977–10984 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Henley JM & Wilkinson KA Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci 17, 337–350 (2016). [DOI] [PubMed] [Google Scholar]
- 144.Lee K et al. AMPA receptors as therapeutic targets for neurological disorders. Adv. Protein Chem. Struct. Biol 103, 203–261 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Arai AC & Kessler M Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr. Drug Targets 8, 583–602 (2007). [DOI] [PubMed] [Google Scholar]
- 146.Li J et al. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol. Cell. Neurosci 19, 138–151 (2002). [DOI] [PubMed] [Google Scholar]
- 147.Berry-Kravis E et al. Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: a controlled trial. J. Child Adolesc. Psychopharmacol 16, 525–540 (2006). [DOI] [PubMed] [Google Scholar]
- 148.Davenport MH, Schaefer TL, Friedmann KJ, Fitzpatrick SE & Erickson CA Pharmacotherapy for fragile X syndrome: progress to date. Drugs 76, 431–445 (2016). [DOI] [PubMed] [Google Scholar]
- 149.Ligsay A & Hagerman RJ Review of targeted treatments in fragile X syndrome. Intractable Rare Dis. Res 5, 158–167 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Berry-Kravis E et al. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J. Med. Genet 46, 266–271 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01348087 (2016).
- 152.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01433354 (2016).
- 153.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01894958 (2017).
- 154.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02126995 (2016).
- 155.Paribello C et al. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 10, 91 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leigh MJ et al. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile x syndrome. J. Dev. Behav. Pediatr 34, 147–155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01013480 (2012).
- 158.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01555333 (2013).
- 159.Erickson CA et al. Impact of acamprosate on behavior and brain-derived neurotrophic factor: an open-label study in youth with fragile X syndrome. Psychopharmacology 228, 75–84 (2013). [DOI] [PubMed] [Google Scholar]
- 160.Kesler SR, Lightbody AA & Reiss AL et al. Cholinergic dysfunction in fragile X syndrome and potenial intervention. Am. J. Med. Genet. A 149A, 403–407 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ligsay A et al. A randomized double-blind, placebo-controlled trial of ganaxolone in children and adolescents with fragile X syndrome. J. Neurodev. Disord 9, 26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01120626 (2016).
- 163.Sahu JK et al. Effectiveness and safety of donepezil in boys with fragile x syndrome: a double-blind, randomized, controlled pilot study. J. Child Neurol 28, 570–575 (2013). [DOI] [PubMed] [Google Scholar]