Abstract
Background
Peanut allergy causes severe and fatal reactions. Current food allergen labelling fails to address these risks adequately against the burden of restricting food choice for allergic individuals because of limited data on thresholds of reactivity and the influence of everyday factors.
Objective
We estimated peanut threshold doses for a UK peanut-allergic population and examined the effect of sleep deprivation and exercise.
Methods
In a crossover study, following blinded challenge, peanut-allergic participants underwent three open peanut challenges in random order: with exercise following each dose, with sleep deprivation preceding challenge, and with no intervention. Primary outcome was the threshold dose triggering symptoms (mg protein). Primary analysis estimated the difference between non-intervention challenge and each intervention in log threshold (as % change). Dose distributions were modelled deriving eliciting doses in the peanut-allergic population.
Result
Baseline challenges were performed in 126 subjects, 100 were randomized and 81 (mean age 25y) completed at least one further challenge. The mean (SD) threshold was 214 mg (330mg) for non-intervention challenges and this was reduced by 45% (95% confidence interval 21,61 p=0.001) and 45% (22,62 p=0.001) for exercise and sleep deprivation, respectively. Mean (95% confidence interval) estimated eliciting doses for 1% of the population were 1.5mg (0.8,2.5) during non-intervention challenge (n=81), 0.5mg (0.2,0.8) following sleep and 0.3mg (0.1,0.6) following exercise.
Conclusion
Exercise and sleep deprivation each significantly reduce the threshold of reactivity in people with peanut allergy, putting them at greater risk of a reaction. Adjusting reference doses using these data will improve allergen risk-management and labelling to optimize protection of peanut-allergic consumers.
ClinicalTrials.gov Identifier: NCT01429896
Keywords: Peanut, allergy, thresholds, exercise and sleep deprivation
Introduction
IgE-mediated peanut allergy is a significant public health concern, being the commonest cause of severe and sometimes fatal allergic reactions to food.(1,2) The current standard of care for the management of peanut allergy is complete avoidance of peanut(3) but this is difficult to achieve and inadvertent reactions are common. To assist peanut-allergic individuals in the safe management of their allergy, the presence of food allergen can be indicated on food labelling. The labelling of deliberately added allergens as ingredients is legally mandated in the European Union and United States. However, allergens can also accidentally contaminate foods during production methods and some manufacturers utilise voluntary precautionary allergen labelling (PAL), such as ‘May contain traces of…’, warning patients about allergen contamination. Studies show that PAL may bear no relationship to the presence of allergen, with some PAL labelled foods containing no allergen at all, and other unlabelled foods containing residual amounts of allergen.(4,5) These confusing and vague statements affect peanut-allergic individuals, restricting their food choices and impairing their quality of life.(6)
The identification of reference doses for food allergens considered safe for the majority of food allergic individuals, would inform risk assessment and provide guidance on when PAL should be used. A consensus on levels of allergens that are low risk is lacking. Studies on doses of allergen which elicit reactions in allergic individuals have been performed and attempts have been made using dose distribution modelling to define doses of allergenic protein which are likely to elicit a reaction in a proportion of the population. Recently, single dose challenges have been used to validate these doses helping to move the debate forward,(7) but concerns remain about the general applicability of such levels and how they might be modified by everyday lifestyle factors (co-factors).(8) The involvement of sleep deprivation as a co-factor in modulating allergic reactions has so far relied on anecdotal reports as well as retrospective surveys of individuals suffering from anaphylaxis which is subject to recall bias. There is good evidence that exercise may exacerbate allergic reactions to wheat and other foods although this has not been formally explored in relation to peanut(9),(10) There are also indications from peanut immunotherapy studies that co-factors may be responsible for a loss of tolerance during maintenance therapy.(11) Food challenges from which threshold data are derived are usually performed under ‘ideal’ test conditions that do not reflect everyday exposure conditions.(12) Furthermore, the effects of co-factors have not been investigated in a prospective study. If co-factors can affect the threshold dose at which allergic reactions are elicited, then there is a need to account for this in population threshold modelling. Our aims were to conduct a robust, prospective examination of the threshold of peanut reactivity in allergic adults and examine the influence of each of two important co-factors, exercise and sleep deprivation.
Methods
Trial design
A multicentre randomised crossover study was performed between 2013 and June 2016 at the NIHR/Welcome Trust Cambridge Clinical Research Facility (Cambridge, UK) and Royal Brompton & Harefield NHS Foundation Trust Clinical Research Facility (London, UK). Following confirmation of allergy by a double-blind, placebo-controlled (DBPC) peanut challenge (baseline challenge), eligible participants underwent three further open peanut challenges in a randomly assigned, balanced order: one with exercise, one with sleep deprivation on the night preceding the challenge and one with neither intervention (termed non-intervention).
Participants
Participants were recruited from the UK general adult peanut-allergic population both nationally (through advertisements in the media and through national patient support groups, (Anaphylaxis Campaign and Allergy UK)) and locally (allergy clinics and local media). Interested participants registered on the study website where they were asked initial screening questions about their allergy. Eligible participants underwent telephone screening. If they fulfilled criteria on pre-screening, they were invited for face to face screening visit. Participants were included in the study if they were aged 18-45 years with a history of an immediate systemic allergic reaction after peanut ingestion with evidence of sensitisation to peanut and the diagnosis confirmed by positive DBPC peanut challenge. Sensitisation was defined as a positive skin prick test to peanut (extract ALK-Abello, Hørsholm, Denmark), skin weal of ≥3mm greater than the negative control or serum specific IgE to peanut >0.35 kUA/L (ImmunoCAP). Volunteers were excluded if they gave a history suggestive solely of oral allergy syndrome to peanut (a different, milder disorder). They were also excluded if they had previous life-threatening reactions to peanut, poorly controlled asthma, a significant drop in lung function with exercise or a diagnosis of mastocytosis. A full list of inclusion and exclusion criteria is included in the supplementary material (Table E1).
The study was approved by the national research ethics (NRES) committee East of England (12 EE02/89) and performed with each participant’s written informed consent. The UK Food Standards Agency funded the study.
Randomisation
The baseline challenge consisted of one active peanut and one placebo challenge on separate days, at an interval of one week. The order of these challenges was randomly assigned, and both the participant and investigator were blinded to the order. Participants then underwent three further challenges at three-month intervals in a randomised open fashion. Two of the challenges were interventional; one combined with exercise and one following sleep deprivation prior to the day of the challenge. A third challenge with no intervention was also undertaken, and termed the ‘non-intervention’ challenge. The randomised challenge sequence for each patient was determined using a secure online tool with audit trail (randomizer.au). Randomization was to one of six blocks containing all permutations of challenge combinations. Randomization was stratified according to centre, age and presence of asthma.
Food challenges
Prior to the commencement of challenges participants were physically examined and the control of co-existent atopic conditions was assessed using the Asthma Control Test(13) and spirometry for asthma, the POEM score for eczema(14) and Total Nasal Symptom score for rhinitis. Challenges were postponed if these conditions were inadequately controlled or if the patient was unwell with an infective illness. The challenges were undertaken using a harmonised protocol in accordance with best practice where participants ingested increasing doses of the validated Europrevall dessert food matrix(12) either alone (placebo) or containing peanut allergen (active, 12.5% fat, light roast peanut flour from the Golden Peanut Company, Alphretta, GA, USA) until they developed an objective allergic reaction (definition below). An unblinded scientist with no interaction with the participant or the study team was responsible for the randomisation of subjects and preparation of the challenge material. During the active and intervention challenges participants consumed increasing doses of peanut protein in the form of partially defatted peanut flour in a challenge matrix. The dosing regimen was: 3µg, 30µg, 300µg, 3mg, 30mg, 100mg, 300mg and 1g peanut protein (1 gram peanut protein is equivalent to approximately 8 peanuts) (15). The doses were delivered at 30-minute intervals although the investigator could extend the interval to a maximum of 1 hour if symptoms were evolving. A dose could be repeated if the participant was nearing their threshold and the investigator deemed it appropriate not to escalate by a full dose increment. Challenges were performed in a harmonised manner across both centres using a common approach to score and stop challenges with site training. Using a modified version of the PRACTALL criteria (11), symptoms were assigned a green, yellow or red colour code (Table E2). Challenges were stopped when participants reached an objective threshold of 3 concurrent yellow symptoms or one red symptom. After piloting the established PRACTALL challenge criteria on 6 participants (data not shown) it was decided by Trial Steering Committee consensus that further refinement of the criteria was needed to enhance safety. Greater discrimination was added to lower respiratory symptoms defining milder airway symptoms and peak flow was incorporated as a functional measurement to detect rapid progression to severe symptoms. Gastrointestinal symptoms were also further defined in terms of their persistence (>/=30minutes). Detail on the modification of the criteria are shown in Table E2. Participants who developed symptoms were given treatment as appropriate. The intervention challenges were run in the same way, and modified as follows. The exercise challenge regimen, optimised during pilot testing, consisted of a 10-minute bout of exercise on a static bike at an intensity of 85% VO2 max (maximum exercise capacity, determined during screening) 5 minutes after each dose. In London the investigator supervised the challenge and exercise, whereas in Cambridge the investigator supervised the challenge and a physiologist supervised the exercise. For the sleep deprivation challenge, participants were admitted to the research ward on the night preceding the challenge and were allowed to sleep for a maximum of 2 hours and then kept awake until the challenge. Prior to the challenges, participants were encouraged to keep a sleep diary and if they had experienced a disruption to their normal sleep pattern (<30% normal sleep in the two weeks preceding the challenge) appointments were postponed. The non-intervention challenge was run in exactly the same way as the initial challenges, except that, like the Interventional Challenges, the challenge was open, with only one ‘active’ challenge taking place (see Protocol Changes section).
Outcomes
The primary outcome was the peanut threshold in each individual (or dose triggering symptoms) and defined as the Lowest Observed Adverse Effect Level (LOAEL), the lowest cumulative dose that causes an objective allergic reaction (defined below). This was measured in mg peanut protein and summarised by challenge type and timing of challenge. As secondary outcomes, threshold dose distribution curves were derived for the different challenge types and probability distribution modelling was used to determine population thresholds, the cumulative dose of peanut protein predicted to provoke reactions in different percentages of the peanut-allergic population (Eliciting Dose-EDx%). The number and type of adverse events were reported. A summary of terms and their definitions are detailed in Table I.
Table I. Terms and their definitions.
| Term | Definition |
|---|---|
| Primary Outcome | Peanut threshold or lowest observed adverse effect level (LOAEL) which is the lowest cumulative dose causing an objective allergic reaction. Determined for each individual in mg peanut protein following each challenge |
| Primary Analysis | Difference in log-threshold between non-intervention challenge and each intervention challenge also expressed as percentage change |
| Secondary outcome | Eliciting dose (EDx) or population threshold cumulative eliciting dose (ED) predicted to provoke a reaction in a defined proportion of the population (x) |
| Full analysis population | Individuals who received at least one post baseline intervention challenge |
| Extended analysis population | All individuals who received a baseline peanut challenge |
| Baseline challenge | Initial double-blind placebo-controlled challenge to confirm diagnosis of peanut allergy |
| Non-intervention challenge | Open challenge to determine threshold when no intervention applied |
| Intervention challenge | Open challenge to determine threshold with either exercise or sleep deprivation intervention |
Reaction severity was not measured as a pre-planned main outcome in this study. However, a detailed post-hoc analysis of reaction severity and symptom pattern and discussion of development of a severity score will be reported in a separate manuscript.
Analysis populations
The primary analysis population was the full-analysis set, which was defined as all participants who had completed at least one post-baseline challenge. Analyses on the per-protocol population, defined as participants who completed all three post-baseline challenges were also performed (data not shown). The extended analysis set consisted of all patients who received a baseline challenge. The safety population consisted of all participants who underwent at least one challenge.
Sample size
As there were no published data on intra-individual variation in thresholds over time from repeat challenges, we considered different scenarios (described in protocol), with power assessed by simulation. In the most conservative scenario investigated (within-person correlation=0.5 and variance=4), 72 participants would mean 80% power (5% two-sided significance level) to detect a minimum change in threshold (logged) of -0.9 (i.e. a 60% reduction in threshold from baseline).
Protocol changes
The initial protocol specified blinded food challenge (DBPC) for all challenges. However, in view of the complexity of the protocol and excessive time burden on participants a decision was made by the Trial Steering Committee to change to open challenges for the final three challenges for each participant. Eighteen blinded challenges with interventions were performed, and a sensitivity analysis showed no difference in threshold between challenges with and without placebo.
Statistical analyses
All analyses were planned prospectively and detailed in a statistical analysis plan. The primary outcome was expressed as a mean (SD). The primary analysis estimated the difference in log-threshold between the non-intervention challenge and each intervention challenge (exercise and sleep deprivation) using a linear mixed-effects model along with 95% confidence interval and p-value for whether the difference in log threshold was significant. Changes in threshold were also expressed as percentage change. Fixed effects included the challenge type (exercise, sleep deprivation, with non-intervention as reference), age, sex, order of challenge, baseline log threshold, presence of asthma, centre and baseline Ara h 2. non-intervention. For the secondary outcome of constructing the population threshold curves, a parametric interval-censored survival analysis method described by Taylor(16) was used. The threshold values were included as interval censored data between the threshold dose one below and at which the reaction occurred. Thresholds were expressed as cumulative doses unless otherwise specified. If a participant reacted on the first dose of the challenge the data was left censored at the first dose. If no reaction took place for any dose the data were right censored at the final dose. The Survival package (‘survreg’ function in ’R’)(17) was used to fit log-normal, log-logistic and Weibull distributions. The model that fitted the data best according to the Akaike information criterion was chosen. The model was used to find the eliciting dose (and 95% CI) predicted to provoke reactions in different proportions (as percentage) of the peanut-allergic population (EDx%). For example, the ED10 is the dose which provokes a reaction in 10% of the peanut-allergic population. For the baseline population threshold curve the extended analysis population was used which included all participants who underwent a baseline challenge (excluding those who were subsequently determined to be non-allergic). All other population threshold curves were based on the full analysis population.
Results
Participants
We screened 222 participants aged 18-45y (Figure 1). Of these, 123 underwent both baseline challenges and 100 participants were randomised to undergo the interventional challenges (median 25.0 years F:53). The most frequent reason for non-randomisation was tolerance of all challenge doses (14 subjects) and hence inability to identify a threshold, with other reasons being severity of reactions, non-compliance and quota of randomised patients already being complete (9). During placebo challenges the majority of symptoms experienced by participants were mild green symptoms or infrequently yellow symptoms usually abdominal pain or persistent nausea occurring in isolation however none of these symptoms met the stopping criteria in any participant. The baseline characteristics of the randomised participants are listed in Table II. The full analysis population completed at least one post-baseline challenge and consisted of 81 participants. Sixty-four participants completed all three post-baseline challenges (per-protocol set) (Data not shown).
Figure 1. Consort diagram.
*one was excluded after review on the grounds that it had been stopped prematurely, resulting in a full analysis population of 81 participants
Table II. Baseline characteristics for study populations.
For binary variables, number and percentage (in parentheses) are shown; for continuous variables the mean and standard variation (in parentheses) are shown.
| Characteristic | All randomised (n=100) | Full analysis set (n=81) |
|---|---|---|
| Age (years) | 24.7 (6.6) | 25.2 (7) |
| Gender: Female | 53 (53 %) | 43 (53 %) |
| Site: Cambridge | 53 (53 %) | 46 (57 %) |
| Index reaction Adrenaline use | 34 (34 %) | 30 (37 %) |
| Index reaction wheeze | 45 (45%) | 38 (47%) |
| Presence of Asthma | 55 (55%) | 45 (56%) |
| Rhinitis | 80 (80%) | 65 (80%) |
| Eczema | 53 (53%) | 46 (57%) |
| Peanut SPT wheal (mm) | 11.5 (4.2) | 11.2 (3.8) |
| V02 max (ml/min/kg) | 34.5 (11) | 34 (10) |
| Peanut specific IgE (kUA/L) | 30 (34) | 31.6 (35) |
| Ara h 2 specific IgE (kUA/L) | 20.6 (28) | 21.3 (29) |
| FEV1 (l) | 3.9 (0.8) | 3.9 (0.78) |
| FEV1 (l, % predicted) | 105.8 (12) | 106 (13) |
| Number of historical reactions | 8.6 (3.4) | 8.7 (3.5) |
| Baseline LOAEL (mg protein) | 304 (410) | 330.1 (420) |
| PEFR (l/min) | 511.8 (110) | 506.7 (110) |
Primary outcome: Peanut thresholds and the effect of co-factors
The mean (SD) cumulative threshold for baseline challenges was 330mg (424mg) peanut protein for the full analysis population, 191mg (358mg) for exercise challenges, 157mg (300mg) for sleep deprivation challenges and 214mg (330mg) for non-intervention challenges (n=81). When assessing the impact of each intervention on threshold, the estimated change in (natural) log threshold for the sleep deprivation challenge compared to the non-intervention challenge was –0.61 (-0.97, -0.25; p=0.0011) and for the exercise challenge was -0.60 (-0.95, -0.24; p=0.0013). Both changes equate to a reduction in threshold of 45% shown in Figure 2 and Table III. No patient reacted on the first dose (3µg protein), therefore there were no left-censored participants.
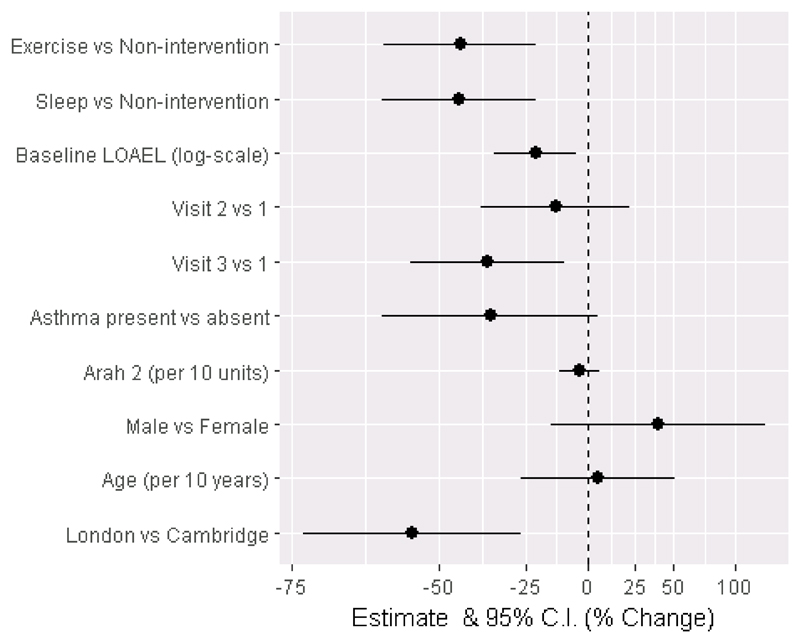
Figure 2. Percentage change in threshold (logged) for each covariate.
Full-analysis population n=81. Visits 1-3 refer to the chronological order of post-baseline challenge days. LOAEL = lowest observed adverse effect level is the reactive threshold in mg peanut protein during baseline challenge
Table III. Estimated effect shown in log and percentage scale, 95% confidence interval and p-value for each term in the linear mixed effects model. Full-analysis population, n=81.
Visits 1-3 refer to the chronological order of post-baseline challenge days. LOAEL = lowest observed adverse effect level is the reactive threshold in mg peanut protein during baseline challenge. The estimates for binary variables indicate the modelled difference from reference category in log LOAEL (and absolute percentage change). The estimates for continuous variables (Arah2, Age and baseline LOAEL) indicate the modelled change in log LOAEL per one-unit increase.
| Variables | Estimate (log-scale) |
CI | Estimate (absolute change in %) |
CI | p-value |
|---|---|---|---|---|---|
| Baseline LOAEL (log-scale) | -0.244 | (-0.436,-0.052) | -22 | (-35,-5) | 0.014 |
| Non-intervention | Reference | ||||
| Exercise | -0.596 | (-0.953,-0.239) | -45 | (-61,-21) | 0.0013 |
| Sleep | -0.599 | (-0.959,-0.239) | -45 | (-62,-21) | 0.0013 |
| Post baseline visit 1 | Reference | ||||
| Post baseline visit 2 | -0.148 | (-0.497,0.2) | -14 | (-39,+22) | 0.40 |
| Post baseline visit 3 | -0.469 | (-0.83,-0.107) | -37 | (-56,-10) | 0.011 |
| Cambridge | Reference | ||||
| London | -0.820 | (-1.33,-0.309) | -56 | (-74,-27) | 0.002 |
| No asthma at baseline | Reference | ||||
| Asthma at baseline | -0.456 | (-0.963,0.051) | -37 | (-62,+5) | 0.077 |
| Arah2 (per 10 units) | -0.039 | (-0.133,0.055) | -4 | (-12,+6) | 0.41 |
| Female | Reference | ||||
| Male | 0.332 | (-0.173,0.838) | +39 | (-16,+131) | 0.19 |
| Age (per 10 years) | 0.050 | (-0.308,0.408) | +5 | (-27,+50) | 0.78 |
Secondary outcomes: Threshold distribution modelling for peanut
Full analysis population
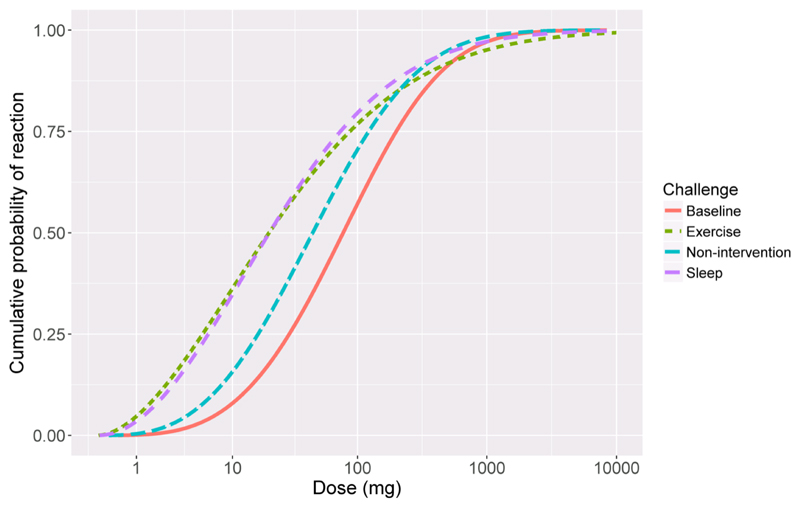
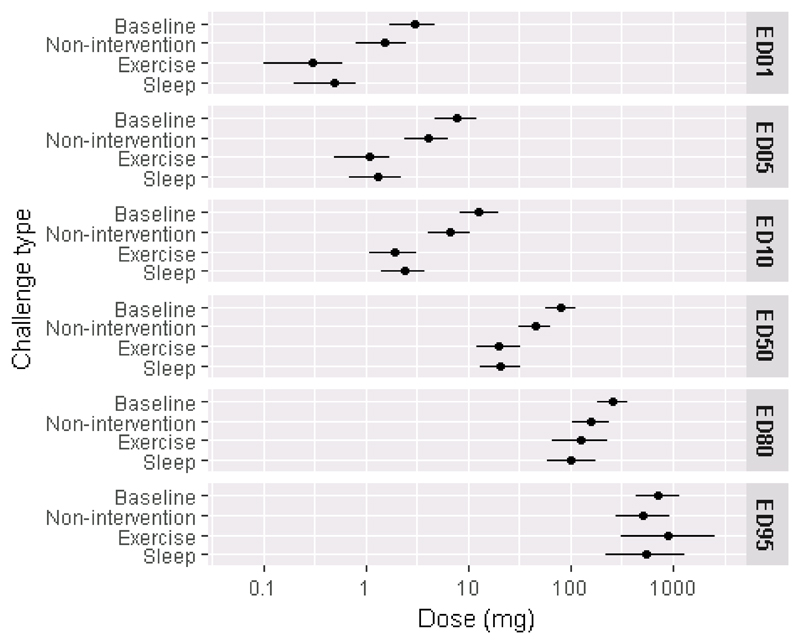
The mean (95% confidence interval) eliciting doses for the full-analysis population during non-intervention challenge were ED1 = 1.5mg (0.8,2.5), ED5 = 4.0mg (2.4,6.4) and ED10 = 6.7mg (4.1,10.5) peanut protein respectively. Compared with the threshold dose distribution curves (TDC) for the non-intervention challenges, the curves for exercise and sleep deprivation were significantly different and shifted to the left (Figure 3). Thus, during exercise or sleep deprivation challenges, participants reacted at a lower dose than when no intervention was applied. For example, the ED1 for no intervention was 1.5mg (0.8,2.5), for sleep deprivation was 0.5mg (0.2,0.8) and for exercise was 0.3mg (0.1,0.6). The effect was most pronounced at lower eliciting doses, but not noticeable at higher eliciting doses (ED50 – ED95) (Figure 4; Table IV).
Figure 3. Threshold dose distribution model.
Doses given in mg peanut protein, per challenge type, showing cumulative probability of reacting against dose in peanut protein in milligrams. Full analysis population, n=81
Figure 4. Eliciting dose estimates (mg peanut protein) derived from threshold distribution curve;
mean (95% CI) by challenge type for eliciting doses (ED) for 1, 5, 10, 50, 80 and 95% of the full analysis population, n=81 are shown.
Table IV. Predicted dose (and 95% CI) that gives different probability of reactions (EDx = dose that gives x% probability of reaction), full-analysis set n=81.
| Dose | Baseline challenge, (n=81) | Non-intervention challenge, (n=71) | Sleep challenge, (n=71) | Exercise challenge, (n=73) |
|---|---|---|---|---|
| ED1 | 3 (1.7,4.8) | 1.5 (0.8,2.5) | 0.5 (0.2,0.8) | 0.3 (0.1,0.6) |
| ED5 | 7.6 (4.7,12) | 4 (2.4,6.4) | 1.3 (0.7,2.2) | 1.1 (0.5,1.7) |
| ED10 | 12.8 (8.2,19.8) | 6.7 (4.1,10.5) | 2.4 (1.4,3.8) | 1.9 (1.1,3.1) |
| ED50 | 80.6 (57.9,112) | 44.6 (30.8,64.5) | 20.4 (12.9,31.9) | 19.7 (12,32) |
| ED80 | 255 (180.2,360.8) | 156.2 (103.5,235.5) | 101.8 (58.4,176.9) | 123.6 (65.3,233.3) |
| ED95 | 715.9 (441.9,1159.4) | 502 (276.9,909.3) | 537 (223.6,1287.6) | 894.7 (308.4,2592.2) |
Extended analysis population
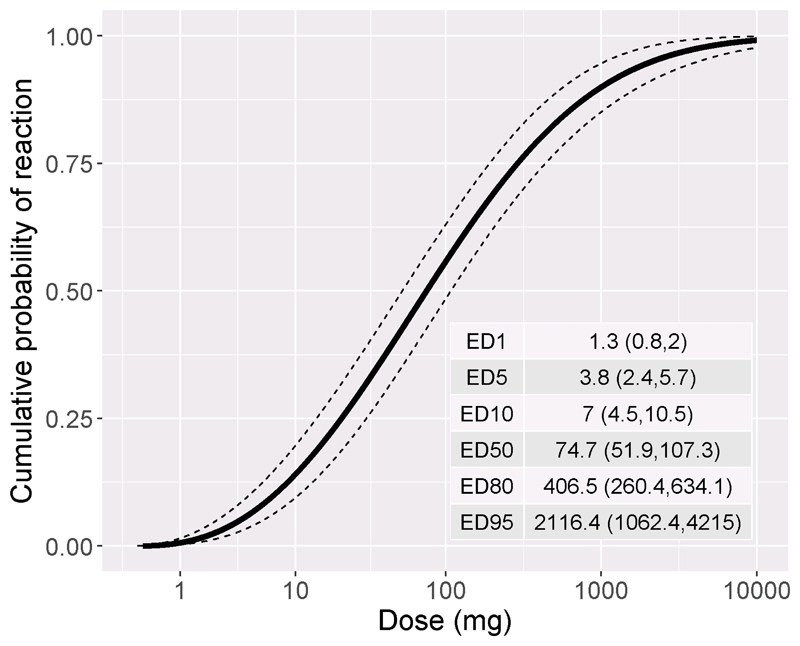
The dose distribution curve for the extended analysis population, which included all individuals who received a baseline challenge, is shown in Figure 5 (n=123). The mean (95% confidence interval) eliciting doses were ED1 =1.3 mg (0.8,2.0), ED5 =3.8mg (2.4,5.7) and ED10 =7mg (4.5,10.5) peanut protein. Fourteen participants did not reach challenge stopping criteria during baseline challenge and their data were therefore right censored at the maximum dose. An independent expert reviewed their cases and on the basis of their history, sensitisation patterns and challenge symptoms deemed that they were clinically allergic with likely thresholds greater than 1 gram protein. They were therefore included in the extended analysis population but excluded from randomisation.
Figure 5. Dose distribution curve for extended analysis population (n=123) with 95% confidence intervals.
Dose is mg peanut protein. Eliciting doses (ED) in mg with 95% CI for 1, 5, 10, 50, 80 and 95% of the extended analysis population are shown as an inset table.
Covariates
No significant effects on threshold were observed for other variables including presence of asthma, sex, age, or IgE against Ara h 2 levels (Table III and Figure 2).
There was a trend towards reduction in threshold for each successive intervention visit which became significant only for the third post-baseline challenge versus the first post-baseline challenge: threshold (logged) = -0.47 (95% CI -0.83,-0.11); p=0.011.
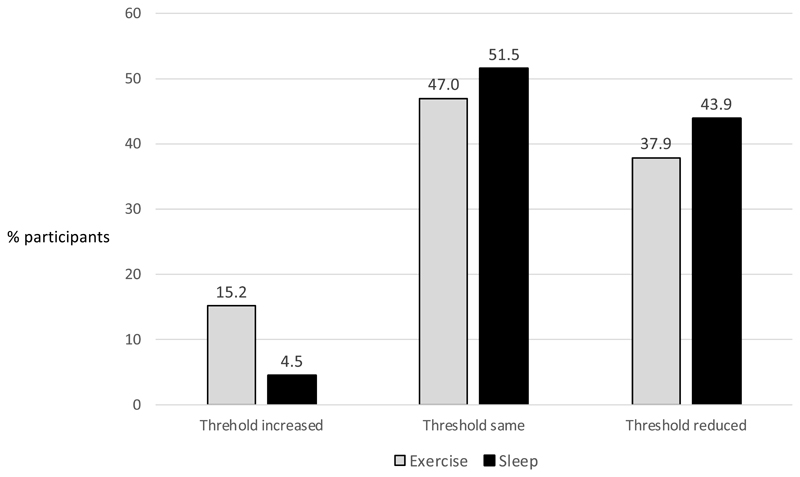
A post-hoc descriptive responder analysis was undertaken on those the participants who undertook an exercise and non-intervention challenge or sleep deprivation and non-intervention challenge (n=66) (Figure 6).
Figure 6. Descriptive analysis of participants whose dose threshold increased, decreased or remained the same following exercise and sleep deprivation (n=66).
Numbers show percentage of participants in each group (of a total of n=66 who undertook an exercise and non-intervention challenge, or sleep deprivation and non-intervention challenge). This was a post –hoc analysis therefore no statistical test was applied.
A significant effect of centre was also observed. Compared with Cambridge, London participants had a lower threshold (logged) across post-baseline challenges. In particular with regard to exercise a marginally non-significant difference in effect of exercise challenge vs non-intervention was observed between centres (threshold (logged) -0.78 (95% CI - 1.59,0.03) p=0.061). However, the exercise versus non-intervention point estimate was consistent with the overall estimate (i.e. the direction of effect was the same within each centre). Overall a a threshold lowering effect of both interventions was seen independently at both sites. Pre-specified analysis of the primary outcome was adjusted for both site and challenge order.
Safety
There was a single serious adverse reaction, one patient was admitted overnight following a challenge after developing hypotension and required two doses of adrenaline and intravenous fluids. Intramuscular adrenaline was delivered in 52/342 (15%) challenges. Two doses of intramuscular adrenaline were delivered to stabilise the participants in 6/342 (2%) challenges Nebulised adrenaline was administered in 3/342 (1%) challenges.
Discussion
We have defined a mean reactivity threshold of 214mg peanut protein for an individual, approximately equivalent to one peanut(15), and have demonstrated that both exercise and sleep deprivation caused a 45% reduction in an individual’s threshold. To our knowledge these findings provide the first systematically generated data on peanut allergy thresholds in a UK adult peanut-allergic population, and the first prospectively collected data to show that co-factors significantly reduce allergic thresholds in peanut allergy.
To determine a population threshold we used threshold dose distribution modelling, to estimate the amounts of peanut protein that would elicit a reaction in 1, 5 and 10% of the peanut-allergic population. These eliciting doses were 1.5mg, 4mg and 6.7mg peanut protein respectively. Eliciting dose values for the extended analysis population were not significantly different, even when including the right-censored individuals who had no threshold identified. Several groups have established peanut threshold distribution data on children, although none have been elicited for UK adults. Furthermore, these studies have often included individuals with milder phenotypes, and have excluded participants with a history of anaphylaxis. Our estimate for ED10 (6.7mg) was higher when compared to some other previous estimates, which range from 0.7-4.42mg(18)(19)(20)(21) (22). Although some studies have often used subjective symptoms as stopping criteria leading to lower threshold estimates(19), many have not(21,23). The most likely explanation for the higher ED10 in this study is the use of more robust stopping criteria employed in our study, where three concurrent objective symptoms were required to stop the challenge and establish the threshold. Klemans at al who used threshold data derived from diagnostic food challenges estimated an ED10 of 13.7 (4.37-42.8 95% CI) mg peanut protein in adults, although the confidence intervals were wide(23).
We show for the first time that co-factors lower the reactivity threshold in allergic reactions. Sleep deprivation may exert its effect at least partly through a stress response affecting the immune and gastrointestinal systems. In animal models of inflammatory bowel disease, stress results in enhanced intestinal permeability (24,25) potentially associated with a significant increase in permeability of the epithelium to macromolecules, which may account for the reduction in threshold. Similarly under-perfusion of the gut may occur during exercise leading to ischaemia with resultant damage to tight junction integrity and increased permeability to food allergens. (26) Co-factors such as exercise, alcohol and non-steroidal anti-inflammatory drugs, are increasingly being implicated in food anaphylaxis. (27)
This study is the first to establish population eliciting doses for peanut when participants are deliberately subjected to the co-factors sleep deprivation and exercise. Further, we are able to relate these to a reference threshold when no co-factor (non-intervention) is applied to calculate the magnitude of the effect. Current allergen risk assessment by food industry and regulators involves defining an eliciting dose (e.g. ED1 or ED5) representing an exposure that is likely to be safe for the population. Hourihane et al have recently validated the proposed ED5 for peanut of 1.5mg peanut protein by performing single dose peanut challenges on 378 children and observed that only 8 participants (2.1%) experienced objective symptoms (all mild), only half of whom required treatment with oral antihistamines(7). Further studies are required to validate proposed ED5 and ED1 doses, particularly in the adult population. The food industry can then use these validated eliciting doses to develop guidelines for the use of voluntary precautionary food labelling (reference doses). Previously a reference dose of 0.2mg peanut protein, based on the ED1, has been proposed by the VITAL group. (8) However, the group acknowledge in their study that further application of an uncertainty or safety factor to this reference dose may be necessary to account for individual factors which may potentially affect this dose estimate. Due to a paucity of clinical data, the application of safety factors has traditionally followed toxicology practice accounting for (10-fold) inter-species (for thresholds defined in non-human models) and (a further 10-fold) intra-individual variation in response. In practice, such large safety factors result in very low reference doses which, being near or below the limit of detection of available assays, are difficult to measure with accuracy, rendering them impractical for the food industry to implement. This results in over-cautious food labelling. We show in this study, that a safety factor can be many magnitudes smaller. Sleep deprivation lowers the ED1 from 1.5mg (for the non-intervention dose distribution) to 0.5mg; this is equivalent to applying a safety factor of 0.33 to the ED1 calculated from the non-intervention dose distribution. Similarly exercise lowers the ED1 from 1.5mg (non-intervention) to 0.3mg equivalent to a safety factor of 0.2. The derivation of reference doses which use evidence-based safety factors such as those which are provided by our study will enhance the allergen risk assessment process. This should encourage better industry engagement with evidence-based voluntary food labelling reducing excessive, overly cautious precautionary allergen labelling and provide allergic consumers with greater assurance that foods without precautionary allergen labelling are safe for the majority to consume.
The safety data in this trial show that the overall adrenaline use across all challenges was 15%, broadly reflecting the rate of adrenaline use in positive food challenges in other studies. Jarvinen et al reported its use in 11% of positive food challenges(28) and Lieberman in 9% of positive food challenges.(29) The use of multiple doses of adrenaline was infrequent, and only occurred in 2% of challenges.
We found no association between threshold and other factors such as the presence of asthma, the level of peanut specific IgE, Ara h 2 or gender. Previous studies have noted an inverse correlation between Ara h 2 specific IgE and elicitation threshold, but we did not replicate this finding in our study.(20)
A potential limitation of this study is that our eliciting dose estimate is based on a volunteer peanut-allergic population. Although participants with a history of anaphylaxis and historical adrenaline use were included, those who have suffered the most severe reactions in the community may be under-represented, being possibly reluctant to volunteer for the study. This could introduce bias only if participants who suffered more severe reactions in the community represent the more sensitive (i.e. lower dose) reactors. However a previous study has shown that minimum eliciting dose distributions for participants with histories of more severe reactions did not differ significantly from those participants with histories of milder reactions.(21). Our study population had a low average age of 25 years. Fatal anaphylaxis episodes occur more commonly in this age group (30) perhaps due to more risk taking behaviour(31), thus in defining a threshold for the whole population, it is of benefit that the model is based on this age group.
A significant centre effect was observed with participants in London having overall lower thresholds than those in Cambridge, though a threshold lowering effect of both interventions was seen independently at both sites, reinforcing the generalisability of our findings. No differences were observed in the baseline characteristics of the study populations to account for the centre effect. The most likely explanation is variation between investigators in the interpretation of clinical symptoms and decision about when to stop the challenge and administer treatment. Attempts were made to standardise practice across both sites through common stopping criteria for challenges and cross-site training to minimise this. Variability in the interpretation of clinical symptoms by clinical experts is known to occur in food challenges and has been reported in another study. (32) All analyses were adjusted for centre.
Another potential weakness was the use of open challenges following the blinded baseline food challenge. We observed an apparent lowering of threshold linked with an increasing number of challenges. Although this may be a true phenomenon it is also possible that the open study design may have contributed to this, by participants and investigators ‘learning’ reactions over time and anticipating the development of more severe symptoms. However, the study was designed to minimise this bias by ensuring that the participant was deemed to have reached their reaction threshold with only the appearance of pre-specified objective symptoms, and the balanced design means that the two interventions were spread equally across the order of challenge days.
In conclusion, our study identified eliciting dose estimates from a well characterised adult peanut-allergic population. Also, for the first time it has been shown that co-factors such as sleep deprivation and exercise lower allergen reactivity thresholds, and the magnitude of their effect has been defined. This study, funded by the FSA, has important public health implications helping food policy makers and the food industry provide harmonised guidance on allergen labelling, which will ultimately benefit all peanut allergic individuals.
This study was commissioned by the UK Food Standards Agency (FSA), UK Government.
Extended Data
Table E1. Study inclusion and exclusion criteria.
Inclusion criteria
|
Exclusion criteria
|
Table E2. Modification and explanation of existing PRACTALL CRITERIA.
| Existing PRACTALL CRITERIA | Modified PRACTALL CRITERIA | Explanation of modification made |
|---|---|---|
| Mild, occasional scratching [Green] | Pruritus -Occasional scratching [Green] | |
| Moderate -scratching continuously for > 2 minutes at a time [Green] | Pruritus-scratching continuously for >2 mins at a time [Green] | |
| Severe hard continuous scratching excoriations [Yellow] | Hard continuous scratching causing excoriations [Yellow] | |
| Mild < 3 hives, or mild lip edema [Yellow] | Urticaria-<3 hives or mild lip oedema [Yellow] | |
| Moderate - < 10 hives but >3, or significant lip or face edema [Red] | Urticaria- <10 hives ≥ 3or significant lip or face oedema [Red] | |
| Severe generalized involvement [Red] | Urticaria-generalised involvement [Red] | |
| Mild few areas of faint erythema [Green] | Rash- Few areas of faint erythema [Green] | |
| Moderate areas of erythema [Yellow] | Rash- Areas of erythema [Yellow] | |
| Severe generalized marked erythema (>50%) [Red] | Rash- Generalised marked erythema>50% [Red] | |
| Mild rare bursts, occasional sniffing [Green] | Itching in inner ear canal [green] | Itching in inner ear canal was added as it was a common mild symptom identified by many patients during piloting. |
| Rare bursts of sneezing occasional sniffing [green] | ||
| Moderate bursts < 10, intermittent rubbing of nose, and/or eyes or frequent sniffing [Yellow] | Bursts < 10, intermittent rubbing of nose, and/or eyes or frequent sniffing [Yellow] | Rhinitis symptoms downgraded from red to yellow. These were not regarded by the study team as severe enough symptoms singly to warrant stopping challenge. |
| Severe continuous rubbing of nose and/or eyes, periocular swelling and/or long bursts of sneezing, persistent rhinorrhea [Red] | Continuous rubbing of nose and/or eyes, [Yellow] Periocular swelling and/or l ong bursts of sneezing, [Yellow] Persistent rhinorrhoea [Yellow] |
|
| Mild expiratory wheezing to auscultation [Red] | Chest tightness without any fall in PEFR [Green] Chest tightness with a <10% fall in PEFR [green] |
In the existing Practall criteria study team felt that there needed to be representation of milder respiratory symptoms as the existing criteria escalate too rapidly to wheeze which is a clear objective symptoms. Therefore to enhance safety and aid detection, the gradation of lower respiratory symptoms was extended adding milder ones and incorporating functional measurement of PEFR. |
| Moderate inspiratory and expiratory wheezing [Red] | ||
| Severe use of accessory muscles, audible wheezing [Red] | Chest tightness with a 10-20% fall in PEFR [yellow] | |
| Chest tightness with a >20% fall in PEFR [red] | ||
| Expiratory or inspiratory wheeze [Red] | ||
| Use of accessory muscles [Red] | ||
| Mild >3 discrete episodes of throat clearing or cough, or persistent throat tightness/pain [Yellow] | Throat tingling/altered sensation in throat [Green] | Mild oropharyngeal symptoms added |
| Moderate hoarseness, frequent dry cough [Red] | > 3 discrete episodes of throat clearing or cough [Yellow] | Definition of persistence added and defined as symptom present for ≥30 minutes |
| Severe stridor [Red] | Persistent throat tightness [Yellow] | |
| Hoarseness or frequent dry cough [Red] | ||
| Stridor [Red] | ||
| Mild complaints of nausea or abdominal pain, itchy mouth/throat [Yellow] | Oral itching [Green] | Milder and transient abdominal symptoms downgraded |
| Transient nausea [green] | ||
| Moderate frequent c/o nausea or pain with normal activity [Yellow] | Transient abdominal pain [green] | Incorporated duration of abdominal symptoms as a marker of severity. Persistent defined as symptom present ≥30 minutes |
| Severe - notably distressed due to GI symptoms with decreased activity [Yellow] | Persistent nausea [yellow] | |
| Persistent abdominal pain [yellow] | ||
| Objective Mild 1 episode of emesis or diarrhea [Yellow] | Emesis/diarrhoea (1 episode) [Yellow] | |
| Moderate 2-3 episodes of emesis or diarrhea or 1 of each [Red] | Emesis/diarrhoea (more than 1 episode) [Red] | |
| Severe >3 episodes of emesis or diarrhea or 2 of each [Red] | ||
| Mild-subjective response (weak, dizzy), or tachycardia [Yellow] | Weak/dizzy or tachycardia [Yellow] | |
| moderate-drop in blood pressure and/or >20% from baseline, or significant change in mental status. severe-cardiovascular collapse, signs of impaired circulation (unconscious) [Red] |
Drop in BP and/or >20% from baseline [Red] | |
| Cardiovascular collapse/signs of impaired circulation [Red] Altered level of consciousness [Red] |
||
Clinical implications.
Exercise and sleep deprivation each individually lower reaction threshold by approximately half; this needs to be accounted for when defining reference doses for food labelling.
Capsule summary.
We show that co-factors (sleep deprivation and exercise) cause a reduction in reactivity threshold to peanut by 45% and accounting for this variation in population threshold estimates will more accurately guide reference doses for allergen risk management.
Acknowledgements
The authors thank the members of the study Trial Steering Committee: Moira Austin, Phillipa Cauldwell, Victoria Cornelius, Hazel Gowland, Laura Pasea, Steve Till and Yvonne King for their assistance in study design and study oversight, the study team extend particular thanks to Dr Chris Palmer for statistical help during the design of the study. In addition the study team are grateful to the members of the TRACE IDMC: Tina Dixon, David Luyt, Abdel Douri, Gillian Vance for reviewing patient safety and data, the Anaphylaxis Campaign for their assistance with study design and patient recruitment and providing a lay member for the Trial Steering Committee, Sabita Islam, Loraine Foley and Frankie Saunders for their help with challenge meal preparation, all members of the Cambridge NIHR/Wellcome Trust Clinical Research Facility and the Royal Brompton and Harefield Biomedical Research Unit, staff at the University of Manchester in particular Matthew Machin and Kathleen Haigh-Hutchinson for their assistance with data management and the database build and Carol-Ann Costello for her help with challenge meal production and supply, Paul Turner and finally the Food Standards Agency, in particular, Chun-Han Chan, Joelle Buck, Sarah Hardy, Sue Hattersley, Paul Tossell, Ruth Willis and Ross Yarham.
Abbreviations
- DBPCFC
double blind placebo controlled food challenge
- ED
eliciting dose
- ICSA
interval censored survival analysis
- IgE
Immunoglobulin E
- LOAEL
lowest observed adverse effect level
- PAL
precautionary allergen labelling
Footnotes
Disclosure statement
Funded by the Food Standards Agency who had no involvement in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. None of the authors had a conflict of interest relevant to this study.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pumphrey RSH. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30(8):1144–50. doi: 10.1046/j.1365-2222.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 2.Pumphrey RS, Gowland MH. Further fatal allergic reactions to food in the United Kingdom 1999-2006. J Allergy Clin Immunol. 2007;119:1018–9. doi: 10.1016/j.jaci.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Stiefel G, Anagnostou K, Boyle RJ, Brathwaite N, Ewan P, Fox AT, et al. BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin Exp Allergy. 2017;47(6) doi: 10.1111/cea.12957. [DOI] [PubMed] [Google Scholar]
- 4.Remington BC, Baumert JL, Blom WM, Houben GF, Taylor SL, Kruizinga AG. Unintended allergens in precautionary labelled and unlabelled products pose significant risks to UK allergic consumers. Allergy Eur J Allergy Clin Immunol. 2015 doi: 10.1111/all.12625. [DOI] [PubMed] [Google Scholar]
- 5.Robertson ON, Hourihane JO, Remington BC, Baumert JL, Taylor SL. Survey of peanut levels in selected Irish food products bearing peanut allergen advisory labels. Food Addit Contam Part A. 2013;30(9):1467–72. doi: 10.1080/19440049.2013.804953. [Internet] [DOI] [PubMed] [Google Scholar]
- 6.Hefle SL, Furlong TJ, Niemann L, Lemon-Mule H, Sicherer S, Taylor SL. Consumer attitudes and risks associated with packaged foods having advisory labeling regarding the presence of peanuts. J Allergy Clin Immunol. 2007;120(1):171–6. doi: 10.1016/j.jaci.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Hourihane JOB, Allen KJ, Shreffler WG, Dunngalvin G, Nordlee JA, Zurzolo GA, et al. Peanut Allergen Threshold Study (PATS): Novel single-dose oral food challenge study to validate eliciting doses in children with peanut allergy. J Allergy Clin Immunol. 2017;139(5):1583–90. doi: 10.1016/j.jaci.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 8.Allen KJ, Remington BC, Baumert JL, Crevel RWR, Houben GF, Brooke-Taylor S, et al. Allergen reference doses for precautionary labeling (VITAL 2.0): Clinical implications. J Allergy Clin Immunol. 2014;133(1):156–64. doi: 10.1016/j.jaci.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Christensen MJ, Eller E, Mortz CG, Brockow K, Bindslev-Jensen C. Exercise Lowers Threshold and Increases Severity, but Wheat-Dependent, Exercise-Induced Anaphylaxis Can Be Elicited at Rest. J Allergy Clin Immunol Pract. 2018;6:514–20. doi: 10.1016/j.jaip.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Brockow K, Kneissl D, Valentini L, Zelger O, Grosber M, Kugler C, et al. Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 2015;135(4):977–984.e4. doi: 10.1016/j.jaci.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383(9925):1297–304. doi: 10.1016/S0140-6736(13)62301-6. [Internet]. Anagnostou et al Open Access article distributed under the terms of CC BY-NC-ND, Available from: http://linkinghub.elsevier.com/retrieve/pii/S0140673613623016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, Sicherer S, Teuber SS, Burks AW, et al. J Allergy Clin Immunol. 6. Vol. 130. Elsevier Ltd; 2012. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology–European Academy of Allergy and Clinical Immunology PRACTALL consensus report; pp. 1260–74. [Internet] Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674912016636. [DOI] [PubMed] [Google Scholar]
- 13.Jia CE, Zhang HP, Lv Y, Liang R, Jiang YQ, Powell H, et al. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131(3):695–703. doi: 10.1016/j.jaci.2012.08.023. [Internet] Available from: http://www.jacionline.org/article/S0091674912013863/fulltext. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt J, Langan S, Williams HC. What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol. 2007;120(6):1389–98. doi: 10.1016/j.jaci.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Tonnis B, Pinnow D, Barkley N, Pederson G. Progress on screening the USDA cultivated peanut germplasm collection for variability in seed weight, seed-coat color, oil content and fatty acid composition) 2015 [Internet]. Available from: https://www.ars.usda.gov/research/publications/publication/?seqNo115=317089.
- 16.Taylor SL, Crevel RWR, Sheffield D, Kabourek J, Baumert J. Threshold dose for peanut: Risk characterization based upon published results from challenges of peanut-allergic individuals. Food Chem Toxicol. 2009;47(6):1198–204. doi: 10.1016/j.fct.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 17.R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 18.Zhu J, Pouillot R, Kwegyir-Afful EK, Luccioli S, Gendel SM. A retrospective analysis of allergic reaction severities and minimal eliciting doses for peanut, milk, egg, and soy oral food challenges. Food Chem Toxicol. 2015;80:92–100. doi: 10.1016/j.fct.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Ballmer-Weber BK, Fernandez-Rivas M, Beyer K, Defernez M, Sperrin M, Mackie AR, et al. How much is too much? Threshold dose distributions for 5 food allergens. J Allergy Clin Immunol. 2015;135(4):964–71. doi: 10.1016/j.jaci.2014.10.047. [DOI] [PubMed] [Google Scholar]
- 20.Blumchen K, Beder A, Beschorner J, Ahrens F, Gruebl A, Hamelmann E, et al. J Allergy Clin Immunol. 2. Vol. 134. Elsevier Ltd; 2014. Modified oral food challenge used with sensitization biomarkers provides more real-life clinical thresholds for peanut allergy; pp. 390–398.e4. [Internet] [DOI] [PubMed] [Google Scholar]
- 21.Taylor SL, Moneret-Vautrin DA, Crevel RWR, Sheffield D, Morisset M, Dumont P. Threshold dose for peanut: Risk characterization based upon diagnostic oral challenge of a series of 286 peanut-allergic individuals. Food Chem Toxicol. 2010;48(3):814–9. doi: 10.1016/j.fct.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Blom WM, Vlieg-Boerstra BJ, Kruizinga AG, Van Der Heide S, Houben GF, Dubois AEJ. J Allergy Clin Immunol. 1. Vol. 131. Elsevier Ltd; 2013. Threshold dose distributions for 5 major allergenic foods in children; pp. 172–9. [Internet] [DOI] [PubMed] [Google Scholar]
- 23.Klemans RJB, Blom WM, van Erp FC, Masthoff LJN, Rubingh CM, van der Ent CK, et al. Objective eliciting doses of peanut-allergic adults and children can be combined for risk assessment purposes. Clin Exp Allergy. 2015;45(7):1237–44. doi: 10.1111/cea.12558. [DOI] [PubMed] [Google Scholar]
- 24.Million M, Taché Y, Anton P. Susceptibility of Lewis and Fischer rats to stress-induced worsening of TNB-colitis: protective role of brain CRF. Am J Physiol. 1999;276(4 Pt 1):G1027–36. doi: 10.1152/ajpgi.1999.276.4.G1027. [Internet] [DOI] [PubMed] [Google Scholar]
- 25.Gue M, Bonbonne C, Fioramonti J, More J, Rio-Lacheze C, Comera C, et al. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. AmJPhysiol. 1997;272(0002-9513):G84–91. doi: 10.1152/ajpgi.1997.272.1.G84. [DOI] [PubMed] [Google Scholar]
- 26.Robson-Ansley P, Toit Du G. Pathophysiology, diagnosis and management of exercise-induced anaphylaxis. Current Opinion in Allergy and Clinical Immunology. 2010:312–7. doi: 10.1097/ACI.0b013e32833b9bb0. [DOI] [PubMed] [Google Scholar]
- 27.Wölbing F, Fischer J, Köberle M, Kaesler S, Biedermann T. About the role and underlying mechanisms of cofactors in anaphylaxis. Allergy: European Journal of Allergy and Clinical Immunology. 2013 doi: 10.1111/all.12193. [DOI] [PubMed] [Google Scholar]
- 28.Järvinen KM, Amalanayagam S, Shreffler WG, Noone S, Sicherer SH, Sampson HA, et al. Epinephrine treatment is infrequent and biphasic reactions are rare in food-induced reactions during oral food challenges in children. J Allergy Clin Immunol. 2009;124(6):1267–72. doi: 10.1016/j.jaci.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieberman JA, Cox AL, Vitale M, Sampson HA. Outcomes of office-based, open food challenges in the management of food allergy. Journal of Allergy and Clinical Immunology. 2011:1120–2. doi: 10.1016/j.jaci.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yun J, Katelaris CH. Food allergy in adolescents and adults. Intern Med J. 2009;39(7):475–8. doi: 10.1111/j.1445-5994.2009.01967.x. [Internet] [DOI] [PubMed] [Google Scholar]
- 31.Marrs T, Lack G. Why do few food-allergic adolescents treat anaphylaxis with adrenaline? - Reviewing a pressing issue. Pediatric Allergy and Immunology. 2013:222–9. doi: 10.1111/pai.12013. [DOI] [PubMed] [Google Scholar]
- 32.Van Erp FC, Knulst AC, Meijer Y, Gabriele C, Van Der Ent CK. Standardized food challenges are subject to variability in interpretation of clinical symptoms. 2014:1–6. doi: 10.1186/s13601-014-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]