Abstract
Background and study aims Colonic angioectasia are the most common vascular lesions in the gastrointestinal tract and are among the most common causes for chronic or recurrent lower gastrointestinal bleeding. Endoscopic treatment involves a variety of techniques, all of which focus on destruction of the mucosal abnormality. However, recurrent bleeding after endoscopic treatment is common, with more than one treatment frequently necessary. We report a technique for definitive treatment of colonic angioectasia by targeting the feeding submucosal vessel.
Patients and methods Analogous to endoscopic mucosal resection, a submucosal injection is made beneath the target lesion which is then removed by electrocautery snare resection of the mucosal lesion. The exposed feeding vessel is then destroyed by application of coagulation current. The resection defect is closed by clips.
Results Six patients with a total of 14 colonic angioectasia were treated over the study period. All lesions were destroyed without adverse events.
Conclusion Elevation, hot snare resection and coagulation (ESC) of the visible vessel for treating colonic angioectasia appears safe and effective. Larger prospective comparative studies are required to assess its specific role.
Introduction
Colonic angioectasia are the most common vascular lesions in the gastrointestinal tract and are among the most common causes for chronic or recurrent lower gastrointestinal bleeding 1 2 . Angioectasia are an acquired vascular malformation associated with advanced age. Pathogenesis of colonic angioectasia formation is multifactorial and commonly attributed to mild chronic venous obstruction and to chronic mucosal hypoxemia resulting in increased vascular endothelial growth factor (VEGF) expression 3 . Low-grade, intermittent, obstruction of venous drainage, hypothesized to be related to colonic luminal dilation, results in dilatation of submucosal veins. This in turn leads to mucosal venule and capillary dilatation and when the pre-capillary sphincter loses competency, arteriovenous fistula form, resulting in formation of the characteristic mucosal lesion 4 .
Colonic angioectasia are most commonly found in the right colon (54 % – 89 % located in cecum and ascending colon). Prevalence of colonic angioectasia is estimated to be 0.8 % – 6.2 % 2 and is higher in patients with comorbidities such as aortic stenosis, Von Willebrand Disease (vWD) and chronic kidney disease (CKD). Colonic angioectasia more often presents as multiple lesions rather than a single lesion (40 % – 60 %) 5 .
Most colonic angioectasia are found incidentally, are asymptomatic and do not require treatment 2 . However, they have been implicated as the source of bleeding in 3 % to 15 % of lower gastrointestinal bleeding 6 and up to 30 % in cases of severe hematochezia 7 .
Multiple endoscopic modalities have been reported for treatment of colonic angioectasia. The most commonly used endoscopic treatment is argon plasma coagulation (APC) but other modalities have also been described, including bipolar or heater probe coagulation, endoscopic clips, and injection sclerotherapy 3 . APC is simple to use and widely accepted but it is not complication-free with a perforation rate of 1 % 8 . Post-treatment rebleeding rates of up to 34 % have been described 9 and many patients will need ongoing iron supplementation on long-term follow-up. Most patients need more than one course of endoscopic treatment 10 .
All existing endoscopic therapies target the mucosal expression of the underlying vascular lesion. We propose a technique that targets the submucosal “feeding” vessel to definitively treat colonic angioectasia.
Methods
Elevate, snare resect, coagulate (ESC) method
Video 1 Elevate, snare resection, coagulate (ESC) of a proximal ascending colon angioectasia. Elevation with chromogelofusion solution is followed by snare resection. Bleeding is encountered from the submucosal feeding vessel and is treated immediately by snare tip soft coagulation followed by coagulation forceps soft coagulation. The defect is then closed by clips.
The mucosal lesion is resected by endoscopic mucosal resection (EMR). Submucosal injection is made with normal saline or succinylated gelatin (“Gelofusine”; B. Braun, Crissier, Switzerland) 11 and adrenaline at a final concentration of 1:100 000. Inert dye (methylene blue or indigo carmine) can be added to the solution to enhance delineation of the colonic angioectasia margin. The mucosal part of the colonic angioectasia is resected en bloc with fractionated electrocautery set to EndoCut Q, effect 3 (ERBE, Tübingen, Germany). The underlying feeding vessel is then identified and cauterized with coagulation forceps using soft coagulation, effect 4, 80 watts (ERBE, Tübingen, Germany). The defect is then closed with endoscopic clips ( Fig. 1 , Fig. 2 , Video 1 ). Specimens were retrieved for histopathological examination ( Fig. 3 ). Snare tip soft coagulation with the same energy settings can be used for small flat vessels within the defect that are difficult to grasp.
Fig. 1 a.
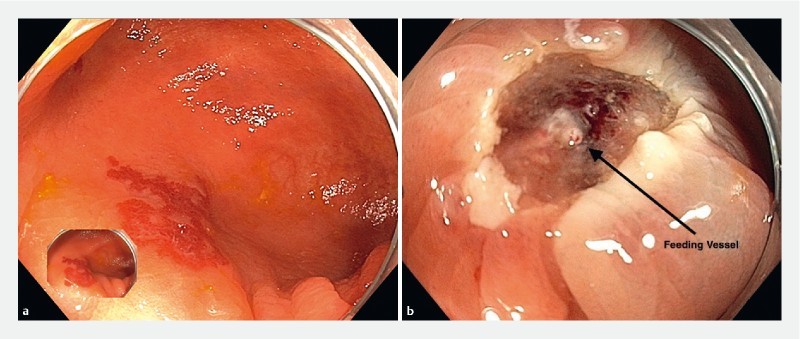
Cecal angioectasia. Elevation with saline without dye. b, arrow Feeding vessel clearly seen after mucosal resection.
Fig. 2 a.
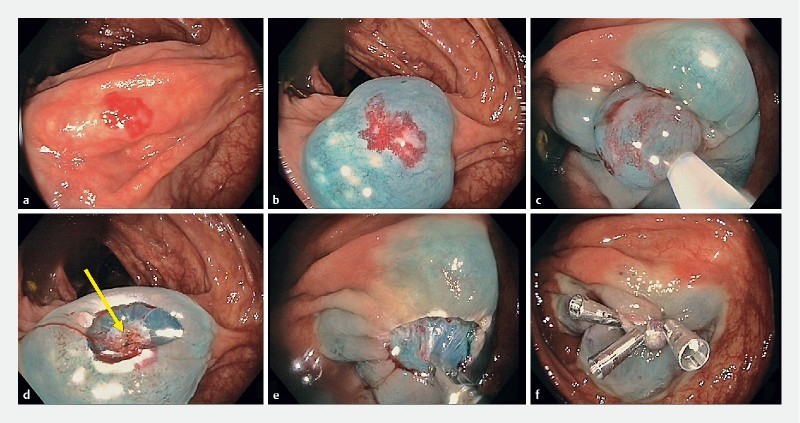
Elevate, snare resection, coagulate (ESC) of a proximal ascending colon angioectasia. b Elevation with chromogelofusion solution is followed by c snare resection. d The submucosal feeding vessel is identified (yellow arrow) and e treated by soft coagulation. f The defect is then closed by clips.
Fig. 3.
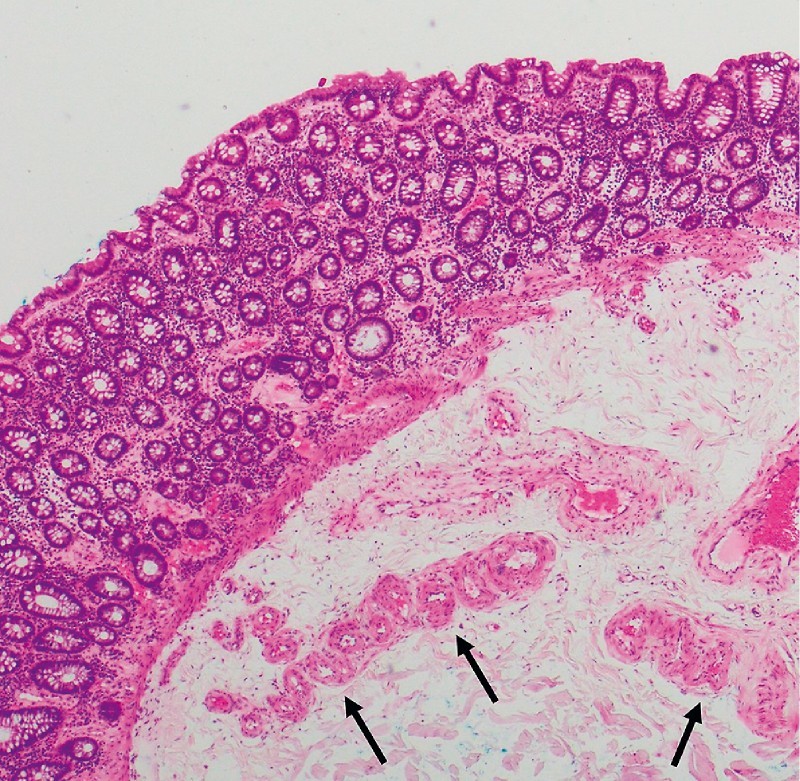
Histopathology from resected colonic angioectasia. Hematoxylin & eosin stain × 40. Dilated, clustered vessels in mucosa and submucosa, with tortuous feeding vessel at the resection base (marked with black arrows).
Results
Between May 2016 and Nov 2018, six patients with a total of 14 colonic angioectasia were treated with ESC ( Table 1 ). The most common indication was iron deficiency anemia. Other indications were recurrent lower gastrointestinal bleeding. Mean size of the treated lesions was 6 mm (3 mm to 10 mm). All lesions were in the right colon with the majority in the cecum. No immediate or delayed complications occurred. Mean follow up is 10 months (range 3 – 18). None of the treated patients have required blood or iron products nor required repeat endoscopies for ongoing blood loss.
Table 1. Patients characteristics.
| Patient | Age | Sex | Indication | Previous treatment | No. of lesions | Location |
| 1 | 79 | Male | IDA, LGIB | APC | 3 | Cecum, ascending |
| 2 | 81 | Male | IDA | APC | 1 | Cecum |
| 3 | 84 | Female | IDA | None | 1 | Cecum |
| 4 | 58 | Female | IDA | None | 2 | Cecum |
| 5 | 66 | Male | IDA | None | 4 | Cecum, ascending |
| 6 | 66 | Male | IDA | None | 3 | Cecum |
IDA, iron-deficiency anemia; LGIB, lower gastrointestinal bleeding; APC, argon plasma coagulation.
Discussion
Colonic angioectasia are an important etiology of chronic, recurrent and acute gastrointestinal bleeding. While the majority of these lesions are asymptomatic, treatment is usually warranted for overt bleeding and occult bleeding leading to iron deficiency anaemia.
In 2019 the majority of colonic angioectasia are treated with APC. This targets mucosal expression of the primary pathology located in the deeper submucosal layer. Scientific and clinical data attest to APC’s lack of precision. Accurate lesion targeting is suboptimal, even when en-face, and depth of tissue destruction is not standardized or predictable and subject to the angle of influence of the argon beam to the mucosal surface. An en-face position delivers deeper, possibly dangerous tissue destruction, whilst a tangential approach is less injurious and possibly suboptimal. Moreover, immediate APC-induced bleeding is common and formation of a carbonized coagulum of blood over the lesion limits energy penetration and the ability to achieve complete mucosal layer destruction, as manifested by APCʼs failure to prevent recurrent adenoma 12 . These aspects may explain the high rebleeding rate and need for multiple procedures 9 . The majority of colonic angioectasia are located in the right colon where risk of perforation with thermal ablative therapies is highest.
Targeting submucosal vessels for treatment of vascular pathologies elsewhere in the gastrointestinal tract has been described, primarily with polidocanol submucosal injection. This sclerosing agent, historically used for variceal 13 and ulcer treatment 14 , has also been used for angioectasia treatment in the small bowel 15 . However, it has been associated with delayed perforations in the esophagus and stomach as the sclerosing tissue effect can be unpredictable 16 .
These issues challenge endoscopists to develop a standardized, predictable, and reproducible method to treat the primary pathology of colonic angioectasia.
ESC targets the primary pathology of colonic angioectasia, the submucosal feeding vessel or vessels by a modification of standard EMR. Basic EMR forms part of the core endoscopic training and, even in the right colon, is proven to be safe with a reported perforation rate of 0.05 % to 0.07 % 17 . In addition, EMR for these small vascular lesions, < 10 mm, is inherently safe due to the small lesion size, however, it easily exposes the primary submucosal pathology to facilitate precisely targeted therapy. Snare tip soft coagulation has also proven to be a safe and effective method of securing hemostasis for bleeding during colonic EMR and polypectomy and can be used safely in this submucosal context as previously shown 18 . Clip closure of small mucosal defects is well established and adds an additional safety measure against delayed perforation or bleeding 19 . Defect closure may not be necessary, but additional study is required and there seems little down side when it is easily achieved with few clips.
Because our study describes a novel technique and introduces the concept of endoscopic targeting of the colonic angioectasia’s feeding vessel, we did not compare outcomes to current treatment options, such as APC. This limitation will need to be addressed in future research.
Conclusion
In conclusion, ESC appears to be safe and effective for treatment of colonic angioectasia. It specifically targets the primary pathological feeding vessel(s) underlying the mucosal lesion allowing for definitive destruction of the colonic angioectasia. This treatment skillset is within that of most endoscopists who remove polyps and thus it is readily available. Large prospective randomized trials are required to establish the role of ESC in treatment of colonic angioectasia. These may prove to be logistically challenging to conduct; however, ESC has the potential to replace APC as the standard of care for symptomatic colonic angioectasia.
Footnotes
Competing interests None
References
- 1.Foutch P G. Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol. 1993;88:807–818. [PubMed] [Google Scholar]
- 2.Foutch P G, Rex D K, Lieberman D A. Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 1995;90:564–567. [PubMed] [Google Scholar]
- 3.Sami S S, Al-Araji S A, Ragunath K. Review article: gastrointestinal angiodysplasia - pathogenesis, diagnosis and management. Aliment Pharmacol Therap. 2014;39:15–34. doi: 10.1111/apt.12527. [DOI] [PubMed] [Google Scholar]
- 4.Boley S J, Sammartano R, Adams A et al. On the nature and etiology of vascular ectasias of the colon. Degenerative lesions of aging. Gastroenterol. 1977;72:650–660. [PubMed] [Google Scholar]
- 5.Steger A C, Galland R B, Hemingway A et al. Gastrointestinal haemorrhage from a second source in patients with colonic angiodysplasia. B J Surgery. 1987;74:726–727. doi: 10.1002/bjs.1800740824. [DOI] [PubMed] [Google Scholar]
- 6.ASGE Standards of Practice Committee . Pasha S F, Shergill A et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc. 2014;79:875–885. doi: 10.1016/j.gie.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Jensen D M, Machicado G A. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterol. 1988;95:1569–1574. doi: 10.1016/s0016-5085(88)80079-9. [DOI] [PubMed] [Google Scholar]
- 8.Olmos J A, Marcolongo M, Pogorelsky V et al. Long-term outcome of argon plasma ablation therapy for bleeding in 100 consecutive patients with colonic angiodysplasia. Dis Colon Rectum. 2006;49:1507–1516. doi: 10.1007/s10350-006-0684-1. [DOI] [PubMed] [Google Scholar]
- 9.Jackson C S, Gerson L B.Management of gastrointestinal angiodysplastic lesions (GIADs): a systematic review and meta-analysis Am J Gastroenterol 2014109474–483.; quiz 84 [DOI] [PubMed] [Google Scholar]
- 10.Jensen D M, Machicado G A. Colonoscopy for diagnosis and treatment of severe lower gastrointestinal bleeding. Routine outcomes and cost analysis. Gastrointest Endosc Clin North Am. 1997;7:477–498. [PubMed] [Google Scholar]
- 11.Moss A, Bourke M J, Metz A J. A randomized, double-blind trial of succinylated gelatin submucosal injection for endoscopic resection of large sessile polyps of the colon. The Am J Gastroenterol. 2010;105:2375–2382. doi: 10.1038/ajg.2010.319. [DOI] [PubMed] [Google Scholar]
- 12.Moss A, Williams S J, Hourigan L F et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57–65. doi: 10.1136/gutjnl-2013-305516. [DOI] [PubMed] [Google Scholar]
- 13.Technology Assessment Committee . Croffie J, Somogyi L et al. Sclerosing agents for use in GI endoscopy. Gastrointest Endosc. 2007;66:1–6. doi: 10.1016/j.gie.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Soehendra N, Grimm H, Stenzel M. Injection of nonvariceal bleeding lesions of the upper gastrointestinal tract. Endoscopy. 1985;17:129–132. doi: 10.1055/s-2007-1018481. [DOI] [PubMed] [Google Scholar]
- 15.Igawa A, Oka S, Tanaka S et al. Major predictors and management of small-bowel angioectasia. BMC Gastroenterol. 2015;15:108. doi: 10.1186/s12876-015-0337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J G, Lieberman D A. Complications related to endoscopic hemostasis techniques. Gastrointest Endosc Clin North Am. 1996;6:305–321. [PubMed] [Google Scholar]
- 17.ASGE Standards of Practice Committee . Fisher D A, Maple J T et al. Complications of colonoscopy. Gastrointest Endosc. 2011;74:745–752. doi: 10.1016/j.gie.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Fahrtash-Bahin F, Holt B A, Jayasekeran V et al. Snare tip soft coagulation achieves effective and safe endoscopic hemostasis during wide-field endoscopic resection of large colonic lesions (with videos) Gastrointest Endosc. 2013;78:158–163 e1. doi: 10.1016/j.gie.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Pohl H, Grimm I S, Moyer M T et al. Clip closure prevents bleeding after endoscopic resection of large colon polyps in a randomized trial. Gastroenterology. 2019 doi: 10.1053/j.gastro.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


