Abstract
Background
Gatifloxacin is used for the treatment of multidrug-resistant tuberculosis (MDR-TB). The optimal dose is unknown.
Methods
We performed a 28-day gatifloxacin hollow-fiber system model of tuberculosis (HFS-TB) study in order to identify the target exposures associated with optimal kill rates and resistance suppression. Monte Carlo experiments (MCE) were used to identify the dose that would achieve the target exposure in 10000 adult patients with meningeal or pulmonary MDR-TB. The optimal doses identified were validated using probit analyses of clinical data from 2 prospective clinical trials of patients with pulmonary and meningeal tuberculosis. Classification and regression-tree (CART) analyses were used to identify the gatifloxacin minimum inhibitory concentration (MIC) below which patients failed or relapsed on combination therapy.
Results
The target exposure associated with optimal microbial kill rates and resistance suppression in the HFS-TB was a 0–24 hour area under the concentration-time curve-to-MIC of 184. MCE identified an optimal gatifloxacin dose of 800 mg/day for pulmonary and 1200 mg/day for meningeal MDR-TB, and a clinical susceptibility breakpoint of MIC ≤ 0.5 mg/L. In clinical trials, CART identified that 79% patients failed therapy if MIC was >2 mg/L, but 98% were cured if MIC was ≤0.5 mg/L. Probit analysis of clinical data demonstrated a >90% probability of a cure in patients if treated with 800 mg/day for pulmonary tuberculosis and 1200 mg/day for meningeal tuberculosis. Doses ≤400 mg/day were suboptimal.
Conclusions
Gatifloxacin doses of 800 mg/day and 1200 mg/day are recommended for pulmonary and meningeal MDR-TB treatment, respectively. Gatifloxacin has a susceptible dose-dependent zone at MICs 0.5–2 mg/L.
Keywords: Monte Carlo experiments, hollow-fiber system model, machine learning, susceptibility breakpoint, probit regression
The emergence of multidrug-resistant tuberculosis (MDR-TB) has been well chronicled, as have the potential reasons [1]. In patients with MDR-TB treated with gatifloxacin-containing regimens, the treatment success rate was 87% in gatifloxacin-susceptible tuberculosis versus 51% with high-level gatifloxacin resistance [2]. Successful treatment outcomes in extensively drug-resistant tuberculosis (XDR-TB), which is MDR-TB with resistance to quinolones and aminoglycosides, are a dismal 28% [3]. Thus, the use of fluoroquinolones in treating MDR-TB is crucial for therapy success. While preclinical pharmacokinetics/pharmacodynamics (PK/PD) work has been performed for moxifloxacin, no PK/PD–derived target exposure for gatifloxacin has been derived for Mycobacterium tuberculosis (Mtb) [4]. Since high-dose gatifloxacin could be less arrhythmogenic than high-dose moxifloxacin, it is imperative that equivalent dosing be established for high-dose gatifloxacin [5]. Given that quinolone- and aminoglycoside-acquired drug resistance (ADR) arise in 9–14% of patients after an average of 5 months of combination therapy for MDR-TB, it is crucial to identify a gatifloxacin dose that suppresses ADR [6, 7]. Here, we performed PK/PD studies for gatifloxacin microbial kill rates and ADR suppression in the hollow-fiber model of tuberculosis (HFS-TB).
The HFS-TB, with repetitive sampling for drug concentrations and bacterial burdens, allows the derivation of the relationships between drug exposure, microbial kill rates, and resistance suppression for monotherapy and for combination therapy [4, 8–11]. Delineation of these PK/PD relationships allows for the estimation of clinical doses [8, 12–15]. Since drug concentrations achieved in patients with tuberculosis are a major driver of both therapy failure and ADR, the estimation of clinical doses takes into account the pharmacokinetic variability [16–21]. Similarly, the antibiotic minimum inhibitory concentration (MIC) is also an important determinant of a patient’s outcome, with a dramatic fall in the patient response once a breakpoint MIC is achieved [21–26]. Here, we utilized population pharmacokinetics (and thus variability) of gatifloxacin from 169 patients with tuberculosis, gatifloxacin MICs from 243 Mtb isolates, and the PK/PD target derived from the HFS-TB to identify the gatifloxacin doses likely to be effective in pulmonary and meningeal tuberculosis, and the MIC breakpoint above which therapy is expected to fail, in Monte Carlo experiments (MCE) [27, 28]. The dose versus outcome relationships and MIC breakpoints derived thereof were then validated using clinical trial data from actual patients. Currently, doses of 200 mg to 1200 mg are given in MDR-TB.
METHODS
Materials, Isolates, and Reagents
Stock Mtb H37Ra (American Type Culture Collection # 25177) culture stored at −80°C in Middlebrook 7H9 broth was thawed before each experiment and grown into logarithmic growth phase (log-phase) in a Middlebrook 7H9 broth supplemented with 10% oleic acid, dextrose, and catalase at 37°C under 5% CO2, under shaking conditions. Gatifloxacin powder was purchased from Sigma-Aldrich and moxifloxacin-13Cd3 from United States Pharmacopeia (Rockville, MD). Hollow-fiber cartridges were purchased from FiberCell (Frederick, MD). We utilized the BACTEC MGIT 960 Mycobacterial Growth Indicator Tube (MGIT) System for monitoring time-to-positivity (TTP; Franklin Lakes, NJ).
Minimum Inhibitory Concentration and Screening for Gatifloxacin Intracellular Effect
MICs of the laboratory strain were identified using the standard macrobroth dilution reference method and the MGIT assay, at concentrations of 0.03125–16 mg/L on a 2-fold dilution scale, as described previously [29, 30]. We used the 1% proportion method. Next, we examined the microbial kill characteristics of gatifloxacin, at the same concentrations, in extracellular and intracellular Mtb over 7 days of co-incubation, as described in detail in prior studies [29, 30].
Hollow-fiber System Model of Tuberculosis and Pharmacokinetics/Pharmacodynamics Modeling
The construction of the HFS-TB and descriptions of how it works have been extensively published, as detailed in the introduction paper in this supplement [5, 8, 15, 17, 31]. Log-phase growth Mtb cultures were inoculated into 7 HFS-TB units, and 24 hours later were started on treatment with gatifloxacin doses to achieve daily AUC 0–24/MIC exposures of 0, 18, 37, 83, 171, 389, and 678 at a half-life of 11 hours for 28 days. The central compartment of each HFS-TB was sampled at 0, 1, 4, 8, 12, 22, and 23.5 hours post–first dose, and gatifloxacin concentrations were measured. The peripheral compartment was sampled for colony forming units (CFU) on Middlebrook 7H10 agar supplemented with 10% oleic acid, dextrose, and catalase and for MGIT TTP [5, 9, 30, 32, 33]. CFUs of a gatifloxacin-resistant subpopulation growing on agar supplemented with gatifloxacin 3-times MIC were identified. The gatifloxacin-resistant colonies underwent whole genome sequencing (WGS), with specific mutations confirmed by Sanger sequencing [30, 33].
Gatifloxacin AUC0-24/MIC versus total Mtb log10 CFU/mL was examined using the inhibitory sigmoid Emax model, and the exposures associated with 50% (EC50) and 80% (EC80) of the maximal kill (Emax) were identified [8]. The AUC0-24/MIC versus the size of the ADR population was examined using the quadratic equation, as described in the introduction paper in this supplement [8, 34]. The EC80 and exposure-suppressing ADR were defined as target exposures.
Dose Finding Using Monte Carlo Experiments
We performed 10000 patient MCEs for gatifloxacin doses of 200, 400, 600, and 800 mg/day, using ADAPT 5 software [35]. We used the gatifloxacin parameter estimates shown in Table 1 and identified by others [28]. The gatifloxacin concentration in tuberculosis cavities-to-serum was assumed to be 1.3-fold, based on epithelial lining fluid and bronchial mucosa studies and the caseum protein unbound proportion in tuberculous cavities [36–38]. Sensitivity testing was performed for a 1-fold change (ie, similar concentration to serum) in lung cavities. The gatifloxacin AUC0-24 penetration ratio into the cerebrospinal fluid (CSF) in tuberculous meningitis (TBM) was 0.48, as identified in both patients and rabbits [25, 39].
Table 1.
Comparison of Pharmacokinetic Parameters in Simulation to Patients
| Domain of Input in Subroutine PRIOR | 10000 Simulated Subjects | FDA Gatifloxacin Label Information | |
|---|---|---|---|
| Clearance in L/hr (mean ± SD) | 6.2 ± 2.0 | 6.1 ± 2.0 | 8.8 ± 1.5a |
| Volume in L (mean ± SD) | 141.0 ± 31.0 | 141.0 ± 8.8 | ... |
| Absorption constant (hr-1) | 1.3 ± 0.4 | 1.3 ± 0.6 | ... |
| 200 mg oral | |||
| AUC0-24 mg*hr/L | ... | 20.8 ± 2.8 | 14.2 ± 0.4b |
| Peak concentration in mg/L | ... | 1.3 ± 0.1 | 2.0 ± 0.4b |
| 400 mg oral | |||
| AUC0-24 mg*hr/L | ... | 41.6 ± 5.6 | 51.3 ± 20.4a |
| Peak concentration in mg/L | ... | 2.5 ± 0.2 | 4.2 ± 1.9a |
Abbreviations: AUC, area under the concentration-time curve; FDA, Food and Drug Administration; SD, standard deviation.
aMultiple dose in patients with non-tuberculous infection.
bHealthy volunteers, single dose.
We validated the MCE using recommended steps [8], including a comparison to the concentrations achieved by doses of 200 mg and 400 mg, used for Food and Drug Administration licensing (https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21061s023,024,21062s026,037lbl.pdf). We calculated the target attainment probability (TAP) at each MIC in the 243 isolates from Spain [27]. This MIC distribution from Spain was based on Middlebrook 7H11 agar, which could have higher MICs by 1-tube dilution, compared to MGIT. Therefore, in a further sensitivity analysis, to account for differences in the methods used to identify MIC, we also utilized a distribution derived in the MGIT by Isaeva, et al. [40]. Cumulative fraction of response (CFR) was calculated by summing over this MIC range, as described elsewhere [8]. The CFR is the proportion of 10000 patients treated with a specified dose who achieved the target exposure over the MIC range.
External Validation of Monte Carlo Experiment Dose and Minimum Inhibitory Concentration Findings Using Clinical Trials Data
Data on the relationship between gatifloxacin dose, MIC, CSF exposure, and patient outcomes in 15 patients with TBM have been published by Thwaites and colleagues [25]. We used those patient-level data to examine the relationship between the dose (mg/kg) and the proportion of patients with a specified outcome in probit regression modeling. The outcomes examined were (1) patient survival, (2) death or disability, (3) relapse, and (4) a composite therapeutic success or good outcome, defined as not experiencing death, disability, failure, or relapse.
Similarly, we examined the clinical outcomes in 161 patients in Bangladesh with confirmed pulmonary MDR-TB who were treated with varying gatifloxacin doses of 200–800 mg by weight band [26]. The main outcomes and patient characteristics in this study have been reported before [3, 26]. Patient isolates at therapy commencement had gatifloxacin MICs identified based on the Löwenstein-Jensen medium. Mutations in the deoxyribonucleic acid gyrase A (gyrA) and gyrase B (gyrB) subunits genes were also identified. We performed probit regression for the relationship between a cure and the gatifloxacin dose in mg/kg. Next, we utilized a machine-learning algorithm, classification, and regression tree analysis (CART) to identify the MIC above which combination therapy fails, following steps in our prior work [23, 24]. We then performed a probit analysis of dose versus good clinical outcome for patients with MICs above and below this threshold.
RESULTS
Gatifloxacin Microbial Kill of Intracellular and Extracellular Mtb
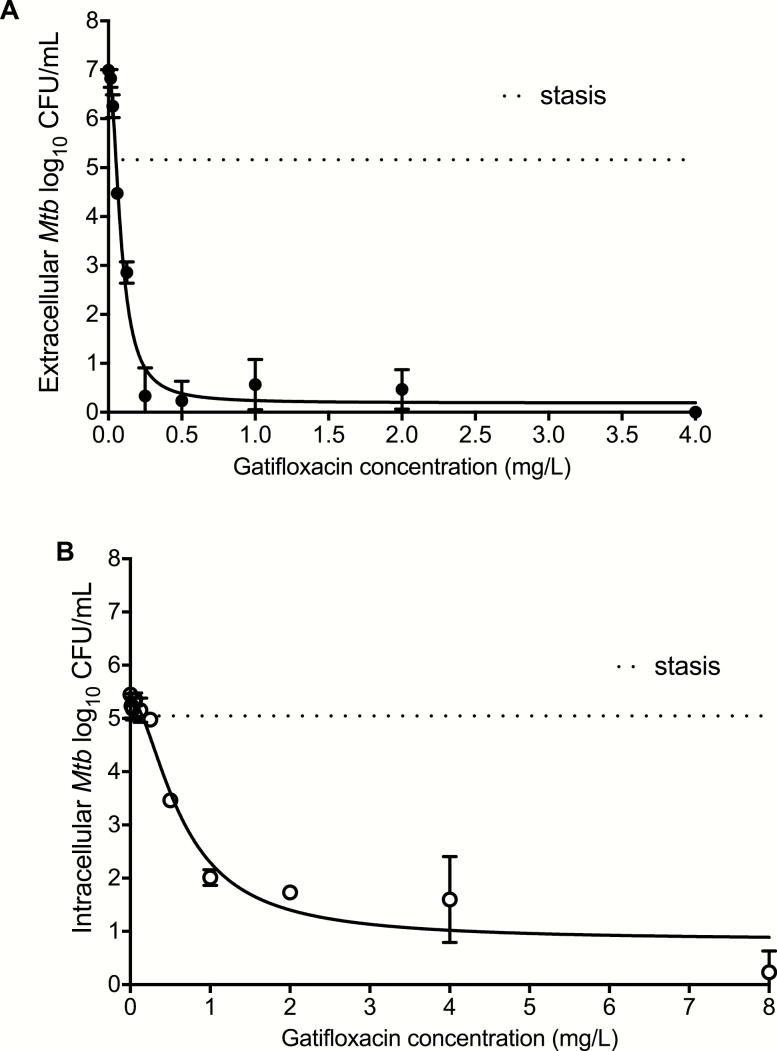
The gatifloxacin MIC of Mtb H37Ra was 0.06 mg/L with the MGIT assay, but 0.125 mg/L by the reference microbroth-dilution method. Therefore, we adopted an MIC of 0.125 mg/L; however, for sensitivity analyses for dose findings, we compared the findings to those using the MGIT-derived MICs (see below). Next, we compared the microbial kill rates of intracellular and extracellular Mtb by gatifloxacin, with results shown in Figure 1. Gatifloxacin achieved an Emax of 6.79 log10 CFU/mL (95% confidence interval [CI] 6.30–7.32) against the log-phase growth extracellular Mtb in 7 days (Figure 1). The EC50 was 0.08 mg/L (95% CI: 0.07–0.10; r2 = 0.98). Figure 1 also shows the results of the intracellular Mtb study. The Emax was 4.55 mg/L (95% CI: 3.94–5.65) and the EC50 was 0.64 mg/L (95% CI: 0.50–1.01; r2 = 0.95).
Figure 1.
Gatifloxacin effect against extracellular and intracellular Mtb at static concentrations. Concentrations were examined in triplicate, and results shown are for mean and standard deviations, with an error bar. The dotted line indicates the starting (day 0) bacterial burden. A, After 7 days of co-incubation, gatifloxacin completely eliminated the log-phase growth of extracellular Mtb, with a kill of 5.16 ± 0.03 log10 CFU/mL below stasis. B, Gatifloxacin showed a considerable effect against intracellular Mtb, with a kill of 4.82 ± 0.40 log10 CFU/mL below stasis. However, this was slightly less than that observed against extracellular Mtb. Abbreviations: CFU, colony forming units; Mtb, Mycobacterium tuberculosis.
Hollow-fiber Model of Tuberculosis Study Results
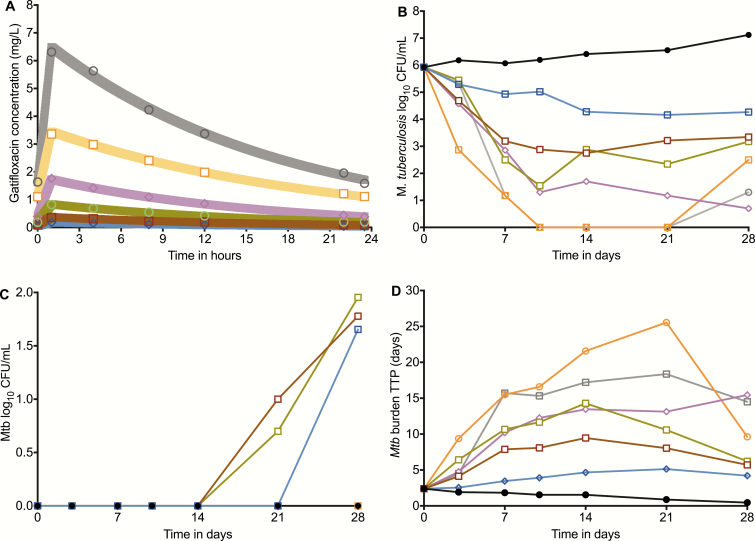
The gatifloxacin concentration-time profiles achieved in the HFS-TB are shown in Figure 2. Pharmacokinetic modeling revealed a mean ± standard deviation elimination-rate constant of 0.06 ± 0.01 hr-1 and a volume of distribution of 0.52 ± 0.05 L. The pharmacokinetic model–predicted versus –observed concentrations had a slope of 1.00 ± 0.01 (r2 > 0.99), indicating no bias. Figure 2 demonstrates the time-kill curves in the HFS-TB based on the CFU/mL readout. At AUC0-24/MIC > 171.2, gatifloxacin completely sterilized the HFS-TB on day 10, but rebounded by the end of the study. Figure 2 also shows that a gatifloxacin-resistant subpopulation arose in the 3 lowest gatifloxacin exposures, starting after day 10, and was responsible for the rebound. However, there was a gatifloxacin exposure above which no drug-resistant subpopulation arose. Figure 2 shows the kill curves based on TTP, which confirmed that the Mtb population was actually not all killed on day 10.
Figure 2.
Gatifloxacin pharmacokinetics and time-kill curves in the HFS-TB. A, The pharmacokinetic model–predicted gatifloxacin concentrations are shown with the shaded graph lines, while the concentrations observed on direct measurement are shown as the symbols. The concentrations achieved were used to calculate the 24 hr AUC and AUC/MICs. B, Time-kill curves based on log10 CFU/mL versus the AUC/MIC achieved in each HFS-TB unit revealed a biphasic decline at each exposure. C, There was an emergence of a gatifloxacin-resistant subpopulation on days 14 and 21 in the 3 lowest exposures, while higher exposures suppressed resistance. In the non-treated controls, the percentage of the subpopulation that was gatifloxacin-resistant did not change and remained close to 0%. D, When bacterial burden was expressed as TTP, the bacterial burden demonstrated the same biphasic pattern, but starting at day 14 and with rebound or regrowth in virtually all systems. None of the HFS-TB units demonstrated complete sterilization with monotherapy. Abbreviations: AUC, area under the concentration-time curve; CFU, colony forming units; HFS-TB, hollow fiber system model of tuberculosis; MIC, minimum inhibitory concentration; Mtb, Mycobacterium tuberculosis; TTP, time-to-positivity.
Pharmacokinetics/Pharmacodynamics Modeling for Microbial Kill and Acquired Drug Resistance Suppression
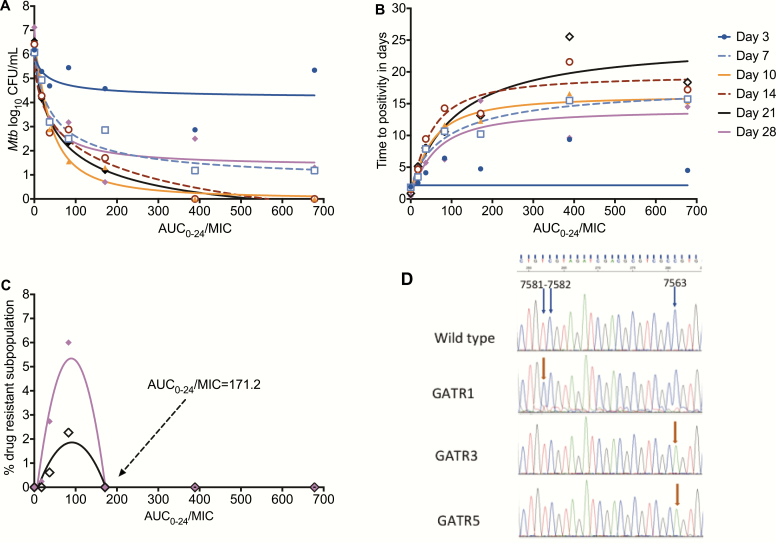
Figure 3 shows exposure versus the total Mtb population. Given the rebound growth in the 3 lowest doses on day 28, the model achieved a moderate fit at the end of the experiment (r2 = 0.89), with an EC80 that was an AUC0-24/MIC of 82. On the other hand, the TTP assay–based results demonstrated better model fits (Figure 3), with the lowest Akaike Information Criteria score noted on day 10 and associated with an EC80 that was an AUC0-24/MIC of 147, while the EC80 at the end of the experiment was an AUC0-24/MIC of 184. The percentage of the gatifloxacin-resistant subpopulation versus exposure is shown in Figure 3. The AUC0-24/MIC associated with the suppression of ADR was 171.2. Taken together, the gatifloxacin exposure that would achieve both a maximal kill rate and suppression of ADR was an AUC0-24/MIC ≥ 184.
Figure 3.
Exposure versus effect models for microbial kill and acquired resistance. A, Inhibitory sigmoid Emax curves for gatifloxacin microbial kill in log10 CFU/mL for each day of sampling. The EC80s can be read off the graphs for each day at the inflection point of each curve. B, The same inhibitory sigmoid curves, based on TTP readout. The model fit was better with this readout (except on day 3) than with log10 CFU/mL. C, Quadratic function model for acquired drug resistance revealed a model fit associated with an r2 = 0.82 on day 21 (black) and r2 = 0.90 on day 28 (magenta). The AUC/MIC of 171.2 is shown, which was associated with resistance suppression. D, Illustration of mutations in gyrA of some gatifloxacin-resistant isolates. The mutations cluster at position 7563 of gyrA in all resistant isolates but 1. Abbreviations: AUC, area under the concentration-time curve; CFU, colony forming units; EC80, exposure associated with 80% of maximal kill; Emax, maximal kill exposure; gyrA, gyrase subunit A; MIC, minimum inhibitory concentration; Mtb, Mycobacterium tuberculosis; TTP, time-to-positivity.
WGS of the gatifloxacin-resistant isolates identified several gyrA mutations. Sanger sequencing confirmation results are shown in Figure 3. The gatifloxacin-resistant isolates carried either an A>G Asp94Gly, G>A Asp94Asn, or G>T Gly88Cys gyrA mutation, all in the quinolone resistance–determining region [41].
Monte Carlo Experiment Dose-finding for Pulmonary and Meningeal Tuberculosis
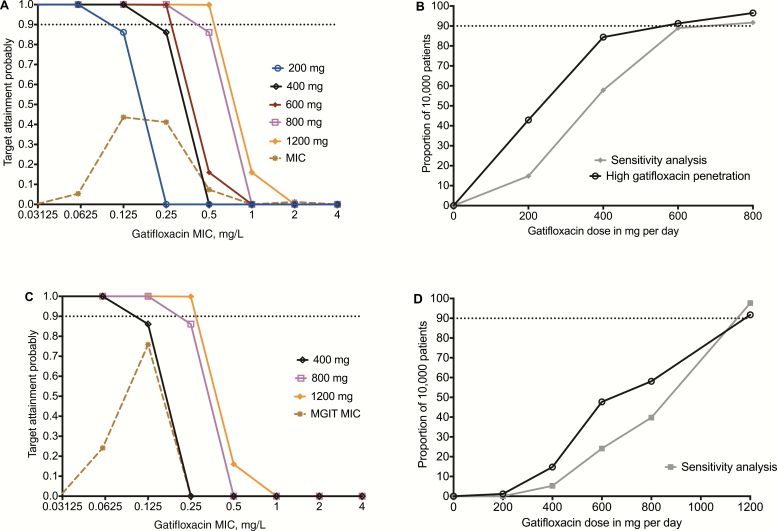
The TAPs at different MICs by different doses in 10000 patients with pulmonary tuberculosis are shown in Figure 4. The TAP for the dose of 400 mg/day fell precipitously at MIC = 0.25 mg/L; with 800 mg/day this occurred at 0.5 mg/L. When the TAP was summated over the MIC range of the 243 Mtb Spanish isolates, the proportion of patients who achieved target exposures (ie, CFR) were as shown in Figure 4. The optimal dose was 600 mg/day, which achieved the target exposure in 91% of cases. However, in a sensitivity analysis assuming a 1:1 penetration ratio, the 600 mg dose fell just short of 90% and the optimal dose was 800 mg/day. At this dose, the gatifloxacin TAP fell just short of 90% at the MIC of 0.5 mg/L, based on the Middlebrook 7H11 agar dilution test. In a second sensitivity analysis, we utilized the MGIT-derived MICs of our laboratory isolate (0.0625 mg/L), which increased the PK/PD exposure targets by a factor of 2 from an AUC0-24/MIC ≥ 184 to ≥368: together, the MGIT-derived MIC distribution of Mtb isolates from Russian patients [40]. Results are shown in Figure 4.
Figure 4.
Performance of different gatifloxacin doses in Monte Carlo experiments. A, TAP for the AUC0-24/MIC ≥ 184 target at Middlebrook 7H11 medium MICs of 243 isolates from Spain [27]. B, The CFR, with and without sensitivity analysis, demonstrated that 800 mg/day was the minimal dose to consistently achieve the CFR > 90% in pulmonary tuberculosis. C, Sensitivity analyses based on MGIT MICs from Russia identified similar doses and MIC breakpoints as those based on the Middlebrook 7H11 medium. D, The CFR in patients with TBM revealed that a gatifloxacin dose of 1200 mg/day would be required to achieve target exposures in >90% of patients, even on sensitivity analyses. Abbreviations: AUC, area under the concentration-time curve; CFR, cumulative fraction of response; MGIT, BACTEC MGIT 960 Mycobacterial Growth Indicator Tube System; MIC, minimum inhibitory concentration; TAP, target attainment probability; TBM, tuberculous meningitis.
Next, we examined the performance of doses in suppressing ADR target exposure in TBM, as shown in Figure 4. The dose of 400 mg/day was associated with a mean CSF AUC0-24/MIC of 208.6, with a range of 1.9 to 901.0. The CFR for 400 mg was 15%, while that for 600 mg was 48%. In a sensitivity analysis, the dose of 1200 mg/day remained as the best for TBM.
Validation of Monte Carlo Experiment–identified Doses With Clinical Data
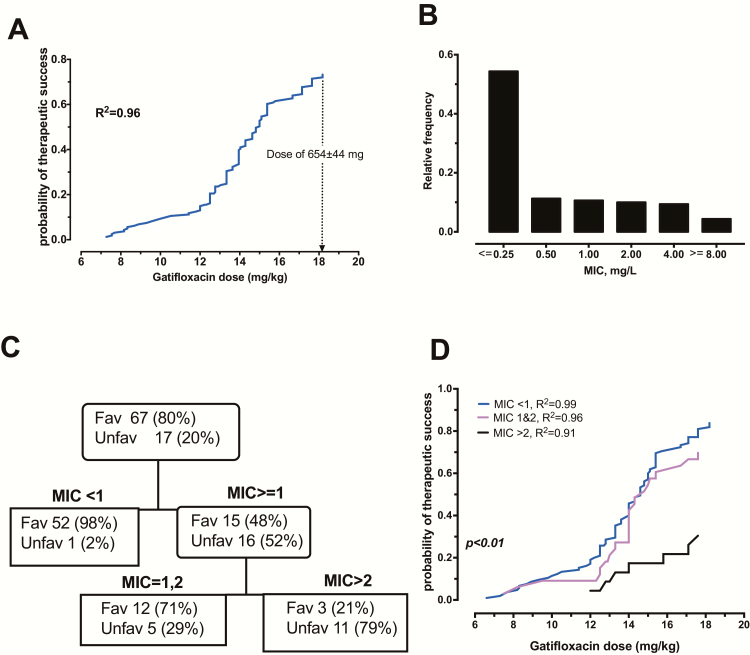
Next, we examined the clinical outcomes in 161 Bangladesh patients treated with gatifloxacin plus ethambutol, pyrazinamide, clofazimine, kanamycin, prothionamide, and isoniazid during the first 4 months, followed by gatifloxacin, ethambutol, pyrazinamide, and clofazimine for 5 months. Their clinical characteristics are shown in Table 2. Probit analyses of doses in mg/kg versus the probability of a favorable outcome (cure without relapse) revealed the results shown in Figure 5. The figure shows a relatively flat response between doses of 6–12 mg/kg, with favorable outcomes of less than 20%, followed by a steep dose-versus-success curve up until the dose of 18.18 mg/kg, with a probability of success of 73%. The curve is still on an upswing, such that, based on the shape of the curve, a 90% cure would be achieved by a dose of 801.37 mg, given the weights of patients in study. That dose is virtually the same as that identified by MCE.
Table 2.
Demographic and Clinical Characteristics of 161 Patients With Multidrug-resistant Tuberculosis
| Clinical or Laboratory Feature | Estimate |
|---|---|
| Gender | |
| Age | |
| Weight in kg, median (range) | 43 (26–65) |
| Pulmonary tuberculosis, n (%) | 161 (100%) |
| Gatifloxacin dose, median (range) | 600 (400–800) |
| Gatifloxacin dose mg/kg, median (range) | 14.0 (6.6–18.2) |
| Minimum inhibitory concentration in mg/L, median (range) | 0.25 (<0.25 to >8) |
| Gyrase mutations in pretreatment isolates | |
| Wild type | 108 (67%) |
| Gyrase subunit A mutations | 53 (33%) |
| Outcomes | |
| Cure | 131 (81%) |
| Failure | 23 (14%) |
| Relapse | 7 (4%) |
Figure 5.
Gatifloxacin MIC and doses predictive of microbiologic cure in pulmonary tuberculosis patients. A, Probability of therapeutic success (cure without relapse) increases as gatfloxacin doses increase (r2 = 0.96). A dose of 654 mg would achieve success of about 73%, while that of 801.37 mg would achieve a probability of success of 90%. B, Löwenstein–Jensen agar-based MIC distribution in 161 Mtb clinical isolates at the start of therapy. About 30% of the MDR-TB isolates had MICs of 1.0 to 4 mg/L. C, A pruned classification and regression in the test-sample tree shows that the highest node that separated patients who succeeded (98%) was an MIC of <1.0 mg/L (ie, 0.5 mg/L and below), with the rest having favorable outcomes in only 48%. The second node was of MICs of 1 and 2 mg/L, with a favorable outcome in 71% of patients versus 21% of patients when MIC > 2.0 mg/L, which are exactly the success rates for XDR-TB. Half of the data was used for training the model, which is why 84 patients are shown in this figure. D, There are, in fact, 3 different dose response curves on probits (P < .01): 1 for patients with Löwenstein-Jensen–based MICs < 1.0 mg/L; 1 at MICs of 1 and 2 mg/L; and 1 at MIC > 2 mg/L, indicating resistance. Abbreviations: Fav, favorable outcome/therapeutic success; MDR-TB, multidrug-resistant tuberculosis; MIC, minimum inhibitory concentration; Mtb, Mycobacterium tuberculosis; Unfav, unfavorable outcomes/therapeutic failures; XDR-TB, extensively drug-resistant tuberculosis.
The distribution of gatifloxacin MICs in the 161 MDR-TB patients is shown in Figure 5; 78% of isolates were wild-type, while 22% had gyrA and or gryB mutations. We divided the 161-patient data set randomly into 2 equal halves, trained a CART model to identify the gatifloxacin MIC above which patients with pulmonary tuberculosis failed the combination therapy or relapsed after a cure, and then used the second dataset for validation. The validated CART model shown in Figure 5 had a receiver operating characteristic curve score of 0.86, a precision of 0.96, and a specificity of 0.87. Since the receiver operating characteristic curve on the learn model after 10-fold cross-validation was 0.91 ± 0.04, this CART model will be highly reproducible when applied to future independent data. The CART model shown in Figure 5 identified 2 breakpoints: the primary node was MIC < 1.0 mg/L and the secondary node was MICs 1–2 mg/L. Thus, there is a susceptibility breakpoint of 0.5 mg/L and an intermediate susceptibility zone of 1–2 mg/L. The odds ratio for failure/relapse at an MIC > 1 mg/L versus MICs < 1 mg/L for the entire data was 21.74 (95% CI: 7.09–66.67; P < .001). Given these findings, we performed a probit analysis of mg/kg dose in patients infected with Mtb that had MICs ≤ 0.5 mg/L versus 1.0–2.0 mg/L versus ≥ 1 mg/L, which revealed the results shown in Figure 5. At the dose of 800mg/day, favorable outcomes were observed in 54% of patients with MIC > 1.0 mg/L versus 84% with MIC < 1.0 mg/L. There were 13 patients with gatifloxacin MIC < 1 who were cured but had the weight and gatifloxacin dose data missing; otherwise, the proportion cured in this group would have been 96%.
Gatifloxacin Dose Versus Cure in Patients With Meningeal Tuberculosis
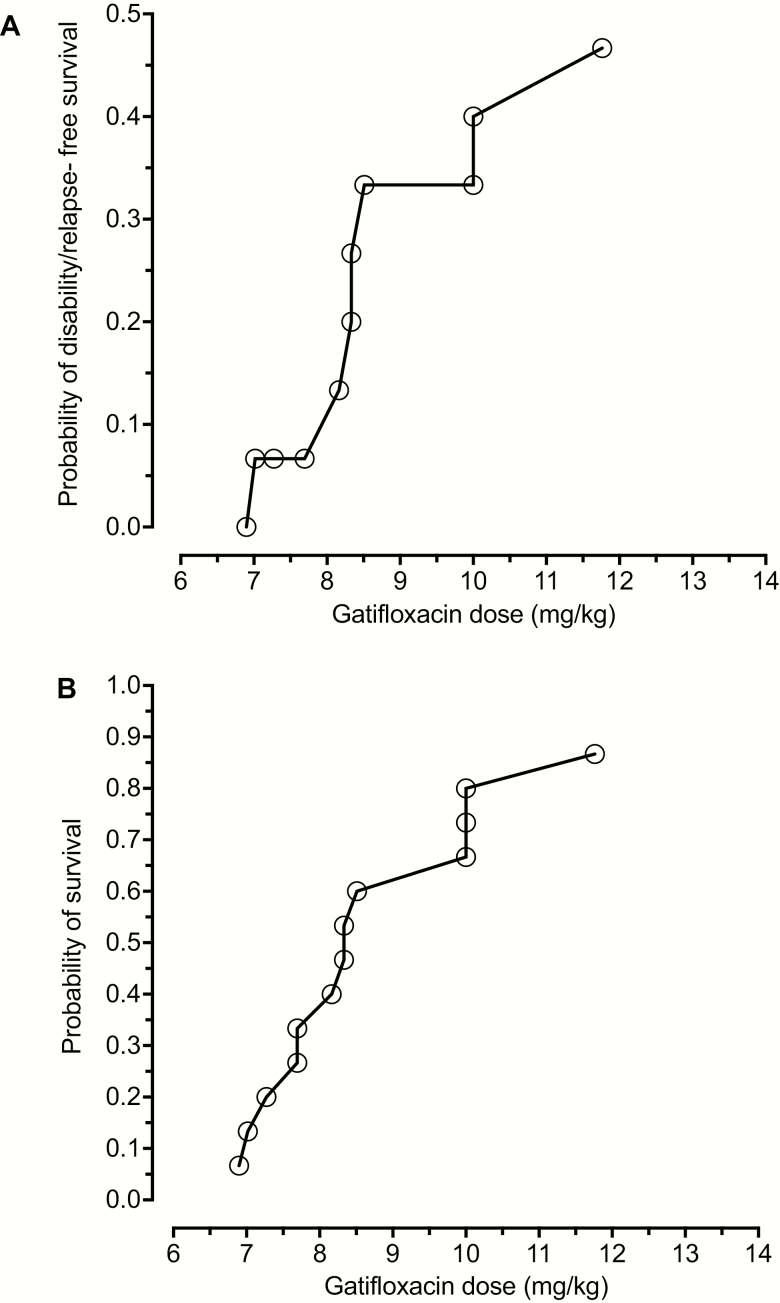
A group of 15 Vietnamese patients with TBM were treated with gatifloxacin at 400 mg/day for the first 60 days of treatment; full clinical details of these patients have been published elsewhere [25] and further treatment details are in Supplementary Table 1. The gatifloxacin dose achieved a mean CSF AUC0-24/MIC of 148.2 (95% CI: 74.4–222.3); the 95% CI is within the values we identified in MCE with this dose. The overall favorable outcome (no disability, death, or relapse) at this dose was 7/15 (47%). Probit analyses of dose mg/kg versus outcome were as shown in Figure 6 (r2 = 0.95); the probability of disability/relapse–free survival at the median dose of 8.33 mg/kg was 20.0–26.7%. The curves mirror those for MCE in TBM patients. However, the confidence intervals in the probits were too wide to accurately calculate the dose associated with a 90% probability of a favorable response.
Figure 6.
Gatifloxacin dose versus probability of favorable outcome in tuberculous meningitis. A, Plot of probability of gatifloxacin dose (mg/kg) versus survival based on relapse- and disability-free survival in 15 patients treated with 400mg of gatifloxacin for tuberculous meningitis. Any neurological disability was taken as a poor outcome, regardless of severity, as were death and relapse. B, Plot of probability of survival versus dose in mg/kg. In both cases, confidence intervals for the EC50 were too wide to be able to calculate an optimal dose with precision.
DISCUSSION
Firstly, we found that gatifloxacin kill curves and Emax were reminiscent of those of moxifloxacin, while the time to ADR was better than that documented in the past for moxifloxacin in the same HFS-TB model [4]. MCE identified a gatifloxacin dose of 800 mg/day that could minimize the commonly-observed ADR rates in MDR-TB treatment, while optimizing the microbial kill rate. This dose has been given before to patients with MDR-TB, and was well tolerated by patients [2, 26]. In the probit analyses, a similar dose was calculated to be associated with at least a 90% probability of a cure in MDR-TB patients with pulmonary TB. Thus, the gatifloxacin doses of 200–400 mg/day for pulmonary tuberculosis currently utilized by some are suboptimal; that of 600 mg/day is good, but not optimal; and that of 800 mg would be the best, especially when used for the replacement of high-dose moxifloxacin. An equivalent dose for levofloxacin for the same target has been derived elsewhere [42].
Secondly, the MCE performances of different doses to achieve target exposures for efficacy and suppression of resistance in CSF were examined. Thwaites, et al., have demonstrated that quinolone AUC/MIC mapped with a favorable outcome in patients with TBM is a U-shaped curve, with an optimal zone of CSF AUC0-24/MIC of 14–252 for survival outcome, 0–240 for post-treatment disability, and 94–352 for relapse [25]. Interestingly, our target exposure AUC0-24/MIC of 184 falls squarely in the middle of this optimal zone of exposure ranges. Probit analyses curves mirrored MCEs, confirming the dose-response relationship. We found that the dose of 1200 mg/day would be best able to achieve the target exposure in TBM. This is a high dose, however, and would only be applicable to patients with MDR-TB in whom there are otherwise no other good second drugs that penetrate the CSF and achieve a sufficient kill rate [43]. With drug-susceptible TB, other drugs that penetrate CSF would also be able to kill the Mtb, and the gatifloxacin optimal dose could be less under those circumstances [44].
The proposed high gatifloxacin doses could increase the rates of adverse events. As an example, dysglycemia is already encountered in 2–9% of MDR-TB patients on 400 mg/day and is especially problematic in the elderly [45, 46]. Moreover, in a large, retrospective study of 605127 patients on standard doses for mundane bacteria that were treated with a shorter duration than that used for MDR-TB, the adjusted rate ratios (aRR) of serious arrhythmia were highest with gatifloxacin (aRR = 7.38), followed by moxifloxacin (aRR = 3.30) and ciprofloxacin (aRR = 2.15) [47]. However, in a randomized, controlled trial of 1602 patients in which half received 400 mg/day of gatifloxacin for tuberculosis, there was no difference in the corrected QT interval lengthening (QTC) at 1, 2, and 4 months of therapy on or off gatilfloxacin, and concentrations did not correlate with QTc [5].
Thirdly, we propose a gatifloxacin susceptibility breakpoint of 0.25 mg/L in MGIT assays and 0.5 mg/L with macrobroth dilution assays at the 800mg/day dose. Interestingly, the agnostic CART algorithm identified a susceptibility breakpoint MIC of 0.5 mg/L on Löwenstein-Jensen agar based on patient outcomes. Further, based on the findings from the clinical study, we defined an intermediate-susceptibility dose-dependent zone of 1–2 mg/L on Löwenstein-Jensen agar, while the MCE-based zone would be 0.5–1 mg/L in broth, at which 1200 mg of gatifloxacin could lead to a microbial kill. Elsewhere, we have introduced a similar concept for first-line anti-tuberculosis drugs [48].
The gatifloxacin-resistant isolates in the HFS-TB had either a Asp94Gly, Asp94Asn, or Gly88Cys gyrA mutation, which are the most commonly encountered in fluoroquinolone-resistant MDR-TB strains [41]. These quinolone resistance–determining region mutations confer high-level fluoroquinolones resistance, while Gly88Cys has been associated with intermediate susceptibilities [49]. The ability to repetitively sample the HFS-TB through time in order to document an evolution of resistance—and the WGS—allowed us to catch the “antibiotic resistance arrow of time” in process [10, 11]. Mutations arose at specific “resistance amplifying” exposures, but not at high exposures, suggesting that high enough concentrations can shut down, or at least delay, quinolone resistance.
There are several limitations to our studies. First, more precise PK/PD exposure targets could be identified if different Mtb isolates were used in the HFS-TB experiments. However, the MCE take into account isolates with a large range of MICs in calculating the optimal dose. Moreover, the clinical validation portion of our study suggests that the findings have clinical meaning. A second limitation is that optimal exposures and doses are regimen-specific; lower gatifloxacin doses may be needed in the case of a regimen with drugs that are synergistic or have high efficacies [21, 32]. In addition, the HFS-TB does not include the immune system, and the contribution of the immune system to therapy success could reduce the requirement for a large dose. Thus, our dose choices should be considered a worst-case scenario. However, the validation study suggests that in the case of gatifloxacin, the doses derived using the HFS-TB reflect clinical reality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. T. G. and D. D. designed the study; T. G., D. D., and P. B. performed the hollow fiber studies; D. D. wrote the first draft of the manuscript; T. K. performed deoxyribonucleic acid extraction; T. G. and S. S. performed the whole genome sequencing analysis; T. G. performed pharmacokinetics/pharmacodynamics modeling and Monte Carlo experiments; W. S. and H. M. performed the gatifloxacin population pharmacokinetic analyses; J. G. P. and T. G. performed Probit and classification and regression tree analyses of clinical data; S. M. B., P. G. A., H. M., G. T., and A. vD. performed the clinical studies and wrote the paper with special emphasis of clinical details from the clinical studies; M. G. and A. vD. performed minimum inhibitory concentration and gyrA sequencing in the 161 clinical isolates; D. D., J. G. P., S. S., and T. G. wrote the manuscript; and all authors edited and contributed to the final version of the manuscript.
Acknowledgments. The authors thank Dr. Claudio Köser for many insightful comments into the paper and for discussion on susceptibilities and minimum inhibitory concentrations.
Financial support. This work was supported by the Baylor Research Institute and the Wellcome Trust, United Kingdom.
Supplement sponsorship. This supplement is sponsored by the Baylor Institute of Immunology Research of the Baylor Research Institute.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dheda K, Gumbo T, Maartens G, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med 2017; pii: S2213-2600(17)30079–6. doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 2. Aung KJ, Van Deun A, Declercq E, et al. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 2014; 18:1180–7. [DOI] [PubMed] [Google Scholar]
- 3. Migliori GB, Sotgiu G, Gandhi NR, et al. ; The Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. Drug resistance beyond extensively drug-resistant tuberculosis: individual patient data meta-analysis. Eur Respir J 2013; 42:169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis 2004; 190:1642–51. [DOI] [PubMed] [Google Scholar]
- 5. Olliaro PL, Merle C, Mthiyane T, et al. Effects on the QT Interval of a Gatifloxacin-containing regimen versus standard treatment of pulmonary tuberculosis. Antimicrob Agents Chemother 2017; 61:e01834–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cegielski JP, Dalton T, Yagui M, et al. ; Global Preserving Effective TB Treatment Study (PETTS) Investigators. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2014; 59:1049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kempker RR, Kipiani M, Mirtskhulava V, Tukvadze N, Magee MJ, Blumberg HM. Acquired drug resistance in Mycobacterium tuberculosis and poor outcomes among patients with multidrug-resistant tuberculosis. Emerg Infect Dis 2015; 21:992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gumbo T, Alffenaar JWC. An introduction to pharmacokinetics/pharmacodynamics methods and scientific evidence base for dosing of second line tuberculosis drugs. Clin Infect Dis 2018; 67(Suppl 3):S267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deshpande D, Srivastava S, Nuermberger E, Pasipanodya JG, Swaminathan S, Gumbo T. A faropenem, linezolid, and moxifloxacin regimen for both drug-susceptible and multidrug-resistant tuberculosis in children: FLAME path on the milky way. Clin Infect Dis 2016; 63:S95–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmalstieg AM, Srivastava S, Belkaya S, et al. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother 2012; 56:4806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pasipanodya JG, Nuermberger E, Romero K, Hanna D, Gumbo T. Systematic analysis of hollow fiber model of tuberculosis experiments. Clin Infect Dis 2015; 61(Suppl 1):S10–7. [DOI] [PubMed] [Google Scholar]
- 12. Gumbo T, Pasipanodya JG, Romero K, Hanna D, Nuermberger E. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin Infect Dis 2015; 61(Suppl 1):S25–31. [DOI] [PubMed] [Google Scholar]
- 13. Pasipanodya J, Gumbo T. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob Agents Chemother 2011; 55:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gumbo T, Angulo-Barturen I, Ferrer-Bazaga S. Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J Infect Dis 2015; 211(Suppl 3):S96–S106. [DOI] [PubMed] [Google Scholar]
- 15. Gumbo T, Louie A, Liu W, et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother 2007; 51:2329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pasipanodya JG, Gumbo T. A meta-analysis of self-administered vs directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin Infect Dis 2013; 57:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis 2011; 204:1951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pasipanodya JG, Srivastava S, Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis 2012; 55:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rockwood N, Pasipanodya JG, Denti P, et al. Concentration-dependent antagonism and culture conversion in pulmonary tuberculosis. Clin Infect Dis 2017; 64:1350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swaminathan S, Pasipanodya JG, Ramachandran G, et al. Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis 2016; 63:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chigutsa E, Pasipanodya JG, Visser ME, et al. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 2015; 59:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gumbo T. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob Agents Chemother 2010; 54:1484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gumbo T, Pasipanodya JG, Wash P, Burger A, McIlleron H. Redefining multidrug-resistant tuberculosis based on clinical response to combination therapy. Antimicrob Agents Chemother 2014; 58:6111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gumbo T, Chigutsa E, Pasipanodya J, et al. The pyrazinamide susceptibility breakpoint above which combination therapy fails. J Antimicrob Chemother 2014; 69:2420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thwaites GE, Bhavnani SM, Chau TT, et al. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother 2011; 55:3244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rigouts L, Coeck N, Gumusboga M, et al. Specific gyrA gene mutations predict poor treatment outcome in MDR-TB. J Antimicrob Chemother 2016; 71:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodríguez JC, Ruiz M, López M, Royo G. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents 2002; 20:464–7. [DOI] [PubMed] [Google Scholar]
- 28. Smythe W, Merle CS, Rustomjee R, et al. Evaluation of initial and steady-state gatifloxacin pharmacokinetics and dose in pulmonary tuberculosis patients by using Monte Carlo simulations. Antimicrob Agents Chemother 2013; 57:4164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deshpande D, Srivastava S, Pasipanodya JG, et al. Linezolid for infants and toddlers with disseminated tuberculosis: first steps. Clin Infect Dis 2016; 63:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deshpande D, Srivastava S, Chapagain M, et al. Ceftazidime-avibactam has potent sterilizing activity against highly drug-resistant tuberculosis. Sci Adv 2017; 3:e1701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gumbo T, Lenaerts AJ, Hanna D, Romero K, Nuermberger E. Nonclinical models for antituberculosis drug development: a landscape analysis. J Infect Dis 2015; 211(Suppl 3):S83–95. [DOI] [PubMed] [Google Scholar]
- 32. Deshpande D, Srivastava S, Nuermberger E, Pasipanodya JG, Swaminathan S, Gumbo T. Concentration-dependent synergy and antagonism of linezolid and moxifloxacin in the treatment of childhood tuberculosis: the dynamic duo. Clin Infect Dis 2016; 63:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Srivastava S, Magombedze G, Koeuth T, et al. Linezolid dose that maximizes sterilizing effect while minimizing toxicity and resistance emergence for tuberculosis. Antimicrob Agents Chemother 2017; 61:e00751–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gumbo T, Dona CS, Meek C, Leff R. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother 2009; 53:3197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. D’Argenio DZ, Schumitzky A, Wang X.. ADAPT 5 user’s guide: Pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles, CA: Biomedical Simulations Resource, 2009. [Google Scholar]
- 36. Honeybourne D, Banerjee D, Andrews J, Wise R. Concentrations of gatifloxacin in plasma and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother 2001; 48:63–6. [DOI] [PubMed] [Google Scholar]
- 37. Kikuchi J, Yamazaki K, Kikuchi E, Ishizaka A, Nishimura M. Pharmacokinetics of gatifloxacin after a single oral dose in healthy young adult subjects and adult patients with chronic bronchitis, with a comparison of drug concentrations obtained by bronchoscopic microsampling and bronchoalveolar lavage. Clin Ther 2007; 29:123–30. [DOI] [PubMed] [Google Scholar]
- 38. Pienaar E, Sarathy J, Prideaux B, et al. Comparing efficacies of moxifloxacin, levofloxacin and gatifloxacin in tuberculosis granulomas using a multi-scale systems pharmacology approach. PLoS Comput Biol 2017; 13:e1005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perrig M, Acosta F, Cottagnoud M, Gerber CM, Täuber MG, Cottagnoud P. Efficacy of gatifloxacin alone and in combination with cefepime against penicillin-resistant Streptococcus pneumoniae in a rabbit meningitis model and in vitro. J Antimicrob Chemother 2001; 47:701–4. [DOI] [PubMed] [Google Scholar]
- 40. Isaeva Y, Bukatina A, Krylova L, Nosova E, Makarova M, Moroz A. Determination of critical concentrations of moxifloxacin and gatifloxacin for drug susceptibility testing of Mycobacterium tuberculosis in the BACTEC MGIT 960 system. J Antimicrob Chemother 2013; 68:2274–81. [DOI] [PubMed] [Google Scholar]
- 41. Maruri F, Sterling TR, Kaiga AW, et al. A systematic review of gyrase mutations associated with fluoroquinolone-resistant Mycobacterium tuberculosis and a proposed gyrase numbering system. J Antimicrob Chemother 2012; 67:819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deshpande D, Pasipanodya JG, Mpagama SG, et al. Levofloxacin pharmacokinetics-pharmacodynamics, dosing, susceptibility breakpoints, and AI in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2018; 67(Suppl 3):S312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Akkerman OW, Odish OF, Bolhuis MS, et al. Pharmacokinetics of bedaquiline in cerebrospinal fluid and serum in multidrug-resistant tuberculous meningitis. Clin Infect Dis 2016; 62:523–4. [DOI] [PubMed] [Google Scholar]
- 44. Pouplin T, Bang ND, Toi PV, et al. Naïve-pooled pharmacokinetic analysis of pyrazinamide, isoniazid and rifampicin in plasma and cerebrospinal fluid of Vietnamese children with tuberculous meningitis.BMC Infect Dis 2016; 16:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park-Wyllie LY, Juurlink DN, Kopp A, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med 2006; 354:1352–61. [DOI] [PubMed] [Google Scholar]
- 46. Chiang CY, Van Deun A, Rieder HL. Gatifloxacin for short, effective treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2016; 20:1143–7. [DOI] [PubMed] [Google Scholar]
- 47. Lapi F, Wilchesky M, Kezouh A, Benisty JI, Ernst P, Suissa S. Fluoroquinolones and the risk of serious arrhythmia: a population-based study. Clin Infect Dis 2012; 55:1457–65. [DOI] [PubMed] [Google Scholar]
- 48. Zuur MA, Pasipanodya JG, van Soolingen D, van der Werf TS, Gumbo T, Alffenaar JC. Intermediate susceptibility dose- dependent breakpoints for high dose rifampicin, isoniazid and pyrazinamide treatment in multidrug-resistant tuberculosis programmes. Clin Infect Dis 2018; 67:1743–9. [DOI] [PubMed] [Google Scholar]
- 49. Willby M, Sikes RD, Malik S, Metchock B, Posey JE. Correlation between GyrA substitutions and ofloxacin, levofloxacin, and moxifloxacin cross-resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2015; 59:5427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.