Human immunization with a polymorphic malaria vaccine candidate, MSP2, induced functional cross-reactive antibodies targeting conserved epitopes. This contrasts with naturally acquired antibodies, which target polymorphic epitopes, mediating immune escape. Findings reveal potential to overcome antigenic diversity for effective malaria vaccines.
Keywords: malaria, merozoite surface protein 2, vaccine, opsonic phagocytosis, antibody cross-reactivity, complement
Abstract
Background
Overcoming antigenic diversity is a key challenge in the development of effective Plasmodium falciparum malaria vaccines. Strategies that promote the generation of antibodies targeting conserved epitopes of vaccine antigens may provide protection against diverse parasites strains. Understanding differences between vaccine-induced and naturally acquired immunity is important to achieving this goal.
Methods
We analyzed antibodies generated in a phase 1 human vaccine trial, MSP2-C1, which included 2 allelic forms of MSP2, an abundant vaccine antigen on the merozoite surface. Vaccine-induced responses were assessed for functional activity against multiple parasite strains, and cross-reactivity of antibodies was determined using competition ELISA and epitope mapping approaches.
Results
Vaccination induced cytophilic antibody responses with strain-transcending opsonic phagocytosis and complement-fixing function. In contrast to antibodies acquired via natural infection, vaccine-induced antibodies were directed towards conserved epitopes at the C-terminus of MSP2, whereas naturally acquired antibodies mainly targeted polymorphic epitopes. Functional activity of C-terminal–targeted antibodies was confirmed using monoclonal antibodies that promoted opsonic phagocytosis against multiple parasite strains.
Conclusion
Vaccination generated markedly different responses to polymorphic antigens than naturally acquired immunity and targeted conserved functional epitopes. Induction of antibodies targeting conserved regions of malaria antigens provides a promising vaccine strategy to overcome antigenic diversity for developing effective malaria vaccines.
(See the Editorial commentary by Angrisano and Blagborough, on pages 5–6.)
There is a continuing strong need for an effective malaria vaccine to enhance control and elimination efforts. This is highlighted by recent estimates suggesting that the global burden of malaria has not declined in the last 3 years with approximately 220 million cases annually [1]. Persisting challenges in malaria vaccine development are polymorphisms and antigenic diversity in leading vaccine candidates, which impacts on vaccine efficacy [2]. For the most advanced vaccine, RTS,S, protective efficacy was significantly higher against Plasmodium falciparum infections with vaccine-similar strains than infections with vaccine-dissimilar strains [3]. Phase 2 trials of candidate vaccines containing the merozoite antigens AMA1 or MSP2 [4], or trials with whole-cell P. falciparum attenuated sporozoites [5], also showed evidence of strain-specific efficacy. Naturally acquired immunity to malaria is typically slow to develop and requires repeated exposure to malaria over time; this slow acquisition is attributed in part to the requirement for the development of a sufficiently broad repertoire of antibodies against diverse strains [6, 7].
Vaccine strategies to overcome antigenic diversity and vaccine escape include incorporating multiple alleles, developing antigens with modified sequences to address antigenic diversity, or targeting conserved or less-diverse regions and epitopes [2]. However, there is currently limited knowledge on the specificity and cross-reactivity of naturally acquired or vaccine-induced human immune responses to vaccine candidates. Further, the conditions and approaches that favor the development of cross-reactive responses, which may overcome or limit immune escape through antigenic diversity, are unknown. While some strategies have been explored in animal models [8–12], little is known about induction of cross-reactive or variant-specific responses in humans. Understanding this would greatly assist the development of highly efficacious vaccines to meet the vaccine efficacy target of 75% set by the World Health Organization [13].
We investigated these questions in a phase 1 clinical trial of the P. falciparum malaria vaccine candidate MSP2 [14], an abundant protein on the surface of merozoites that infect erythrocytes during blood-stage replication [15, 16]. MSP2 is a target of naturally acquired antibodies that function through promoting opsonic phagocytosis by monocytes [17, 18] and activating complement to inhibit merozoite invasion and cause merozoite lysis [19]. A prior phase 2 trial of an MSP2-based vaccine, known as Combination B, demonstrated efficacy against P. falciparum parasitemia, but this effect was principally strain specific [4]. The vaccine showed significant efficacy against infections with 3D7-like alleles (vaccine strain), but not against infections with alternate alleles. MSP2 occurs as 2 predominant allelic types (3D7 and FC27) and polymorphisms are mostly contained within the central variable region, which also includes highly polymorphic tandem repeats [20, 21]. In addition, there is a short N-terminal sequence, which contains some polymorphic residues, and a longer conserved C-terminal region. To address the issue of vaccine escape demonstrated in the trial by Genton et al [4], a vaccine containing a combination of 3D7 and FC27 alleles was manufactured (MSP2-C1) and tested in a phase 1 trial.
In this study, we used MSP2 as a model to better understand the specificity and function of human antibodies generated by vaccines compared with naturally acquired responses, to determine whether the MSP2 combination vaccine successfully generated functional antibodies to different P. falciparum strains, and to determine the specificity of vaccine-induced antibodies. Surprisingly, we found that the vaccine-generated antibodies predominantly targeting conserved C-terminal epitopes, in striking contrast to allele-specific naturally acquired antibodies that predominantly targeted polymorphic epitopes. This provided the first clear evidence that a P. falciparum subunit vaccine in humans can redirect responses to conserved and functional epitopes.
METHODS
More detailed description of methods can be found in Supplementary Methods.
Study Cohorts
MSP2-C1 Vaccine Trial Sera
Samples from a total of 29 participants were obtained from a phase 1 trial of the MSP2-C1 vaccine [14]. Twenty-three participants were in vaccine groups and 6 participants were in the placebo groups.
Naturally Acquired Antibody Samples—Mugil Cohort
Samples were collected from a total of 206 school-age (5–14 years) children as part of a treatment reinfection study conducted in Papua New Guinea in 2004 [22].
Naturally Acquired Samples—XMX Cohort
Plasma samples were available from a cross-sectional study of adults (n = 49, median age 28 years) conducted in Madang Province, Papua New Guinea in 2007 [23].
Ethics Statement
Written informed consent was obtained from all study participants, or in the case of children, parents or guardians. For human vaccine trial samples, the study was approved by the Queensland Institute of Medical Research Human Research Ethics Committee and by the Western Institutional Review Board, which is the designated research ethics board for PATH Malaria Vaccine Initiative, the sponsor of the trial. The study was conducted in accordance with Declaration of Helsinki principles for the conduct of clinical trials and the International Committee of Harmonization Good Clinical Practice Guidelines as recognized by the Australian Therapeutic Goods Administration (TGA). The trial was conducted with regulatory oversight by the TGA under the Clinical Trial Notification scheme, and registered at the Australian and New Zealand Clinical Trials registry (www.anzctr.org.au/ACTRN12607000552482.aspx). For Mugil and XMX cohorts, approvals were obtained from the Papua New Guinea Medical Research Advisory Council, the Human Research and Ethics Committees of the Alfred Hospital, and the Walter and Eliza Hall Institute.
Direct ELISA
Vaccine trial serum samples were tested by standard enzyme-linked immunosorbent assay (ELISA) methods to determine antibody reactivity to MSP2 recombinant antigens. Samples were tested for IgG1, IgG2, IgG3, and IgG4, and total IgM against vaccine-grade recombinant MSP2 3D7 and FC27 antigens, as described previously [14, 24].
Competition ELISAs
To quantify the levels of allele-specific and cross-reactive antibodies to MSP2, serum samples (day 28 and day 112) from 16 vaccine trial subjects who were highly responsive in direct ELISA were tested. In addition, 18 sera samples from the Mugil cohort with high levels of MSP2 antibodies were tested. Competition ELISA was performed as previously described [23, 25]. Briefly, competing antigens were added to sera prior adding the sera to antigen-coated plates for a direct ELISA. The level of antibody reactivity that was out-competed by the competing antigen was calculated as measurement of antibody cross-reactivity.
Epitope Mapping Peptide Array
A pool of serum samples from high responders of the MSP2 vaccine trial (n = 10, day 112) and a pool of serum samples from naturally exposed children (n = 10, Mugil cohort) were tested against an MSP2 peptide array as previously described [26]. The array contained 84 biotinylated 13-mer peptides covering the entire mature MSP2-3D7 and MSP2-FC27 sequences with overlap of 8 residues [26]. Additionally, samples from 3 MSP2-C1 vaccine recipients and 2 naturally exposed subjects were tested as individual samples.
Cell Culture
P. falciparum lines D10, 3D7, CS2, and W2mef were maintained in continuous culture and synchronized by sorbitol treatment as previously described [17]. THP1 monocytic cells were maintained as previously described [17].
Opsonic Phagocytosis of Merozoites by THP-1 Cells
Opsonic phagocytosis activity using undifferentiated THP-1 monocytes was performed and quantified as previously described [17].
C1q Fixation Assay
Measurement of the level of C1q fixation to recombinant MSP2-3D7 and MSP2-FC27 was performed according to a previous published method [19] with modifications. Briefly, Maxisorp 96-well plates (Nunc, Roskilde, Denmark) were coated with recombinant MSP2 3D7 or FC27 at 0.5μg/mL in phosphate-buffered saline (PBS) and incubated overnight at 4°C. Plates were blocked for 2 hours at 37°C with 1% casein-PBS. Serum samples were diluted at 1/100 in 0.1% casein and incubated for 2 hours in duplicates. Plates were incubated with human C1q (10 μg/mL in 0.1% casein-PBS, 30 minutes). C1q deposition was detected with rabbit anti-C1q antibodies (Dako; 1/1000), followed by anti-rabbit-horseradish peroxidase at 1/2500 (Millipore, North Ryde, Australia). Enzymatic activity was detected using 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) liquid substrate with reactions stopped after 30 minutes to 1 hour with 1% sodium dodecyl sulfate (SDS). The level of background signal was subtracted from each sample before the mean of each sample was calculated.
IgG Purification
Both pre- and postvaccination serum samples from 13 (10 high responders and 3 placebos) individuals with high total IgG responses at day 112 were selected for IgG purification using Melon Gel IgG Spin Purification Kit (Thermo Fisher, Scoresby, Australia). Assays were performed according to the manufacturer’s user manual.
RESULTS
Human Vaccination Induces Cytophilic Antibodies Against 3D7 and FC27 Alleles of MSP2
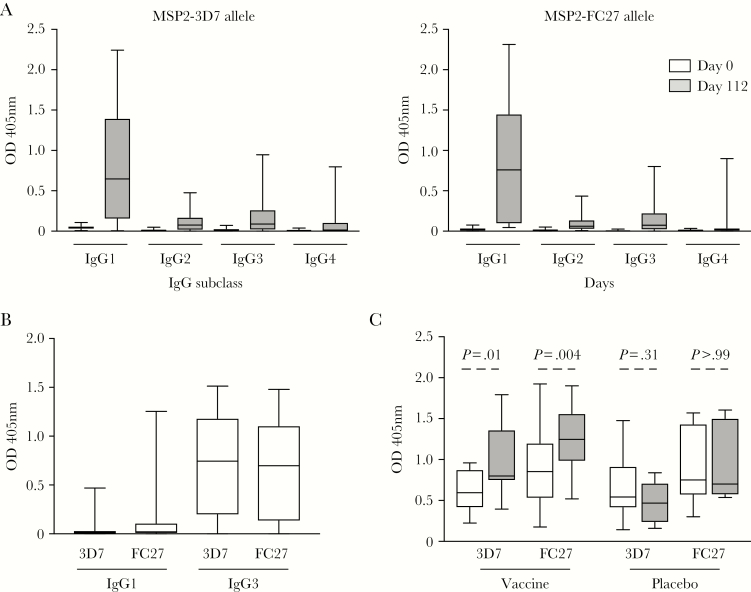
We previously showed that immunization with the MSP2-C1 vaccine induced IgG against both MSP2 alleles (MSP2-3D7 and MSP2-FC27), with responses peaking at day 112 [14]. Here, we further assessed the induction of specific IgG subclasses, along with IgM, against MSP2-3D7 and MSP2-FC27 recombinant proteins. Vaccine-induced antibodies were predominantly IgG1, with some IgG3 and very little IgG2 or IgG4 responses (Figure 1A). All responses peaked at day 112 (Supplementary Figure 1) and no statistically significant differences were observed in the magnitude of IgG1 and IgG3 responses between cohort 1 (three 10-μg doses) and cohort 2 (two 40-μg doses) (Supplementary Figure 2). In contrast to vaccine-induced antibodies, naturally acquired antibody responses against MSP2 were predominantly IgG3 (Figure 1B) [24]. Although the baseline level of IgM to MSP2 was high, which could be due to nonimmune IgM binding to MSP2, there was a substantial increase in IgM following immunization (Figure 1C). This increase was not observed with the placebo controls (Figure 1C). No difference in the magnitude of IgM levels following immunization was observed between cohort 1 and cohort 2 (Supplementary Figure 2). There was also significant induction of IgA antibodies; however, the magnitude of response was low compared to IgG1, IgG3, and IgM (Supplementary Figure 3).
Figure 1.
Human vaccine-induced and naturally acquired IgG subclass and IgM antibodies to MSP2. A, IgG subclasses (IgG1–IgG4) were assessed in sera from MSP2-C1 vaccine recipients prior to vaccination (day 0) and at day 112 postvaccination (peak response) against MSP2 3D7 (left) and FC27 (right) alleles. B, Naturally acquired IgG subclass responses to MSP2. Samples were obtained from malaria-exposed children residing in Papua New Guinea (ages 5–14, n = 206) [24]. C, IgM responses were measured in samples from MSP2-C1 vaccine (n = 29) and placebo (n = 6) recipients prior to vaccination (day 0, white boxes) and postvaccination (day 112, grey boxes) against both 3D7 and FC27 alleles. There was a significant increase in IgM responses against both alleles in vaccine recipients, but no difference was observed between day 0 and day 112 with the placebo controls. P values were calculated using the Wilcoxon matched-pairs signed rank test for the comparison between day 0 and day 112 samples.
Vaccination-Induced Antibodies Promote the Opsonic Phagocytosis of Multiple P. falciparum Strains
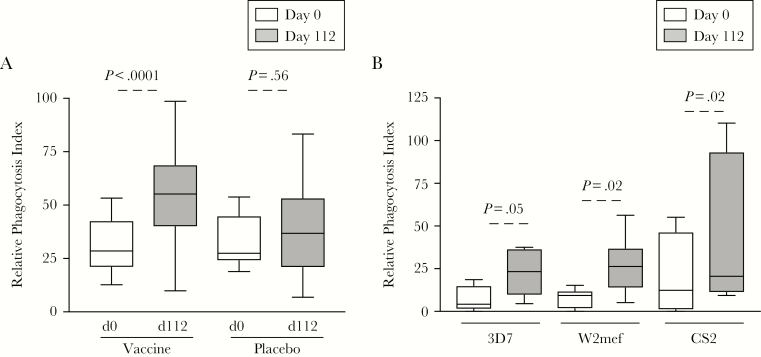
To assess whether cytophilic antibodies induced by vaccination were functional, we measured the capacity of antibodies to mediate opsonic phagocytosis of D10 merozoites (expressing MSP2-FC27 allele). There was a significant increase in the level of opsonic phagocytosis activity following vaccination in sera from patients receiving the active vaccine, but not with placebo controls (Figure 2A). The phagocytosis index was positively correlated with the level of MSP2-FC27–specific IgG and IgG subclass responses (Spearman ρ 0.48, 0.54, and 0.50 for total IgG, IgG1, and IgG3, respectively; P < .001 for all) but not IgM (Spearman ρ 0.10, P = .47) or IgA responses (Spearman ρ 0.29, P = .13) (Supplementary Figure 4).
Figure 2.
Human vaccination with MSP2 induced antibodies that promote merozoite opsonic phagocytosis against multiple parasite strains. A, The relative phagocytosis index was measured in samples from prevaccination (day 0) and 112 days postvaccination. Samples tested were from vaccine (n = 27) and placebo (n = 6) participants against D10 merozoites (expressing MSP-FC27). P values were calculated using the Wilcoxon matched-pairs signed rank test. Relative phagocytosis index represents activity relative to an IgG pool from malaria-exposed adults. B, The relative phagocytosis index was measured to 3D7 and W2mef parasites (expressing the MSP2-3D7 allele) and CS2 (expressing the MSP2-FC27allele). Samples tested were from prevaccination (day 0) and 112 days postvaccination using IgG purified sera from high vaccine responders, based on IgG reactivity by enzyme-linked immunosorbent assay (ELISA; n = 8). P values were calculated using Wilcoxon matched-pairs signed rank test comparing day 0 to day 112. Data are from 1 assay performed in duplicate.
To assess whether MSP2 antibodies induced by vaccination were functional against genetically diverse P. falciparum strains, we purified IgG from serum samples with high antibody levels to MSP2 following vaccination and tested these for opsonic phagocytosis activity against merozoites from genetically distinct parasite strains (CS2 parasites expressing the FC27 allele and 3D7 and W2mef parasites expressing the 3D7 allele). There was a significantly increased opsonic phagocytosis activity in postvaccination antibodies for all parasite strains (Figure 2B). Thus, vaccine-induced antibodies effectively mediated the opsonic phagocytosis of merozoites isolated from 2 parasite lines expressing the FC27 allele (D10 and CS2), and 2 isolates expressing the 3D7 allele (3D7 and W2mef) of MSP2.
Human Vaccination Induces Antibodies That Fix Complement
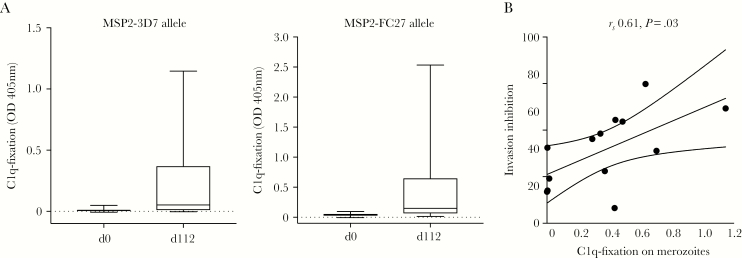
We previously showed that vaccination with MSP2-C1 induced complement-dependent invasion-inhibitory antibodies [19]. To quantify the ability of vaccine-induced antibodies to activate complement via the classical (antibody-dependent) complement pathway, we measured C1q fixation by serum samples from MSP2-C1 vaccine recipients to MSP2 protein. Vaccination induced C1q-fixing antibodies to MSP2-3D7 and MSP2-FC27 alleles, with a major increase in C1q fixation in postvaccination serum (Figure 3A). A subset of 10 serum samples with the highest total IgG to MSP2-FC27 from the vaccinees and 3 serum samples from day 0 was tested for invasion-inhibitory capacity [19]. The level of C1q-fixing antibodies to the surface of D10 merozoites (MSP2-FC27 expressing) correlated with complement-dependent invasion-inhibition activity using fresh serum as a source of complement (Spearman ρ = 0.61, P = .03; Figure 3B).
Figure 3.
MSP2 vaccination induced C1q-fixing antibodies to MSP2 that correlated with complement-dependent invasion inhibition activity. A, Serum samples derived from the MSP2 vaccine trial were assayed for C1q-fixing antibodies against the 3D7 or FC27 alleles of MSP2. Data are median optical density (OD) levels and interquartile ranges, day 0 prevaccination and 112 days postvaccination samples. C1q-fixation is reported as OD values measured by enzyme-linked immunosorbent assay (ELISA). B, C1q-fixing antibodies from postvaccination samples (day 112) were correlated with complement-dependent invasion inhibition activity.
Human Vaccination Induces Antibodies That Are Predominately Cross-Reactive Between MSP2 Alleles
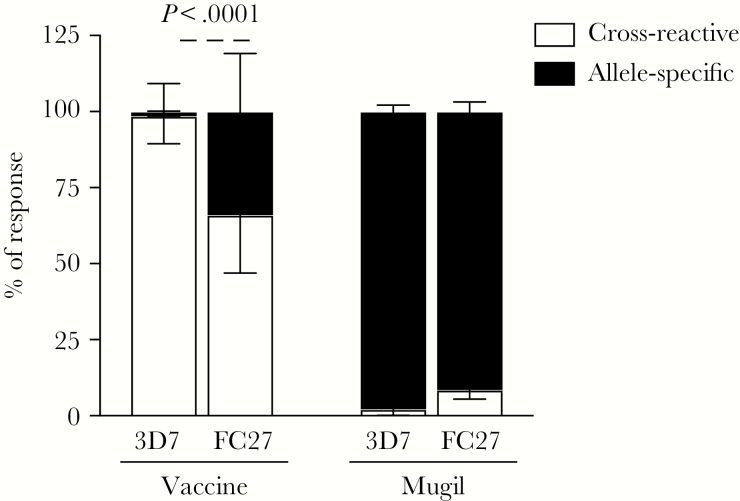
We assessed whether vaccine-induced antibodies were allelic specific or cross-reactive by measuring antibody responses using competition ELISA. Naturally acquired MSP2 antibodies are largely directed towards allele-specific regions [27]. Surprisingly, we found that the majority of antibody responses in vaccine recipients were highly cross-reactive to both 3D7 and FC27 alleles of MSP2. This was demonstrated by the ability of the FC27 allele to out-compete antibody binding to the 3D7 allele, and vice versa (Figure 4). The level of cross-reactive antibody responses was higher to 3D7 compared to the FC27 allele (3D7 99%, interquartile range [IQR] 89%–100%; FC27 66%, IQR 47%–78%; P < .001). Sera from 7/16 of subjects had antibodies to the 3D7 allele that were completely cross-reactive to FC27, while all FC27 responses were at least in part allele specific (Supplementary Figure 5). In stark contrast, the great majority of MSP2 antibodies from naturally exposed individuals from Papua New Guinea were allele specific; incubation of sera with the MSP2 FC27 allele could not effectively out-compete antibody binding to MSP-3D7 allele, and vice versa (Figure 4; MSP2-3D7 98%, IQR 77%–100%; MSP2-FC27 92%, IQR 88%–95%; Supplementary Figure 5).
Figure 4.
MSP2 cross-reactive and allele-specific responses induced by vaccination or acquired through natural malaria exposure. MSP2 antibody responses from vaccine trial recipients (Vaccine; n = 16) and individuals with naturally acquired antibodies (Mugil; n = 18) were tested in competition enzyme-linked immunosorbent assay (ELISA) and the percentage of response that was cross-reactive and allele specific was calculated. Data from tested selected individual samples are presented in Supplementary Figure 5. P values were calculated using the Wilcoxon matched-pairs signed rank test.
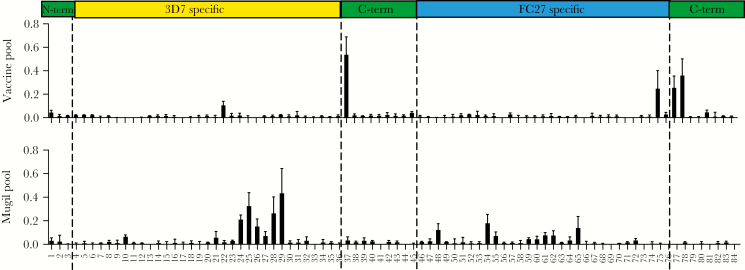
Human Immunization Generates Antibodies That Target Conserved C-Terminal Epitopes
To further understand the molecular basis of the cross-reactivity of vaccine-induced antibodies, and how it differs from naturally acquired antibodies, we epitope-mapped antibodies using a MSP2-peptide array [26] and pools of high responders from vaccinees or malaria naturally exposed individuals. Antibodies in the vaccine pool (n = 10) were reactive to several peptides from the conserved C-terminal region of MSP2 (Figure 5). In contrast, in the pool of sera from naturally exposed individuals, antibodies were directed to peptides within the 3D7 or FC27 allele-specific region (Figure 5). Peptide reactivity profiles of antibodies were further confirmed using individual samples from 3 vaccinees, and 2 naturally exposed individuals residing in Papua New Guinea (Supplementary Figure 6). In 1 of the vaccinated individuals, there were also antibodies directed to the 3D7 and FC27 allele-specific regions and a minor antibody response to 1 peptide in the N-terminal region (Supplementary Figure 6). Among naturally exposed individuals, antibodies to the conserved C-terminal were also observed, but at much lower levels than those seen to strain-specific regions or seen among vaccine samples (Supplementary Figure 6).
Figure 5.
Epitope mapping of vaccine-induced and naturally acquired antibodies. Antibodies were tested in an overlapping peptide array of MSP2. Vaccine pool represents 10 high-responding donors within the MSP2-C1 trial. Mugil pool represents sera from 10 malaria-exposed donors from the Mugil cohort in Papua New Guinea. Bars indicate mean reactivity from 2 experiments performed in duplicate; error bars represent SEM. Regions of the MSP2 protein corresponding to the peptide array are indicated.
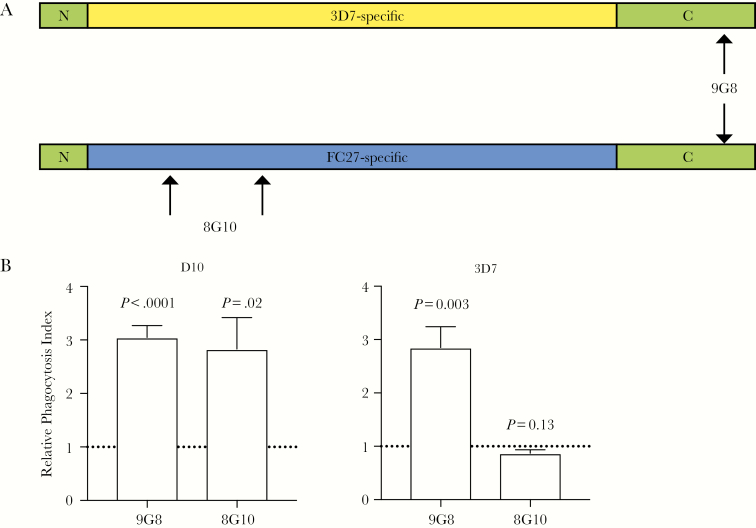
Antibodies to the Conserved C-Terminal Region of MSP2 Can Promote Opsonic Phagocytosis
To confirm that antibodies to C-terminal epitopes are functional, we assessed the ability of a monoclonal antibody (MAb) that is specific to the conserved C-terminal region of MSP2 (9G8; mouse IgG2b subclass, which can interact with human Fc-receptors) to promote opsonic phagocytosis (Figure 6A). For comparison, a MAb directed against the allele-specific variable region of MSP2-FC27 was tested (8G10, mouse IgG2b subclass) [26]. Assays were performed with both 3D7 parasites (expressing the MSP2-3D7 allele) or D10 parasites (expressing MSP-FC27). The C-terminal–targeted MAb, 9G8, promoted opsonic phagocytosis of merozoites from both 3D7 and D10 parasites (Figure 6B). In contrast, the allele-specific MAb 8G10 only promoted opsonic phagocytosis of the FC27 strain (D10), but not 3D7. Therefore, these results support the conclusion that human vaccination with MSP2 induced functional C-terminal targeted cross-reactive antibodies.
Figure 6.
Opsonic phagocytosis activity of a monoclonal antibody (MAb) targeting the conserved C-terminal region of MSP2. A, Schematic diagram of MSP2 with the binding sites of MAbs 8G10 and 9G8 indicated by arrows. B, MAbs to the C-terminus of MSP2 (9G8) and MSP2-FC27 allelic specific region (8G10) were tested for their opsonic phagocytosis activity against D10 parasites (expressing MSP2-FC27; left) and 3D7 parasites (expressing MSP2-3D7; right). The MAb 9G8 mediated the opsonic phagocytosis of both parasite strains, while 8G10 mediated the opsonic phagocytosis against D10 only. Unopsonized parasites were used as a negative control and the level of phagocytosis is calculated as a fold increase from unopsoniszed parasites. The line at 1 represents no fold change. Assays were performed thrice with samples tested in duplicate; bars represent the mean and SEM of the fold change values.
DISCUSSION
Here, we show that vaccination in humans with a bivalent vaccine induced cross-reactive and functional antibodies to the polymorphic malaria antigen MSP2, contrasting with the highly allele-specific nature of naturally acquired antibodies. Cross-reactive antibodies predominately targeted the conserved C-terminal region of MSP2. Using a C-terminal–targeted MAb, we confirmed that this region of MSP2 is the target of functional antibodies, and we showed that vaccine-induced antibodies had functional activity against multiple P. falciparum strains. The dominance of cross-reactive antibodies generated by vaccination is in contrast to the dominance of antibodies to the variable region acquired from natural infection. To the best of our knowledge, this is the first report of the successful generation of vaccine-induced cross-reactive antibodies with strain-transcending functional activity to a P. falciparum malaria antigen in humans. Additionally, our findings provide important new insights into how vaccine-induced responses in humans can differ markedly from naturally acquired immunity, which will inform future strategies for developing highly efficacious malaria vaccines.
Antigenic diversity in malaria vaccine antigens has been highlighted as a key impediment to vaccine efficacy [2]. Indeed, extensive antigen polymorphism, particularly in regions of high immunogenicity, are hypothesized to be a key immune evasion mechanism of the parasite [6]. MSP2 is a naturally immunogenic protein and significant antibodies can be acquired in young children after limited malaria exposure [28, 29], and polymorphisms are therefore consistent with MSP2 being an important target of naturally acquired immunity. The prior phase 2 trial of the MSP2-based vaccine, Combination B, contained a single allele of MSP2 and clearly demonstrated that MSP2 vaccine efficacy was highly strain specific [4]. Strain-specific efficacy in clinical trials has been seen for other malaria vaccine candidates based on single alleles such as AMA1 [30] and CSP [3]. To overcome the limitation of strain-specific efficacy, the MSP2-C1 vaccine combined 2 alleles of MSP2, resulting in generation of antibodies to both allelic families [14]. However, in contrast to the immunodominance of allele-specific variable regions in naturally exposed populations [27], our data clearly show that the majority of vaccine-induced antibodies were cross-reactive and directed to conserved C-terminal epitopes. Increased induction of cross-reactive antibodies has been previously reported in mice and rabbits using multiallele vaccination with AMA1 [10, 11]. However, it was not shown that this strategy resulted in antibodies targeting conserved epitopes; studies of naturally acquired AMA1 responses showed a strong allele-specific component of antibodies [23, 25]. Further, such an effect has not been previously demonstrated in humans, and some data have been reported suggesting that the nature of vaccine-induced antibody responses to malaria antigens can vary between humans and laboratory animals [31], highlighting the need for human studies of naturally acquired and vaccine-induced immunity to clearly inform vaccine development.
Our data show that epitopes in polymorphic and conserved C-terminal regions of MSP2 are immunogenic in humans; however, the immunodominance of these epitopes contrasted between vaccine-induced and naturally acquired responses. The mechanisms mediating the preferential induction of cross-reactive antibodies by vaccination remain unclear, but may include: (1) differences in antigenic load of conserved versus polymorphic epitopes when different alleles are used as mixtures; (2) conformational differences in the antigens when presented in a vaccine (recombinant soluble antigen) versus natural infection (membrane bound on the merozoite surface); and/or (3) adjuvant-driven changes. A recent study on influenza responses in mice indicated that route of antigen administration (nasal infection verses subcutaneous vaccination) and form of antigen modulated the immunodominance of specific antigen regions in the antibody response [32]. While further studies are needed to understand these mechanisms, our data indicate that it is possible to induce cross-reactive antibodies to conserved epitopes via vaccination in humans and this provides a strong rationale for future studies to understand the immunologic mechanisms underlying this effect that could be exploited in vaccine development.
Cross-reactive antibodies induced by the MSP2-C1 vaccination were functional, showing a capacity to promote opsonic phagocytosis of merozoites expressing different MSP2 alleles, and fix complement to MSP2. In contrast, vaccine-induced antibodies had no direct growth-inhibitory function [14], highlighting the importance of Fc-mediated effector function for antibodies targeting MSP2. Recent studies have indicated that these functional antibodies are associated with protection from malaria [17, 19, 33, 34]. Cytophilic antibodies IgG1 and IgG3 have a higher capacity to fix complement and mediate opsonic phagocytosis via Fc-receptor interactions than IgG2 and IgG4 [35]. MSP2-C1 vaccination induced both IgG1 and IgG3. While IgG1 was dominant over IgG3, in contrast to the pattern seen in highly exposed populations [24, 27], functional activity of vaccine-induced antibodies was still substantial. The functional significance of the difference in IgG1:IgG3 ratio remains to be determined. We further confirmed that antibodies to the C-terminal region were likely to be mediating the functional activity seen with vaccine-induced antibodies by demonstrating that a MAb to a conserved epitope of the C-terminal region of MSP2 could promote opsonic phagocytosis of merozoites from different parasite strains.
With the stalling of progress in reducing the global malaria burden despite ongoing control efforts [1], strategies to develop highly effective malaria vaccines are a high priority. In particular, strategies that overcome antigenic diversity will be essential in overcoming parasite polymorphisms and vaccine escape. Here, for the first time, we show that vaccination in humans can result in the induction of cross-reactive and functional antibodies targeting conserved epitopes of a P. falciparum polymorphic antigen, and contrast the polymorphic targets of naturally acquired immunity. Our data highlight the importance of detailed analyses of vaccine-induced responses in humans to fully understand the fine specificities and functional mechanisms of these responses. Further studies are needed to assess whether similar cross-reactive responses can be induced in children with prior malaria exposure. Indeed, findings from RTS,S suggest that differences in vaccine-induced responses may exist in those with a history of malaria [36]. Nevertheless, our findings advance the understanding of vaccine-induced antibodies and mechanisms allowing for the rational design of second-generation highly effective malaria vaccines, and support the development of vaccines based on polymorphic antigens.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all the study participants and the staff involved in the study from Papua New Guinea Institute of Medical Research, Madang, and all staff and participants involved in the MSP2-C1 clinical trial.
Financial support. This work was supported by the National Health and Medical Research Council of Australia (NHMRC; Program Grant and Senior Research Fellowship to J. G. B. and I. M., Project Grant, Early Career Fellowship, and Career Development Award to M. J. B.); the Australian Research Council (Future Fellowship to J. G. B.); and the Wellcome Trust (F. O.). The Burnet Institute is supported by the NHMRC Independent Research Institutes Infrastructure Support Scheme and the Victorian State Government Operational Infrastructure Support. The MSP2-C1 clinical trial was funded by the PATH Malaria Vaccine Initiative.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Malaria in American Society of Tropical Medicine and Hygiene Annual Meeting, Washington, DC, 2013; Lorne Infection and Immunity Conference, Lorne, Australia, 2015; Lorne Infection and Immunity Conference, Lorne, Australia, 2016; International Congress of Immunology, Melbourne, Australia, 2016; Malaria in Melbourne, Melbourne, Australia, 2015; and Molecular Approaches to Malaria, Lorne, Australia, 2016.
References
- 1. World Health Organization (WHO). World Malaria Report 2017. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 2. Ouattara A, Barry AE, Dutta S, Remarque EJ, Beeson JG, Plowe CV. Designing malaria vaccines to circumvent antigen variability. Vaccine 2015; 33:7506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neafsey DE, Juraska M, Bedford T, et al. Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N Engl J Med 2015; 373:2025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Genton B, Betuela I, Felger I, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis 2002; 185:820–7. [DOI] [PubMed] [Google Scholar]
- 5. Epstein JE, Paolino KM, Richie TL, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2017; 2:e89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conway DJ, Cavanagh DR, Tanabe K, et al. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat Med 2000; 6:689–92. [DOI] [PubMed] [Google Scholar]
- 7. Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med 1998; 4:358–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krishnarjuna B, Andrew D, MacRaild CA, et al. Strain-transcending immune response generated by chimeras of the malaria vaccine candidate merozoite surface protein 2. Sci Rep 2016; 6:20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drew DR, Hodder AN, Wilson DW, et al. Defining the antigenic diversity of Plasmodium falciparum apical membrane antigen 1 and the requirements for a multi-allele vaccine against malaria. PLoS One 2012; 7:e51023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dutta S, Dlugosz LS, Drew DR, et al. Overcoming antigenic diversity by enhancing the immunogenicity of conserved epitopes on the malaria vaccine candidate apical membrane antigen-1. PLoS Pathog 2013; 9:e1003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kusi KA, Faber BW, Thomas AW, Remarque EJ. Humoral immune response to mixed PfAMA1 alleles; multivalent PfAMA1 vaccines induce broad specificity. PLoS One 2009; 4:e8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Remarque EJ, Faber BW, Kocken CH, Thomas AW. A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect Immun 2008; 76:2660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. Malaria vaccine technology roadmap. http://www.who.int/immunization/topics/malaria/vaccine_roadmap/en/. Accessed 2 April 2018. [Google Scholar]
- 14. McCarthy JS, Marjason J, Elliott S, et al. A phase 1 trial of MSP2-C1, a blood-stage malaria vaccine containing 2 isoforms of MSP2 formulated with Montanide® ISA 720. PLoS One 2011; 6:e24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyle MJ, Langer C, Chan JA, et al. Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum. Infect Immun 2014; 82:924–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilson PR, Nebl T, Vukcevic D, et al. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics 2006; 5:1286–99. [DOI] [PubMed] [Google Scholar]
- 17. Osier FH, Feng G, Boyle MJ, et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 2014; 12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flueck C, Frank G, Smith T, et al. Evaluation of two long synthetic merozoite surface protein 2 peptides as malaria vaccine candidates. Vaccine 2009; 27:2653–61. [DOI] [PubMed] [Google Scholar]
- 19. Boyle MJ, Reiling L, Feng G, et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015; 42:580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fenton B, Clark JT, Khan CM, et al. Structural and antigenic polymorphism of the 35- to 48-kilodalton merozoite surface antigen (MSA-2) of the malaria parasite Plasmodium falciparum. Mol Cell Biol 1991; 11:963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smythe JA, Coppel RL, Day KP, et al. Structural diversity in the Plasmodium falciparum merozoite surface antigen 2. Proc Natl Acad Sci U S A 1991; 88:1751–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michon P, Cole-Tobian JL, Dabod E, et al. The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg 2007; 76:997–1008. [PMC free article] [PubMed] [Google Scholar]
- 23. Terheggen U, Drew DR, Hodder AN, et al. Limited antigenic diversity of Plasmodium falciparum apical membrane antigen 1 supports the development of effective multi-allele vaccines. BMC Med 2014; 12:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stanisic DI, Richards JS, McCallum FJ, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 2009; 77:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drew DR, Wilson DW, Elliott SR, et al. A novel approach to identifying patterns of human invasion-inhibitory antibodies guides the design of malaria vaccines incorporating polymorphic antigens. BMC Med 2016; 14:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adda CG, MacRaild CA, Reiling L, et al. Antigenic characterization of an intrinsically unstructured protein, Plasmodium falciparum merozoite surface protein 2. Infect Immun 2012; 80:4177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor RR, Smith DB, Robinson VJ, McBride JS, Riley EM. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect Immun 1995; 63:4382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCallum FJ, Persson KE, Fowkes FJ, et al. Differing rates of antibody acquisition to merozoite antigens in malaria: implications for immunity and surveillance. J Leukoc Biol 2017; 101:913–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanisic DI, Fowkes FJ, Koinari M, et al. Acquisition of antibodies against Plasmodium falciparum merozoites and malaria immunity in young children and the influence of age, force of infection, and magnitude of response. Infect Immun 2015; 83:646–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thera MA, Doumbo OK, Coulibaly D, et al. Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial. PLoS One 2008; 3:e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miura K, Zhou H, Muratova OV, et al. In immunization with Plasmodium falciparum apical membrane antigen 1, the specificity of antibodies depends on the species immunized. Infect Immun 2007; 75:5827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Angeletti D, Gibbs JS, Angel M, et al. Defining B cell immunodominance to viruses. Nat Immunol 2017; 18:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hill DL, Eriksson EM, Li Wai Suen CS, et al. Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLoS One 2013; 8:e74627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joos C, Marrama L, Polson HE, et al. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS One 2010; 5:e9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol 2015; 67:171–82. [DOI] [PubMed] [Google Scholar]
- 36. RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.